Abstract
People with schizophrenia have a higher prevalence of obesity than the general population. Many people with this illness struggle with weight gain, due, in part, to medications and other factors that act as obstacles to exercise and healthy eating. Several studies have shown the benefits of behavioral weight loss programs targeting eating and/or exercise in people with schizophrenia. Fewer studies have used competitive events as a goal for an exercise program. The current study tested the feasibility of preparing, using an exercise program, for a 5-kilometer (5K) event in people with schizophrenia. The exercise program was a 10-week training program consisting of three supervised walking/jogging sessions per week and a weekly educational meeting on healthy behaviors. Almost 65% (11/17) of the subjects participated in all of the training sessions, and 82% (14/17) participated in the 5K event. Participants did not gain a significant amount of weight during the exercise program (median weight change = 0.7 kg; 25th percentile 0.5, 75th percentile 3.9, p = .10). This study suggests that using an achievable goal, such as a 5K event, promotes adherence to an exercise program and is feasible in a population of people with chronic schizophrenia.
Keywords: schizophrenia, weight gain, weight management, 5K, healthy behavior
People with schizophrenia are vulnerable to weight gain, and as a population, have a higher prevalence of obesity than the general population (American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, & North American Association for the Study of Obesity, 2004; Newcomer, 2007b). Many people with this illness struggle with weight gain due, in part, to their medications and psychological factors that act as obstacles to exercise and healthy eating. People with schizophrenia also tend to have lifestyles that increase their risk for physical disease (McCreadie, 2003; Strassnig, Brar, & Ganguli, 2003). These lifestyle factors include lack of motivation to exercise, poor diet, and cigarette smoking, complicated by limited resources for nutritional and educational services (Bushe, Haddad, Peveler, & Pendlebury, 2005). Treatment-naive patients with schizophrenia, as well as their family members, appear to be at risk for metabolic complications, consistent with the fact that the illness itself increases the risk for metabolic disorders and obesity (Spelman, Walsh, Sharifi, Collins, & Thakore, 2007). Limiting weight gain and obesity, however, are important in schizophrenia, as obesity-related complications are known to include type II diabetes and cardiovascular disease as well as other diseases (Allison et al., 2009).
Recent progress in the treatment for schizophrenia has resulted in a new class of antipsychotic drug, introduced in the 1990s and referred to as second-generation antipsychotics (SGAs). SGAs are currently prescribed as first-line therapy in people with schizophrenia. These new antipsychotics induce less of the serious side effects that occur with first-generation antipsychotics (FGAs), such as extrapyramidal symptoms and tardive dyskinesia (TD). However, SGAs are associated with other adverse effects (Newcomer, 2007a), of which weight gain is one of the more serious. Between 40 and 80% of patients taking an antipsychotic medication experience significant weight gain (Maayan & Vakhrusheva, 2010; Novick, Haro, Perrin, Suarez, & Texeira, 2009). A large 3-year observational study, the Schizophrenia Health Outcomes Study (SOHO), showed that 44% of patients on olanzapine, 39% of patients on risperidone, 36% of patients on an “other” SGA, and 17% of patients on an FGA gained at least 7% of their body weight (Haro, Novick, Suarez, & Roca, 2009). Weight gain associated with antipsychotic use may increase the risk for obesity-related consequences and the metabolic syndrome (Newcomer, 2008). The American Heart Association lists the following metabolic risk factors as characteristics of the metabolic syndrome: abdominal obesity, atherogenic dyslipidemia, elevated blood pressure, glucose intolerance, a prothrombotic state, and a proinflammatory state (American Heart Association, 2010; Newcomer, 2007). The metabolic syndrome is associated with higher rates of cardiovascular disease and associated mortality (American Heart Association, 2010; National Cholesterol Education Program, 2001). The risks for cardiovascular morbidity and mortality are known to be higher in schizophrenia and other serious mental illness than in the general population (Kelly et al., 2010). Whether there is increased mortality associated with particular antipsychotic treatments, however, remains controversial (Daumit et al., 2008; De Hert, Correll, & Cohen, 2010; Kelly et al., 2010; Tiihonen et al., 2009) and will be more complex to elucidate due to many potentially confounding environmental and disease factors.
Independent from its effect on adiposity, an increased level of physical activity lowers all-cause mortality, the risk of cardiovascular disease and metabolic diseases, and certain cancers (Hainer, Toplak, & Stich, 2009). To achieve potential survival benefits, it is recommended that unfit people with cardiovascular risk factors engage in exercise programs of sufficient duration and intensity to improve fitness (Mandic, Myers, Oliveira, Abella, & Froelicher, 2009). Being physically fit and maintaining a normal waist girth, combined with not smoking, have been associated with a lowered risk of cardiovascular related events and all-cause mortality in men (Lee, Sui, & Blair, 2009). People with schizophrenia generally have low levels of regular exercise and often have low motivation to engage in physical activity. For example, Smith and colleagues (2007) recently reported that, in 966 people with mental illness, 37% did not engage in exercise at all, and only 31% engaged in at least 90 min of exercise per week. In the general population, dropout from exercise programs is around 50% after 6 months (Dishman, 1991). In a 6-month exercise program (free access to a fitness facility; N = 20) for people with schizophrenia, dropout rates were 90% after 6 months and 70% after 9 months (Archie, Wilson, Osborne, Hobbs, & McNiven, 2003). Thus, while people with schizophrenia may have even greater needs than the general population to increase physical activity, dropout from exercise programs in this group is at least as high as in the general population. In fact, motivation to exercise may be especially reduced among people with schizophrenia, due in part to avolition, a lack of motivation or drive, which is a key player in the phenomenology of this illness (Bleuler, 1950; Kraepelin, 1919).
Interestingly, people with schizophrenia have been reported to respond positively to the attention and support they are given during an exercise intervention (Beebe, 2006). Several studies have demonstrated mental (Gimino & Levin, 1984; Pajonk et al., 2010; Pelham, Campagna, Ritvo, & Birnie, 1993) and physical (Archie et al., 2003; Ball, Coons, & Buchanan, 2001; Beebe et al., 2005; Bushe, McNamara, Haley, McCrossan, & Devitt, 2008; Centorrino et al., 2006; Chen, Chen, & Huang, 2009; Fogarty, Happell, & Pinikahana, 2004; Gaitero Calleja, Santed German, Rullas Trincado, & Grande de Lucas, 2007; Jean-Baptiste et al., 2007; Kwon et al., 2006; Lee, Choi, & Kwon, 2008; Menza et al., 2004; Pendlebury, Haddad, & Dursun, 2005; Vreeland et al., 2003; Weber & Wyne, 2006) benefits of behavioral weight management programs targeting eating and/or exercise in people with schizophrenia. In addition, physical activity has been associated with increased cerebral gray matter volume (Floel et al., 2010), and it has been suggested that aerobic exercise training may inhibit gray matter volume loss in the insula (Gondoh et al., 2009). Increased fitness has also been associated with lower levels of atrophy of the medial temporal lobe volume in Alzheimer’s patients (Honea et al., 2009). Hippocampal volume may increase in people with schizophrenia as well as in healthy controls in response to exercise (Pajonk et al., 2010). Much of the emerging literature on the effectiveness of, and level of adherence to, behavioral platforms includes an exercise education component for preventing weight gain and/or decreasing weight in people with severe mental illness (Bushe et al., 2008; Gaitero Calleja et al., 2007; Jean-Baptiste et al., 2007; Kwon et al., 2006; Lee et al., 2008; Pendlebury et al., 2005; Weber & Wyne, 2006). Other investigators have reported specific behavioral interventions with detailed exercise components (Ball et al., 2001; Beebe et al., 2005; Centorrino et al., 2006; Chen et al., 2009; Fogarty et al., 2004; Menza et al., 2004; Vreeland et al., 2003) that have benefits in aerobic fitness, physical improvement, improved psychiatric symptoms as well as reductions in body fat and body mass index (BMI).
Archie et al. (2003) reported that lack of motivation was the most frequent reason given by people with schizophrenia for poor adherence at the exercise facility used in their program. Thus, motivational techniques and goal setting to improve exercise may lead to improvements in exercise. Menza and colleagues (2004) included a motivational counseling piece their 52-week exercise and weight loss program. The retention rate was 66%, and weight and BMI decreased significantly, suggesting higher success with improved motivating factors. Pendlebury, Bushe, Wildgust, and Holt (2007) reported successful weight loss in self-referred, self-motivated participants (N = 93) with a dropout rate of 23% in the first 8 weeks. Beebe et al. (2009) recently published the design and rationale of an ongoing randomized study with a motivational group (1 hr/1 week) for 4 weeks prior to starting a 16-week walking program. They hypothesize that this motivational session will increase participation and attendance in the exercise program. People with schizophrenia may have improved participation and adherence to an exercise program if they are given a specific, tangible, and achievable goal, as opposed to the seemingly difficult and broad goals of weight loss or improved health.
Our aim in the current study was to examine the participation and adherence of people with schizophrenia in a 10-week exercise program preparing them for a 5-kilometer (5K) walking/jogging event. We selected a 5K event because it is an achievable and realistic exercise option that provides a goal for which people can strive. Events like these are held routinely throughout the year in many towns and cities across the United States. Introducing people with schizophrenia to this type of exercise opportunity opens up a door to numerous opportunities for physical activity.
Materials and Method
Study Design and Setting
We used a single-group design to test the feasibility of an exercise program in preparation for a 5K event. The study took place from May to October 2008 on the grounds of a State of Maryland inpatient and outpatient mental health facility on a sprawling campus in the suburbs of Baltimore, Maryland. This site provided ample space for walking in a safe, traffic-free, environment. The University of Maryland and Department of Health and Mental Hygiene (DHMH) Institutional Review Boards approved this study.
Human Participants
Inpatients and outpatients being treated at a research facility on the grounds of the campus were eligible. We gave outpatients the option of free transportation to the site so that cost would not affect participation. Eligible participants included those with a diagnosis of schizophrenia or schizoaffective disorder (according to the criteria established in the Diagnostic and Statistical Manual of Mental Disorders [4th ed.; DSM-IV]) who were between the ages of 18 and 64 years. All participants went through an extensive diagnostic evaluation involving collection of relevant information from family members and former treatment providers and the completion of the Structured Clinical Interview for DSM Disorders (SCID; First, Spitzer, Gibbon, & Williams, 1997). Pregnant women were excluded. Otherwise, with the approval of the treating psychiatrist, all patients were eligible to participate, regardless of their medication regimen, diabetes status, or BMI. Patients’ clinicians and the research staff informed patients of study. We gave all interested, potential participants a detailed description of the study. If they were still interested and understood the study risks and benefits, we asked them if they would like to consent. Research staff obtained witnessed consent using an evaluation to consent form to ensure that the participants had the capacity to consent and understood the possible risks and benefits of the research (DeRenzo, Conley, & Love, 1998).
Exercise Program
The exercise program lasted 10 weeks and consisted of three supervised walking/jogging sessions per week plus one 30-min group session per week providing education on healthy behaviors. Duration of walking sessions started at 20 min and increased by 2–5 min per week to allow the participants to gradually build up to completing a 5K (3.1 mile) course. Two to three clinical or research staff members accompanied each walking group in order to supervise people of all walking speeds. Participants were encouraged to engage in the exercise sessions but were not coerced. There was a friendly, as opposed to boot camp-like, atmosphere during the exercise sessions. Participants were permitted to jog if they desired, and they had the option to end early or take a rest break.
Research staff taught participants how to measure their pulse, and they did so before and after each walking session. We gave each participant a pedometer to log her or his steps and a water bottle to use during exercise sessions. Weekly, 30-min healthy behavior lessons consisted of topics related to healthy eating and exercise, as outlined in Table 1. We adapted these lessons from Ganguli and Brar’s “A Behavioral Group-Based Treatment for Weight Reduction in Schizophrenia and Other Severe Mental Illnesses,” which were taught in 17 weekly, hour-long classes (Brar et al., 2005). Participants maintained a weekly diet and activity log that gauged progress outside the sessions and was used as a teaching tool during the weekly classes. We gave participants a lunch voucher to the campus café after each exercise session. Prior to the beginning of the program, we instructed all participants on the need to stay hydrated and on the signs and symptoms of overexertion and instructed them to report such symptoms to the staff accompanying them during exercise sessions should they occur. The 2008 Physical Activity Guidelines for Americans recommends a minimum of 150 min of physical activity per week for adults (U.S. Department of Health and Human Services, 2008). For inactive people and people with low levels of physical activity, an exercise program should begin with relatively moderate-intensity aerobic activity increasing by 20-min each week. Some participants in the current study did increase their intensity to moderate intensity (brisk walking or jogging) as the study progressed. The exercise program did not exceed The 2008 Physical Activity Guidelines, therefore minimizing safety concerns. Number of steps and duration were recorded for each individual for each exercise session.
Table 1.
Topics for Weekly 30-Min Healthy Behavior Lessons
| Week | Topic |
|---|---|
| 1 | Self-monitoring: Awareness of body weight and what one eats |
| 2 | Burning calories by exercise |
| 3 | The 5K race |
| 4 | Decreasing food cues to overeat and snack |
| 5 | Developing good eating habits |
| 6 | Self-control of overeating |
| 7 | Changing snack habits |
| 8 | Controlling urges to overeat and snack |
| 9 | Dressing for not-so-ideal-weather exercise |
| 10 | Review of topics 1–9 |
Note: 5K = 5 kilometer.
Laboratory and Vital Sign Measures
At baseline, a physical examination, blood chemistries (glucose, cholesterol [very low, low and high-density lipoprotein (VLDL, LDL, and HDL)], triglycerides, aspartate aminotransferase [AST], alanine aminotransferase [ALT], and bilirubin) and electrocardiogram (EKG) were performed to determine the safety of participation in an exercise program at the discretion of the physician. We measured height using a wall-mounted stadiometer and weight on a digital scale after participants had removed shoes, outerwear, and pocket contents. We took vital signs, including weight, heart rate, blood pressure, and respiratory rate, weekly, before one of the exercise sessions. We repeated baseline measurements at the end of the study. All laboratory tests were to be fasting; however, three subjects had nonfasting levels drawn.
Clinical Assessments
We performed clinical ratings for psychiatric symptoms at baseline and at the end of the 10-week study. We used the Brief Psychiatric Rating Scale (BPRS) total score (20 items, each scored 1–7; Overall & Gorham, 1962) to assess general psychopathology. The BPRS positive symptom item score (sum of conceptual disorganization, hallucinations, unusual thought content, and suspiciousness) and the BPRS anxiety/depression factor measure changes in positive symptoms and affective symptoms, respectively. We used the Scale for the Assessment of Negative Symptoms (SANS) to measure negative symptom change. SANS item scores range from 0 to 5, with a total score ranging from 0 to 90 (Andreasen, 1982). We used the Clinical Global Impression (CGI) severity of illness item to measure global changes (Guy, 1976). The BPRS, SANS, and CGI were completed by research and clinical staff that had completed both initial and ongoing reliability training to assure interrater reliability. The intraclass correlation coefficient (ICC) for all assessments was maintained at a minimum of .80. These ratings were performed by trained clinical interviewers who passed reliability assessments on each rating.
The 5K Event
The 5K event, organized by the investigators, was held on a Friday at 9:00 a.m. The 5K course was the same course on which participants had been training. They received a T-shirt and race number. T-shirt’s were made of dry-fit material, were designed by Maryland Psychiatric Research Center patients, and included sponsor logos on the back. Local sponsors provided bottled water, sports bottles, and funding for end-of-race food. Local police blocked off streets and Emergency Management Technicians (EMT) were on site in case of emergency. A balloon arch signified the start and finish of the race course and music and amplifiers/microphones were provided. Volunteers passed out water in cups at water stations located at Miles 1 and 2. Each participant was monitored to see if they finished the race, and the time of all finishers was clocked. Water, bagels, and fresh fruit were provided at the finish. Family members, staff, and community members were encouraged to attend the race. The event was also advertised on http://www.active.com to encourage individuals outside the study to participate. Each study participant received a medal for participation.
Statistical Tests
We used SAS® to analyze data. We assessed frequencies of exercise participation, performed paired t tests on baseline and end-of-study physiological measures, calculated Spearman rank correlations for each participant between exercise session and performance measures (walking time and number of steps), and used Mantel-Haenszel chi-squares, stratified by participant, to test for the significance of the correlation between session and performance measures (Arndt, Davis, Miller, & Andreasen, 1993).
Results
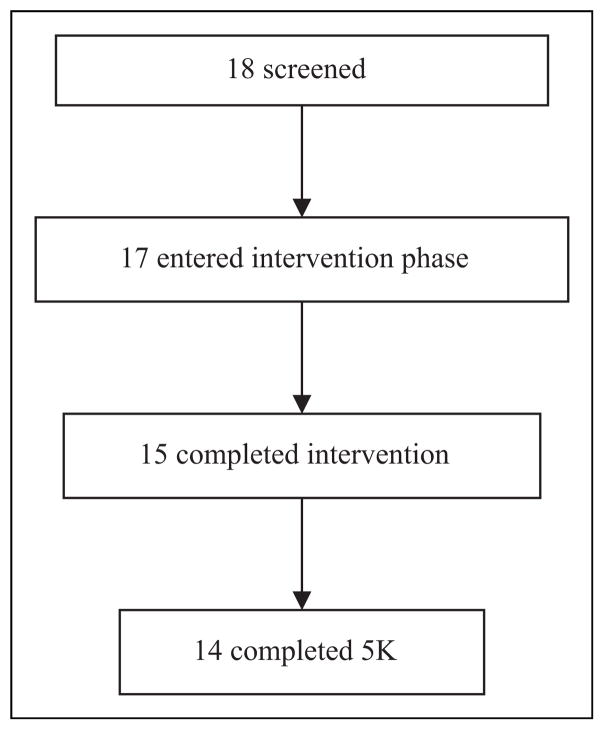
Eighteen (6 outpatients and 12 inpatients) participants (ages 21 to 51 years) gave informed consent. One participant was excluded due to participation in another weight-related study that was a potential conflict with this study. Therefore, 17 (94% of those interested) participants were enrolled. The demographic and clinical characteristics of the 17 participants are listed in Table 2. Of the 17 participants, 14 (82%) participated in both the training sessions and completed the 5K, with a M ± SD race time of 55 min, 35 s ± 13 min, 40 s (range: 33 min, 15 s–96 min, 30 s). Additionally, one subject participated in the training sessions and attended race day but did not participate in the race. The remaining two participants dropped from the training sessions and did not participate in the event (see Figure 1).
Table 2.
Baseline Demographic and Clinical Characteristics of Participating Patients with Schizophrenia (N = 17)
| Baseline Characteristics | N or M (SD) |
|---|---|
| Age (years; M [SD]) | 39.9 (10.1) |
| Sex (male; n) | 11 |
| Race (White; n) | 9 |
| Antipsychotic treatment | |
| Clozapine | 7 |
| Olanzapine | 3 |
| Risperidone | 3 |
| Other or polytherapy | 9 |
| Age of illness onset (years; M [SD]) | 19.3 (3.8) |
| BPRS (M [SD]) | |
| Total | 36.4 (10.2) |
| Psychosis | 9.4 (4.7) |
| Anxiety/depression | 8.1 (3.2) |
| Hostility | 5.9 (2.8) |
| SANS total (M [SD]) | 32.7 (13.2) |
Note. BPRS = Brief Psychiatric Rating Scale; SANS = Scale for the Assessment of Negative Symptoms.
Figure 1.
CONSORT flow chart of patient recruitment and study completion. After consent, one participant did not meet criteria for inclusion. During the intervention, two participants dropped from the study due to lack of interest, while one participant completed the entire intervention and attended the 5K event but did not participate in the 5K.
Of the 17 subjects, 11 (64.7%) participated in all training sessions, 14 (82%) participated in 50% or more of the sessions, and all subjects participated in 25% or more of the sessions. Additionally, three participants opted to jog, generally in 1- to 2-min bouts separated by 5-min walking intervals. No participants opted to take rests. On three occasions, two participants opted to end early. The weekly mean session length and steps are listed in Table 3. As planned in the protocol, as the program progressed, participants successfully achieved an increased number of steps by pedometer rating and an increased duration of walking (average within-participant correlation between exercise session and number of steps, r = .31, Mantel-Haenszel χ2 = 38.24, df = 1, p < .001; correlation between exercise session and walking duration, r = .75, Mantel-Haenszel χ2 = 163.08, df = 1, p < .001).
Table 3.
Weekly Mean Exercise Session Duration and Steps
| Week | Exercise Session | Duration (min) | Steps (Pedometer Readings) |
|---|---|---|---|
| 1 | 1–3 | 23.33 | 2423.25 |
| 2 | 4–6 | 24.15 | 2141.46 |
| 3 | 7–9 | 28.68 | 2765.18 |
| 4 | 10–12 | 30.72 | 2324.43 |
| 5 | 13–15 | 32.65 | 2137.93 |
| 6 | 16–18 | 37.15 | 1959.93 |
| 7 | 19–21 | 43.91 | 3186.67 |
| 8 | 22–24 | 38.87 | 3188.83 |
| 9 | 25–27 | 43.24 | 2769.83 |
| 10 | 28–30 | 44.86 | 3868.58 |
Summary data on laboratory and physiologic variables are shown in Table 4. There were no significant changes in any of these variables during the 10 weeks of training, other than pulse, which increased significantly over the 10 weeks (t = 2.42, p < .05). During the study there were no significant changes in the BPRS total (p = .192), positive symptom (p = .418), anxiety/depression (p = .366), SANS (p = .428), or CGI severity (p = .189) scores. No adverse events occurred during the study. Median weight change during the study was small (+0.7 kg), although 3 participants gained ≥7 kg. The largest weight gains were seen in inpatients, and we speculate that food vouchers for a campus café may have afforded these participants an opportunity to add extra calories to their diet that would have otherwise been unavailable.
Table 4.
Baseline to 10-Week Comparisons in Laboratory and Physiological Variables in Exercise-Group Participants
| Variable | Baseline
|
10-Week
|
Mean Difference
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Median | IQR | n | Median | IQR | na | Median | IQR | |
| Weight (kg) | 15 | 88.5 | 75.3–112.9 | 15 | 96.2 | 77.6–111.6 | 13 | 0.7 | −0.5–3.9 |
| Weight, outliers removed (kg) | 12 | 103.8 | 77.3–122.1 | 12 | 98.0 | 78.0–115.9 | 10 | −0.3 | −0.7–1.8 |
| M | SD | M | SD | M | SD | ||||
| BMI (kg/m2) | 14 | 33.2 | 9.1 | 15 | 33.5 | 8.4 | 13 | 0.8 | 1.6 |
| Triglycerides | 15 | 127.3 | 42.7 | 14 | 171.5 | 134.9 | 14 | 49.5 | 135.7 |
| Fasting lipids (cholesterol) | 15 | 175.4 | 38.8 | 14 | 191.9 | 55.2 | 14 | 16.7 | 35.3 |
| HDL | 15 | 47.0 | 12.7 | 14 | 43.6 | 10.6 | 14 | −4.2 | 10.7 |
| LDL | 15 | 102.9 | 34.5 | 13 | 109.4 | 34.2 | 13 | 6.7 | 13.0 |
| VLDL | 15 | 25.5 | 8.4 | 13 | 27.9 | 13.5 | 13 | 3.2 | 11.2 |
| Fasting glucose | 15 | 101.7 | 15.8 | 14 | 122.1 | 64.4 | 14 | 20.0 | 60.3 |
| Pre-exercise blood pressure | |||||||||
| Systolic | 15 | 117.1 | 9.4 | 15 | 123.5 | 14.7 | 13 | 6.2 | 14.6 |
| Diastolic | 15 | 75.9 | 12.8 | 15 | 77.5 | 9.4 | 13 | 2.6 | 8.0 |
| Pulse (bpm) | 15 | 84.9 | 12.6 | 15 | 90.6 | 11.6 | 13 | 9.0* | 13.4 |
| Respirations (rpm) | 15 | 20.0 | 3.1 | 15 | 19.6 | 2.6 | 13 | −0.5 | 3.3 |
| Waist (inches) | 11 | 43.0 | 5.7 | 13 | 43.4 | 5.7 | 11 | −0.1 | 2.5 |
Note. BMI = body mass index; HDL = high-density lipoproteins; IQR = interquartile range (25th–75th percentile); LDL = low-density lipoproteins; VLDL = very low-density lipoproteins.
Number of participants for whom there were both baseline and end-of-study data.
Significant at the .05 level.
Discussion
This study tested (a) the feasibility of having people with schizophrenia participate in an exercise program in preparation for a 5K event and (b) the level of participation and adherence to the exercise program. Overall, 82% of participants participated in the 5K and approximately 65% participated in all of the exercise sessions, a significant accomplishment considering this included 30 walking/jogging sessions over 10 weeks. The duration of exercise and number of steps increased over time. Adherence rate to the exercise program was high, possibly due to the motivational effect of the 5K event, though no systematic data were collected to evaluate the motivational role of the event.
This study was not associated with any adverse events from the exercise program, and no significant changes in laboratory measures were observed in the study. We did see an unexpected and unexplained increase in pulse rate which was not clinically significant in any patient. This finding may have been due to chance, such as can be expected when examining many variables. Out of 17 participants, 3 gained over 7 kg during the duration of this study; these were inpatients who may have made more extensive use of café food vouchers than the outpatients who receive them regularly for study participation. The increase in weight may also have been due to measurement error. For instance, during their weight measurements, participants may have been wearing heavier clothes in October than in May. In the future, we will control for this possible source of error by having participants wear paper gowns during weight measurements.
Both the exercise sessions and the 5K involved both patients and staff and were a means for participants with schizophrenia to interact with others in a destigmatizing manner. Limitations of this study include the following. First, we did not systematically measure the effects of the exercise program on subject motivation to exercise or quality of life. We will use validated measures of well-being, such as the Quality of Life Scale (Heinrichs, Hanlon, & Carpenter, 1984), in future studies. Next, the study was not intended as a weight loss intervention, as caloric restriction was not included. Future studies including both dietary and exercise interventions should be considered. We also did not intend for lunch vouchers, customarily given out to outpatient study participants, to be so enticing to the inpatients. Future studies will not include lunch vouchers. We did not examine physiological measures of physical fitness nor did we assess changes in cognitive function, which may improve with increasing exercise duration. Also, a few blood chemistry measures in our study were not fasting; however, we did not anticipate finding significant improvements in blood chemistries in a 10-week trial without a dietary component.
In conclusion, we found it possible to achieve a high rate of adherence in patients with schizophrenia to an exercise program conducted in preparation for a 5K event. The exercise program used in this study did not include a dietary intervention to reduce caloric intake and in fact included an unintentional food incentive after exercise which may have increased caloric intake in some inpatients, as noted in the results. It is, therefore, not surprising that this program, despite success in increasing exercise, did not result in significant weight loss. Future investigations will include motivational assessments and measurement of neurocognitive functioning and quality of life assessments in relation to exercise. Changes in objective measures of physical fitness, such as submaximum heart rate, resting metabolic rate, and body composition, should also be measured in a future investigation to see if exercise programs do, indeed, improve physical health. Less structured opportunities to continue exercise after a 5K event may be used to assess the impact of training for such an event on long-term physical fitness and readiness to engage in exercise and healthy behaviors.
Acknowledgments
Funding
This project was funded by the National Institutes of Mental Health (NIMH R03 MH069871-01; Kelly, PI) and the Advanced Centers for Intervention and Services Research (NIMH P50 MH40279; Carpenter, PI).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no conflicts of interest with respect to the authorship and/or publication of this article.
References
- Allison DB, Newcomer JW, Dunn AL, Blumenthal JA, Fabricatore AN, Daumit GL, Alpert JE. Obesity among those with mental disorders: A National Institute of Mental Health meeting report. American Journal of Preventive Medicine. 2009;36:341–350. doi: 10.1016/j.amepre.2008.11.020. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists & North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27:596–601. doi: 10.2337/diacare.27.2.596. [DOI] [PubMed] [Google Scholar]
- American Heart Association. Metabolic syndrome. 2010 Retrieved March 1, 2010, from http://www.americanheart.org/presenter.jhtml?identifier=4756.
- Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Archives of General Psychiatry. 1982;39:784–788. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- Archie S, Wilson JH, Osborne S, Hobbs H, McNiven J. Pilot study: Access to fitness facility and exercise levels in olanzapine-treated patients. Canadian Journal of Psychiatry. 2003;48:628–632. doi: 10.1177/070674370304800910. [DOI] [PubMed] [Google Scholar]
- Arndt S, Davis CS, Miller DD, Andreasen NC. Effect of antipsychotic withdrawal on extrapyramidal symptoms: Statistical methods for analyzing single-sample repeated-measures data. Neuropsychopharmacology. 1993;8:67–75. doi: 10.1038/npp.1993.8. [DOI] [PubMed] [Google Scholar]
- Ball MP, Coons VB, Buchanan RW. A program for treating olanzapine-related weight gain. Psychiatric Services. 2001;52:967–969. doi: 10.1176/appi.ps.52.7.967. [DOI] [PubMed] [Google Scholar]
- Beebe LH. Walking tall: A person with schizophrenia on a journey to better health. Journal of Psychosocial Nursing and Mental Health Services. 2006;44:53–55. doi: 10.3928/02793695-20060601-08. [DOI] [PubMed] [Google Scholar]
- Beebe LH, Burk R, McIntyre KB, Smith K, Velligan DI, Resnick B, Dessieux O. Motivating persons with schizophrenia spectrum disorders to exercise: Rationale and design. Clinical Schizophrenia and Related Psychoses. 2009;3:111–116. doi: 10.3371/CSRP.3.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe LH, Tian L, Morris N, Goodwin A, Allen SS, Kuldau J. Effects of exercise on mental and physical health parameters of persons with schizophrenia. Issues in Mental Health Nursing. 2005;26:661–676. doi: 10.1080/01612840590959551. [DOI] [PubMed] [Google Scholar]
- Bleuler E. Dementia praecox or the group of schizophrenias. New York, NY: International Universities Press; 1950. [Google Scholar]
- Brar JS, Ganguli R, Pandina G, Turkoz I, Berry S, Mahmoud R. Effects of behavioral therapy on weight loss in overweight and obese patients with schizophrenia or schizoaffective disorder. Journal of Clinical Psychiatry. 2005;66:205–212. doi: 10.4088/jcp.v66n0208. [DOI] [PubMed] [Google Scholar]
- Bushe C, Haddad P, Peveler R, Pendlebury J. The role of lifestyle interventions and weight management in schizophrenia. Journal of Psychopharmacology. 2005;19:28–35. doi: 10.1177/0269881105058682. [DOI] [PubMed] [Google Scholar]
- Bushe CJ, McNamara D, Haley C, McCrossan MF, Devitt P. Weight management in a cohort of Irish inpatients with serious mental illness (SMI) using a modular behavioural programme. A preliminary service evaluation. BMC Psychiatry. 2008;8:76. doi: 10.1186/1471-244X-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centorrino F, Wurtman JJ, Duca KA, Fellman VH, Fogarty KV, Berry JM, Baldessarini RJ. Weight loss in overweight patients maintained on atypical antipsychotic agents. International Journal of Obesity (Lond) 2006;30:1011–1016. doi: 10.1038/sj.ijo.0803222. [DOI] [PubMed] [Google Scholar]
- Chen CK, Chen YC, Huang YS. Effects of a 10-week weight control program on obese patients with schizophrenia or schizoaffective disorder: A 12-month follow up. Psychiatry and Clinical Neurosciences. 2009;63:17–22. doi: 10.1111/j.1440-1819.2008.01886.x. [DOI] [PubMed] [Google Scholar]
- Daumit GL, Goff DC, Meyer JM, Davis VG, Nasrallah HA, McEvoy JP, Lieberman JA. Antipsychotic effects on estimated 10-year coronary heart disease risk in the CATIE schizophrenia study. Schizophrenia Research. 2008;105:175–187. doi: 10.1016/j.schres.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hert M, Correll CU, Cohen D. Do antipsychotic medications reduce or increase mortality in schizophrenia? A critical appraisal of the FIN-11 study. Schizophrenia Research. 2010;18:18–24. doi: 10.1016/j.schres.2009.12.029. [DOI] [PubMed] [Google Scholar]
- DeRenzo EG, Conley RR, Love R. Assessment of capacity to give consent to research participation: State-of-the-art and beyond. Journal of Health Care Law Policy. 1998;1:66–87. [PubMed] [Google Scholar]
- Dishman R. Increasing and maintaining exercise and physical activity. Behavioral Therapy. 1991;21:345–373. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM–IV axis I disorders (SCID-I/P) New York, NY: Psychiatric Institute, Biometrics Research Department; 1997. [Google Scholar]
- Floel A, Ruscheweyh R, Kruger K, Willemer C, Winter B, Volker K, Knecht S. Physical activity and memory functions: Are neurotrophins and cerebral gray matter volume the missing link? Neuroimage. 2010;49:2756–2763. doi: 10.1016/j.neuroimage.2009.10.043. [DOI] [PubMed] [Google Scholar]
- Fogarty M, Happell B, Pinikahana J. The benefits of an exercise program for people with schizophrenia: A pilot study. Psychiatric Rehabilitation Journal. 2004;28:173–176. doi: 10.2975/28.2004.173.176. [DOI] [PubMed] [Google Scholar]
- Gaitero Calleja AM, Santed German MA, Rullas Trincado M, Grande de Lucas A. Cognitive behavioral weight-loss program for individuals with psychotic mental diseases. Psicothema. 2007;19:640–645. [PubMed] [Google Scholar]
- Gimino FA, Levin SJ. The effects of aerobic exercise on perceived self-image in post-hospitalized schizophrenic patients. Medicine and Science in Sports and Exercise. 1984;16:139. [Google Scholar]
- Gondoh Y, Sensui H, Kinomura S, Fukuda H, Fujimoto T, Masud M, Takekura H. Effects of aerobic exercise training on brain structure and psychological well-being in young adults. Journal of Sports Medicine and Physical Fitness. 2009;49:129–135. [PubMed] [Google Scholar]
- Guy W. Clinical global impressions. In: Guy W, editor. ECDEU assessment manual for psychopharmacology. Rockville, MD: National Institute for Mental Health; 1976. pp. 218–222. Revised DHEW Publication no. [ADM]: 76–338. [Google Scholar]
- Hainer V, Toplak H, Stich V. Fat or fit: What is more important? Diabetes Care. 2009;32:S392–S397. doi: 10.2337/dc09-S346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haro JM, Novick D, Suarez D, Roca M. Antipsychotic treatment discontinuation in previously untreated patients with schizophrenia: 36-month results from the SOHO study. Journal of Psychiatric Research. 2009;43:265–273. doi: 10.1016/j.jpsychires.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Heinrichs DW, Hanlon TE, Carpenter WT., Jr The Quality of Life Scale: An instrument for rating the schizophrenic deficit syndrome. Schizophrenia Bulletin. 1984;10:388–398. doi: 10.1093/schbul/10.3.388. [DOI] [PubMed] [Google Scholar]
- Honea RA, Thomas GP, Harsha A, Anderson HS, Donnelly JE, Brooks WM, Burns JM. Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer disease. Alzheimer Disease and Associated Disorders. 2009;23:188–197. doi: 10.1097/WAD.0b013e31819cb8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Baptiste M, Tek C, Liskov E, Chakunta UR, Nicholls S, Hassan AQ, Wexler BE. A pilot study of a weight management program with food provision in schizophrenia. Schizophrenia Research. 2007;96:198–205. doi: 10.1016/j.schres.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Kelly DL, McMahon RP, Liu F, Love RC, Wehring HJ, Shim JC, Conley RR. Cardiovascular disease mortality in patients with chronic schizophrenia treated with clozapine: A retrospective cohort study. Journal of Clinical Psychiatry. 2010;71:304–311. doi: 10.4088/JCP.08m04718yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepelin E. Dementia praecox and paraphrenia. Edinburgh, UK: Livingstone; 1919. [Google Scholar]
- Kwon JS, Choi JS, Bahk WM, Yoon Kim C, Hyung Kim C, Chul Shin Y, Geun Oh C. Weight management program for treatment-emergent weight gain in olanzapine-treated patients with schizophrenia or schizoaffective disorder: A 12-week randomized controlled clinical trial. Journal of Clinical Psychiatry. 2006;67:547–553. doi: 10.4088/jcp.v67n0405. [DOI] [PubMed] [Google Scholar]
- Lee CD, Sui X, Blair SN. Combined effects of cardiorespiratory fitness, not smoking, and normal waist girth on morbidity and mortality in men. Archives of Internal Medicine. 2009;169:2096–2101. doi: 10.1001/archinternmed.2009.414. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Choi EJ, Kwon JS. A naturalistic multicenter trial of a 12-week weight management program for overweight and obese patients with schizophrenia or schizoaffective disorder. Journal of Clinical Psychiatry. 2008;69:555–562. doi: 10.4088/jcp.v69n0406. [DOI] [PubMed] [Google Scholar]
- Maayan LA, Vakhrusheva J. Risperidone associated weight, leptin, and anthropometric changes in children and adolescents with psychotic disorders in early treatment. Human Psychopharmacology. 2010;25:133–138. doi: 10.1002/hup.1097. [DOI] [PubMed] [Google Scholar]
- Mandic S, Myers J, Oliveira RB, Abella J, Froelicher VF. Characterizing differences in mortality at the low end of the fitness spectrum in individuals with cardiovascular disease. European Journal of Cardiovascular Prevention and Rehabilitation. 2009;17:289–295. doi: 10.1097/HJR.0b013e32833163e2. [DOI] [PubMed] [Google Scholar]
- McCreadie RG. Diet, smoking and cardiovascular risk in people with schizophrenia: Descriptive study. British Journal of Psychiatry. 2003;183:534–539. doi: 10.1192/bjp.183.6.534. [DOI] [PubMed] [Google Scholar]
- Menza M, Vreeland B, Minsky S, Gara M, Radler DR, Sakowitz M. Managing atypical antipsychotic-associated weight gain: 12-month data on a multimodal weight control program. Journal of Clinical Psychiatry. 2004;65:471–477. [PubMed] [Google Scholar]
- National Cholesterol Education Program. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Newcomer JW. Antipsychotic medications: Metabolic and cardiovascular risk. Journal of Clinical Psychiatry. 2007a;68:8–13. [PubMed] [Google Scholar]
- Newcomer JW. Metabolic syndrome and mental illness. American Journal of Managed Care. 2007b;13:170–177. [PubMed] [Google Scholar]
- Newcomer JW. Introduction: Cardiovascular disease and metabolic risk factors in patients with mental illness. CNS Spectrums. 2008;13:1–14. [PubMed] [Google Scholar]
- Novick D, Haro JM, Perrin E, Suarez D, Texeira JM. Tolerability of outpatient antipsychotic treatment: 36-month results from the European Schizophrenia Outpatient Health Outcomes (SOHO) study. European Neuropsychopharmacology. 2009;19:542–550. doi: 10.1016/j.euroneuro.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:812. [Google Scholar]
- Pajonk FG, Wobrock T, Gruber O, Scherk H, Berner D, Kaizl I, Falkai P. Hippocampal plasticity in response to exercise in schizophrenia. Archives of General Psychiatry. 2010;67:133–143. doi: 10.1001/archgenpsychiatry.2009.193. [DOI] [PubMed] [Google Scholar]
- Pelham TW, Campagna PD, Ritvo PG, Birnie WA. The effects of exercise therapy on clients in a psychiatric rehabilitation program. Psychosocial Rehabilitation Journal. 1993;16:75–84. [Google Scholar]
- Pendlebury J, Bushe CJ, Wildgust HJ, Holt RI. Long-term maintenance of weight loss in patients with severe mental illness through a behavioural treatment programme in the UK. Acta Psychiatrica Scandinavica. 2007;115:286–294. doi: 10.1111/j.1600-0447.2006.00906.x. [DOI] [PubMed] [Google Scholar]
- Pendlebury J, Haddad P, Dursun S. Evaluation of a behavioural weight management programme for patients with severe mental illness: 3 year results. Human Psychopharmacology. 2005;20:447–448. doi: 10.1002/hup.707. [DOI] [PubMed] [Google Scholar]
- Smith S, Yeomans D, Bushe CJ, Eriksson C, Harrison T, Holmes R, Sullivan G. A well-being programme in severe mental illness. Baseline findings in a UK cohort. International Journal of Clinical Practice. 2007;61:1971–1978. doi: 10.1111/j.1742-1241.2007.01605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spelman LM, Walsh PI, Sharifi N, Collins P, Thakore JH. Impaired glucose tolerance in first-episode drug-naive patients with schizophrenia. Diabetic Medicine. 2007;24:481–485. doi: 10.1111/j.1464-5491.2007.02092.x. [DOI] [PubMed] [Google Scholar]
- Strassnig M, Brar JS, Ganguli R. Body mass index and quality of life in community-dwelling patients with schizophrenia. Schizophrenia Research. 2003;62:73–76. doi: 10.1016/s0920-9964(02)00441-3. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Lonnqvist J, Wahlbeck K, Klaukka T, Niskanen L, Tanskanen A, Haukka J. 11-year follow-up of mortality in patients with schizophrenia: A population-based cohort study (FIN11 study) Lancet. 2009;374:620–627. doi: 10.1016/S0140-6736(09)60742-X. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. 2008;2010 Retrieved from http://www.health.gov/paguidelines. [Google Scholar]
- Vreeland B, Minsky S, Menza M, Rigassio Radler D, Roemheld-Hamm B, Stern R. A program for managing weight gain associated with atypical antipsychotics. Psychiatric Services. 2003;54:1155–1157. doi: 10.1176/appi.ps.54.8.1155. [DOI] [PubMed] [Google Scholar]
- Weber M, Wyne K. A cognitive/behavioral group intervention for weight loss in patients treated with atypical antipsychotics. Schizophrenia Research. 2006;83:95–101. doi: 10.1016/j.schres.2006.01.008. [DOI] [PubMed] [Google Scholar]