Abstract
Four new labdane-type diterpenoids: hedychicoronarin (1), peroxycoronarin D (2), 7β-hydroxycalcaratarin A (3), and (E)-7β-hydroxy-6-oxo-labda-8(17),12-diene-15,16-dial (4), have been isolated from the rhizomes of Hedychium coronarium, together with 13 known compounds (5–17). The structures of these new compounds were determined through spectroscopic and MS analyses. Compounds 3, 5, 6, and 10 exhibited inhibition (IC50 values ≤4.52 μg/mL) of superoxide anion generation by human neutrophils in response to formyl-L-methionyl-L-leucyl-L-phenylalanine/cytochalasin B (fMLP/CB). Compounds 3–6, 10, and 11 inhibited fMLP/CB-induced elastase release with IC50 values ≤6.17 μg/mL.
Keywords: Hedychium coronarium, Zingiberaceae, labdane-type diterpenoid, anti-inflammatory activity
1. Introduction
Hedychium coronarium Koenig (Zingiberaceae) is a perennial herb distributed in India, Southeast Asian countries, southern China, Japan, and Taiwan [1]. H. coronarium, popularly called “White Butterfly Flower” or “Butterfly Ginger”, is used as a folk medicine for treatment of headache, contusion, inflammation, insomnia, stomach disorders, and sharp pain due to rheumatism in China [2,3].
Labdane-type diterpenes [3–9], farnesane-type sesquiterpenes [2], and their derivatives were isolated from this plant in previous studies. Many of these compounds were found to exhibit antiallergic [2], cytotoxic [3,4,9], anti-inflammatory [6,7], and hepatoprotective [8] activities. Granule proteases (e.g., elastase, cathepsin G, and proteinase-3) and reactive oxygen species (ROS) (e.g., superoxide anion (O2•−) and hydrogen peroxide) produced by human neutrophils are involved in the pathogenesis of a variety of inflammatory diseases.
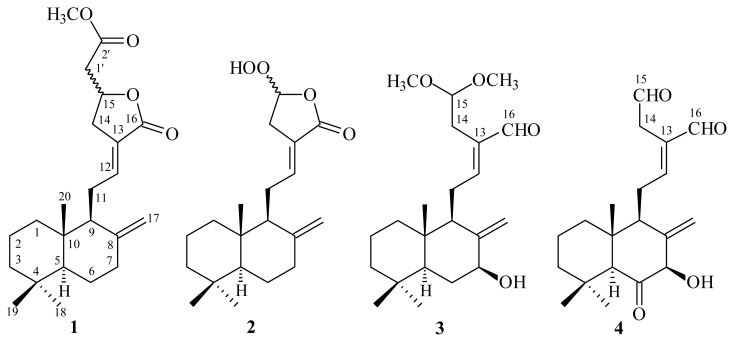
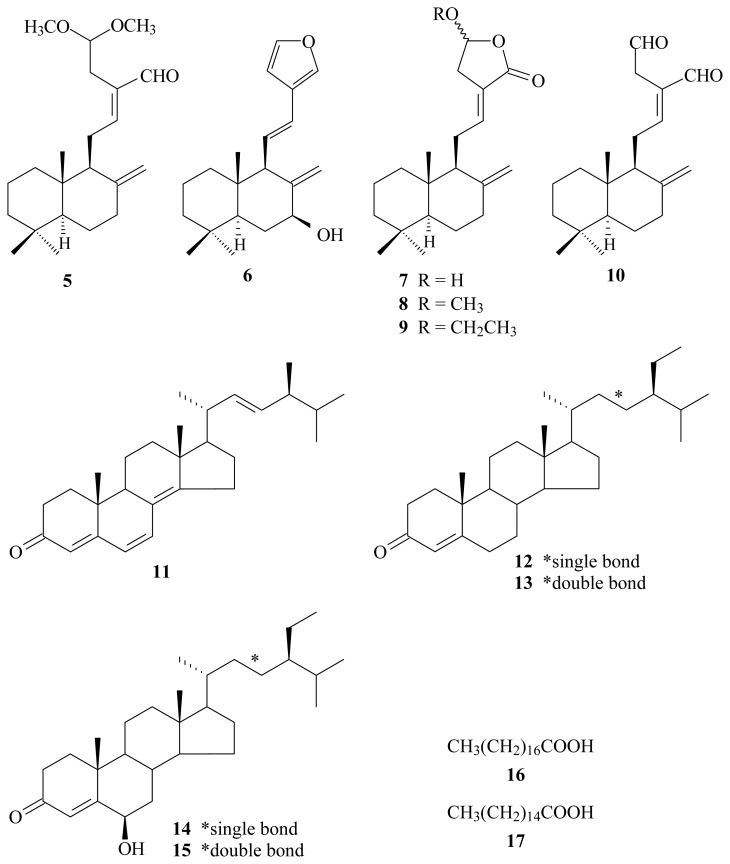
In our studies on the anti-inflammatory constituents of Formosan plants, many species have been screened for in vitro inhibitory activity on neutrophil pro-inflammatory responses, and H. coronarium has been found to be an active species. The MeOH extract of the rhizomes of H. coronarium showed potent inhibitory effects on superoxide anion generation and elastase release by human neutrophils in response to formyl-l-methionyl-l-leucyl-l-phenylalanine/cytochalasin B (fMLP/CB). Figure 1 illustrates the structures of four new labdane-type diterpenoids: hedychicoronarin (1), peroxycoronarin D (2), 7β-hydroxycalcaratarin A (3), and (E)-7β-hydroxy-6-oxo-labda-8(17),12-diene-15,16-dial (4). Thirteen known compounds (5–17), have been isolated and identified from the rhizomes of H. coronarium and their structures are depicted in Figure 2.
Figure 1.
The chemical structures of new compounds 1–4 isolated from H. coronarium.
Figure 2.
The chemical structures of known compounds 5–17 isolated from H. coronarium.
This paper describes the structural elucidation of the compounds numbered 1 through 4, and the inhibitory activities of all isolates on superoxide generation and elastase release by neutrophils.
2. Results and Discussion
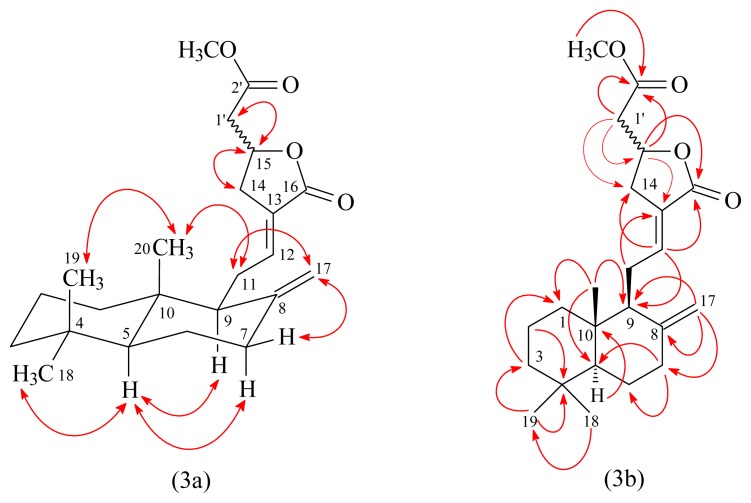
Hedychicoronarin (1) was isolated as an optically active colorless oil ([α]D25 = +13.8) with molecular formula C23H34O4 as determined by positive-ion HRESIMS, showing an [M + Na]+ ion at m/z 397.2353 (calcd for C23H34O4Na: 397.2355). The presence of two carbonyl groups was revealed by the bands at 1746 and 1757 cm−1 in the IR spectrum, and was confirmed by the resonances at δ 170.0 and 170.3 in the 13C-NMR spectrum. The 1H- and 13C-NMR spectra of 1 showed signals assignable to a methoxy [δ 3.72 (3H, s)], three methyls [δ 0.71, 0.81, 0.88 (each 3H, each s, H-20, 19, and 18)], an exo-methylene [δ 4.35 (1H, br s, H-17), 4.81 (1H, d, J = 1.2 Hz, H-17)], and an olefin [δ 6.72 (1H, m, H-12)], together with eight methylenes (H2-1, 2, 3, 6, 7, 11, 14, and 1′), two methines (H-5 and 9), an oxymethine (H-15), and six quaternary carbons (C-4, 8, 10, 13, 16, and 2′). The 1H- and 13C-NMR data of 1 was similar to those of coronarin D methyl ether (8) [10], except that the 2-methoxy-2-oxoethyl group [δH 2.64 (1H, dd, J = 16.0, 7.2 Hz, H-1’a), 2.85 (1H, dd, J = 16.0, 6.4 Hz, H-1’b), and 3.72 (3H, s, OMe-2′); δC 40.6 (C-1′), 52.0 (OMe), and 170.0 (C-2′)] at C-15 of 1 replaced the 15-methoxy group [δH 3.52/3.53 (3H, s, OMe-15); δC 56.54 (OMe-15)] of 8. This was supported by (i) HMBC correlation observed between H-1′ (δ 2.64, 2.85) and C-14 (δ 31.3), C-15 (δ 73.0), and C-2′ (δ 170.0); and (ii) HMBC correlation observed between OMe-2′ (δ 3.72) and C-2′ (δ 170.0). The relative stereochemistry of 1 was elucidated on the basis of NOESY experiments (Figure 3). The NOESY cross-peaks between H-5/H-7α, H-5/H-9, H-5/H3-18, H2-11/H3-20, and H3-19/H3-20 suggested that H-5, H-7α, H-9, and H3-18 are α-oriented, and H3-19 and H3-20 are β-oriented. The occurrence of epimers of labdane diterpenes at C-15 position has been previously reported [3,10–12]. These labdane diterpenes with C-15 substituent were usually isolated as C-15 epimeric mixtures, which could not be separated. The presence of duplicated resonances of 13C-NMR signals of 1 at C-7 (δ 37.74/37.76), C-8 (δ 148.03/148.06), C-9 (δ 56.12/56.15), C-10 (δ 39.40/39.41), C-12 (δ 143.16/143.18), and C-17 (δ 107.36/107.43) as in the cases of coronarin D [10] and coronarin D methyl ether [10] suggested that it was isolated as a C-15 epimeric mixture. On the basis of the evidence above, the structure of 1 was elucidated as methyl 2-((E)-5-oxo-4-(2-((1S,8aS)-5,5,8atrimethyl- 2-methylenedecahydronaphthalen-1-yl)ethylidene)tetrahydrofu-ran-2-yl)acetate, named hedychicoronarin. This was further confirmed by 1H–1H COSY and NOESY experiments (Figure 3). The assignment of 13C-NMR resonances was confirmed by DEPT, HSQC and HMBC techniques (Figure 3).
Figure 3.
Key NOESY (3a) and HMBC (3b) correlations of 1.
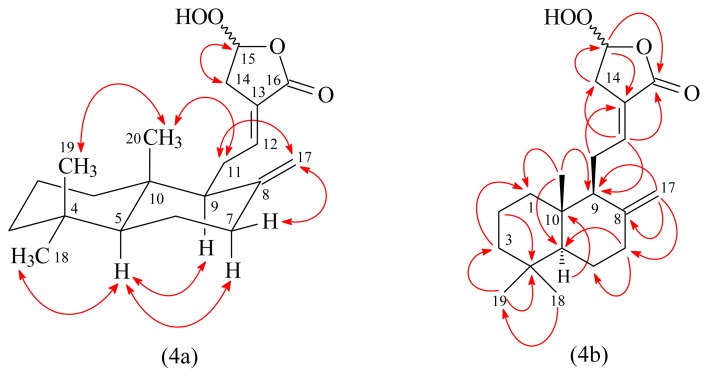
Peroxycoronarin D (2) was obtained as an optically active colorless oil ([α]D25 = +15.8). Its molecular formula, C20H30O4, was determined on the basis of the negative HRESIMS at m/z 333.2057 [M–H]− (calcd 333.2066) and supported by the 1H, 13C, and DEPT NMR data. The IR spectrum showed the presence of OH (3413 cm−1), exo-methylene (3081, 1643, 889 cm−1), C=C (1678 cm−1), and carbonyl (1762 cm−1) groups. The 1H-NMR spectrum of 2 showed three methyl signals at δH 0.71, 0.81, and 0.88 (each 3H, each s, H-20, H-19, and H-18) and an exomethylene group at δH 4.41 and 4.82 (each 1H, each d, J = 1.2 Hz). These were characteristic of C-8 exomethylene labdane diterpenoids [13]. The 1H- and 13C-NMR data of 2 were similar to coronarin D (7) [10], except that the 15-hydroperoxy group of 2 replaced 15-hydroxy group of coronarin D (7). This was supported by (i) the MS (molecular weight of 2 was m/z +16 (O) more than coronarin D); (ii) the chemical shifts of C-15 (δC 100.4) of 2 appeared at relatively low field [C-15 (δC 95.9) of coronarin D], due to the electron-withdrawing effect of 15-OOH group of 2; and (iii) the HMBC correlation (Figure 4) between H-15 (δH 5.61) and C-13 (δC 124.4), C-14 (δC 33.2), and C-16 (δC 169.9). However, the 1H- and 13C-NMR data of 2 showed two sets of signals at H-15 (δH 5.59/5.61), H2-17 (δH 4.34/4.41 and 4.80/4.82) and C-1 (δC 39.21/39.26), C-4 (δC 33.29/33.31), C-6 (δC 24.05/24.07), C-8 (δC 147.81/148.09), C-12 (δC 142.56/142.73), C-14 (δC 33.12/33.17), C-17 (δC 107.31/107.64), and C-20 (δC 14.30/14.34), which suggest that it was isolated as a C-15 epimeric mixture [3,10–12]. In addition, the relative stereostructure of 2 was elucidated by NOESY experiment, in which the NOESY correlations (Figure 4) were observed between the following proton pairs (H-5/H-7α, H-5/H-9, H-5/H3-18, H2-11/H3-20, and H3-19/H3-20). The full assignment of 1H and 13C-NMR resonances was confirmed by 1H–1H COSY, NOESY (Figure 4), DEPT, HSQC, and HMBC (Figure 4) techniques. Based on the data above, the structure of 2 was elucidated as (E)-5-hydroperoxy-3-(2-((1S,4aS,8aS)-5,5,8a-trimethyl-2-methylenedecahydronaphthalen-1-yl)ethylidene)dihydrofuran-2(3H)-one, named peroxycoronarin D.
Figure 4.
Key NOESY (4a) and HMBC (4b) correlations of 2.
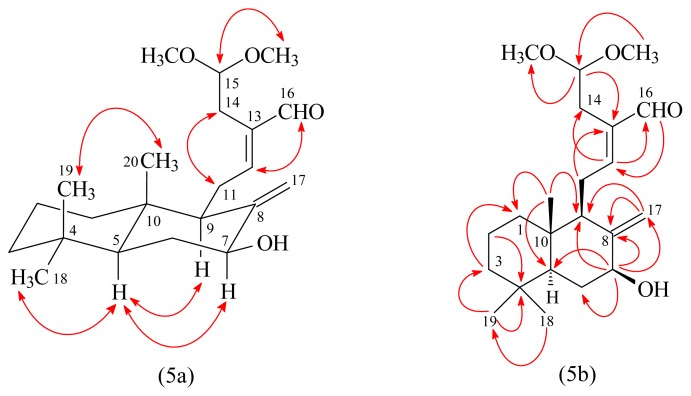
7β-Hydroxycalcaratarin A (3) was isolated as an optically active colorless oil ([α]D25 +11.9). The molecular formula C22H36O4 was deduced from a sodium adduct ion at m/z 387.2513 [M + Na]+ (calcd 387.2511) in the HRESI mass spectrum. The presence of hydroxy and carbonyl groups was revealed by the bands at 3429 and 1683 cm−1, respectively, in the IR spectrum. The 1H-NMR data of 3 was similar to those of calcaratarin A (5) [14], except that the 7β-hydroxy group of 3 replaced H-7β [δ 2.39 (1H, ddd, J = 12.9, 4.2, 2.4 Hz)] of calcaratarin A (5). This was supported by (i) the chemical shifts of H-7α (δH 4.00) and C-7 (δC 73.6) appeared at relatively low field, due to the electron-withdrawing effect of 7β-OH group; and (ii) the HMBC correlation (Figure 5) between H-7α (δH 4.00) and C-5 (δC 53.0), C-8 (δC 150.1), C-9 (δC 54.4), and C-17 (δC 104.4). The NOESY correlations between H-11 and H-14 and between H-12 and H-16 suggested 12E-configuration of 3. The relative stereochemistry of 3 at chiral centers was based on the analysis of NOESY spectrum, which displayed NOESY correlations (Figure 5) between H-5/H-7, H-5/H-9, H-5/H3-18, and H3-19/H3-20 and suggested that H-5, H-7, H-9, and H3-18 are α-oriented, and H3-19, H3-20, and OH-7 are β-oriented. The structure elucidation of 3 was supported by 1H–1H COSY and NOESY (Figure 5) experiments, and 13C NMR assignments were confirmed by DEPT, HSQC, and HMBC (Figure 5) techniques.
Figure 5.
Key NOESY (5a) and HMBC (5b) correlations of 3.
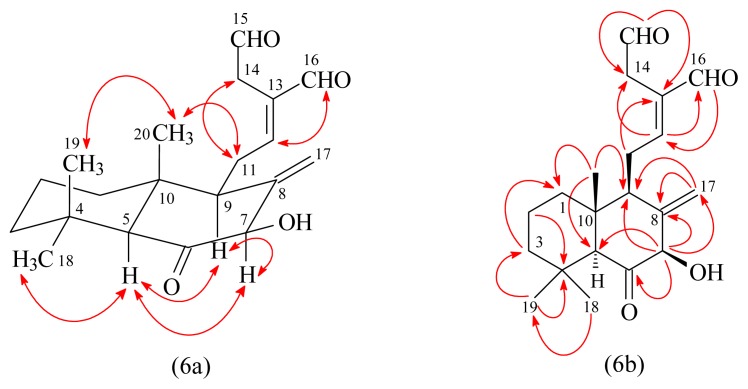
(E)-7β-Hydroxy-6-oxo-labda-8(17),12-diene-15,16-dial (4) had the molecular formula C20H28O4, as indicated by the sodiated HRESIMS ion peak at m/z = 355.1882 [M + Na]+ (calcd for C20H28O4Na, 355.1885). IR absorption for a hydroxy function (3485 cm−1) was observed. The presence of three carbonyl groups was revealed by the bands at 1684, 1716, and 1728 cm−1 in the IR spectrum, which was confirmed by the resonances at δ 193.4, 197.2, and 207.9 in the 13C-NMR spectrum. The 1H-NMR spectrum of 4 showed three methyl signals at δ 0.68 (3H, s, H3-20), 1.00 (3H, s, H3-19), and 1.26 (3H, s, H3-18), and an exomethylene moiety at δ 4.67, 5.44 (each 1H, each br s, H2-17), which were characteristic of a labdane-type diterpenoid. Comparison of the 1H and 13C-NMR data of 4 with those of (E)-7β-hydroxy-labda-8(17),12-diene-15,16-dial [3] suggested that their structures were closely related except that the C-6 carbonyl group [δC 207.9] of 4 replaced the C-6 methylene group [δH 2.12 (1H, m, H-6α), 1.29 (1H, br t, J = 12.0 Hz, H-6β) and δC 33.7 (C-6)] of (E)-7β-hydroxy-labda-8(17),12-diene-15,16-dial. This was supported by HMBC correlation (Figure 6) observed between H-5 (δ 2.26) and C-6 (δ 207.9) and between H-7 (δ 4.50) and C-6 (δ 207.9). The NOESY correlation of the signal of the formyl group at δH 9.40 (H-16) and the olefinic signal at δH 6.75 (H-12) indicated this double bond to be in the E-configuration. The relative stereochemistry of 4 was elucidated on the basis of NOESY experiments (Figure 6). The NOESY cross-peaks between H-5/H-7, H-5/H-9, H-7/H-9, H-5/H3-18, and H3-19/H3-20 suggested that H-5, H-7, H-9, and H3-18 are α-oriented, and H3-19, H3-20, and OH-7 are β-oriented. The structure elucidation of 4 was further confirmed by 1H–1H COSY, NOESY (Figure 6), DEPT, HSQC, and HMBC experiments (Figure 6).
Figure 6.
Key NOESY (6a) and HMBC (6b) correlations of 4.
The known isolates were readily identified by a comparison of physical and spectroscopic data (UV, IR, 1H NMR, [α]D, and MS) with corresponding authentic samples or literature values, and this included six labdane-type diterpenoids, calcaratarin A (5) [14], coronarin A (6) [4], coronarin D (7) [4,10], coronarin D methyl ether (8) [15], coronarin D ethyl ether (9) [16], and (E)-labda-8(17),12-diene-15,16-dial (10) [4,5], five steroids, ergosta-4,6,8(14),22-tetraen-3-one (11) [17], a mixture of β-sitostenone (12) and β-stigmasta-4,22-dien-3-one (13) [18], and a mixture of 6β-hydroxystigmast-4-en-3-one (14) and 6β-hydroxystigmasta-4,22-dien-3-one (15) [19], and a mixture of stearic acid (16) and palmitic acid (17) [20].
Human neutrophils are known to play crucial roles in host defence against microorganisms and in pathogenesis of different diseases, such as rheumatoid arthritis, chronic obstructive pulmonary disease (COPD), asthma, and ischemia-reperfusion injury [21–24]. In response to diverse stimuli, activated neutrophils secrete a series of cytotoxins, such as the superoxide anion radical (O2•−), a precursor to other reactive oxygen species (ROS), granule proteases, bioactive lipids, and neutrophil elastase, a major contributor to destruction of tissue in chronic inflammatory disease [21,25,26]. Suppression of the extensive or inappropriate activation of neutrophils by drugs has been proposed as a method to ameliorate inflammatory diseases. The effects on neutrophil pro-inflammatory responses of compounds isolated from the rhizomes of H. coronarium were evaluated by suppressing fMet-Leu-Phe/cytochalasin B (fMLP/CB)-induced superoxide anion (O2•−) generation and elastase release by human neutrophils. The inhibitory activity data on neutrophil pro-inflammatory responses are summarized in Table 1. LY294002, a phosphatidylinositol-3-kinase inhibitor, was used as a positive control for superoxide anion generation and elastase release. Diphenyleneiodonium, an NADPH oxidase inhibitor, was used as a positive control for superoxide anion generation. From the results of our biological tests, the following conclusions can be drawn: (a) 7β-Hydroxycalcaratarin A (3), calcaratarin A (5), coronarin A (6), and (E)-labda-8(17),12-diene-15,16-dial (10) displayed potent inhibition (IC50 ≤ 4.52 μg/mL) of superoxide anion (O2•−) generation by human neutrophils in response to fMLP/CB. (b) 7β-Hydroxycalcaratarin A (3), (E)-7β-hydroxy-6-oxo-labda-8(17),12-diene-15,16-dial (4), calcaratarin A (5), coronarin A (6), (E)-labda-8(17),12-diene-15,16-dial (10), and ergosta-4,6,8(14),22-tetraen-3-one (11) exhibited potent inhibition (IC50 ≤ 6.17 μg/mL) against fMLP-induced elastase release. (c) Calcaratarin A (5), with 14-dimethoxymethyl group, exhibited more effective inhibition than its analogue, (E)-labda-8(17),12-diene-15,16-dial (10) (with 14-formyl group) against fMLP-induced O2•− generation and elastase release. (d) Calcaratarin A (5), without any substituent at C-7, exhibited more effective inhibition than its analogue, 7β-hydroxycalcaratarin A (3) (with 7β-hydroxy group) against fMLP-induced O2•− generation and elastase release. (e) (E)-Labda-8(17),12-diene-15,16-dial (10), without any substituent at C-6 and C-7, showed more effective inhibition than its analogue, (E)-7β-hydroxy-6-oxo-labda-8(17),12-diene-15,16-dial (4) (with 7β-hydroxy-6-oxo group) against fMLP-induced O2•− generation and elastase release. (f) Among the labdane-type diterpenoid analogues (1, 2, and 6–9), coronarin A (6) (with a hydroxy group at C-7 and a furan-3-yl group at C-12) exhibited more effective inhibition than its analogues, 1, 2, and 7–9 (without any substituent at C-7 and with a 2-oxo-5-substituted-tetrahydrofuran-3-yl group at C-12) against fMLP-induced O2•− generation and elastase release. (g) Calcaratarin A (5) displayed the most effective among the isolates, with IC50 values of 2.25 ± 0.42 and 2.36 ± 0.41 μg/mL, respectively, against fMLP-induced O2•− generation and elastase release. Our study suggests H. coronarium and its isolates (especially 3, 5, 6, and 10) could be further developed as potential candidates for the treatment or prevention of various inflammatory diseases. Thus, the detailed mechanism of action of these compounds appears worthy of further investigation.
Table 1.
Inhibitory effects of compounds 1–17 from the rhizome of H. coronarium on superoxide radical anion generation and elastase release by human neutrophils in response to fMet-Leu-Phe/cytochalasin B a.
| Compounds | Superoxide anion | Elastase |
|---|---|---|
| IC50 (μg/mL) b | IC50 (μg/mL) b | |
| Hedychicoronarin (1) | >10 | >10 |
| Peroxycoronarin D (2) | >10 | >10 |
| 7β-Hydroxycalcaratarin A (3) | 4.52 ± 1.14 e | 3.86 ± 0.98 g |
| (E)-7β-Hydroxy-6-oxo-labda-8(17),12-diene-15,16-dial (4) | >10 | 5.36 ± 1.23 |
| Calcaratarin A (5) | 2.25 ± 0.42 e | 2.36 ± 0.41 e |
| Coronarin A (6) | 3.31 ± 2.41 | 2.67 ± 1.68 |
| Coronarin D (7) | >10 | >10 |
| Coronarin D methyl ether (8) | >10 | >10 |
| Coronarin D ethyl ether (9) | >10 | >10 |
| (E)-Labda-8(17),12-diene-15,16-dial (10) | 3.21 ± 1.24 | 3.70 ± 1.18 f |
| Ergosta-4,6, 8(14),22-tetraen-3-one (11) | >10 | 6.17 ± 1.30 e |
| Mixture of β-sitostenone (12) and β-stigmasta-4,22-dien-3-one (13) | >10 | >10 |
| Mixture of 6β-hydroxystigmast-4-en-3-one (14) and 6β-hydroxystigmasta-4,22-dien-3-one (15) | >10 | >10 |
| Mixture of stearic acid (16) and palmitic acid (17) | >10 | >10 |
| LY294002 c | 0.36 ± 0.04 | 0.63 ± 0.07 |
| Diphenyleneiodonium d | 0.55 ± 0.24 |
Results are presented as averages ± SEM (n = 4).
Concentration necessary for 50% inhibition (IC50).
LY294002, a phosphatidylinositol-3-kinase inhibitor, was used as a positive control for superoxide anion generation and elastase release.
Diphenyleneiodonium, an NADPH oxidase inhibitor, was used as a positive control for superoxide anion generation.
p < 0.05 compared with the control.
p < 0.01 compared with the control.
p < 0.001 compared with the control.
3. Experimental Section
3.1. General Experimental Procedures
Melting points were determined on a Yanaco micro-melting point apparatus and were uncorrected. Optical rotations were measured using a Jasco DIP-370 polarimeter in CHCl3. Ultraviolet (UV) spectra were obtained on a Jasco UV-240 spectrophotometer. Infrared (IR) spectra (neat or KBr) were recorded on a Perkin Elmer 2000 FT-IR spectrometer. Nuclear magnetic resonance (NMR) spectra, including correlation spectroscopy (COSY), nuclear Overhauser effect spectrometry (NOESY), heteronuclear multiple-bond correlation (HMBC), and heteronuclear single-quantum coherence (HSQC) experiments, were acquired using a Varian Unity 400 or a Varian Unity Plus-600 spectrometer operating at 400 or 600 MHz (1H) and 100 or 150 MHz (13C), respectively, with chemical shifts given in ppm (δ) using tetramethylsilane (TMS) as an internal standard. Electrospray ionisation (ESI) and high-resolution electrospray ionization (HRESI)-mass spectra were recorded on a Bruker APEX II or a VG Platform Electrospray ESI/MS mass spectrometer. Silica gel (70–230, 230–400 mesh, Merck) was used for column chromatography (CC). Silica gel 60 F-254 (Merck, Darmstadt, Germany) was used for thin-layer chromatography (TLC) and preparative thin-layer chromatography (PTLC).
3.2. Plant Material
The rhizomes of H. coronarium were collected from Taitung District Agricultural Research and Extension Station, Taitung County, Taiwan, in June 2009 and identified by J. F. Chen. A voucher specimen (Chen 3011) was deposited in the Department of Pharmacy, Tajen University, Pingtung, Taiwan.
3.3. Extraction and Isolation
The dried rhizomes (6.2 kg) of H. coronarium were extracted three times with MeOH (30 L each) for 3 days. The MeOH extracts were concentrated under reduced pressure at 35 °C, and the residue (638 g) was partitioned between n-hexane and H2O (1:1). The n-hexane layer was concentrated to give a residue (fraction A, 112 g). The water layer was further extracted with n-BuOH, and the n-BuOH-soluble part (fraction B, 245 g) and the water-solubles (fraction C, 258 g) were separated. Fraction A (112 g) was chromatographed on silica gel (70–230 mesh, 5.2 kg), eluting with n-hexane, gradually increasing the polarity with EtOAc and MeOH to give 11 fractions: A1 (2 L, n-hexane), A2 (2 L, n-hexane/EtOAc, 50:1), A3 (2 L, n-hexane/EtOAc, 30:1), A4 (3 L, n-hexane/EtOAc, 10:1), A5 (6 L, n-hexane/EtOAc, 5:1), A6 (3 L, n-hexane/EtOAc, 3:1), A7 (6 L, n-hexane/EtOAc, 1:1), A8 (5 L, n-hexane/EtOAc, 1:2), A9 (2 L, n-hexane/EtOAc, 1:4), A10 (5 L, EtOAc), A11 (2 L, MeOH). Fraction A4 (9.4 g) was separated by column chromatography on silica gel (230–400 mesh, 395 g) eluting with CHCl3/acetone (20:1) to yield 10 fractions (A4-1–A4-10). Fraction A4-4 (155 mg) was purified by preparative TLC (silica gel, n-hexane/EtOAc, 6:1) to afford a mixture of 12 and 13 (7.4 mg) (Rf = 0.45). Fraction A4-5 (410 mg) was separated by MPLC (silica gel column, n-hexane/acetone, 8:1) to give 8 fractions (each 150 mL, A4-5-1–A4-5-8). Fraction A4-5-3 (98 mg) was purified by preparative TLC (silica gel, CHCl3/EtOAc, 50:1) to obtain 10 (3.8 mg) (Rf = 0.56) and 11 (5.8 mg) (Rf = 0.71). Fraction A5 (10.2 g) was chromatographed further on silica gel (230–400 mesh, 460 g) eluting with CHCl3/acetone (10:1) to give 12 fractions (each 1.2 L, A5-1–A5-12). Fraction A5-6 (510 mg) was purified by MPLC (silica gel column, 230–400 mesh, n-hexane/acetone, 5:1) to afford 10 fractions (each 160 mL, A5-6-1–A5-6-10). Fraction A5-6-5 (85 mg) was purified by preparative TLC (silica gel, CHCl3/EtOAc, 50:1) to afford 1 (4.2 mg) (Rf = 0.58). Fraction A5-7 (87 mg) was purified by preparative TLC (silica gel, n-hexane/acetone, 10:1) to obtain 5 (2.5 mg) (Rf = 0.57). Fraction A5-10 (162 mg) was purified by preparative TLC (silica gel, CHCl3/acetone, 50:1) to afford a mixture of 16 and 17 (5.7 mg) (Rf = 0.50). Fraction A6 (9.5 g) was chromatographed further on silica gel (230–400 mesh, 430 g) eluting with n-hexane/acetone (8:1) to give 14 fractions (each 900 mL, A6-1–A6-14). Fraction A6-5 (132 mg) was purified by preparative TLC (silica gel, n-hexane/acetone, 6:1) to afford 3 (2.8 mg) (Rf = 0.53). Fraction A6-6 (245 mg) was purified by preparative TLC (silica gel, n-hexane/EtOAc, 5:1) to obtain 6 (43 mg) (Rf = 0.62) and 8 (57 mg) (Rf = 0.45). Fraction A6-10 (175 mg) was purified by preparative TLC (silica gel, CH2Cl2/MeOH, 15:1) to yield a mixture of 14 and 15 (5.3 mg) (Rf = 0.67). Fraction A7 (10.8 g) was chromatographed further on silica gel (230–400 mesh, 485 g) eluting with n-hexane/acetone (5:1) to give 12 fractions (each 850 mL, A7-1–A7-12). Fraction A7-4 (178 mg) was purified by preparative TLC (silica gel, n-hexane/acetone, 5:1) to afford 2 (2.6 mg) (Rf = 0.71) and 7 (3.4 mg) (Rf = 0.64). Fraction A7-5 (165 mg) was purified by preparative TLC (silica gel, CHCl3/MeOH, 15:1) to yield 9 (4.6 mg) (Rf = 0.62). Fraction A7-6 (138 mg) was purified by preparative TLC (silica gel, CH2Cl2/MeOH, 12:1) to obtain 4 (3.1 mg) (Rf = 0.59).
3.3.1. Hedychicoronarin (1)
Colorless oil. [α]D25: +13.8 (c 0.12, CHCl3). UV (MeOH): λmax (log ɛ) = 225 (4.06) nm. IR (neat): υmax = 1757 (C=O), 1746 (C=O), 1676 (C=C), 3077, 1644, 887 (exo-methylene bonds) cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 0.71 (3H, s, H-20), 0.81 (3H, s, H-19), 0.88 (3H, s, H-18), 1.07 (1H, ddd, J = 12.4, 12.4, 4.0 Hz, H-1α), 1.12 (1H, dd, J = 12.8, 2.8 Hz, H-5), 1.19 (1H, ddd, J = 13.2, 13.2, 4.2 Hz, H-3α), 1.33 (1H, dddd, J = 13.2, 12.8, 12.8, 4.4 Hz, H-6β), 1.41 (1H, br d, J = 13.2 Hz, H-3β), 1.51 (1H, m, H-2α), 1.58 (1H, ddddd, J = 13.2, 13.2, 12.4, 3.6, 3.2 Hz, H-2β), 1.68 (1H, br d, J = 12.4 Hz, H-1β), 1.73 (1H, dddd, J = 12.8, 5.0, 2.8, 2.4 Hz, H-6α), 1.86 (1H, br d, J = 10.8 Hz, H-9), 1.99 (1H, ddd, J = 13.2, 12.8, 5.0 Hz, H-7α), 2.38 (1H, ddd, J = 12.8, 4.4, 2.4 Hz, H-7β), 2.56 (1H, br d, J = 16.8 Hz, H-14), 2.64 (1H, dd, J = 16.0, 7.2 Hz, H-1’a), 2.85 (1H, dd, J = 16.0, 6.4 Hz, H-1’b), 3.10 (1H, dd, J = 16.8, 8.2 Hz, H-14), 3.72 (3H, s, OCH3), 4.35 (1H, br s, H-17a), 4.81 (1H, d, J = 1.2 Hz, H-17b), 4.91 (1H, ddd, J = 8.2, 7.2, 6.4 Hz, H-15), 6.72 (1H, m, H-12). 13C-NMR (CDCl3, 100 MHz, doubled signals due to epimers at C-15): δ = 19.3 (C-2), 24.1 (C-6), 25.5 (C-11), 31.3 (C-14), 33.5 (C-4), 37.74/37.76 (C-7), 39.3 (C-1), 39.40/39.41 (C-10), 40.6 (C-1′), 42.0 (C-3), 55.3 (C-5), 56.12/56.15 (C-9), 73.0 (C-15), 124.6 (C-13), 143.16/143.18 (C-12), 148.03/148.06 (C-8), 170.0 (C-2′), 170.3 (C-16). ESI-MS: m/z = 397 [M + Na]+. HR-ESI-MS: m/z = 397.2353 [M + Na]+ (calcd for C23H34O4Na: 397.2355).
3.3.2. Peroxycoronarin D (2)
Colorless oil. [α]D25: +15.8 (c 0.14, CHCl3). UV (MeOH): λmax (log ɛ) = 224 (4.01) nm. IR (neat): υmax 3413 (OH), 1762 (C=O), 1678 (C=C), 3081, 1643, 889 (exo-methylene bonds) cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 0.71 (3H, s, H-20), 0.81 (3H, s, H-19), 0.88 (3H, s, H-18), 1.06 (1H, ddd, J = 13.0, 12.6, 4.0 Hz, H-1α), 1.12 (1H, m, H-5), 1.18 (1H, ddd, J = 13.6, 12.8, 4.0 Hz, H-3α), 1.32 (1H, dddd, J = 13.0, 13.0, 12.8, 4.2 Hz, H-6β), 1.40 (1H, br d, J = 13.6 Hz, H-3β), 1.49 (1H, m, H-2α), 1.57 (1H, ddddd, J = 14.0, 12.8, 12.6, 3.0, 3.0 Hz, H-2β), 1.68 (1H, br d, J = 13.0 Hz, H-1β), 1.73 (1H, m, H-6α), 1.85 (1H, br d, J = 10.4 Hz, H-9), 1.99 (1H, ddd, J = 13.0, 12.8, 5.0 Hz, H-7α), 2.17 (1H, m, H-11), 2.33 (1H, m, H-11), 2.38 (1H, ddd, J = 12.8, 4.2, 2.4 Hz, H-7β), 2.66 (1H, br d, J = 17.4 Hz, H-14), 2.98 (1H, br d, J = 17.4 Hz, H-14), 4.34/4.41 (1H, d, J = 1.2 Hz, H-17a of C-15 epimers), 4.80/4.82 (1H, d, J = 1.2 Hz, H-17b of C-15 epimers), 5.59/5.61 (1H, dd, J = 2.0, 1.2 Hz, H-15 of C-15 epimers), 6.72 (1H, m, H-12). 13C-NMR (CDCl3, 100 MHz, doubled signals due to epimers at C-15): δ = 14.30/14.34 (C-20), 19.3 (C-2), 21.7 (C-18), 24.05/24.07 (C-6), 25.4 (C-11), 33.12/33.17 (C-14), 33.29/33.31 (C-4), 33.5 (C-19), 37.8 (C-7), 39.21/39.26 (C-1), 39.4 (C-10), 42.0 (C-3), 55.28/55.34 (C-5), 56.1 (C-9), 100.29/100.40 (C-15), 107.31/107.64 (C-17), 124.4 (C-13), 142.56/142.73 (C-12), 147.81/148.09 (C-8), 169.87/169.90 (C-16). ESI-MS: m/z = 333 [M − H]−. HR-ESI-MS: m/z = 333.2057 [M − H]− (calcd for C20H29O4: 333.2066).
3.3.3. 7β-Hydroxycalcaratarin A (3)
Colorless oil. [α]D25: +11.9 (c 0.15, CHCl3). UV (MeOH): λmax (log ɛ) = 234 (4.01) nm. IR (KBr): υmax = 3429 (OH), 1683 (C=O) cm−1. 1H-NMR (CDCl3, 600 MHz): δ = 0.73 (3H, s, H-20), 0.83 (3H, s, H-19), 0.92 (3H, s, H-18), 1.08 (1H, ddd, J = 13.2, 12.6, 4.2 Hz, H-1α), 1.17 (1H, dd, J = 12.6, 2.4 Hz, H-5), 1.21 (1H, ddd, J = 13.8, 13.2, 4.2 Hz, H-3α), 1.30 (1H, ddd, J = 12.8, 12.6, 11.4 Hz, H-6β), 1.45 (1H, br d, J = 13.2 Hz, H-3β), 1.53 (1H, m, H-2α), 1.58 (1H, m, H-2β), 1.73 (1H, br d, J = 12.6 Hz, H-1β), 1.88 (1H, br d, J = 10.8 Hz, H-9), 2.11 (1H, ddd, J = 12.8, 5.4, 2.4 Hz, H-6α), 2.46 (1H, ddd, J = 17.4, 10.8, 6.0 Hz, H-11), 2.57 (1H, dd, J = 13.2, 5.4 Hz, H-14), 2.60 (1H, dd, J = 13.2, 5.4 Hz, H-14), 2.64 (1H, ddd, J = 17.4, 6.0, 3.0 Hz, H-11), 3.35 (6H, s, OMe-15 × 2), 4.00 (1H, dd, J = 11.4, 5.4 Hz, H-7), 4.45 (1H, t, J = 5.4 Hz, H-15), 4.57 (1H, br s, H-17), 5.20 (1H, br s, H-17), 6.56 (1H, t, J = 6.0 Hz, H-12), 9.34 (1H, s, H-16). 13C-NMR (CDCl3, 150 MHz): δ = 14.4 (C-20), 19.3 (C-2), 21.6 (C-19), 24.8 (C-11), 29.0 (C-14), 33.5 (C-4), 33.5 (C-6), 33.6 (C-18), 39.1 (C-1), 39.2 (C-10), 41.9 (C-3), 53.0 (C-5), 54.3 (OMe-15 × 2), 54.4 (C-9), 73.6 (C-7), 103.9 (C-15), 104.4 (C-17), 138.1 (C-13), 150.1 (C-8), 160.0 (C-12), 195.0 (C-16). ESI-MS: m/z = 387 [M + Na]+. HR-ESI-MS: m/z = 387.2513 [M + Na]+ (calcd for C22H36O4Na: 387.2511).
3.3.4. (E)-7β-Hydroxy-6-oxo-labda-8(17),12-diene-15,16-dial (4)
Colorless oil. [α]D25: +22.6 (c 0.21, CHCl3). UV (MeOH): λmax (log ɛ) = 234 (3.97) nm. IR (neat): υmax = 3485 (OH), 1728 (C=O), 1716 (C=O), 1684 (C=O) cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 0.68 (3H, s, H-20), 1.00 (3H, s, H-19), 1.16 (1H, ddd, J = 13.6, 13.2, 4.4 Hz, H-3α), 1.26 (3H, s, H-18), 1.27 (1H, ddd, J = 13.2, 12.4, 4.0 Hz, H-1α), 1.43 (1H, br d, J = 13.2 Hz, H-3β), 1.55–1.63 (2H, m, H-2α and H-2β), 1.79 (1H, br d, J = 12.4 Hz, H-1β), 2.26 (1H, br s, H-5), 2.38 (1H, br d, J = 10.8 Hz, H-9), 2.39 (1H, ddd, J = 16.8, 10.8, 6.4 Hz, H-11), 2.52 (1H, ddd, J = 16.8, 6.4, 3.0 Hz, H-11), 3.44 (2H, br s, H-14), 4.50 (1H, br s, H-7), 4.67 (1H, br s, H-17), 5.44 (1H, br s, H-17), 6.75 (1H, t, J = 6.4 Hz, H-12), 9.40 (1H, s, H-16), 9.65 (1H, br s, H-15). 13C-NMR (CDCl3, 100 MHz): δ = 15.6 (C-20), 18.9 (C-2), 21.9 (C-19), 24.4 (C-11), 32.5 (C-18), 32.8 (C-4), 38.8 (C-1), 39.3 (C-14), 42.2 (C-3), 42.4 (C-10), 53.7 (C-9), 64.1 (C-5), 79.9 (C-7), 107.8 (C-17), 135.1 (C-13), 145.7 (C-8), 159.0 (C-12), 193.4 (C-16), 197.2 (C-15), 207.9 (C-6). ESI-MS: m/z = 355 [M + Na]+. HR-ESI-MS: m/z = 355.1882 [M + Na]+ (calcd for C20H28O4Na: 355.1885).
3.4. Biological Assay
The effect of the isolated compounds on neutrophil pro-inflammatory response was evaluated by monitoring the inhibition of superoxide anion generation and elastase release in fMLP/CB-activated human neutrophils in a concentration-dependent manner. The purity of the tested compounds was >98% as identified by NMR and MS. LY294002 (purity >99%, Sigma, St. Louis, MO, USA) was used as a positive control.
3.4.1. Preparation of Human Neutrophils
Human neutrophils from venous blood of healthy, adult volunteers (20–28 years old) were isolated using a standard method of dextran sedimentation prior to centrifugation in a Ficoll Hypaque gradient and hypotonic lysis of erythrocytes [27]. Purified neutrophils containing >98% viable cells, as determined by the trypan blue exclusion method [28], were re-suspended in a calcium (Ca2+)-free HBSS buffer at pH 7.4 and were maintained at 4 °C prior to use.
3.4.2. Measurement of Superoxide Anion Generation
The assay for measurement of superoxide anion generation was based on the SOD-inhibitable reduction of ferricytochrome c [29,30]. In brief, after supplementation with 0.5 mg/mL ferricytochrome c and 1 mM Ca2+, neutrophils (6 × 105/mL) were equilibrated at 37 °C for 2 min and incubated with different concentrations (10–0.01 μg/mL) of compounds or DMSO (as control) for 5 min. Cells were incubated with cytochalasin B (1 μg/mL) for 3 min prior to the activation with 100 nM formyl-l-methionyl-l-leucyl-l-phenylalanine for 10 min. Changes in absorbance with the reduction of ferricytochrome c at 550 nm were continuously monitored in a double-beam, six-cell positioner spectrophotometer with constant stirring (Hitachi U-3010, Tokyo, Japan). Calculations were based on differences in the reactions with and without SOD (100 U/mL) divided by the extinction coefficient for the reduction of ferricytochrome c (ɛ = 21.1/mM/10 mm).
3.4.3. Measurement of Elastase Release
Degranulation of azurophilic granules was determined by measuring elastase release as described previously [30]. Experiments were performed using MeO-Suc-Ala-Ala-Pro-Val-p-nitroanilide as the elastase substrate. Briefly, after supplementation with MeO-Suc-Ala-Ala-Pro-Val-p-nitroanilide (100 μM), neutrophils (6 × 105/mL) were equilibrated at 37 °C for 2 min and incubated with compounds for 5 min. Cells were stimulated with fMLP (100 nM)/CB (0.5 μg/mL), and changes in absorbance at 405 nm were monitored continuously in order to assay elastase release. The results were expressed as the percent of elastase release in the fMLP/CB-activated, drug-free control system.
3.4.4. Statistical Analysis
Results are expressed as the mean ± SEM, and comparisons were made using Student’s t-test. A probability of 0.05 or less was considered significant. The software SigmaPlot was used for the statistical analysis.
4. Conclusions
Seventeen compounds, including four new labdane-type diterpenoids 1–4, were isolated from the rhizome of H. coronarium. The structures of these compounds were established on the basis of spectroscopic data. Reactive oxygen species (ROS) [e.g., superoxide anion (O2•−), hydrogen peroxide] and granule proteases (e.g., elastase, cathepsin G) produced by human neutrophils contribute to the pathogenesis of inflammatory diseases. The effects on neutrophil pro-inflammatory responses of isolates were evaluated by suppressing fMLP/CB-induced O2•− generation and elastase release by human neutrophils. The results of anti-inflammatory experiments indicate that compounds 3–6, 10, and 11 can significantly inhibit fMLP-induced O2•− generation and/or elastase release. Among the isolates, calcaratarin A (5) was the most effective among the isolated compounds, with IC50 values of 2.25 ± 0.42 and 2.36 ± 0.41 μg/mL, respectively, against fMLP-induced O2•− generation and elastase release. The above isolated compounds might support the traditional use of H. coronarium for the treatment of inflammatory processes. Our study suggests H. coronarium and its isolates (especially 3, 5, 6, and 10) could be further developed as potential candidates for the treatment or prevention of various inflammatory diseases.
Acknowledgments
This research was supported by grants from the National Science Council of the Republic of China (No. NSC 98-2320-B-127-001-MY3 and NSC 101-2320-B-127-001-MY3), awarded to J.-J. Chen. We also thank the National Center for High-Performance Computing (NCHC, Taiwan) for providing computer resources and chemical database services.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Wang J.C. Zingeraceae. In: Huang T.C., editor. Flora of Taiwan. 2nd ed. Vol. 5. Editorial Committee of the Flora of Taiwan; Taipei, Taiwan: 2000. pp. 707–724. [Google Scholar]
- 2.Morikawa T., Matsuda H., Sakamoto Y., Ueda K., Yoshikawa M. New farnesane-type sesquiterpenes, hedychiols A and B 8,9-diacetate, and inhibitors of degranulation in RBL-2H3 cells from the rhizome of Hedychium coronarium. Chem. Pharm. Bull. 2002;50:1045–1049. doi: 10.1248/cpb.50.1045. [DOI] [PubMed] [Google Scholar]
- 3.Chimnoi N., Sarasuk C., Khunnawutmanotham N., Intachote P., Seangsai S., Saimanee B., Pisutjaroenpong S., Mahidol C., Techasakul S. Phytochemical reinvestigation of labdane-type diterpenes and their cytotoxicity from the rhizomes of Hedychium coronarium. Phytochem. Lett. 2009;2:184–187. [Google Scholar]
- 4.Itokawa H., Morita H., Katou I., Takeya K., Cavalheiro A.J., de Oliveira R.C.B., Ishige M., Motidome M. Cytotoxic diterpenes from the rhizomes of Hedychium coronarium. Planta Med. 1988;54:311–315. doi: 10.1055/s-2006-962442. [DOI] [PubMed] [Google Scholar]
- 5.Nakatani N., Kikuzaki H., Yamaji H., Yoshio K., Kitora C., Okada K., Padolina W.G. Labdane diterpenes from rhizomes of Hedychium coronarium. Phytochemistry. 1994;37:1383–1388. [Google Scholar]
- 6.Matsuda H., Morikawa T., Sakamoto Y., Toguchida I., Yoshikawa M. Antiinflammatory principles and three new labdane-type diterpenes, hedychilactones A, B, and C, from the rhizome of Hedychium coronarium Koeng. Heterocycles. 2002;56:45–50. [Google Scholar]
- 7.Matsuda H., Morikawa T., Sakamoto Y., Toguchida I., Yoshikawa M. Labdane-type diterpenes with inhibitory effects on increase in vascular permeability and nitric oxide production from Hedychium coronarium. Bioorg. Med. Chem. 2002;10:2527–2534. doi: 10.1016/s0968-0896(02)00121-9. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura S., Okazaki Y., Ninomiya K., Morikawa T., Matsuda H., Yoshikawa M. Medicinal flowers. XXIV. Chemical structures and hepatoprotective effects of constituents from flowers of Hedychium coronarium. Chem. Pharm. Bull. 2008;56:1704–1709. doi: 10.1248/cpb.56.1704. [DOI] [PubMed] [Google Scholar]
- 9.Suresh G., Reddy P.P., Babu K.S., Shaik T.B., Kalivendi S.V. Two new cytotoxic labdane diterpenes from the rhizomes of Hedychium coronarium. Bioorg. Med. Chem. Lett. 2010;20:7544–7548. doi: 10.1016/j.bmcl.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 10.Chimnoi N., Pisutjareonpong S., Ngiwsara L., Dechtrirut D., Chokchaichamnankit D., Khunnawutmanotham N., Mahidol C., Techasakul S. Labdane diterpenes from the rhizomes of Hedychium coronarium. Nat. Prod. Res. 2008;22:1249–1256. doi: 10.1080/14786410701726434. [DOI] [PubMed] [Google Scholar]
- 11.Taveira F.N., Oliveira A.B., Souza Filho J.D., Braga F.C. Epimers of labdane diterpenes from the rhizomes of Hedychium coronarium J. Koenig. Braz. J. Pharm. 2005;15:55–59. [Google Scholar]
- 12.Zhao Q., Qing C., Hao X.J., Han J., Zuo G.Y., Zou C., Xu G.L. Cytotoxicity of labdane-type diterpenoids from Hedychium forrestii. Chem. Pharm. Bull. 2008;56:210–212. doi: 10.1248/cpb.56.210. [DOI] [PubMed] [Google Scholar]
- 13.Mohamad H., Lajis H.N., Abas F., Ali M.A., Sukari A.M., Kikuzaki H., Nakatani N. Antioxidative constituents of Etlingera elatior. J. Nat. Prod. 2005;68:285–288. doi: 10.1021/np040098l. [DOI] [PubMed] [Google Scholar]
- 14.Kong L.Y., Qin M.J., Niwa M. Diterpenoids from rhizomes of Alpinia calcarata. J. Nat. Prod. 2000;63:939–942. doi: 10.1021/np9904962. [DOI] [PubMed] [Google Scholar]
- 15.Singh S., Gray A.I., Waterman P.G. 14,15,16-Trinorlabda-8(17),11-(E)-dien-13-al: A trinorlabdane diterpene from the rhizome of Hedychium coronarium. Nat. Prod. Lett. 1993;3:163–166. [Google Scholar]
- 16.Singh S., Gray A.I., Skelton B.W., Waterman P.G., White A.H. (+)-14β-Hydroxylabda-8(17),12-dieno-16,15-lactone [(+)-isocoronarin-D]: A new diterpene from Hedychium coronarium (Zingiberaceae) Aust. J. Chem. 1991;44:1789–1793. [Google Scholar]
- 17.Price M.J., Worth G.K. The occurrence of ergosta-4,6,8(14),22-tetraen-3-one in several fungi. Aust. J. Chem. 1974;27:2505–2507. [Google Scholar]
- 18.Chen J.J., Hung H.C., Sung P.J., Chen I.S., Kuo W.L. Aporphine alkaloids and cytotoxic lignans from the roots of Illigera luzonensis. Phytochemistry. 2011;72:523–532. doi: 10.1016/j.phytochem.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 19.Georges P., Sylvestre M., Ruegger H., Bourgeois P. Ketosteroids and hydroxyketosteroids, minor metabolites of sugarcane wax. Steroids. 2006;71:647–652. doi: 10.1016/j.steroids.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Lee C.K., Lee P.H., Kou Y.H. The chemical constituents from the aril of Cassia fistula L. J. Chin. Chem. Soc. 2001;48:1053–1058. [Google Scholar]
- 21.Witko-Sarsat V., Rieu P., Descamps-Latscha B., Lesavre P., Halbwachs-Mecarelli L. Neutrophils: Molecules, functions and pathophysiological aspects. Lab. Invest. 2000;80:617–653. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 22.Okajima K., Harada N., Uchiba M. Ranitidine reduces ischemia/reperfusion-induced liver injury in rats by inhibiting neutrophil activation. J. Pharmacol. Exp. Ther. 2002;301:1157–1165. doi: 10.1124/jpet.301.3.1157. [DOI] [PubMed] [Google Scholar]
- 23.Ennis M. Neutrophils in asthma pathophysiology. Curr. Allergy Asthma Rep. 2003;3:159–165. doi: 10.1007/s11882-003-0029-2. [DOI] [PubMed] [Google Scholar]
- 24.Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc. Res. 2004;61:481–497. doi: 10.1016/j.cardiores.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Borregaard N. The human neutrophil. Function and dysfunction. Eur. J. Haematol. 1998;41:401–413. doi: 10.1111/j.1600-0609.1988.tb00219.x. [DOI] [PubMed] [Google Scholar]
- 26.Roos D., van Bruggen R., Meischl C. Oxidative killing of microbes by neutrophils. Microbes. Infect. 2003;5:1307–1315. doi: 10.1016/j.micinf.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Invest. 1968;97:77–89. [PubMed] [Google Scholar]
- 28.Jauregui H.O., Hayner N.T., Driscoll J.L., Williams-Holland R., Lipsky M.H., Galletti P.M. Trypan blue dye uptake and lactate dehydrogenase in adult rat hepatocytes-freshly isolated cells, cell suspensions, and primary monolayer cultures. In Vitro. 1981;17:1100–1110. doi: 10.1007/BF02618612. [DOI] [PubMed] [Google Scholar]
- 29.Babior B.M., Kipnes R.S., Curnutte J.T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Invest. 1973;52:741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hwang T.L., Leu Y.L., Kao S.H., Tang M.C., Chang H.L. Viscolin, a new chalcone from Viscum coloratum, inhibits human neutrophil superoxide anion and elastase release via a cAMP-dependent pathway. Free Radic. Biol. Med. 2006;41:1433–1441. doi: 10.1016/j.freeradbiomed.2006.08.001. [DOI] [PubMed] [Google Scholar]