Preeclampsia is a disease carrying high risks of mortality and morbidity among pregnant mothers and infants worldwide. Many immune mechanisms stimulated in response to reductions in uterine perfusion pressure (RUPP) have been identified as key players in the disease progress.
Abstract
Preeclampsia is associated with hypertension and increased infant and maternal morbidity and mortality. The underlying cause of preeclampsia is largely unknown, but it is clear that an immunological component plays a key pathophysiological role. This review will highlight immunological key players in the pathology of preeclampsia and discuss their role in the pathophysiology observed in the reduced placental perfusion (RUPP) rat model of preeclampsia.
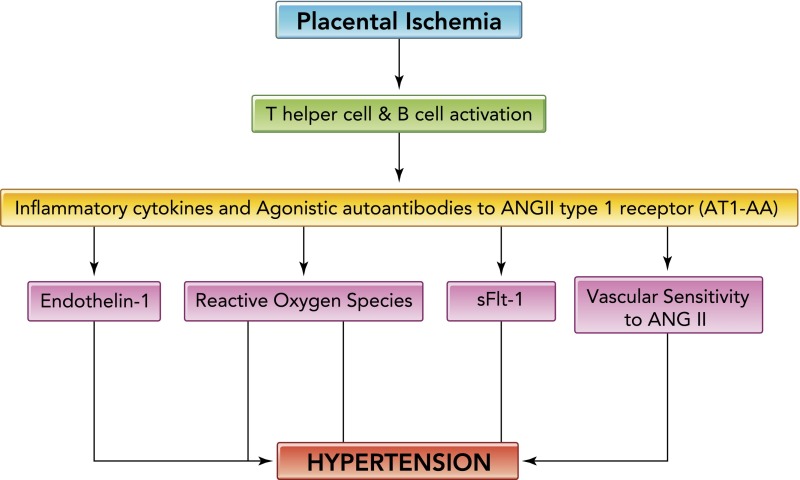
Preeclampsia is characterized by elevation in maternal blood pressure and decline in renal function, intrauterine growth restriction, chronic immune activation, and multi-organ dysfunction (38, 39, 41). The disease is defined as newly developed hypertension with proteinuria during pregnancy and affects ∼8% of pregnancies in the U.S. The theory used to explain the origin of disease is that inadequate uteroplacental vascular remodeling leads to decreased placental blood flow that over time results in placental hypoxia and ischemia (37, 43, 45, 46). The ischemic placenta is associated with a dysregulation of natural killer cells, activation of CD4+ T lymphocytes, and the release of anti-angiogenic and proinflammatory factors such as the soluble VEGF receptor-1 (sFlt-1) and s endoglin, the angiotensin II type-1 receptor autoantibody (AT1-AA), and cytokines such as TNF-α and IL-6 and IL-17 (9, 13–18, 36, 37, 44–47, 52, 57). Through various studies by our laboratory and others, many of these factors have been shown to stimulate maternal endothelial dysfunction, circulating and local endothelin (ET-1), reactive oxygen species (ROS), or enhanced vascular sensitivity to angiotensin II, which have been shown to contribute to the decrease in renal function and/or to the hypertension in pregnant animal models of this disease (FIGURE 1) (6–8, 13, 14, 16, 18, 20–28, 32, 34, 35, 51, 52, 54, 56, 58, 59). Understanding the link between immune activation, placental ischemia, endothelial dysfunction, and hypertension during pregnancy should lead to better prediction, prevention, and treatment strategies for women and children affected by this devastating disease.
FIGURE 1.
Hypertension in response to placental ischemia
Hypertension in response to placental ischemia proceeds via immune activation, CD4+ T-cells mediating the release of angiotensin II type-1 receptor autoantibody (AT1-AA), and inflammatory cytokines that contribute to the increased vasoactive peptide ET-1 increased sensitivity to ANGII, oxidative stress, and sFlt-1, all known players in the pathophysiology of preeclampsia.
An Animal Model of Preeclampsia: Reduced Uterine Perfusion Pressure During Pregnancy
The Reduced Placental Perfusion Model
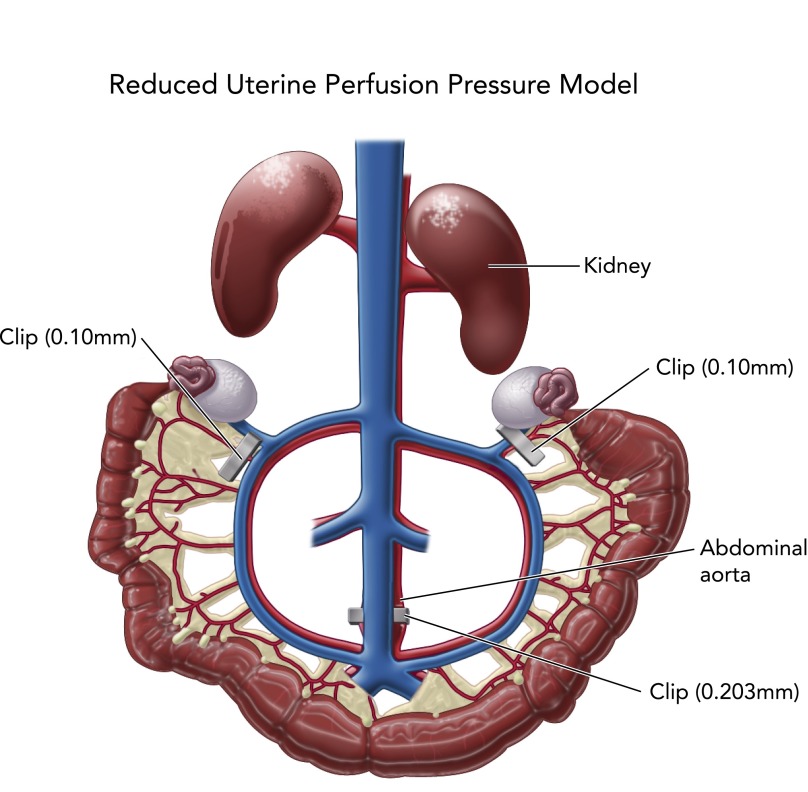
Because of the difficulties in ascertaining cause-and-effect relationship in preeclamptic patients, animal models mimicking this complex disease are necessary. It is believed that preeclampsia is caused by abnormal trophoblast invasion of the spiral arteries, thus leading to a reduction in uterine blood flow. To date, no animal model spontaneously developing a reduction in uterine perfusion pressure similar to preeclamptic women has proven to be adequate to study mechanisms of this disease. Therefore, to test the hypothesis that a reduction in uterine perfusion pressure leads to a preeclampsia-like state, many investigators have utilized the reduced placental perfusion (RUPP) rat model. The RUPP rat model of preeclampsia is performed by placing silver surgical clips (0.203 mm ID) around the abdominal aorta above the iliac bifurcation (FIGURE 2) and around both right and left ovarian arteries (silver clip, 0.100 mm ID) feeding the uterine horns. This procedure is performed on day 14 of gestation in the rat, and hypertension, pup weight, and soluble and genetic factors are measured on day 19 of gestation (11, 13, 14, 21, 22, 27, 28, 34). The RUPP rat mimics numerous physiological features of preeclampsia in women. Some of these important pathophysiological characteristic include chronic immune activation, increased mean arterial pressure, impaired renal function, and fetal growth reduction with decreased litter number and pup weight. Both RUPP rats and preeclamptic patients have significant reductions in glomerular filtration rate and renal plasma flow compared with normal pregnancy, which is oftentimes associated with proteinuria.
FIGURE 2.
Reduced uterine perfusion pressure model
Reduced uterine perfusion pressure model is utilized to induce placental ischemia in pregnant rats on day 14 of gestation; blood pressure and soluble factors are collected on day 19 of gestation.
Findings from recent molecular and cellular studies suggest that, similar to women with preeclampsia, RUPP rats have increased AT1-AAs that bind to and activate the AT1R (angiotensin II type I receptor) and contribute to hypertension in the model (28, 53, 56). We performed a study similar to that published previously by Taylor et al. (44), in which cultured endothelial cells were exposed to sera from preeclamptic patients and secreted ET-1 was compared with ET-1 secreted from cells exposed to sera from normal pregnant (NP) patients. These investigators found that ET-1 secretion was greatly enhanced from cells following exposure to preeclamptic sera compared with ET-1 from cells exposed to NP sera. In our study, similar to findings with preeclamptic sera, sera from RUPP rats caused greater endothelial cell secretion of ET-1 compared with sera from NP rats (46). This effect was attenuated by AT1R blockade, thereby suggesting that the AT1-AA circulating in sera from RUPP rats binds to and activates the AT1R on the vascular endothelial cells in culture, thus resulting in greater ET-1 secretion compared with NP rat sera. RUPP rats also exhibit increased tissue vasoconstrictor peptide ET-1, and chronic treatment with an ETA receptor antagonists attenuated the blood pressure increases observed in this model (13, 14). As discussed previously, many investigators have shown that women with preeclampsia have increased circulating levels and/or placental expression of pro-inflammatory cytokines (5, 6, 9, 36, 37, 44, 45). In RUPP rats, serum levels of TNF-α, IL-6, and IL-17 are increased, and we and others have shown that infusion of either TNF-α, IL-6, or IL-17 into NP rats increased blood pressure, suggesting an important role for immune pathways in mediating hypertension in response to placental ischemia during pregnancy (8, 11, 22). Lastly, as seen in women with preeclampsia, RUPP rats exhibit greater circulating and placental levels of sFlt-1, which increased blood pressure when infused into NP rats (29, 32). SFlt-1-induced hypertension was attenuated by administration of an ETA receptor antagonist (32).
Although the RUPP rat model has many pathophysiological features similar to that of preeclamptic women, it is not a good model to utilize when examining deficiencies in trophoblast migration, implantation, or uterine artery remodeling. The procedure is induced on day 14 of gestation, well past the point in early pregnancy at which all of these key pathologies have occurred in the preeclamptic women. Although there is no ideal laboratory-manufactured animal model mimicking human disease, designing and utilizing such models are essential to learning, identifying, and understanding mechanisms responsible for pathophysiological conditions in both men and women. Knowledge gained from these types of studies is the only way we can better understand pathways and elucidate treatment strategies for improving patient care.
Hypoxia-Induced Pathways Associated With Hypertension During Pregnancy
The chronic reduction in blood flow delivering oxygen and nutrients to the placenta is hypothesized to contribute to placental hypoxia. Hypoxia stimulates HIf-1α, a transcription factor regulating sFlt-1, which binds to vascular endothelial growth factor and placental growth factor, having a negative impact on placental vasculogenesis during preeclampsia (31, 33). Endoglin is another hypoxia-inducible protein that is a component of the TGF-β receptor complex and is associated with cellular proliferation and NO signaling (29). s-Endoglin behaves as an anti-angiogenic factor by impairing TGF-β1 binding to cell surface receptors. Recently, studies have reported that increased sFlt-1 and s-endoglin may have predictive value in diagnosing preeclampsia as concentrations seem to increase before manifestation of symptoms.
Additional studies indicated that alterations in HIf-1α regulatory pathway leads to symptoms similar to those seen in preeclamptic women (33). Decreased regulators acting on HIf-1α such as 2 methoxyestradiol (2ME) could be an indicator of hypoxic events leading to elevated levels of HIf-1α, which in turn stimulated sFlt-1 and s-endoglin. Preeclamptic women display low levels of 2ME compared with women with normal pregnancies. Furthermore, genetically modified knockdown of catechol-O-methyltransferase (COMT) decreased 2ME during pregnancy and mimicked pathophysiological characteristics of preeclampsia such as hypertension, proteinuria, elevated sFlt-1, and placental hypoxia. Interestingly, in addition to hypoxia, activation of various immune pathways has been shown to lead to increased sFlt-1 or s-endoglin such as T lymphocytes, TNF-α, and AT1-AA during pregnancy (18, 51, 57). Although the decrease in oxygen delivery may contribute to placental hypoxia, which stimulates important factors known to play a role in preeclampsia, the major focus of this review will be inflammatory factors stimulated in response to placental ischemia and how these factors contribute to the development of hypertension during pregnancy.
Immune Pathways Mediating Hypertension During Pregnancy
Natural Killer Cells
Natural killer (NK) cells play an important role in the innate immune response providing viral protection and efficiently killing tumor cells by secreting granulosymes and cytotoxins. Of late, we have learned of an important role for NK cells in reproductive success. NK cells exists in the circulation as well as compose a large portion of uterine lymphocytes from which they are distinguished by specific markers such as CD56 bright (10, 56). These cells secrete angiogenic cytokines and proteins such as angiopoietin 1 and 2 and vascular endothelial growth factor (VEGF) and placenta growth factor (PlGF) (56). Co-culture studies reveal that uterine NK cells prefer close association with the trophoblasts and secrete cytokines that play an important role in trophoblasts differentiation, growth, and spiral arteriole invasion during normal pregnancy and thus contribute to the success of trophoblasts invasion (10, 56).
Many investigators believe that a shift in the NK1/NK2 functional profile may be used to predict pregnancy outcomes. Importantly, uterine NK cells can induce lysis of trophoblast cells lacking specific cellular surface antigens that would normally invade the spiral arteries; this mechanism has been noted as an important cause of miscarriage early in pregnancy (10, 56). Incomplete loss of such cells results in the shallow trophoblast invasion and thus the deficient oxygen and nutrient supply to the developing placenta, which has been described as the genesis of preeclampsia. Furthermore, we know that, during preeclampsia, inadequate vasculogensis of the placenta leads to hypoxia, thus stimulating production of the VEGF and PlGF antagonist sFlt, thereby stimulating a viscous cycle of events that worsens throughout the pregnancy. These data indicate the importance of the functional profile of the uterine NK cell to either maintain or compromise a potentially healthy pregnancy.
It has been shown that peripheral NK1 cells secreting TNF-α predominates in preeclamptic women (10). Moreover, it has been reported that the NK cells in the periphery that produce VEGF were significantly lower in preeclamptic women compared with NP women. However, examining peripheral NK cells may not reveal the uterine NK-cell population. Moreover, the interaction between peripheral NK vs. uterine NK cells and the trophoblast is controversial and not well understood. However, it is understood that appropriate balance of the NK1 vs. NK2 cells is important to maintain a healthy pregnancy (10, 56). A dysregulation among NK-cell cytotoxicity, cytokines, or angiogenic factor secretion strongly correlates with reproductive failures and/or preeclampsia.
CD4+ T Helper Lymphocytes
Preeclampsia is associated with chronic immune activation, and multiple immune factors have been shown to play a role in mediating endothelial dysfunction and hypertension during pregnancy (5, 13, 20, 23, 24, 34, 42, 48, 51, 52, 57, 58, 59). Many investigators believe that partial failure of the maternal immune tolerance mechanisms precedes the development of placental oxidative stress and ischemia, which we know to be major players in the pathophysiology of preeclampsia (2, 6, 9, 38, 45). This maternal immune tolerance involves crucial interactions between regulatory CD4+ T cells and uterine NK cells recognizing and accepting the fetal antigens and facilitating placental growth. Complete failure leads to spontaneous miscarriage, whereas partial failure of this crucial step leads to poor placentation and dysfunctional placental perfusion and chronic immune activation originating from the placenta (36, 37, 43–47, 54, 55). Importantly, analysis of blood collected from preeclamptic women has demonstrated a decrease in the proportion of circulating regulatory CD4+ T cells (36, 44). Our data echo these findings in the rat RUPP model of preeclampsia (51). Findings from our previous study revealed that pregnant dams that have undergone the RUPP procedure have a 47% decrease in regulatory CD4+ T cells in the peripheral circulation compared with NP rats (51). T-helper 17 cells, which are upregulated in autoimmune disorders including lupus, psoriasis, and multiple sclerosis, are also increased in preeclamptic women, and we have recently shown them to be increased in the RUPP rat model (51). These data support the hypothesis that hypertension in response to placental ischemia represents a shift from the normal anti-inflammatory state of pregnancy to a pro-inflammatory state.
Most recently, we have demonstrated a role for CD4+ T cells in hypertension in response to placental ischemia induced by RUPP in pregnant rats (34, 51, 52). We have shown that RUPP-induced CD4+ T cells increased blood pressure and decreased glomerular filtration rate when adoptively transferred into NP rats (34). Hypertension that developed in response to adoptive transfer of RUPP CD4+ T cells was associated with elevated TNF-α, sFlt-1, AT1-AA, and ET-1 in NP recipient rats, none of which were elevated in NP control rats (34, 51, 52). Our previous studies have shown an important role for ETA receptor blockade to attenuate hypertension in response to RUPP or chronically infused TNF-α, sFlt-1, and AT1-AA (13, 14, 21, 23, 32). Therefore, we administered an ETA receptor antagonist to NP recipient rats of RUPP CD4+ T cells. The hypertension in this model was attenuated, thus indicating the importance of ET-1 activation as a mediator of hypertension when inflammatory CD4+ T cells are stimulated in response to placental ischemia (52). Additionally, we demonstrated that circulating factors were present in the sera of recipients of RUPP CD4+ T cells that stimulated ET-1 secretion from cultured endothelial cells similar to what we had previously shown in RUPP controls and in preeclamptic women (40). These data suggest that circulating factors were stimulated in response to RUPP CD4+ T cells that were important in causing endothelial cells to secrete ET-1.
To further examine the role of CD4+ T cells in the pathophysiology of preeclampsia, we suppressed T cells by administration of abatacept (Orencia), which is a fusion molecule of CTLA-4. CTLA-4 is a marker on T cells used to stimulate an immune response to antigens (2). Orencia was administered to pregnant rats on gestational day 13, before placental insult. RUPP was induced on day 14, and blood pressure and soluble factors were collected on day 19 (Richards, LaMarca B, unpublished observations). Administration of Orencia decreased T cells and the blood pressure response to RUPP in pregnant rats, thereby confirming our hypothesis that T cells are important in causing hypertension in response to placental ischemia.
B Lymphocytes
An important function of CD4+ T cells is to facilitate the B lymphocyte memory immune response and specific antibody production toward a single antigen. This process is known as the T-cell-dependent antibody response (2). Auto-antibodies are produced during preeclampsia, suggesting an important role for B lymphocytes in the pathogenesis of this disease. Moreover, Liao et al. demonstrated that the percentage of circulating memory B lymphocytes were significantly greater in preeclamptic women than in the NP cohort (30). B-2 B lymphocytes are the conventional memory B cells that undergo antigen processing via recognition of MHC class II peptide complex with the activated CD4+ T lymphocyte (2). For B-cell maturation and IgG production, several co-stimulatory signals must occur between the antibody producing B lymphocyte and CD4+ T-helper cell (2). One of these includes stimulation of the CD20 receptor on the surface of the B cell. This recognition stimulates the B cell to enter the circulation and produce antigen-specific immunoglobulin. Another necessary co-stimulatory molecule for B-cell maturation is the CD40 on the surface of the B cell. This molecule binds with the CD40 ligand on the surface of the T cell (2). B cells then proceed through proliferation, differentiation, and internal isotype switching, leading to production of specific antigen-stimulated antibodies, which leads to the formation of short-lived plasma cells that secrete antibody and memory B cells residing in the germinal lymph node centers, which will be available for future interactions with specific T cells.
To treat various autoimmune diseases, many therapeutic agents inhibiting specific interactions between immune molecules on cells have been developed. In a recent study, we utilized a chemotherapeutic agent that has shown efficacy among autoimmune patients by blocking the CD20 (Rutiximab) co-stimulatory molecule (4, 27). Rutiximab is used to inhibit B lymphocytes from entering the circulation and secreting antibody, a process known as B-cell depletion (2, 4). We found that, with B-cell depletion, RUPP rats had lower blood pressure, circulating TNF-α, autoantibodies, and tissue ET-1 than control RUPP rats (27). We exposed endothelial cells to serum from B-cell-depleted RUPP rats and found that ET-1 secretion was completely attenuated compared with control RUPP sera. These data supported the hypothesis that memory B lymphocytes stimulated through T-cell interaction in response to placental ischemia in pregnant rats played an important role to increase blood pressure, circulating inflammatory cytokines, and ET-1, possibly via secreting autoantibodies, during pregnancy.
Although this study demonstrated a role for memory B2 B lymphocytes in the pathogenesis of hypertension in response to placental ischemia, it did not clarify antigenic stimulation or examine the role for the other B-cell subtypes in the progression of this disease. B lymphocytes can be characterized as either B1 or B2 cells, each having distinct markers and roles in facilitating immune reactions. B1 lymphocytes can be divided into B1a or B1b cells (2, 30). These cells express IgM in greater quantities than IgG and are the primary source of natural antibodies produced in the absence of antigenic stimulation. These antibodies are polyreactive and cross-react with multiple antigens such as autoantigens, other immunoglobulins, and bacterial polysaccharides (2, 19). B1 B cells have been implicated in the progression of autoimmune diseases and are elevated in the lupus and rheumatoid arthritis. B1 B cells are present in low numbers in the circulation, lymph nodes, and spleen and are predominantly found in the peritoneal and pleural cavities. B1 B lymphocytes are responsible for T-cell-independent antibody production.
Recently, Jensen et al. uncovered an important role for B1 lymphocytes in the progression of preeclampsia (19). Preeclamptic placentas stained positive for markers of B1 B lymphocytes (CD19+CD5+). Furthermore, these authors demonstrated that B1 B lymphocytes were stimulated to produce AT1-AA when co-cultured with sera from preeclamptic women but not from NP women. This study further illustrated the importance of B cells in the preeclamptic placenta and their stimulation by a soluble factor to produce AT1-AA and contribute to the progression of this disease. Furthermore, high levels of B1 cells is yet another important characteristic that preeclamptic women share with patients presenting with autoimmune diseases.
Activating Autoantibodies to the Angiotensin II Type I Receptor
Many studies in preeclamptic women have demonstrated increased circulating concentrations of an agonistic autoantibody to the angiotensin type 1 receptor (AT1-AA) (7, 15, 16, 19, 53, 54, 56, 58, 59). In addition to being elevated during preeclampsia, the AT1-AA continues to be produced postpartum (17). Utilizing a cardiomyocyte contraction bioassay, the epitope of the AT1-AA was identified to be within the second extracellular loop of the AT1-receptor and comprised the amino acids AFHYESQ (7, 14, 45). Confocal microscopy and co-immunoprecipitation confirmed the binding of these autoantibodies to the AT1-receptor. AT1-AA have been detected in pregnancies with abnormal uterine perfusion and increased uterine-resistive index as well as in patients with systemic sclerosis and renal allograft rejection; however, the most investigated role of AT1-AA has been its role in causing hypertension during pregnancy (51–54). The specific mechanisms that lead to excess production of AT1-AA may be T-cell dependent or independent and are being investigated. Furthermore, the mechanism whereby AT1-AA increases blood pressure during pregnancy has been a recent focus of much investigation.
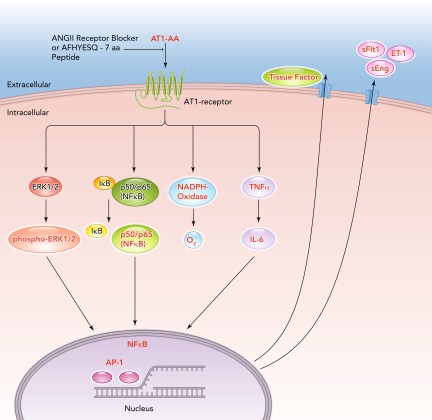
AT1-AAs are implicated as a central mediator of several pathophysiological mechanisms in preeclampsia. During preeclampsia, AT1-AA induce NADPH oxidase and the MAPK/ERK pathway leading to NF-κB and tissue factor activation (7, 15, 16, 46, 47, 53, 54, 58, 59). AT1-AA with the same epitope binding region have also been detected in animal models of preeclampsia (7, 18, 22, 34, 58, 59) (FIGURE 3). By utilizing animal and cell culture models, the AT1-AA have been shown to be responsible for a variety of effects in several different tissues and cells. For example, AT1-AA were shown to stimulate sFlt-1 expression from trophoblast cells and IL-6 production from mesangial cells (7, 15, 16, 22, 58, 59). They have been shown to cause increased intracellular Ca2+ signaling in platelets in women who went on to develop preeclampsia (53, 54, 56, 58, 59). Moreover, we have reported that increasing levels of AT1-AA to levels observed in preeclamptic women in pregnant rats increased blood pressure, ET-1, sFlt-1, and placental oxidative stress (7, 18, 24, 25, 27, 34, 35, 56, 58, 59). In addition, we found chronic AT1-AA caused renal endothelial dysfunction in the isolated renal interlobar artery (35). Furthermore, AT1-AA-induced hypertension in pregnant rats can be attenuated by either AT1-receptor antagonist losartan, an ET-type A-receptor antagonist, or the super oxide dismutase mimetic Tempol (16, 24, 28).
FIGURE 3.
Signal cascades of the angiotensin II type 1 receptor autoantibodies
The agonistic angiotensin II type 1 receptor autoantibodies (AT1-AA) induce signaling by the angiotensin II type 1 receptor (AT1-receptor), inhibited by AT1-receptor blocker (ARB) or the seven-amino acid peptide (AFHYESQ) mimicking the epitope of the AT1-AA in the second extracellular domain of the AT1-receptor. Intracellular cascades and promoter activations in the nucleus lead to an upregulation of endothelin-1 (ET-1), tissue factor, soluble fms-like tyrosine kinase-1 (sFlt1), soluble endoglin (sEng), and oxidative stress.
Our most recent studies indicated an important interaction between AT1-AA and ANG II that occurred at the level of the AT1R. Many years ago, Gant et al. demonstrated that women who developed pregnancy-induced hypertension displayed greater vascular sensitivity to lower concentrations of infused ANG II than women who progressed to a normal pregnancy had to the same concentrations (12). With the finding of the AT1-AA, we and others believe this heightened sensitivity to ANG II during preeclampsia is caused by the AT1-AA. In a recent study, we demonstrated, with the combination AT1-AA plus ANG II-induced hypertension, proteinuria, intrauterine growth retardation, and arteriolosclerosis in the uteroplacental unit (56). Moreover, acute vascular infusion of ANG II into AT1-AA-induced hypertensive pregnant rats sharply increased blood pressure above that of NP rats acutely infused with ANG II. In addition, we found that, although AT1-AA or ANG II alone induced ET-1 secretion from cultured endothelial cells, the combination of ANG II with AT1-AA drastically increased ET-1 secretion compared with control or with AT1-AA or ANG II-treated endothelial cells. This study supported the hypothesis that the AT1-AA could play an integral role to enhance ANG II-induced vascular sensitivity during preeclampsia.
As stated previously, AT1-AA are produced in many animal models of preeclampsia. We have shown that placental ischemia in pregnant rats is associated with increased levels of the AT1-AA (28). Furthermore, we have shown that chronic infusion of TNF-α, IL-6, or IL-17 into pregnant rats stimulates production of the AT1-AA, while having no effect in nonpregnant rats (8, 26, 28). These studies illustrate the importance of elevated cytokines to drive the maternal immune response toward a pro-inflammatory Th1 response in the pregnant rat, which mimics that seen in preeclamptic women. Moreover, we found that the hypertension in each of these animal models was markedly attenuated by antagonism of the AT1 receptor and ETA receptor or by the superoxide dismutase mimetic Tempol.
Furthermore, as in the case of B-cell-depleted RUPP rats, AT1-AA was significantly lower than control RUPPs, B-cell-depleted RUPP rats had lower tissue ET-1 transcript in renal cortices and placentas, circulating TNF-α, and hypertension in response to placental ischemia than seen in RUPP control rats. This study suggests that AT1-AA is an important hypertensive mechanisms occurring in response to placental ischemia (27); however, this study could not distinguish between the effects of lower AT1-AA or B-cell depletion on the hypertension and pathophysiology occurring in response to placental ischemia. Therefore, future studies with a specific AT1-AA-blocking peptide in animal models of placental ischemia or immune activation will be important to ascertain specific modalities of AT1-AA-induced pathophysiology from that of not only B cells or other B-cell products but also T cells and T-cell products stimulated in RUPP rats, cytokine-infused rats, or recipients of RUPP CD4+ T cells.
Inflammatory Cytokines
Several groups have shown an important role for inflammatory cytokines in the etiology of preeclampsia (5–11, 20, 22, 23, 26, 44, 45, 58, 59). Changes in inflammatory markers during pregnancy and the current inflammatory status of women who previously had preeclampsia against matched controls was previously examined. Importantly, preeclamptic women displayed short- and long-term changes in inflammatory status, thus suggesting that chronic inflammation exists postpartum in preeclamptic women (9). Chronically produced autoimmune cytokines, such as IL-17, and increased circulating TH17 and B lymphocytes producing AT1-AA and placental B1 B cells all strongly suggests that preeclampsia is similar to other autoimmune diseases.
Although inflammatory cytokines such as IL-6, IL-17, and TNF-α may be elevated in preeclamptic women, studies in animal models have been important to show that moderate, long-term increases in cytokines during pregnancy increases blood pressure and compromises renal function. Mechanisms of hypertension during pregnancy in response to elevated cytokines appear to involve activation of ET-1, increased oxidative stress, and activation of AT1 receptors by AT1-AA. In addition, many laboratories have shown that TNF-α directly stimulates endothelial cells in culture to secrete ET-1 and sICAM, which would attract leukocytes to adhere to vascular tissues and play a role in edema, which could lead to temporary increases in blood pressure (2, 5, 6). TNF-α mRNA is increased in preeclamptic placentas and thus could directly increase ET-1 in the placental unit (55). We have previously demonstrated that chronic TNF-α infusion into pregnant rats not only stimulates AT1-AA but also activates the ET-1 system as a mechanism of hypertension during pregnancy (20, 23). Recent studies indicate that inhibition of TNF-α decreased blood pressure, ET-1, sFlt-1, and AT1-AA in pregnant rodent models of preeclampsia (18). Studies from the Xia laboratory demonstrated the AT1-AA to increase TNF-α in the circulation of AT1AA-injected pregnant mice but not in nonpregnant mice. Moreover, TNF-α blockade in AT1-AA-injected pregnant mice attenuated the key features of preeclampsia, such as hypertension, albuminuria, and circulating sFlt-1 and s-endoglin. These data demonstrated an important role for TNF-α production subsequent to AT1-AA activation of the AT1 receptor to mediate hypertension during pregnancy.
We have previously demonstrated an important role for IL-6 to increase blood pressure in pregnant rats (11, 26). IL-6 is important in both anti-inflammatory and pro-inflammatory processes and is a pivotal cytokine to influence activation of B cells as well as effector or regulatory T cells (2). IL-6 is elevated in preeclamptic women, the RUPP rat, and AT1-AA-induced hypertensive pregnant mice. Zhou et. al. demonstrated that IL-6 blockade decreased blood pressure and downstream ET-1 production in the AT1-AA-induced hypertensive pregnant mice (59). We have recently shown that infusion of IL-6 into pregnant rats increased blood pressure and plasma renin activity, decreased renal function, and stimulated AT1-AA (11, 26). However, infusion of IL-6 into nonpregnant rats had no effect on blood pressure or AT1-AA production, again highlighting a very different effect of chronic inflammatory cytokines during pregnancy compared with the nonpregnant state.
IL-17 is a cytokine that has mostly been associated with autoimmune diseases but has recently gained attention in preeclamptic research (3, 8, 44). Recent studies have shown that circulating IL-17 secreting TH17 cells are increased in preeclamptic patients compared with NP patients (44). More recent studies have revealed an important role for TH17 cells and IL-17 in the clearing of bacterial infections (3). One important function of IL-17 producing TH17 cells is to recruit neutrophils and other phagocytic cells to a site of infection. IL-17 stimulates neutrophil activation, production of antimicrobial substances such as defensins and ROS, and phagocytosis of microbes or necrotic tissues (3). Macrophages and neutrophils convert molecular oxygen into ROS by the phagocyte NADPH oxidase system. Activated neutrophils cause injury to normal host tissues, such as the placental unit, by the release of lysosomal enzymes, ROS, or nitric oxide. Preeclamptic women display oxidative stress, increased NADPH oxidase subunits within the placental unit, and elevated blood, urinary, and placental 8 isoprostanes, an indicator of whole body oxidative stress (38, 39, 41). We recently showed that IL-17, when infused into pregnant rats, increased blood pressure, placental oxidative species, urinary isoprostanes, and AT1-AA (8). However, infusion of IL-17 into nonpregnant rats had no effect to increase blood pressure. Furthermore, we found that administration of Tempol not only attenuated the placental oxidative stress but also decreased blood pressure and, surprisingly, AT1-AA produced in response to IL-17. These data indicate the importance of IL-17 and ROS as signaling molecules between damaged tissues and immune cells as mediators of the pathology associated with preeclampsia.
An additional cytokine gaining attention in the area of preeclamptic research is CD40/CD40 ligand. The CD40 antigen binds to the CD40 ligand on T cells and is important to stimulate B-lymphocyte proliferation, as previously mentioned (1, 2). A recent study compared the effect of maternal serum from preeclamptic patients and NP patients to induce apoptosis in cultured endothelial cells (57). This study showed that endothelial dysfunction may be induced by this CD40/CD40 ligand. These authors found altered morphology, decreased cell growth, and increased apoptosis were greater with CD40/CD40 ligand increased expression following exposure to preeclamptic sera vs. that from healthy NP women. However, important in vivo studies revealing an important role for CD40/CD40 ligand during pregnancy are lacking. Furthermore, inhibition of this interaction would inhibit T cell-B cell communication and could clarify the role of either memory B cells vs. nonmemory B cells in the production of AT1-AA, as mentioned previously. Nonmemory B cells do not require this interaction with T cells for antibody secretion. Therefore, this could be a defining study revealing a role for the memory immune response as well as the route of production of AT1-AA during preeclampsia. Moreover, in vivo studies overexpressing CD40 ligand and thereby stimulating greater CD40/CD40 ligand interaction and T cell-B cell communication could stimulate many characteristics of preeclampsia in a pregnant animal model. These types of in vivo studies are important for preeclamptic researchers to identify a safe alternative to treating preeclampsia in the patient population. Knowledge gained from these types of studies are essential for developing better prediction strategies as well as for moving patient treatment options forward and improving patient outcome for both babies and mothers suffering from preeclampsia.
Conclusions
Preeclampsia is a disease of pregnancy that is typically diagnosed during the latter gestational months and is characterized as new onset hypertension with proteinuria. However, the onset of this disease is hypothesized to occur early in placentation, leading to an overall decrease in placental blood flow and increased uterine artery resistance index. The pregnancy proceeds with progressive inflammation and ischemia in the placental unit. We have highlighted studies indicating the importance of inflammatory cells and products to cause the characteristic rise in blood pressure and decline in renal function that occur during preeclampsia. Efficacy of anti-inflammatories to decrease immune cell activation or interrupt cytokine pathways have proven beneficial in animal models of preeclampsia. However, potential use of such therapies remain highly questionable in the pregnant patient. Therefore, further research is necessary to identify potential safe therapies and provide answers to important questions in preeclamptic research.
Acknowledgments
The authors thank Dr. Florian Herse for use of FIGURE 3.
Footnotes
This work was supported by American Heart Association Grant SDG0835472N and National Institutes of Health Grants HL-78147, HL-51971, and HD-067541.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: B.B.D.L. and K.W. conception and design of research; B.B.D.L. and K.W. performed experiments; B.B.D.L. and K.W. analyzed data; B.B.D.L. interpreted results of experiments; B.B.D.L. and K.W. prepared figures; B.B.D.L. drafted manuscript; B.B.D.L. and D.C. edited and revised manuscript; B.B.D.L., K.W., and D.C. approved final version of manuscript.
References
- 1. Abbus A, Lichtman A. Cellular and Molecular Immunology. General Properties of the Immune Response, Cells and Tissues of the Immune System. Philadelphia, PA: Elsevier, 2005, p. 163–188 [Google Scholar]
- 2. Abbus A, Lichtman A. Cellular and Molecular Immunology. General Properties of the Immune Response, Cells and Tissues of the Immune System. Philadelphia, PA: Elsevier, 2005, p. 189–215 [Google Scholar]
- 3. Abbus A, Lichtman A. Cellular and Molecular Immunology. General Properties of the Immune Response, Cells and Tissues of the Immune System. Philadelphia, PA: Elsevier, 2005, p. 243–274 [Google Scholar]
- 4. Cianchini G, Corona R, Frezzolini A, Ruffelli M, Didona B, Puddu P. Treatment of severe pemphigus with rituximab: report of 12 cases and a review of the literature. Arch Dermatol 143: 1033–1038, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Cid MC, Kleinman HK, Grant DS, Schnaper HW, Fauci AS, Hoffman GS. Estradiol enhances leukocyte binding to tumor necrosis factor (TNF)-stimulated endothelial cells via an increase in TNF-induced adhesion molecules E-selectin, intercellular adhesion molecule type 1, and vascular cell adhesion molecule type 1. J Clin Invest 93: 17–25, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol 37: 240–249, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Dechend R, Gratze P, Wallukat G, Shagdarsuren E, Plehm R, Brasen JH, Fiebeler A, Schneider W, Caluwaerts S, Vercruysse L, Pijnenborg R, Luft FC, Muller DN. Agonistic autoantibodies to the AT1 receptor in a transgenic rat model of preeclampsia. Hypertension 45: 742–746, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Dhillion P, Wallace K, Herse F, Scott J, Wallukat G, Heath J, Mosely J, Martin JN, Jr, Dechend R, LaMarca B. IL-17-mediated oxidative stress is an important stimulator of AT1-AA and hypertension during pregnancy. Am J Physiol Regul Integr Comp Physiol 303: R353–R358, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Freeman DJ, McManus F, Brown EA, Cherry L, Norrie J, Ramsay JE, Clark P, Walker ID, Sattar N, Greer IA. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension 44: 708–714, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Fukui A, Yokota M, Funamizu A, Nakamu R, Fukuhara R, Yamada K, Kimura H, Fukuyama A, Kamoi M, Tanaka K, Mizunuma H. Changes of NK cells in preeclampsia. Am J Reprod Immunol 67: 278–286, 2012 [DOI] [PubMed] [Google Scholar]
- 11. Gadonski G, LaMarca BB, Sullivan E, Bennett W, Chandler D, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of interleukin 6. Hypertension 48: 711–716, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PCA. Study of angiotensin I. I. pressor response throughout primigravid pregnancy. J Clin Invest 52: 2682–2689, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of hypertension during preeclampsia: Linking placental ischemia with endothelial dysfunction. Hypertension 38: 718–722, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Granger JP, Alexander BT, Bennett WA, Khalil RA. Pathophysiology of pregnancy-induced hypertension. Microcirculation 9: 147–160, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Herse F, Dechend R, Harsem NK, Wallukat G, Janke J, Qadri F, Hering L, Muller DN, Luft FC, Staff AC. Dysregulation of the circulating and tissue-based renin-angiotensin system in preeclampsia. Hypertension 49: 604–611, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Herse F, Lamarca B. Angiotensin II type 1 receptor autoantibody (at1-aa)-mediated pregnancy hypertension. Am J Reprod Immunol 69: 413–418, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hubel CA, Wallukat G, Wolf M, Herse F, Rajakumar A, Roberts JM, Markovic N, Thadhani R, Luft FC, Dechend R. Agonistic angiotensin ii type 1 receptor autoantibodies in postpartum women with a history of preeclampsia. Hypertension 49: 612–617, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Irani RA, Zhang Y, Zhou CC, Blackwell SC, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Autoantibody-mediated angiotensin receptor activation contributes to preeclampsia through tumor necrosis factor-alpha signaling. Hypertension 55: 1246–1253, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jensen F, Wallukat G, Herse F, Budner O, El-Mousleh T, Costa SD, Dechend R, Zenclussen AC. CD19+CD5+ cells as indicators of preeclampsia. Hypertension 59: 861–868, 2012 [DOI] [PubMed] [Google Scholar]
- 20. Keiser SD, Veillon EW, Parrish MR, Bennett W, Cockrell K, Fournier L, Granger JP, Martin JN, Jr, LaMarca BB. Effects of 17-hydroxyprogesterone on tumor necrosis factor-alpha-induced hypertension during pregnancy. Am J Hypertens 22: 1120–1125, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. LaMarca B, Alexander B, Gilbert J, Ryan M, Sedeek M, Granger J. Pathophysiology of hypertension in response to placental ischemia during pregnancy: a central role for endothelin? Gender Medicine 5: S133–S138, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension 46: 1022–1025, 2005 [DOI] [PubMed] [Google Scholar]
- 23. LaMarca BB, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of Endothelin in Mediating Tumor Necrosis Factor-Induced Hypertension in Pregnant Rats. Hypertension 46: 82–86, 2005 [DOI] [PubMed] [Google Scholar]
- 24. LaMarca BB, Parrish M, Ray LF, Murphy SR, Roberts L, Wallukat PGG, Wenzel K, Cockrell K, Martin JN, Jr, Ryan MJ, Dechend R. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin-1. Hypertension 54: 905–909, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. LaMarca B, Parrish MR, Wallace K. Agonistic autoantibodies to the angiotensin ii type i receptor cause pathophysiologic characteristics of preeclampsia. Gend Med 9: 139–146, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. LaMarca BB, Speed J, Ray LF, Cockrell K, Wallukat G, Dechend R, Granger J. Hypertension in response to IL-6 during pregnancy: role of AT1-receptor activation of interferon, cytokine, and mediator. Inter J Res 3: 65–70, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. LaMarca B, Wallace K, Herse F, Wallukat G, Weimer A, Dechend R. Hypertension in response to placental ischemia during pregnancy: role of B lymphocytes. Hypertension 57: 865–871, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. LaMarca BB, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Elevated agonistic autoantibodies to the angiotensin type 1 (AT1-AA) receptor in response to placental ischemia and TNF alpha in pregnant rats. Hypertension 52: 7–11, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, Karumanchi SA. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med 355: 992–1005, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Liao AH, Liu LP, Ding WP, Zhang L. Functional changes of human peripheral B lymphocytes in preeclampsia. Am J Reprod Immunol 61: 313–321, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–658, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murphy SR, LaMarca BB, Cockrell K, Granger JP. Soluble fms-like tyrosine-1 induced hypertension: role of endothelin. Hypertension 55: 394–398, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of anti-angiogenic factors and implications for later cardiovascular disease. Circulation 123: 2856–2869, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Novotny SR, Wallace K, Heath J, Moseley J, Dhillon P, Weimer A, Wallukat G, Herse F, Wenzel K, Martin JN, Jr, Dechend R, LaMarca B. Activating autoantibodies to the angiotensin receptor are important in mediating hypertension in response to adoptive transfer of RUPP CD4+ T Lymphocytes. Am J Physiol Regul Integr Comp Physiol 302: R1197–R1201, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marc R, Parrish M, Ryan MJ, Glover P, Brewer J, Ray L, Dechend R, Martin JN, Jr, LaMarca BB. Angiotensin II type 1 autoantibody induced hypertension during pregnancy is associated with renal endothelial dysfunction. Gen Med 8: 184–188, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prins J, Boelens H, Heimweg J, Van der Heide S, Dubois A, Van Oosterhout A, Erwich J. Preeclampsia is associated with lower percentages of regulatory T cells in maternal blood. Hypertension Pregnancy 28: 300–311, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Redman CW, Sargent IL. Immunology of pre-eclampsia. Am J Reprod Immunol 63: 534–543, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Roberts JM, Gammill H. Insulin resistance in preeclampsia. Hypertension 47: 341–342, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension 46: 1243–1249, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Roberts L, LaMarca D, Fournier L, Bain J, Cockrell K, Granger JP. Enhanced endothelin synthesis by endothelial cells exposed to sera from pregnant rats with decreased uterine perfusion. Hypertension 47: 615–618, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI working group on research on hypertension during pregnancy. Hypertension 41: 437–445, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol 161: 1200–1204, 1989 [DOI] [PubMed] [Google Scholar]
- 43. Roberts JM, Von Versen-Hoeynck F. Maternal fetal/placental interactions and abnormal pregnancy outcomes. Hypertension 49: 15–16, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Santner-Nanan B, Peek M, Khanam R, Richarts L, Zhu E, Fazekas de St G, Nanan R. Systemic increase in the ration between FoxP3+ and IL-17 producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol 183: 7023–7030, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Sargent I, Borzychowski A, Redman C. Immunoregulation in normal pregnancy and preeclampsia: an overview. Reprod Biomed Online 13: 680–686, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Stepan H, Faber R, Wessel N, Wallukat G, Schultheiss HP, Walther T. Relation between circulating angiotensin ii type 1 receptor agonistic autoantibodies and soluble fms-like tyrosine kinase 1 in the pathogenesis of preeclampsia. J Clin Endocrinol Metab 91: 2424–2427, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Stepan H, Walther T. Questionable role of the angiotensin ii receptor subtype 1 autoantibody in the pathogenesis of preeclampsia. Hypertension 50: e3, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Taylor RN, de Groot CJ, Cho YK, Lim KH. Circulating factors as markers and mediators of endothelial cell dysfunction in preeclampsia. Semin Reprod Endocrinol 16: 17–31, 1998 [DOI] [PubMed] [Google Scholar]
- 49. Thadhani RI, Johnson RJ, Karumanchi SA. Hypertension during pregnancy: a disorder begging for pathophysiological support. Hypertension 46: 1250–1251, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Vacca P, Mingari MC, Moretta L. Natural killer cells in human pregnancy. J Rep Imm 97: 14–19, 2013 [DOI] [PubMed] [Google Scholar]
- 51. Wallace K, Novotny S, Weimer A, Martin JN, LaMarca B. CD4+ T helper cells stimulated in response to placental ischemia mediate hypertension in pregnant rats. Hypertension 57: 949–955, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wallace K, Novotny S, Heath J, Moseley J, Martin JN, Jr, Owens MY, LaMarca B. Hypertension in response to CD4+ T cells from reduced uterine perfusion pregnant rats is associated with activation of the endothelin-1 system. Am J Physiol Regul Integr Comp Physiol 303: R144–R149, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest 103: 945–952, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Walther T, Wallukat G, Jank A, Bartel S, Schultheiss HP, Faber R, Stepan H. Angiotensin II type 1 receptor agonistic antibodies reflect fundamental alterations in the uteroplacental vasculature. Hypertension 46: 1275–1279, 2005 [DOI] [PubMed] [Google Scholar]
- 55. Wang Y, Walsh SW. TNF alpha concentrations and mRNA expression are increased in preeclamptic placentas. J Reprod Immunol 32: 157–169, 1996 [DOI] [PubMed] [Google Scholar]
- 56. Wenzel K, Rajakumar A, Haase H, Geusens N, Hubner N, Schulz H, Brewer J, Roberts L, Hubel CA, Herse F, Hering L, Qadri F, Lindschau C, Wallukat G, Pijnenborg R, Heidecke H, Riemekasten G, Luft FC, Muller DN, Lamarca B, Dechend R. Angiotensin II type 1 receptor antibodies and increased angiotensin II sensitivity in pregnant rats. Hypertension 58: 77–84, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wu CF, Huang FD, Sui RF, Sun JX. Preeclampsia serum upregulates CD40/CD40L expression and induces apoptosis in human umbilical cord endothelial cells. Reprod Biol Endocrinol 10: 28, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Xia Y, Ramin SM, Kellems RE. Potential roles of angiotensin receptor-activating autoantibody in the pathophysiology of preeclampsia. Hypertension 50: 269–275, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhou CC, Irani RA, Dai Y, Blackwell SC, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Autoantibody-mediated IL6-dependent endothelin-1 elevation underlies pathogenesis in a mouse model of preeclampsia. J Immunol 186: 6024–6034, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]