Abstract
Ideal oncology drugs would be curative after a short treatment course if they could eliminate epithelium-originated carcinomas at their non-invasive, pre-malignant stages. Such ideal molecules, which are expected to molecularly abrogate all the instrumental mechanisms acquired by migrating cancer stem cells (CSCs) to by-pass tumour suppressor barriers, might already exist. We here illustrate how system biology strategies for repositioning existing FDA-approved drugs may accelerate our therapeutic capacity to eliminate CSC traits in pre-invasive intraepithelial neoplasias. First, we describe a signalling network signature that overrides bioenergetics stress- and oncogene-induced senescence (OIS) phenomena in CSCs residing at pre-invasive lesions. Second, we functionally map the anti-malarial chloroquine and the anti-diabetic metformin (“old drugs”) to their recently recognized CSC targets (“new uses”) within the network. By discussing the preclinical efficacy of chloroquine and metformin to inhibiting the genesis and self-renewal of CSCs we finally underscore the expected translational impact of the “old drugs–new uses” repurposing strategy to open a new CSC-targeted chemoprevention era.
Keywords: Breast cancer, DCIS, Autophagy, Hypoxia, EMT, Cancer stem cells, OIS, Oncogene-induced senescence
1. Introduction
An effective reduction of breast cancer mortality largely depends on our therapeutic ability to successfully intervene in the critical transition from non-invasive ductal carcinoma in situ (DCIS) to life-threatening invasive breast cancer (IBC). Our ever increasing knowledge of molecular and pathway biology in DCIS lesions can facilitate hypothesis-driven therapeutic strategies aimed to arrest invasion at the pre-malignant state of BC disease (Espina and Liotta, 2011). Because DCIS cells should adapt to survive in the highly stressful microenvironment of the intraductal niche (Gatenby and Gillies, 2008; Menendez and Lupu, 2007), they must circumvent hypoxia-induced apoptotic death while avoiding nutrient stress-induced senescence. Beyond evading biophysical constraints, DCIS cells must make use of alternative sources of energy such as the autophagic pathway, a major catabolic process that may permit DCIS starving cells to recycling intracellular components during periods of metabolic stress to maintain homeostasis and viability (Lum et al., 2005; Mathew et al., 2007). Remarkably, this metabolic adaptation appears to occur in DCIS tumour-founding progenitor cells because pre-malignant, cytogenetically abnormal DCIS spheroid-forming cells directly isolated from human DCIS lesions have increased expression of autophagy-associated proteins that persist in culture and in tumours generated by these cells in immunosupressed NOD/SCID mice (Espina et al., 2010). Given that: (a) the anti-autophagy small-molecule chloroquine has been found to kill DCIS progenitor spheroids and prevent their tumorigenicity in mice via reduced expression of autophagy-associated proteins (Espina et al., 2010) and (b) the BC invasive phenotype is already genetically programmed at pre-invasive stages of disease progression (i.e. DCIS lesions), pharmacological abrogation of autophagy may be viewed as a novel therapeutic strategy for BC chemoprevention. This scenario strongly supports the Preventing Invasive Neoplasia with Chloroquine (PINC) trial (NCT01023477), which will measure the effectiveness of chloroquine administration to patients with low-grade, intermediate-grade or high-grade DCIS to directly test the hypothesis that pharmacological blockade of autophagy is an effective treatment for DCIS (Espina and Liotta, 2011).
Developing a known drug (e.g. chloroquine, which is the drug of choice used for the prophylaxis treatment of malaria because it is effective, low toxic to humans, and inexpensive) for another clinical purpose (e.g. prevention of the invasive progression of pre-malignant lesions such as DCIS in BC) is termed repositioning or repurposing. This approach can be very effective to develop new oncology therapeutics since many existing drugs have been studied for their pharmacokinetics and safety profiles and often have already been approved by the regulatory agencies FDA (US), EMEA (Europe) and MHLW (Japan). Here, we exemplify how system biology strategies for repositioning regulatory agencies-approved drugs chloroquine and metformin may accelerate our therapeutic capacity to prevent invasive progression of pre-invasive intraepithelial neoplasias by eliminating cancer stem cell (CSC) traits (Fig. 1). First, we illustrate a signalling network signature that CSCs should necessarily acquire to successfully override intrinsic tumour suppressor barriers (e.g. metabolic- and oncogene-induced senescence) activated in pre-malignant lesions. Second, we functionally map the anti-malarial chloroquine and the anti-diabetic metformin (the “old drugs”) to their presumed CSC molecular targets (the “new uses”) within the network: chloroquine, by inhibiting autophagy, is expected to impede a crucial manner of energy production that allows CSCs to survive hypoxic and nutrient-deprived microen-vironments. Metformin, by preventing the molecular transition of epithelial tumour cells to embryonic mesenchymal pheno-types (EMT), is expected to block an essential senescence escape mechanism while nullifying EMT-driven CSC features. We finally discuss the preclinical efficacy of the repositioned drugs to inhibiting the genesis and self-renewal of CSCs, thus underscoring the translational impact of the “old drugs–new uses” repurposing strategy, which may rapidly provide us with ideal, curative oncology drugs able to arrest epithelium-originated carcinomas at their noninvasive, pre-malignant stages.
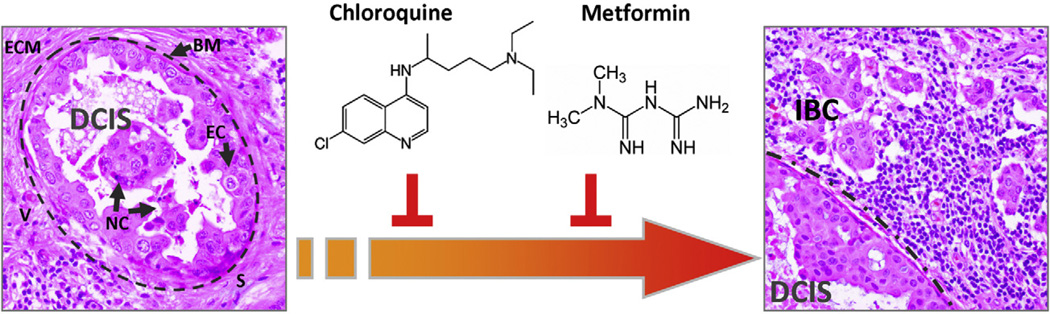
Fig. 1.
Prevention and treatment of pre-malignant lesions for accelerated development of existing anti-CSC drugs. Cell adaptation to chronic stressful conditions that occur in oxygen-and nutrient-starved areas of pre-malignant DCIS lesions (left) could lead to the generation of CSC within the mass of cells accumulating in the duct before the onset of BC invasion (right). Repositioning pre-existing drugs such as chloroquine and metformin to molecularly impede all the instrumental mechanisms acquired by migrating CSCs to by-pass tumour suppressive barriers may open a new chemoprevention era aimed to curatively inhibit the genesis and self-renewal of CSCs (BM: basement membrane -dashed line; EC: epithelial cells; ECM: extracellular matrix; IBC: invasive breast cancer; S: stroma; V: blood vessels).
2. Autophagy and oncogene-induced senescence (OIS): more than friends
Besides biophysical stress-induced senescence, DCIS lesions should circumvent also oncogene-induced senescence (OIS) (Braig and Schmitt, 2006; Collado and Serrano, 2010; Serrano, 2010) before they develop into IBC. Permanent activation of certain oncogenic pathways causes cell senescence by default and many human transformed cells, before reaching full malignancy, stop proliferating and undergo senescence at the pre-malignant (non-invasive) stage, at which senescence-inducing signals (e.g. oncogenic proteins, oxidative stress, persistent DNA damage) reach sufficient intensity to be effective (Braig and Schmitt, 2006; Collado and Serrano, 2010; Serrano, 2010). Proliferating IBC cells with activated oncogenes, therefore, truly represent progeny of tumour cells that have acquired mechanisms to suppress OIS in earlier stages of BC pathogenesis (e.g. DCIS). In this regard, landmark studies have revealed that autophagy is a causal pre-requisite for senescence (Young and Narita, 2010) and, accordingly, interference with autophagy impedes stress-induced senescence and significantly attenuates the extent of OIS. This scenario might appear to contradict a recent suggestion that autophagy is a main determinant of DCIS cell fate because it allows DCIS cells to survive and proliferate by evading cell cycle arrest/senescence responses to the high-stress microenvironment of the intraductal space (Espina et al., 2010). In fact, although autophagy does contribute to tumour suppression by actively controlling the senescence phenotype, if tumour cells somehow bypass OIS, autophagy would then help such cells to survive DCIS-associated biophysical stresses, unwittingly facilitating their full transformation. A causal relationship between autophagy and cell survival in DCIS lesions has been illustrated by the fact that ATG6/Beclin-1 (a haploinsufficient tumour suppressor protein that is essential for autophagy (Karantza-Wadsworth et al., 2007; Liang et al., 1999)) is upregulated in human comedo-DCIS at the viable rim of intraductal cells within the hypoxic ductal niche. On the contrary, deletion or monosomy of the ATG6/Beclin-1 gene significantly associates with ERBB2 oncogene amplification (both on 17q21) in a subset of BC (Negri et al., 2010). Because ERBB2 overexpression can be found in ~25% of invasive/metastastic BC, but it takes place in 50–60% of DCIS in general and 60–70% of high-grade DCIS, these findings altogether raise the interesting question about whether an enhanced autophagic flux, while indispensable in terms of DCIS cell survival (Espina and Liotta, 2011; Espina et al., 2010; Gatenby and Gillies, 2008; Lum et al., 2005; Mathew et al., 2007; Menendez and Lupu, 2007), is also necessary and/or sufficient to promote progression from non-invasive to IBC in different cell origin subtypes of DCIS (Hannemann et al., 2006; Muggerud et al., 2010).
2.1. Loss of autophagic genes: a chance to escape from the senescence prison
The apparent paradox that a metastasis-promoting oncoprotein (e.g. ERBB2) is more frequently overexpressed in non-invasive DCIS than in IBC is consistent with the prevailing view that a single oncogene is insufficient to drive IBC progression. Because ERBB2 can be expected to trigger stress-induced premature senescence and growth arrest of pre-malignant DCIS lesions, ERBB2 overexpression likewise requires a collaborating molecular scenario (“second hits”) to convert the cytostatic nature of the senescence program into a pro-survival, mitogenic, and invasive process (Lu et al., 2009; Muthuswamy et al., 2001). Defective autophagy imposed by loss of ATG6/Beclin-1 could promote the occurrence of ERBB2 gene amplification by increasing genomic instability (Karantza-Wadsworth et al., 2007). Loss of ATG6/Beclin-1 may provide also an efficient mechanism to bypass ERBB2-induced OIS, thus enhancing the ability of ERBB2 to promote DCIS progression to IBC (Vazquez-Martin et al., 2011a,b,c). Does this mean that chloroquine will be ineffective when used against ERBB2-positive/ATG6-negative DCIS? We can only answer that the transition of subsets of (ERBB2-gene amplified) DCIS to (ERBB2-gene amplified) IBC may occur through evolutionary molecular mechanisms involving defective autophagy but maintaining an intact cell senescence response. Accordingly, ERBB2-positive/ATG6-defective advanced BC have been found to significantly retain a functional cell senescence pathway in terms of CDKN2A (p16INK4a) expression, further supporting the notion that pivotal genes controlling autophagy and cell senescence might obey a “principle of exclusiveness” (i.e. only one gene is generally deregulated among those with similar function and belonging to the same pathway (Vogelstein and Kinzler, 2004)). This scenario may explain the following facts: (a) treatment with the anti-ERBB2 antibody trastuzumab induces both the activation of autophagy (Vazquez-Martin et al., 2009a,b) and the restoration of tumour suppressor responses to induce cell senescence (Arnal-Estape et al., 2010) in ERBB2/ATG6-positive models of advanced BC in vitro, and (b) loss of ATG6/Beclin-1 in ERBB2-positive BC predicts better responses to trastuzumab alone or in combination with cytotoxics in the presence of competent apoptosis or senescence pathways in advanced BC in vivo (Negri et al., 2010).
Whereas in bona fide ERBB2-positive BC, the ERBB2 oncoprotein is overexpressed in cancer stem cells (CSCs) and in bulk tumour populations, it is also possible that the ERBB2 oncoprotein will be selectively overexpressed in CSC but not bulk BC cell populations (Liu and Wicha, 2010; Magnifico et al., 2009). The crucial findings by Magnifico et al. (2009) revealing that BCSCs express the highest level of ERBB2 oncoprotein regardless ERBB2 gene amplification status and that high levels of ERBB2 protein are indispensable for BCSC survival in non ERBB2-gene amplified cell populations, may provide an alternative scenario in which upregulation of autophagic (e.g. Beclin-1) and oncogenic (e.g. ERBB2) proteins may concurrently occur in biophysically stressed DCIS tumour-founding progenitor cells in the absence of OIS-like responses. It would be relevant to assess whether specific, transcriptional enhancement of ERBB2 expression in tumour-founding DCIS progenitor cells is part of the genetic program triggered by a high-stress intraductal microenvironment. Moreover, could we detect tumour-funding DCIS progenitors by immunohistochemically assessing co-upregulation of beclin-1 and ERBB2 proteins at the viable cell rim within the hypoxic, nutrient-deprived ductal niche? Further studies are required to evaluate the utility of immunohistochemistry in the identification of autophagic tumour cells that might express the BCSC mesenchymal phenotype CD44hiCD24low/− (Al-Hajj et al., 2003), the frequency of which is actively enhanced by ERBB2 (Wang et al., 2010a), in autophagic zones of hypoxic stress in pre-invasive intraductal carcinomas.
3. Epithelial-to-mesenchymal transition (EMT): bypassing oncogene- and metabolic stress-induced senescence to create cancer stem cell traits
We are beginning to recognize the existence of a complex functional interplay between OIS, autophagy and the ability of (normal and transformed) epithelial cells to acquire tumour-initiating, stem cell-like features via induction of the epithelial-to-mesenchymal transition (EMT) genetic program (Fig. 2). These crossing paths certainly merit to be carefully considered as they may underlie the generation of so-called CSCs during tumour initiation (i.e. DCIS) and invasive/metastatic dissemination (Ansieau et al., 2008; Kalluri and Weinberg, 2009; Mani et al., 2008; Morel et al., 2008; Smit and Peeper, 2010; Weinberg, 2008). As mentioned above, additional genetic/epigenetic events are needed for oncogene-harbouring DCIS cells to gain invasive capability that can efficiently drive progression of DCIS into IBC. Ansieau et al. demonstrated that whereas ectopically expressed ERBB2 induces senescence (i.e. OIS), co-overexpression of both the transcription factor Twist, a prototypic EMT driver, and ERBB2 triggers EMT and allows for senescence bypass in human (Ansieau et al., 2008). By being able to inhibit the expression of p16INK4a and p21WAF1/CIP1 tumour suppressor proteins, EMT-inducing transcription factors are instrumentally exploited by evolving premalignant cells to avoid or escape OIS. This molecular scenario in which the cellular transdifferentiation EMT program functions early in tumour progression by preventing OIS can also drive the acquisition of bona fide stem and tumorigenic features characteristics of CSCs. This crucial linkage of the EMT process with the CSC traits (i.e. high-grade malignancy, notably invasive and metastatic powers) operates through two prerequisites of tumour cell invasion, namely increased cell migration and loss/reduction of cell–cell adhesion, which are required by migrating CSC to obtain the “capability” (migration) and the “freedom” (dissemination) to escape from the original rigid constraints of the surrounding tissue architecture (Kalluri and Weinberg, 2009; Lu et al., 2009; Smit and Peeper, 2010).
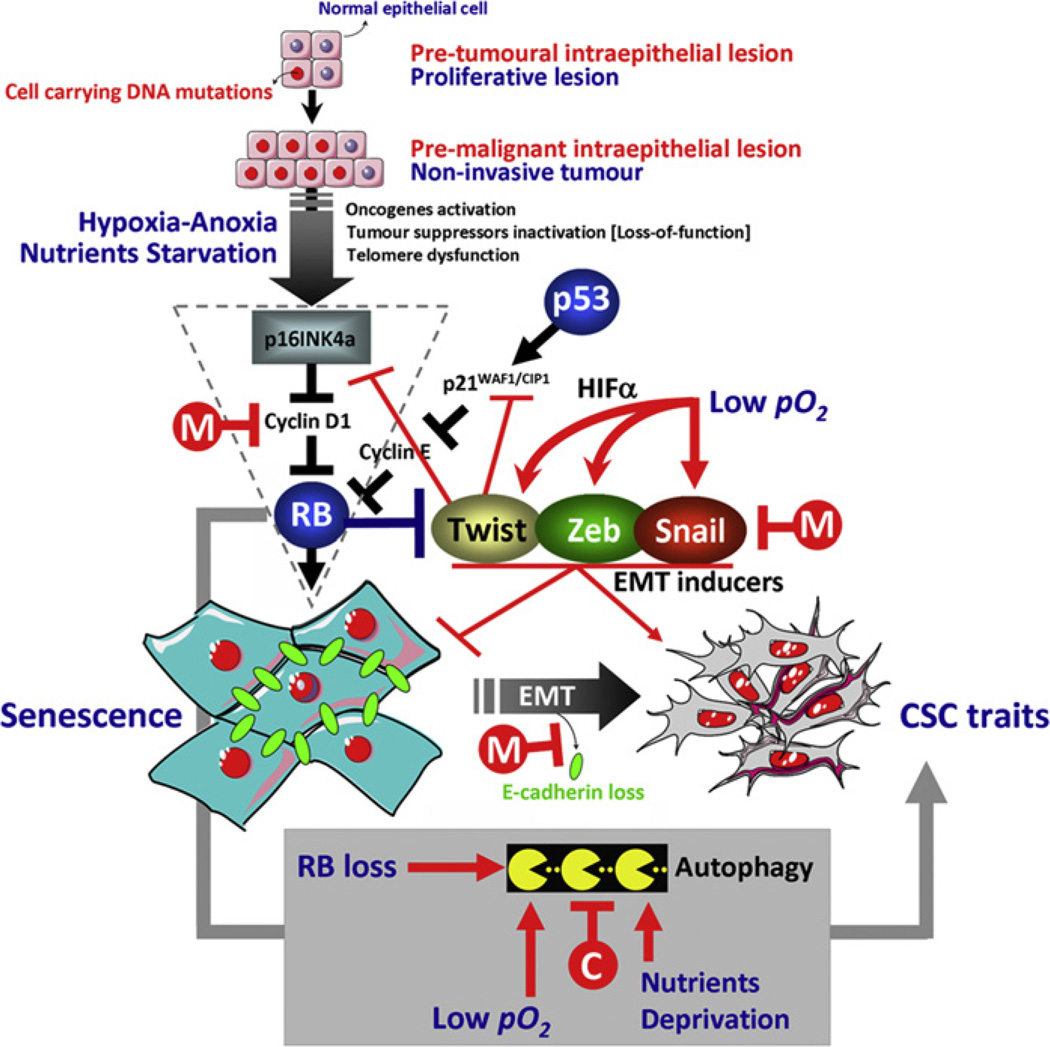
Fig. 2.
The bioenergetics-EMT-cancer initiating cell axis in the pathogenesis of epithelium-originated carcinomas: a roadmap in the pursuit of targeted chemo-prevention. Convergence of EMT-driven acquisition of stem cell traits with bioenergetics stress-induced autophagy in pre-malignant phenotypes may yield cancer-initiating cells that efficiently couple invasive/metastatic spread to the bypass of metabolic stress- and oncogene-induced cellular senescence. Overgrowth in intraepithelial pre-malignant lesions with an increased autophagic activity may be taken as the expansion of hypoxia-adapted, EMT-enriched CSCs populations with increased metabolic demands and, presumably, of aggressive behaviour. Drugs such as chloroquine and metformin able to simultaneously modulate the RB tumour suppressor pathway, the EMT phenomenon and/or the autophagy machinery can deliver a multiple inhibitory effect to efficiently prevent invasive progression of pre-malignant lesions (C: chloroquine; M: metformin; RB: retinoblastoma).
Loss of expression/function of the tumour invasion suppressor E-cadherin (CDH1), which is essential for the maintenance of adherent junctions between neighbouring cells and thus confers physical integrity on epithelial cells, represents the central molecular process required for EMT-mediated increase in invasion/metastasis. If activation of EMT generates migrating CSCs by directly linking E-cadherin loss-related acquisition of cellular motility with the maintenance of tumour-initiating (stemness) capacity, it could be envisioned that the hypoxic, nutrient-deprived intraductal microenvironment might directly promote the derepression of the invasive phenotype via EMT activation/E-cadherin suppression. Indeed, hypoxia has been demonstrated to efficiently promote EMT through direct regulation of Twist expression through the hypoxic response mediator hypoxia inducible factor-1 (HIF-1) (Peinado and Cano, 2008; Yang et al., 2008). Other authors have suggested, however, that hypoxic BC cells are only partially pushed towards EMT because hypoxia fails to universally promote a migratory phenotype despite it notably induces the expression of the transcription factor Snail, an other inducer of EMT (Lundgren et al., 2009). Recent studies have confirmed that hypoxia increases the expression of transcription factors Snail and Slug and decreases E-cadherin expression, two hallmarks of the EMT phenomenon (Chen et al., 2010). Thus, hypoxia’s ability to simultaneously inhibit senescence and maintain mesenchymal stem cell properties through down-regulation of p21WAF1/CIP1 via HIF-Twist1 is not redundant in relation to other hypoxia upregulated EMT regulators (e.g. Snail) (Chen et al., 2010; Lundgren et al., 2009; Tsai et al., 2011; Yang and Wu, 2008). Furthermore, new approaches that experimentally mimicked chronic exposure to hypoxia and reoxygenation occurring in solid tumours due to an irregular or a-vascular microenvironment, have demonstrated that stemlike BC cell subpopulations could be expanded through repetitive hypoxia/reoxygenation cycles without genetic manipulation (Louie et al., 2010). Of note, tumorigenic breast CSCs populations enriched by hypoxia/reoxygenation treatments exhibited both stem-like and EMT phenotypes (Louie et al., 2010). These findings strongly suggest that DCIS-associated hypoxia favours EMT, allowing the emergence of stem-like properties in subpopulations of DCIS cells capable of developing metabolic tolerance to limiting availability of oxygen and nutrients.
4. DCIS→ IBC “funnel factors”: cellular stress sensors to predict recurrence
An intriguing possibility relates to the occurrence of DCIS → IBC “funnel factors” (Armengol et al., 2007) through which all the pro-invasive and pro-senescence signals would inevitably have to pass. These would act as a “bottleneck” through which biophysical stress-induced senescence, OIS and EMT promoters can converge to channel the invasive/metastatic signals regardless of the upstream-specific metabolic/transforming alteration. In other words, these funnel factors should integrate a cross-talk between senescence and EMT in response to energy metabolism and oncogenic alterations to reflect the invasive potential of DCIS lesions. In this regard, a compromised retinoblastoma (RB) pathway (e.g. via loss of the RB1 itself, loss of the p16INK4A locus, translocation of cyclin D1, etc.) (Burkhart and Sage, 2008; Weinberg, 1995) would permit DCIS cells not only to disregard bioenergetics stress signals (Espina and Liotta, 2011) but also to bypass the senescence program triggered by unscheduled oncogenic signalling (i.e. OIS). OIS relies on the activation of tumour suppressor gene networks such as RB and various mechanisms aimed to directly or indirectly inactivate the senescence function of RB (Chicas et al., 2010) can be observed in a subset of intrinsically aggressive DCIS (Gauthier et al., 2007; Simpson et al., 2007). Moreover, abrogation of the RB tumour suppressor activity may cooperate with oncogenic signalling to promote EMT-related tumour cell invasion by suppressing, at the transcriptional level, the expression of CDH1 (E-cadherin) gene (Arima et al., 2008; Batsche et al., 1998). Indeed, inhibition of EMT can be considered a novel tumour suppressor function of RB because:
RB depletion reduces E-cadherin expression, disrupts cell–cell adhesion and induces a mesenchymal-like phenotype in cultured BC cells by upregulating EMT-related transcription factors (e.g. Zeb, Snail) (Arima et al., 2008).
RB overexpression blocks the ability of the master regulator of EMT TGFβ to induce mesenchymal-like morphologies and to suppress E-cadherin expression in breast epithelial cells (Batsche et al., 1998).
Concurrent down-regulation of RB and E-cadherin expression takes place in mesenchymal-like invasive BC (Jiang et al., 2010b).
By providing a joining point between senescence and EMT in DCIS lesions, the RB/p16INK4a tumour-suppressive pathway could be efficiently used by DCIS cells to avoid bioenergetics/oncogenic stress-induced senescence, allowing uncontrolled cell proliferation, cell motility and luminal filling and can be considered instrumental in the transition of oncogene-harbouring DCIS cells to a malignant (locally invasive and metastasizing) stage. Inhibition of the RB pathway can contribute to the invasive progression of pre-malignant lesions due to not only loss of cell proliferation control (Knudsen and Knudsen, 2008) and decreased senescence (Chicas et al., 2010) but also reprogramming of somatic tumour cells to an undifferentiated CSC-like phenotype. Of note, EMT repression as a novel tumour suppressor function of RB that impedes conversion to an invasive phenotype can be linked to RB’s ability to concomitantly regulate autophagy (i.e. transfer of RB to RB-null cells results in the induction of autophagy) (Ciavarra and Zacksenhaus, 2011; Jiang et al., 2010a,b; Tracy et al., 2007). While it remains to be ascertained whether RB-mediated autophagy is required for RB-regulated senescence, RB enforced cell–cell contact inhibition appears to efficiently prevent cell outgrowth into structures where cells with CSC traits can be generated from differentiated somatic cells in advancing invasive carcinomas (Liu et al., 2009b). Accordingly, the expression of biomarkers indicative of an intact “cellular stress response” has been found to strongly associate with a disease-free prognosis in women diagnosed with DCIS whereas the expression of biomarkers indicative of an abrogated response to cellular stress predicts DCIS with worse outcome. Likewise the RB/p16INK4a pathway was identified as the most accurate predictor of recurrence and DCIS progression to EMT-enriched basal-like invasive BC (Gauthier et al., 2007).
5. The ideal DCIS→ IBC inhibitory drug should impact both pro-EMT signals and pro-survival energy metabolism
Ideally, rather than affecting tumour cell proliferation or apoptosis, DCIS → IBC blockers should prevent the occurrence of the mesenchymal, motile, invasive phenotype characteristic of CSCs (Tan et al., 2009), because mammary epithelial cells and BC epithelial cells undergoing EMT induced by TGFβ exhibit better mammosphere-forming capabilities (Creighton et al., 2010; Singh and Settleman, 2010; Taylor et al., 2010; Wang et al., 2010b) and EMT inducers such as TGFβ are not able to activate the EMT genetic program when cells are locked into a senescence state (Ansieau et al., 2010; Ohashi et al., 2010). Thus optimally, DCIS→ IBC blockers should drive the differentiation of mammary stem cells intoductal cells and/or convert invasive, pre-malignant DCIS progenitor cells to a more epithelioid, non-proliferating, non-metastatic phenotype (Fig. 3). On the other hand, they should inhibit adaptive changes in cellular energy metabolism, including autophagy, which facilitates a higher tolerance to limiting oxygen/nutrients availability in DCIS lesions. Agents possessing such properties, like chloroquine and metformin, will be discussed below.
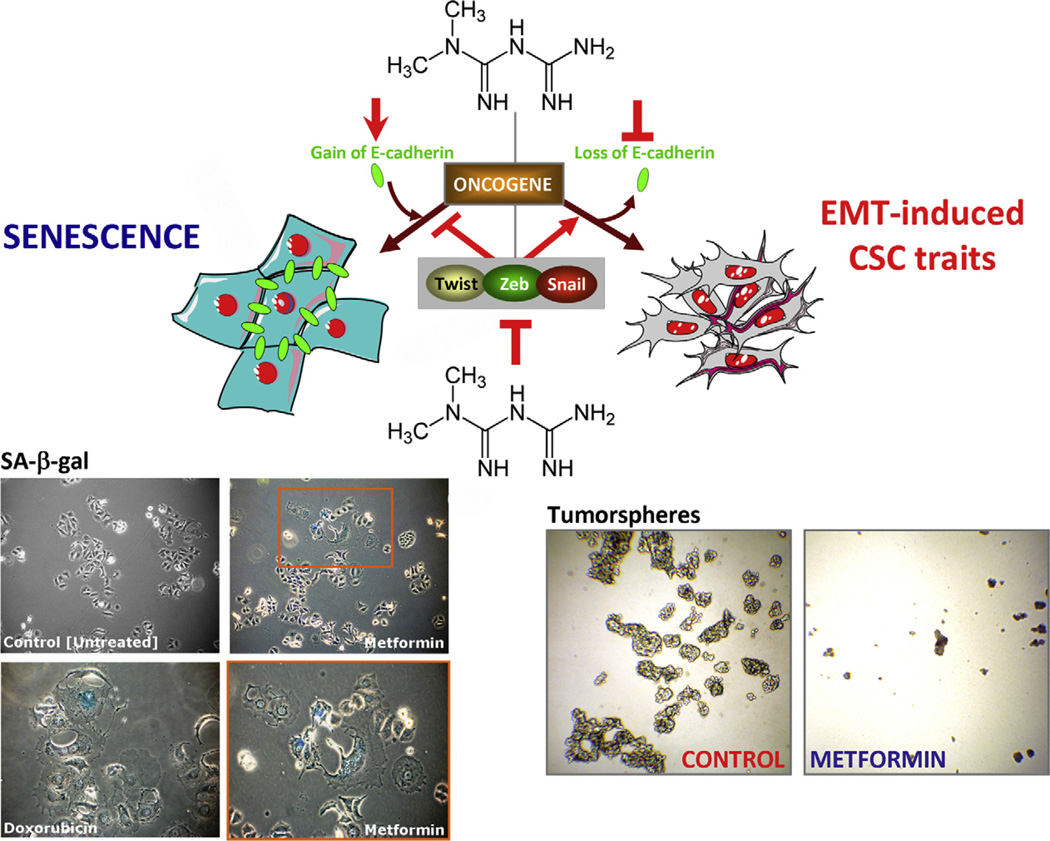
Fig. 3.
Working model schematically depicting how the anti-diabetic drug metformin can alter the cross-path linking EMT and senescence to prevent cancer progression. Oncogenic stimuli can either induce senescence or EMT, depending on the cellular context. Conversely, EMT-inducing transcription factors can simultaneously suppress the oncogene-induced senescence response and induce an EMT, both phenomena contributing to malignant progression. Metformin-based strategies avenues targeting the major players promoting EMT and senescence may have a double inhibitory impact on tumour progression. Figure illustrates that metformin can function as a senescence-inducing stressor in MCF-7 breast epithelial cells (left) while inhibiting both the maintenance and the renewal of CSCs (right) - which are capable of surviving, proliferating and forming discrete clusters of cells termed tumorspheres (Dontu et al., 2003) - in MCF-7 cells engineered to stably overexpress the ERBB2 oncogene (MCF-7/HER2 cells; Menendez et al., 2009).
5.1. Chloroquine
An impaired autolysosomal degradation and accumulation of damaged autolysosomes imposed by the lysosomotropic drug chloroquine or other chloroquine-like agents interfering with the lysosomal degradation function can be expected to cause accelerated cell death under autophagy-inducing conditions (Boya et al., 2005; Kroemer and Jaattela, 2005). Indeed, a crucial biological advantage of pharmacologically arresting autophagy to successfully prevent invasive progression of DCIS lesions relates to the fact that blocking autolysosomal degradation, e.g. by chloroquine, while allowing the autophagic sequestration to continue (e.g. in response to severe metabolic, oxidative, and chronic hypoxic stress occurring in a non-vascular intraductal space) may lead to a vicious cycle of excessive cytoplasmic vacuolization.
In this scenario, engagement of DCIS cells in a chronic autophagic response may be an Achilles heel that sensitizes DCIS progenitors to agents targeting the later steps of the autophagic process of bulk lysosomal degradation and recycling of cytoplasmic material and organelles. Of note, chloroquine-induced lysosomal dilatation not only would render autophagic recycling unproductive but it also may prevent, at least in part, the occurrence of the EMT process. TGFβ can cooperate with oncogenic stimuli to cause EMT and tumour progression by favouring endocytic internalization of E-cadherin, which facilitates the dissolution of adherens junctions and lysosomal degradation of ubiquitinated E-cadherin, which impedes E-cadherin being recycled back to the lateral membrane (Palacios et al., 2005). E-cadherin trafficking to the lysosome serves as a means to ensure that epithelial cells do not reform their cell–cell contacts and phenotypically remain motile or mesenchymal. Although chloroquine-inhibited lysosomal activity cannot block the loss of E-cadherin from the junctional complexes, it can efficiently prevent E-cadherin degradation thus causing accumulation of E-cadherin vesicles (Janda et al., 2006). Whereas loss of E-cadherin via transcriptional repression is a chloroquine-unresponsive late event in EMT, the lysosomal targeting of E-cadherin is a chloroquine-responsive post-translational downregulatory mechanism to deplete E-cadherin during early stages of oncogene-induced EMT. Further studies are warranted to evaluate whether the effect of inhibiting autophagy by chloroquine has also a significant role in TGFβ and/or oncogene-induced EMT and subsequent dedifferentiation to stem cell-like states in DCIS cells.
5.2. Metformin
The biguanide derivative N′,N′-dimethyl-biguanide metformin, an orally administered drug widely used to lower blood glucose concentration in patients with type 2 diabetes and metabolic syndrome, may be a novel prototype drug for preventing invasive progression of DCIS lesions via regulation of CSC biology and DCIS energy metabolism. Many preclinical studies offer molecular explanations for the effects of metformin on the proliferation and survival of fully transformed BC cells (Ben Sahra et al., 2010b; Berstein, 2010; Gonzalez-Angulo and Meric-Bernstam, 2010; Jalving et al., 2010; Martin-Castillo et al., 2010b; Pollak, 2010; Vazquez-Martin et al., 2010a,b). Initial clinical data have shown that metformin use positively associates with enhanced response to neoadjuvant therapy in BC (Jiralerspong et al., 2009). In addition, observational studies suggest that metformin may be useful in BC prevention (Ben Sahra et al., 2010a; Berstein, 2010; Giovannucci et al., 2010; Gonzalez-Angulo and Meric-Bernstam, 2010; Jalving et al., 2010; Martin-Castillo et al., 2010b; Pollak, 2010; Vazquez-Martin et al., 2010a,b). Confirming and expanding a landmark study that reported a reduction in the risk of subsequent cancer diagnosis (including BC) in patients with type 2 diabetes who received metformin and that metformin’s protective effect increased with greater metformin use (i.e. dose and/or time) (Evans et al., 2005), a recently conducted retrospective study has reported an impressive 56% decrease in BC risk among diabetics receiving metformin compared with diabetics treated with other therapies (Bodmer et al., 2010). Because reductions in cancer mortality related to metformin use are similar in magnitude to reductions in cancer incidence (Pollak, 2010), it might be that metformin’s anti-cancer effects largely depend on or are restricted to its preventive effects.
6. Mechanisms of metformin activity
6.1. Systemic actions of metformin to prevent DCIS → IBC
It has been proposed (Horwitz and Sartorius, 2008), that progestins-induced reactivation of CSCs in pre-existing BC may explain why hormone replacement therapies significantly increase BC risk in some women. Because the median prevalence of invasive BC at death was 1.3% and the median prevalence of pre-invasive DCIS lesions was 8.9% in autopsy data from women over 40 years who did not have known BC during life (Welch and Black, 1997), this reservoir of undetected, pre-invasive BC lesions (or dormant CSCs within DCIS) can also underlie the apparent implausibility of increased BC risk within 2 years among diabetic women receiving the insulin analogue glargine (Hemkens et al., 2009; Jonasson et al., 2009; Smith and Gale, 2009). It is biologically plausible that hormonal factors influencing the natural history of BC disease (e.g. oestrogen, insulin, insulin-like growth factor-I [IGF-1]) could manifest their effects in unexpected short timescales considering that they do not reflect the initiation of new tumours but rather the growth of subclinical malignant lesions (Pollak and Russell-Jones, 2010). Besides genetic alterations, progression of DCIS to IBC also appears to involve environmental factors and a role has been postulated for metabolic-endocrine changes (Stoll, 2000; Stoll, 2002). Confirmed markers of high risk for BC in women such as hyperinsulinaemia and the concomitant increase in circulating levels of bioactive IGF-1 and free oestradiol positively associates also with an increased presence of DCIS lesions. Because high levels of IGF-1 and oestrogen can favour the generation or maintenance of mammary tissue-specific stem cells (Bendall et al., 2007; Fillmore et al., 2010; Savarese et al., 2006), pharmacological measures aimed to enhance insulin sensitivity, such as metformin (Goodwin et al., 2008) might reduce the risk of invasive progression in DCIS lesions. If we speculate that regulators of breast stem cell niches – which not only supports the self-renewal and maintenance of stem cell identity but also control stem cell number and proliferation (Li and Neaves, 2006) – such as IGF-1 and oestradiol - similarly function as mitogens for DCIS tumour progenitor cells, than metformin’s ability to decrease circulating insulin and IGF-1 via inhibition of hepatic gluconeogenesis (Pollak, 2008) and to inhibit ovarian steroidogenesis and, hence, systemic concentrations of oestradiol via inhibition of aromatase expression in granulosa cells (Rice et al., 2009), should impose a strict control of the number and proliferation rate of tumour-founding DCIS progenitors. This provides an efficient preventive molecular mechanism against IBC in pre- and post-menopausal women.
6.2. Cell autonomous actions of metformin to prevent DCIS→ IBC
Hirsch and colleagues demonstrated that non-cytotoxic, clinically relevant doses of metformin inhibited cellular transformation and selectively killed the CD44hiCD24low/− CSCs (Hirsch et al., 2009). Taking advantage of the ability of BCSCs to form multicellular “microtumours” in non-adherent and non-differentiating conditions (i.e. “mammospheres”), we have confirmed that metformin treatment reduced both mammosphere-forming efficiency (MSFE) and mammosphere size which indirectly reflects metformin’s ability to suppress both stem self-renewal and progenitor cell proliferation in HER2-positive BC cell populations (Vazquez-Martin et al., 2011a,b,c) (Fig. 3). Moreover, metformin treatment significantly altered the genetic and/or epigenetic plasticity of BCSCs because it re-sensitized mammosphere-initiating, TGFβ-overexpressing CSCs to ERBB2-targeted drugs (Vazquez-Martin et al., 2011a,b,c). This unexpected specific effect of metformin on CSCs within heterogeneous BC populations might be exploited to block the progression of DCIS provided that it disrupts a molecular link between self-renewal, EMT, and acquisition of a migratory phenotype in DCIS tumour progenitor cells.
6.2.1. Metformin as anti-EMT agent
Because TGFβ-induced EMT promotes the ontogenesis of the CD44hiCD24low/− BCSC phenotype (Massague, 2008), we envisioned that metformin treatment might directly impede the BCSC mesenchymal phenotype by transcriptionally repressing the stem cell property EMT triggered in the unusual metabolic situation in DCIS cells growing in the hypoxic microenvironment of pre-malignant BC stages (Vazquez-Martin et al., 2010a,b, 2011a,b,c). Indeed, metformin dynamically suppressed the CD44hiCD24low/− BCSC phenotype via transcriptional repression of the pivotal inducers and drivers of the EMT machinery TGFβs, Zeb, Twist and Slug in highly metastatic basal-like BC cells (Honeth et al., 2008; Vazquez-Martin et al., 2010a,b).
6.2.2. Metformin as anti-invasive agent
Because loss of E-cadherin expression is a hallmark of cells undergoing EMT and invasive/metastatic progression of BC can be viewed as a process of progressive dedifferentiation by EMT (Peter, 2009), we proposed that metformin might prevent breast carcinogenesis by promoting a differentiated epithelial state of BC cells in an E-cadherin-related manner. In the presence of metformin, TGFβ failed to downregulate membranous E-cadherin in epithelial MCF-7 BC cells (Cufi et al., 2010). Metformin-targeted expression of E-cadherin largely prevented the TGFβ-induced scattered, fibroblast-like, spindle-shaped, elongated mesenchymal morphologies as well as the appearance of the mesenchymal marker vimentin (Cufi et al., 2010). Moreover, metformin induced accumulation of E-cadherin at sites of cell–cell contact to promote a more cuboidal appearance of epithelial MCF-7 BC cell cultures, which grew in a more compact, homogeneous structured monolayer (Cufi et al., 2010). The mechanistic explanation might be that metformin acts via regulation of specific micro(mi)RNAs, such as lethal-7 (let-7) (Fig. 4). Expression of the tumour suppressor miRNA let-7 is induced at late stages of development and remains upreg-ulated in the adult organism to ensure permanent repression of embryonic genes in most terminally differentiated tissues (Mineno et al., 2006; Park et al., 2007). Accordingly let-7 is a marker of fully differentiated cells and downregulation of let-7 is a marker of less-differentiated cancer and let-7 expression is undetectable in CSCs (Shell et al., 2007; Yu et al., 2007). The ability of let-7 to regulate self-renewal of tumorigenic BC cells has suggested that restoration or enhancement of let-7 expression can be viewed as a useful therapeutic option in cancer prevention (Yu et al., 2007; Ibarra et al., 2007; Peter, 2009; Boyerinas et al., 2010; Wang et al., 2010c). Metformin has been shown to involve upregulation and maintenance of high levels of let-7a in well-differentiated, epithelioid BC cells exposed to the EMT inducer TGFβ, thus epigenetically preserving the differentiated phenotype of mammary epithelium while preventing EMT-related CSC self-renewal (Oliveras-Ferraros et al., 2011).
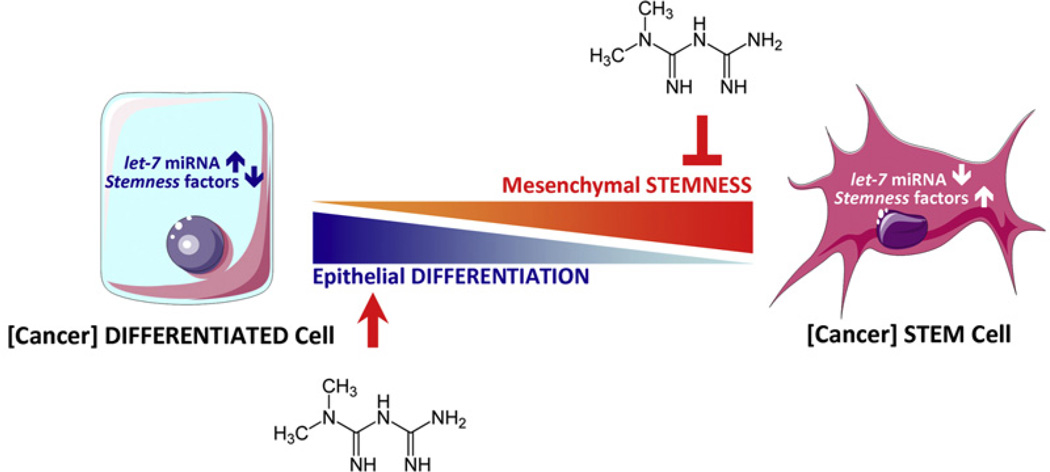
Fig. 4.
Working model schematically depicting how the anti-diabetic drug metformin can alter miRNA-regulated cell differentiation to prevent cancer progression. Inva-sive/metastatic progression of BC can be viewed as a miRNA let-7-regulated continuum of progressive dedifferentiation (i.e. EMT) with a cell at the endpoint that has stem cell-like properties (Peter, 2009). Metformin-enhanced let-7 expression in pre-malignant cells may efficiently push them to become less “embryonic” (i.e. mesenchymal stem-like cells) and more “normal” (non-stem differentiated epithelial cells), thus blocking the dynamic nature of cellular transformation and CSC formation in response not only to oncogenes but also to the stressful local ductal tissue microenvironment.
6.2.3. Metformin as senescence-inducing agent
On the one hand, it cannot be excluded that E-cadherin has a direct role in the induction of cell senescence because several senescence players such as p16INK4a harbour E-boxes in their promoters (Zheng et al., 2004). On the other hand, the transcription factors Twist and Zeb1 can simultaneously suppress the senescence program while inducing EMT, thus coupling invasive/metastatic spread to the bypass of senescence in response to environmental cues of primary tumour cells residing at the invasive edge (Ansieau et al., 2008; Smit and Peeper, 2008). Twist suppresses, at the promoter level, the expression of genes that are central in senescence signalling including p16INK4a and CDKN1A, which encodes the cell-cycle-inhibitory protein p21Waf1/Cip1 (Martin and Cano, 2010; Shiota et al., 2008; Yang et al., 2010). This indirectly suggests that cells with an intact senescence program could not undergo a full EMT. Mesenchymal BC cells refractory to the cell growth inhibitory effects of metformin express significantly lower levels of p21Waf1/Cip1 than metformin-sensitive epithelial BC cells and ectopic overexpression of p21Waf1/Cip1 re-sensitizes mesenchymal BC cells to the cell cycle arresting effects of metformin (Zhuang and Miskimins, 2008). While activation of EMT is linked to suppression of cellular senescence (Liu et al., 2008; Brabletz and Brabletz, 2010), when cancer epithelial cells are locked in a senescent state they cannot undergo EMT (Ohashi et al., 2010). Not surprisingly, cellular senescence is being increasingly recognized as a critical feature of mammalian cells to suppress tumorigenesis (Collado and Serrano, 2010) and, therefore, activating the program of senescence in tumour cells seems an attractive approach to cancer treatment (Roninson, 2003). To evaluate whether metformin can function as a senescence-inducing stressor owing its ability to modulate CDK inhibitory kinases such as p21Waf1/Cip1 and p27Kip1 (Zhuang and Miskimins, 2008; Campaner et al., 2010; Serrano, 2010), we have recently tested the hypothesis that metformin’s ability to increase expression of epithelial proteins such as E-cadherin via inhibition of the “E-cadherin repressor interac-tome” (Hugo et al., 2011) may accelerate tumour cell senescence (Fig. 3). We assessed the occurrence of replicative senescence following acute exposure to metformin (4 h) using the MCF-7 cell line, which retains characteristics of differentiated mammary epithelium. β-Galactosidase expression, a marker of cellular senescence (Dimri et al., 1995; Lee et al., 2006) was present in the MCF-7 cells as early as 3 days after acute exposure to metformin (data no shown). After 1 week, histochemical staining was intense in a significant number of cultured cells. Cells expressing the senescence marker β-galactosidase were typically much larger in size and multinucleated, both of which are morphological features indicative of a senescent state (Rodier and Campisi, 2011). Acute exposure to doxorubicin (1 µmol/L) used as a positive control (Elmore et al., 2002) induced intense β-galactosidase activity in virtually every cell of the culture. Because one could argue that metformin-promoted β-galactosidase staining in MCF-7 cells might only reflect a lack of cell division rather than true senescence, MCF-7 cells were held in a non-dividing state by serum removal for 7 days and stained for β-galactosidase. In contrast to metformin-treated cells, β-galactosidase expression was detected only very rarely in non-dividing MCF-7 cells, consistent with that observed for untreated MCF-7 cells. The EMT process, by crucially contributing to override the tumour suppressive function of cellular senescence, has been implicated in metastatic dissemination, therapeutic resistance and generation of tumour cells with stem/progenitor cell properties (Mani et al., 2008; Polyak and Weinberg, 2009). In this scenario, metformin-based strategies avenues inhibiting the major players promoting EMT to encourage senescence of transformed epithelial cells (Menendez et al., 2011) may have a multiple inhibitory impact on the core molecular machinery driving metastatic tumour progression.
6.2.4. Metformin as multi-targeted agent
Metformin’s ability to target major molecular players promoting EMT and senescence may offer even more augmented benefit for preventing the invasive progression of DCIS lesions for several reasons:
The full manifestation of both senescence bypass and activation of EMT requires the collaboration of EMT-regulating transcription factors with oncogenic signals like ERBB2. Thus, metformin’s ability to decrease ERBB2 tyrosine kinase activity (Alimova et al., 2009) and ERBB2 oncoprotein expression (Vazquez-Martin et al., 2009a,b) might be highly effective against full malignant transformation by simultaneously targeting invasion, motility, and senescence in pre-invasive DCIS lesions.
A compromised function of RB such as by loss-of-heterozygosity is further altered by the expression status of proteins regulating RB phosphorylation and function in cell cycle arrest (commonly observed BC-associated p16INK4a loss and cyclin D1 amplification (Bosco and Knudsen, 2007)). Because of the link between EMT transcription factor overexpression and loss-of-function of the RB pathway, metformin’s ability to promote cyclin D1 mRNA loss (Zhuang and Miskimins, 2008) and strongly reduce cyclin D1 protein level (Ben Sahra et al., 2008; Liu et al., 2009a) might efficiently restore the tumour-suppressive nature of the p16INK4a/Cyclin D1/RB pathway thus promoting senescence of DCIS cells in response to bioenergetics stresses.
An early activation of de novo (endogenous) fatty acid (FA) biosynthesis catalysed by the lipogenic enzymes acetyl-CoA carboxylase (ACACA) and FA synthase (FASN) occurs in hypoxia-tolerant DCIS cells as the need for more oxidizing power when oxygen is limiting. The FASN pathway is used to balance the cellular redox potential through its ability to consume reducing equivalents (i.e. NADPH) as part of its normal function (Esslimani-Sahla et al., 2007; Hochachka, 1980). Metformin-induced activation of the AMP-activated protein kinase (AMPK) rapidly induces phosphorylation of ACACA and thus blocks the formation of malonyl-CoA, the first committed molecule in the endogenous pathway of FA biosynthesis (Brunet et al., 2008). Besides metformin’s ability to acutely shutting-down of the functioning of the lipogenic pathway in an ACACA-related manner, metformin-induced activation of AMPK may chronically decrease the expression of the lipogenic transcription factor SREBP1c (Eberle et al., 2004), thus suppressing the synthesis of FASN and other enzymes that participate actively in endogenous FA biogenesis pathway (Swinnen et al., 2005). Because in order to successfully adapt to an anoxic or unstable oxic-hypoxic acidic microenvironment DCIS cells should necessarily favour synthesis and chain elongation of endogenous FA to make up for the shortfall in oxidizing power imposed by aerobic glycolysis and to allow their autonomy for their own anabolic metabolism despite surrounding starving conditions (Hochachka et al., 2002), metformin’s ability to suppress the lipogenic phenotype may significantly delay progression of DCIS lesions into invasive BC (Algire et al., 2010; Alli et al., 2005; Lu and Archer, 2005; Menendez and Lupu, 2007; Menendez et al., 2004).
Metformin’s ability to activate the metabolic-stress-sensing protein kinase AMPK is expected to stimulate autophagy because activation of AMPK will inhibit mTOR activity (Dowling et al., 2007; Meley et al., 2006; Zakikhani et al., 2006; Zhou et al., 2001) and blocking of mTOR is known stimulate autophagy (Rubinsztein et al., 2007), Only one study has shown that metformin induces autophagy in cancer cells (Buzzai et al., 2007) whereas other AMPK activating drugs such as AICAR have been reported to inhibit autophagy instead (Samari and Seglen, 1998). Although it remains to be elucidated whether the effects of AMPK activating drugs on autophagy might be dose-, cell type-and/or context-dependent, it should be noted that metformin has been recently found to inhibit 2-deoxyglucose (2DG)-induced autophagy, decrease expression of beclin-1 and trigger a switch from an autophagic survival process to apoptotic cell death (Ben Sahra et al., 2010a,c). Treatment with the non-metabolizable glucose analogue 2DG mimics nutrient deprivation of tumour cells addicted to high glucose influxes (the Warburg effect). Therefore, metformin’s ability to suppress Beclin 1-driven blockade of the onset of the apoptotic cascade highlights its potential use as a therapeutic metabolic perturbator in DCIS lesions as it may efficiently trigger cell death in DCIS tumour-founding progenitor cells engaged in the survival autophagic response induced by chronic exposure to hypoxia and nutrient starvation.
7. Metformin as a preventative cancer drug: a clinical perspective
The understanding of the molecular, cellular and microenvironmental factors that contribute to the pathophysiological functioning of pre-malignant lesions may offer new inroads for arresting cancer invasion and metastasis at the pre-malignant stage. In this regard, the interest on cancer-associated metabolic perturbations, such as the Warburg effect, lipid metabolism and autophagy is growing from the cancer prevention perspective. We have learned that new drugs aimed to prevent cancer need to have proven safety for long-term administrations and must first be shown to be efficacious in reducing cancer incidence or mortality (Kelloff and Sigman, 2007). Metformin has a wellestablished safety and tolerability profile and epidemiological data have repeatedly shown a lower risk of cancer onset (incidence) and mortality of patients with type 2 diabetes who use metformin compared with non-users. This fact, together with the ever-growing description of metformin’s anti-cancer actions in rodents and in cultured tumour cells, appears to delineate by far the most favourable clinical scenario for metformin-based cancer prevention trials. However, it has been recently suggested (http://clinicaltrials.gov/ct2/results?term=metformin+cancer), that the use of metformin in ongoing and planned clinical trials (Anisimov, 2010; Goodwin et al., 2011; Martin-Castillo et al., 2010a) would be restricted to subpopulations defined by host insulin levels and/or loss of the AMPK kinase LKB1 (Algire et al., 2011; Memmott and Dennis, 2009). Regardless LKB1 status, metformin treatment was found to abolish the excess tumour growth associated with a high-fat diet and hyperinsulinaemia (Algire et al., 2011). Conversely, the anti-neoplastic activity of metformin, regardless insulin levels, was confined to LKB1-deficient tumours in mice without diet-induced hyperinsulinaemia (Algire et al., 2011). In this scenario, metformin-based large-scale cancer prevention trials would be more justifiable if we could provide criteria to specify high-risk populations in which metformin is expected to provide a greater benefit (Pollak, 2010). We now propose that, beyond insulin- and/or LKB1/AMPK-dependency of the anti-proliferative effects of metformin against proliferating, differentiated tumour cells, metformin’s ability to regulate CSC biology might rather explain beneficial effects of metformin in cancer prevention. From a CSC-oriented perspective, and given that DCIS lesions do contain pre-existing carcinoma precursor cells (Espina and Liotta, 2011; Espina et al., 2010), it would be of interest to evaluate whether metformin use in non-diabetic women may reduce the expected progression rate (12–15%) of DCIS lesions to invasive BC. By utilizing precedent trial designs for tamoxifen-based neoadjuvant therapy of DCIS (Fisher et al., 1998), it might be reasonable to open a clinical trial for metformin-based neoadjuvant therapy of DCIS in which metformin will be administered after diagnosis by a primary biopsy but before the commencement of standard-of-care surgical therapy. By employing precedent trial designs aimed to evaluate the anti-CSC activity of molecularly targeted drugs in a neoadjuvant setting (Li et al., 2008; Lacerda et al., 2010), paired core biopsies would be obtained from patients with DCIS before and after treatment with neoadjuvant tamoxifen plus/minus metformin (oestrogen receptor [ER]-positive DCIS) or metformin alone (ER-negative DCIS). Cells isolated from biopsy samples taken before and after this therapy would be assayed for the proportion of tumorigenic cells and the ability to form mammospheres in vivo as an indication of self-renewal. This approach may be particularly relevant when considering that autophagy has a primary pro-survival role during tamoxifen challenge and that autophagy knockdown may be useful in a combination therapy setting to sensitize tumour cells to tamoxifen therapy (Qadir et al., 2008; Samaddar et al., 2008; Schoenlein et al., 2009).
8. Conclusion
Cancer prevention remains the most promising strategy to reduce the burden of cancer. The ideal prevention strategy of the future would employ a limited course of low toxicity therapy to suppress or eradicate pre-malignant lesions in high-risk cancer patients (Espina and Liotta, 2011). If treating BC before it can become invasive is indeed analogous to the prevention of colon cancer by the removal of pre-malignant polyps (Kelloff and Sigman, 2007; O’Shaughnessy et al., 2002), the recent knowledge gained from a pilot clinical trial that provided evidence that short-term, low-dose metformin (250 mg once daily for 1 month versus typical 500 mg three times daily in diabetes) safely and directly suppresses both colorectal epithelial proliferation and aberrant crypt formation (Hosono et al., 2010), might provide a strong support basis for the clinical application of metformin and other biguanides aimed at suppressing or eradicating pre-malignant lesions.
Chloroquine, the old anti-malarial medication, and metformin, the old anti-diabetes medication, appear to represent successful examples of drug repurposing aimed to eliminate CSC traits in pre-malignant intraepithelial lesions. Although many pharmaceutical companies are currently developing anti-CSC drugs following traditional de novo drug discovery and development – a process that can take 10–17 years from idea to marketed drug (Ashburn and Thor, 2004; Reichert, 2003) – both the development time and risk can be significantly reduced using anti-CSCs repositioning candidates that have been through several stages of clinical development and therefore have well-known safety and pharmacokinetic profiles (Ashburn and Thor, 2004). A further exploration of the unexpected anti-CSC repurposing opportunities of chloroquine and metformin, however, might be problematic because these drugs are both off-patent and therefore generic. Mechanisms proposed to justify investment of new indications for old medicines (Ashburn and Thor, 2004; Morgan, 2010) should now ensure that our ever increasing knowledge of molecular and pathway biology for successful and specific targeting of CSCs may rapidly guide new hypothesis-driven clinical trials aimed to successfully arrest metastatic progression of human malignancies early in the course of the disease. The recent recognition that the malignant phenotype of epithelium-originated carcinomas already exists in cells within intraepithelial pre-malignant lesions (Espina et al., 2010) should accelerate our scanning of existing pharmacopoeia for rapidly repositioning FDA-approved drugs to target biologically distinct regulatory events responsible for the emergence of cancer stemness and cellular invasiveness within a chronically stressed pre-invasive niche.
Acknowledgments
Work at the laboratory of Javier A. Menendez is supported by the Instituto de Salud Carlos III (Ministerio de Sanidad y Consumo, Fondo de Investigación Sanitaria (FIS), Spain, Grants CP05–00090, PI06–0778 and RD06–0020–0028), the Fundación Científica de la Asociación Española Contra el Cáncer (AECC, Spain), and by the Ministerio de Ciencia e Innovación (SAF2009–11579, Plan Nacional de I+D+ I, MICINN, Spain). Alejandro Vazquez-Martin is the recipient of a “Sara Borrell” post-doctoral contract (CD08/00283, Ministerio de Sanidad y Consumo, Fondo de Investigación Sanitaria (FIS), Spain). Sílvia Cufí is the recipient of a Research Fellowship (Formación de Personal Investigador, FPI) by the Ministerio de Ciencia e Innovación (MICINN, Spain).
Footnotes
Conflicts of interest
None to declare.
References
- Algire C, Amrein L, Bazile M, David S, Zakikhani M, Pollak M. Diet and tumor LKB1 expression interact to determine sensitivity to antineoplastic effects of metformin in vivo. Oncogene. 2011;30:1174–1182. doi: 10.1038/onc.2010.483. [DOI] [PubMed] [Google Scholar]
- Algire C, Amrein L, Zakikhani M, Panasci L, Pollak M. Metformin blocks the stimulative effect of a high-energy diet on colon carcinoma growth in vivo and is associated with reduced expression of fatty acid synthase. Endocr. Relat. Cancer. 2010;17:351–360. doi: 10.1677/ERC-09-0252. [DOI] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. U.S.A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alimova IN, Liu B, Fan Z, Edgerton SM, Dillon T, Lind SE, et al. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle. 2009;8:909–915. doi: 10.4161/cc.8.6.7933. [DOI] [PubMed] [Google Scholar]
- Alli PM, Pinn ML, Jaffee EM, Mcfadden JM, Kuhajda FP. Fatty acid synthase inhibitors are chemopreventive for mammary cancer in neu-N transgenic mice. Oncogene. 2005;24:39–46. doi: 10.1038/sj.onc.1208174. [DOI] [PubMed] [Google Scholar]
- Anisimov VN. Metformin for aging and cancer prevention. Aging (Albany, NY) 2010;2:760–774. doi: 10.18632/aging.100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 2008;14:79–89. doi: 10.1016/j.ccr.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Ansieau S, Morel AP, Hinkal G, Bastid J, Puisieux A. TWISTing an embryonic transcription factor into an oncoprotein. Oncogene. 2010;29:3173–3184. doi: 10.1038/onc.2010.92. [DOI] [PubMed] [Google Scholar]
- Arima Y, Inoue Y, Shibata T, Hayashi H, Nagano O, Saya H, et al. Rb depletion results in deregulation of E-cadherin and induction of cellular phenotypic changes that are characteristic of the epithelial-to-mesenchymal transition. Cancer Res. 2008;68:5104–5112. doi: 10.1158/0008-5472.CAN-07-5680. [DOI] [PubMed] [Google Scholar]
- Armengol G, Rojo F, Castellvi J, Iglesias C, Cuatrecasas M, Pons B, et al. 4E-binding protein 1: a key molecular “funnel factor” in human cancer with clinical implications. Cancer Res. 2007;67:7551–7555. doi: 10.1158/0008-5472.CAN-07-0881. [DOI] [PubMed] [Google Scholar]
- Arnal-Estape A, Tarragona M, Morales M, Guiu M, Nadal C, Massague J, et al. HER2 silences tumor suppression in breast cancer cells by switching expression of C/EBPss isoforms. Cancer Res. 2010;70:9927–9936. doi: 10.1158/0008-5472.CAN-10-0869. [DOI] [PubMed] [Google Scholar]
- Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004;3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- Batsche E, Muchardt C, Behrens J, Hurst HC, Cremisi C. RB and c-Myc activate expression of the E-cadherin gene in epithelial cells through interaction with transcription factor AP-2. Mol. Cell. Biol. 1998;18:3647–3658. doi: 10.1128/mcb.18.7.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Sahra I, Laurent K, Giuliano S, Larbret F, Ponzio G, Gounon P, et al. Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res. 2010a;70:2465–2475. doi: 10.1158/0008-5472.CAN-09-2782. [DOI] [PubMed] [Google Scholar]
- Ben Sahra I, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P, et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576–3586. doi: 10.1038/sj.onc.1211024. [DOI] [PubMed] [Google Scholar]
- Ben Sahra I, Le Marchand-Brustel Y, Tanti JF, Bost F. Metformin in cancer therapy: a new perspective for an old antidiabetic drug? Mol. Cancer Ther. 2010b;9:1092–1099. doi: 10.1158/1535-7163.MCT-09-1186. [DOI] [PubMed] [Google Scholar]
- Ben Sahra I, Tanti JF, Bost F. The combination of metformin and 2-deoxyglucose inhibits autophagy and induces AMPK dependent apoptosis in prostate cancer cells. Autophagy. 2010c;6:670–671. doi: 10.4161/auto.6.5.12434. [DOI] [PubMed] [Google Scholar]
- Bendall SC, Stewart MH, Menendez P, George D, Vijayaragavan K, Werbowetski-Ogilvie T, et al. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature. 2007;448:1015–1021. doi: 10.1038/nature06027. [DOI] [PubMed] [Google Scholar]
- Berstein LM. Modern approach to metabolic rehabilitation of cancer patients: biguanides (phenformin and metformin) and beyond. Future Oncol. 2010;6:1313–1323. doi: 10.2217/fon.10.87. [DOI] [PubMed] [Google Scholar]
- Bodmer M, Meier C, Krahenbuhl S, Jick SS, Meier CR. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care. 2010;33:1304–1308. doi: 10.2337/dc09-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco EE, Knudsen ES. RB in breast cancer: at the crossroads of tumorigenesis and treatment. Cell Cycle. 2007;6:667–671. doi: 10.4161/cc.6.6.3988. [DOI] [PubMed] [Google Scholar]
- Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, et al. Inhibition of macroautophagy triggers apoptosis. Mol. Cell. Biol. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyerinas B, Park SM, Hau A, Murmann AE, Peter ME. The role of let-7 in cell differentiation and cancer. Endocr. Relat. Cancer. 2010;17:F19–F36. doi: 10.1677/ERC-09-0184. [DOI] [PubMed] [Google Scholar]
- Brabletz S, Brabletz T. The ZEB/miR-200 feedback loop—a motor of cellular plasticity in development and cancer? EMBO Rep. 2010;11:670–677. doi: 10.1038/embor.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braig M, Schmitt CA. Oncogene-induced senescence: putting the brakes on tumor development. Cancer Res. 2006;66:2881–2884. doi: 10.1158/0008-5472.CAN-05-4006. [DOI] [PubMed] [Google Scholar]
- Brunet J, Vazquez-Martin A, Colomer R, Grana-Suarez B, Martin-Castillo B, Menendez JA. BRCA1 and acetyl-CoA carboxylase: the metabolic syndrome of breast cancer. Mol. Carcinog. 2008;47:157–163. doi: 10.1002/mc.20364. [DOI] [PubMed] [Google Scholar]
- Burkhart DL, Sage J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat. Rev. Cancer. 2008;8:671–682. doi: 10.1038/nrc2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzai M, Jones RG, Amaravadi RK, Lum JJ, Deberardinis RJ, Zhao F, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–6752. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- Campaner S, Doni M, Hydbring P, Verrecchia A, Bianchi L, Sardella D, et al. Cdk2 suppresses cellular senescence induced by the c-myc oncogene. Nat. Cell Biol. 2010;12:54–59. doi: 10.1038/ncb2004. [DOI] [PubMed] [Google Scholar]
- Chen J, Imanaka N, Griffin JD. Hypoxia potentiates Notch signaling in breast cancer leading to decreased E-cadherin expression and increased cell migration and invasion. Br. J. Cancer. 2010;102:351–360. doi: 10.1038/sj.bjc.6605486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicas A, Wang X, Zhang C, Mccurrach M, Zhao Z, Mert O, et al. Dissecting the unique role of the retinoblastoma tumor suppressor during cellular senescence. Cancer Cell. 2010;17:376–387. doi: 10.1016/j.ccr.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciavarra G, Zacksenhaus E. Direct and indirect effects of the pRb tumor suppressor on autophagy. Autophagy. 2011:7. doi: 10.4161/auto.7.5.15056. [DOI] [PubMed] [Google Scholar]
- Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nat. Rev. Cancer. 2010;10:51–57. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton CJ, Chang JC, Rosen JM. Epithelial–mesenchymal transition (EMT) in tumor-initiating cells and its clinical implications in breast cancer. J. Mammary Gland Biol. Neoplasia. 2010;15:253–260. doi: 10.1007/s10911-010-9173-1. [DOI] [PubMed] [Google Scholar]
- Cufi S, Vazquez-Martin A, Oliveras-Ferraros C, Martin-Castillo B, Joven J, Menendez JA. Metformin against TGFbeta-induced epithelial-to-mesenchymal transition (EMT): from cancer stem cells to aging-associated fibrosis. Cell Cycle. 2010;9:4461–4468. doi: 10.4161/cc.9.22.14048. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. U.S.A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804–10812. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- Eberle D, Hegarty B, Bossard P, Ferre P, Foufelle F. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie. 2004;86:839–848. doi: 10.1016/j.biochi.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Elmore LW, Rehder CW, Di X, McChesney PA, Jackson-Cook CK, Gewirtz DA, Holt SE. Adriamycin-induced senescence in breast tumor cells involves functional p53 and telomere dysfunction. J. Biol. Chem. 2002;277:35509–35515. doi: 10.1074/jbc.M205477200. [DOI] [PubMed] [Google Scholar]
- Espina V, Liotta LA. What is the malignant nature of human ductal carcinoma in situ? Nat. Rev. Cancer. 2011;11:68–75. doi: 10.1038/nrc2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espina V, Mariani BD, Gallagher RI, Tran K, Banks S, Wiedemann J, et al. Malignant precursor cells pre-exist in human breast DCIS and require autophagy for survival. PLoS One. 2010;5:e10240. doi: 10.1371/journal.pone.0010240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esslimani-Sahla M, Thezenas S, Simony-Lafontaine J, Kramar A, Lavaill R, Chal-bos D, et al. Increased expression of fatty acid synthase and progesterone receptor in early steps of human mammary carcinogenesis. Int. J. Cancer. 2007;120:224–229. doi: 10.1002/ijc.22202. [DOI] [PubMed] [Google Scholar]
- Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore CM, Gupta PB, Rudnick JA, Caballero S, Keller PJ, Lander ES, et al. Estrogen expands breast cancer stem-like cells through paracrine FGF/Tbx3 signaling. Proc. Natl. Acad. Sci. U.S.A. 2010;107:21737–21742. doi: 10.1073/pnas.1007863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl. Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- Gatenby RA, Gillies RJ. A microenvironmental model of carcinogenesis. Nat. Rev. Cancer. 2008;8:56–61. doi: 10.1038/nrc2255. [DOI] [PubMed] [Google Scholar]
- Gauthier ML, Berman HK, Miller C, Kozakeiwicz K, Chew K, Moore D, et al. Abrogated response to cellular stress identifies DCIS associated with subsequent tumor events and defines basal-like breast tumors. Cancer Cell. 2007;12:479–491. doi: 10.1016/j.ccr.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. CA CancerJ. Clin. 2010;60:207–221. doi: 10.3322/caac.20078. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Angulo AM, Meric-Bernstam F. Metformin: a therapeutic opportunity in breast cancer. Clin. Cancer Res. 2010;16:1695–1700. doi: 10.1158/1078-0432.CCR-09-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin PJ, Pritchard KI, Ennis M, Clemons M, Graham M, Fantus IG. Insulin-lowering effects of metformin in women with early breast cancer. Clin. Breast Cancer. 2008;8:501–505. doi: 10.3816/CBC.2008.n.060. [DOI] [PubMed] [Google Scholar]
- Goodwin PJ, Stambolic V, Lemieux J, Chen BE, Parulekar WR, Gelmon KA, et al. Evaluation of metformin in early breast cancer: a modification of the traditional paradigm for clinical testing of anti-cancer agents. Breast Cancer Res. Treatm. 2011;126:215–220. doi: 10.1007/s10549-010-1224-1. [DOI] [PubMed] [Google Scholar]
- Hannemann J, Velds A, Halfwerk JB, Kreike B, Peterse JL, Van De Vijver MJ. Classification of ductal carcinoma in situ by gene expression profiling. Breast Cancer Res. 2006;8:R61. doi: 10.1186/bcr1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemkens LG, Grouven U, Bender R, Gunster C, Gutschmidt S, Selke GW, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia. 2009;52:1732–1744. doi: 10.1007/s00125-009-1418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–7511. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka PW. Living Without Oxygen. Cambridge, USA: Harvard University Press; 1980. pp. 1–181. [Google Scholar]
- Hochachka PW, Rupert JL, Goldenberg L, Gleave M, Kozlowski P. Going malignant: the hypoxia-cancer connection in the prostate. Bioessays. 2002;24:749–757. doi: 10.1002/bies.10131. [DOI] [PubMed] [Google Scholar]
- Honeth G, Bendahl PO, Ringner M, Saal LH, Gruvberger-Saal SK, Lovgren K, et al. The CD44+/CD24- phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008;10:R53. doi: 10.1186/bcr2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz KB, Sartorius CA. Progestins in hormone replacement therapies reactivate cancer stem cells in women with preexisting breast cancers: a hypothesis. J. Clin. Endocrinol. Metab. 2008;93:3295–3298. doi: 10.1210/jc.2008-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosono K, Endo H, Takahashi H, Sugiyama M, Sakai E, Uchiyama T, et al. Metformin suppresses colorectal aberrant crypt foci in a short-term clinical trial. Cancer Prev. Res. (Phila) 2010;3:1077–1083. doi: 10.1158/1940-6207.CAPR-10-0186. [DOI] [PubMed] [Google Scholar]
- Hugo HJ, Kokkinos MI, Blick T, Ackland ML, Thompson EW, New-green DF. Defining the E-cadherin repressor interactome in epithelial–mesenchymal transition: the PMC42 model as a case study. Cells Tissues Organs. 2011;193:23–40. doi: 10.1159/000320174. [DOI] [PubMed] [Google Scholar]
- Ibarra I, Erlich Y, Muthuswamy SK, Sachidanandam R, Hannon GJ. A role for microRNAs in maintenance of mouse mammary epithelial progenitor cells. Genes Dev. 2007;21:3238–3243. doi: 10.1101/gad.1616307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalving M, Gietema JA, Lefrandt JD, De Jong S, Reyners AK, Gans RO, et al. Metformin: taking away the candy for cancer? Eur. J. Cancer. 2010;46:2369–2380. doi: 10.1016/j.ejca.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Janda E, Nevolo M, Lehmann K, Downward J, Beug H, Grieco M. Raf plus TGFbeta-dependent EMT is initiated by endocytosis and lysosomal degradation of E-cadherin. Oncogene. 2006;25:7117–7130. doi: 10.1038/sj.onc.1209701. [DOI] [PubMed] [Google Scholar]
- Jiang H, Martin V, Gomez-Manzano C, Johnson DG, Alonso M, White E, et al. The RB-E2F1 pathway regulates autophagy. Cancer Res. 2010a;70:7882–7893. doi: 10.1158/0008-5472.CAN-10-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Deng T, Jones R, Li H, Herschkowitz JI, Liu JC, et al. Rb deletion in mouse mammary progenitors induces luminal-B or basal-like/EMT tumor subtypes depending on p53 status. J. Clin. Invest. 2010b;120:3296–3309. doi: 10.1172/JCI41490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiralerspong S, Palla SL, Giordano SH, Meric-Bernstam F, Liedtke C, Barnett CM, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J. Clin. Oncol. 2009;27:3297–3302. doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson JM, Ljung R, Talback M, Haglund B, Gudbjornsdottir S, Steineck G. Insulin glargine use and short-term incidence of malignancies—a population-based follow-up study in Sweden. Diabetologia. 2009;52:1745–1754. doi: 10.1007/s00125-009-1444-2. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial–mesenchymal transition. J. Clin. Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karantza-Wadsworth V, Patel S, Kravchuk O, Chen G, Mathew R, Jin S, et al. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007;21:1621–1635. doi: 10.1101/gad.1565707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelloff GJ, Sigman CC. Assessing intraepithelial neoplasia and drug safety in cancer-preventive drug development. Nat. Rev. Cancer. 2007;7:508–518. doi: 10.1038/nrc2154. [DOI] [PubMed] [Google Scholar]
- Knudsen ES, Knudsen KE. Tailoring to RB: tumour suppressor status and therapeutic response. Nat. Rev. Cancer. 2008;8:714–724. doi: 10.1038/nrc2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Jaattela M. Lysosomes and autophagy in cell death control. Nat. Rev. Cancer. 2005;5:886–897. doi: 10.1038/nrc1738. [DOI] [PubMed] [Google Scholar]
- Lacerda L, Pusztai L, Woodward WA. The role of tumor initiating cells in drug resistance of breast cancer: implications for future therapeutic approaches. Drug Resist. Updates. 2010;13:99–108. doi: 10.1016/j.drup.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Lee BY, Han JA, Im JS, Morrone A, Johung K, et al. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 2006;5:187–195. doi: 10.1111/j.1474-9726.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66:4553–4557. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]
- Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J. Natl. Cancer Inst. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- Liu B, Fan Z, Edgerton SM, Deng XS, Alimova IN, Lind SE, et al. Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell Cycle. 2009a;8:2031–2040. doi: 10.4161/cc.8.13.8814. [DOI] [PubMed] [Google Scholar]
- Liu S, Wicha MS. Targeting breast cancer stem cells. J. Clin. Oncol. 2010;28:4006–4012. doi: 10.1200/JCO.2009.27.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Clem B, Zuba-Surma EK, El-Naggar S, Telang S, Jenson AB, et al. Mouse fibroblasts lacking RB1 function form spheres and undergo reprogramming to a cancer stem cell phenotype. Cell Stem Cell. 2009b;4:336–347. doi: 10.1016/j.stem.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, El-Naggar S, Darling DS, Higashi Y, Dean DC. Zeb1 links epithelial–mesenchymal transition and cellular senescence. Development (Cambridge, England) 2008;135:579–588. doi: 10.1242/dev.007047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie E, Nik S, Chen JS, Schmidt M, Song B, Pacson C, et al. Identification of a stem-like cell population by exposing metastatic breast cancer cell lines to repetitive cycles of hypoxia and reoxygenation. Breast Cancer Res. 2010;12:R94. doi: 10.1186/bcr2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Guo H, Treekitkarnmongkol W, Li P, Zhang J, Shi B, et al. 14-3-3zeta Cooperates with ErbB2 to promote ductal carcinoma in situ progression to invasive breast cancer by inducing epithelial–mesenchymal transition. Cancer Cell. 2009;16:195–207. doi: 10.1016/j.ccr.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Archer MC. Fatty acid synthase is a potential molecular target for the chemoprevention of breast cancer. Carcinogenesis. 2005;26:153–157. doi: 10.1093/carcin/bgh278. [DOI] [PubMed] [Google Scholar]
- Lum JJ, Deberardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat. Rev. Mol. Cell Biol. 2005;6:439–448. doi: 10.1038/nrm1660. [DOI] [PubMed] [Google Scholar]
- Lundgren K, Nordenskjold B, Landberg G. Hypoxia, Snail and incomplete epithelial–mesenchymal transition in breast cancer. Br. J. Cancer. 2009;101:1769–1781. doi: 10.1038/sj.bjc.6605369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnifico A, Albano L, Campaner S, Delia D, Castiglioni F, Gasparini P, et al. Tumor-initiating cells of HER2-positive carcinoma cell lines express the highest oncoprotein levels and are sensitive to trastuzumab. Clin. Cancer Res. 2009;15:2010–2021. doi: 10.1158/1078-0432.CCR-08-1327. [DOI] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Cano A. Tumorigenesis: Twist1 links EMT to self-renewal. Nat. Cell Biol. 2010;12:924–925. doi: 10.1038/ncb1010-924. [DOI] [PubMed] [Google Scholar]
- Martin-Castillo B, Dorca J, Vazquez-Martin A, Oliveras-Ferraros C, Lopez-Bonet E, Garcia M, et al. Incorporating the antidiabetic drug metformin in HER2-positive breast cancer treated with neoadjuvant chemotherapy and trastuzumab: an ongoing clinical-translational research experience at the Catalan Institute of Oncology. Ann. Oncol. 2010a;21:187–189. doi: 10.1093/annonc/mdp494. [DOI] [PubMed] [Google Scholar]
- Martin-Castillo B, Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. Metformin and cancer: doses, mechanisms and the dandelion and hormetic phenomena. Cell Cycle. 2010b;9:1057–1064. doi: 10.4161/cc.9.6.10994. [DOI] [PubMed] [Google Scholar]
- Massague J. TGFbeta in cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat. Rev. Cancer. 2007;7:961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meley D, Bauvy C, Houben-Weerts JH, Dubbelhuis PF, Helmond MT, Codogno P, et al. AMP-activated protein kinase and the regulation of autophagic proteolysis. J. Biol. Chem. 2006;281:34870–34879. doi: 10.1074/jbc.M605488200. [DOI] [PubMed] [Google Scholar]
- Memmott RM, Dennis PA. LKB1 and mammalian target of rapamycin as predictive factors for the anticancer efficacy of metformin. J. Clin. Oncol. 2009;27:e226. doi: 10.1200/JCO.2009.25.3963. Author reply e227. [DOI] [PubMed] [Google Scholar]
- Menendez JA, Cufí S, Oliveras-Ferraros C, Vellon L, Joven J, Vazquez-Martin A. Gerosuppressant metformin: less is more. 2011 doi: 10.18632/aging.100316. Aging (Albany, NY) (April 4) (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- Menendez JA, Mehmi I, Verma VA, Teng PK, Lupu R. Pharmacological inhibition of fatty acid synthase (FAS): a novel therapeutic approach for breast cancer chemoprevention through its ability to suppress Her-2/neu (erbB-2) oncogene-induced malignant transformation. Mol. Carcinog. 2004;41:164–178. doi: 10.1002/mc.20054. [DOI] [PubMed] [Google Scholar]
- Menendez JA, Vazquez-Martin A, Oliveras-Ferraros C, Garcia-Villalba R, Carrasco-Pancorbo A, et al. Extra-virgin olive oil polyphenols inhibit HER2 (erbB-2)-induced malignant transformation in human breast epithelial cells: relationship between the chemical structures of extra-virgin olive oil secoiridoids and lignans and their inhibitory activities on the tyrosine kinase activity of HER2. Int. J. Oncol. 2009;34:43–51. [PubMed] [Google Scholar]
- Mineno J, Okamoto S, Ando T, Sato M, Chono H, Izu H, et al. The expression profile of microRNAs in mouse embryos. Nucleic Acids Res. 2006;34:1765–1771. doi: 10.1093/nar/gkl096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial–mesenchymal transition. PLoS One. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan G. More patent protection for medicines with a new purpose. Nature. 2010;465:1005. doi: 10.1038/4651005b. [DOI] [PubMed] [Google Scholar]
- Muggerud AA, Hallett M, Johnsen H, Kleivi K, Zhou W, Tahmasebpoor S, et al. Molecular diversity in ductal carcinoma in situ (DCIS) and early invasive breast cancer. Mol. Oncol. 2010;4:357–368. doi: 10.1016/j.molonc.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat. Cell Biol. 2001;3:785–792. doi: 10.1038/ncb0901-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negri T, Tarantino E, Orsenigo M, Reid JF, Gariboldi M, Zambetti M, et al. Chromosome band 17q21 in breast cancer: significant association between beclin 1 loss and HER2/NEU amplification. Genes Chromosomes Cancer. 2010;49:901–909. doi: 10.1002/gcc.20798. [DOI] [PubMed] [Google Scholar]
- Ohashi S, Natsuizaka M, Wong GS, Michaylira CZ, Grugan KD, Stairs DB, et al. Epidermal growth factor receptor and mutant p53 expand an esophageal cellular subpopulation capable of epithelial-to-mesenchymal transition through ZEB transcription factors. Cancer Res. 2010;70:4174–4184. doi: 10.1158/0008-5472.CAN-09-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveras-Ferraros C, Cufí S, Vazquez-Martin A, Torres-Garcia VZ, Del Barco S, Martin-Castillo B, Menendez JA. Micro(mi)RNA expression profile of breast cancer epithelial cells treated with the anti-diabetic drug metformin: Induction of the tumor suppressor miRNA let-7a and suppression of the TGFFJ-induced oncomiR miRNA-181a. Cell Cycle. 2011;10:1144–1151. doi: 10.4161/cc.10.7.15210. [DOI] [PubMed] [Google Scholar]
- O’Shaughnessy JA, Kelloff GJ, Gordon GB, Dannenberg AJ, Hong WK, Fabian CJ, et al. Treatment and prevention of intraepithelial neoplasia: an important target for accelerated new agent development. Clin. Cancer Res. 2002;8:314–346. [PubMed] [Google Scholar]
- Palacios F, Tushir JS, Fujita Y, D’souza-Schorey C. Lysosomal targeting of E-cadherin: a unique mechanism for the down-regulation of cell–cell adhesion during epithelial to mesenchymal transitions. Mol. Cell. Biol. 2005;25:389–402. doi: 10.1128/MCB.25.1.389-402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SM, Shell S, Radjabi AR, Schickel R, Feig C, Boyerinas B, et al. Let-7 prevents early cancer progression by suppressing expression of the embryonic gene HMGA2. Cell Cycle. 2007;6:2585–2590. doi: 10.4161/cc.6.21.4845. [DOI] [PubMed] [Google Scholar]
- Peinado H, Cano A. A hypoxic twist in metastasis. Nat. Cell Biol. 2008;10:253–254. doi: 10.1038/ncb0308-253. [DOI] [PubMed] [Google Scholar]
- Peter ME. Let-7 and miR-200 microRNAs: guardians against pluripotency and cancer progression. Cell Cycle. 2009;8:843–852. doi: 10.4161/cc.8.6.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- Pollak M. Metformin and other biguanides in oncology: advancing the research agenda. Cancer Prev. Res. (Phila) 2010;3:1060–1065. doi: 10.1158/1940-6207.CAPR-10-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak M, Russell-Jones D. Insulin analogues and cancer risk: cause for concern or cause celebre? Int. J. Clin. Pract. 2010;64:628–636. doi: 10.1111/j.1742-1241.2010.02354.x. [DOI] [PubMed] [Google Scholar]
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat. Rev. Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- Qadir MA, Kwok B, Dragowska WH, To KH, Le D, Bally MB, et al. Macroautophagy inhibition sensitizes tamoxifen-resistant breast cancer cells and enhances mitochondrial depolarization. Breast Cancer Res. Treatm. 2008;112:389–403. doi: 10.1007/s10549-007-9873-4. [DOI] [PubMed] [Google Scholar]
- Reichert JM. Trends in development and approval times for new therapeutics in the United States. Nat. Rev. Drug Discov. 2003;2:695–702. doi: 10.1038/nrd1178. [DOI] [PubMed] [Google Scholar]
- Rice S, Pellatt L, Ramanathan K, Whitehead SA, Mason HD. Metformin inhibits aromatase via an extracellular signal-regulated kinase-mediated pathway. Endocrinology. 2009;150:4794–4801. doi: 10.1210/en.2009-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F, Campisi J. Four faces of cellular senescence. J. Cell Biol. 2011;192:546–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roninson IB. Tumor cell senescence in cancer treatment. Cancer Res. 2003;63:2705–2715. [PubMed] [Google Scholar]
- Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ. Potential therapeutic applications of autophagy. Nat. Rev. Drug Discov. 2007;6:304–312. doi: 10.1038/nrd2272. [DOI] [PubMed] [Google Scholar]
- Samaddar JS, Gaddy VT, Duplantier J, Thandavan SP, Shah M, Smith MJ, et al. A role for macroautophagy in protection against 4-hydroxytamoxifen-induced cell death and the development of antiestrogen resistance. Mol. Cancer Ther. 2008;7:2977–2987. doi: 10.1158/1535-7163.MCT-08-0447. [DOI] [PubMed] [Google Scholar]
- Samari HR, Seglen PO. Inhibition of hepatocytic autophagy by adenosine, aminoimidazole-4-carboxamide riboside, and N6-mercaptopurine riboside. Evidence for involvement of amp-activated protein kinase. J. Biol. Chem. 1998;273:23758–23763. doi: 10.1074/jbc.273.37.23758. [DOI] [PubMed] [Google Scholar]
- Savarese TM, Low HP, Baik I, Strohsnitter WC, Hsieh CC. Normal breast stem cells, malignant breast stem cells, and the perinatal origin of breast cancer. Stem Cell Rev. 2006;2:103–110. doi: 10.1007/s12015-006-0016-9. [DOI] [PubMed] [Google Scholar]
- Schoenlein PV, Periyasamy-Thandavan S, Samaddar JS, Jackson WH, Barrett JT. Autophagy facilitates the progression of ERalpha-positive breast cancer cells to antiestrogen resistance. Autophagy. 2009;5:400–403. doi: 10.4161/auto.5.3.7784. [DOI] [PubMed] [Google Scholar]
- Serrano M. Cancer: a lower bar for senescence. Nature. 2010;464:363–364. doi: 10.1038/464363a. [DOI] [PubMed] [Google Scholar]
- Shell S, Park SM, Radjabi AR, Schickel R, Kistner EO, et al. Let-7 expression defines two differentiation stages of cancer. Proc. Natl. Acad. Sci. U.S.A. 2007;104:11400–11405. doi: 10.1073/pnas.0704372104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota M, Izumi H, Onitsuka T, Miyamoto N, Kashiwagi E, Kidani A, et al. Twist and p53 reciprocally regulate target genes via direct interaction. Oncogene. 2008;27:5543–5553. doi: 10.1038/onc.2008.176. [DOI] [PubMed] [Google Scholar]
- Simpson PT, Da Silva LM, Lakhani SR. In situ carcinoma—can we predict which patient will come back with a recurrence? CancerCell. 2007;12:409–411. doi: 10.1016/j.ccr.2007.10.026. [DOI] [PubMed] [Google Scholar]
- Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit MA, Peeper DS. Deregulating EMT and senescence: double impact by a single twist. CancerCell. 2008;14:5–7. doi: 10.1016/j.ccr.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Smit MA, Peeper DS. Epithelial-mesenchymal transition and senescence: two cancer-related processes are crossing paths. Aging (Albany, NY) 2010;2:735–741. doi: 10.18632/aging.100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith U, Gale EA. Does diabetes therapy influence the risk of cancer? Dia-betologia. 2009;52:1699–1708. doi: 10.1007/s00125-009-1441-5. [DOI] [PubMed] [Google Scholar]
- Stoll BA. Biological mechanisms in breast cancer invasiveness: relevance to preventive interventions. Eur. J. Cancer Prev. 2000;9:73–79. doi: 10.1097/00008469-200004000-00002. [DOI] [PubMed] [Google Scholar]
- Stoll BA. Oestrogen/insulin-like growth factor-I receptor interaction in early breast cancer: clinical implications. Ann. Oncol. 2002;13:191–196. doi: 10.1093/annonc/mdf059. [DOI] [PubMed] [Google Scholar]
- Swinnen JV, Beckers A, Brusselmans K, Organe S, Segers J, Timmermans L, et al. Mimicry of a cellular low energy status blocks tumor cell anabolism and suppresses the malignant phenotype. Cancer Res. 2005;65:2441–2448. doi: 10.1158/0008-5472.CAN-04-3025. [DOI] [PubMed] [Google Scholar]
- Tan AR, Alexe G, Reiss M. Transforming growth factor-beta signaling: emerging stem cell target in metastatic breast cancer? Breast Cancer Res. Treatm. 2009;115:453–495. doi: 10.1007/s10549-008-0184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MA, Parvani JG, Schiemann WP. The pathophysiology of epithelial–mesenchymal transition induced by transforming growth factor-beta in normal and malignant mammary epithelial cells. J. Mammary Gland Biol. Neoplasia. 2010;15:169–190. doi: 10.1007/s10911-010-9181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy K, Dibling BC, Spike BT, Knabb JR, Schumacker P, Macleod KF. BNIP3 is an RB/E2F target gene required for hypoxia-induced autophagy. Mol. Cell. Biol. 2007;27:6229–6242. doi: 10.1128/MCB.02246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CC, Chen YJ, Yew TL, Chen LL, Wang JY, Chiu CH, et al. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood. 2011;117:459–469. doi: 10.1182/blood-2010-05-287508. [DOI] [PubMed] [Google Scholar]
- Vazquez-Martin A, Cufi S, Oliveras-Ferraros C, Martin-Castillo B, Del Barco S, Lopez-Bonet E, et al. Expression status of the autophagy-regulatory gene ATG6/BECN1 in ERBB2-positive breast carcinomas: bypassing ERBB2-induced oncogenic senescence to regulate the efficacy of ERBB2-targeted therapies. Genes Chromosomes Cancer. 2011a;50:284–290. doi: 10.1002/gcc.20846. [DOI] [PubMed] [Google Scholar]
- Vazquez-Martin A, Oliveras-Ferraros C, Barco SD, Martin-Castillo B, Menendez JA. The anti-diabetic drug metformin suppresses self-renewal and proliferation of trastuzumab-resistant tumor-initiating breast cancer stem cells. Breast Cancer Res. Treatm. 2011b;126:355–364. doi: 10.1007/s10549-010-0924-x. [DOI] [PubMed] [Google Scholar]
- Vazquez-Martin A, Oliveras-Ferraros C, Cufi S, Del Barco S, Martin-Castillo B, Lopez-Bonet E, et al. The anti-diabetic drug metformin suppresses the metastasis-associated protein CD24 in MDA-MB-468 triple-negative breast cancer cells. Oncol. Rep. 2011c;25:135–140. [PubMed] [Google Scholar]
- Vazquez-Martin A, Oliveras-Ferraros C, Cufi S, Del Barco S, Martin-Castillo B, Menendez JA. Metformin regulates breast cancer stem cell ontogeny by transcriptional regulation of the epithelial–mesenchymal transition (EMT) status. Cell Cycle. 2010a;9:3807–3814. [PubMed] [Google Scholar]
- Vazquez-Martin A, Oliveras-Ferraros C, Cufi S, Martin-Castillo B, Menendez JA. Metformin and energy metabolism in breast cancer: from insulin physiology to tumour-initiating stem cells. Curr. Mol. Med. 2010b;10:674–691. doi: 10.2174/156652410792630625. [DOI] [PubMed] [Google Scholar]
- Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. The antidiabetic drug metformin suppresses HER2 (erbB-2) oncoprotein overexpression via inhibition of the mTOR effector p70S6K1 in human breast carcinoma cells. Cell Cycle. 2009a;8:88–96. doi: 10.4161/cc.8.1.7499. [DOI] [PubMed] [Google Scholar]
- Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. Autophagy facilitates the development of breast cancer resistance to the anti-HER2 monoclonal antibody trastuzumab. PLoS One. 2009b;4:e6251. doi: 10.1371/journal.pone.0006251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat. Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- Wang KH, Kao AP, Chang CC, Lee JN, Hou MF, Long CY, et al. Increasing CD44+/CD24(−) tumor stem cells, and upregulation of COX-2 and HDAC6, as major functions of HER2 in breast tumorigenesis. Mol. Cancer. 2010a;9:288. doi: 10.1186/1476-4598-9-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yu Y, Tsuyada A, Ren X, Wu X, Stubblefield K, et al. Transforming growth factor-beta regulates the sphere-initiating stem cell-like feature in breast cancer through miRNA-181 and ATM. Oncogene. 2010b;30:1470–1480. doi: 10.1038/onc.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Li Y, Ahmad A, Azmi AS, Kong D, Banerjee S, Sarkar FH. Targeting miRNAs involved in cancer stem cell and EMT regulation: an emerging concept in overcoming drug resistance. Drug Resist. Updat. 2010c;13:109–118. doi: 10.1016/j.drup.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- Weinberg RA. Twisted epithelial–mesenchymal transition blocks senescence. Nat. Cell Biol. 2008;10:1021–1023. doi: 10.1038/ncb0908-1021. [DOI] [PubMed] [Google Scholar]
- Welch HG, Black WC. Using autopsy series to estimate the disease “reservoir” for ductal carcinoma in situ of the breast: how much more breast cancer can we find? Ann. Intern. Med. 1997;127:1023–1028. doi: 10.7326/0003-4819-127-11-199712010-00014. [DOI] [PubMed] [Google Scholar]
- Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY, Yang WH, et al. Bmi1 is essential in Twist1-induced epithelial–mesenchymal transition. Nat. Cell Biol. 2010;12:982–992. doi: 10.1038/ncb2099. [DOI] [PubMed] [Google Scholar]
- Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, et al. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat. Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- Yang MH, Wu KJ. TWIST activation by hypoxia inducible factor-1 (HIF-1): implications in metastasis and development. Cell Cycle. 2008;7:2090–2096. doi: 10.4161/cc.7.14.6324. [DOI] [PubMed] [Google Scholar]
- Young AR, Narita M. Connecting autophagy to senescence in pathophysiology. Curr. Opin. Cell Biol. 2010;22:234–240. doi: 10.1016/j.ceb.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–10273. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- Zheng W, Wang H, Xue L, Zhang Z, Tong T. Regulation of cellular senescence and p16(INK4a) expression by Id1 and E47 proteins in human diploid fibroblast. J. Biol. Chem. 2004;279:31524–31532. doi: 10.1074/jbc.M400365200. [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Y, Miskimins WK. Cell cycle arrest in metformin treated breast cancer cells involves activation of AMPK, downregulation of cyclin D1, and requires p27Kip1 or p21Cip1. J. Mol. Signal. 2008;3:18. doi: 10.1186/1750-2187-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]