Abstract
There is increasing evidence that the cholinergic habenulo-interpeduncular pathway and the dopaminergic mesolimbic pathway may jointly mediate the reinforcing properties of addictive drugs. However, the effects of addictive drug on the functioning of the habenulo-interpeduncular pathway have not been well-characterized. Thus, several drugs of abuse (i.e., nicotine, cocaine, amphetamine) have been shown to alter the morphology of the habenulo-interpeduncular pathway, causing selective degeneration of the cholinergic neurons in this area. On the other hand, morphine was shown to alter the neurochemistry of the habenulo-interpeduncular pathway, inducing biphasic changes in acetylcholine release in the interpeduncular nucleus. In order to determine the effects of cocaine, amphetamine and nicotine on cholinergic neurotransmission in the habenulo-interpeduncular pathway, levels of acetylcholine were assessed during microdialysis in freely-moving rats. Nicotine (0.1 and 0.4 mg/kg s.c.) produced a dose-dependent decrease in extracellular levels of acetylcholine, while methamphetamine (1 and 4 mg/kg i.p.) produced an increase in acetylcholine release in the interpeduncular nucleus. Cocaine (5 and 20 mg/kg i.p.) produced a biphasic effect on extracellular acetylcholine release, i.e., a low dose enhanced the release of acetylcholine and a high dose decreased its release. These results suggest that the habenulo-intepeduncular pathway may be a common target for drugs of abuse and, by modulating the mesolimbic pathway, may mediate unique aspects of the rewarding effects of different drugs.
Keywords: acetylcholine, microdialysis, methamphetamine, nicotine, cocaine, interpeduncular nucleus, HPLC
1. Introduction
The habenulo-interpeduncular pathway is the largest cholinergic pathway in the brain, and it projects from the medial habenula (MHb) in the diencephalon to the interpeduncular nucleus (IPN) in the midbrain [1]. The habenulo-interpeduncular pathway was described by Blander and Wise (1989) as a distinct system supporting self-stimulation reward [2]. Subsequently, it has been shown to be important for mediation of drug reward [3]. This latter function of the habenulo-interpeduncular pathway involves α3β4 nicotinic receptors densely expressed in the medial habenula and the interpeduncular nucleus [3;4].
The habenulo-interpeduncular pathway was shown to be anatomically and functionally interrelated with the mesolimbic pathway [5]. The two pathways are thought to be parts of a brain reward circuitry [6], both contributing to the processing of neurochemical and reinforcing effects of addictive drugs. Consistent with this premise, the habenulo-interpeduncular pathway was previously shown to modulate the sensitized release of dopamine in the nucleus accumbens of morphine-treated rats [7]. This effect was mediated by α3β4 nicotinic receptors in the MHb and IPN. Morphine was also shown to have biphasic effects on acetylcholine levels in the IPN [8]. To explore the generality of this phenomenon, it is important to study changes in acetylcholine release in the IPN during exposure to other drugs of abuse.
The habenulo-interpeduncular pathway is a known target for nicotine and cocaine [9;10]. For example, both the MHb and the IPN have been demonstrated to have the highest levels of nicotine binding in the brain [11]. In addition, locomotor-depressant effects of nicotine have been shown to be attenuated by lesions of cholinergic terminals in the IPN in rats [10]. Furthermore, repeated cocaine injections have been shown to induce tolerance to the effects of cocaine in the MHb, as measured with 2-deoxyglucose autoradiography [9]. Although several models have been utilized to assess morphological changes in the habenulo-interpeduncular pathway in response to these drugs, their neurochemical effects in this pathway are not known [12;13]. Thus, in the present experiments, the effects of acute systemic administration of nicotine, methamphetamine and cocaine were assessed on the release of acetylcholine in the interpeduncular nucleus during microdialysis in freely-moving animals.
2. Methods
2.1 Animals
Naïve female Sprague-Dawley rats (Taconic, Germantown, NY), weighing 250–310 g, were housed individually and maintained on a normal 12:12-h light/dark cycle (light on 7 a.m., light off at 7 p.m.). Food and water were provided at libitum. All experiments were performed in accordance with the United States Public Health Service Guide for the Care and Use of Laboratory Animals and were approved by the Albany Medical College Institutional Animal Care and Use Committee.
2.2 Drugs
Nicotine hydrogen bitartrate, methamphetamine sulfate, and cocaine hydrochloride (Sigma, St. Louis, MO) were dissolved in sterile saline. Nicotine (0.1 and 0.4 mg/kg, free base) was administered subcutaneously (s.c.), while methamphetamine (1 and 4 mg/kg) and cocaine (5 and 20 mg/kg) were administered intraperitoneally (i.p.).
2.3 Stereotaxic surgery
The brain cannulation surgery was performed according to the previously described protocol [8]. Briefly, the animals were anesthetized with sodium pentobarbital (0.052mg/kg) and placed in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA). Body temperature was maintained with a heating pad. The rats were implanted unilaterally with microdialysis guide cannula (CMA Microdialysis, North Chelmsford, MA) such that when a dialysis probe(s) were inserted, the tip was located in the interpeduncular nucleus (in mm, AP=−6.3; ML=±2.5; DV=−9.2 using a 15° angle) [14]. Rats were allowed to recover from surgery for four to five days.
2.4 In vivo microdialysis
The microdialysis was conducted, as previously described by Taraschenko et al (2007), in an environmentally controlled room with a normal 12:12-h light/dark cycle. On the afternoon prior to the dialysis day, the animal was placed in a custom made Plexiglas chamber and a calibrated 1 mm-microdyalysis probe was inserted through the guide cannula into its IPN. The probe was then continuously perfused with an artificial cerebrospinal fluid (aCSF) containing 0.1 µM neostigmine at a rate of 1µl/min by means of an infusion pump (Harvard Apparatus, Holliston, MA). The aCSF consisted of 146 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, and 1.0 mM MgCl, pH=7.3. On the following day, fifteen 20-min samples (i.e., six baseline samples and nine post-injection samples) were collected. The samples were analyzed using high performance liquid chromatography (HPLC).
2.4 HPLC
The HLPC system consisted of a CMA200 autosampler, an ESA solvent delivery system and an ESA Coulochem II electrochemical detector. Chromatograms were analyzed with EZChrom Elite software (ESA). CSF samples were run after each sample to minimize the background contamination from the previous samples. The mobile phase containing 50mM NaH2PO4, 0.5 mM Na2EDTA and 50 µl of 0.005% Proclin, adjusted to pH=8.3 with 6N NaOH, was delivered at a flow rate 120 µl/min using a HPLC pump (ESA). After separation in the analytical column (UniJet 50x1 mm, BAS, West Lafayette, IN), acetylcholine was enzymatically converted to hydrogen peroxide in the miniature post-column enzyme reactor (BAS) and electrochemically detected by an ESA 5041 analytical cell containing peroxidase-redox polymer-coated glassy carbon target electrode maintained at a potential of −200mV.
2.5 Verification of the probe placement
Following the completion of each experiment, rats were euthanized and decapitated. The brains were frozen at − 80 °C using tissue freezing medium (TBS, Durham, NC) and brain sections of 20–30 µm were cut with a cryostat. Only the data from animals with probes located within the boundaries of the IPN were accepted for analysis.
2.6 Statistical Analysis
The basal levels of acetylcholine expressed in fmol/15µl of perfusate were analyzed using repeated measures analysis of variance (ANOVA) with treatment as a main factor and time as a repeated measure variable. Subsequent analysis was performed using percentage of mean baseline values using ANOVA with treatment as a main factor and time as a repeated measure followed by post-hoc comparison tests (Fisher LSD tests) when appropriate.
3. Results
3.1 Basal levels of extracellular acetylcholine in the IPN
The average basal levels of acetylcholine in the IPN of rats treated with nicotine, methamphetamine, cocaine and saline are shown in Table 1. The saline-treated group was comprised of rats that received either intraperitoneal injections (n=6) or subcutaneous injections (n=4) of saline. Since there were no significant differences between the responses in the two subgroups, they were combined in a single group for all further analyses (F14, 84 = 0.23, P>0.99). The average basal levels of acetylcholine in animals treated with either nicotine, methamphetamine or cocaine were not significantly different from those in saline-treated controls (for nicotine: F2,17 =0.45, P>0.60; for methamphetamine: F2,16=0.003, P>0.99; for cocaine: F2,14= 2.69, P>0.10).
Table 1.
Average basal levels of extracellular acetylcholine in the IPN of rats treated with nicotine, methamphetamine, cocaine or saline.
| Treatment | # of animals | Mean ± SEM (fmol/15ml) |
|---|---|---|
| Saline | 9 | 124.53 ± 14.26 |
| Nicotine 0.4mg/kg | 6 | 126.23 ± 30.08 |
| Nicotine 0.1mg/kg | 5 | 97.26 ± 14.6 |
| Methamphetamine 4mg/kg | 4 | 127.54 ± 46.45 |
| Methamphetamine 1mg/kg | 6 | 125.54 ± 20.22 |
| Cocaine 20mg/kg | 4 | 220.71 ± 54.05 |
| Cocaine 5mg/kg | 4 | 132.67 ± 17.04 |
3.2 Nicotine inhibits the release of acetylcholine in the IPN
As shown in Fig. 1, administration of nicotine to naïve animals produced a dose-dependent decrease of acetylcholine release in the IPN (Treatment effect: F2,17=4.75, P<0.02, Treatment × Time interaction: F28,238=1.51, P<0.05). The lower dose of nicotine (i.e., 0.1 mg/kg, s.c.) produced a transient decrease of acetylcholine release apparent at 20 and 60 minutes after injection; the reduction was maximal (73.4 ± 3.4 % of baseline) at 60 minutes after treatment. On the other hand, the higher dose of nicotine (i.e., 0.4 mg/kg, s.c.) significantly reduced acetylcholine release at 20, 60 and 80–160 min after injection. The maximum reduction of 62.1 ± 14.0 % of baseline was observed at 100 min after drug administration.
Figure 1.
Time-course of extracellular acetylcholine release (mean ± S.E.M.) in the interpeduncular nucleus of rats treated with nicotine. Zero point indicates injection of nicotine or saline. ‡, vs saline for both groups; #, vs saline for one group (P<0.05, LSD test).
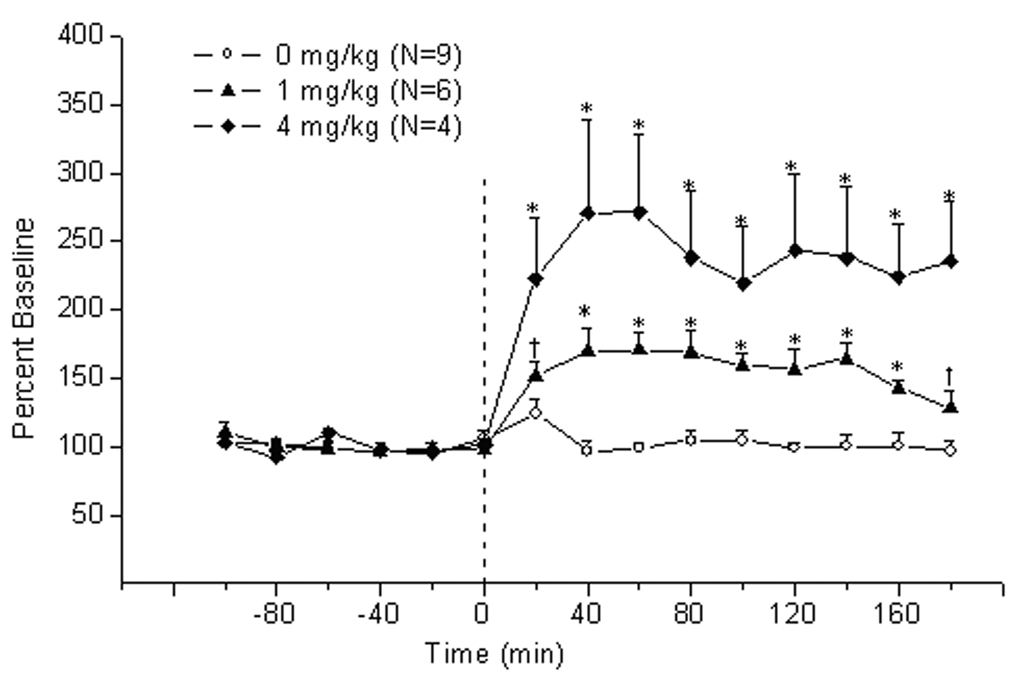
3.3 Methamphetamine enhances the release of acetylcholine in the IPN
As shown in Fig. 2, methamphetamine treatment produced a dose-dependent increase of extracellular acetylcholine levels in the IPN (Treatment effect: F2,16=12.24, P<0.006; Treatment × Time interaction F28,224=7.25, P<0.00001). Further analysis revealed that the effect of lower dose of methamphetamine (i.e., 1 mg/kg, i.p.) was apparent at 40–160 min after injection and was maximal (i.e., 170.6 ± 12.7 % of baseline) at 60 min post-treatment. The higher dose of the drug (i.e., 4 mg/kg, i.p.) increased the release of the acetylcholine at all time points after injection; a maximal increase of 270.9 ± 67.7% of baseline occurred at 40 min after treatment.
Figure 2.
Time-course of extracellular acetylcholine release (mean ± S.E.M.) in the interpeduncular nucleus of rats treated with methamphetamine. Zero point indicates injection of methamphetamine or saline.*, vs saline and the other group; †, vs other group (P<0.05, LSD test)
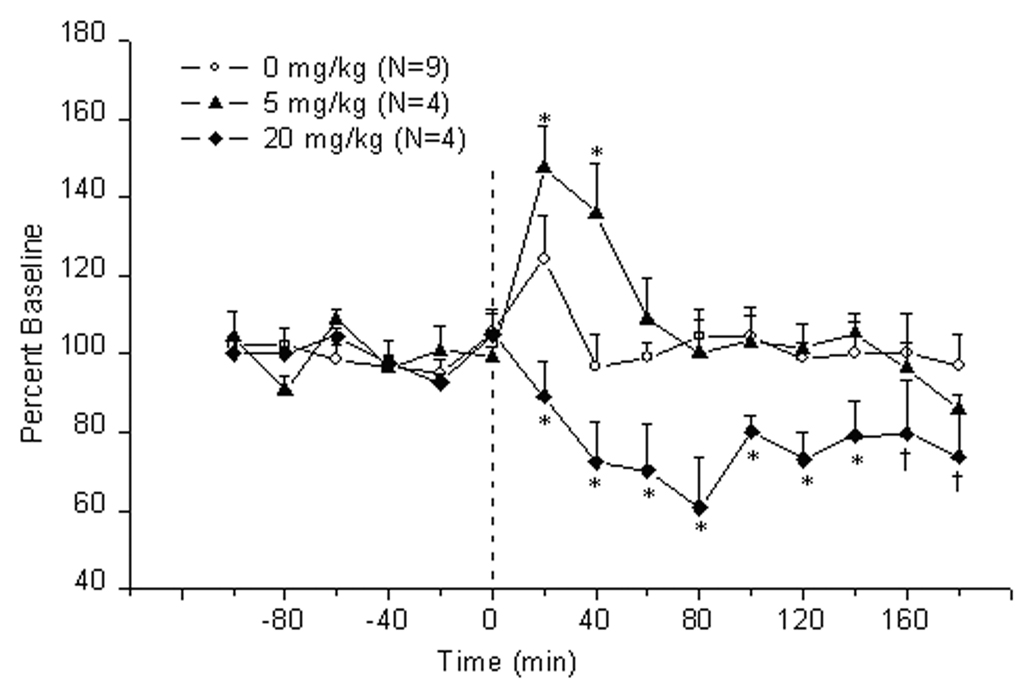
3.4. Cocaine produced a biphasic effect on acetylcholine release in the IPN
The effects of cocaine administration on release of acetylcholine in the IPN are shown in Fig. 3. Cocaine produced a biphasic effect on acetylcholine release (Treatment effect: F2,14=8.77, P<0.003; Treatment × Time interaction F28,196 =2.34, P<0.0003). Specifically, the lower dose of cocaine (i.e., 5 mg/kg, i.p.) produced a significant increase of acetylcholine levels at 20–40 min after injection. The maximal levels were 147.5 ± 10.5 % of baseline at 20 min post-treatment. In contrast, the higher dose of cocaine (i.e., 20 mg/kg, i.p.) decreased the release of acetylcholine; this effect was significant at all time points and was maximal (i.e., 60.9 ± 12.5% of baseline) at 80 min following injection.
Figure 3.
Time-course of extracellular acetylcholine release (mean ± S.E.M.) in the interpeduncular nucleus of rats treated with cocaine. Zero point indicates injection of cocaine or saline.*, vs saline and the other group; †, vs saline (P<0.05, LSD test)
4. Discussion
The present study demonstrates that nicotine (0.1 and 0.4 mg/kg, s.c.) and the higher dose of cocaine (20 mg/kg, i.p.) decreased acetylcholine release in the IPN, and that methamphetamine (1 and 4 mg/kg, i.p.) and the lower dose of cocaine (5 mg/kg, i.p.) increased the release of acetylcholine in the IPN. While our data are consistent with the premise that the habenulo-interpeduncular pathway is a common target of drugs of abuse, more work is needed to determine whether such involvement is direct or indirect, e.g., mediated by connections with the mesolimbic pathway.
In the medial habenula, the majority of nicotinic receptors (predominantly of the α3β4 subtype) are located on cholinergic cells; nicotinic receptor-mediated activation of the latter cells and the consequent increase of acetylcholine release have been demonstrated in vitro[4;15]. Likewise, acetylcholine release in the IPN could be mediated by presynaptic α3β4 nicotinic receptors located on the axons of cholinergic habenular afferents [16]. By acting on receptors in the MHb and/or the IPN, nicotine was expected to increase the release of acetylcholine from axonal terminals [16]. The demonstrated decrease of acetylcholine release in the present study could be due to nicotine’s predominant action on GABA-ergic terminals within the MHb [17]. By analogy with other brain areas, presynaptic nicotinic receptors located on GABA-ergic terminals in the MHb are likely to have an α4β2 composition; their activation would lead to an increase of GABA release from axonal terminals [cf. [18]]. Subsequent postsynaptic inhibition of the habenular cells could cause a decrease of acetylcholine release in the IPN. The dose-dependent decrease of acetylcholine release is consistent with nicotine’s relatively lower potency at α4β2 receptors than at α3β4 receptors [cf. [18]]. An alternative mechanism explaining the effect of nicotine on acetylcholine release in the IPN could involve projections from the nucleus accumbens to the MHb, which are presumably GABA-ergic [19]. It is therefore conceivable that activation of the mesolimbic pathway in response to nicotine can occur in parallel with reduced neurotransmission in the habenulo-interpeduncular pathway. The nicotine-induced decrease in acetylcholine release in the IPN way reflect a feedback effect of mesolimbic activation, perhaps contributing to the nicotine’s incentive value; pharmacological interference with this feedback might reduce nicotine’s reinforcing efficacy.
The mechanism of the methamphetamine-induced increase in acetylcholine release in the IPN may involve dopaminergic projections from the mesolimbic system (i.e., VTA) to the MHb [20]. Methamphetamine-induced activation of dopamine transmission in the mesolimbic system is well-characterized [21]. By increasing dopamine release from terminals in the MHb, methamphetamine could induce excitation of cholinergic cells projecting to the IPN, causing the increase of acetylcholine release in the IPN. Although the co-expression of dopamine and nicotinic receptors has never been described for habenular cells, joint functionality of presynaptic D2 receptors and nicotinic heteroreceptors has been observed in dopaminergic terminals in the striatum [22]. It thus appears that the methamphetamine-induced activation of the mesolimbic pathway occurs in parallel with its activation of the habenulo-intepeducular pathway. Two pathways may interact when methamphetamine is given such that pharmacological manipulations that reduce the output of the IPN onto the VTA or NAC could lead to a reduced rewarding effect of methamphetamine. Consistent with this idea, recent data have shown that local administration of α3β4 nicotinic antagonist in the IPN reduces methamphetamine self-administration (Glick et al., unpublished results).
Cocaine acts by binding to the monoamine uptake transporter and blocking it, causing increases in extracellular levels of dopamine [23]. Although the effects of cocaine on central cholinergic neurotransmission are largely mediated by dopamine [24], direct effects of cocaine on acetylcholine have also been described [cf. [25]]. Acetylcholine and muscarinic cholinergic receptors in the VTA has been shown to be involved in mediating the reinforcing properties of cocaine [26]. In the nucleus accumbens, increased levels of extracellular acetylcholine were observed in rats self-administering cocaine; this effect was thought to be mediated by dopamine [27]. The biphasic changes in acetylcholine release in the IPN demonstrated in the present study may involve complex interactions of dopamine (arriving from the VTA) and GABA (arriving from the septal area and the NAC) in the MHb [17;20]. Thus, it is conceivable that the lower dose of cocaine preferentially increases dopamine release in the MHb, causing excitation of the habenular cells and increases in acetylcholine in the IPN. On the other hand, the higher dose of cocaine could increase both dopamine and GABA release in the MHb. Thus, if the latter neurotransmitter prevailed, there would be an overall inhibitory tone in the MHb, resulting in decreased acetylcholine release in the IPN. Finally, it is possible that cocaine could directly affect either nicotinic or muscarinic receptors on cholinergic terminals in the IPN, and depending on the dose, produce either increased or decreased acetylcholine release. A similar switch from nicotinic receptor-mediated facilitation to muscarinic receptor-mediated inhibition of glutamate release in the IPN has previously been demonstrated with nicotine treatment [28]. Although no data exist on direct effects of cocaine on central nicotinic receptor subtypes, cocaine-induced blockade of peripheral nicotinic receptors has been previously described [29].
Some comment about the differential effects of the two stimulants (i.e., cocaine and methamphetamine) on acetylcholine release in the IPN should be noted. Although the two drugs have similar effects on monoamine transmission, they can differentially modulate GABAergic transmission in the brain. Thus, the anticonvulsants gabapentin and valproate, which are both known to enhance GABAergic function in the brain, have been shown to attenuate locomotor sensitization to methamphetamine, but not to cocaine [30;31]. An alternative explanation for the different effects of methamphetamine and cocaine could involve the direct effect of cocaine on cholinergic receptors [cf.[25]]; such a mechanism has never been reported for methamphetamine.
In summary, the results from the present study suggest that acetylcholine release in the habenulo-interpeduncular pathway can be altered by acute injections of nicotine, methamphetamine and cocaine. Such alterations may be due to either direct actions of the drugs on the latter pathway or to indirect modulation by the mesolimbic pathway. These mechanisms, as well as overall role of the habenulo-interpeduncular pathway in addiction, obviously warrant further studies.
Acknowledgements
This research was supported by NIDA Grant DA 016283
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci. Biobehav. Rev. 1982;6:1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- 2.Blander A, Wise RA. Anatomical mapping of brain stimulation reward sites in the anterior hypothalamic area: special attention to the stria medullaris. Brain Res. 1989;483:12–16. doi: 10.1016/0006-8993(89)90029-2. [DOI] [PubMed] [Google Scholar]
- 3.Glick SD, Ramirez RL, Livi JM, Maisonneuve IM. 18-Methoxycoronaridine acts in the medial habenula and/or interpeduncular nucleus to decrease morphine self-administration in rats. Eur. J. Pharmacol. 2006;537:94–98. doi: 10.1016/j.ejphar.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 4.Quick MW, Ceballos RM, Kasten M, McIntosh JM, Lester RA. Alpha3beta4 subunit-containing nicotinic receptors dominate function in rat medial habenula neurons. Neuropharmacology. 1999;38:769–783. doi: 10.1016/s0028-3908(99)00024-6. [DOI] [PubMed] [Google Scholar]
- 5.Nishikawa T, Fage D, Scatton B. Evidence for, and nature of, the tonic inhibitory influence of habenulointerpeduncular pathways upon cerebral dopaminergic transmission in the rat. Brain Res. 1986;373:324–336. doi: 10.1016/0006-8993(86)90347-1. [DOI] [PubMed] [Google Scholar]
- 6.Ellison G. Neural degeneration following chronic stimulant abuse reveals a weak link in brain, fasciculus retroflexus, implying the loss of forebrain control circuitry. Eur. Neuropsychopharmacol. 2002;12:287–297. doi: 10.1016/s0924-977x(02)00020-2. [DOI] [PubMed] [Google Scholar]
- 7.Taraschenko OD, Shulan JM, Maisonneuve IM, Glick SD. 18-MC acts in the medial habenula and interpeduncular nucleus to attenuate dopamine sensitization to morphine in the nucleus accumbens. Synapse. 2007;61:547–560. doi: 10.1002/syn.20396. [DOI] [PubMed] [Google Scholar]
- 8.Taraschenko OD, Rubbinaccio HY, Shulan JM, Glick SD, Maisonneuve IM. Morphine-induced changes in acetylcholine release in the interpeduncular nucleus and relationship to changes in motor behavior in rats. Neuropharmacology. 2007;53:18–26. doi: 10.1016/j.neuropharm.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammer RP, Jr, Cooke ES. Gradual tolerance of metabolic activity is produced in mesolimbic regions by chronic cocaine treatment, while subsequent cocaine challenge activates extrapyramidal regions of rat brain. J. Neurosci. 1994;14:4289–4298. doi: 10.1523/JNEUROSCI.14-07-04289.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hentall ID, Gollapudi L. The interpeduncular nucleus regulates nicotine's effects on free-field activity. Neuroreport. 1995;6:2469–2472. doi: 10.1097/00001756-199512150-00008. [DOI] [PubMed] [Google Scholar]
- 11.Mugnaini M, Tessari M, Tarter G, Merlo PE, Chiamulera C, Bunnemann B. Upregulation of [3H]methyllycaconitine binding sites following continuous infusion of nicotine, without changes of alpha7 or alpha6 subunit mRNA: an autoradiography and in situ hybridization study in rat brain. Eur. J. Neurosci. 2002;16:1633–1646. doi: 10.1046/j.1460-9568.2002.02220.x. [DOI] [PubMed] [Google Scholar]
- 12.Carlson J, Noguchi K, Ellison G. Nicotine produces selective degeneration in the medial habenula and fasciculus retroflexus. Brain Res. 2001;906:127–134. doi: 10.1016/s0006-8993(01)02570-7. [DOI] [PubMed] [Google Scholar]
- 13.Ellison G. Continuous amphetamine and cocaine have similar neurotoxic effects in lateral habenular nucleus and fasciculus retroflexus. Brain Res. 1992;598:353–356. doi: 10.1016/0006-8993(92)90207-p. [DOI] [PubMed] [Google Scholar]
- 14.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego, California: Academic Press Inc.; 1986. [Google Scholar]
- 15.McCormick DA, Prince DA. Acetylcholine causes rapid nicotinic excitation in the medial habenular nucleus of guinea pig, in vitro. J. Neurosci. 1987;7:742–752. doi: 10.1523/JNEUROSCI.07-03-00742.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grady SR, Meinerz NM, Cao J, Reynolds AM, Picciotto MR, Changeux JP, McIntosh JM, Marks MJ, Collins AC. Nicotinic agonists stimulate acetylcholine release from mouse interpeduncular nucleus: a function mediated by a different nAChR than dopamine release from striatum. J. Neurochem. 2001;76:258–268. doi: 10.1046/j.1471-4159.2001.00019.x. [DOI] [PubMed] [Google Scholar]
- 17.Contestabile A, Fonnum F. Cholinergic and GABAergic forebrain projections to the habenula and nucleus interpeduncularis: surgical and kainic acid lesions. Brain Res. 1983;275:287–297. doi: 10.1016/0006-8993(83)90989-7. [DOI] [PubMed] [Google Scholar]
- 18.Vizi ES, Lendvai B. Modulatory role of presynaptic nicotinic receptors in synaptic and non-synaptic chemical communication in the central nervous system. Brain Res. Brain Res. Rev. 1999;30:219–235. doi: 10.1016/s0165-0173(99)00016-8. [DOI] [PubMed] [Google Scholar]
- 19.Powell EW, Leman RB. Connections of the nucleus accumbens. Brain Res. 1976;105:389–403. doi: 10.1016/0006-8993(76)90589-8. [DOI] [PubMed] [Google Scholar]
- 20.Phillipson OT, Pycock CJ. Dopamine neurones of the ventral tegmentum project to both medial and lateral habenula. Some implications for habenular function. Exp. Brain Res. 1982;45:89–94. doi: 10.1007/BF00235766. [DOI] [PubMed] [Google Scholar]
- 21.Izawa J, Yamanashi K, Asakura T, Misu Y, Goshima Y. Differential effects of methamphetamine and cocaine on behavior and extracellular levels of dopamine and 3,4-dihydroxyphenylalanine in the nucleus accumbens of conscious rats. Eur. J. Pharmacol. 2006;549:84–90. doi: 10.1016/j.ejphar.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 22.Quarta D, Ciruela F, Patkar K, Borycz J, Solinas M, Lluis C, Franco R, Wise RA, Goldberg SR, Hope BT, Woods AS, Ferre S. Heteromeric nicotinic acetylcholine-dopamine autoreceptor complexes modulate striatal dopamine release. Neuropsychopharmacology. 2007;32:35–42. doi: 10.1038/sj.npp.1301103. [DOI] [PubMed] [Google Scholar]
- 23.Feldman R, Meyer J, Quenzer L. Principles of Neuropsychopharmacology. Sunderland, Massachusetts: Sinauer Associates, Inc., Publishers; 1997. Stimulants: Amphetamine and Cocaine; pp. 549–590. [Google Scholar]
- 24.Consolo S, Caltavuturo C, Colli E, Recchia M, Di CG. Different sensitivity of in vivo acetylcholine transmission to D1 receptor stimulation in shell and core of nucleus accumbens. Neuroscience. 1999;89:1209–1217. doi: 10.1016/s0306-4522(98)00309-1. [DOI] [PubMed] [Google Scholar]
- 25.Williams MJ, Adinoff B. The Role of Acetylcholine in Cocaine Addiction. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fink-Jensen A, Fedorova I, Wortwein G, Woldbye DP, Rasmussen T, Thomsen M, Bolwig TG, Knitowski KM, McKinzie DL, Yamada M, Wess J, Basile A. Role for M5 muscarinic acetylcholine receptors in cocaine addiction. J. Neurosci. Res. 2003;74:91–96. doi: 10.1002/jnr.10728. [DOI] [PubMed] [Google Scholar]
- 27.Mark GP, Hajnal A, Kinney AE, Keys AS. Self-administration of cocaine increases the release of acetylcholine to a greater extent than response-independent cocaine in the nucleus accumbens of rats. Psychopharmacology (Berl) 1999;143:47–53. doi: 10.1007/s002130050918. [DOI] [PubMed] [Google Scholar]
- 28.Girod R, Role LW. Long-lasting enhancement of glutamatergic synaptic transmission by acetylcholine contrasts with response adaptation after exposure to low-level nicotine. J. Neurosci. 2001;21:5182–5190. doi: 10.1523/JNEUROSCI.21-14-05182.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swanson KL, Albuquerque EX. Nicotinic acetylcholine receptor ion channel blockade by cocaine: the mechanism of synaptic action. J. Pharmacol. Exp. Ther. 1987;243:1202–1210. [PubMed] [Google Scholar]
- 30.Li JX, Han R, Deng YP, Chen SQ, Liang JH. Different effects of valproate on methamphetamine- and cocaine-induced behavioral sensitization in mice. Behav. Brain Res. 2005;161:125–132. doi: 10.1016/j.bbr.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Itzhak Y, Martin JL. Effect of riluzole and gabapentin on cocaine- and methamphetamine-induced behavioral sensitization in mice. Psychopharmacology (Berl) 2000;151:226–233. doi: 10.1007/s002130000394. [DOI] [PubMed] [Google Scholar]