Abstract
CTLA-4 is a potent co-inhibitory molecule that is critical for peripheral T cell tolerance. A report in this issue reveals the autoantigen-specific nature of CTLA-4-deficient CD4+ T cells within target tissue. In vivo studies demonstrate that CTLA-4 expression by self-reactive effector cells is sufficient to limit their infiltration into target tissue and that regulatory T cells expressing CTLA-4 can control autoantigen-specific effector T cells. Thus, these findings provide evidence that CTLA-4 exerts its critical immunoregulatory functions by controlling conventional T cells as well as regulatory T cells.
It has been appreciated for some time that the Cytotoxic T lymphocyte antigen-4 (CTLA-4) receptor, a homolog of CD28, is an indispensable negative regulator of peripheral T cell function1. The fatal lymphoproliferative phenotype of CTLA-4−/− mice revealed the critical negative regulatory function of CTLA-4 and provided the first evidence that costimulatory receptors could provide negative, as well as positive, second signals2,3. CTLA-4−/− mice develop severe myocarditis and pancreatitis and die within the first month of life. This pathology resembled an autoimmune disease and gave impetus to studies investigating the role of CTLA-4 in T cell tolerance and autoimmunity4. While the role of CTLA-4 in regulating peripheral T cell tolerance has become firmly established, there are still many questions regarding how CTLA-4 exerts its key immunoregulatory functions. Among them is the unresolved question of whether or not T cells that infiltrate various tissues in the CTLA-4−/− mice are autoreactive and tissue-specific. In this issue of Nature Immunology, Ise et al. show for the first time that the hyperproliferative and destructive T cell populations in CTLA-4-deficient mice are not on autopilot, but require specific signals provided by autoantigens to cause tissue damage5. Their work points to an important role for CTLA-4 expression by effector T cells in restraining tissue-specific CD4+ T cells from infiltrating, expanding and/or surviving in target organs and provides evidence that CTLA-4 can control pathogenicity of self-reactive T cell at multiple levels.
Ise et al. were able to analyze the TCR repertoire of CTLA-4−/− T cells by fixing the TCRβ chain of CTLA-4−/− mice (DOβCTLA-4−/− mice). DOβCTLA-4−/− mice exhibited similar, but somewhat delayed, pathology in comparison with CTLA-4−/− mice. The antigen-specific nature of pathogenic CTLA-4−/− T cells was suggested by homing studies where CD4+ T cells from the spleen or affected organs of DOβCTLA-4−/− mice were introduced into Rag-2−/− recipients. While transfer of splenic T cells recapitulated the multi-organ CTLA-4−/− phenotype, T cells transferred from affected organs (eg. lungs, heart or pancreas) tended to accumulate in the corresponding organ of the recipient and only caused pathology in this tissue. Ise et al. identified acinar cell-specific protein disulfide isomerase associated 2 (Pdia2) as a specific autoantigen that drives pancreatic pathology in DOβCTLA-4−/− mice and found anti-Pdia2 antibodies in sera from CTLA-4−/− mice. They cloned a Pdia-2-reactive TCR that endowed naïve T cells with pancreas-specific reactivity and compared the functions of CTLA-4+/+ and CTLA-4−/− Pdia2-specific T cells
Notably, CTLA-4+/+ and CTLA-4−/− Pdia2-specific T cells migrated and accumulated comparably in pancreatic lymph nodes, but CTLA-4 deficiency markedly enhanced infiltration of these T cells into the pancreas. These findings point to a novel function for CTLA-4 expressed by antigen-specific effector T cells in regulating peripheral tolerance at the level of the tissue. Their work indicates that CTLA-4 governs a critical tolerance checkpoint that controls self reactive T cell entry, expansion and/or survival in target tissue. A role for CTLA-4 in modulating the dynamics of T cell-antigen presenting cell (APC) interactions has been previously demonstrated, but the focus has been mainly on interactions within the lymph node, rather than trafficking into tissues6. Further studies are needed to understand how CTLA-4 protects the tissues from pathogenic self reactive T cells.
The work of Ise et al. also addresses the controversial question of whether expression of CTLA-4 is important only on regulatory T cells (Treg) or whether conventional T cells (Tconv) also require expression of this molecule. Treg act in trans to control the responsiveness of Tconv and other components of the immune system. A role for CTLA-4 in facilitating Treg function was first suggested by bone marrow chimera studies7. Recently, specific deletion of CTLA-4 in FoxP3+ Treg and sophisticated blastocyst chimera experiments unambiguously demonstrated the crucial role for CTLA-4 in Treg biology 8,9. However, a lack of CTLA-4 on Treg does not fully account for the phenotype of CTLA-4 null mice, as Foxp3-specific CTLA-4 deficiency results in a fatal inflammatory pathology that is delayed in onset and more restricted as compared to CTLA-4 null mice8.
Ise et al. provide evidence to support a role for CTLA-4 in controlling Tconv, exclusive of its role on Treg. CTLA-4-expressing, Pdia2-specific T cells were studied in a Rag-2−/− environment and did not express Foxp3, suggesting that CTLA-4 expression on Tconv is sufficient to prevent these cells from becoming pathogenic. However, CTLA-4-expressing Treg could also suppress the pathogenicity of CTLA-4-deficient Pdia2-specific T cells. Thus, this work points to dual functions for CTLA-4, acting both on Tconv and Treg.
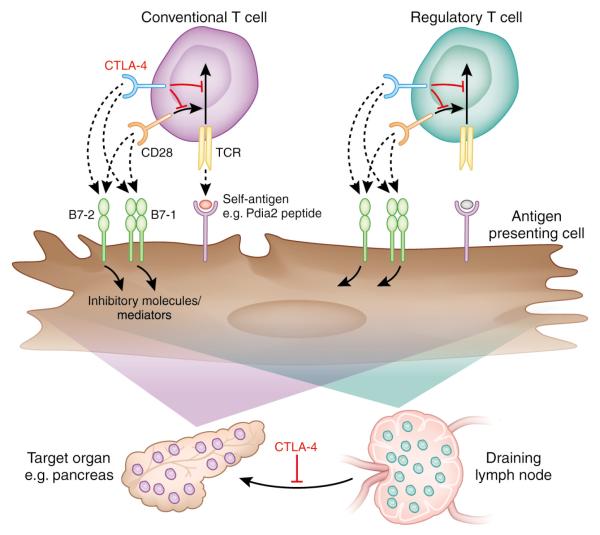
The work of Ise et al. suggests that particular self-reactive T cells that escape negative selection must either express CTLA-4 themselves or become subject to peripheral control by Treg that also depend on CTLA-4 for their function (Figure 1). It is not yet settled whether CTLA-4 acts directly on the T cell that expresses it, or acts on the APC, either by binding of B7 by CTLA-4, which leads to back signaling into APC 10,11 or by down-modulating B7 expression8. The finding that CTLA-4 governs a critical tolerance checkpoint that controls T cell entry into and accumulation within target organs opens a novel area of investigation. It will be important to understand how CTLA-4 regulates the balance between pathogenic effector and protective Treg responses within non-lymphoid organs. Since B7-1 (CD80) and B7-2 (CD86) can be expressed on some non-hematopoietic cells, as well as hematopoietic cells, there may exist as yet unappreciated cellular interactions by which CTLA-4 defends tissues from pathogenic self reactive T cells, which would provide insights into immunoregulation in specific tissue microenvironments.
Figure 1.
CTLA-4 limits autoantigen-specific T cells at multiple checkpoints. Self-antigen-specific T cells that escape negative selection must either express CTLA-4 themselves or become subject to peripheral control by Treg that also depend on CTLA-4 for their function. As well as restraining activation events in the lymph node, CTLA-4 also prevents T cell infiltration, expansion and survival at the level of the target tissue.
Further understanding of how CTLA-4 regulates tolerance has therapeutic as well as fundamental importance. The critical role of CTLA-4 in human autoimmune disease is underscored by genome-wide association studies that have identified CTLA-4 as a key gene associated with risk of developing multiple human autoimmune diseases12. In addition, CTLA-4 is an important therapeutic target in cancer immunotherapy13. The work of Ise et al. suggests a new means by which CTLA-4 may contribute to the multiple barriers that prevent anti-tumor responses and shield tumors from immune eradication. Better understanding of the levels at which CTLA-4 exerts its inhibitory functions will facilitate design of therapeutic strategies that target CTLA-4 to treat cancer, autoimmunity, graft rejection and infectious diseases.
Footnotes
Our apologies to those authors whose work we could not cite due to space limitations.
References
- 1.Fife BT, Bluestone JA. Immunol Rev. 2008;224:166–82. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 2.Tivol EA, et al. Immunity. 1995;3:541–7. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 3.Waterhouse P, et al. Science. 1995;270:985–8. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 4.Perez VL, et al. Immunity. 1997;6:411–7. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 5.Ise W, et al. Nat Immunol. (This issue) [Google Scholar]
- 6.Schneider H, et al. Science. 2006;313:1972–5. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 7.Bachmann MF, Kohler G, Ecabert B, Mak TW, Kopf M. J Immunol. 1999;163:1128–31. [PubMed] [Google Scholar]
- 8.Wing K, et al. Science. 2008;322:271–5. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 9.Friedline RH, et al. J Exp Med. 2009;206:421–34. doi: 10.1084/jem.20081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fallarino F, et al. Nat Immunol. 2003;4:1206–12. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 11.Dejean AS, et al. Nat Immunol. 2009;10:504–13. doi: 10.1038/ni.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueda H, et al. Nature. 2003;423:506–11. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 13.Pentcheva-Hoang T, Corse E, Allison JP. Immunol Rev. 2009;229:67–87. doi: 10.1111/j.1600-065X.2009.00763.x. [DOI] [PubMed] [Google Scholar]