Abstract
Rumination is an established cognitive vulnerability for depression. Despite substantial work on the environmental origins of rumination, the heritability of rumination has not been examined and it is not known whether rumination accounts for some of the genetic vulnerability associated with depression. 756 adolescent twins ages 12–14 years completed the Response Styles Questionnaire and multiple measures of depressive symptoms. Brooding correlated positively and distraction correlated negatively with concurrent depressive symptoms. Estimated heritabilites were 54% for depression, 21% for brooding, 37% for reflection, and 30% for distraction. Bivariate genetic analyses suggested that (1) individual differences in distraction share both genetic and environmental sources of variation with depression; and (2) although the heritable influences on brooding are small, these heritable influences account for the majority of the relationship between brooding and depression (h2 = .62).
Keywords: Rumination, depression, distraction, response styles, behavioral genetics, twins
Roughly 1 in 10 adolescents will experience major depression or dysthymia by age 18 (Merikangas et al., 2010). Adolescents with depressive symptoms who do not meet DSM-IV criteria for depression nonetheless experience significant impairment (e.g., González-Tejera et al., 2005), and are at elevated risk for later development of major depression and suicidal behaviors (Fergusson, Horwood, Ridder, & Beautrais, 2005; Klein, Shankman, Lewinsohn, & Seeley, 2009). To inform intervention for depression, we must rigorously examine risk factors and the developmental origins of these risk factors during this critical adolescent stage. Rumination, “the process of thinking perseveratively about one’s feelings and problems,” (Nolen-Hoeksema, Wisco, & Lyubomirsky, 2008, p. 400) is an established cognitive vulnerability for depression. Higher levels of rumination are associated with greater risk for onset of depression, longer duration and increasing severity of symptoms (Abela & Hankin, 2011; Nolen-Hoeksema et al., 2008). Given rumination’s predictive power for the onset of depression, researchers have focused on the origins of rumination. Despite a growing body of research on the environmental origins of rumination (Hankin et al., 2009), little research on possible genetic origins of rumination exists. The lack of attention to genetic factors is understandable, given that the study of rumination began within the coping framework, which was not biologically oriented. However, the evidence that adolescent depressive symptoms are moderately heritable (Lau & Eley, 2006; Lau & Eley, 2010; Rice, Harold, & Thapar, 2002) invites the question of whether rumination differences account for some of the genetic vulnerability associated with depression. Thus, we aimed to illuminate the genetic and environmental influences on rumination and its relationship to a composite measure of depressive symptoms using a community sample of adolescent twins. Understanding the magnitude of the genetic and environmental overlap between rumination and depression first requires analysis of the heritability of rumination. Initial analyses are best conducted in a community sample, which provides a baseline for future genetic study in diagnostic groups.
We have observed the recent refinement in studies of rumination to remove depression-confounded items and to recognize subcomponents that differentially relate to depression: brooding and reflection (Armey et al., 2009; Joormann, Dkane, & Gotlib, 2006; Treynor, Gonzalez, & Nolen-Hoeksema, 2003). The brooding items reflect “moody pondering” (Treynor et al., 2003, p.252), or passively focusing on one’s problems and their consequences, whereas the reflection items represent a purposeful attempt to understand and overcome one’s problems. Brooding is more strongly associated with concurrent depression than is reflection and also predicts the development of depressive symptoms and diagnoses (Armey et al., 2009; Gibb, Grassia, Stone, Uhrlass, & McGreary, 2012; Treynor et al. 2003).
The literature on the development of rumination has focused on environmental factors. Children who experience over-controlling parenting and a negative-submissive style of family interaction tend to brood more as adolescents (Hilt, Armstrong, & Essex, 2011). Parenting in both childhood and adolescence appears to play an important role in the development of rumination. Adolescent girls whose mothers encourage emotional expression are more likely to ruminate (Cox, Mezulis, & Hyde, 2010). Although children of depressed mothers brood more than children of non-depressed mothers, this difference does not appear to be due to modeling of the mother’s rumination (Gibb et al., 2012). However, examinations of familial resemblance for rumination that are not genetically informative neglect possible passive and evocative gene-environment correlations (e.g., Lau & Eley, 2008b) Additionally, the inconsistent nature of findings regarding two specific genetic variants and rumination (Beevers et al., 2009; Hilt et al., 2007; cf. Clasen et al., 2011; Gibb et al., 2012) point to a need to establish the overall magnitude of the genetic influences on rumination prior to embarking on candidate gene studies, or at least to provide a framework for interpretation of candidate gene and other more comprehensive genomic approaches.
Although rumination has garnered substantial empirical support as a risk factor for depression, the relationship between other response styles and depression has been inconsistent. In the original response styles theory, Nolen-Hoeksema (1991) argued that the use of positive distractions was an adaptive alternative to rumination. However, distraction and depression correlate inconsistently in both cross-sectional and longitudinal studies (Nolen-Hoeksema et al., 2008; Rood, Roelofs, Bögels, Nolen-Hoeksema, & Schouten, 2009). A ratio approach (rumination:distraction) appears to be more predictive of changes in depressive symptoms than either of the subscales alone (Abela, Aydin, & Auberach, 2007; Hilt, McLaughlin, & Nolen-Hoeksema, 2010), suggesting that individuals who have both a greater tendency to ruminate and a lesser tendency to distract are at the greatest risk for experiencing depressive symptoms. Some researchers have speculated that the more specific reflection construct may be an adaptive form of rumination (Watkins, 2008). Although the relation between reflection and depression is positive concurrently (Joormann et al., 2006), it is negative longitudinally (Treynor et al., 2003), suggesting that reflection may be adaptive in the long term. Although no other study has examined the heritability of distraction or reflection as measured by the Response Styles Questionnaire (RSQ; Nolen-Hoeksema & Morrow, 1991), two twin studies of distraction using the Coping Inventory for Stressful Situations (Endler & Parker, 1990) do exist. However, these studies report conflicting findings: data from Polish adult twins showed that one-third of the variance in distraction subscale scores were associated with genetic differences (Kozak, Strelau, & Miles, 2005) whereas data from Canadian adult twins on the same subscale showed exclusively environmental effects (Jang, Thordarson, Stein, Cohan, & Taylor, 2007). We use the RSQ, which is central in the literature and which allows us to study reflection and distraction as well as rumination, and we focus on the developmentally crucial early adolescent period.
In summary, our main objective was to analyze brooding, reflection, distraction, and depressive symptoms in adolescent twins to determine whether response styles account for some of the genetic vulnerability associated with depression. Integrating the genetics of response styles with the genetics of depression is paramount to understanding the etiology of depression.
Methods
Participants
Participants were drawn from the Wisconsin Twin Project, a statewide, birth register-based twin sample that was mildly enriched for internalizing and externalizing symptoms when the twins were seven years old (Lemery-Chalfant, Goldsmith, Schmidt, Arneson & Van Hulle, 2006). Families with twin births in Wisconsin from 1989–2004 joined the project by responding to one of two recruitment letters. Eighty-one percent of families contacted responded favorably. The University of Wisconsin Institutional Review Board approved this study. Parents provided consent and adolescents provided assent for their participation. All participants were paid. Our sample comprised 756 adolescent twins ages 12–14 years (M = 13.1, SD = 1.3; 53% female). The sample was 88.6% Caucasian, representative of Wisconsin’s population. 35.8% of the sample was monozygotic (MZ), 33.8% same-sex dizygotic (DZ), and 30.4% opposite-sex DZ.
Measures
Depression composite
We created a composite of depressive symptoms based on four depression-relevant self-report scales or sub-scales. The Children’s Depression Inventory (CDI; Kovacs, 1985) measures negative mood, interpersonal difficulties, negative self-esteem, ineffectiveness, and anhedonia during the previous two weeks differentiates emotionally distressed children from non-distressed children (Kovacs, 1981; Saylor, Finich, Spirito, & Bennett, 1984; Smucker, Craighead, Craighead, & Green, 1986). The Early Adolescent Temperament Questionnaire-Revised (EATQ-R; Capaldi & Rothbart, 1992; Ellis & Rothbart, 2001) measures social-emotional functioning and aspects of temperament related to self-regulation, reactivity, and emotionality. The depressed mood subscale of the EATQ-R measures unpleasant affect, lowered mood, and loss of enjoyment and interest and is associated with higher levels of temperamental negative affectivity (Ellis & Rothbart, 2001). The depression subscale of the Health and Behavior Questionnaire (Armstrong & Goldstein, 2003) measures mental health symptoms, physical health, and academic and social functioning; it discriminates between clinic-referred children and controls (Ablow et al., 1999) and corresponds well with DSM-IV symptoms and diagnoses in children (Lemery-Chalfant et al., 2007). Finally, we included symptoms of Major Depressive Disorder from the Diagnostic Interview Schedule for Children, Version IV (DISC; Fisher et al., 1997). Of the 756 participants, 97% had data from at least 3 measures (with 79% having data from all 4 measures). Each measure was standardized and all available measures for each participant were averaged to create a depression composite.
Response styles
Response styles were assessed using the 22-item Ruminative Response Scale (RRS: brooding, reflection, and depression-confounded items) and the 11-item Distraction Scale from the RSQ. Participants indicate how frequently they engage in ruminative or distractive thoughts/activities when they are upset on a scale from 1 = almost never to 4 = almost always. The 5 brooding items assess moody pondering (e.g., “I think ‘What am I doing to deserve this?’”). The 5 reflection items assess neutral pondering (e.g., “I go away by myself and think about why I feel this way”). The 12 depression-confounded items are items similar to depressive symptoms (e.g., “I think about how hard it is to concentrate”); given the focus of this paper, we dropped the depression-confounded items from our analyses. The distraction items assess actions or plans to engage in actions that divert one’s attention when upset (e.g., “I do something fun with a friend”). Internal consistencies were comparable to those reported in previous work (Hilt et al., 2010; Mezulis et al., 2011): .71 for brooding, .72 for reflection, and .87 for distraction.
Zygosity
Zygosity was determined at multiple phases using multiple methods including maternal report on the Zygosity Questionnaire for Young Twins (Goldsmith, 1991), observer ratings, and genotyping.
Socioeconomic Status
We standardized and averaged mother and father years of education and annual family income to create a composite SES score. Mothers had an average education of 14.6 years and fathers had an average education of 14.0 years; median family income was $60,000-$70,000.
Genetic Analyses
Twin studies utilize the varying degree of genetic similarity of monozygotic (MZ) and dizygotic (DZ) twins to parse genetic and environmental contributions to individual differences in behavior. Covariation between twins is due to correlated genetic factors (coefficient 1.0 for MZ and .5 for DZ twins) and correlated shared environments (coefficient of 1.0 for both MZ and DZ twins). If MZ twins are more similar than DZs on the variable of interest, genetic variance is implicated. If, however, MZ and DZ twins are comparably similar, shared environmental influences are implicated. Non-shared environmental influences are non-genetic factors that make siblings different and are implied to the degree that MZ twins are dissimilar (Plomin, DeFries, McClearn, & Rutter, 2008).
We used maximum likelihood estimation techniques to fit structural equation models (Neale & Cardon, 1992) to partition the observed variation into latent additive genetic (A; additive effect of multiple genes), shared environmental (C), and non-shared environmental (E; also includes measurement error) components, including 95% confidence intervals for each component. Model fitting proceeded in a stepwise fashion: individual parameters were dropped and the sub-model fit compared to the fit of the full model using the difference in log-likelihoods (LL), which is distributed as a chi-square (χ2difference = −2LLsubmodel−2LLfullmodel), with degrees of freedom equal to the difference in the number of estimated parameters. A significant change in chi-square indicates that the sub-model should be rejected in favor of the full model. In general, the model that explains the most variance with the fewest parameters is preferred (Neale & Cardon, 1992). All models were fit using the statistical program OpenMx (Boker et al., 2011).
Results
Phenotypic Analyses
Means and standard deviations for the depression composite and response styles are included in supplementary materials. There was a modest gender difference in the depression composite with girls (M = 0.06, SD = 0.88) scoring higher than boys (M = −0.06, SD = 0.75, Welch’s t (752) = 1.94, p = 0.052, d = .15). There was a moderate gender difference in reflection with girls (M = 2.17, SD = 0.68) reflecting significantly more than boys (M = 1.92, SD = 0.58, Welch’s t (746) = 5.36, p < 0.001, d = .38). The lack of a gender difference in brooding is unsurprising, as the gender difference in rumination may not emerge until age 15 (Cox, Mezulis, & Hyde, 2010). Subscale intercorrelations were run using one randomly selected twin from each twin pair (full results are in supplementary materials). The depression composite correlated with all response styles: r = .47, p <.001 for brooding; r = .14, p <.05 for reflection; r = −.40, p <.001 for distraction; and r = .66, p <.001 for the brooding:distraction ratio. Reflection correlated positively with both brooding and distraction (r = .48 and r = .45, respectively, ps <.001), but brooding and distraction were not correlated (r = .09, n.s.). Age correlated positively with brooding and the brooding:distraction ratio (r = .12, p <.05 for both correlations). SES correlated negatively with the depression composite (r = −.47, p <.001).
Genetic Analyses
Prior to genetic analyses, all measures were log-transformed to correct for non-normal distributions. Residualized scores accounted for age, sex, and SES effects (McGue & Bouchard, 1984). Twin intraclass correlations for the residualized variables were: depression composite (rMZ = .53; rDZ = .26, ps<.01), brooding (rMZ = .22, p<.05; rDZ = .10, n.s.), reflection (rMZ = .40, p<.001; rDZ = .14, p<.05), and distraction (rMZ = .40, p<.05; rDZ = .02, n.s.). Doubling the difference between MZ and DZ intraclass correlations roughly estimates heritability (Falconer & Mackay, 1996).
Univariate models examined the depression composite, brooding, reflection, and distraction independently (see Table for model fit). We found no evidence for shared environmental influences on any of the variables. Significant additive genetic influences were implicated for all variables. The estimated heritability of the depression composite was 54% (95% CI: 42%–63%), brooding was 21% (95% CI: 7%–34%), reflection was 37% (95% CI: 24%–49%), and distraction was 30% (95% CI: 17%–42%). The remainder of the variability could be accounted for by nonshared environmental factors: 46% for depression composite (95% CI: 37%–58%), 79% for brooding (95% CI: 66%–93%), 63% for reflection (95% CI: 51%–76%), and 70% for distraction (95% CI: 58%–83%).
Table.
Fit Statistics for Univariate Models
| Variable | Model | −2LL | AIC | Δχ2 | df | p |
|---|---|---|---|---|---|---|
| Depression | ACE | 2079.95 | 575.94 | |||
| CE | 2092.09 | 586.09 | 12.15 | 1 | ||
| AE | 2079.95 | 575.94 | 0 | 1 | 1.0 | |
| Brooding | ACE | 1377.60 | −110.40 | |||
| CE | 1378.76 | −111.24 | 1.16 | 1 | .28 | |
| AE | 1377.60 | −112.4 | 0 | 1 | 1.0 | |
| E | 1386.08 | −105.92 | 8.47 | 2 | .01 | |
| Reflection | ACE | 1410.53 | −77.48 | |||
| CE | 1417.19 | −72.81 | 6.66 | 1 | .01 | |
| AE | 1410.53 | −79.47 | 0 | 1 | 1.0 | |
| Distraction | ACE | 1315.72 | −172.79 | |||
| CE | 1323.45 | −166.55 | 7.73 | 1 | .01 | |
| AE | 1315.72 | −174.28 | 0 | 1 | 1.0 |
Note. Most parsimonious models are in bold. All scores residualized on age, gender, and SES. A = additive genetic influences, C = shared environment influences, and E = non-shared environment influences and error.
Bivariate Genetic Analyses
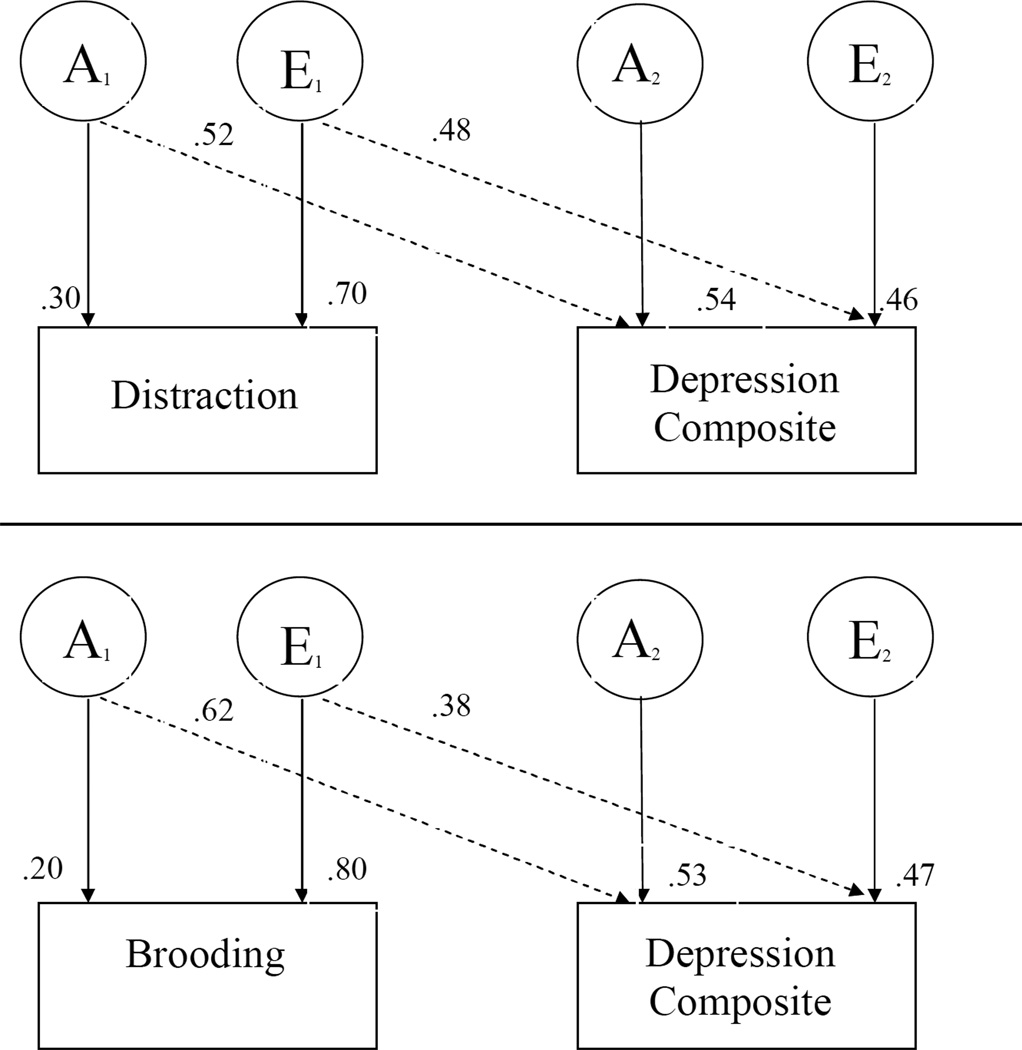
Bivariate genetic analyses examined the unique and shared genetic and environmental influences on two correlated variables. Because reflection did not strongly correlate phenotypically with the depression composite, we limited our analyses to two models: brooding and the depression composite and distraction and the depression composite. Fitting the bivariate Cholesky model revealed substantial additive genetic (h2 = .62) and non-shared environment (e2 = .38) influences on the relationship between brooding and the depression composite, as well as unique genetic and non-shared environmental influences on each (-2LL = 3306.50, AIC = 314). The genetic correlation was .83 and the environmental correlation was .27, indicating substantial genetic contributions to the covariance between brooding and depression. Model fitting also revealed substantial additive genetic (h2 = .48) and non-shared environmental (e2 = .52) influences on the relationship between distraction and the depression composite, as well as unique genetic and non-shared environmental influences on each (-2LL = 3304.10, AIC = 312). The genetic correlation was −.40 and the environmental correlation was −.30.
Discussion
We estimated the magnitude of the genetic and environmental effects on three components of response styles (brooding, reflection, and distraction) and the magnitude of the genetic and environmental contributions to the covariance between response styles and depression. Brooding, reflection, and distraction have small to moderate heritable components but are mostly influenced by individual-specific environmental factors (h2 = .21 for brooding, .37 for reflection, and .30 for distraction). Consistent with our results, many twin studies of psychopathology report no evidence for shared environmental effects by adolescence (Kendler et al., 2008; Rhee & Waldman, 2002). Our estimates of genetic and non-shared environment influences on depression were similar to those reported by Lau and Eley (2006), suggesting that our depression findings are representative.
Our key contribution to understanding pathways to a depressive phenotype is our estimation of the magnitude of the genetic and environmental overlap between response styles and depression. The bivariate analysis of distraction and depression, coupled with the negative correlation between distraction and depression, suggests that the same genetic factors that contribute to distraction may protect against concurrent depression (h2 =.48). Likewise, non-shared environmental influences that lead one to use distraction could also protect against depression (e2=.52). Showing that this developmental interpretation of our cross-sectional findings is valid should be a priority for future research. Although brooding has a low heritability (i.e., is most strongly influenced by environmental factors unique to each individual), the bivariate analysis indicated that most of the covariance between brooding and depression is due to a common set of underlying genetic influences (h2 =.62, genetic correlation = .83). One interpretation of this result is that part of the genetic effect on depression is mediated by the maladaptive tendency to brood when confronted with loss and goal blockage.
The results of this study suggest four potential avenues for future research. 1) Given brooding’s low heritability, candidate gene studies of brooding should not be a high priority, at least for the measures and type of sample that we used. However, the strong genetic correlation between brooding and depressive symptoms suggests that a novel candidate gene design targeting depressive brooders (individuals high on both depression and brooding compared with those who are low on both) might illuminate the shared genetic vulnerability. 2) As noted above, future research should examine genetic and environmental contributions to the relationship between response styles and depression longitudinally. As all constructs in this study were measured concurrently, it remains unclear from our results alone whether brooding is a risk factor for depression or is merely associated with depressed mood concurrently. However, our demonstrating genetic underpinnings of this association does support the argument that the association is not an artifact of measurement. A longitudinal approach would also illuminate how the heritability of response styles and their relationships to depression might change at different developmental stages (see, for example, Kendler et al., 2008 and Lau & Eley, 2006). 3) It will be important to conduct genetically informative analyses using more nuanced measures of rumination. The entire realm of brooding-like behavior is likely not captured by five items on the RSQ. The RSQ brooding items, which focus on typical responses to being upset, are qualitatively different from ruminating about depressed affect (Nolen-Hoeksema, 1991) and ruminating about negative life events (Mezulis, Abramson, & Hyde, 2002; Robinson & Alloy, 2003). 4) Finally, future research should identify the unique and shared relationships between distinct cognitive vulnerabilities and depression. Attributional style is also heritable (Lau & Eley, 2008) and associated with the BDNF Val66Met genetic polymorphism (Haeffel, Eastman, & Grigorenko, 2012). To inform both etiological understanding and intervention, it will be important to determine whether different cognitive vulnerabilities share distinct or overlapping genetic variance with depression; this could be determined using multivariate Cholesky analyses. Extended family designs could also serve to provide added strength to identify shared environmental influences, as well as help clarify possible gene-environment interactions and correlations.
Supplementary Material
Figure.
Bivariate genetic models of distraction and depression, and brooding and depression.
Acknowledgments
This work was supported by NIMH R01 MH59785 (to Goldsmith and Lemery-Chalfant) and a Conte Neuroscience Center (P50 MH084051). Infrastructure support was provided by the Waisman Center via P30 HD03352.
Contributor Information
Mollie N. Moore, Department of Psychology, University of Wisconsin–Madison.
Rachel H. Salk, Department of Psychology, University of Wisconsin–Madison.
Carol A. Van Hulle, Waisman Center, University of Wisconsin–Madison.
Lyn Y. Abramson, Department of Psychology, University of Wisconsin–Madison.
Janet S. Hyde, Department of Psychology, University of Wisconsin–Madison.
Kathryn Lemery-Chalfant, Department of Psychology, Arizona State University..
H. Hill Goldsmith, Waisman Center & Department of Psychology, University of Wisconsin–Madison..
References
- Abela JRZ, Hankin BL. Rumination as a vulnerability factor to depression during the transition from early to middle adolescence: A multiwave longitudinal study. Journal of Abnormal Psychology. 2011;120:259–271. doi: 10.1037/a0022796. [DOI] [PubMed] [Google Scholar]
- Abela JRZ, Aydin CM, Auerbach RP. Responses to depression in children: Reconceptualizing the relation among response styles. Journal of Abnormal Child Psychology. 2007;35:913–927. doi: 10.1007/s10802-007-9143-2. [DOI] [PubMed] [Google Scholar]
- Ablow JC, Measelle JR, Kraemer HC, Harrington R, Luby J, Smider N, Dierker L, Clark V, Dubicka B, Heffelfinger A, Essex MJ, Kupfer DJ. The MacArthur Three-City Outcome Study: Evaluating multi-informant measures of young children's symptomatology. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;12:1580–1590. doi: 10.1097/00004583-199912000-00020. [DOI] [PubMed] [Google Scholar]
- Armey MF, Fresco DM, Moore MT, Turk CL, et al. Brooding and pondering: Isolating the active ingredients of depressive rumination with exploratory factor analysis and structural equation modeling. Assessment. 2009;16:315–327. doi: 10.1177/1073191109340388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JM, Goldstein LH The MacArthur Working Group on Outcome Assessment. MacArthur Foundation Research Network on Psychopathology and Development. Pittsburgh: University of Pittsburgh Press; 2003. Manual for the MacArthur Health and Behavior Questionnaire (HBQ 1.0). [Google Scholar]
- Beevers CG, Wells TT, McGeary JE. The BDNF Val66Met polymorphism is associated with rumination in healthy adults. Emotion. 2009;9:579–584. doi: 10.1037/a0016189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T, Fox J. OpenMx: An open source extended structural equation modeling framework. Psychometrika. 2011;76(2):306–317. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi DM, Rothbart MK. Development and validation of an early adolescent temperament measure. Journal of Early Adolescence. 1992;12:153–173. [Google Scholar]
- Clasen PC, Wells TT, Knopik VS, McGeary JE, Beevers CG. 5-HTTLPR and BDNF Val66Met polymorphisms moderate effects of stress on rumination. Genes, Brain and Behavior. 2011;10:740–746. doi: 10.1111/j.1601-183X.2011.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox SJ, Mezulis AH, Hyde JS. The influence of child gender role and maternal feedback to child stress on the emergence of the gender difference in depressive rumination in adolescence. Developmental Psychology. 2010;46:842–852. doi: 10.1037/a0019813. [DOI] [PubMed] [Google Scholar]
- Endler N, Parker J. Coping Inventory for Stressful Situations Manual. North Tonawanda, NY: Multi-Health Systems; 1990. [Google Scholar]
- Ellis LK, Rothbart MK. Revision of the Early Adolescent Temperament Questionnaire; Poster presented at the 2001 Biennial Meeting of the Society for Research in Child Development; Minneapolis, Minnesota. 2001. [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to quantitative genetics. London: Longman; 1996. [Google Scholar]
- Fergusson DM, Horwood LJ, Ridder EM, Beautrais AL. Subthreshold depression in adolescence and mental health outcomes in adulthood. Archives of General Psychiatry. 2005;62:66–72. doi: 10.1001/archpsyc.62.1.66. [DOI] [PubMed] [Google Scholar]
- Fisher P, Lucas C, Schaffer D, Schwab-Stone M, Dulcan M, Graae F, Lichtman J, Willoughby S, Gerald J. Diagnostic Interview Schedule for Children, Version IV: Test-restest reliability in a clinical sample; Paper presented at the 44th Annual Meeting of the American Academy of Child and Adolescent Psychiatry; Toronto. 1997. [Google Scholar]
- Gibb BE, Grassia M, Stone LB, Uhrlass DJ, McGeary JE. Brooding rumination and risk for depressive disorders in children of depressed mothers. Journal of Abnormal Child Psychology. 2012;40:317–326. doi: 10.1007/s10802-011-9554-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith HH. A zygosity questionnaire for young twins: A research note. Behavior Genetics. 1991;21:257–269. doi: 10.1007/BF01065819. [DOI] [PubMed] [Google Scholar]
- González-Tejera G, Canino G, Ramírez R, Chávez L, Shrout P, Bird H, Bravo M, Martínez-Taboas A, Ribera J, Bauermeister J. Examining minor and major depression in adolescents. Journal of Child Psychology and Psychiatry. 2005;46:888–899. doi: 10.1111/j.1469-7610.2005.00370.x. [DOI] [PubMed] [Google Scholar]
- Haeffel GJ, Eastman M, Grigorenko EL. Using a cognitive endophenotype to identify risk genes for depression. Neuroscience Letters. 2012;510:10–13. doi: 10.1016/j.neulet.2011.12.060. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Oppenheimer C, Jenness J, Barrocas A, Shapero BG, Goldband J. Developmental origins of cognitive vulnerabilities to depression: Review of processes contributing to stability and change across time. Journal of Clinical Psychology. 2009;65:1327–1338. doi: 10.1002/jclp.20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilt LM, Sander LC, Nolen-Hoeksema S, Simen AS. The BDNF Val66Met polymorphism predicts rumination and depression differently in young adolescent girls and their mothers. Neuroscience Letters. 2007;429:12–16. doi: 10.1016/j.neulet.2007.09.053. [DOI] [PubMed] [Google Scholar]
- Hilt LM, Mclaughlin KA, Nolen-Hoeksema S. Examination of the response styles theory in a community sample of young adolescents. Journal of Abnormal Child Psychology. 2010;38:545–556. doi: 10.1007/s10802-009-9384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilt LM, Armstrong JM, Essex MJ. Early family context and development of adolescent ruminative style: Moderation by temperament. Cognition & Emotion. 2011;37:41. doi: 10.1080/02699931.2011.621932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang KL, Thordarson DS, Stein MB, Cohan SL, Taylor S. Coping styles and personality: A biometric analysis. Anxiety, Stress, and Coping: An International Journal. 2007;20:17–24. doi: 10.1080/10615800601170516. [DOI] [PubMed] [Google Scholar]
- Joormann J, Dkane M, Gotlib IH. Adaptive and maladaptive components of rumination? Diagnostic specificity and relation to depressive biases. Behaviour Therapy. 2006;37:269–280. doi: 10.1016/j.beth.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Juhasz G, Dunham JS, McKie S, Thomas E, Downey D, Chase D, et al. The CREB1-BDNF-NTRK2 pathway in depression: multiple gene-cognition-environment interactions. Biological Psychiatry. 2011;69:762–771. doi: 10.1016/j.biopsych.2010.11.019. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Annas P, Neale MC, Eaves LJ, Lichtenstein P. A longitudinal twin study of fears from middle childhood to early adulthood: Evidence for a developmentally dynamic genome. Archives Of General Psychiatry. 2008;65(4):421–429. doi: 10.1001/archpsyc.65.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D, Shankman SA, Lewinsohn PM, Seeley JR. Subthreshold depressive disorder in adolescents: Predictors of escalation to full-syndrome depressive disorders. Journal of the American Academy of Child and Adolescent Psychiatry . 2009;48:703–710. doi: 10.1097/CHI.0b013e3181a56606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. Rating scales to assess depression in school-aged children. International Journal of Child and Adolescent Psychiatry. 1981;46:305–315. [PubMed] [Google Scholar]
- Kovacs M. The Children’s Depression Inventory (CDI) Psychopharmacology Bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- Kozak B, Strelau J, Miles JNV. Genetic determinants of individual differences in coping styles. Anxiety, Stress, and Coping. 2005;18:1–15. [Google Scholar]
- Lau JY, Eley TC. The genetics of mood disorders. Annual Review of Clinical Psychology. 2010;6:313–337. doi: 10.1146/annurev.clinpsy.121208.131308. [DOI] [PubMed] [Google Scholar]
- Lau JYF, Eley TC. Attributional style as a risk marker of genetic effects for adolescent depressive symptoms. Journal of Abnormal Psychology. 2008a;117:849–859. doi: 10.1037/a0013943. [DOI] [PubMed] [Google Scholar]
- Lau JYF, Eley TC. Disentangling gene environment correlations and interactions in adolescent depression. Journal of Child Psychology & Psychiatry. 2008b;42:142–150. doi: 10.1111/j.1469-7610.2007.01803.x. [DOI] [PubMed] [Google Scholar]
- Lau JYF, Eley TC. Changes in genetic and environmental influences on depressive symptoms across adolescence and young adulthood. The British Journal of Psychiatry. 2006;189:422–427. doi: 10.1192/bjp.bp.105.018721. [DOI] [PubMed] [Google Scholar]
- Lemery-Chalfant K, Schreiber JE, Schmidt NL, Van Hulle CA, Essex MJ, Goldsmith HH. Assessing internalizing, externalizing, and attention problems in young children: Validation of the MacArthur HBQ. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:1315–1323. doi: 10.1097/chi.0b013e3180f616c6. [DOI] [PubMed] [Google Scholar]
- Lemery-Chalfant K, Goldsmith HH, Schmidt NL, Arneson CL, Van Hulle CA. Wisconsin Twin Project: Current directions and findings. Twin Research and Human Genetics. 2006;9:1030–1037. doi: 10.1375/183242706779462363. [DOI] [PubMed] [Google Scholar]
- McGue M, Bouchard TJ. Adjustment of twin data for the effects of age and sex. Behavior Genetics. 1984;14:325–343. doi: 10.1007/BF01080045. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J. Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A) Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezulis AH, Priess H, Hyde JS. Rumination mediates the relationship between infant temperament and adolescent depressive symptoms. Depression Research and Treatment. 2011;2011:487873. doi: 10.1155/2011/487873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezulis A, Abramson LY, Hyde JS. Domain specificity of gender differences in rumination. Journal of Cognitive Psychotherapy. 2002;16:421–434. [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht, The Netherlands: Kluwer Academic Press; 1992. [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology. 1991;100:569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspectives on Psychological Science. 2008;3:400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral genetics. 5th ed. New York: Worth Publishers; 2008. [Google Scholar]
- Raes F, Hermans D. On the mediating role of subtypes of rumination in the relationship between childhood emotional abuse and depressed mood: brooding versus reflection. Depression and Anxiety. 2008;25:1067–1070. doi: 10.1002/da.20447. [DOI] [PubMed] [Google Scholar]
- Rhee S, Waldman ID. Genetic and environmental influences on antisocial behavior: A meta-analysis of twin and adoption studies. Psychological Bulletin. 2002;128(3):490–529. [PubMed] [Google Scholar]
- Robinson MS, Alloy LB. Negative cognitive styles and stress-reactive rumination interact to predict depression: A prospective study. Cognitive Therapy & Research. 2003;27:275–292. [Google Scholar]
- Rood L, Roelofs J, Bögels SM, Nolen-Hoeksema S, Schouten E. The influence of emotion-focused rumination and distraction on depressive symptoms in non-clinical youth: A meta-analytic review. Clinical Psychology Review. 2009;29:607–616. doi: 10.1016/j.cpr.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Saylor CF, Finch AJ, Spirito A, Bennett B. The Children’s Depression Inventory: A systematic evaluation of psychometric properties. Journal of Consulting and Clinical Psychology. 1984;52:955–967. doi: 10.1037//0022-006x.52.6.955. [DOI] [PubMed] [Google Scholar]
- Smucker MR, Craighead WE, Craighead LW, Green BJ. Normative and reliability data for the Children’s Depression Inventory. Journal of Abnormal Child Psychology. 1986;14:25–39. doi: 10.1007/BF00917219. [DOI] [PubMed] [Google Scholar]
- Spasojevic J, Alloy LB. Who becomes a depressive ruminator? Developmental antecedents of ruminative response style. Journal of Cognitive Psychotherapy: An International Quarterly. 2002;16:405–419. [Google Scholar]
- Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research. 2003;27:247–259. [Google Scholar]
- Verstraeten K, Vasey MW, Raes F, Bijttebier P. Temperament and risk for depressive symptoms in adolescence: mediation by rumination and moderation by effortful control. Journal of Abnormal Child Psychology. 2009;37:349–361. doi: 10.1007/s10802-008-9293-x. [DOI] [PubMed] [Google Scholar]
- Watkins E. Constructive and unconstructive repetitive thought. Psychological Bulletin. 2008;134:163–206. doi: 10.1037/0033-2909.134.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.