Abstract
Bacteriochlorins are attractive candidates as photosensitizers for photodynamic therapy (PDT) due to their intense absorption in the near-infrared (NIR) region of the spectrum where light transmission through tissue is maximal. Many naturally occurring bacteriochlorins are inherently unstable due to adventitious atmospheric oxidation. A de novo synthesis affords bacteriochlorins that contain a geminal dimethyl group in each reduced pyrrole ring to increase stability against oxidation. Here, three new synthetic bacteriochlorins, each bearing a single side-chain containing one or two positive charges, were investigated for their in vitro PDT activity against HeLa human cancer cells. All bacteriochlorins were active at low nanomolar concentration when activated with NIR light; those bearing a single positive charge exhibited faster uptake and higher activity. The bacteriochlorins were localized in mitochondria, lysosomes and endoplasmic reticulum as shown by organelle specific fluorescent probes. Cell death was via apoptosis as shown by cell morphology and nuclear condensation. Taken together, the results show the importance of appropriate peripheral groups about a photosensitizer for effective PDT applications.
Keywords: photodynamic therapy, HeLa cancer cells, bacteriochlorins, confocal microscopy, subcellular localization, apoptosis
INTRODUCTION
Photodynamic therapy (PDT) is a successful and clinically approved anti-cancer modality that involves three basic components, namely, a photosensitizer, light and oxygen [1]. Individually, none of these species is toxic, but when combined together produce reactive oxygen species such as singlet oxygen, superoxide and hydroxyl radicals. Selectivity for tumors is provided by two mechanisms: the tendency of intravenously injected photosensitizer to accumulate in tumors, and the ability to spatially confine the light delivery to the area of the lesion [2–4]. The production of reactive oxygen species inside the malignant cells leads to cell necrosis via apoptosis or autophagy depending on the cell type, structure of the photosensitizer and the light parameters employed [5]. Though many tetrapyrrole-based photosensitizers, such as porphyrins have been clinically approved for some types of cancer, such molecules suffer from various limitations. One limitation is the low absorption of light in the near-infrared (NIR) region of the spectrum where tissue transmission of light is highest. Therefore, PDT using currently available photosensitizers penetrates only the first 2–3 mm of tissue. To overcome this limitation, bacteriochlorins have gained considerable interest as potential photosensitizers. Bacteriochlorins differ from porphyrins by the reduction of two opposite pyrrole rings. The reduction of the number of π-electrons from 22 to 18 raises the energy (and changes the nature) of the HOMO, and thus narrows the HOMO–LUMO energy gap. This change in the HOMO–LUMO energy gap causes a bathochromic and hyperchromic effect (i.e. redshift and intensification) on the Q-band. A bacteriochlorin therefore has an intense absorption band (~100,000 M-1.cm-1) in the 720–850 nm spectral region where light penetration through tissue is maximal.
Naturally derived bacteriochlorins have been investigated for their in vitro and in vivo PDT efficacy in animal models, and a Pd-bacteriopheophorbide derivative called TOOKAD has been tested in clinical trials for prostate cancer [6, 7]. A major limitation of naturally derived bacteriochlorins is their instability in the presence of oxygen. To overcome such limitations, a number of groups have developed routes to synthetic bacterichlorins. The synthesis of bacteriochlorins has been the subject of a number of reviews over the past decade [8–11]. Notable recent advances include (1) Brückner's two-fold OsO4-mediated dihydroxylation of meso-tetraarylporphyrins followed by ring-expansion of the resulting tetrahydroxybacteriochlorins to give morpholinobacteriochlorins [12], and (2) Pereira's scalable diimide-mediated reduction of meso-tetraarylporphyrins to give the corresponding bacteriochlorins [13]. Our own contribution in this area entails a de novo synthetic pathway to bacteriochlorins wherein a geminal dimethyl group is located in each reduced pyrrole ring [14, 15]. The chemical robustness of the synthetic bacteriochlorins and the versatility of the synthetic methodology enable wavelength tunability and substituent tailorability (e.g. lipophilicity, molecular asymmetry) as needed for PDT applications [16, 17]. In an earlier study, three somewhat lipophilic synthetic bacteriochlorins were found to overcome the resistance of melanoma to PDT [18]. Members of a set of 12 synthetic bacteriochlorins with varying peripheral substituents, including four bacteriochlorins bearing two or four positive charges, were examined as photosensitizers for killing HeLa human cervical cancer cells, which led to quantitative structure-function relationships [19]. Furthermore, three cationic bacteriochlorins (bearing two, four or six positive charges) proved effective in antimicrobial PDT [20]. Each of these bacteriochlorins was substituted with positively charged groups at two opposite sites on the macrocycle.
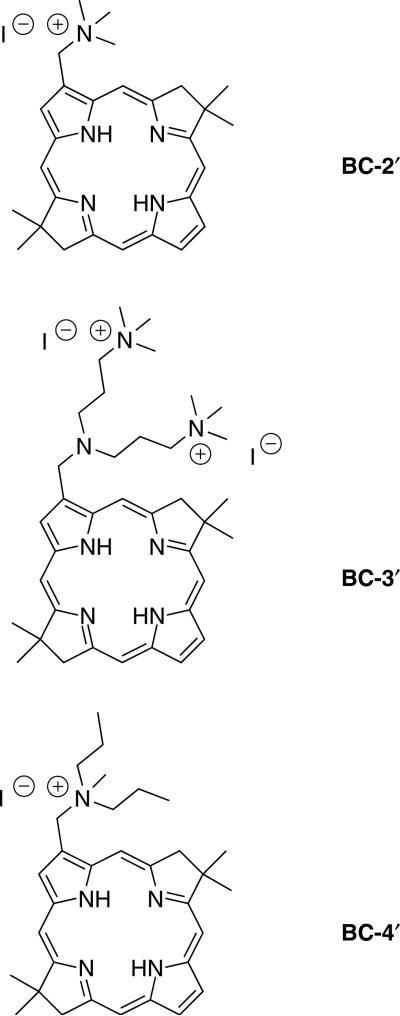
In the present study we describe the synthesis of three cationic bacteriochlorins bearing one rather than two charged substituents. Furthermore, the three bacteriochlorins are examined for their photodynamic efficacy in killing HeLa human cervical cancer cells. This cell line was chosen for two reasons: (1) to facilitate comparisons with our prior studies of the PDT activity of other sets of bacteriochlorins against HeLa cells, including analogs that contain two (rather than one) of the same charged groups, and (2) HeLa cells are by far the most common human cancer cell line used in cancer research studies. The bacteriochlorins were derived from a monoformylbacteriochlorin [16] by reductive amination and quaternization. The three bacteriochlorins are shown in Chart 1. By changing the amine employed in the reductive amination, the number of sites for quaternization and the lipophilicity of the resulting cationic alkylammonium group can be controlled. The placement of singly or doubly charged groups at one site at the periphery of the hydrophobic bacteriochlorin macrocycle affords amphiphilic character. Numerous cationic (and amphiphilic) chlorins have been prepared by derivatization of chlorophyll-related compounds (for leading references see Refs. 21–27). On the other hand, to our knowledge only one example of an analogous cationic derivative of bacteriochlorophyll has been reported [28].
Chart 1.
Synthetic, cationic bacteriochlorin amphiphiles examined herein
RESULTS AND DISCUSSION
Molecular design
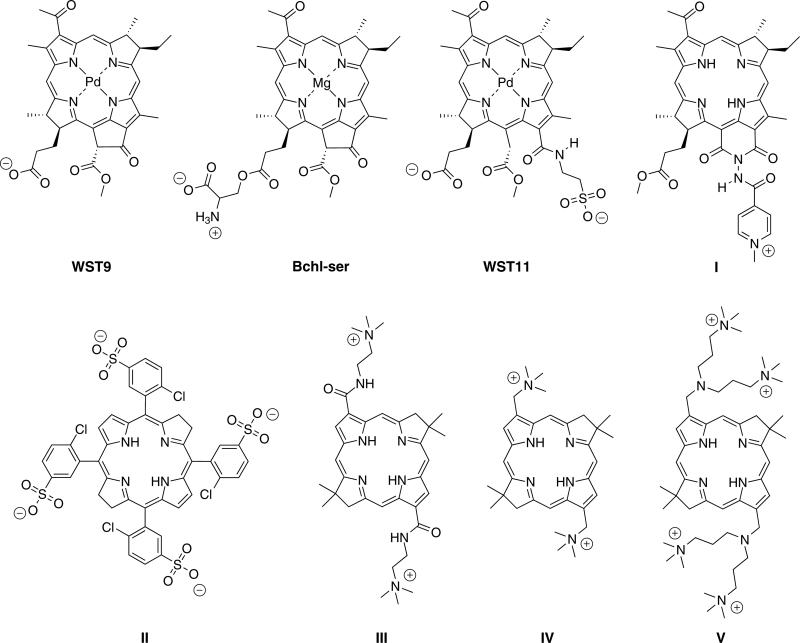
Bacteriochlorins have emerged relatively recently as candidates for PDT. The bacteriochlorin examined most extensively to date is WST9 (Tookad), a palladium chelate derived from bacteriochlorophyll a (Chart 2). Tookad bears one anionic group at physiological pH and hence is amphiphilic in character. Analogs in this series include the serine conjugate of bacteriochlorophyllide (Bchl-ser) [29], a zwitterionic compound, and WST-11 (TOOKAD soluble) [30], which bears two anionic groups. Each compound is amphiphilic given the location of the charged groups with respect to the hydrophobic bacteriochlorin macrocycle. A naturally derived bacteriochlorin-imide containing a single positive charge (I) has been prepared [28], but to our knowledge has not yet been examined for PDT activity, and a number of non-cationic analogs also have been prepared [31]. A family of tetraanionic bacteriochlorins, of which II is the parent member, and aryl sulfonamide analogs, have been designed and subjected to extensive PDT studies [32–34]. Such compounds are not amphiphilic in the traditional sense given the rectilinear placement of four anionic substituents. Previously, we prepared nine cationic bacteriochlorins [16, 19, 20] of which three representative structures (III–V) are displayed in Chart 2. The bacteriochlorins prepared in this manner bear two, four or six charges, and in each case the macrocycles are substituted at both pyrrole moieties.
Chart 2.
Representative bacteriochlorins (counterions are omitted for clarity)
The molecular design employed herein (BC-2′, BC-3′, BC-4′) centers broadly around analogs of IV and V. The analogs contain a single site of substitution rather than two sites. Changing the composition of the aminoalkyl moiety allows tuning of (i) the number of charges, (ii) the spatial disposition of the charges, and (iii) the lipophilic character of the positively charged moiety. The resulting compounds have a polar head group (positively charged nitrogen) and a hydrophobic tail (bacteriochlorin macrocycle), and as such are well-suited for comparison with their disubstituted analogs (III–V and other members), which carry a positive charge on each side of the bacteriochlorin macrocycle.
Synthesis of bacteriochlorins
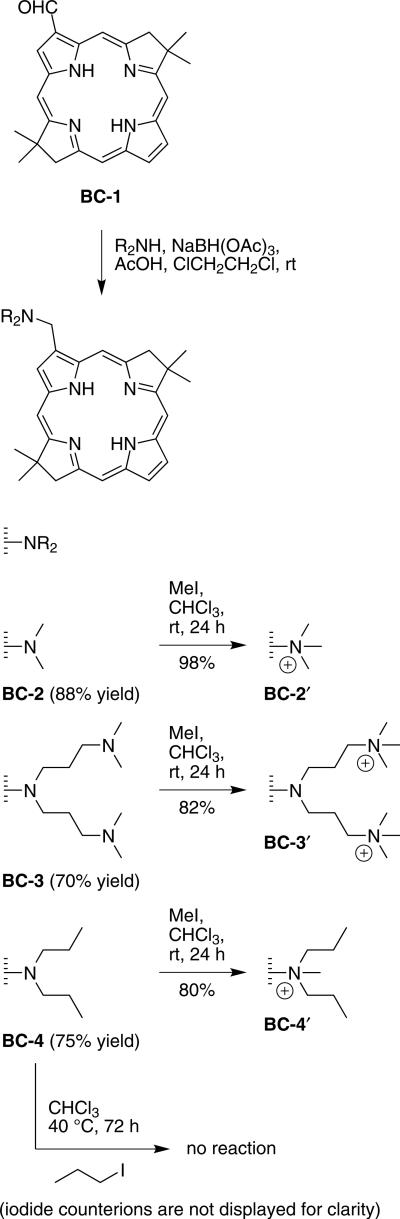
Reaction of 3,13-dibromo-8,8,18,18-tetramethylbacterio chlorin with Pd(PPh3)4 in toluene/DMF at 70 °C under an atmosphere of CO for 2 h and subsequent treatment with Bu3SnH afforded the corresponding 3,13-diformyl bacteriochlorin in 60% yield along with 3-formyl-13-desbromobacteriochlorin BC-1 in 25% yield [16]. By this method a total of 100 mg of the 3,13-diformylbacteriochlorin and 30 mg of BC-1 were prepared. The 3,13-diformyl bacteriochlorin was used previously in reductive amination reactions to afford a variety of disubstituted bacteriochlorins [16]. While not a rational method of synthesis, the availability of the monoformylbacteriochlorin BC-1 presented an opportunity to prepare a small family of amphiphilic bacteriochlorins. A complementary approach was recently reported by Yu and Ptaszek [35], who showed that a 3,13-dibromo-5-methoxybacteriochlorin underwent sequential Pd-mediated derivatization reactions. One of the resulting unsymmetrically substituted bacteriochlorins has been employed for in vivo imaging of ovarian cancer [36].
In the work described herein, reductive amination has been carried out with BC-1 to afford monosubstituted bacteriochlorins. The reductive amination of BC-1 was carried out at modest concentrations (12 mM), using sodium triacetoxyborohydride [37] in 1,2-dichloroethane containing acetic acid. Reductive amination with dimethylamine afforded 3-(dimethylaminomethyl) bacteriochlorin BC-2 in 88% yield after column chromatography. Similar treatment with bis(3-(dimethylamino)propyl)amine or dipropylamine afforded BC-3 or BC-4 in 70% or 75% yield, respectively (Scheme 1). Each bacteriochlorin was characterized by 1H NMR spectroscopy, mass spectrometry (LD-MS or ESI-MS) and absorption spectroscopy.
Scheme 1.
Synthesis of amphiphilic bacteriochlorins
To impart amphiphilic character, the aminoalkyl bacteriochlorins were subjected to conditions for quaternization [38]. Quaternization using methyl iodide in CHCl3 at room temperature is known to alkylate the alkyl amino substituents rather than the pyrrolic or pyrrolinic nitrogens [16, 38]. Thus, the overnight reaction of bacteriochlorin BC-2 with excess methyl iodide in CHCl3 at room temperature resulted in the iodide salt BC-2′. Similarly, bacteriochlorin BC-3 or BC-4 was converted to the corresponding iodide or diiodide salt in excellent yield (Scheme 1). Bacteriochlorin BC-4′ was designed to extend the hydrophobic alkyl chains on the quaternized nitrogen to potentially facilitate insertion into a phospholipid bilayer, a design strategy that has been explored with porphyrins [39, 40].
Bacteriochlorin BC-3 contains three tertiary amines. However, treatment of BC-3 only methylated the two distal nitrogens and not the proximal nitrogen. Evidence in support of this interpretation stems from ESI-MS and 1H NMR spectroscopy: (1) MS data shows a molecular ion peak at m/z = 299.7338, which for a doubly charged ion corresponds to a parent molecule of mass 599.4676 Da, and (2) the 1H NMR spectrum of BC-3, shows a singlet at 2.20 ppm (12 protons), which is attributed to the N-methyl group; whereas in BC-3′, the singlet appears at 3.09 ppm and integrates for 18 protons. More complex spectra would be expected if the proximal nitrogens were methylated preferentially, and a greater number of charges if both distal and proximal nitrogens were quaternized. These observations are consistent with previous observations on disubstituted bacteriochlorins [16]. Attempted alkylation of BC-4 using excess propyl iodide in CHCl3 at 45 °C for 72 h gave recovered starting material rather than the expected tripropylaminomethyl bacteriochlorin.
Each cationic bacteriochlorin was purified by washing the crude product with organic solvents such as ether and CH2Cl2/hexanes to give the corresponding quaternary salts, which were clean by 1H NMR spectroscopy. Purification of BC-4′ was also achieved by silica gel chromatography [CH2Cl2/MeOH (9:1)].
Characterization of bacteriochlorins
A. Structural proof
The cationic bacteriochlorins were characterized by ESI-MS, 1H NMR spectroscopy (in CDCl3 or DMSO-d6) and absorption spectroscopy. The ESI-MS spectrum of each bacteriochlorin BC-2, BC-2′, BC-4 and BC-4′ only showed a molecular ion peak at m/z = 383.22, which is attributed to benzyl-type cleavage at the 3-CH2-NR2 or 3-CH2-NR3 bond to yield the bacteriochlorin-CH2 molecular ion. BC-3 and BC-3′ each displayed a molecular ion peak at m/z = 383.22, along with the expected parent molecular ion peak.
B. Solubility
Each bacteriochlorin (regardless of neutral or charged) was readily soluble at room temperature in CH2Cl2 or CHCl3. In addition BC-3′, which contains two positive charges, was also soluble in H2O at modest concentrations (~0.1 mM).
C. Absorption spectral properties
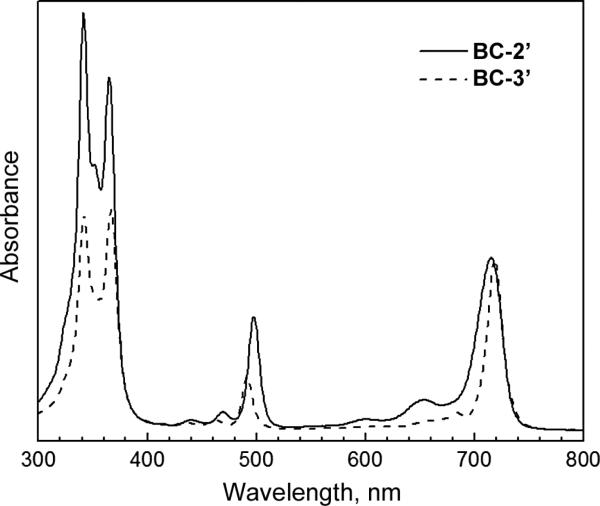
The absorption spectra for all bacteriochlorins were collected in CH2Cl2. Representative spectra are shown in Fig. 1. The absorption spectra were characteristic of bacteriochlorins [41, 42], with Qy bands ranging from 715–719 nm and relatively little difference between the neutral bacteriochlorins and their cationic derivatives. BC-2 and BC-4 showed a small (2–3) nm hypsochromic shift (718 nm to 715 nm; 717 nm to 715 nm) of the Qy band upon quaternization, whereas BC-3 showed a slight (1 nm) bathochromic shift (718 nm to 719 nm) upon quaternization. In BC-2′ and BC-4′, the positively charged nitrogens and the bacteriochlorin π-system are separated by only a single methylene group.
Fig. 1.
Absorption spectra of BC-2′ (solid) and BC-3′ (dashed) in CH2Cl2, normalized in the Qy band
The absorption spectrum for BC-3′ was also collected in H2O. The absorption spectra of BC-3′ taken in CH2Cl2 and H2O were comparable and showed only slight broadening of the Qy band in H2O (full-width-at-half-maximum increased from 16 nm in CH2Cl2 to 22 nm in H2O). In both solvents the λmax for the Qy band was at 719 nm with the other absorption bands varying only little between the two solvents. This observation further illustrates that a given tetrapyrrole chromophore exhibits very similar spectral features in organic and aqueous media if homogenously dispersed [43, 44]. Synthetic bacteriochlorins related to those described herein typically have a molar absorptivity of ~120,000 M-1cm-1 at the Qy maximum [14]. Such features are in keeping with the spectral characteristics of the natural bacteriochlorin photosynthetic chromophores [41] once one considers the typically larger spectral widths of the latter, giving generally similar integrated band intensities.
A few interesting comparisons can be made concerning the above-noted spectral effects of quaternization for the monosubstituted bacteriochlorins vs. the disubstituted analogs prepared previously [16]. Quaternization to form disubstituted 3,13-bis(trimethylammoniomethyl) bacteriochlorin IV (Chart 2) in CH2Cl2 results in a 15-nm bathochromic shift in the Qy band (722 to 737) nm, which is slightly larger than the 11-nm shift found when H2O is used as the solvent for the quaternized species IV (which introduces a potential solvent effect). In both cases, the bathochromic shifts are opposed to the small hypsochromic shifts (2–3 nm in CH2Cl2) for quaternization to form BC-2′ (or BC-4′). Similarly, quaternization of the disubstituted bacteriochlorin V (726 nm in H2O; not soluble in CH2Cl2) gives a 4-nm bathrochromic shift vs. the neutral parent bacteriochlorin (722 nm in CH2Cl2) compared to the 1-nm bathochromic shift for the monosubstituted BC-3/BC-3′ pair in the same media.
Collectively, the spectral differences found for quaternization of monosubstituted vs. disubstituted bacteriochlorins, and effects of solvent, may in part reflect the fact that quaternization of monosubstituted bacteriochlorins gives the molecule a (substantially larger) permanent dipole moment whereas quaternization of the symmetric, disubstituted analogs does not.
Photobleaching
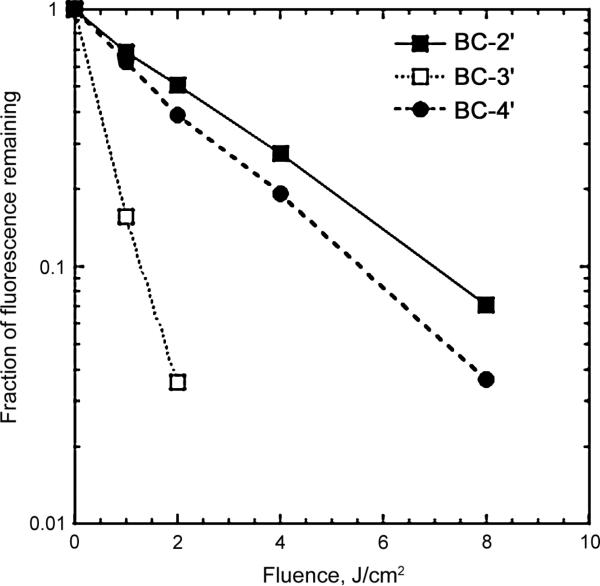
Significant photobleaching of BC-3′ was observed upon illumination with very low amounts of 700–850-nm light (1–2 J/cm2) as measured by reduction of fluorescence (Fig. 2). Bacteriochlorins BC-2′ and BC-4′ were more photostable but still were photobleached upon illumination with 8 J/cm2. No attempts were made to identify any photoproducts formed in the photobleaching.
Fig. 2.
Photobleaching of bacteriochlorins (1 μM in methanol) using 400-nm excitation and monitored by reduction in fluorescence (600-800 nm)
In vitro PDT on HeLa cells
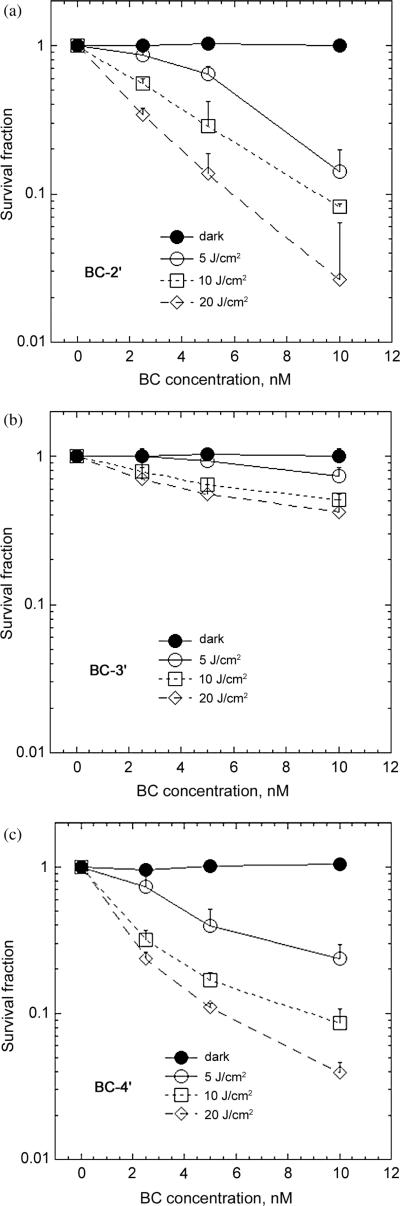
In vitro photoinactivation studies were carried out with HeLa cells after incubating with bacteriochlorins in complete medium for 24 h followed by exposure to NIR light (700–850 nm). The results are shown in Fig. 3. Initial studies had shown an extremely high PDT activity and necessitated reducing the concentrations of bacteriochlorins several times in order to find measurable cell survival after light delivery. Eventually a range of concentrations from 1–10 nM allowed LD50 concentration values (in nM) to be calculated after 5, 10 or 20 J/cm2 of 700–850 nm light had been delivered. The effectiveness for PDT activity was higher for BC-2′ and BC-4′ than for BC-3′ (compare Figs 3a and 3c with Fig. 3b).
Fig. 3.
In vitro PDT killing of HeLa cells incubated with increasing concentrations of bacteriochlorin for 24 hours in complete medium and illuminated with NIR light (5, 10 and 20 J/cm2). (a) BC-2′; (b) BC-3′; (c) BC-4′
The LD50 values in Table 1 were 1.5 to 6.0 nM for BC-2′ and 1.5 nM to 5.0 nM for BC-4′ compared to 7.5 nM to >10 nM for BC-3′. Dark toxicity was negligible or very low for all compounds (up to 0.5 μM) and there was no evident cytotoxicity of the 10 J/cm2 NIR light alone (i.e. without any bacteriochlorin). This is the first study showing effective LD50 phototoxicity at such a low (1.5–5.0 nM) concentration of the photosensitizer. In our previous study with 12 synthetic bacteriochlorins [19], phototoxicity at nanomolar concentrations was observed after 10 J/cm2 of NIR light, but the lowest LD50 (out of 12 compounds tested) was 15 nM and two of the present compounds are therefore 5–10 times more active.
Table 1.
LD50 values of bacteriochlorins against HeLa cells after various fluences of lighta
| 5 J/cm2 | 10 J/cm2 | 20 J/cm2 | |
|---|---|---|---|
| BC-2′ | 6.0 nM | 3.0 nM | 1.5 nM |
| BC-3′ | N/D (>10 nM) | 10 nM | 7.5 nM |
| BC-4′ | 5.0 nM | 2.5 nM | 1.5 nM |
Values were obtained from the data in Fig. 1. N/D, not determined.
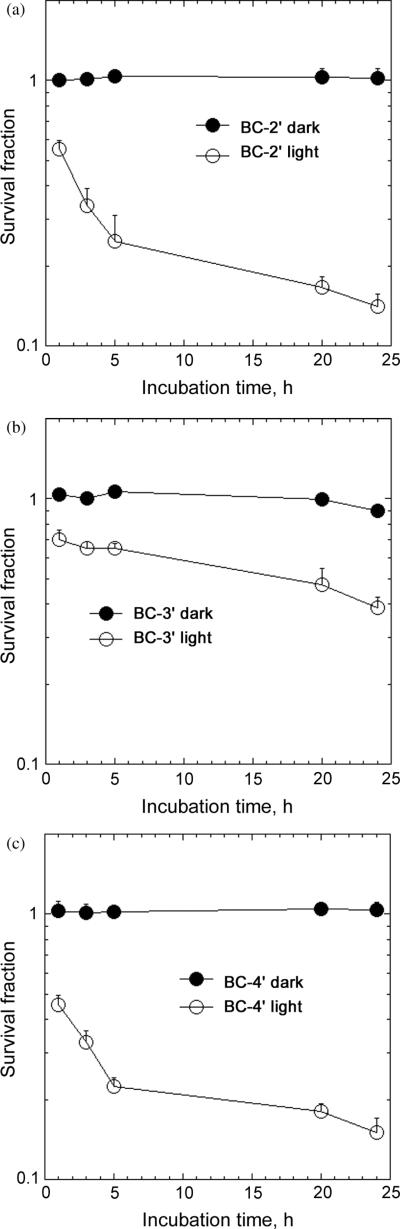
The effect of incubation time on phototoxicity was tested to assess the rate at which the cells became photosensitive when incubated with the bacteriochlorin. The most active compounds (BC-2′ and BC-4′) exhibited faster kinetics of photosensitization with significant killing apparent after 3–5 h, while the less active BC-3′ also had slower kinetics, with PDT killing still increasing after 24 h (Fig. 4). One explanation for these observations is that the more lipophilic nature of a singly charged bacteriochlorin (BC-2′ and BC-4′) enables more rapid entry into cells by diffusion, while the more polar and slightly bulkier doubly charged bacteriochlorins (BC-3′ and others studied previously) are predominantly taken-up into the cells by the slower process of endocytosis.
Fig. 4.
Effect of incubation time on PDT killing. Bacteriochlorins were incubated at 10 nM, and 10 J/cm2 of NIR light was used. (a) BC-2′; (b) BC-3′; (c) BC-4′
Subcellular localization
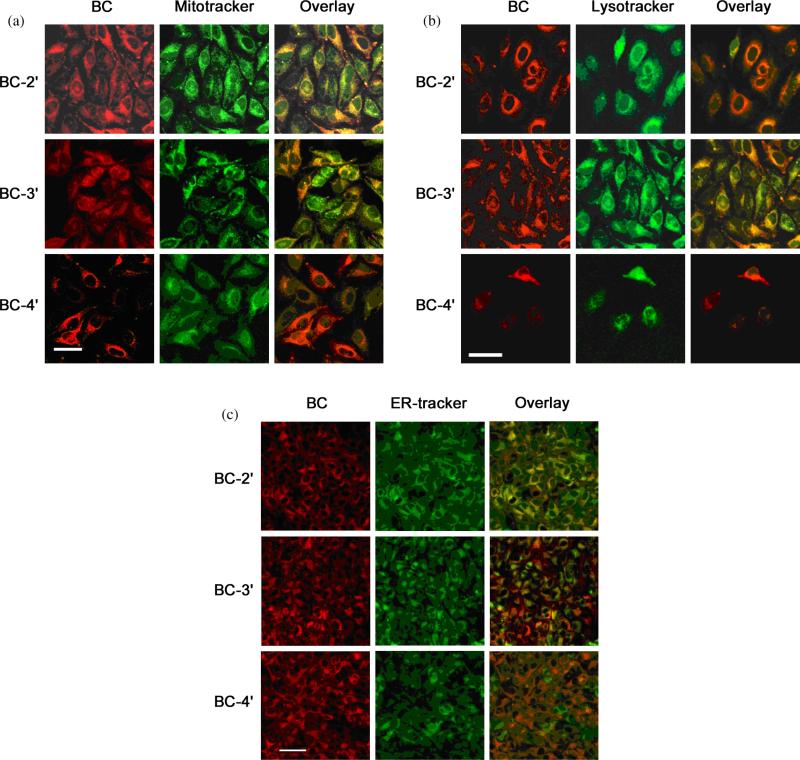
Different subcellular localizations of the bacteriochlorins might be expected, if the singly charged bacteriochlorins BC-2′ and BC-4′ were taken-up more by diffusion while the doubly charged bacteriochlorin BC-3′ was taken-up more by endocytosis. Therefore, each of the three bacteriochlorins (BC-2′, BC-3′, BC-4′) was incubated with HeLa cells and examined using confocal microscopy to determine the subcellular localization of the compounds. For these studies the bacteriochlorin was coincubated with green-fluorescent probes specific for mitochondria (MitoTracker, Fig. 5a), lysosomes (LysoTracker, Fig. 5b), or endoplasmic reticulum (ER Tracker, Fig. 5c). The fluorescence of the bacteriochlorin is shown in red and the fluorescence of the organellespecific probe is shown in green.
Fig. 5.
Subcellular localization by confocal microscopy. For all panels (a–c), the left columns display bacteriochlorin red fluorescence, the middle columns display green fluorescence from organelle-specific probes, and the right columns display the overlays of the bacteriochlorin and probe fluorescence. Bacteriochlorins were used at 250 nM for 24 h and probes at 5 μg/mL for 30 min. (a) MitoTracker; (b) LysoTracker; (c) ER-Tracker. Scale bar = 50 μM for a and b, and 100 μm for c
Bacteriochlorin BC-2′ localized mainly in the mitochondria and endoplasmic reticulum and to a lesser extent in the lysosomes, while BC-3′ showed good overlap with lysosomal and less overlap with mitochondria and poor overlap with the ER probe. Bacteriochlorin BC-4′ was found to localize more in the mitochondria and lysosomes and to a lesser extent in the endoplasmic reticulum. This affinity for different organelles can be explained on the basis of lipophilicity [45]. Intracellular localization of the photosensitizer is considered to be important for maximizing PDT-mediated cell toxicity [46]. In this regard, the accumulation of the photosensitizer in certain specific organelles (mitochondria and endoplasmic reticulum) is thought to lead to more efficient triggering of cell death upon illumination [47–49]. On the other hand, accumulation in other locations (lysosomes and plasma membrane) that are not thought to be as sensitive to photodamage tends to lessen PDT efficiency [50, 51]. The specific localization of the photosensitizer within the cell can depend on incubation time [52], aggregation state of the photosensitizer [53] and can change after light delivery has begun [54].
The subcellular localization of BC-2′ in endoplasmic reticulum and mitochondria, and that of BC-4′ predominantly in mitochondria correlates with the finding that both BC-2′ and BC-4′ are more active photosensitizers than BC-3′. This correlation supports the hypothesis that mitochondria are highly sensitive sites for photodamage while lysosomes are less sensitive to PDT effects. The PDT-induced oxidative damage in mitochondria and the ensuing change in mitochondrial membrane potential have been shown to correlate with induction of apoptosis by PDT agents [55, 56].
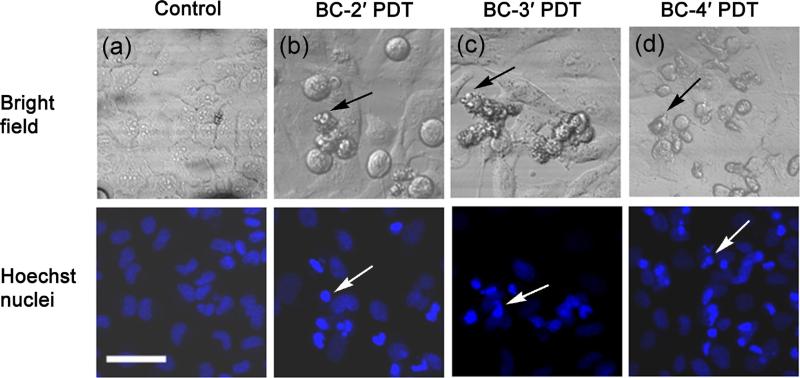
The cellular and nuclear morphology studies carried out after PDT showed an apoptotic mode of cell death in all the bacteriochlorins examined as shown in Fig. 6. The bright-field images in the PDT-treated cells showed apoptotic bodies (black arrows), and the Hoechst nuclear stain showed condensed nuclei (white arrows).
Fig. 6.
Cellular morphology by bright-field fluorescence microscopy of PDT-treated cells. (a) Control cells; (b) BC-2′, PDT treated; (c) BC-3′, PDT treated; (d) BC-4′, PDT treated. Nuclear morphology of PDT-treated cells by Hoechst fluorescence staining of nuclei: (a) control cells; (b) BC-2′, PDT treated; (c) BC-3′, PDT treated; (d) BC-4′, PDT treated. Scale bar = 100 μm
Lipophilic cationic photosensitizers are known to localize in mitochondria [57] while less lipophilic cationic photosensitizers localize in lysosomes [58]. The second positive charge present on BC-3′ increases the cationic character and thus apparently reduces the lipophilicity sufficiently to alter the localization from mitochondria to lysosomes and thus decreases the PDT efficacy to some extent; however, even BC-3′ still has very high activity versus comparable compounds. The mono-substituted molecular framework in the present compounds was much more active than the symmetrical disubstituted framework we reported previously [19]. Bacteriochlorins bearing two positive charges (one on each side) had LD50 values ranging from 3000 nM to 800 nM, corresponding to ≥ 2 orders of magnitude lower activity than the similar mono-substituted bacteriochlorins reported here. The explanation undoubtedly resides in the amphiphilic character of the mono-substituted bacteriochlorins. Insertion of tetrapyrrole photosensitizers into cell membranes may be critically involved in the sub-cellular mechanism [59], and it is expected that amphiphilic, mono-substituted bacteriochlorins would do so more easily than the symmetrical disubstituted bacteriochlorins studied previously.
EXPERIMENTAL
General synthesis procedures
1H NMR (400 MHz) spectra were collected at room temperature in CDCl3 unless noted otherwise. Absorption spectra were obtained in CH2Cl2 or water at room temperature. Bacteriochlorins were analyzed by laser desorption mass spectrometry (LD-MS) in the absence of a matrix [60]. Electrospray ionization mass spectroscopy (ESI-MS) data are reported for the molecular ion or protonated molecular ion. 3-Formyl-8,8,18,18-tetramethylbacteriochlorin (BC-1) was prepared as described previously [16].
Synthesis
3-(dimethylaminomethyl)-8,8,18,18-tetramethyl-bacteriochlorin (BC-2)
Following a procedure for reductive amination [37], a solution of BC-1 (5.0 mg, 0.012 mmol) in 1,2-dichloroethane (1.0 mL) was treated with dimethylamine (32 μL, 0.062 mmol, 2.0 M in THF). The mixture was stirred at room temperature under argon for 5 min before adding NaBH(OAc)3 (5.0 mg, 0.025 mmol) all at once, followed by glacial acetic acid (1.4 μL, 0.024 mmol). The reaction was complete after 6 h as determined by TLC (silica, CH2Cl2). The mixture was quenched by the addition of saturated aqueous NaHCO3 (2 mL) and ethyl acetate. The organic layer was separated, dried (Na2SO4), and concentrated. The crude product was subjected to column chromatography [silica, CH2Cl2/MeOH (99:1)] to yield a green solid (4.7 mg, 88%). 1H NMR: δ, ppm -2.37 (brs, 1 H), -2.24 (brs, 1 H), 1.96 (s, 12 H), 2.59 (s, 6 H), 4.45 (s, 2 H), 4.50 (s, 2 H), 4.68 (s, 2 H), 8.65 (s, 1 H), 8.69 (m, 1 H), 8.71 (s, 1 H), 8.72 (m, 1 H), 8.73 (m, 1 H), 8.80 (s, 1 H), 8.95 (s, 1H). LD-MS: m/z obsd. 427.9, calcd. 427.2 (C27H33N5). ESI-MS: m/z obsd. 383.2230, calcd. 383.2236 ([M′]+, C25H27N4, where M′ = M - N(CH3)2, M = C27H33N5). UV-vis (CH2Cl2): λabs, nm 341, 366, 490, 718.
3-(trimethylammoniomethyl)-8,8,18,18-tetramethylbacteriochlorin iodide (BC-2′)
Following a procedure for amine quaternization [16, 38], a solution of BC-2 (7.6 mg, 0.018 mmol) in CHCl3 (1 mL, stabilized with EtOH) was treated with MeI (12 μL, 0.18 mmol, 10 equiv) under argon. The mixture was stirred at room temperature for 24 h. Excess methyl iodide and solvent were removed under reduced pressure at ambient temperature. Purification of the crude product was achieved by adding anhydrous diethyl ether to the crude product (5.0 mL). The mixture was sonicated for 2 min in a benchtop sonication bath. The solid was filtered and washed with dichloromethane/hexanes [2 mL (1:3)] to yield the title compound (10 mg, 98%). 1H NMR: δ, ppm -1.55 (brs, 1 H), -1.37 (brs, 1 H), 1.81 (s, 6 H), 1.85 (s, 6 H), 3.69 (s, 9 H), 4.29 (s, 2 H), 4.53 (s, 2 H), 6.25 (s, 2 H), 8.47 (s, 1 H), 8.49 (s, 1 H), 8.57 (s, 1 H), 8.67 (dd, J = 5.36, 1.79 Hz, 2 H), 8.77 (d, J = 2.20 Hz, 1 H), 9.43 (s, 1 H). ESI-MS: m/z obsd. 383.2222, calcd. 383.2236 ([M′]+, C25H27N4, where M′ = M - N(CH3)3, M = C28H36N5). UV-vis (CH2Cl2): λabs, nm 341, 365, 498, 715.
3-[bis(3-(dimethylamino)propyl)aminomethyl]-8, 8,18,18-tetramethylbacteriochlorin (BC-3)
A solution of BC-1 (5.0 mg, 0.012 mmol) in 1,2-dichloroethane (1.0 mL) was treated with bis(3-(dimethylamino)propyl) amine (14 μL, 0.062 mmol). The mixture was stirred at room temperature under argon for 5 min before adding NaBH(OAc)3 (5.0 mg, 0.025 mmol) all at once, followed by glacial acetic acid (1.4 μL, 0.024 mmol). The reaction was complete after 16 h as determined by TLC (silica, CH2Cl2). The mixture was quenched by the addition of saturated aqueous NaHCO3 (2 mL) and ethyl acetate. The organic layer was separated, dried (Na2SO4), and concentrated. The crude product was subjected to column chromatography [silica, CH2Cl2/MeOH (95:5)] to yield a green solid (5.0 mg, 70%). 1H NMR: δ, ppm -2.38 (brs, 1 H), -2.22 (brs, 1 H), 1.90 (m, 4 H), 1.96 (s, 6 H), 1.97 (s, 6 H), 2.20 (s, 12 H), 2.35 (t, J = 7.15 Hz, 4 H), 2.81 (t, J = 7.15 Hz, 4 H), 4.44 (s, 2 H), 4.47 (s, 2 H), 4.81 (s, 2 H), 8.63 (s, 1 H), 8.67 (m, 2 H), 8.70 (s, 1 H), 8.72 (m, 1 H), 8.80 (s, 1 H), 9.04 (s, 1 H). LD-MS: m/z obsd. 570.4. ESI-MS: m/z obsd. 285.7175, calcd. 285.7175 ([M + 2H]2+, M = C35H51N7). UV-vis (CH2Cl2): λabs, nm 342, 367, 491, 718.
3-[bis(3-(trimethylammonio)propyl)aminomethyl]-8,8,18,18-tetramethylbacteriochlorin diiodide (BC-3′)
Under conditions similar to those for BC-2′, BC-3 (5.0 mg) afforded a green solid (6.3 mg, 82%). 1H NMR (DMSO-d6): δ, ppm –2.51 (brs, 1 H), –2.42 (brs, 1 H), 1.93 (s, 6 H), 1.94 (s, 6 H), 2.12 (m, 4 H), 2.82 (m, 4 H), 3.09 (s, 18 H), 3.38 (m, 4 H, overlapped by H2O signal), 4.44 (s, 2 H), 4.48 (s, 2 H), 4.90 (s, 2 H), 8.88 (brs, 1 H), 8.89 (s, 1 H), 8.91 (d, J = 1.65 Hz, 1 H), 8.93 (s, 1 H), 8.95 (s, 1 H), 8.97 (brs, 1 H), 9.07 (s, 1 H). ESI-MS: m/z obsd. 299.7338, calcd. 299.7332 ([M]2+, M = C37H57N7). UV-vis (H2O): λabs, nm 339, 364, 493, 719. UV-vis (CH2Cl2): λabs, nm 342, 367, 491, 719.
3-(dipropylaminomethyl)-8,8,18,18-tetramethylbacteriochlorin (BC-4)
A solution of BC-1 (7.1 mg, 0.018 mmol) in 1,2-dichloroethane (1.0 mL) was treated with dipropylamine (15 μL, 0.090 mmol). The mixture was stirred at room temperature under argon for 5 min before adding NaBH(OAc)3 (10. mg, 0.05 mmol) all at once, followed by glacial acetic acid (1.4 μL, 0.024 mmol). The reaction was complete after 16 h as determined by TLC (silica, CH2Cl2). The mixture was quenched by the addition of saturated aqueous NaHCO3 (2 mL) and ethyl acetate. The organic layer was separated, dried (Na2SO4), and concentrated. The crude product was subjected to column chromatography [silica, CH2Cl2/MeOH (99:1)] to yield a green solid (6.3 mg, 75%). 1H NMR: δ, ppm -2.33 (brs, 1 H), -2.17 (brs, 1 H), 0.95 (t, J = 7.29, 6 H), 1.76 (m, 4 H), 1.96 (s, 6 H), 1.97 (s, 6 H), 2.74 (t, J = 7.29, 4 H), 4.43 (s, 2 H), 4.47 (s, 2 H), 4.82 (s, 2 H), 8.63 (s, 1 H), 8.67 (m, 1 H), 8.69 (s, 2 H), 8.72 (m, 1 H), 8.79 (s, 1 H), 9.04 (s, 1 H). LD-MS: m/z obsd. 483.5, calcd. 483.3 (C231H41N5). ESI-MS: m/z obsd. 383.2230, calcd. 383.2236 ([M′]+, C25H27N4, where M′ = M - N(CH2CH2CH3)2, M = C31H41N5). UV-vis (CH2Cl2): λabs, nm 341, 367, 491, 717.
3-(N-methyl-N,N-dipropylammoniomethyl)-8,8,18,18-tetramethylbacteriochlorin (BC-4′)
Under conditions similar to those for BC-2′, BC-4 (5.0 mg) afforded a green solid (5.0 mg, 80%). 1H NMR: δ, ppm -1.44 (brs, 1 H), -1.28 (brs, 1 H), 1.11 (t, J = 7.15 Hz, 6 H), 1.87 (s, 6 H), 1.89 (s, 6 H), 1.93–2.18 (m, 4 H), 3.43 (s, 3 H), 3.57–3.74 (m, 2 H), 3.74–3.92 (m, 2 H), 4.31 (s, 2 H), 4.54 (s, 2 H), 5.99 (s, 2 H), 8.46 (s, 1 H), 8.51 (s, 1 H), 8.56 (d, J = 1.93 Hz, 1 H), 8.58 (s, 1 H), 8.62–8.74 (m, 2 H), 9.41 (s, 1 H). ESI-MS: m/z obsd. 383.2230, calcd. 383.2236 ([M′]+, C25H27N4, where M′ = M - N[(CH3)(CH2CH2CH3)2], M = C32H44N5). UV-vis (CH2Cl2): λabs, nm 341, 365, 498, 715.
Cell culture
HeLa cancer cells (ATCC Manassas, VA) were cultured in RPMI medium with L-glutamine and NaHCO3 (Sigma, St Louis, MO) supplemented with 10% fetal bovine serum, penicillin/streptomycin (100 U/mL) (Sigma) at 37 °C in 5% CO2-humidified atmosphere in 75 cm2 flasks (Falcon, Invitrogen, Carlsbad, CA). On reaching 80% confluence, the cells were washed with phosphate-buffered saline (PBS) and harvested with 2 mL of 0.25% trypsin-EDTA solution (Sigma). Cells were then centrifuged and counted in Trypan Blue to ensure viability and plated at a density of 10,000/well in flat-bottom 96-well plates (Fisher Scientific, Pittsburgh, PA).
In vitro PDT studies
After 24 h growth the cells were incubated with different concentration of bacteriochlorins diluted in RPMI medium (containing 10% serum, 100 U/mL penicillin and 100 μg/mL streptomycin), and further incubated for an additional 24 h. The cells were then washed twice with PBS, the medium was replaced with fresh complete medium, and 10 J/cm2 of illumination was delivered with a Lumacare lamp (Newport Beach, CA) (700–850 nm range). Control groups were as follows: no bacteriochlorin and light treatment, light alone, and bacteriochlorin alone (at the same dilution used for PDT experiments). For kinetics studies, incubations were carried out with 10 nM of bacteriochlorins BC-2′, BC-3′, and BC-4′ for various times, and subsequently light with 10 J/cm2 of light was delivered.
Biological assays
MTT assay
Following PDT treatment the cells were returned to the incubator. After overnight incubation, the cell-culture media was removed and replaced with 500 μg/mL of MTT diluted in serum-free media. The cells were incubated for 4 h. At the end of incubation the media was removed, and the cells were dissolved in DMSO. The plate was read at 570 nm using a microplate spectrophotometer (Spectra Max 340 PC, Molecular Devices, Sunnyvale, CA). Each experiment was repeated 3 times.
Subcellular localization by fluorescence microscopy
Cells were plated in an optically transparent 96-well black-sided plate and grown for 24 h. The cells were then incubated with 250 nM bacteriochlorins in complete medium for another 24 h. Cells were washed in PBS and 5 μg/mL of (i) LysoTracker green DND-26, (ii) MitoTracker green FM, or (iii) ER-Tracker green (Molecular Probes Invitrogen, Carlsbad, CA) was added and incubated for 30 min at 37 °C. Cells were again washed in PBS and 5–10 min later observed on an Olympus confocal FV1000 microscope and analyzed using the Olympus Fluoview software (Olympus America, Inc. Center Valley, PA). The microscope used excitation with a 488-nm argon laser and emission using either a band pass filter (525 ± 10 nm) or a 580-nm long pass filter and a 63x1.20 NA water immersion lens.
Nuclear condensation studies
The cells after PDT treatment were kept at 37 °C for 24 h and then stained with Hoechst 33342 (0.5 μg/mL). Cells were again washed in PBS and the confocal laser fluorescence microscope was used to image the cells at a resolution of 800 × 800 pixels. The microscope employed excitation with a 405-nm laser and emission using either a band pass filter (470 ± 10 nm) or a 480-nm long pass filter.
Statistical analysis
Each value is the mean of three separate experiments with an error bar that reflects the standard deviation or standard error of the mean for that determination.
CONCLUSION
A de novo synthesis has afforded stable bacteriochlorins that bear a single side-chain containing one or two positive charges. The compounds with one positive charge, BC-2′ and BC-4′, have LD50 values in the low nanomolar range. These values indicate that the two compounds are substantially more efficacious than any of the bacteriochlorins that we have studied previously. Additionally to our knowledge, they appear to be among the most powerful photosensitizers against cancer cells in vitro that have so far been reported. This high activity, relatively low dark toxicity and the ability to be activated by NIR light (which affords excellent tissue penetration), suggest that these bacteriochlorin motifs should be further investigated to mediate PDT in animal models of cancer.
Acknowledgements
This work was supported by a grant from the NIH (R01AI050875 to M.R.H.). M.K. was supported by the Jimmy V NCSU Cancer Therapeutics Training Program. L.H. was supported by a grant (R41AI072854) from the National Institute of Allergy and Infectious Diseases to NIRvana Pharmaceuticals, Inc. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or the NIH. Mass spectra were obtained at the NCSU Department of Chemistry Mass Spectrometry Facility. Funding for the facility was obtained from the North Carolina Biotechnology Center and the NCSU Department of Chemistry.
REFERENCES
- 1.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J. CA Cancer J. Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castano AP, Demidova TN, Hamblin MR. Photodiagn. Photodyn. Ther. 2004;1:279–293. doi: 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castano AP, Demidova TN, Hamblin MR. Photodiagn. Photodyn. Ther. 2005;2:1–23. doi: 10.1016/S1572-1000(05)00030-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castano AP, Demidova TN, Hamblin MR. Photodiagn. Photodyn. Ther. 2005;2:91–106. doi: 10.1016/S1572-1000(05)00060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kessel D, Oleinick NL. Methods Mol. Biol. 2010;635:35–46. doi: 10.1007/978-1-60761-697-9_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Z, Chen Q, Dole KC, Barqawi AB, Chen YK, Blanc D, Wilson BC, Hetzel FW. Photochem. Photobiol. Sci. 2007;6:1318–1324. doi: 10.1039/b705984a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson SRH, Weersink RA, Haider MA, Gertner MR, Bogaards A, Giewercer D, Scherz A, Sherar MD, Elhilali M, Chin JL, Trachtenberg J, Wilson BC. Phys. Med. Biol. 2009;54:2293–2313. doi: 10.1088/0031-9155/54/8/003. [DOI] [PubMed] [Google Scholar]
- 8.Brückner C, Samankumara L, Ogikubo J. In: Handbook of Porphyrin Science. Kadish K, Smith KM, Guilard R, editors. Vol. 17. World Scientific; Singapore: 2012. pp. 1–112. [Google Scholar]
- 9.Grin MA, Mironov AF, Shtil AA. Anti-Cancer Agents Med. Chem. 2008;8:683–697. [PubMed] [Google Scholar]
- 10.Galezowski M, Gryko DT. Curr. Org. Chem. 2007;11:1310–1338. [Google Scholar]
- 11.Chen Y, Li G, Pandey RK. Curr. Org. Chem. 2004;8:1105–1134. [Google Scholar]
- 12.Samankumara L, Wells S, Zeller M, Acuña AM, Röder B, Brückner C. Angew. Chem. Int. Ed. 2012;51:5757–5760. doi: 10.1002/anie.201201124. [DOI] [PubMed] [Google Scholar]
- 13.Pereira MM, Abreu AR, Goncalves NPF, Calvete MJF, Simoes AVC, Monteiro CJP, Arnaut LG, Eusébio ME, Canotilho J. Green Chem. 2012;14:1666–1672. [Google Scholar]
- 14.Kim HJ, Lindsey JS. J. Org. Chem. 2005;70:5475–5486. doi: 10.1021/jo050467y. [DOI] [PubMed] [Google Scholar]
- 15.Taniguchi M, Cramer DL, Bhise AD, Kee HL, Bocian DF, Holten D, Lindsey JS. New J. Chem. 2008;32:947–958. [Google Scholar]
- 16.Ruzié C, Krayer M, Balasubramanian T, Lindsey JS. J. Org. Chem. 2008;73:5806–5820. doi: 10.1021/jo800736c. [DOI] [PubMed] [Google Scholar]
- 17.Krayer M, Ptaszek M, Kim HJ, Meneely KR, Fan D, Secor K, Lindsey JS. J. Org. Chem. 2010;75:1016–1039. doi: 10.1021/jo9025572. [DOI] [PubMed] [Google Scholar]
- 18.Mroz P, Huang YY, Szokalska A, Zhiyentayev T, Janjua S, Nifli AP, Sherwood ME, Ruzié C, Borbas KE, Fan D, Krayer M, Balasubramanian T, Yang E, Kee HL, Kirmaier C, Diers JR, Bocian DF, Holten D, Lindsey JS, Hamblin MR. FASEB J. 2010;24:3160–3170. doi: 10.1096/fj.09-152587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang YY, Mroz P, Zhiyentayev T, Sharma SK, Balasubramanian T, Ruzié C, Krayer M, Fan D, Borbas KE, Yang E, Kee HL, Kirmaier C, Diers JR, Bocian DF, Holten D, Lindsey JS, Hamblin MR. J. Med. Chem. 2010;53:4018–4027. doi: 10.1021/jm901908s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang L, Huang YY, Mroz P, Tegos GP, Zhiyentayev T, Sharma SK, Lu Z, Balasubramanian T, Krayer M, Ruzié C, Yang E, Kee HL, Kirmaier C, Diers JR, Bocian DF, Holten D, Lindsey JS, Hamblin MR. Antimicrob. Agents Chemother. 2010;54:3834–3841. doi: 10.1128/AAC.00125-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Losev A, Mauzerall D. Photochem. Photobiol. 1983;38:355–361. [Google Scholar]
- 22.Bonnett R, Nizhnik AN, White SG. J. Photochem. Photobiol. B: Biol. 1990;6:29–37. doi: 10.1016/1011-1344(90)85071-4. [DOI] [PubMed] [Google Scholar]
- 23.Taima H, Okubo A, Yoshioka N, Inoue H. Tetrahedron Lett. 2005;46:4161–4164. [Google Scholar]
- 24.Nazarova A, Ignatova A, Feofanov A, Karmakova T, Pljutinskaya A, Mass O, Grin M, Yakubovskaya R, Mironov A, Maurizot JC. Photochem. Photobiol. Sci. 2007;6:1184–1196. doi: 10.1039/b706921a. [DOI] [PubMed] [Google Scholar]
- 25.Pereira MM, Monteiro CJP, Simoes AV, Pinto SMA, Arnaut LG, Sa GFF, Silva EFF, Rocha LB, Simoes S, Formosinho SJ. J. Porphyrins Phthalocyanines. 2009;13:567–573. [Google Scholar]
- 26.Huang L, Zhiyentayev T, Xuan Y, Azhibek D, Kharkwal GB, Hamblin MR. Lasers Surg. Med. 2011;43:313–323. doi: 10.1002/lsm.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li JZ, Wang JJ, Yoon I, Cui BC, Shim YK. Bioorg. Med. Chem. Lett. 2012;22:1846–1849. doi: 10.1016/j.bmcl.2012.01.088. [DOI] [PubMed] [Google Scholar]
- 28.Mironov AF, Grin MA, Tsiprovskii AG, Titeev RA, Nizhnik EA, Lonin IS. Mendeleev Commun. 2004:204–207. [Google Scholar]
- 29.Meerovich IG, Kubasova IY, Oborotova NA, Meerovich GA, Demura SA, Brandis A, Rosenbach-Belkin V, Baryshnikov AY, Scherz A. Proc. SPIE. 2005;5973:59730G1–G11. [Google Scholar]
- 30.Ashur I, Goldschmidt R, Pinkas I, Salomon Y, Szewczyk G, Sarna T, Scherz A. J. Phys. Chem. A. 2009;113:8027–8037. doi: 10.1021/jp900580e. [DOI] [PubMed] [Google Scholar]
- 31.Mironov AF, Grin MA, Tsiprovskii AG, Segenevich AV, Dzardanov DV, Golovin KV, Tsygankov AA, Shim YK. Russ. J. Bioorg. Chem. 2003;29:190–197. doi: 10.1023/a:1023272718636. [DOI] [PubMed] [Google Scholar]
- 32.Dabrowski JM, Arnaut LG, Pereira MM, Monteiro CJ, Urbanska K, Simoes S, Stochel G. ChemMedChem. 2010;5:1770–1780. doi: 10.1002/cmdc.201000223. [DOI] [PubMed] [Google Scholar]
- 33.Dabrowski JM, Urbanska K, Arnaut LG, Pereira MM, Abreu AR, Simoes S, Stochel G. ChemMedChem. 2011;6:465–475. doi: 10.1002/cmdc.201000524. [DOI] [PubMed] [Google Scholar]
- 34.Dabrowski JM, Arnaut LG, Pereira MM, Urbanska K, Simoes S, Stochel G, Cortes L. Free Radic. Biol. Med. 2012;52:1188–1200. doi: 10.1016/j.freeradbiomed.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 35.Yu Z, Ptaszek M. Org. Lett. 2012 doi: 10.1021/ol3015545. DOI 10.1021/ol3015545. [DOI] [PubMed] [Google Scholar]
- 36.Alexander VM, Sano K, Yu Z, Nakajima T, Choyke PL, Ptaszek M, Kobayashi H. Bioconjugate Chem. 2012 doi: 10.1021/bc3002419. DOI 10.1021/bc3002419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdel-Magid AF, Carson KG, Harris BD, Maryanoff CA, Shah RD. J. Org. Chem. 1996;61:3849–3862. doi: 10.1021/jo960057x. [DOI] [PubMed] [Google Scholar]
- 38.Okada E, Tone H, Tsukushi N, Otsuki Y, Takeuchi H, Hojo M. Heterocycles. 1997;45:339–345. [Google Scholar]
- 39.Reddi E, Ceccon M, Valduga G, Jori G, Bommer JC, Elisei F, Latterini L, Mazzucato U. Photochem. Photobiol. 2002;75:462–470. doi: 10.1562/0031-8655(2002)075<0462:ppaaao>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 40.Orlandi VT, Caruso E, Banfi S, Barbieri P. Photochem. Photobiol. 2012;88:557–564. doi: 10.1111/j.1751-1097.2012.01122.x. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi M, Akiyama M, Kano H, Kise H. In: Chlorophylls and Bacteriochlorophylls — Biochemistry, Biophysics, Functions and Applications. Grimm B, Porra RJ, Rüdiger W, Scheer H, editors. Vol. 25. Springer; Dordrecht, The Netherlands: 2006. pp. 79–94. [Google Scholar]
- 42.Yang E, Kirmaier C, Krayer M, Taniguchi M, Kim HJ, Diers JR, Bocian DF, Lindsey JS, Holten D. J. Phys. Chem. B. 2011;115:10801–10816. doi: 10.1021/jp205258s. [DOI] [PubMed] [Google Scholar]
- 43.Borbas KE, Chandrashaker V, Muthiah C, Kee HL, Holten D, Lindsey JS. J. Org. Chem. 2008;73:3145–3158. doi: 10.1021/jo7026728. [DOI] [PubMed] [Google Scholar]
- 44.Kee HL, Bhaumik J, Diers JR, Mroz P, Hamblin MR, Bocian DF, Lindsey JS, Holten D. J. Photochem. Photobiol. A: Chem. 2008;200:346–355. doi: 10.1016/j.jphotochem.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tejedor-Estrada R, Nonell S, Teixido J, Sagrista ML, Mora M, Villanueva A, Canete M, Stockert JC. Curr. Med. Chem. 2012;19:2472–2482. doi: 10.2174/092986712800269290. [DOI] [PubMed] [Google Scholar]
- 46.Oliveira CS, Turchiello R, Kowaltowski AJ, Indig GL, Baptista MS. Free Radic. Biol. Med. 2011;51:824–833. doi: 10.1016/j.freeradbiomed.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 47.Teiten MH, Bezdetnaya L, Morlière P, Santus R, Guillemin F. Br. J. Cancer. 2003;88:146–152. doi: 10.1038/sj.bjc.6600664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Francois A, Marchal S, Guillemin F, Bezdetnaya L. Int. J. Oncol. 2011;39:1537–1543. doi: 10.3892/ijo.2011.1174. [DOI] [PubMed] [Google Scholar]
- 49.Shahzidi S, Cunderlikova B, Wiedlocha A, Zhen Y, Vasovic V, Nesland JM, Peng Q. Photochem. Photobiol. Sci. 2011;10:1773–1782. doi: 10.1039/c1pp05169e. [DOI] [PubMed] [Google Scholar]
- 50.Pogue BW, Ortel B, Chen N, Redmond RW, Hasan T. Cancer Res. 2001;61:717–724. [PubMed] [Google Scholar]
- 51.Haywood-Small SL, Vernon DI, Griffiths J, Schofield J, Brown SB. Biochem. Biophys. Res. Commun. 2006;339:569–576. doi: 10.1016/j.bbrc.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 52.Marchal S, François A, Dumas D, Guillemin F, Bezdetnaya L. Br. J. Cancer. 2007;96:944–951. doi: 10.1038/sj.bjc.6603631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacDonald IJ, Morgan J, Bellnier DA, Paszkiewicz GM, Whitaker JE, Litchfield DJ, Dougherty TJ. Photochem. Photobiol. 1999;70:789–797. [PubMed] [Google Scholar]
- 54.Wood SR, Holroyd JA, Brown SB. Photochem. Photobiol. 1997;65:397–402. doi: 10.1111/j.1751-1097.1997.tb08577.x. [DOI] [PubMed] [Google Scholar]
- 55.Buytaert E, Dewaele M, Agostinis P. Biochim. Biophys. Acta. 2007;1776:86–107. doi: 10.1016/j.bbcan.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 56.Dewaele M, Verfaillie T, Martinet W, Agostinis P. Methods Mol. Biol. 2010;635:7–33. doi: 10.1007/978-1-60761-697-9_2. [DOI] [PubMed] [Google Scholar]
- 57.Hügli D, Seiffert W, Zimmermann HW. J. Photochem. Photobiol. B: Biol. 1995;31:145–158. doi: 10.1016/1011-1344(95)07191-1. [DOI] [PubMed] [Google Scholar]
- 58.Kessel D, Luguya R, Vicente MGH. Photochem. Photobiol. 2003;78:431–435. doi: 10.1562/0031-8655(2003)078<0431:lapeot>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 59.Dror SB, Bronshtein I, Garini Y, O'Neal WG, Jacobi PA, Ehrenberg B. Photochem. Photobiol. Sci. 2009;8:354–361. doi: 10.1039/b814970d. [DOI] [PubMed] [Google Scholar]
- 60.Srinivasan N, Haney CA, Lindsey JS, Zhang W, Chait BT. J. Porphyrins Phthalocyanines. 1999;3:283–291. [Google Scholar]