Abstract
The avian haemosporidian parasites (phylum Apicomplexa) are taxonomically diverse and cosmopolitan in distribution; infecting most bird families. Sources of concern are reports of clinical haemosporidian infections in birds kept as part of zoo and aviary collections. Recently, severe and acute mortality episodes have been reported in masked bobwhite quail (Colinus virginianus ridgwayi), an endangered subspecies from the American Southwest. Two hundred and five eggs of the captive flock held in Arivaca, Arizona, were hatched at a zoo in the American Southwest. Thirty four sub-adult or adult animals had lesions associated with tissue phases of hemoparasites, especially vasculitis, ventricular leiomyositis and ulcerative pododermatitis. Molecular techniques applied to blood collected from the zoo’s last twelve remaining animals resulted in the detection of a Plasmodium juxtanucleare-like and Haemoproteus sp. parasites. A Raven (Corvus corax), in a contiguous exhibit, was positive for the same Plasmodium juxtanucleare-like parasite, but remained asymptomatic for three years following detection. These findings indicate that other birds in the exhibit within the zoo premises could act as reservoirs. We conclude that haemosporidian infections could be a factor in the demise of the captive masked bobwhite quails housed at the zoo. We suggest that active surveillance for haemoporidian parasites should be incorporated as a precaution to ex-situ conservation efforts of susceptible endangered species.
Keywords: Masked Bobwhite Quail, Haemosporidia, Plasmodium, Haemoproteus, Cytochrome b gene, vasculitis, ventricular leiomyositis, ulcerative pododermatitis
1. Introduction
Avian haemosporidian infections have been increasingly studied as model systems for investigating different aspects of the host-parasite interactions in ecology, evolution, and conservation biology. The avian haemosporidian parasites (phylum Apicomplexa) are taxonomically diverse, cosmopolitan in distribution, and infect most bird families (Valkiunas, 2005). The three commonly reported avian hemoprotozoa belong to the genera Plasmodium, Haemoproteus, and Leucocytozoon. Regardless of their broad host and geographic distributions, information on their pathogenicity is almost completely based on laboratory experiments with domesticated birds (canaries, chickens, ducklings, pigeons, turkey poults) (Valkiunas, 2005). However, some haemosporidioses are well studied and thoroughly documented in zoos and rehabilitation centers. Based on such assessments, we know that these parasites are associated with a broad spectrum of clinical disease manifestations, from asymptomatic to acutely fatal (Cardona et al., 2002, Valkiūnas, 2005; Ferrell et al., 2007, Grim et al. 2008). Among haemosporidian parasites, Plasmodium is responsible for most frequently observed outbreaks of especially severe malaria in domestic and wild birds in zoos and aviaries (Valkiūnas, 2005, Fix et al., 1988, Cranfield et al., 1990, Ferrell et al., 2007, Alley et al., 2008, Olias et al., 2011). However, some species of Haemoproteus can be highly pathogenic and cause severe myositis in avian hosts (Cardona et al., 2002, Olias et al. 2011). For example, chronic outbreaks of Haemoproteus lophortyx infection have been reported in captive bobwhite quail (Cardona et al., 2002).
Recently, severe and acute mortality episodes have been reported in masked bobwhite quail (Colinus virginianus ridgwayi, Odontophoridae) at breeding facilities. Masked bobwhite quail, a subspecies of the Northern bobwhite, is the only endangered quail in North America. Although the birds never had an extensive distribution in the U.S., they were plentiful and common in Northern Mexico during the 1860’s (Brown and Ellis, 1977). Excessive grazing pressure, coupled with extended drought during the early 1890s, deteriorated the grasslands of Southern Arizona and caused the masked bobwhite population to shrink (Brown, 1900). This endangered sub-species is currently restricted to one captive population in the Buenos Aires National Wildlife Refuge (Altar Valley, Southern Arizona) and two known native populations in private ranches in north-central Sonora, Mexico (Kuvlesky et al. 2000). The current captive flock of masked bobwhite quail may represent the only hope for this highly endangered subspecies (Hernández et al. 2006).
In this study, we explore the possibility that the recent mortality episodes in masked bobwhite quail at the zoos could be related, in part, to outbreaks of Haemosporidian infections. In this investigation we report both Haemoproteus sp. and Plasmodium juxtanucleare-like parasites for the first time in captive masked bobwhite quail hatched and raised in a zoo. We describe new pathological signs such as vascular damage, especially in the ventriculus and skin associated with the haemosporidian infection.
2. Materials and methods
2.1. Samples description
In 2006, a Southwest zoo received a total of 206 fertile masked bobwhite quail eggs for hatching. Two dozen masked bobwhite quail were placed on exhibit, and the rest of the animals were placed in separate and isolated wire mesh holding facilities. The birds placed on exhibit were housed with other native regional wildlife in large connected flight cages. These birds were in contact with a flock of 14 thick billed parrots (Rhynchopsitta pachyrhyncha). A small stream ran through the exhibit. A contiguous flight cage housed two bald eagles (Haliaeetus leucocephalus), two black vultures (Cathartes aura), two common ravens (Corvus corax), and an American porcupine (Erethizon dorstatum). Gambel’s quail (Callipepla gambelii), and two golden eagles (Aquila chrysaetos) were exhibited in other remote parts of the zoo.
The Zoo keepers noted that many quails appeared weak, listless and unresponsive while still in adequate body condition, and many had ulcerative skin lesions on the dorsum of the feet and around the tarsometatarsal-phalangeal joint. Blood smears of clinically ill birds consistently showed numerous large intra-erythrocytic hemosporidian parasites. Affected birds would be found dead soon after initial clinical signs. Necropsy would usually reveal generalized pallor. Tissues from five dead masked bobwhite quail were fixed in 10% neutral buffered formalin and submitted for histologic examination to two separate laboratories. By March 2007 only 10 adults quail remained alive; thus, the success rate of this breeding facility was only 5% from the original 206 fertile eggs. Those 10 adults were the subject of this investigation in an effort to understand why such failure in maintaining this endangered species in that particular location.
2.2 Cytology and histopathology
Histopathologic and cytologic investigation was conducted at Northwest ZooPath and at the Arizona Veterinary Diagnostic Laboratory on five of the dead quail. Following necropsy, sections of brain, heart, lung, liver, kidney, spleen, pancreas, alimentary tract at all levels, reproductive tract, and skin of the feet were fixed in 10% neutral buffered formalin, processed routinely, sectioned at 5 microns and stained with hematoxylin and eosin (HE). Blood films from each bird were stained with Wright-Giemsa technique.
2.3. Detection of Haemosporidian parasites by polymerase chain reaction (PCR)
Zoo animals close to the exhibited masked bobwhite quail were bled as part of their annual medical exam. A total volume of 0.25 to 0.5 ml of whole blood was collected in EDTA from each animal and frozen prior to testing. Blood samples were drawn from the remaining ten masked bobwhite quail, six Gambel’s quail (Callipepla gambelii, Odontophoridae), two common raven (Corvus corax, Corvidae), four thick-billed parrot (Rhynchopsitta pachyrhyncha, Psittacidae), one bald eagle (Haliaeetus leucocephalus, Accipitridae), and two golden eagles (Aquila chrysaetos, Accipitridae). In addition, we collected and analyzed blood samples from 38 masked bobwhite quail at the Buenos Aires National Wildlife Refuge. Three blood samples of the greater roadrunner (Geococcyx californianus) were also analyzed; one from the zoo, and two from Liberty Wildlife, a wildlife rehabilitation center in the same region. All samples were frozen at − 4.0°C and banked for future testing.
Using 100 μl of thawed blood samples from each of the birds, deoxyribonucleic acid (DNA) was extracted using QiAamp® DNA Blood Mini Kit (Qiagen GmbH, Hilden, Germany). Each DNA sample was screened for haemosporidian parasites by using a nested polymerase chain reaction (PCR) protocol that targets mitochondrial markers in the parasite. Mitochondrial markers were chosen given that they have been widely used in avian malaria ecology and there is a relatively extensive dataset available in the Genebank (National Center for Biotechnology Information, National Institutes of Health), allowing us to compare our results with those from previous studies. In order to amplify an 1800 base pair (bp) fragment of the complete mitochondrial cytochrome b (cyt b), specific primers forward-TGTAATGCCTAGACGTATTCC and reverse-GTCAAWCAAACATGAATATAGAC were designed by using the published sequences of Haemoproteus and Plasmodium mitochondrial DNA genome (mtDNA). PCR amplifications were carried out in a 50 μl volume, 20 ng of total genomic DNA, 3 mM MgCl2, 1X PCR buffer, 1.25 mM of each deoxynucleoside triphosphate, 0.4 mM of each primer, and 0.03U/μL AmpliTaq polymerase (Applied Biosystems, Roche-USA). The PCR conditions were: a partial denaturation at 94 °C for 4 min and 35 cycles with 1 min at 94 °C, 1 min at 55°C and 2 min extension at 72 °C, a final extension of 10 min was added in the last cycle.
Those samples that tested negative in the first PCR amplification were studied with a second, nested PCR step using 1 μl of the first amplification as the template. The PCR conditions were identical but with internal primer forward-TCTATTAATTTAGYWAAAGCAC and reverse-GCTTGGGAGCTGTAATCATAAT. After electrophoresis, all amplified products were excised from the gels, purified by the QIAmp Gel Extraction Kit (Qiagen), cloned using the pGEM®-T Easy Vector Systems I (Promega, USA), and sequenced using an Applied Biosystems 3730 capillary sequencer. Both strands were sequenced from at least four clones. Sequence identity was confirmed using BLAST by comparing the unknown Plasmodium and Haemoproteus sequences to known mtDNA sequences available in the GenBank.
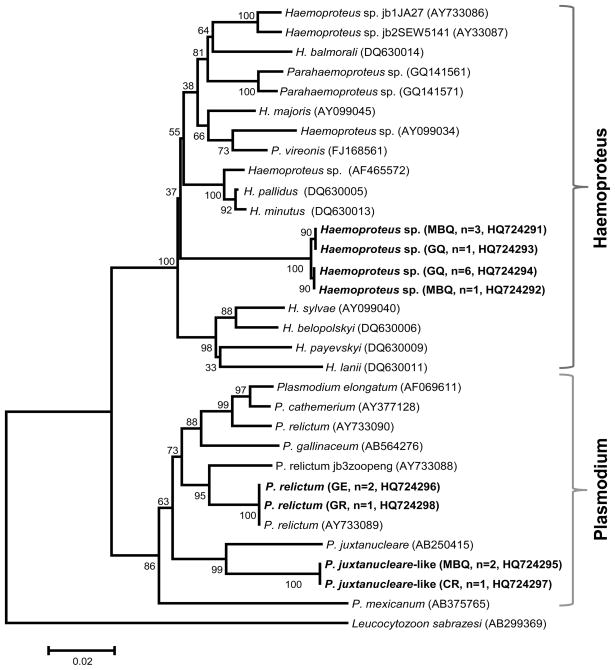
Previously reported sequences from other haemosporina and ours were aligned using the ClustalX software version 2.0.12 (available at http://www.clustal.org/) with manual editing. We used MEGA 4 software (Tamura et al., 2007) to generate a Neighbor-Joining tree using Tamura 3-parameter distance. The reliability of the nodes in the NJ tree was assessed by bootstrap method with 1000 pseudo-replications. The sequences reported in our study are deposited in the GenBank under the accession numbers HQ724291 - HQ724298, and the accession numbers of all sequences previously reported and included in this study are given in Figure 3.
Figure 3.
Neighbor-Joining tree of the Hemosporidian parasites found in birds sampled in this study using Tamura 3-parameter distance. The numbers on nodes of the tree are percent of bootstrap values based on 1000 pseudo-replications. (MBQ) Masked Bobwhite Quail, (GQ) Gambel’s Quail, (CR) Common Raven, (GE) Golden Eagle, and (GR) Greater Roadrunner. Numbers of positive individual are indicated within parenthesis. GenBank accession numbers are provided for our and the sequences previously reported.
3. Results
3.1. Cytology and histopathology
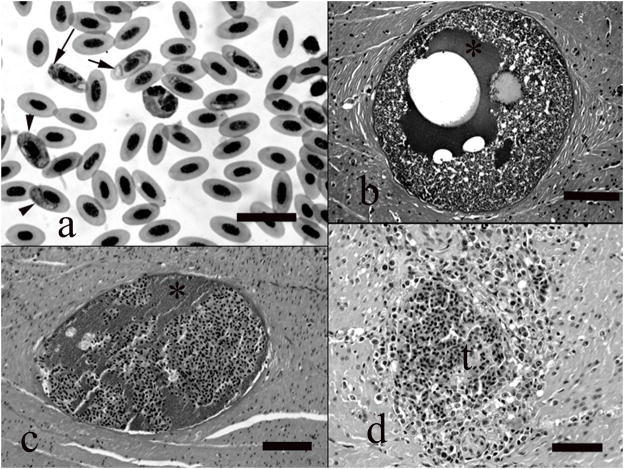
All five masked bobwhite quail had gametogenous stages of hemoparasites in circulating erythrocytes, and these parasites were detected in Wright-Giemsa stained blood films, as well as in histologic section. In the blood films the developing parasites were clearly intracytoplasmic and often there were more than one parasite in the infected cell. The parasites appeared to develop as small dark spherical nondescript structures 1–2 microns in greatest dimension, gradually enlarging to include spherical or slightly ovoid ring forms with displaced nuclei and clear cytoplasm. Larger elongated forms had central nuclei and partially wrapped around, displaced, or flattened the erythroid nucleus. Scattered low numbers of dark brown granules could be seen in the cytoplasm of the larger gametes, but no schizonts were detected in erythrocytes (Fig. 1a).
Figure 1.
a) Blood film showing numerous erythrocytes that contain intracytoplasmic protozoan gametes, including small dark bodies (long arrow), ring forms (short arrow), and elongated forms (arrowheads). Wright-Giemsa, bar = 25 μm. b) Megaloschizont filling lumen of a blood vessel in the smooth muscle tunic of the ventriculus. Note clear vacuole, proteinaceous fluid (*), and zoites. HE, bar = 220 μm. c) Degenerative cyst in ventricular smooth muscle tunic. Note that the remnant cyst contains proteinaceous fluid (*) of the parasite admixed with host erythrocytes. HE, bar = 190 μm. d) Focus of mixed inflammation and hemorrhage following megaloschizont rupture and vascular obliteration. HE, bar = 210 μm.
All five birds had megaloschizonts in endothelial cells. Megaloschizonts were found primarily in the ventricular muscular tunics, but were also seen in the heart (Fig 1). The megaloschizonts developed within endothelial cells of small veins or venules within the fascial planes of the ventricular muscle and in the interstitium between myofibers of the myocardium. The cysts were generally spherical and ranged in size from 100 – 250 μm in diameter. The cysts had a thin outer capsular membrane. Cyst lumina contained the developing merozoites, often circumferentially delineating the inner surface of the capsule. Centrally the cysts were clear or contained proteinaceous fluid. The outer membrane of the cyst was sometimes ruptured and the cyst lumen suffused with blood, and the vessel lumen in these areas contained fibrinocellular thrombi. Affected vessels were sometimes necrotic, inflamed or unapparent. The vessel region was sometimes surrounded or replaced by a narrow zone of macrophages and lymphocytes (Fig. 1b-d).
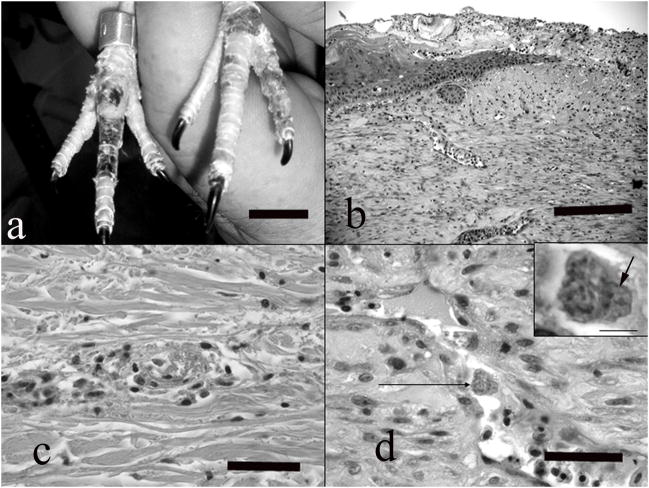
Skin lesions were observed in the dorsal aspect of the foot or tarsometatarsal-phalangeal joint. In these sections, endothelial cells lining the dermal veins and venules sometimes contained intracytoplasmic schizonts. These schizonts caused mild hypertrophy of the cell cytoplasm and there was a continuum in the development of the parasite. Sometimes the cells contained amorphous blue basophilic bodies, or clearly discernable clusters of zoites. This form was associated with generalized endothelial cell hypertrophy and occasionally with transmural lymphoplasmacytic or heterophilic inflammation, thrombosis, fibrinoid degeneration of the vessel wall, and perivascular inflammatory cell cuffs. Vascular lesions were accompanied by superficial and deep dermal edema, and epidermal coagulative necrosis, ulceration, regeneration and hyperplasia. Ulcerated regions were frequently lined by thick serocellular crusts. Adjacent to ulcerated regions, some dermal scarring and neovascularization were also noted (Fig. 2a-d).
Figure 2.
a) Note numerous foci of dark discoloration and ulceration on the dorsal aspects of the feet, corresponding to vascular lesions detected histologically. Bar = 2 cm. b). Full thickness section of skin from the dorsal aspect of the toe, showing chronic lesions. Note ulceration, crust formation, epidermal hyperplasia adjacent to the ulcer, and dermal scarring. HE, bar = 300 μm. c) A venule in the dermis showing acute fibrinoid necrosis with thrombosis and mild perivascular inflammatory infiltrate. HE, bar = 150 μm. d) Small vein in the dermis showing endothelial cell hypertrophy, necrosis and exfoliation. Note intracytoplasmic schizont in a hypertrophied endothelial cell (arrow). HE, bar = bar = 60 μm. Inset) higher magnification of the infected endothelial cell. Note the numerous zoites fill the cytoplasm and displace the host nucleus (arrow). HE, bar = 10 μm.
Additional histologic lesions included amyloidosis, and one each of bacterial septicemia, bacterial air sac granuloma, interstitial pneumonia, lymphocytic periportal hepatitis, and myocarditis associated with Sarcocystis-like cysts in the sarcoplasm of the myofibers.
3.2. Molecular analysis of blood samples
Table 1 shows the number of individuals screened in this study that were positive for haemosporidian parasites by using PCR and nested PCR. All 38 masked bobwhite quail from the Buenos Aires National Wildlife Refuge were negative for haemosporidia. However, six of the ten masked bobwhite quail collected at the zoo were positive, along with all of the zoo’s Gambel’s quail (Table 1). Two of the 10 masked bobwhite quail died during this study, but were found to be negative by PCR. The genetic analysis suggests that four of the zoo’s masked bobwhite quail were infected with Haemoproteus sp, and two with a Plasmodium juxtanucleare-like organism. (Table 1, Figure 3). We could not find evidence of co-infection with both Plasmodium sp. and Haemoproteus sp. in any quail. In the case of Gambel’s quail resident on the zoo grounds, all six were infected with the same genotype of Haemoproteus sp. found in the zoo’s masked bobwhite quail. We also found mixed infections, with two genotypes of Haemoproteus sp. in both masked bobwhite and Gambel’s quail suggesting two different species of parasites co-infecting those individuals. Gambel’s quail showed no clinical signs of disease.
Table 1.
Hemosporidian parasites found in the blood samples included this study by using a nested polymerase chain reaction (PCR) protocol targeting the mitochondrial cytochrome b (cyt b) of the protozoa. (H.) Haemoproteus sp. and (P.) Plasmodium sp.
| Bird family, Common name and Scientific name | n | Negative | Positive | Parasite species | Locality | |
|---|---|---|---|---|---|---|
| H. | P. | |||||
| Odontophoridae | ||||||
| Masked Bobwhite Quail | 10 | 4 | 4 | 2 | Haemoproteus sp. Plasmodium juxtanucleare-like | Zoo |
| Colinus virginianus ridwayi | ||||||
| 38 | 38 | 0 | 0 | - | Buenos Aires National Wildlife Refuge, Arizona | |
| Gambel’s Quail | ||||||
| Callipepla gambelii | 6 | 0 | 6 | 0 | Haemoproteus sp. | Zoo |
| Corvidae | ||||||
| Common Raven | 2 | 1 | 0 | 1 | Plasmodium juxtanucleare-like | Zoo |
| Corvus corax | ||||||
| Psittacidae | ||||||
| Thick-billed Parrot | 4 | 4 | 0 | 0 | - | Zoo |
| Rhynchopsitta pachyrhyncha | ||||||
| Accipitridae | ||||||
| Bald Eagle | 1 | 1 | 0 | 0 | - | Zoo |
| Haliaeetus leucocephalus | ||||||
| Golden Eagle | 2 | 0 | 0 | 2 | Plasmodium relictum | Zoo |
| Aquila chrysaetos | ||||||
| Cuculidae | 1 | 1 | 0 | 0 | - | Zoo |
| Greater Roadrunner | ||||||
| Geococcyx californianus | 2 | 1 | 0 | 1 | Plasmodium relictum | Liberty Wildlife, Arizona |
Of all the other birds housed near the zoo’s masked bobwhite quail and tested, looking for a potential reservoir of Haemosporidian parasites, only one common raven and the two golden eagles were positive by PCR. The parasite sequence obtained from the common raven was 100% identical to the sequence of Plasmodium juxtanucleare-like found in the masked bobwhite quail (Table 1, Fig. 3). One bald eagle appeared to be infected with Plasmodium relictum. In addition, the same genotype indentified as P. relictum was found in a greater roadrunner from a regional wildlife rehabilitation center in Scottsdale, Arizona. It is worth noting that the P. relictum sequence found in this study was identical to P. relictum, isolate ATCC 30141 from a mourning dove (Zenaida macroura, Columbidae) sampled in Nebraska [AY733089] (Fig. 3). The common raven and the bald eagle showed no clinical signs of malaria (no weakness, progressive weight loss, severe anemia, and/or diarrhea as described in Valkiūnas, 2005).
4. Discussion
Avian hemosporidian parasites, including species of Haemoproteus, Plasmodium, and Leucocytozoon, are transmitted by hematophagous dipteran insects (Diptera) and are cosmopolitan in their distribution (Valkiūnas, 2005). Given that these birds hatched in the zoo, they must have acquired the infection there. Indeed, the area of the zoo that the masked bobwhite quail were housed in had a history of acute West Nile virus infections in mammals, suggesting a high insect vector population in the area that could allow active transmission of these parasites. Although most hemosporidian infections are subclinical, some avian species, as well as stressed or immune-compromised individuals, could suffer severe or fatal infections. To our knowledge, this is the first report of a Plasmodium juxtanucleare-like parasite in captive masked bobwhite quail hatched and raised in a zoo. Among malaria parasites, P. juxtanucleare is responsible for observed outbreaks of particularly severe malaria in domestic birds (Valkiūnas, 2005) and penguins (Grim et al., 2003). It primarily infects chickens (Gallus gallus domesticus) and other species of the Phasianidae (Massard and Massard, 1981). As is the case with several naturally occurring avian haemosporidia infections, there are rarely any clinical signs associated with P. juxtanucleare infections (Mota, 1998). However, with immune-suppression or stress, high parasitemia has been reported. Several clinical signs usually appear shortly before death; when birds become weak, move with difficulty, show progressive weight loss, pale mucous membranes, severe anemia, and diarrhea (Valkiūnas, 2005).
Histologic evaluation of affected quail revealed asexual stages of at least one hemoparasite in all 5 of the birds. In all of these birds, infection was associated with vascular damage, especially in the ventriculus and skin. The gross skin lesion involving the feet appeared to be due to dermal vascular damage associated with schizogenous stages of the parasite. Although this manifestation of hemoparasite infection is rarely reported, dermal vascular manifestations of malaria has been observed in penguins, teals and various species of Passeriformes (unpublished observation). Based on the cytologic and histologic findings in these quail it is considered likely that the hemoparasitism contributed significantly to morbidity and mortality in this collection.
Concurrent disease processes in these birds included amyloidosis, bacterial infection and sarcocystosis, and were considered secondary or unrelated disease processes. Amyloidosis is a common finding in granivorous birds associated with chronic inflammation and stress (Landman et al. 1998). It is likely that parasitism contributed to the development of amyloidosis in some of these birds. Concurrent bacterial or nonspecific inflammation was also seen in these birds, and likely also contributed to clinical morbidity and amyloidosis.
As indicated by our molecular analysis, the common raven could serve as a reservoir of Plasmodium juxtanucleare-like parasite at this zoo given that the DNA sequence of this parasite is identical to the one found in masked bobwhite quail. This result is consistent with previous observations indicating that passerines are frequently infected with Plasmodium parasites and could be asymptomatic carriers, exhibiting a low degree of host specificity (Bensch et al. 2000, Valkiūnas, 2005, Szymanski and Lovette 2005, Križanauskienė et al. 2006, Krone et al., 2008). Whereas reservoir hosts within the zoo collection could facilitate transmission of the disease to sensitive birds, we cannot determine the importance of unidentified wild birds within the zoo as reservoir hosts (Valkiūnas, 2005, Fix et al., 1988, Cranfield et al., 1990, Ferrell et al., 2007, Alley et al., 2008). Indeed, similar observations have been reported in aviaries from Germany and Switzerland where the bird hosted at those facilities, in this case parrots, were affected by Haemoproteus sp. commonly found in wild European songbirds that appear to act as reservoirs of the disease (Olias et al., 2011).
In addition to the P. juxtanucleare-like species found in this study, some masked bobwhite quail were infected with at least two different species of Haemoproteus spp. as suggested by our genetic analyses (Fig. 3). The same parasite was found in the Gambel’s quail resident on the zoo without clinical signs of disease. Infections by Haemoproteus species have low pathogenicity and are rarely considered a cause of avian mortality except in quail, turkey, Columbiformes, and parrots (Atkinson et al. 1988, Rae, 1995, Cardona et al., 2002, Olias et al., 2011). Thus, in the case of this endangered quail, Haemoproteus species should be considered a serious risk for ex-situ breeding programs. A case report describes severe mortality associated with outbreaks of Haemoproteus lophortyx in captive bobwhite quail (Colinus virginianus) (Cardona et al. 2002). Clinically, the signs of infection included reluctance to move, ruffled appearance, prostration, and death. These signs were associated with parasitemia, anemia, and the presence of large megaloschizonts in skeletal muscles; particularly those of the thighs and back (Cardona et al. 2002). Unfortunately, there are no DNA sequences for H. lophortyx reported in the GenBank that can be used for comparison.
In recent reports, PCR analyses of affected liver identified Haemoproteus sp. and Plasmodium sp. megaloschizont stages with identical histologic features as the likely etiologic agents of fatal hepatopathies in a zoological collection of green jays (Cyanocorax yncas glaucescens), lesser Flamingos (Phoeniconaias minor) and oropendulas (Psarocolius montezuma) (Ferrell et al. 2007). This observation suggests that different hemoparasites may have similar or identical light microscopic features, especially during asexual development. This may explain the PCR identification of more than one hemoparasite in quails with similar lesions.
An important finding for conservation effort is that the 38 masked bobwhite quail from the Buenos Aires National Wildlife Refuge were negative for haemosporidian parasites by nested PCR. It appears that the birds in the reserve are not infected. However, because some Plasmodium spp. can be recrudescent (Valkiūnas, 2005), detection of infection by blood film microscopy or PCR could be difficult. Thus, in cases like this where the species are endangered, sequential screening of birds may be indicated in order to rule out possible infections whenever new and potentially susceptible species are going to be incorporated as part of a zoo or aviary collection.
We also found that two golden eagles, housed away from the masked bobwhite quail at the zoo, appeared to be infected with P. relictum. Although this species was not found infecting bobwhite quails; its presence reinforces our observation that there is active malaria transmission. The same strain was found in a wild greater roadrunner from a regional wildlife rehabilitation facility, indicating that it may be present in the state of Arizona in a variety of bird species. The P. relictum sequence found in both bird species from Arizona was identical to one isolate from Nebraska. These results indicate that this malarial parasite is actively transmitted among genetically distant birds and in a broad geographic area. Plasmodium parasites have been described as causing disease and mortality in birds of prey (Greiner et al., 1981, Krone et al. 2001, 2008) and have been frequently recorded in many zoos of North America, Europe, and Asia (Cranfield et al., 1990, Valkiūnas, 2005, Ferrell et al. 2007, Grim et al. 2008). Specifically, P. relictum is considered the most common pathogenic parasite associated with clinical disease in falcons, gyrfalcons, kestrels, and other Falconiformes and Strigiformes (Kingston et al., 1976, Remple, 2004). This finding highlights the importance of active surveillance of zoo collections in order to detect potentially harmful parasites that could start circulating in the area and affect susceptible birds.
Based on our results, we propose that haemosporidian infections were a contributing factor in the demise of the captive masked bobwhite quails housed at the zoo. Therefore, the impact of hemosporozoan infections on current ex-situ conservation efforts should be evaluated. This study highlights the importance of periodic health examination of avian collections, as well as the application of proper quarantine periods in zoos when receiving and managing susceptible group of birds.
Acknowledgments
The authors are grateful to K. Orr (Liberty Wildlife), S. Gall, M. Hunnicutt, D. Cohan, and A. Witman of the Buenos Aires Wildlife Refuge for their valuable contributions to this investigation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alley MR, Fairley RA, Martin Howe DGL, Atkinson T. An outbreak of avian malaria in captive yellowheads/mohua (Mohoua ochrocephala) New Zeland Veterinary Journal. 2008;56:247–251. doi: 10.1080/00480169.2008.36842. [DOI] [PubMed] [Google Scholar]
- Atkinson CT, Forrester DJ, Greiner EC. Pathogenicity of Haemoproteus meleagridis (Haemosporina: Haemoproteidae) in experimentally infected turkeys. Journal of Parasitology. 1988;74:228–239. [PubMed] [Google Scholar]
- Bensch S, Stjernman M, Hasselquist D, Östman Ö, Hansson B, Westerdahl H, Pinheiro RT. Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proceedings of the Royal Society of London B. 2000;267:1583–1589. doi: 10.1098/rspb.2000.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. Conditions governing bird life in Arizona. Auk. 1900;17:31–34. [Google Scholar]
- Brown DE, Ellis DH. Status summary and recovery plan for the Masked Bobwhite. U.S. Fish and Wildlife Service; Albuquerque, New Mexico, USA: 1977. [Google Scholar]
- Cardona CJ, Ihejirika A, McClellan L. Haemoproteus lophortyx infection in Bobwhite Quail. Avian Diseases. 2002;46:249–255. doi: 10.1637/0005-2086(2002)046[0249:HLIIBQ]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Cranfield MR, Shaw M, Beall F, Skjoldager M, Ialeggio D. A review and update of avian malaria in the African penguin (Spheniscus demersus) Proceedings American Association of Zoo Veterinarians. 1990:243–248. [Google Scholar]
- Ferrell ST, Snowden K, Marlar AB, Garner M, Lung NP. Fatal Haemoprotozoal infections in multiple avian species in a zoological park. Journal of Zoo and Wildlife Medicine. 2007;38:309–316. doi: 10.1638/1042-7260(2007)038[0309:FHIIMA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Fix AS, Waterhouse C, Greiner EC, Stoskopf MK. Plasmodium relictum as a cause of avian malaria in wild-caught Magellanic Penguins (Spheniscus magellanicus) 1988 doi: 10.7589/0090-3558-24.4.610. [DOI] [PubMed] [Google Scholar]
- Grim KCh, Van der Merwe E, Sullivan M, Parsons N, McCutchan T, Sullivan M, Cranfield MR. Plasmodium juxtanucleare associated with mortality in Black-footed penguins (Spheniscus demersus) admitted to a rehabilitation center. Journal of Zoo and Wildlife Medicine. 2003;34:250–255. doi: 10.1638/02-070. [DOI] [PubMed] [Google Scholar]
- Grim KCh, McCutchan T, Sullivan M, Cranfield MR. Unidentified Plasmodium species in Australian Black Swans (Cygnus atratus) hatched and raised in North America. Journal of Zoo and Wildlife Medicine. 2008;39:216–220. doi: 10.1638/2007-0110R.1. [DOI] [PubMed] [Google Scholar]
- Greiner EC, Black DJ, Iverson WO. Plasmodium in a Bald Eagle (Haliaeetus leucocephalus) in Florida. Journal of Wildlife Diseases. 1981;17:555–558. doi: 10.7589/0090-3558-17.4.555. [DOI] [PubMed] [Google Scholar]
- Hernández F, Kuvlesky WP, Jr, DeYoung RW, Brennan LA, Gall SA. Recovery of rare species: case study of the Masked Bobwhite. Journal of Wildlife Management. 2006;70:617–631. [Google Scholar]
- Kingston N, Remple JD, Burnham W, Stabler RM, McGhee B. Malaria in a captive-produced F1 Gyrfalcon and in two F. peregrine falcons. Journal of Wildlife Diseases. 1976;12:562–565. doi: 10.7589/0090-3558-12.4.562. [DOI] [PubMed] [Google Scholar]
- Križanauskien, Hellgren O, Kosarev V, Sokolov L, Bensch S, Valkinas G. Variation in host specificity between species of avian hemosporidian parasites: evidence from parasite morphology and cytochrome b gene sequences. Journal of Parasitology. 2006;92:1319–1324. doi: 10.1645/GE-873R.1. [DOI] [PubMed] [Google Scholar]
- Krone O, Priemer J, Streich J, Sömmer P, Langgemach T, Lessow O. Haemosporida of birds of prey and owls from Germany. Acta Protozoologica. 2001;40:281–289. [Google Scholar]
- Krone O, Waldenström J, Valki nas G, Lessow O, Müller K, Lezhova TA, Fickel J, Bensch S. Haemosporidian blood parasites in European birds of prey and owls. Journal of Parasitology. 2008;94:709–715. doi: 10.1645/GE-1357.1. [DOI] [PubMed] [Google Scholar]
- Kuvlesky WP, Gall SA, Jr, Dobrott SJ, Tolley S, Guthery FS, Destefano SA, King N, Nolte KR, Silvy NJ, Lewis JC, Gee G, Camou Liders G, Engel-Wilson R. The status of endangered masked bobwhite recovery in the United States and Mexico. Proceeding of the National Quail Symposium. 2000;4:42–57. [Google Scholar]
- Landman WJ, Gruys E, Gielkens AL. Avian amyloidosis. Avian Pathology. 1998;27:437–49. doi: 10.1080/03079459808419367. [DOI] [PubMed] [Google Scholar]
- Massard CL, Massard CA. Aspectos biológicos: do Plasmodium Novyella juxtanucleare Versiani e Gomes, 1941 em aves no Brasil. Revista Brasileira de Medicina Veteriária. 1981;4:3–24. [Google Scholar]
- Mota RA, Cunha ELP, Soares C, Alves LC, Massard CL. Variáveis hematológicas em Gallus gallus domesticus, Linneaus, 1758, de criações rústicas da região metropolitana do Recife, naturalmente infectados com Plasmodium Novyella juxtanucleare Versiani e Gomes, 1941. Ciência Veterinária tropical Recife. 1998;1:76–80. [Google Scholar]
- Olias P, Wegelin M, Zenker W, Freter S, Gruber AD, Klopfleisch R. Avian malaria deaths in parrots, Eurpoe. Emerging Infectious Diseases. 2011;17:950–952. doi: 10.3201/eid1705.101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae M. Hemoprotozoa of caged and aviary birds. Seminars Avian and Exotic Pet Medicine. 1995;4:131–137. [Google Scholar]
- Remple JD. Intracellular hematozoa of raptors: a review and update. Journal of Avian Medicine and Surgery. 2004;18:75–88. [Google Scholar]
- Szymanski MM, Lovette IJ. High lineage diversity and host sharing of malarial parasites in a local avian assemblage. Journal of Parasitology. 2005;91:768–774. doi: 10.1645/GE-417R1.1. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tomlinson RE. Current status of the endangered Masked Bobwhite quail. Transactions of the North America Wildlife Conference. 1972;37:294–311. [Google Scholar]
- Valkiūnas G. Avian malaria parasites and other haemosporidia. CR press; USA: 2005. [Google Scholar]