Summary
Plasmacytoid urothelial carcinoma is a rare but aggressive variant of bladder cancer with no clear therapeutic guidelines. Dysregulation of the mammalian target of rapamycin (mTOR) pathway has been linked to oncogenesis in conventional bladder cancer. Several antineoplastic agents targeting mTOR pathway are currently available. This study assesses mTOR pathway status as well as c-myc and p27 expression. We retrieved 19 archival cases of plasmacytoid urothelial carcinoma from two institutions. Whole tissue sections were evaluated for immunoexpression of phosphatase and tensin homolog (PTEN), phosphorylated mTOR, phosphorylated protein kinase B (AKT), phosphorylated S6, c-myc, and p27. We evaluated intensity (0 to 3+) and extent (0%–100%) of expression for all markers. An H score was calculated as the sum of products of intensity and extent for each marker and used during analysis. In addition, PTEN loss was defined as absence of expression in >10% of tumor cells. We encountered PTEN loss in 28%. Higher H score for nuclear phosphorylatedAKT and a lowerHscore for phosphorylated S6 was encountered in muscle invasive tumors compared to non-muscle invasive tumors (P = .007 and P = .009, respectively). Although a trend for negative prognostic impact on overall survival for higher phosphorylated mTOR expression was noted (P = .051), markers expression levels failed to predict survival in our cohort. We found dysregulation of mTOR pathway members in urinary bladder plasmacytoid urothelial carcinoma, suggesting that the use of mTOR pathway inhibitors might be beneficial for patients with this aggressive tumor.
Keywords: Mammalian target of rapamycin, PTEN, Plasmacytoid urothelial carcinoma, Bladder
1. Introduction
Divergent differentiation is a relatively common feature of invasive urothelial carcinoma with the most common variants being those with squamous and or glandular differentiation [1]. Other histologic variants have also been described, including plasmacytoid urothelial carcinoma. This rare variant was initially described by Sahin et al [2] and Zukerberg et al [3] in 1991 and is currently recognized in the 2004 World Health Organization classification of urothelial neoplasms [1]. The reported incidence of plasmacytoid urothelial carcinoma ranges from less than 1% to 3% [4,5].
Despite advances in multidisciplinary treatment approach, muscle-invasive bladder cancer continues to inflict a high mortality rate [6]. In recent years, a greater understanding of the molecular pathways involved in urothelial oncogenesis has been achieved. Dysregulation of the mammalian target of rapamycin (mTOR) pathway has been linked to oncogenesis in several malignancies, including conventional bladder cancer [7]. This pathway plays an important role in cell growth, migration, and proliferation and offers a potential target of therapy [8]. Activation of mTOR pathway occurs by upstream activation of phosphatidylinositol 3-kinase (PI3K) and protein kinase B (AKT) as well as inactivation of phosphatase and tensin homolog (PTEN) tumor suppressor gene. This will result in up-regulation of protein translation via two main downstream effectors: phosphorylated S6 protein and eukaryotic translation initiation factor 4E-BP1 (4E-binding protein-1) [9]. Furthermore, cell cycle progression through p27kip1 (p27) depletion [10] and cell proliferation through c-myc up-regulation [11] also result from activation of this pathway. Recently, several reports have described the clinico-pathological and immunohistochemical characteristics of plasmacytoid urothelial carcinoma emphasizing its high histological grade, advanced stage at presentation and the shorter overall survival of patients suffering from this variant compared to conventional invasive urothelial carcinoma [4,5,12–15].
The rarity of plasmacytoid urothelial carcinoma makes the evaluation of therapeutic modalities in this type of tumor a difficult endeavor. Identifying new therapeutic targets is therefore of interest. The current study assesses the expression status of the mTOR pathway related biomarkers (PTEN, phosphorylated AKT, phosphorylated mTOR, phosphorylated S6 protein, p27 and c-myc) in plasmacytoid urothelial carcinoma.
2. Materials and methods
This study was approved by the Johns Hopkins University School of Medicine and Emory University School of Medicine Institutional Review Boards.
2.1. Patient cohort
The surgical pathology files of Johns Hopkins Hospital, Emory University School of Medicine and the personal consult service of two of the authors were queried for all cases with a diagnosis of plasmacytoid urothelial carcinoma. Twenty eight cases of plasmacytoid urothelial carcinoma were retrieved, and slides were reviewed by a senior uropathologist for confirmation of the original diagnosis, according to the 2004 World Health Organization criteria. Briefly, the criteria (DX criteria %) included presence of malignant cells showing abundant eosinophilic cytoplasm and eccentrically placed nuclei with small nucleoli and a discohesive single cell growth pattern. Paraffin blocks were available in 19 cases for immunohistochemistry. All medical records were reviewed for pertinent clinical information, including age, sex, stage, and outcome.
2.2. Immunohistochemistry
A representative block from each case was selected, and standard immunohistochemical (IHC) staining was performed on whole sections of formalin-fixed, paraffin-embedded tissue. In tumors showing additional component of invasive conventional or divergent urothelial carcinoma, a section with pure plasmacytoid carcinoma was used for IHC. IHC stains were performed using antibodies against the following mTOR pathway members: PTEN, phosphorylated-mTOR (phos-mTOR), phosphorylated-AKT (phos-AKT), and phosphorylated-S6 protein (phos-S6); AKT-regulated markers p27 and c-myc were also evaluated. Antibody dilutions, clones, and vendors are specified in Table 1.
Table 1.
Summary of antibody specifications
| Vendor | Clone | Pre-treatment | Dilution | |
|---|---|---|---|---|
| PTEN | Cell Signaling (Beverly, MA) | D4.3 | EDTA, 45 min | 1:100 |
| c-MYC | Epitomics (Burlingame, CA) | Y69 | EDTA, 45 min | 1:300 |
| p27 | Transduction Lab | 57 | Citrate, 25 min | 1:4000 |
| Phos-AKTa | Cell Signaling | 736E11 | EDTA, 45 min | 1:50 |
| Phos-S6 b | Cell Signaling | Polyclonal | EDTA, 45 min | 1:200 |
| Phos-mTOR | Cell Signaling | 49F9 | EDTA, 45 min | 1:50 |
Phosphorylation site at Ser473.
Phosphorylation site at Ser235/236.
Immunostaining was performed using a Novocastra Power-Vision Poly-HRP IHC Detection Systems (Leica Microsystems, Bannockburn, IL). Sections were deparaffinized, rehydrated, and subjected to heat-induced antigen retrieval with a buffer solution using a steamer. Sections were then incubated with appropriate primary antibody. After the application of a secondary polyclonal rabbit antibody (except for c-myc, for which the Dako Catalyzed Signal Amplification System Kit was used), slides were developed using 3-3′-diaminobenzidine chromogen and counterstained with hematoxylin. Proper cell lines were used as external controls [16].
2.3. Scoring system
Cytoplasmic PTEN expression was evaluated by two approaches: (i) in each case we identified three 400× power fields with lowest percentage of expression (“cold spots”) where an H score was calculated as the sum of the products of the intensity (0, 1+, 2+, and 3+) and the extent of percentage of positive cells (0%–100%); an average H score was used per case during analysis. (ii) In the second approach, a tumor was categorized as showing PTEN loss when complete loss of any expression (intensity 0) was found in >10% of the tumor cells (<90% expression present), in the presence of adequate internal control (see Fig. 1) [16].The remaining markers (cytoplasmic phos-mTOR and phos-S6, cytoplasmic and nuclear phos-AKT and nuclear expression of c-myc and p27) were evaluated by identifying three 400× power fields with highest percentage of positivity (“hot spots”) in each tumor where an H score was calculated as the sum of the products of the intensity (0, 1+, 2+, and 3+) and the extent of percentage of positive cells (0%–100%); here again, an average H Score was used per case during analysis.
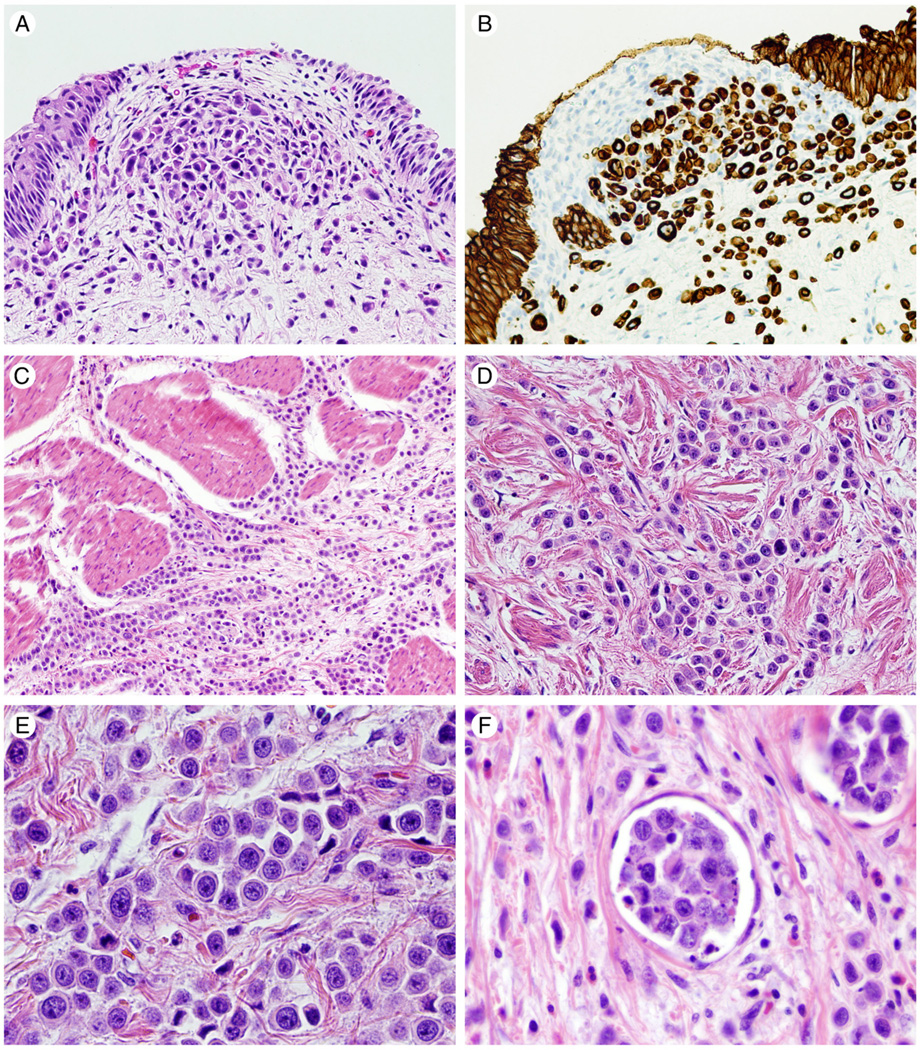
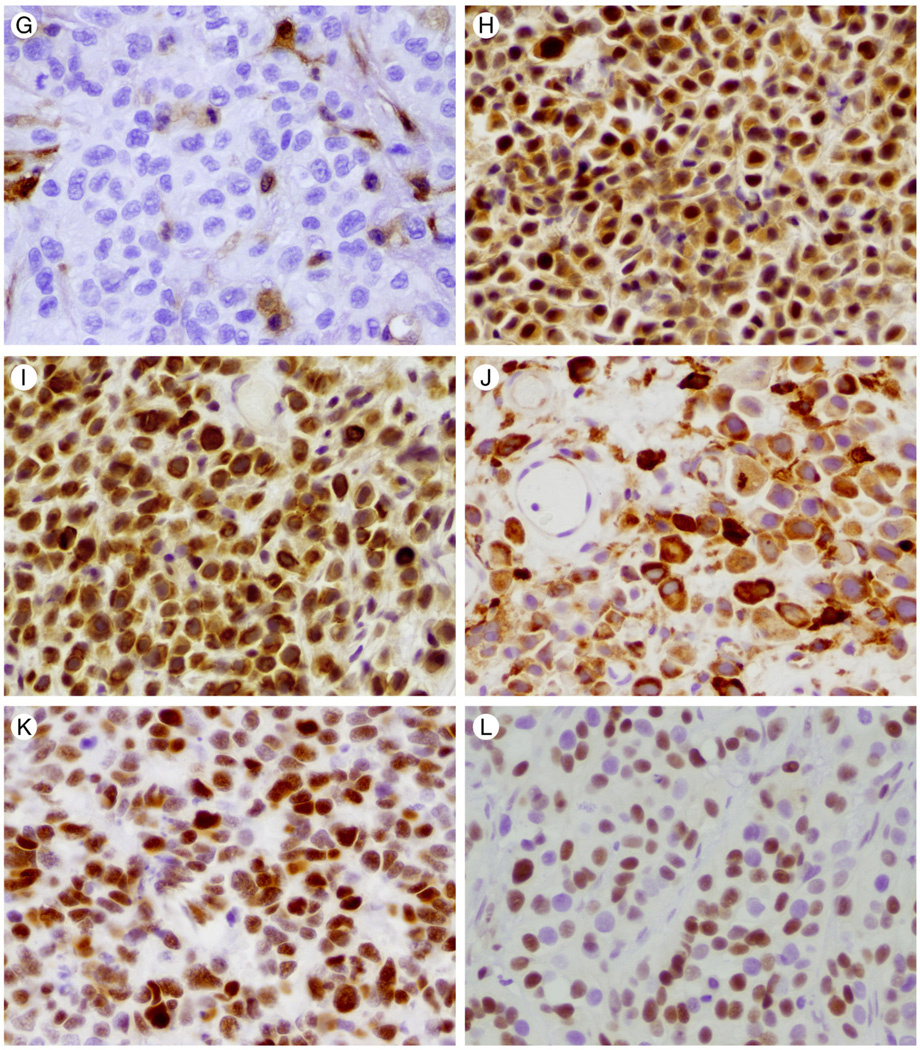
Fig. 1.
Hematoxylin and eosin stained photomicrographs of plasmacytoid urothelial carcinoma are shown in panels A, C, D, E, F. Muscularis propria invasion is shown in C and lymphovascular invasion in F. (×100 and ×400, respectively). B reveals plasmacytoid urothelial carcinoma immunohistochemical positivity for CK8/18 (×200). Representative immunohistochemical staining for PTEN, mTOR pathway members, c-myc, and p27 in tumor cells are illustrated in 1G-1L (×400). G illustrates loss of PTEN staining. Positive PTEN staining in endothelial cells is used as an internal control. H reveals nuclear and cytoplasmic staining for phos-AKT. Cytoplasmic staining for phos-mTOR and phos-S6 is shown in I and J respectively. K and L depict nuclear staining for c-myc and p27, respectively.
H score is used as a surrogate for measuring “total” amount of a marker protein expression in examined population of tumor cells. Given the usually encountered variability in intensity of expression among tumor cells, the H Score method is expected to better reflect the amount of protein expression by factoring in the proportional spectrum of different intensities rather than assigning a tumor marker expression level based only on cells with highest (or lowest) intensity.
2.4. Statistical analysis
Findings were analyzed using the SPSS Statistics 17.0 (SPSS, Chicago, IL) software package. Continuous variables were expressed as median and range; categorical variables were expressed as percentages. The parametric Pearson’s correlation coefficient test and the non-parametric Spear-man’s correlation coefficient test were calculated to evaluate correlations among IHC expression of pathway markers. Associations between markers and the clinico-pathological characteristics were assessed using Wilcoxon rank-sum test, Fisher exact test, χ2 test and Kruskal-Wallis test. Overall survival (OS) and disease-specific survival (DSS) intervals were estimated using Kaplan-Meier method and were calculated from the date of diagnosis to the date of death. The log-rank test was performed to compare the survival distributions with different levels of markers expression. P < .05 was considered to indicate statistical significance.
3. Results
3.1. Patient cohort
Of the 19 patients, 16 (84%) were men with a median age at diagnosis of 68 years (56–93 years). Thirteen patients (68%) were white. Thirteen of the 19 patients (68%) presented with pT2+ disease (6 pT2 on TURB; 4 pT3a and 1 pT3b on cystectomy and 2 clinically non-resectable T4). The remaining 6 TURB revealed invasion of lamina propria where muscularis propria was not sampled. Median length of follow-up was 242 days (31–792 days). Lymph node status was available in 6 patients, 4 were stage pN0, and the remaining 2 were stage pN2. Fourteen patients died during follow-up (74%); 8 documented to be dead of disease. Information on metastasis status was available on 15 patients. Visceral metastases were documented in 3 patients (19%). Metastatic sites included the omentum and colon, anorectal region and peritoneum, and small bowel; 1 patient each. All 3 metastases were biopsy-proven showing similar plasmacytoid morphologic characteristics to their primary counterparts. Clinicopathologic characteristics are summarized in Table 2.
Table 2.
Cohort clinico-pathological characteristics and outcome
| Parameter | n (%) |
|---|---|
| Median age (y), median (range) | 68 (56–93) |
| Ethnicity | |
| White | 13 (68) |
| African-American | 1 (5) |
| Unknown | 5 (26) |
| Gender | |
| Male | 16 (84) |
| Female | 3 (16) |
| Pathologic T stage | |
| pT1 | 6 (32) |
| pT2 | 6 (32) |
| pT3a/T3b | 5 (26) |
| pT4 | 2 (10) |
| Lymph node metastasis | |
| No (pN0) | 4/6 (67) |
| Yes (>pN0) | 2/6 (33) |
| Visceral metastasis | |
| No | 13/16 (81) |
| Yes | 3/16 (19) |
| Overall survival | |
| Alive | 5 (26) |
| Dead | 14 (74) |
| Disease-specific survival | |
| Alive | 5/13 (38) |
| Dead of disease | 8/13 (62) |
The invasive urothelial carcinoma was purely of the plasmacytoid variant in all cases with the exception of three tumors; one also showing conventional high grade urothelial carcinoma and the remaining 2 showing nested and rhabdoid variants each. Six tumors demonstrated urothelial carcinoma in situ; 3 cases had noninvasive high-grade papillary urothelial carcinoma, and 1 case contained a noninvasive low-grade papillary urothelial carcinoma.
3.2. Biomarkers of immunohistochemical expression
Table 3 summarizes all markers’ expression. Cytoplasmic PTEN expression was evaluable in 18 cases. All cases showed some degree of PTEN expression with a median PTEN H score of 100 (60–118). PTEN loss was encountered in 5 (28%) cases using the >10% loss of expression approach (method “ii” above).
Table 3.
Biomarkers H score levels of expression
| Biomarker | Median H-score (range) |
Mean H score ± SD |
|---|---|---|
| Cytoplasmic phos-AKT | 228 (67–295) | 211 ± 66 |
| Nuclear phos-AKT | 189 (0–277) | 151 ± 93 |
| Phos-mTOR | 238 (97–285) | 218 ± 54 |
| Phos-S6 | 262 (164–298) | 248 ± 39 |
| c-myc | 7 (0–223) | 51 ± 75 |
| PTENa | 100 (60–118) | 93 ± 45 |
| p27 | 28 (0–225) | 61 ± 78 |
PTEN loss: 5/18 (28%), defined as absence of expression in >10% of tumor cells, see Material and methods.
Cytoplasmic and nuclear phos-AKT expression was evaluated in all 19 cases. While cytoplasmic expression was present in all (100%) tumors, nuclear expression was present only in 16 (84%). The median H score was 228 (67–295) for cytoplasmic and 189 (0–277) for nuclear expression.
Cytoplasmic phos-mTOR expression was evaluable in all 19 cases. All cases showed some degree of phos-mTOR expression with a median H score of 238 (97–285).
Cytoplasmic phos-S6 expression was evaluable in 19 cases. All cases showed some level of phos-S6 expression. The median H score was 262 (164–298).
C-myc was evaluated in 16 cases. Eleven cases (69%) showed some degree of positivity. The median expression was 7 (0–223).
p27 was evaluated in 17 cases. Six cases (35%) were negative (H score=0) for p27. The median expression was 28 (0–225).
3.2.1. Correlation of biomarker expression with clinico-pathological parameters
Table 4 summarizes the distribution of biomarkers expression in the relevant clinico-pathological categories including gender, pT stage and pT stage groups. With the exception of higher H score in nuclear phos-AKT expression and a lower H score for phos-S6 expression in muscle invasive tumors compared to non-muscle invasive tumors (P = .007 and P = .009, respectively), no other statistically significant association between markers expression levels and assessed parameters was found.
Table 4.
Associations between biomarkers expression and relevant clinico-pathological characteristics
| PTEN |
Phos-AKT |
Phos-mTOR | Phos-S6 | c-myc | p27 | |||
|---|---|---|---|---|---|---|---|---|
| Loss a (%) |
H Score | Cytoplasmic | Nuclear | |||||
| Gender | ||||||||
| Male | 3/15 (20) | 100 (13–174) | 217 (67–295) | 203 (0–277) | 247 (97–285) | 263 (164–298) | 6 (0–223) | 28 (0–225) |
| Female | 2/3 (67) | 97 (67–131) | 246 (175–268) | 64 (59–185) | 156 (150–238) | 203 (197–251) | 25 (6–44) | 87 (0–175) |
| P value | .172 | .983 | .817 | .301 | .171 | .138 | .792 | .985 |
| pT stage | ||||||||
| pT1 | 2/5 (40) | 100 (13–157) | 200 (67–230) | 26 (0–217) | 243 (97–270) | 276 (264–291) | 5 (0–154) | 26 (0–225) |
| pT2 | 0/6 (0) | 120 (39–174) | 237 (68–280) | 217 (136–240) | 246 (191–275) | 235 (174–282) | 6 (0–187) | 50 (0–175) |
| pT3 | 3/5 (60) | 97 (67–114) | 268 (175–295) | 192 (59–277) | 170 (150–285) | 238 (164–298) | 77 (0–223) | 1 (0–107) |
| pT4 | 0/2 (0) | 66 (33–99) | 248 (246–250) | 212 (189–236) | 248 (240–256) | 253 (244–263) | 28 (0–57) | 134 (48–221) |
| P value | .108 | .417 | .285 | .051 | .598 | .086 | .772 | .520 |
| pT stage groups: | ||||||||
| Muscle invasive | ||||||||
| No (pT1) | 2/5 (40) | 100 (13–157) | 200 (67–230) | 26 (0–217) | 243 (97–270) | 276 (264–291) | 5 (0–154) | 26 (0–225) |
| Yes (pT2+) | 3/13 (23) | 99 (33–174) | 246 (68–295) | 215 (59–277) | 238 (150–285) | 244 (164–298) | 8 (0–223) | 40 (0–221) |
| P value | .583 | .616 | .068 | .007 * | .831 | .009 * | .806 | .700 |
| Organ confined | ||||||||
| Yes (bpT3) | 2/11 (18) | 113 (13–174) | 203 (67–280) | 160 (0–240) | 246 (97–275) | 264 (174–291) | 5 (0–187) | 28 (0–225) |
| No (≥pT3) | 3/7 (43) | 97 (33–114) | 250 (175–295) | 192 (59–277) | 195 (150–285) | 244 (164–298) | 50 (0–223) | 25 (0–221) |
| P value | .326 | .296 | .125 | .260 | .592 | .261 | .467 | .949 |
| Lymph node metastasis | ||||||||
| No (pN0) | 2/4 (50) | 98.5 (67–107) | 276 (200–295) | 159 (64–277) | 175 (148–285) | 271 (164–298) | 24 (0–223) | 53.50 (0–225) |
| Yes (NpN0) | 1/2 (50) | 90.50 (67–114) | 177 (175–179) | 148 (59–238) | 160 (150–170) | 217 (197–238) | 110 (110–110) | 3 (3–3) |
| P value | 1.000 | .814 | .064 | .643 | .643 | .355 | .480 | 1.000 |
| Visceral metastasis | ||||||||
| No | 3/13 (23) | 113 (13–174) | 228 (68–284) | 215 (0–240) | 240 (150–275) | 247 (164–290) | 11 (0–223) | 32 (0–175) |
| Yes | 1/2 (50) | 98 (97–99) | 246 (132–268) | 64 (53–189) | 231 (156–256) | 251 (244–271) | 44 (5–57) | 26 (0–221) |
| P value | .476 | .838 | .916 | .230 | .611 | .611 | 1.000 | 1.000 |
Statistically significant.
Lymph node and visceral metastasis status was available in only 3 and 4 of the 5 cases with PTEN loss, respectively.
3.2.2. Correlation between biomarkers
There was no significant correlation between PTEN and the remaining markers expression levels. This was true with either method of evaluating PTEN.
H score of cytoplasmic phos-mTOR correlated with that of cytoplasmic phos-AKT expression (coefficient of correlation [cc] = 0.54 “moderate”, P = .017). H score of cytoplasmic phos-S6 expression was inversely correlated with that of nuclear c-myc expression (cc = −0.67 “moderate”, P = .005).
In the 5 cases that demonstrated PTEN loss, correlative higher than median expression of downstream pathway phos-S6 was found in 2 cases with concomitant elevation of phos-mTOR and phos-AKT (nuclear and cytoplasmic) in 1. Two additional tumors revealed higher than median expression of phos-AKT (cytoplasmic and or nuclear). The fifth case did not reveal the expected elevation of downstream members of the mTOR pathway.
3.2.3. Outcome analysis
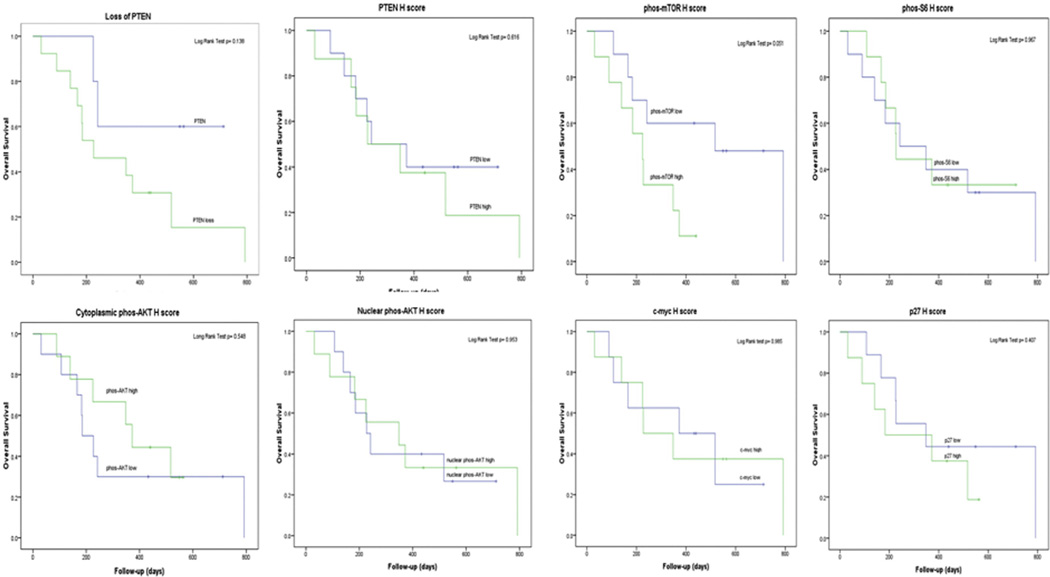
On follow-up, OS of 37% and 29% was observed at 1 and 2 years post diagnosis. DSS rates were 54% and 43% at 1 and 2 years, respectively. As illustrated in Table 5, there was no statistically significant association between levels of expression of any of the analyzed markers and OS or DSS on univariate analysis. While a trend for higher median H score for phos-mTOR expression was noted in association with death (and death of disease), this was not statistically significant. As shown in Fig. 2, no statistically significant correlation was found between markers expression and outcome on Kaplan-Meier survival curve analysis. Again the trend for negative prognostic impact on OS for higher than median phos-mTOR H Score expression only approached statistical significance (P = .051).
Table 5.
Association between biomarkers expression and tumor stage, OS and DSS
| OS |
P | DSS |
P | |||
|---|---|---|---|---|---|---|
| Alive | Dead | Alive | Dead | |||
| pT stage a | .146 | .254 | ||||
| pT1 | 2/6 (33) | 4/6 (67) | 2/3 (67) | 1/3 (33) | ||
| pT2 | 0/6 (0) | 6/6 (100) | 0/3 (0) | 3/3 (100) | ||
| pT3 | 3/5 (60) | 2/5 (40) | 3/5 (60) | 2/5 (40) | ||
| pT4 | 0/2 (0) | 2/2 (100) | 0/2 (0) | 2/2 (100) | ||
| pT Stage Groups: | ||||||
| Muscle invasive Status b | 1.000 | .510 | ||||
| No (pT1) | 2/6 (33) | 4/6 (67) | 2/3 (67) | 1/3 (33) | ||
| Yes (pT2+) | 3/13 (23) | 10/13 (77) | 3/10 (30) | 7/10 (70) | ||
| Organ confined b | .305 | 1.000 | ||||
| Yes (<pT3) | 2/12 (17) | 10/12 (83) | 2/6 (33) | 4/6 (67) | ||
| No (≥ pT3) | 3/7 (43) | 4/7 (57) | 3/7 (43) | 4/7 (57) | ||
| Lymph node metastasis b | ||||||
| No (pN0) | 4/4 (100) | 0/4 (0) | .067 | 4/4 (100) | 0/4 (0) | .067 |
| Yes (>pN0) | 0/2 (0) | 2/2 (100) | 0/2 (0) | 2/2 (100) | ||
| Biomarkers | ||||||
| PTEN loss (%)b | 3/5 (60) | 2/5 (40) | .099 | 3/4 (75) | 1/4 (25) | .222 |
| PTEN H scorec | 97 (23–107) | 113 (13–174) | .277 | 97 (23–107) | 99 (33–131) | .558 |
| phos-AKT cytoplc | 268 (67–295) | 217 (68–280) | .309 | 268 (67–295) | 204 (132–250) | .267 |
| phos-AKT nuclearc | 127 (0–277) | 202 (0–240) | .686 | 127 (0–277) | 204 (53–240) | .622 |
| phos-mTORc | 156 (97–285) | 247 (150–275) | .130 | 156 (97–285) | 234 (150–256) | .284 |
| phos-S6 c | 281 (164–298) | 254 (174–290) | .298 | 281 (164–298) | 231 (174–271) | .171 |
| c-mycc | 5 (0–223) | 8 (0–187) | .758 | 5 (0–223) | 31 (0–187) | .567 |
| p27c | 0 (0–225) | 30 (0–221) | .731 | 0 (0–225) | 48 (3–221) | .427 |
Chi-Square test.
Fisher’s exact test.
Wilcoxon rank-sum test.
Fig. 2.
Graphic Kaplan-Meier overall survival curve analysis. PTEN loss was defined as absence of expression in >10% of tumor cells. For “PTEN H Score” and all remaining markers, high H score was defined as H score > median. Low H score was defined as ≤the median H score.
4. Discussion
Plasmacytoid urothelial carcinoma is a rare but aggressive histological variant of urothelial carcinoma. While 3 cases have been described of non-invasive plasmacytoid urothelial carcinoma [13,17], most are invasive tumors diagnosed at advanced pathological stage and carry a poor prognosis. In our series, all tumors were invasive, with over two-thirds of the cases presenting with pT2 or higher disease. Almost three quarters of our patients died during follow-up. The median age at diagnosis was 68 years, which is in agreement with previous reports [14,15].
Recently, Keck et al. [5] found TP53 mutations in 9 of 31 plasmacytoid urothelial carcinomas (29%) but illustrated lack of FGFR3 and PI3KCA mutations in plasmacytoid urothelial carcinoma. The mTOR pathway is a key regulator of protein translation and cell proliferation that has been shown to be up-regulated in several solid malignancies [18–23] frequently in association with PTEN loss [24–26]. We and others have previously demonstrated PTEN loss and activation of the mTOR pathway in urothelial carcinoma [7,27,28]. In our previous study assessing mTOR in a cohort of 132 urothelial carcinomas from cystectomy patients [7], we detected a generally lower level of expression of PTEN and downstream mTOR pathway members in urothelial carcinomas with divergent histology including those with squamous, sarcomatoid and micropapillary differentiation compared to conventional urothelial carcinoma. The current study is the first to evaluate mTOR pathway expression status in plasmacytoid urothelial carcinoma. In the current cohort, we found PTEN loss of expression in 28% of tumors. The latter is in line with the rate of up to 50% PTEN “reduced expression” previously reported in conventional urothelial carcinoma [27,29] and our prior study finding of low H score of PTEN expression in urothelial carcinoma with divergent histology [7]. Differences in methodology between the current study evaluating whole sections and our previous analysis using tissue microarrays should be taken into consideration [7]. Nevertheless, current plasmacytoid urothelial carcinoma cases revealed overall higher H score expression levels for activated downstream mTOR pathway markers: phos-AKT (mean 151 ± 93) and phos-S6 (mean 248 ±39) compared to urothelial carcinoma cases with divergent histology included in our previous study (3 and 10 respectively) [7].
Loss of PTEN and overexpression of downstream markers in plasmacytoid urothelial carcinoma were not found to be correlated in our current study. This lack of association between PTEN loss and higher levels of expression of downstream markers in our plasmacytoid urothelial carcinoma cases suggests that alternate molecular mechanisms other than the PTEN tumor suppressor gene could be at play in controlling downstream mTOR activation. The latter has been suggested to be at play in other solid tumors [30]. On the other hand, our finding of the biologically expected positive correlation between cytoplasmic phos-mTOR and cytoplasmic phos-AKT is reassuring.
Several mTOR inhibitor agents are now available and are actively under evaluation for the treatment of solid tumors including conventional urothelial carcinoma of the bladder [31–34]. In this regard, our current findings of loss of PTEN and activation of mTOR pathway expression could be viewed as an opportunity to explore the role of mTOR inhibitors in the treatment of plasmacytoid urothelial carcinoma. This would seem especially merited given the disappointing outcome in these tumors with current management protocols.
Our finding of c-myc expression in 69% of the cases should be interpreted with caution given the overall low level of expression encountered (median H score of 7). Additional studies evaluating whether the c-myc oncogene is implicated in plasmacytoid urothelial carcinoma oncogenesis are needed. Our finding of a negative correlation between cytoplasmic phos-S6 and nuclear c-myc expression is in contrast to previously suggested role for c-myc in driving S6 translation [35].
Although a trend for worse OS was suggested in association with higher than median phos-mTOR H score, the trend did not achieve statistical significance. None of the markers analyzed in our study proved to be of prognostic significance in plasmacytoid urothelial carcinoma. The expression levels of mTOR pathway members and pathway related markers p27 and c-myc did not correlate with disease stage, presence of metastasis, OS or DSS. Whether any of the currently studied markers will prove to be of value in predicting therapy response to mTOR inhibitors in plasma-cytoid urothelial carcinoma remains to be seen. Future studies confirming the above observed mTOR pathway alterations at the genomic level would be of value.
A limitation of our study is the small number of cases due to the rarity of this tumor. The limited available follow-up and neo/adjuvant treatment is another potential weakness. Many of our patients were treated and followed up in their local community. Another limitation of our study is the small number of events encountered, which limits the statistical power for identifying prognostic factor.
In conclusion, our study is the first to elucidate immunohistochemical evidence of dysregulation of the mTOR pathway in plasmacytoid urothelial carcinoma of the bladder. Our findings may lend support to explore the utility of mTOR pathway-targeted therapy in these aggressive tumors.
Footnotes
Disclosure: This study was partially supported by the Johns Hopkins Medicine–Patana Fund for Research and Clinical Innovator Award from Flight Attendant Medical Research Institute (FAMRI) Fund.
This study was partially presented at the 2012 Annual Meeting of the American Urological Association in Atlanta, GA.
References
- 1.Eble JN, Sauter G, Epstein JI, Sesterhenn IA. WHO classification of tumors. Lyon: IARC Press; 2004. Pathology and Genetics: tumors of the urinary system and male genital organs. [Google Scholar]
- 2.Sahin AA, Myhre M, Ro JY, Sneige N, Dekmezian RH, Ayala AG. Plasmacytoid transitional cell carcinoma. Report of a case with initial presentation mimicking multiple myeloma. Acta Cytol. 1991;35:277–280. [PubMed] [Google Scholar]
- 3.Zukerberg LR, Harris NL, Young RH. Carcinomas of the urinary bladder simulating malignant lymphoma. A report of five cases. Am J Surg Pathol. 1991;15:569–576. doi: 10.1097/00000478-199106000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Gaafar A, Garmendia M, de Miguel E, et al. Plasmacytoid urothelial carcinoma of the urinary bladder. A study of 7 cases. Actas Urol Esp. 2008;32:806–810. doi: 10.1016/s0210-4806(08)73939-1. [DOI] [PubMed] [Google Scholar]
- 5.Keck B, Stoehr R, Wach S, et al. The plasmacytoid carcinoma of the bladder-rare variant of aggressive urothelial carcinoma. Int J Cancer. 2011;129:346–354. doi: 10.1002/ijc.25700. [DOI] [PubMed] [Google Scholar]
- 6.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 7.Schultz L, Albadine R, Hicks J, et al. Expression status and prognostic significance of mammalian target of rapamycin pathway members in urothelial carcinoma of urinary bladder after cystectomy. Cancer. 2010;116:5517–5526. doi: 10.1002/cncr.25502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 9.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 10.Viglietto G, Motti ML, Fusco A. Understanding p27(kip1) deregulation in cancer: down-regulation or mislocalization. Cell Cycle. 2002;1:394–400. doi: 10.4161/cc.1.6.263. [DOI] [PubMed] [Google Scholar]
- 11.Dominguez-Caceres MA, Garcia-Martinez JM, Calcabrini A, et al. Prolactin induces c-Myc expression and cell survival through activation of Src/Akt pathway in lymphoid cells. Oncogene. 2004;23:7378–7390. doi: 10.1038/sj.onc.1208002. [DOI] [PubMed] [Google Scholar]
- 12.Mai KT, Park PC, Yazdi HM, et al. Plasmacytoid urothelial carcinoma of the urinary bladder report of seven new cases. Eur Urol. 2006;50:1111–1114. doi: 10.1016/j.eururo.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 13.Fritsche HM, Burger M, Denzinger S, Legal W, Goebell PJ, Hartmann A. Plasmacytoid urothelial carcinoma of the bladder: histological and clinical features of 5 cases. J Urol. 2008;180:1923–1927. doi: 10.1016/j.juro.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 14.Nigwekar P, Tamboli P, Amin MB, Osunkoya AO, Ben-Dor D, Amin MB. Plasmacytoid urothelial carcinoma: detailed analysis of morphology with clinicopathologic correlation in 17 cases. Am J Surg Pathol. 2009;33:417–424. doi: 10.1097/PAS.0b013e318186c45e. [DOI] [PubMed] [Google Scholar]
- 15.Raspollini MR, Sardi I, Giunti L, et al. Plasmacytoid urothelial carcinoma of the urinary bladder: clinicopathologic, immunohisto-chemical, ultrastructural, and molecular analysis of a case series. Hum Pathol. 2011;42:1149–1158. doi: 10.1016/j.humpath.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Lotan TL, Gurel B, Sutcliffe S, et al. PTEN protein loss by immunostaining: analytic validation and prognostic indicator for a high risk surgical cohort of prostate cancer patients. Clin Cancer Res. 2011;17:6563–6573. doi: 10.1158/1078-0432.CCR-11-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coyne J, Sim E. Urothelial neoplasia with plasmacytoid morphology. Histopathology. 2006;48:200–201. doi: 10.1111/j.1365-2559.2005.02153.x. [DOI] [PubMed] [Google Scholar]
- 18.Ching CB, Hansel DE. Expanding therapeutic targets in bladder cancer: the PI3K/Akt/mTOR pathway. Lab Invest. 2010;90:1406–1414. doi: 10.1038/labinvest.2010.133. [DOI] [PubMed] [Google Scholar]
- 19.Miyakawa M, Tsushima T, Murakami H, Wakai K, Isozaki O, Takano K. Increased expression of phosphorylated p70S6 kinase and Akt in papillary thyroid cancer tissues. Endocr J. 2003;50:77–83. doi: 10.1507/endocrj.50.77. [DOI] [PubMed] [Google Scholar]
- 20.Feng W, Brown RE, Trung CD, et al. Morphoproteomic profile of mTOR, Ras/Raf kinase/ERK, and NF-kappaB pathways in human gastric adenocarcinoma. Ann Clin Lab Sci. 2008;38:195–209. [PubMed] [Google Scholar]
- 21.Hartman TR, Nicolas E, Klein-Szanto A, et al. The role of the Birt-Hogg-Dube protein in mTOR activation and renal tumorigenesis. Oncogene. 2009;28:1594–1604. doi: 10.1038/onc.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schultz L, Chaux A, Albadine R, et al. Immunoexpression status and prognostic value of mTOR and hypoxia-induced pathway members in primary and metastatic clear cell renal cell carcinomas. Am J Surg Pathol. 2011;35:1549–1556. doi: 10.1097/PAS.0b013e31822895e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armstrong AJ, Netto GJ, Rudek MA, et al. A pharmacodynamic study of rapamycin in men with intermediate- to high-risk localized prostate cancer. Clin Cancer Res. 2010;16:3057–3066. doi: 10.1158/1078-0432.CCR-10-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King JC, Xu J, Wongvipat J, et al. Cooperativity of TMPRSS2-ERG with PI3-kinase pathway activation in prostate oncogenesis. Nat Genet. 2009;41:524–526. doi: 10.1038/ng.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carver BS, Tran J, Gopalan A, et al. Aberrant ERG expression cooperates with loss of PTEN to promote cancer progression in the prostate. Nat Genet. 2009;41:619–624. doi: 10.1038/ng.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pantuck AJ, Seligson DB, Klatte T, et al. Prognostic relevance of the mTOR pathway in renal cell carcinoma: implications for molecular patient selection for targeted therapy. Cancer. 2007;109:2257–2267. doi: 10.1002/cncr.22677. [DOI] [PubMed] [Google Scholar]
- 27.Harris LD, De La Cerda J, Tuziak T, et al. Analysis of the expression of biomarkers in urinary bladder cancer using a tissue microarray. Mol Carcinog. 2008;47:678–685. doi: 10.1002/mc.20420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qian CN, Furge KA, Knol J, et al. Activation of the PI3K/AKT pathway induces urothelial carcinoma of the renal pelvis: identification in human tumors and confirmation in animal models. Cancer Res. 2009;69:8256–8264. doi: 10.1158/0008-5472.CAN-09-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Platt FM, Hurst CD, Taylor CF, Gregory WM, Harnden P, Knowles MA. Spectrum of phosphatidylinositol 3-kinase pathway gene alterations in bladder cancer. Clin Cancer Res. 2009;15:6008–6017. doi: 10.1158/1078-0432.CCR-09-0898. [DOI] [PubMed] [Google Scholar]
- 30.Bose S, Chandran S, Mirocha JM, Bose N. The Akt pathway in human breast cancer: a tissue-array-based analysis. Mod Pathol. 2006;19:238–245. doi: 10.1038/modpathol.3800525. [DOI] [PubMed] [Google Scholar]
- 31.Netto GJ. Molecular biomarkers in urothelial carcinoma of the bladder: are we there yet? Nat Rev Urol. 2011;9:41–51. doi: 10.1038/nrurol.2011.193. [DOI] [PubMed] [Google Scholar]
- 32.Bradley DA, Dunn R, Nanus D, et al. Randomized, double-blind, placebo-controlled phase II trial of maintenance sunitinib versus placebo after chemotherapy for patients with advanced urothelial carcinoma: scientific rationale and study design. Clin Genitourin Cancer. 2007;5:460–463. doi: 10.3816/CGC.2007.n.037. [DOI] [PubMed] [Google Scholar]
- 33.Mansure JJ, Nassim R, Chevalier S, Rocha J, Scarlata E, Kassouf W. Inhibition of mammalian target of rapamycin as a therapeutic strategy in the management of bladder cancer. Cancer Biol Ther. 2009;8:2339–2347. doi: 10.4161/cbt.8.24.9987. [DOI] [PubMed] [Google Scholar]
- 34.Seront E, Rottey S, Sautois B, et al. Phase II study of everolimus in patients with locally advanced or metastatic transitional cell carcinoma of the urothelial tract: clinical activity, molecular response, and biomarkers. Ann Oncol. 2012 doi: 10.1093/annonc/mds057. http://dx.doi.org/10.1093/annonc/mds057. [DOI] [PubMed]
- 35.Schmidt EV, Ravitz MJ, Chen L, Lynch M. Growth controls connect: interactions between c-myc and the tuberous sclerosis complex-mTOR pathway. Cell Cycle. 2009;8:1344–1351. doi: 10.4161/cc.8.9.8215. [DOI] [PMC free article] [PubMed] [Google Scholar]