Abstract
We sought to determine whether depressed myocardial contraction fraction (MCF, the ratio of left ventricular (LV) stroke volume to myocardial volume) predicts cardiovascular disease (CVD) events in initially healthy adults. A subset (N=318, 60±9 yrs, 158 men) of the Framingham Heart Study Offspring cohort free of clinical CVD underwent volumetric cardiovascular magnetic resonance (CMR) imaging in 1998–1999. LV ejection fraction (EF), mass and MCF were determined. “Hard” CVD events comprised cardiovascular death, myocardial infarction, stroke or new heart failure. A Cox proportional hazards model adjusting for Framingham Coronary Risk Score (FCRS) was used to estimate hazard ratios for incident hard CVD events for sex-specific quartiles of MCF, LV mass and LVEF. The lowest quartile of LV mass and highest quartiles of MCF and EF served as referent. Kaplan-Meier survival plots and the log rank test were used to compare event-free survival. MCF was greater in women (0.58±0.13) than men (0.52±0.11), p<0.01. Nearly all (99%) participants had EF ≥ 0.55. Over up to 9-year (median 5.2) follow-up, 31 participants (10%) experienced an incident hard CVD event. Lowest-quartile MCF was 7 times more likely to develop hard CVD (hazard ratio 7.11, p=0.010) compared to the lowest quartile, and the elevated hazards persisted even after adjustment for LV mass (hazard ratio=6.09, p=0.020). The highest-quartile LV mass/height2.7 had nearly five-fold risk (hazard ratio 4.68, p=0.016). Event-free survival was shorter in lowest-quartile MCF, p = 0.0006, but not in lowest-quartile LVEF. Conclusion: In a cohort of adults initially without clinical CVD, lowest-quartile MCF conferred an increased hazard for hard CVD events after adjustment for traditional CVD risk factors and LV mass.
Keywords: magnetic resonance imaging, myocardial contraction fraction, risk factors, left ventricular function
Introduction
King et al. proposed a novel volumetric index, the ratio of LV stroke volume to LV myocardial volume, which they termed “myocardial contraction fraction” (MCF), and used MCF to distinguish between patients with hypertensive LV hypertrophy and athletes with physiologic hypertrophy [1]. It is not known whether MCF has prognostic value regarding major or “hard” adverse cardiovascular disease (CVD) events. We used volumetric cardiovascular magnetic resonance (CMR) to investigate whether decreased MCF was predictive of future hard CVD events in a prospectively monitored cohort of community-dwelling adults free of clinical CVD with normal LVEF.
Methods
The Framingham Heart Study Offspring cohort was initiated in 1971 and comprises the children of the original Framingham cohort and their spouses [2]. Offspring undergo comprehensive (“cycle”) examinations every 3-5 years. Among Offspring without history or signs of clinical CVD, a subsample without contraindications to CMR was recruited using a random sampling strategy based on equal strata of decade age, sex, and quintile of Framingham Coronary Risk Score (FCRS) [3], with double sampling of the top quintile. The study was approved by the institutional review boards of the Beth Israel Deaconess Medical Center and Boston University School of Medicine. All participants provided written informed consent.
Clinical covariates were obtained at Offspring examination cycle 6. Height and weight were measured with participants in light clothing. Systolic and diastolic blood pressure were determined as the average of two readings by a physician. Fasting serum lipid levels, including total and high-density lipoprotein (HDL) cholesterol, were obtained. Diabetes was defined as being on any antihyperglycemic medication or having a fasting serum glucose ≥126 mg/dL on any cycle examination. Any pharmacologic treatment for hypertension or dyslipidemia was recorded. Smoking was defined as having smoked regularly in the year prior to the cycle visit. Incident hard CVD events were defined as the first occurrence during post-CMR follow-up of myocardial infarction, stroke, first hospital admission for heart failure or cardiovascular death. Events were adjudicated by a 3-physician panel using previously described criteria [4].
CMR imaging used a segmented k-space gradient echo cine sequence on a 1.5-Tesla scanner (Gyroscan ACS/NT, Philips Medical Systems, Best, NL) as previously described [5]. Contiguous 10-mm thick slices in the LV short-axis orientation were acquired, 1 per breath-hold. Temporal resolution was 39 ms with in-plane spatial resolution of 1.25×2.0-mm2.
Image data were analyzed using a commercially available workstation (EasyScil, Philips). LV endocardial borders were manually traced at end-diastole and end-systole. LV epicardial borders were also traced at end-diastole. For consistency in analysis, LV trabeculations and papillary muscles were considered LV cavitary volume. Stroke volume was the difference between LV end-diastolic and end-systolic volumes. LVEF was stroke volume divided by end-diastolic volume. The MCF was calculated as LV stroke volume divided by LV myocardial volume. LV mass was myocardial volume multiplied by mean density of myocardium, 1.05 g/ml, and indexed to height (HT), an allometric power of height (HT2.7), and body surface area (BSA). The ratio of LV mass to end-diastolic volume (LVM/LVEDV) was used as a measure of LV concentricity.
Baseline participant characteristics are presented by sex, with continuous variables summarized as mean±1 SD. For each participant the sex-specific FCRS was calculated using age, systolic blood pressure and blood pressure-treatment status, diabetes, total and HDL cholesterol, treatment status for dyslipidemia, and smoking status (put ref for FCRS). Between-sex differences were assessed by a two sample t-test for continuous variables and Chi-squared test for categorical variables. Pearson correlation coefficients were used to assess linear relationships between continuous measures. After confirming the assumption of proportionality, a Cox proportional hazards model adjusting for sex and FCRS was used to estimate hazard ratio for hard CVD events in each quartile of MCF versus the referent (highest) quartile of MCF. Similar analysis was performed for LVEF. For LV mass the lowest mass-quartile was used as the referent. As MCF is explicitly related to LV mass (as myocardial volume) and implicitly related to LV concentricity, we also determined hazard ratios for MCF after adjustment for LV mass and for LVM/LVEDV. Finally, Kaplan-Meier survival plots and the log rank test were used to compare event-free survival between the lowest quartile of MCF and the combination of higher MCF quartiles. Similar analyses were performed for LVEF. All statistical analyses were performed using SAS (version 8.1, SAS Institute, Cary, NC).
Results
Of the 318 participants who underwent CMR, images were unevaluable in 14 (4.4%), principally due to difficulty with breath-holding, leaving 304 participants for analysis. Baseline characteristics for each sex are presented in Table 1. Men were taller, heavier, and had greater body mass index than women. Total and HDL cholesterol were higher in women than men.
Table 1.
Baseline participant characteristics by sex.
| Men (n=149) | Women (n=155) | P-value | |
|---|---|---|---|
|
|
|
|
|
| Age (years) | 59±9 | 60±9 | 0.19 |
| Weight (kg) | 89.3±13.2 | 71.7±16.3 | <0.0001 |
| Height (cm) | 175±6 | 161±6 | <0.0001 |
| Body Mass Index (kg/m2) | 29.1±4.3 | 27.6±6.2 | 0.017 |
| Systolic blood pressure (mmHg) | 130±18 | 129±19 | 0.49 |
| Cigarette smoking | 25 (16%) | 19 (12%) | 0.33 |
| Hypertension treatment | 51 (32%) | 42 (26%) | 0.32 |
| Dyslipidemia treatment | 25 (16%) | 24 (15%) | 0.76 |
| Diabetes | 26 (8.6%) | 19 (6.3%) | 0.23 |
| Total cholesterol (mg/dL) | 203±38 | 217±36 | <0.001 |
| High-density lipoprotein cholesterol (mg/dL) | 43±13 | 56±16 | <0.0001 |
| Framingham coronary risk score (points) | 7.7±3.6 | 8.3±5.2 | 0.22 |
LVEF was ≥0.55 in 301 (99%) participants; of the remaining 3 participants, 1 woman had an LVEF of 0.46 and 2 men had LVEFs of 0.52 and 0.54. Overall, LVEF was greater in women than in men (0.72±0.07 vs 0.69±0.09, p=0.002). MCF was also greater in women than in men(0.58±0.13 vs. 0.52±0.11, p<0.01). Men had greater LV mass than women (159±28 vs. 110±22g, p<0.0001) and this difference remained statistically significant after indexation to HT (91±16 vs. 68±14 g/m, p<0.0001), to HT2.7 (35±6 vs. 31±7 g/m2.7, p<0.0001) and to BSA (77±13 vs. 62±10 g/m2, p<0.0001).
Over a median 5.2-year (interquartile range 4.6 – 5.4) follow up, an incident hard CVD event occurred in 31 (10%) participants (17 men)including 5 instances of myocardial infarction and 7 of unstable angina, 13 cerebrovascular events and 6 cases of heart failure. There were also 4 cardiovascular deaths (all were subsequent to one of the incident events above and hence are not included among the incident hard CVD events). Overall, participants who experienced a hard CVD event (hCVD+) during follow-up had significantly lower MCF than those free of hard CVD events (hCVD-), Table 2. LVEF did not differ between hCVD+ and hCVD- participants. There was no statistically significant linear correlation between EF and MCF in hCVD+ participants (r=0.34, p=0.07). In sex-specific analyses, hCVD+ participants had lower MCF than hCVD- participants, but this difference was significant only among women (Table 2). LVEF did not differ between hCVD+ and hCVD- participants for either sex. LV mass was consistently greater in hCVD+ than hCVD- participants for both men and women, but significance of these differences varied with method of indexation. Considering the components of MCF, stroke volume, with and without indexation to BSA, did not differ between hCVD+ and hCVD-participants for either sex (women: p=0.14; men: p=0.91), suggesting that decreased MCF in hCVD+ participants generally was driven by greater LV mass. Finally, FCRS was greater in hCVD+ participants in both sexes, as expected.
Table 2.
Comparison of myocardial contraction fraction, left ventricular ejection fraction, left ventricular mass, and Framingham coronary risk score between participants experiencing hard cardiovascular disease events (hCVD+) and those free of hard CVD events (hCVD-).
| hCVD- | hCVD+ | P | |
|---|---|---|---|
|
|
|
|
|
| Pooled: Myocardial contraction fraction | 0.55±0.12 | 0.49±0.11 | 0.004 |
| Pooled: Left ventricular ejection fraction | 0.70±0.06 | 0.71±0.08 | 0.27 |
| Pooled: Framingham coronary risk score | 7.7±4.3 | 11.3±4.4 | <0.0001 |
| Men: Myocardial contraction fraction | 0.52±0.10 | 0.48±0.16 | 0.13 |
| Men: Left ventricular ejection fraction | 0.69±0.06 | 0.70±0.10 | 0.51 |
| Men: Framingham coronary risk score | 7.5±3.4 | 9.7±3.9 | 0.015 |
| Men: Left ventricular mass, g | 158±28 | 170±28 | 0.076 |
| Men: Left ventricular mass/Height, g/m | 90±15 | 99±17 | 0.023 |
| Men: Left ventricular mass/Height2.7, g/m2.7 | 35±6 | 39±7 | 0.003 |
| Men: Left ventricular mass/body surface area, g/m2 | 76±12 | 83±13 | 0.038 |
| Women: Myocardial contraction fraction | 0.59±0.13 | 0.50±0.07 | 0.015 |
| Women: Left ventricular ejection fraction | 0.71±0.06 | 0.73±0.06 | 0.26 |
| Women: Framingham coronary risk score | 7.9±5.1 | 13.2±8.0 | 0.0002 |
| Women: Left ventricular mass, g | 109±28 | 122±21 | 0.028 |
| Women: Left ventricular mass/Height, g/m | 68±14 | 76±13 | 0.032 |
| Women: Left ventricular mass/Height2.7, g/m2.7 | 30±7 | 34±7 | 0.022 |
| Women: Left ventricular mass/body surface area, g/m2 | 62±10 | 67±8 | 0.075 |
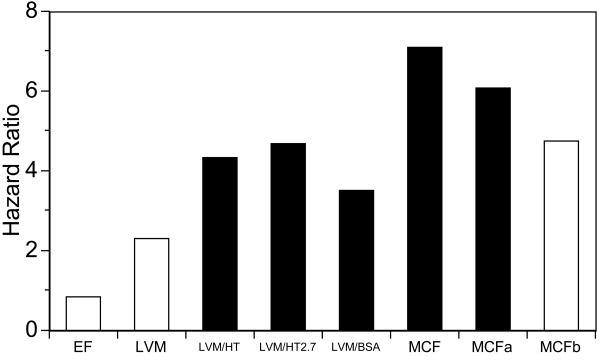
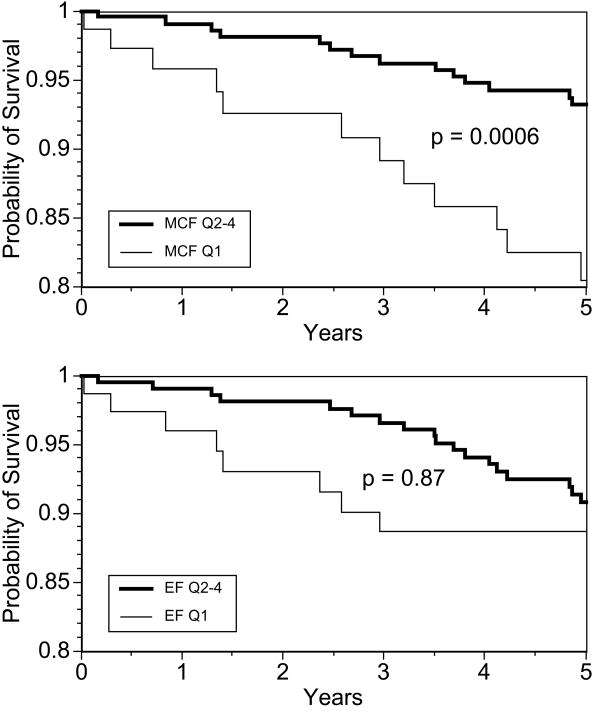
Table 3 shows that that the lowest-quartile MCF had increased hazards of hard CVD events, even after adjustment for sex and FCRS, (hazard ratio 7.11, p=0.010), relative to the referent (highest) quartile of MCF. LVEF and LV mass were not associated with incident hard CVD events. The highest-quartile indexed LV mass had more than four times the hazard for hard CVD events (hazard ratio = 4.34, p=0.023) which remained unchanged with indexation to HT, to HT2.7 and to BSA. After further adjustment for LV mass, the hazard ratio of lowest-quartile MCF remained a significant (hazard ratio = 6.09, p=0.020. The hazard ratios for MCF were no longer significant after adjustment for concentricity (LVM/LVEDV). The hazard ratios for low MCF and LVEF and high LV mass, with and without indexation, are depicted graphically in Figure 1. Kaplan Meir survival plots (Figure 2) showed lower event-free survival for lowest-quartile MCF, but not EF. Results did not change with exclusion of the 3 participants with LVEF < 0.55.
Table 3.
Hazard ratios by quartile for hard cardiovascular events. The lowest quartile (quartile 1) served as the referent for left ventricular mass. The highest quartile (quartile 4) was the referent for left ventricular ejection fraction and myocardial contraction fraction.
| Hazard Ratio | 95% Confidence Intervals | P | |
|---|---|---|---|
|
|
|
|
|
| Left ventricular mass, quartile 2 | 0.84 | [0.23-3.16] | 0.80 |
| Left ventricular mass, quartile 3 | 1.62 | [0.53-4.99] | 0.40 |
| Left ventricular mass, quartile 4 | 2.29 | [0.80-6.51] | 0.12 |
| Left ventricular mass/height, quartile 2 | 1.48 | [0.33-6.66] | 0.61 |
| Left ventricular mass/height, quartile 3 | 3.13 | [0.84-11.65] | 0.09 |
| Left ventricular mass/height, quartile 4 | 4.34 | [1.23-15.35] | 0.023 |
| Left ventricular mass/height 2.7, quartile 2 | 1.13 | [0.23-5.62] | 0.88 |
| Left ventricular mass/height 2.7, quartile 3 | 3.06 | [0.83-11.37] | 0.09 |
| Left ventricular mass/height 2.7, quartile 4 | 4.68 | [1.34-16.32] | 0.016 |
| Left ventricular mass/body surface area, quartile 2 | 1.20 | [0.30-4.81] | 0.80 |
| Left ventricular mass/body surface area, quartile 2 | 2.74 | [0.86-8.80] | 0.09 |
| Left ventricular mass/body surface area, quartile 2 | 3.50 | [1.12-10.95] | 0.031 |
| Left ventricular ejection fraction, quartile 1 | 0.86 | [0.35-2.12] | 0.75 |
| Left ventricular ejection fraction, quartile 3 | 0.53 | [0.20-1.41] | 0.20 |
| Left ventricular ejection fraction, quartile 3 | 0.29 | [0.08-1.01] | 0.051 |
| Myocardial contraction fraction, quartile 1 | 7.11 | [1.60-31.59] | 0.010 |
| Myocardial contraction fraction, quartile 2 | 4.04 | [0.88-18.58] | 0.07 |
| Myocardial contraction fraction, quartile 3 | 2.11 | [0.39-11.60] | 0.39 |
| Myocardial contraction fraction, mass adjusted, quartile 1 | 6.09 | [1.33-27.89] | 0.020 |
| Myocardial contraction fraction, mass adjusted, quartile 2 | 3.75 | [0.81-17.38] | 0.09 |
| Myocardial contraction fraction, mass adjusted, quartile 3 | 1.94 | [0.35-10.68] | 0.45 |
| Myocardial contraction fraction, concentricity adjusted, quartile 1 | 4.76 | [0.73-30.88] | 0.10 |
| Myocardial contraction fraction, concentricity adjusted, quartile 2 | 3.36 | [0.68-16.76] | 0.14 |
| Myocardial contraction fraction, concentricity adjusted, quartile 3 | 1.89 | [0.33-10.64] | 0.47 |
Figure 1.
Hazard ratios for hard cardiovascular disease (CVD) events for Q1 (lowest quartile) MCF and left ventricular ejection fraction (LVEF) and Q4 (highest quartile) left ventricular mass (LVM) versus referent quartiles. LVM/HT, LVM/HT2.7 and LVM/BSA are LVM indexed respectively to height, height2.7 and body surface area. MCFa and MCFb show hazard ratios for MCF adjusted for LVM and LVM/LVEDV, respectively. Q1 MCF and MCFa and Q4 indexed LVM were predictive of future hard CVD events (shaded bars), while Q1 EF and MCFb and Q4 unindexed LVM were not predictive (open bars) Intermediate quartiles were not predictive of hard CVD events and are not shown. MCF = myocardial contraction fraction, EDV = end-diastolic volume.
Figure 2.
Kaplan-Meier survival plots for MCF (top) and LVEF (bottom). Survival free of hard cardiovascular events was significantly lower in the lowest quartile (Q1) of MCF versus the remaining quartiles (Q2-4), p=0.0006, log-rank test. There were no differences in survival between lowest-quartile and Q2-4 EF, p=0.87.
Discussion
In this study of a longitudinally-followed community-dwelling cohort initially free of clinical CVD, we found that decreased MCF, a novel volumetric LV index, predicts hard CVD events over > 5-year follow up. The lowest quartile of MCF was predictive of a major adverse hard CVD event after adjustment for multiple CVD risk factors, including age, systolic blood pressure, dyslipidemia, diabetes, treatment for hypertension or hyperlipidemia, and smoking, as summarized by the FCRS. (The FCRS does not incorporate any CMR results.) Further, decreased MCF remained predictive of future hard CVD events after additional adjustment for LV mass. We also found greater MCF in women versus men.
LVEF is widely used to identify abnormal ventricular function, to perform risk stratification and to guide therapy. However, normal-range LVEF does not necessarily imply low CVD risk, or even freedom from symptomatic CVD. Indeed, heart failure with normal-range LVEF is a common clinical scenario which carries an [6-8]. One means by which LVEF may be preserved despite myocardial contractile dysfunction is via concentric remodeling, where wall thickness increases relative to cavity size [9-10]. Although these concentric changes in LV geometry may allow normalization of the LVEF, concentric remodeling or LV hypertrophy are both associated with excess hard CVD events, as compared to normal LV geometry [11-13]. Given that MCF is the inverse of LV mass multiplied by LV stroke volume and a fixed scaling factor, we accordingly investigated the impact of adjusting for LV mass and found that MCF remained predictive of future hard CVD events. Further, depressed MCF may identify an additional subset of at-risk individuals as compared with increased LV mass alone. In this study cohort, 10% of the hard CVD participants had low MCF but also low LV mass.
Considering MCF mathematically, it can also be expressed as 1.05 × [LVEDV/LVM – LVESV/LVM], where ESV is end-systolic volume. This indicates that MCF is, apart from an overall scaling factor of 1.05 g/ml, the inverse of LV concentricity (LVM/LVEDV ratio) from which the ratio of ESV to LV mass is subtracted. Thus an increased concentricity means a decreased MCF, but MCF will decrease further with larger ESV. This interpretation of MCF suggests that it might be considered an index which encompasses both LV concentricity and systolic function (worse function leading to larger ESV and thus a greater decrease in MCF than would be based on LV concentricity alone). That concentricity is an important component of MCF appears to be reflected by our finding that lowest-quartile MCF was no longer an independent predictor of hard CVD events in a model containing both MCF and concentricity. However, MCF nonetheless trended toward significance, even after adjustment for concentricity, and it may be that lack of statistical power limited our ability to find independent predictive value for MCF in this model.
MCF does not require any additional CMR image acquisition or analyses above those already performed to determine LV mass and volumes and importantly, scanner workflow is not altered. Data acquisition for LV function typically required < 10 minutes in this study using a single breath-hold per image slice, and faster imaging methods are now available [14, 15]. Manual delineation of contours requires approximately 6 minutes, and semi-automated methods can reduce this time further [16]. Computationally, MCF is straightforward, as it is the ratio of stroke volume to myocardial volume. Myocardial volume is related to myocardial mass by a simple scaling factor, the specific gravity of myocardium (1.05 g/cc) but is computationally advantageous over LV mass in that it allows MCF to be a dimensionless ratio, as is LVEF.
The Framingham Heart Study population is largely Caucasian and the Offspring cohort is middle-aged or older; our results may not extend to other ethnic or age groups. Our study used an older (though current at time of CMR scanning) segmented K-space gradient-echo cine method, while LV functional imaging is now performed using steady-state free-precession (SSFP) cine sequences. Compared with gradient-echo, SSFP yields greater LV volumes and lower LV mass [17], and thus higher MCF values. As SSFP is now the overwhelming sequence of choice for assessment of LV size and function, we do not report cut-points for MCF based on our gradient echo data as these would be of little clinical or research value.
In this study, decreased MCF was an independent predictor of future hard CVD events, but the relatively modest number of events during follow-up was insufficiently powered for detailed analyses of the subtypes of hard CVD events. On sex-specific analyses, the difference in MCF between hCVD+ and hCVD- participants was significant only in women, most likely due to sample size and number of events rather than sex-specific predictive value of MCF. We found significantly lower MCF in men than women, but whether sex-specific criteria for identification of depressed MCF are needed is unknown and should be examined in a larger cohort. If CMR is used, this should be done using SSFP imaging. Importantly, MCF is not CMR-specific. Indeed, it was initially described in the context of three-dimensional (3D) echocardiography [1]. King et al. suggested that MCF should be determined using a volumetric (3D) technique, due to the greater accuracy and reproducibility of 3D, vs. 2D, methods for determination of ventricular and myocardial volumes. However, it remains to be investigated as to whether MCF by 2D methods predicts future hard CVD events, as we did not determine biplane CMR LV volumes and mass in the present study. Possible additional relationships between LV mass, concentric remodeling and MCF await further investigation, particularly with regard to persons with both low MCF and low LV mass, but the number of such participants in the present study was too small to permit us to explore this in detail. Also, whether MCF provides additional prognostic value when echocardiographic diastolic function parameters are available also remains to be determined, as there were no contemporaneous echocardiographic data for this study. Finally, the value of MCF in patients who already have clinically overt CVD or depressed LVEF, and whether changes in MCF have prognostic value remains to be investigated.
MCF is a novel, easily-determined volumetric LV index. Decreased MCF is an independent predictor of future CVD morbidity, even in the presence of normal LVEF and after adjustment for both traditional CVD risk factors and LV mass.
Acknowledgments
Funding Sources: Supported in part by grant RO1 AG17509 and by subcontract N01-HC-38038 from the National Institutes of Health.
Footnotes
Financial Disclosures and Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.King DL, El-Khoury Coffin L, Maurer MS. Myocardial contraction fraction: a volumetric index of myocardial shortening by freehand three-dimensional echocardiography. J Am Coll Cardiol. 2002;40:325–329. doi: 10.1016/s0735-1097(02)01944-7. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 3.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 4.Abbott RD, McGee DL. Section 37: The Probability of Developing Certain Cardiovascular Diseases in Eight Years at Specified Values of Some Characteristics. Bethesda, MD: National Heart, Lung, and Blood Institute; 1987. The Framingham Study: An Epidemiological Investigation of Cardiovascular Disease. [Google Scholar]
- 5.Salton CJ, Chuang ML, O'Donnell CJ, Kupka MJ, Larson MG, Kissinger KV, Edelman RR, Levy D, Manning WJ. Gender differences and normal left ventricular anatomy in an adult population free of hypertension. A cardiovascular magnetic resonance study of the Framingham Heart Study Offspring cohort. J Am Coll Cardiol. 2002;39:1055–1060. doi: 10.1016/s0735-1097(02)01712-6. [DOI] [PubMed] [Google Scholar]
- 6.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 7.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 8.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948–1955. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 9.de Simone G, Devereux RB, Celentano A, Roman MJ. Left ventricular chamber and wall mechanics in the presence of concentric geometry. J Hypertens. 1999;17:1001–6. doi: 10.1097/00004872-199917070-00017. [DOI] [PubMed] [Google Scholar]
- 10.Aurigemma GP, Silver KH, Priest MA, Gaasch WH. Geometric changes allow normal ejection fraction despite depressed myocardial shortening in hypertensive left ventricular hypertrophy. J Am Coll Cardiol. 1995;26:195–202. doi: 10.1016/0735-1097(95)00153-q. [DOI] [PubMed] [Google Scholar]
- 11.Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–352. doi: 10.7326/0003-4819-114-5-345. [DOI] [PubMed] [Google Scholar]
- 12.de Simone G, Devereux RB, Koren MJ, Mensah GA, Casale PN, Laragh JH. Midwall left ventricular mechanics. An independent predictor of cardiovascular risk in arterial hypertension. Circulation. 1996;93:259–265. doi: 10.1161/01.cir.93.2.259. [DOI] [PubMed] [Google Scholar]
- 13.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pruessmann KP, Weiger M, Boesiger P. Sensitivity encoded cardiac MRI. J Cardiovasc Magn Reson. 2001;3:1–9. doi: 10.1081/jcmr-100000143. [DOI] [PubMed] [Google Scholar]
- 15.Mascarenhas NB, Muthupillai R, Cheong B, Pereyra M, Flamm SD. Fast 3D cine steady-state free precession imaging with sensitivity encoding for assessment of left ventricular function in a single breath-hold. AJR Am J Roentgenol. 2006;187:1235–1239. doi: 10.2214/AJR.06.0169. [DOI] [PubMed] [Google Scholar]
- 16.Hautvast GLTF, Salton CJ, Chuang ML, Breeuwer M, O'Donnell CJ, Manning WJ. Accurate computer-aided quantification of left ventricular parameters: Experience in 1555 cardiac magnetic resonance studies from the Framingham Heart Study. Magn Reson Med. 2011 doi: 10.1002/mrm/23127. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hudsmith LE, Petersen SE, Tyler DJ, Francis JM, Cheng AS, Clarke K, Selvanayagam JB, Robson MD, Neubauer S. Determination of cardiac volumes and mass with FLASH and SSFP cine sequences at 1.5 vs 3 Tesla: a validation study. J Magn Reson Imaging. 2006;24:312–318. doi: 10.1002/jmri.20638. [DOI] [PubMed] [Google Scholar]