Abstract
Metabolic syndrome is associated with an increased risk of colorectal cancer. This study investigated the impact of hypertension, a component of metabolic syndrome, on azoxymethane (AOM)-induced colorectal carcinogenesis using SHRSP/Izm (SHRSP) non-diabetic/hypertensive rats and SHRSP.Z-Leprfa/IzmDmcr (SHRSP-ZF) diabetic/hypertensive rats. Male 6-week-old SHRSP, SHRSP-ZF, and control non-diabetic/normotensive Wister Kyoto/Izm (WKY) rats were given 2 weekly intraperitoneal injections of AOM (20 mg/kg body weight). Two weeks after the last injection of AOM, the SHRSP and SHRSP-ZF rats became hypertensive compared to the control WKY rats. Serum levels of angiotensin-II, the active product of the renin-angiotensin system, were elevated in both SHRSP and SHRSP-ZF rats, but only the SHRSP-ZF rats developed insulin resistance, dyslipidemia, and hyperleptinemia and exhibited an increase in adipose tissue. The development of AOM-induced colonic preneoplastic lesions and aberrant crypts foci, was significantly accelerated in both SHRSP and SHRSP-ZF hypertensive rats, compared to WKY normotensive rats. Furthermore, induction of oxidative stress and exacerbation of inflammation were observed in the colonic mucosa and systemically in SHRSP and SHRSP-ZF rats. Our findings suggest that hypertension plays a role in the early stage of colorectal carcinogenesis by inducing oxidative stress and chronic inflammation, which might be associated with activation of the renin-angiotensin system.
Keywords: hypertension, colon carcinogenesis, oxidative stress, inflammation, angiotensin-II
1. Introduction
Obesity-related systemic metabolic dysfunctions such as diabetes mellitus, hypertension, and dyslipidemia are collectively known as metabolic syndrome (Mets) and pose serious health problems throughout the world [1,2]. In addition to the morbidity associated with these metabolic disorders, recent studies have revealed that Mets is linked to an increased risk of cancer in several organ sites including the colorectum [3–8]. Several pathophysiological mechanisms for this association have been described, including the emergence of insulin resistance, the state of chronic inflammation, induction of oxidative stress, and occurrence of adipokine imbalance [5,6]. In particular, diabetes is closely associated with the development of colorectal cancer (CRC) as obesity is the main determinant of insulin resistance and hyperinsulinemia [7].
Epidemiological studies have also revealed that hypertension may increase the risk of CRC [3,4]. The renin-angiotensin system is a key regulator of cardiovascular function, and its activation is involved in the etiology of Mets, especially hypertension [9]. There is increasing evidence that the renin-angiotensin system may have paracrine and autocrine functions with regard to tissue oxidative stress and chronic inflammation, as well as cellular proliferation and apoptosis [10–14]. In addition, dysregulation of the renin-angiotensin system has been reported to occur in human malignancies and has been shown to influence cancer cell migration, invasion, and metastasis, all of which are associated with a poor prognosis [10,11,14]. However, the precise mechanisms by which hypertension plays a role in the early stage of colorectal carcinogenesis remain unclear.
The stroke-prone spontaneously hypertensive rat (SHRSP) is a substrain of the spontaneously hypertensive rat (SHR), crossed and further inbred with selected offspring of parents that died of stroke. The SHRSP rats have a higher blood pressure than SHR rats and readily develop apoplexy. The crossing of SHRSP rats with Zucker Fatty (ZF) rats produces SHRSP.Z-Leprfa/IzmDmcr (SHRSP-ZF) rats, which develop hypertension and become obese due to the leptin receptor OB-rb gene mutation carried by ZF rats [15]. SHRSP-ZF rats therefore exhibit a phenotype similar to human Mets and thus may be a useful model to investigate the molecular mechanisms underlying hypertension-related metabolic abnormalities [15,16]. However, colorectal carcinogenesis models using these rats have not been established.
The objective of this study was to determine the susceptibility of SHRSP-ZF and SHRSP rats to azoxymethane (AOM)-induced colorectal carcinogenesis and the utility of these rats as models for Mets, in particular, as models for hypertension-associated colorectal carcinogenesis, that appropriately reflect the pathological conditions of human Mets.
2. Results and Discussion
2.1. General Observations
Table 1 compares the mean body weights, adipose tissue weights, and blood pressures (systolic and diastolic) at the end of the study (10 weeks of age) between 3 groups (Group 1, Wister Kyoto/Izm [WKY] rats; Group 2, SHRSP rats; and Group 3, SHRSP-ZF rats) that received AOM. The mean body weights of WKY (p < 0.001) and SHRSP-ZF (p < 0.05) rats were significantly higher than that of SHRSP rats, but there was no significant difference between the WKY and SHRSP-ZF rats. There was a significant increase in the mean adipose tissue weights in SHRSP-ZF rats compared to WKY (p < 0.001) and SHRSP rats (p < 0.05). The systolic and diastolic blood pressures of SHRSP and SHRSP-ZF rats were markedly higher than those of WKY rats (p < 0.001). However, compared to SHRSP-ZF rats, SHRSP rats had marked hypertension (p < 0.05).
Table 1.
Body, liver and adipose weights, BMI and blood pressure of rats.
| Group NO. | Strain | No. | Body weight (g) | Relative adipose tissue weight (g/100g body weight) a | Blood pressure (mmHg) | |
|---|---|---|---|---|---|---|
|
| ||||||
| Systolic | Diastolic | |||||
| Group 1 | WKY b | 8 | 256.5 ± 11.7 e | 0.72 ± 0.16 | 127 ± 12.8 | 92 ± 4.9 |
| Group 2 | SHRSP c | 8 | 218.9 ± 8.0 f | 0.77 ± 0.16 | 188 ± 12.5 f | 141 ± 10.6 f |
| Group 3 | SHRSP-ZF d | 8 | 270.1 ± 23.4 g | 1.64 ± 0.17 f,g | 169 ± 13.7 f,g | 129 ± 9.0 f,g |
White adipose tissue of the periorchis;
Wister Kyoto/Izm;
stroke-prone spontaneously hypertensive/Izm;
SHRSP.Z-Leprfa/IzmDmcr;
Mean ± SD;
Significantly different from group 1 by Tukey-Kramer Multiple Comparison Test (p < 0.001);
Significantly different from group 2 by Tukey-Kramer Multiple Comparison Test (p < 0.05).
2.2. Serum Parameters of the Experimental Rats
As shown in Table 2, the serum levels of glucose and insulin significantly increased, but the value of QUICKI, a useful index of insulin sensitivity [17], was decreased in SHRSP-ZF rats compared to WKY and SHRSP rats (p < 0.05). The serum levels of leptin, non-esterified fatty acid (NEFA), and triglycerides in SHRSP-ZF rats were also significantly higher than those in WKY and SHRSP rats (p < 0.05). These findings suggest that SHRSP-ZF rats developed insulin resistance, hyperleptinemia, and dyslipidemia, all of which are frequently observed in human Mets patients. There were no significant differences in these serum components between WKY and SHRSP rats. The SHRSP and SHRSP-ZF rats did, however, have significantly elevated levels of serum angiotensin-II (AT-II), the active product of the renin-angiotensin system [18], compared to the WKY rats (p < 0.05), indicating that the renin-angiotensin system is activated in these hypertensive rats.
Table 2.
Serum parameters of the experimental rats.
| Glucose (mg/dL) | Insulin (μIU/mL) | Quicki | Leptin (pg/mL) | NEFA (mEq/L) | Triglyceride (mg/dL) | Angiotensin II (ng/mL) | |
|---|---|---|---|---|---|---|---|
| Group 1 | 85.4 ± 11.7 | 15.81 ± 0.35 | 0.313 ± 0.010 | 11.2 ± 3.6 | 0.459 ± 0.03 | 27.1 ± 7.4 | 352.6 ± 38.1 |
| Group 2 | 83.5 ± 12.3 | 17.00 ± 1.39 | 0.320 ± 0.008 | 12.2 ± 3.4 | 0.419 ± 0.05 | 39.6 ± 14.1 | 494.4 ± 75.6 b |
| Group 3 | 120.0 ± 14.2 b,c | 25.60 ± 8.98 b,c | 0.291 ± 0.010 b,c | 102.7 ± 30.6 b,c | 0.538 ± 0.03 b,c | 257.1 ± 79.4 b,c | 500.9 ± 42.5 b |
Mean ± SD;
Significantly different from group 1 by Tukey-Kramer Multiple Comparison Test (p < 0.05);
Significantly different from group 2 by Tukey-Kramer Multiple Comparison Test (p < 0.05).
2.3. Development of Colonic Preneoplastic Lesions
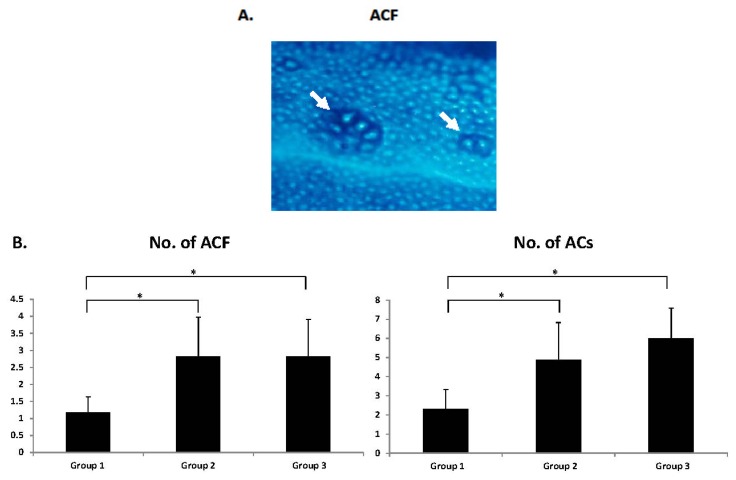
Irrespective of the rat strain, aberrant crypt foci (ACF) (Figure 1A) were observed in the colon of all rats given AOM at the end of the study. However, the number and size (aberrant crypts [ACs] per cm2) of ACF were significantly greater in both the SHRSP and SHRSP-ZF rats than in the WKY rats (Figure 1B; p < 0.05). There was no significant difference in the development of ACF between SHRSP and SHRSP-ZF rats, indicating that hypertension, a common pathophysiological characteristic of these rats, plays a critical role in accelerating the development of colonic preneoplastic lesions.
Figure 1.
ACF developed in the SHRSP, SHRSP-ZF, and WKY rats that received AOM. (A) Representative morphology of ACF (arrows) induced by AOM stained with methylene blue in Group 2. Magnification, 40×; (B) Average number of ACF and ACs (/cm2). Group 1: WKY rats, Group 2: SHRSP rats, and Group 3: SHRSP-ZF rats. The values are expressed as mean ± SD. * p < 0.05.
2.4. Systemic Oxidative Stress and Colonic Epithelial Expression of GPx and CAT mRNA
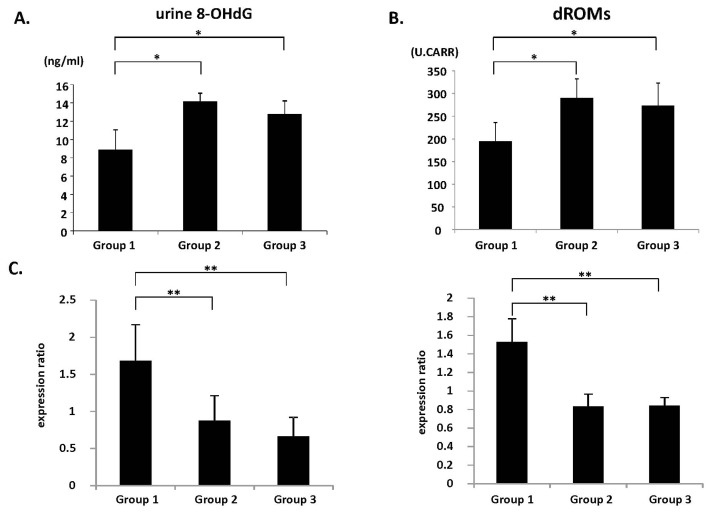
Oxidative stress is implicated in Mets and colorectal tumorigenesis [5]. Therefore, the levels of oxidative stress and antioxidant biomarkers in the experimental rats were assessed. Compared to the WKY rats, the SHRSP and SHRSP-ZF rats had significantly increased levels of urine 8-hydroxy-2′-deoxyguanosine (8-OHdG) (Figure 2A; p < 0.01), a marker of DNA damage induced by oxidative stress, and serum derivatives of reactive oxygen metabolites (d-ROM) (Figure 2B; p < 0.01), which reflects serum hydroperoxide levels. However, the SHRSP and SHRSP-ZF rats also had reduced expression levels of glutathione peroxidase (GPx) and catalase (CAT) mRNA, which encode antioxidant enzymes, in the colonic epithelium (Figure 2C; p < 0.05). These findings suggest that systemic oxidative stress is increased, whereas colonic antioxidant activity is decreased, in both SHRSP and SHRSP-ZF hypertensive rats.
Figure 2.
Measures of oxidative stress and antioxidant biomarkers’ expression. (A) Urine 8-OHdG levels were measured by enzyme immunoassay; (B) Hydroperoxide levels in the serum were determined by the d-ROM test; (C) The expression levels of GPx and CAT mRNA in the colonic epithelium were examined by quantitative real-time RT-PCR using specific primers. The values are expressed as mean ± SD. * p < 0.01, ** p < 0.01.
2.5. Serum and Colonic Epithelial Expression of Inflammatory Markers
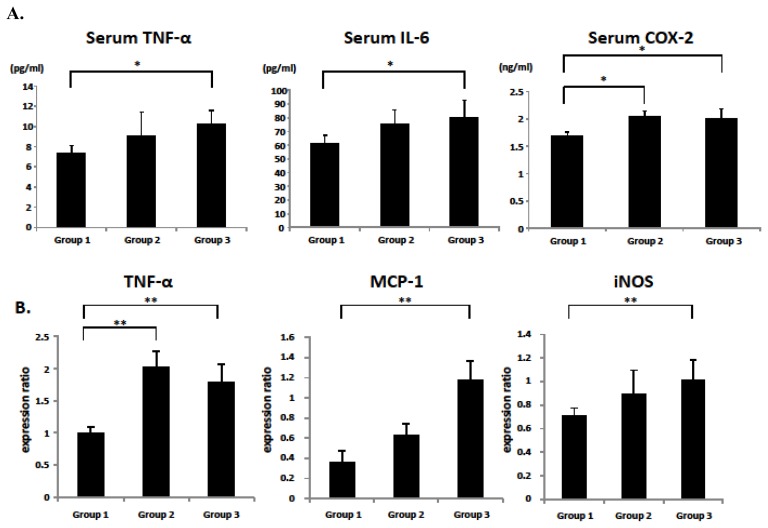
Chronic inflammation plays a critical role in the pathogenesis of Mets and CRC development [5,8]. Therefore, the levels of inflammatory mediators, including tumor necrosis factor (TNF)-α, interleukin (IL)-6, monocyte chemoattractant protein (MCP)-1, inducible nitric oxide synthase (iNOS), and cyclooxygenase (COX)-2 in hypertensive SHRSP and SHRSP-ZF rats were next examined. The serum levels of TNF-α and IL-6 in SHRSP-ZF rats were significantly elevated compared to those in WKY rats (Figure 3A; p < 0.01). The serum levels of COX-2 were also significantly increased in both SHRSP and SHRSP-ZF rats (Figure 3A; p < 0.01). In the colonic epithelium of SHRSP-ZF rats, there was a marked increase in the expression of TNF-α, MCP-1, and iNOS mRNA (Figure 3B; p < 0.05 compared to WKY rats). Compared to the WKY rats, the expression of TNF-α mRNA in the colonic epithelium of SHRSP rats was also significantly increased (Figure 3B; p < 0.05).
Figure 3.
Serum levels of TNF-α, IL-6, and COX-2 and the expression levels of TNF-α, MCP-1, and iNOS mRNA in the colonic epithelium. (A) The serum concentrations of TNF-α, IL-6, and COX-2 were measured by enzyme immunoassay; (B) The expression levels of TNF-α, MCP-1, and iNOS mRNA in the colonic epithelium were examined by quantitative real-time RT-PCR using specific primers. The values are expressed as mean ± SD. * p < 0.01, ** p < 0.05.
2.6. Discussion
Increasing evidence suggests that Mets is involved in the development of CRC, and this continues to be a growing health problem worldwide, especially in developed countries [1–5]. Recent epidemiological studies have suggested that patients with hypertension, a component of Mets [1,2], comprise a high-risk group for CRC [3–5]. However, appropriate animal models to evaluate hypertension-related colorectal carcinogenesis have not yet been generated.
To our knowledge, the present study provides the first evidence that after administration of AOM, SHRSP and SHRSP-ZF rats, both of which present with hypertension, more readily develop colonic preneoplastic lesions than normotensive WKY rats. In particular, we found that SHRSP rats experience accelerated development of ACF. This is significant because these rats did not exhibit insulin resistance, hyperleptinemia, or dyslipidemia and did not have increased adipose tissue, which are involved in the pathophysiology thought to link Mets to CRC [5–8]. These findings, therefore, suggest that hypertension per se might play a critical role in the early events of colorectal carcinogenesis. We have found that the angiotensin converting enzyme inhibitor captopril, an anti-hypertensive drug, significantly prevents the development of ACF in SHRSP-ZF rats [19]. These findings also support our hypothesis that blood pressure elevation per se might be directly involved in the early stage of colorectal carcinogenesis. However, in order to test this hypothesis, further studies are needed to establish whether other anti-hypertensive agents, such as AT-II type 1 receptor blockers and calcium channel blockers, can suppress the development of ACF by lowering blood pressure.
Among the pathophysiological disorders associated with hypertension, an increased level of oxidative stress is thought to be particularly important in CRC development [5,6]. Oxidative stress, defined as the overproduction of oxygen species combined with inadequate anti-oxidative defense mechanisms, can result in DNA damage and, consequently, mutations associated with colorectal carcinogenesis [5,20]. In the present study, the hypertensive SHRSP and SHRSP-ZF rats had significantly elevated urine 8-OHdG levels and serum d-ROM levels, which are associated with increased oxidative stress [21]. However, they also had reduced GPx and CAT mRNA levels, both of which encode antioxidant enzymes, in the colonic epithelium. These findings indicate that both SHRSP and SHRSP-ZF rats are subjected to strong oxidative stress, which might contribute to the development of ACF.
In addition to oxidative stress, the induction of chronic inflammation is also considered to play a critical role in obesity-, diabetes-, and hypertension-related colorectal carcinogenesis [5,6]. In the present study, serum levels of TNF-α and IL-6, as well as colonic expression of MCP-1 and iNOS mRNA, were markedly elevated in SHRSP-ZF obese and diabetic rats. These changes might have been associated with the increase in adipose tissue in SHRSP-ZF rats because excess adipose tissue plays an important role in the exacerbation of systemic inflammation [22,23]. Furthermore, colonic epithelial expression of TNF-α mRNA and serum levels of COX-2 were significantly higher in both the hypertensive SHRSP and SHRSP-ZF rats, although the former did not become obese or develop diabetes. These findings are also significant because the dysregulation of TNF-α, a central mediator of chronic inflammatory diseases, and COX-2 have key roles in the stimulation of tumor growth and the progression of carcinogenesis in several tissues, including the colon and rectum [24,25].
Why did the SHRSP rats, which did not exhibit obesity and insulin resistance, experience an acceleration of oxidative stress, exacerbation of chronic inflammation, and development of ACF to the same extent as SHRSP-ZF rats that are both obese and diabetic? One possible explanation is that the dose of AOM (20 mg/kg body weight) used in the present protocol was considerably greater than that needed to induce ACF development in these hypertensive rats. A lower dose of AOM may therefore result in differences in both the number and size of ACF between SHRSP and SHRSP-ZF rats. It is also possible that an increase in the serum level of AT-II, which is the main effector peptide of the renin-angiotensin system [12,13], might contribute to these phenomena because renin-angiotensin system activation has been implicated in the increase in oxidative stress and the induction of inflammation [11,14,26,27]. Renin-angiotensin system activation induces adipocyte inflammation, as demonstrated by the increased expression of TNF-α and IL-6 in adipose tissue, which in turn is implicated in hypertension [28,29]. In prostate cancer, treatment with AT-II stimulates the secretion of IL-6 and MCP-1 from prostate stromal cells and is associated with the increased proliferation of prostate cancer cells [30]. AT-II also induces the expression of iNOS, an inflammatory marker, along with 8-OHdG in prostate cancer cells [31], suggesting a crosslink between renin-angiotensin system-related inflammation and oxidative stress in cancer tissue.
To date, there is no definitive evidence demonstrating the effectiveness of renin-angiotensin system inhibitors in preventing human malignancies, including CRC, in hypertensive patients [32–35]. However, our findings suggest that targeting hypertension-related metabolic abnormalities, including oxidative stress and chronic inflammation caused by renin-angiotensin system activation, may be an effective strategy to prevent CRC development in patients with Mets, especially those with hypertension. In malignant tissue such as CRC, dysregulation of the renin-angiotensin system is implicated in cancer cell migration, invasion, and metastasis [10,11,13,14,36]. A recent study also showed that treatment with renin-angiotensin system inhibitors could inhibit chemically induced colorectal carcinogenesis in obese and diabetic mice by attenuating chronic inflammation and oxidative stress [37]. In order to test the potential efficacy of renin-angiotensin system inhibitors in preventing CRC development in patients with Mets, additional long-term experiments to evaluate whether these agents can prevent colorectal carcinogenesis in hypertensive rats should be conducted.
3. Experimental Section
3.1. Animals and Chemicals
Five-week-old male SHRSP, SHRSP-ZF, and WKY rats were obtained from Japan SLC (Shizuoka, Japan) and humanely maintained at Gifu University Life Science Research Center in accordance with the Institutional Animal Care Guidelines. The WKY rats are normotensive and not prone to obesity, and thus served as the control group in this study. AOM, which is widely used to mimic sporadic colon carcinogenesis by causing DNA mutations and activating several oncogenic pathways, including the K-ras pathway [38,39], was purchased from Wako (Osaka, Japan).
3.2. Experimental Procedure
After 1 week of acclimatization, the 6-week-old rats were divided into 3 groups of 8 rats each. All rats received an intraperitoneal injection of AOM (20 mg/kg body weight) once a week for 2 weeks. The experimental protocol and dose of AOM were based on previous studies using F344, Sprague-Dawley, or Wister rat strains [40,41]. We did not include non-AOM treated WKY rats as negative controls because no ACF was found to develop in these animals in a preliminary experiment. At the end of the experiment (2 weeks after the last injection of AOM), when the rats were 10 weeks of age, systolic and diastolic blood pressures were measured noninvasively using a tail cuff (SOFTRON BP98A; Softron, Tokyo, Japan). All rats were euthanized by CO2 asphyxiation for colon resection. The third portion of the excised colons (cecum side) was used to extract RNA, and the remaining part was used to determine the number of ACF [42].
3.3. Enumeration of ACF
The frequency of AOM-induced colonic premalignant lesions, ACF, was determined as previously described [42]. Briefly, the colon samples were fixed with 10% buffered formalin, stained with methylene blue (0.5% in distilled water) for 20 s, and then placed on microscope slides to count the number of ACF. The number of ACF was recorded along with the number of ACs in each focus. The data are expressed per unit area (cm2).
3.4. RNA Extraction and Quantitative Real-Time Reverse Transcription-Polymerase Chain Reaction Analysis
The epithelial crypts were isolated from colonic tissue [41]. Total RNA was then extracted from the isolated epithelial crypts using the RNAqueous-4PCR kit (Ambion Applied Biosystems, Austin, TX, USA). cDNA was amplified from 0.2 μg of total RNA using the SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA). Quantitative real-time reverse transcription-PCR (RT-PCR) analysis was performed using specific primers that amplify TNF-α, MCP-1, iNOS, GPx, CAT, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) genes. The sequences of these primers, which were obtained from Primer-BLAST [43], are listed in Table S1. Each sample was analyzed on a LightCycler Nano (Roche Diagnostics, Basel, Switzerland) using FastStart Essential DNA Green Master (Roche Diagnostics). Parallel amplification of GAPDH was used as the internal control.
3.5. Clinical Biochemistry
Blood samples from the inferior vena cava were used for chemical analyses. These samples were obtained at the time of euthanasia, prior to which the rats had fasted for 6 h. The serum levels of TNF-α (R&D Systems, Minneapolis, MN, USA), IL-6 (R & D Systems), insulin (Shibayagi, Gunma, Japan), glucose (BioVision Research Products, Mountain View, CA, USA), leptin (Shibayagi), triglyceride (Wako), NEFA (Wako), AT-II (Phoenix Pharmaceuticals, INC, Burlingame, CA, USA), and COX-2 (MyBioSource, San Diego, CA, USA) were determined using an enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer instructions.
3.6. Oxidative Stress Analysis
Urine 8-OHdG levels were determined using an ELISA kit (NIKKEN SEIL, Shizuoka, Japan). Serum levels of hydroperoxide, a marker for oxidative stress, were evaluated using the d-ROM test (FREE Carpe Diem; Diacron s.r.l., Grosseto, Italy) [21].
3.7. Statistical Analysis
All data are presented as mean ± SD and were analyzed using the GraphPad InStat software program version 3.05 (GraphPad Software, San Diego, CA, USA) for Macintosh. One-way analysis of variance (ANOVA) was used to compare groups. If the ANOVA analysis indicated significant differences, the Tukey-Kramer multiple comparisons test was performed to compare the mean values among the groups. The differences were considered significant when the two-sided p value was less than 0.05.
4. Conclusions
The results of this study indicate that the development of AOM-induced colonic preneoplastic lesions was significantly accelerated in hypertensive rats compared to normotensive rats. This was associated with hypertension-related renin-angiotensin system activation and subsequent induction of oxidative stress and inflammation, suggesting that hypertension plays a critical role in the early stages of CRC.
Supplementary Information
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Alberti K.G., Zimmet P., Shaw J. IDF Epidemiology Task Force Consensus Group. The metabolic syndrome—A new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 2.Grundy S.M., Cleeman J.I., Daniels S.R., Donato K.A., Eckel R.H., Franklin B.A., Gordon D.J., Krauss R.M., Savage P.J., Smith S.C., Jr, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed R.L., Schmitz K.H., Anderson K.E., Rosamond W.D., Folsom A.R. The metabolic syndrome and risk of incident colorectal cancer. Cancer. 2006;107:28–36. doi: 10.1002/cncr.21950. [DOI] [PubMed] [Google Scholar]
- 4.Stocks T., van Hemelrijck M., Manjer J., Bjorge T., Ulmer H., Hallmans G., Lindkvist B., Selmer R., Nagel G., Tretli S., et al. Blood pressure and risk of cancer incidence and mortality in the Metabolic Syndrome and Cancer Project. Hypertension. 2012;59:802–810. doi: 10.1161/HYPERTENSIONAHA.111.189258. [DOI] [PubMed] [Google Scholar]
- 5.Ishino K., Mutoh M., Totsuka Y., Nakagama H. Metabolic syndrome: A novel high-risk state for colorectal cancer. Cancer Lett. 2012 doi: 10.1016/j.canlet.2012.10.01. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu M., Kubota M., Tanaka T., Moriwaki H. Nutraceutical approach for preventing obesity-related colorectal and liver carcinogenesis. Int. J. Mol. Sci. 2012;13:579–595. doi: 10.3390/ijms13010579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuhara H., Steinmaus C., Cohen S.E., Corley D.A., Tei Y., Buffler P.A. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer? Am. J. Gastroenterol. 2011;106:1911–1921. doi: 10.1038/ajg.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donohoe C.L., Pidgeon G.P., Lysaght J., Reynolds J.V. Obesity and gastrointestinal cancer. Br. J. Surg. 2010;97:628–642. doi: 10.1002/bjs.7079. [DOI] [PubMed] [Google Scholar]
- 9.De Kloet A.D., Krause E.G., Woods S.C. The renin angiotensin system and the metabolic syndrome. Physiol. Behav. 2010;100:525–534. doi: 10.1016/j.physbeh.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ager E.I., Neo J., Christophi C. The renin-angiotensin system and malignancy. Carcinogenesis. 2008;29:1675–1684. doi: 10.1093/carcin/bgn171. [DOI] [PubMed] [Google Scholar]
- 11.Deshayes F., Nahmias C. Angiotensin receptors: A new role in cancer? Trends Endocrinol. Metab. 2005;16:293–299. doi: 10.1016/j.tem.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Paul M., Poyan Mehr A., Kreutz R. Physiology of local renin-angiotensin systems. Physiol. Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 13.Fyhrquist F., Saijonmaa O. Renin-angiotensin system revisited. J. Intern. Med. 2008;264:224–236. doi: 10.1111/j.1365-2796.2008.01981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.George A.J., Thomas W.G., Hannan R.D. The renin-angiotensin system and cancer: Old dog, new tricks. Nat. Rev. Cancer. 2010;10:745–759. doi: 10.1038/nrc2945. [DOI] [PubMed] [Google Scholar]
- 15.Hiraoka-Yamamoto J., Nara Y., Yasui N., Onobayashi Y., Tsuchikura S., Ikeda K. Establishment of a new animal model of metabolic syndrome: SHRSP fatty (fa/fa) rats. Clin. Exp. Pharmacol. Physiol. 2004;31:107–109. doi: 10.1111/j.1440-1681.2004.03962.x. [DOI] [PubMed] [Google Scholar]
- 16.Ueno T., Takagi H., Fukuda N., Takahashi A., Yao E.H., Mitsumata M., Hiraoka-Yamamoto J., Ikeda K., Matsumoto K., Yamori Y. Cardiovascular remodeling and metabolic abnormalities in SHRSP.Z-Lepr(fa)/IzmDmcr rats as a new model of metabolic syndrome. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2008;31:1021–1031. doi: 10.1291/hypres.31.1021. [DOI] [PubMed] [Google Scholar]
- 17.Chen H., Sullivan G., Yue L.Q., Katz A., Quon M.J. QUICKI is a useful index of insulin sensitivity in subjects with hypertension. Am. J. Physiol. Endocrinol. Metab. 2003;284:E804–E812. doi: 10.1152/ajpendo.00330.2002. [DOI] [PubMed] [Google Scholar]
- 18.Chrysant S.G., Chrysant G.S., Chrysant C., Shiraz M. The treatment of cardiovascular disease continuum: Focus on prevention and RAS blockade. Curr. Clin. Pharmacol. 2010;5:89–95. doi: 10.2174/157488410791110742. [DOI] [PubMed] [Google Scholar]
- 19.Kochi K., Shimizu M., Ohno T., Baba A., Sumi T., Kubota M., Shirakami Y., Tsurumi H., Tanaka T., Moriwaki H. Department of Internal Medicine, Gifu University Graduate School of Medicine; 2013. Unpublished data. [Google Scholar]
- 20.Tudek B., Speina E. Oxidatively damaged DNA and its repair in colon carcinogenesis. Mutat. Res. 2012;736:82–92. doi: 10.1016/j.mrfmmm.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki Y., Imai K., Takai K., Hanai T., Hayashi H., Naiki T., Nishigaki Y., Tomita E., Shimizu M., Moriwaki H. Hepatocellular carcinoma patients with increased oxidative stress levels are prone to recurrence after curative treatment: A prospective case series study using the d-ROM test. J. Cancer Res. Clin. Oncol. 2013 doi: 10.1007/s00432-013-1389-1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hotamisligil G.S., Shargill N.S., Spiegelman B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 23.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 24.Szlosarek P., Charles K.A., Balkwill F.R. Tumour necrosis factor-alpha as a tumour promoter. Eur. J. Cancer. 2006;42:745–750. doi: 10.1016/j.ejca.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Gupta R.A., Dubois R.N. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat. Rev. Cancer. 2001;1:11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- 26.Cassis P., Conti S., Remuzzi G., Benigni A. Angiotensin receptors as determinants of life span. Pflugers Arch. 2010;459:325–332. doi: 10.1007/s00424-009-0725-4. [DOI] [PubMed] [Google Scholar]
- 27.Smith G.R., Missailidis S. Cancer, inflammation and the AT1 and AT2 receptors. J. Inflam. 2004;1:3. doi: 10.1186/1476-9255-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massiera F., Bloch-Faure M., Ceiler D., Murakami K., Fukamizu A., Gasc J.M., Quignard-Boulange A., Negrel R., Ailhaud G., Seydoux J., et al. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB. 2001;15:2727–2729. doi: 10.1096/fj.01-0457fje. [DOI] [PubMed] [Google Scholar]
- 29.Yvan-Charvet L., Massiera F., Lamande N., Ailhaud G., Teboul M., Moustaid-Moussa N., Gasc J.M., Quignard-Boulange A. Deficiency of angiotensin type 2 receptor rescues obesity but not hypertension induced by overexpression of angiotensinogen in adipose tissue. Endocrinology. 2009;150:1421–1428. doi: 10.1210/en.2008-1120. [DOI] [PubMed] [Google Scholar]
- 30.Uemura H., Ishiguro H., Nagashima Y., Sasaki T., Nakaigawa N., Hasumi H., Kato S., Kubota Y. Antiproliferative activity of angiotensin II receptor blocker through cross-talk between stromal and epithelial prostate cancer cells. Mol. Cancer Ther. 2005;4:1699–1709. doi: 10.1158/1535-7163.MCT-04-0295. [DOI] [PubMed] [Google Scholar]
- 31.Uemura H., Ishiguro H., Ishiguro Y., Hoshino K., Takahashi S., Kubota Y. Angiotensin II induces oxidative stress in prostate cancer. Mol. Cancer Res. MCR. 2008;6:250–258. doi: 10.1158/1541-7786.MCR-07-0289. [DOI] [PubMed] [Google Scholar]
- 32.Lever A.F., Hole D.J., Gillis C.R., McCallum I.R., McInnes G.T., MacKinnon P.L., Meredith P.A., Murray L.S., Reid J.L., Robertson J.W. Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet. 1998;352:179–184. doi: 10.1016/S0140-6736(98)03228-0. [DOI] [PubMed] [Google Scholar]
- 33.Lang L. ACE inhibitors may reduce esophageal cancer incidence. Gastroenterology. 2006;131:343–344. [Google Scholar]
- 34.Sipahi I., Chou J., Mishra P., Debanne S.M., Simon D.I., Fang J.C. Meta-analysis of randomized controlled trials on effect of angiotensin-converting enzyme inhibitors on cancer risk. Am. J. Cardiol. 2011;108:294–301. doi: 10.1016/j.amjcard.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 35.Hallas J., Christensen R., Andersen M., Friis S., Bjerrum L. Long term use of drugs affecting the renin-angiotensin system and the risk of cancer: A population-based case-control study. Br. J. Clin. Pharmacol. 2012;74:180–188. doi: 10.1111/j.1365-2125.2012.04170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimomoto T., Ohmori H., Luo Y., Chihara Y., Denda A., Sasahira T., Tatsumoto N., Fujii K., Kuniyasu H. Diabetes-associated angiotensin activation enhances liver metastasis of colon cancer. Clin. Exp. Metast. 2012;29:915–925. doi: 10.1007/s10585-012-9480-6. [DOI] [PubMed] [Google Scholar]
- 37.Kubota M., Shimizu M., Sakai H., Yasuda Y., Ohno T., Kochi T., Tsurumi H., Tanaka T., Moriwaki H. Renin-angiotensin system inhibitors suppress azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Biochem. Biophys. Res. Commun. 2011;410:108–113. doi: 10.1016/j.bbrc.2011.05.115. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi M., Wakabayashi K. Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents. Cancer Sci. 2004;95:475–480. doi: 10.1111/j.1349-7006.2004.tb03235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen J., Huang X.F. The signal pathways in azoxymethane-induced colon cancer and preventive implications. Cancer Biol. Ther. 2009;8:1313–1317. doi: 10.4161/cbt.8.14.8983. [DOI] [PubMed] [Google Scholar]
- 40.Reddy B.S. Studies with the azoxymethane-rat preclinical model for assessing colon tumor development and chemoprevention. Environ. Mol. Mutagenesis. 2004;44:26–35. doi: 10.1002/em.20026. [DOI] [PubMed] [Google Scholar]
- 41.Raju J. Azoxymethane-induced rat aberrant crypt foci: Relevance in studying chemoprevention of colon cancer. World J. Gastroenterol. 2008;14:6632–6635. doi: 10.3748/wjg.14.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogawa K., Hara T., Shimizu M., Ninomiya S., Nagano J., Sakai H., Hoshi M., Ito H., Tsurumi H., Saito K., et al. Suppression of azoxymethane-induced colonic preneoplastic lesions in rats by 1-methyltryptophan, an inhibitor of indoleamine 2,3-dioxygenase. Cancer Sci. 2012;103:951–918. doi: 10.1111/j.1349-7006.2012.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Primer Blast. [(on accessed 12 July 2012)]. Available online: http://www.ncbi.nlm.nih.gov/tools/primer-blast/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.