Abstract
Purpose
In humans, apolipoprotein E (apoE) is encoded by three major alleles (ε2, ε3, and ε4) and, compared to apoE3, apoE4 increases the risk of developing Alzheimer disease and cognitive impairments following various environmental challenges. Exposure to irradiation, including that of 56Fe, during space missions poses a significant risk to the central nervous system, and apoE isoform might modulate this risk.
Methods and Materials
We investigated whether apoE isoform modulates hippocampus-dependent cognitive performance starting 2 weeks after 56Fe irradiation. Changes in reactive oxygen species (ROS) can affect cognition and are induced by irradiation. Therefore, after cognitive testing, we assessed hippocampal ROS levels in ex vivo brain slices, using the ROS-sensitive fluorescent probe, dihydroethidium (DHE). Brain levels of 3-nitrotyrosine (3-NT), CuZn superoxide dismutase (CuZnSOD), extracellular SOD, and apoE were assessed using Western blotting analysis.
Results
In the water maze, spatial memory retention was impaired by irradiation in apoE2 and apoE4 mice but enhanced by irradiation in apoE3 mice. Irradiation reduced DHE-oxidation levels in the enclosed blade of the dentate gyrus and levels of 3-NT and CuZnSOD in apoE2 but not apoE3 or apoE4 mice. Finally, irradiation increased apoE levels in apoE3 but not apoE2 or apoE4 mice.
Conclusions
The short-term effects of 56Fe irradiation on hippocampal ROS levels and hippocampus-dependent spatial memory retention are apoE isoform-dependent.
Keywords: ApoE, Irradiation, ROS
Introduction
Exposure to radiation, including that of 56Fe, during space missions poses a significant risk to the central nervous system. In rodents, 56Fe irradiation affects cognitive function 3 months or longer following exposure (1). In contrast to that study, few studies have examined potential short-term effects of irradiation on brain function (2).
In the brain, apolipoprotein E (apoE) plays an important role in transport and metabolism of lipids and neuronal repair following injury. In humans, three different alleles encode the apoE gene: ε2, ε3, and ε4. Compared to apoE3, apoE4 increases the risk of developing Alzheimer disease and is associated with worse neurological outcome following various environmental challenges (3). Mice expressing different human apoE isoforms also show effects of apoE2 and apoE4 compared to those of apoE3. ApoE might also be important in modulating the effects of 56Fe irradiation on cognition. Mice lacking apoE are more susceptible than wild-type mice to the effects of 56Fe irradiation on hippocampus-dependent cognitive performance (4). In addition, the effects of 56Fe irradiation on hippocampus-dependent cognition at 13 months after irradiation are dependent on apoE isoform (5). ApoE isoform might also modulate short-term effects of 56Fe on cognition. Brain levels of apoE are apoE isoform-dependent (6), and 56Fe irradiation might also affect apoE levels in an apoE isoform-dependent fashion.
Irradiation can increase brain levels of reactive oxygen species (ROS), such as superoxide, and levels of oxidative stress such as 3-nitrotyrosine (3-NT) (7), a marker of oxidized protein. Oxidative stress can affect tissue by damaging DNA and proteins (8). The antioxidant enzyme superoxide dismutase (SOD) has a critical role in the defense against increases in superoxide (9). There are three isoforms of SOD: SOD1 or CuZnSOD, located in the cytoplasm; SOD2 or MnSOD, located in the mitochondria; and SOD3 or extracellular-SOD (EC-SOD). Mice lacking isoforms of SOD support a role for SOD in modulating cognitive effects of irradiation on brain function (10).
In this study, we assessed short-term effects of 56Fe irradiation in mice expressing human apoE2, apoE3, or apoE4. Mice were cognitively tested starting at 2 to 4 weeks after irradiation. Hippocampal ROS levels were assessed using a novel imaging technique in ex vivo brain slices of cognitively tested mice. Finally, brain levels of 3-NT, CuZnSOD, EC-SOD, and apoE in the tested mice were assessed using Western blot analysis.
Methods and Materials
Animals
Male human apoE2-, apoE3-, and apoE4-targeted replacement mice expressing human apoE under the control of mouse apoE promoter on the C57Bl/6J background were provided for breeding by Dr. Patrick Sullivan (see supplemental application EMMC1 for detailed information). Mice (n = 48) were bred and housed in a 12-h light:12-h dark cycle with food (PicoLab Rodent Diet 20, product no. 5053; PMI Nutrition International, St. Louis, MO) and water provided ad libitum.
56Fe Irradiation
Mice were shipped from Oregon Health and Science University (OHSU) to Brookhaven National Laboratories (BNL) in Upton, NY. After a 1-week acclimation period, mice were either shamirradiated (n = 8 mice per genotype) or received whole-body irradiation (n = 8 mice per genotype) with a 0.5-Gy dose of 56Fe particles at 600 MeV/n. One week after irradiation, the mice were shipped back to OHSU. All procedures were approved by the Institution Animal Care and Use Committee at OHSU and BNL.
Behavioral testing
Behavioral testing began 1 week after mice arrived at OHSU, which was 2 weeks postirradiation. Mice were housed singly starting 3 days before the testing. In the first week, mice were tested for novel object recognition (Days 1–3) and fear conditioning (Days 4–5). In the second week, mice were tested using the water maze test (Days 6–10). Behavioral testing was performed as described in the online supplement material.
Western blot analysis
Mice were killed by cervical dislocation, and their brains were removed. One hemibrain was frozen in liquid nitrogen for Western blot analysis (6), and the other hemibrain was used for ROS analysis using the fluorescent superoxide-sensitive probe dihydroethidium (DHE) as described in the supplemental material. DHE is a fluorescent dye able to detect intracellular and extracellular superoxide and is commonly used for in vitro and in vivo analysis of superoxide.
Primary antibodies against 3-NT (raised in mouse, 1 μg/ml; Millipore, Billerica, MA), apoE (raised in goat, 1:4,000 dilution; Calibiochem, Gibbstown, NJ), CuZnSOD (raised in rabbit, 1:2,000 dilution; LabFrontier), EC-SOD (raised in rabbit, 1 μg/ml, custom made for and graciously provided by T.T. Huang), MnSOD (raised in rabbit, 1:2,000 dilution; Stressgen, Plymouth Meeting, PA), or β-actin antibody (raised in mouse, 0.5 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA) were used. Secondary antibodies were raised against the primary antibody species (donkey anti-mouse-horseradish peroxidase [HRP], 1 μg/ml; donkey anti-rabbit-HRP, 1 μg/ml; or donkey anti-goat HRP, 1 μg/ml [Santa Cruz Biotechnology]) in addition to a secondary antibody for the protein standards (5 μl of Precision protein StrepTactin-HRP [Bio-Rad]).
Statistical analysis
Statistical analysis was performed as described in the online supplemental material.
Results
Novel object recognition
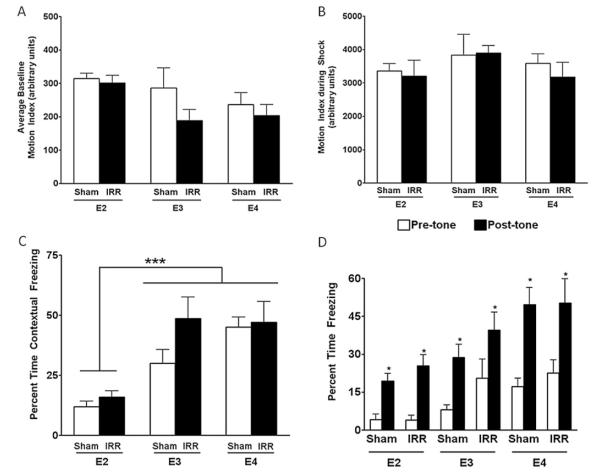
No differences were observed between sham and irradiated mice in the amount of time spent exploring two identical objects on Day 2 (not shown). On Day 3, all groups of mice spent significantly more total time (Fig. 1A) and a fraction of time (Fig. 1B) exploring the novel object compared to the familiar object (p < 0.0001), but there was no effect of genotype or irradiation.
Fig. 1.
Novel object recognition of sham-irradiated and irradiated mice. The total time (sec) (A) and percentage of time exploring the novel object (B) are shown. n = 8 mice/genotype/treatment. ***, p < 0.001 versus familiar object.
Fear conditioning
There was an effect of apoE but not irradiation on the baseline motion index (p = 0.03) (Fig. 2A). ApoE2 mice had higher baseline motion indices than apoE4 mice. Genotype or irradiation did not affect the motion index during the shock (p = 1.0) (Fig. 2B). However, there was an effect of genotype on contextual freezing (p < 0.001) (Fig. 2C) and a trend toward a genotype X irradiation interaction (p = 0.09) (Fig. 2C). In cued fear conditioning, there was a genotype X trial interaction (p = 0.03) (Fig. 2D) but no significant genotype x irradiation interaction. In particular, apoE2 mice spent significantly less time freezing than apoE4 mice.
Fig. 2.
(A) Contextual and cued fear conditioning of sham-irradiated and irradiated mice. (A) Baseline motion index. (B) Motion index during the shock. (C) Contextual fear conditioning. (D) Cued fear conditioning task. n = 8 mice/genotype/treatment. ***, p < 0.001 compared to apoE3 and apoE4 mice; *, p < 0.05 versus the time prior to the tone (pre-tone) during the training was paired with the shock.
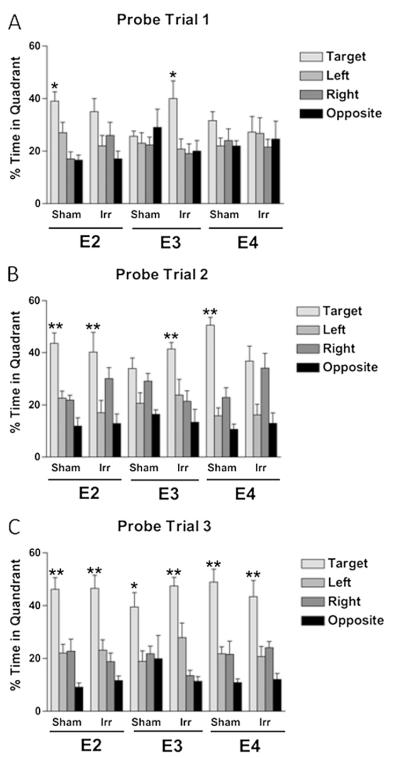
Water maze
There was no effect of genotype or irradiation on ability to locate the visible or hidden platform locations (data not shown). However, when spatial memory retention was assessed in the probe trials, group differences were observed (Fig. 3). In the first probe trial (Fig. 3A), sham-irradiated apoE2 mice showed spatial memory retention, but irradiated apoE2 mice did not. In contrast, while sham-irradiated apoE3 mice did not show spatial memory retention, irradiated apoE3 mice did. Neither sham-irradiated nor irradiated apoE4 mice showed spatial memory retention in the first probe trial. Following an additional day of training, in the second probe trial (Fig. 3B), both sham-irradiated and irradiated apoE2 mice showed spatial memory retention. The pattern in apoE3 mice was the same as that seen in the first probe trial; while sham-irradiated apoE3 mice did not show spatial memory retention, irradiated apoE3 mice did. Sham-irradiated apoE4 mice showed spatial memory retention, but irradiated apoE4 mice did not. Following an additional day of training, in the third probe trial (Fig. 3C), all groups of mice showed a preference for the target quadrant. Together, these data show that irradiation impaired spatial memory retention in apoE2 and apoE4 mice but enhanced spatial memory retention in apoE3 mice.
Fig. 3.
(A) First (A), second (B), and third (C) water maze probe trials. n = 8 mice/genotype/treatment. *, p < 0.05; **, p < 0.01.
Western blot analysis
There was an effect of genotype on apoE isoform levels (p = 0.02) (Fig. 4A). ApoE2 mice had higher brain levels of apoE than apoE3 and apoE4 mice. Interestingly, in apoE3 but not apoE2 or apoE4 mice, there was a trend toward higher apoE levels following irradiation (p = 0.058).
Fig. 4.
Levels of brain apoE (A), 3-NT (B), EC-SOD (C), and CuZnSOD (D) in sham-irradiated and irradiated mice. The 125-kDa 3-NT, 75-kDa CuZnSOD, and 19-kDa EC-SOD bands were used for quantification. The gel lanes match the order of the bar graph. β-Actin was the loading control (not shown). *, p < 0.05; **, p = 0.01 compared to apoE2 irradiated mice.
For 3-NT levels, there was a genotype X irradiation interaction (p = 0.03) (Fig. 4B). ApoE2 sham-irradiated mice had more 3-NT than apoE2 irradiated mice (p = 0.01). Irradiation reduced 3-NT levels in apoE2 but not apoE3 or apoE4 mice. A similar pattern was seen for CuZnSOD levels. There was an effect of genotype X irradiation interaction (p = 0.02) and an effect of irradiation (p = 0.04) for CuZnSOD levels (Fig. 4D). ApoE2 sham-irradiated mice had higher levels of CuZnSOD than irradiated apoE2 mice. Although the pattern was the same, there was no significant effect of genotype or irradiation for EC-SOD levels (Fig. 4C) and only a trend toward an effect of genotype (p = 0.06) and a genotype X irradiation interaction (p = 0.07) for MnSOD levels (data not shown).
Ex vivo analysis of DHE oxidation
There was a significant four-way interaction between time, genotype, irradiation treatment, and hippocampal region for DHE oxidation [F (9,75) = 2.1; p < 0.05]. There was a genotype X irradiation X hippocampal region interaction for the 16-min time point [F (6,76) = 2.5; p < 0.05] but not for the 2- or 8-min time point (not shown) (Fig. 5 shows representative images; Fig. 6A shows a diagram of selected brain regions; Fig. 6B shows quantitative data). At earlier time points, potential group differences might not be revealed because the signal that is based on the accumulation of the stable byproducts of oxidized DHE might be too weak. There was an effect of irradiation in the enclosed blade of the hippocampal dentate gyrus in apoE2 mice (p < 0.05, Student’s t-test) (Fig. 6B); compared to sham-irradiated apoE2 mice, irradiated apoE2 mice had reduced levels of DHE oxidation. In contrast, a trend toward an increase in levels of DHE oxidation following irradiation was seen in the enclosed blade of the dentate gyrus of apoE3 and apoE4 mice. Although not significant, this trend was also observed in the other hippocampal regions of apoE2 mice. In the CA3 region of the hippocampus of apoE3 and apoE4 mice and in the free blade of apoE3 mice, there was also a trend toward decreased levels of DHE oxidation following irradiation.
Fig. 5.
Representative images of DHE oxidation of sham-irradiated (A, C, and E) and irradiated (B, D, and F) mice 16 min after exposure to DHE.
Fig. 6.
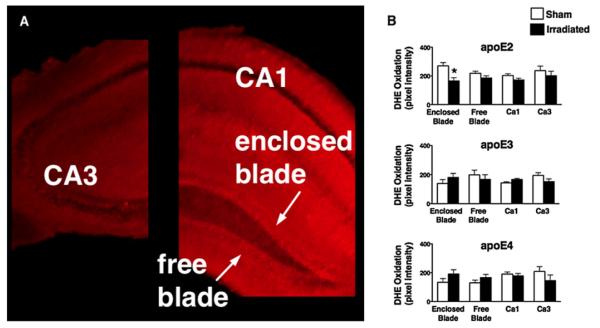
(A) Diagram of hippocampal regions used for DHE analysis. (B) ApoE isoform-dependent effects of irradiation on hippocampal superoxide levels assessed by fluorescent imaging of DHE oxidation. *, p < 0.05. n = 3 to 4 slices per genotype per treatment and region.
Discussion
In this study, we demonstrated apoE isoform-dependent short-term effects of 56Fe irradiation on hippocampal function 2 weeks after the irradiation. While irradiation impaired spatial memory retention in apoE2 and apoE4 mice, it enhanced spatial memory retention in apoE3 mice. In apoE3 mice, the enhanced spatial memory retention following irradiation was associated with higher apoE levels. This might be related to the role of apoE in neuronal repair and involve enhanced secretion of apoE from radiation-activated glia (11) and enhanced signaling involving neuronal apoE receptors. In apoE2 mice, the reduced spatial memory retention following irradiation was associated with lower levels of 3-NT and CuZnSOD levels.
Of the cognitive tests used in this study, the water maze was particularly sensitive for detecting detrimental apoE isoform-dependent hippocampus-dependent effects of 56Fe irradiation on memory retention. Consistent with these data, the water maze test was also sensitive to detection of apoE isoform-dependent effects of apoE at 3 months following 137Cs irradiation (10 Gy; dose rate, 24 Gy/min; 12 or 13 months following 56Fe irradiation) (5).
In contrast to the water maze test, whole-body 56Fe irradiation did not impair contextual fear conditioning in apoE2 and apoE4 mice. Consistent with the water maze probe trial data, there was a trend toward enhanced contextual fear conditioning in apoE3 mice. Consistent with the data for apoE3 mice, brain-only 56Fe irradiation enhanced contextual fear conditioning in C57Bl6/J wild-type mice (13). The direction of the effects of irradiation on contextual fear conditioning might depend on the type of irradiation. In contrast to brain-only 56Fe irradiation, brain-only 137Cs irradiation impaired contextual fear conditioning in C57Bl6/J wild-type mice (10).
The direction of the effects of different apoE isoforms on cognitive performance in the water maze and fear conditioning tests also showed opposite patterns. While sham-irradiated apoE2 mice showed spatial memory retention in the first probe trial following one day of hidden platform training, sham-irradiated apoE3 and apoE4 mice required an additional day of training to show spatial memory retention. In contrast to their responses to the water maze, apoE2 mice showed less contextual fear conditioning than apoE3 and apoE4 mice. While both tests are hippocampus-dependent, differences in additional circuitry involved in the two tests might have contributed to these divergent findings.
Differences in levels of ROS, 3-NT, and SOD isoforms in human apoE mice under baseline conditions and following 56Fe irradiation might have contributed to the paradoxical apoE isoform-dependent effects of 56Fe irradiation on cognitive performance in the water maze and contextual fear conditioning tests. Sham-irradiated apoE2 had higher levels of ROS, 3-NT, and CuZnSOD than sham-irradiated apoE3 and apoE4 mice. In addition, irradiation reduced levels of superoxide, 3-NT, and CuZnSOD only in apoE2 mice. ApoE2 mice might be more susceptible to radiation-induced cognitive impairment due to their higher levels of CuZnSOD prior to radiation. Consistent with this notion, wild-type mice that show lower levels of oxidative stress than mice lacking EC-SOD are more susceptible to radiation-induced cognitive impairments (14). The data that both apoE2 and apoE4 mice showed radiation-induced cognitive impairment whereas irradiation reduced levels of ROS, 3-NT, and CuZnSOD only in apoE2 mice indicate that other measures of oxidative stress might contribute to the cognitive impairments of apoE4 mice. As type III hyperlipoproteinemia is found mainly in homozygous apoE2 carriers and is associated with hyperinsulinemia (15) while apoE4 is strongly associated with increased risk for developing Alzheimer disease (3), these results suggest that the effects on measures of oxidative stress seen in this study might relate more to alterations in lipid metabolism pathways than alterations in other pathways pertinent to Alzheimer disease. We recognize that the detection of ROS using DHE might include products other than superoxide, including hydrogen peroxide, that also play important roles in hippocampus-dependent cognition (16).
The complex relationship between ROS and hippocampus-dependent cognition might be related to the dual role of ROS in the brain, having both positive and negative effects on cognitive performance (12). The SOD isoform might play a role as well. The effects of irradiation on CuZnSOD in apoE2 mice were pronounced. Interestingly, compared to age-matched wild-type mice, 13-month-old mice lacking apoE (Apoe−/−) show reduced hippocampal CuZnSOD levels, while a trend toward reduced levels in 3-month-old mice has been observed (17). Four-month-old Apoe−/− mice also show decreased hippocampal long-term potentiation (LTP) (18) and enhanced oxidative stress following folic acid deficiency (19). The effects of irradiation in apoE2 mice resemble some effects seen in naive Apoe−/− mice, further supporting a role for ROS in the radiation response. CuZnSOD modulates hippocampal neurogenesis (13), and LTP (14) and changes in hippocampal LTP might have contributed to the apoE isoform-dependent effects of 56Fe irradiation on cognitive performance. Based on the relatively short time interval between irradiation and cognitive testing in this study and the anticipated time required for new cells to become functionally integrated, a role for neurogenesis in the observed effects seems unlikely.
Conclusions
In apoE2 mice, irradiation reduced levels of ROS and CuZnSOD, and the same pattern was seen for levels of 3-NT. In contrast, in apoE3 and apoE4 mice, levels of ROS and CuZnSOD were not affected by irradiation and there was trend toward enhanced 3-NT following irradiation. ROS and antioxidant defense mechanisms, like CuZn-SOD, under baseline conditions might be more important than those following irradiation in affecting oxidative damage as assessed by 3-NT levels. In apoE targeted replacement mice comparable in age to the mice used in this study, we (6) and others (20) did not detect differences in overall hippocampal apoE levels. However, we recognize that there might be apoE isoform-dependent differences in apoE levels in distinct hippocampal subfields. Therefore, we cannot distinguish potential effects due to differences in apoE protein levels and protein level-independent apoE isoform-dependent effects. Increased efforts are warranted to assess potential differences in apoE expression in the different hippocampal subregions and whether these differences contribute to the apoE isoform-dependent effects of irradiation on ROS levels and hippocampus-dependent cognitive function.
Supplementary Material
Acknowledgment
We thank Peter Guida and Adam Rusek at BNL for their support with the irradiations and Dr. Ting-Ting Huang for graciously providing the EC-SOD, MnSOD, and CuZnSOD antibodies.
This research was supported by a National Space Biomedical Research Institute postdoctoral fellowship through National Aeronautics and Space Administration (NASA) cooperative agreement NCC 9-58 (to G.E.H.) and NASA grants NNJ06HE63G (to J.R.) and NSCOR NNX10AD59G (to G.N.) and by a Medical Research Foundation research award (to G.E.H.), an American Psychological Association dissertation research award (to L.E.V.), and the development account of Dr Raber.
Footnotes
G.E. Haley and L. Villasana are co-first authors.
Conflict of interest: none.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Raber J. Unintended effects of cranial irradiation on cognitive function. Toxicol Pathol. 2010;38(1):198–202. doi: 10.1177/0192623309352003. [DOI] [PubMed] [Google Scholar]
- 2.Manda K, Ueno M, Anzai K. Space radiation-induced inhibition of neurogenesis in the hippocampal dentate gyrus and memory impairment in mice: ameliorative potential of the melatonin metabolite, AFMK. J Pineal Res. 2008;45(4):430–438. doi: 10.1111/j.1600-079X.2008.00611.x. [DOI] [PubMed] [Google Scholar]
- 3.Poirier J. Apolipoprotein E in animal models of CNS injury and in Alzheimer’s disease. Trends Neurosci. 1994;17(12):525–530. doi: 10.1016/0166-2236(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 4.Higuchi Y, Nelson GA, Vazquez M, et al. Apolipoprotein E expression and behavioral toxicity of high charge, high energy (HZE) particle radiation. J Radiat Res (Tokyo) 2002;43(Suppl):S219–S224. doi: 10.1269/jrr.43.s219. [DOI] [PubMed] [Google Scholar]
- 5.Villasana LE, Benice TS, Raber J. Long-term effects of 56Fe irradiation on spatial memory of mice: Role of sex and apolipoprotein E isoform. Int J Radiat Oncol. 2010;80:567–573. doi: 10.1016/j.ijrobp.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 6.Siegel JA, Haley GE, Raber J. Apolipoprotein E isoform-dependent effects on anxiety and cognition in female TR mice. Neurobiol Aging. 2012;33:345–358. doi: 10.1016/j.neurobiolaging.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riley PA. Free radicals in biology: Oxidative stress and the effects of ionizing radiation. Int J Radiat Biol. 1994;65(1):27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 8.Sardesai VM. Role of antioxidants in health maintenance. Nutr Clin Pract. 1995;10(1):19–25. doi: 10.1177/011542659501000119. [DOI] [PubMed] [Google Scholar]
- 9.Muscoli C, Cuzzocrea S, Riley DP, et al. On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. Br J Pharmacol. 2003;140(3):445–460. doi: 10.1038/sj.bjp.0705430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raber J, Villasana L, Rosenberg J, et al. Irradiation enhances hippocampus-dependent cognition in mice deficient in extracellular superoxide dismutase. Hippocampus. 2011;21(1):72–80. doi: 10.1002/hipo.20724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petegnief V, Saura J, De-Gregorio-Rocasolano N, Paul SM. Neuronal injury-induced expression and release of apolipoprotein E in mixed neuron/glia co-cultures: Nuclear factor kappa B inhibitors reduce basal and lesion-induced secretion of apolipoprotein E. Neuroscience. 2001;104:223–234. doi: 10.1016/s0306-4522(01)00046-x. [DOI] [PubMed] [Google Scholar]
- 12.Villasana L, Acevedo S, Poage C, et al. Sex- and apoE isoform-dependent effects of radiation on cognitive function. Radiat Res. 2006;166(6):883–891. doi: 10.1667/RR0642.1. [DOI] [PubMed] [Google Scholar]
- 13.Raber J, Rosi S, Chakraborti A, et al. Effects of 56Fe cranial radiation on hippocampus-dependent cognition depend on the salience of the environmental stimuli. Radiat Res. 2011;176(4):521–526. doi: 10.1667/rr2635.1. [DOI] [PubMed] [Google Scholar]
- 14.Raber J, Villasana L, Rosenber J, Zou J, Huang TT, Fike JR. Irradiation enhances hippocampus-dependent cognition in mice lacking extracellular superoxide dismutase. Hippocampus. 2011;21(1):72–80. doi: 10.1002/hipo.20724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Beer F, Stalenhoef AFH, Hoogerbrugge N, et al. Expression of type III hyperlipoproteinemia in apolipoprotein E2 (Arg1583Cys) homozygotes is associated with hyperinsulinemia. Arterioscl Thromb Vasc Biol. 2002;22:294–299. doi: 10.1161/hq0202.102919. [DOI] [PubMed] [Google Scholar]
- 16.Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, Murphy MP, Beckman JS. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci U S A. 2006;103(41):15038–15043. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramassamy C, Krzywkowski P, Averill D, et al. Impact of apoE deficiency on oxidative insults and antioxidant levels in the brain. Mol Brain Res. 2001;86:76–83. doi: 10.1016/s0169-328x(00)00268-0. [DOI] [PubMed] [Google Scholar]
- 18.Valastro B, Ghribi O, Poirier J, et al. AMPA receptor regulation and LTP in the hippocampus of young and aged apolipoprotein E-deficient mice. Neurobiol Aging. 2001;22(1):9–15. doi: 10.1016/s0197-4580(00)00177-9. [DOI] [PubMed] [Google Scholar]
- 19.Shea TB, Rogers E. Folate quenches oxidative damage in brains of apolipoprotein E-deficient mice: Augmentation by vitamin E. Mol Brain Res. 2002;108:1–6. doi: 10.1016/s0169-328x(02)00412-6. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan PM, Mace BE, Maeda N, et al. Marked regional differences of brain human apolipoprotein E expression in targeted replacement mice. Neuroscience. 2004;124:725–733. doi: 10.1016/j.neuroscience.2003.10.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.