Abstract
Background: Coenzyme Q10 (CoQ10; also called ubiquinone) is an antioxidant that has been postulated to improve functional status in congestive heart failure (CHF). Several randomized controlled trials have examined the effects of CoQ10 on CHF with inconclusive results.
Objective: The objective of this meta-analysis was to evaluate the impact of CoQ10 supplementation on the ejection fraction (EF) and New York Heart Association (NYHA) functional classification in patients with CHF.
Design: A systematic review of the literature was conducted by using databases including MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, and manual examination of references from selected studies. Studies included were randomized controlled trials of CoQ10 supplementation that reported the EF or NYHA functional class as a primary outcome. Information on participant characteristics, trial design and duration, treatment, dose, control, EF, and NYHA classification were extracted by using a standardized protocol.
Results: Supplementation with CoQ10 resulted in a pooled mean net change of 3.67% (95% CI: 1.60%, 5.74%) in the EF and −0.30 (95% CI: −0.66, 0.06) in the NYHA functional class. Subgroup analyses showed significant improvement in EF for crossover trials, trials with treatment duration ≤12 wk in length, studies published before 1994, and studies with a dose ≤100 mg CoQ10/d and in patients with less severe CHF. These subgroup analyses should be interpreted cautiously because of the small number of studies and patients included in each subgroup.
Conclusions: Pooled analyses of available randomized controlled trials suggest that CoQ10 may improve the EF in patients with CHF. Additional well-designed studies that include more diverse populations are needed.
See corresponding editorial on page 233.
INTRODUCTION
Cardiovascular disease is the leading cause of morbidity and mortality in the United States and worldwide (1), and congestive heart failure (CHF)4 is the third most common cause in this category. In the United States, CHF has an estimated direct and indirect cost of $39.2 billion/y (2). Furthermore, 50% of patients with CHF will die within 5 y (3, 4).
Coenzyme Q10 (CoQ10; also called ubiquinone) is an antioxidant, the main function of which is the production of ATP through the electron transport chain. CoQ10 has been shown in all tissues and organs in the body, with highest concentrations in the heart. The CoQ10 concentration has been inversely related to the severity of CHF (5), and supplementation with CoQ10 has been postulated to improve CHF (6, 7).
Observational studies have reported that the plasma CoQ10 concentration was an independent predictor of mortality in patients with CHF (8). The potential of CoQ10 as a therapeutic agent is of great interest because of the extent of morbidity and mortality that is caused by CHF and has resulted in several clinical trials that investigated the effect of CoQ10 supplementation in patients with CHF with conflicting results (9–11). Several additional studies have been reported since the publication of the most recent meta-analysis (11–13). The objective of this meta-analysis was to examine the association between CoQ10 treatment and CHF outcomes, such as the New York Heart Association (NYHA) classification and left ventricular ejection fraction (EF), from previously published randomized controlled trials.
METHODS
Study selection
A systematic review of the literature was conducted by using electronic databases including MEDLINE (1950 to week 2 of January 2012; http://www.ncbi.nlm.nih.gov/pubmed), EMBASE (http://www.embase.com/home), the Cochrane Central Register of Controlled Trials (http://www.cochrane.org/handbook/6212-cochrane-central-register-controlled-trials-central), and the Cochrane Database of Systematic Reviews (http://www.thecochranelibrary.com/view/0/AboutTheCochraneLibrary.html). The following terms were used as Medical Subject Headings and keywords: (“cardiovascular diseases” or “heart failure”) and (“coenzyme Q10” or “ubiquinone” or “ubidecarenone”) and (“ejection fraction” or “New York Heart Association functional class” or “NYHA functional class” or “hospitalization”). The search was limited to randomized controlled trials in adults (≥19 y of age). The reference sections of all articles selected for inclusion, and previous meta-analyses were hand searched for additional articles. No language restrictions were applied. In addition, 2 unpublished studies were identified by subject-matter knowledge (LAB), and the principal investigator was contacted. However, these 2 studies were not included in this meta-analysis because the studies are still ongoing and not expected to conclude until mid to late 2012.
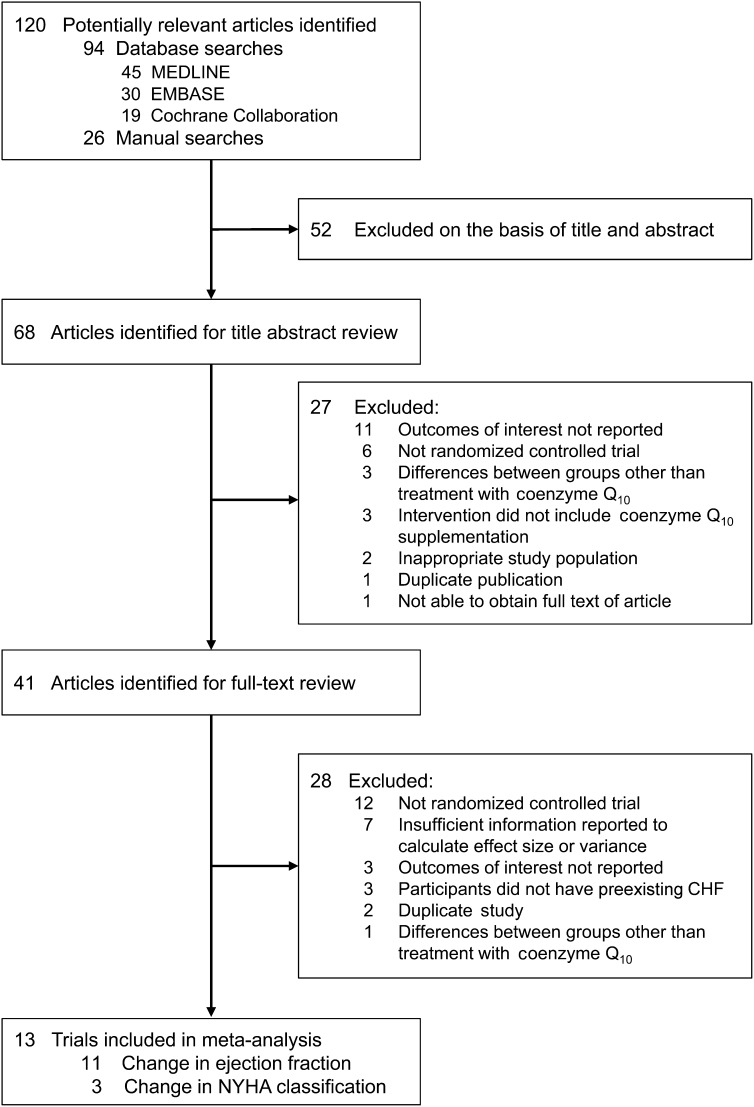
Titles and abstracts of 120 potentially relevant articles were identified and reviewed independently by 2 investigators (ADF and AMT-P) to determine whether they met eligibility criteria for inclusion. Discrepancies regarding study inclusion were resolved by consensus with the third investigator (LAB). Studies were eligible for inclusion if CoQ10 supplementation was the only intervention, a placebo control was used, and the change in at least one outcome of interest was reported or could be estimated from information provided. Primary outcomes of interest included changes in EF, NYHA functional class, and hospitalizations for heart failure. Exclusion criteria included studies in pregnant women, a measure of variance not reported, differences existing between groups other than treatment with CoQ10, and outcomes of interest not reported. Details regarding the selection of trials can be seen in Figure 1. Eleven studies reported changes in EF, and 3 studies reported changes in NYHA functional class (12–24).
FIGURE 1.
Flow diagram of study-selection process. From a total of 120 potentially relevant studies, 13 randomized controlled trials that fit predefined inclusion and exclusion criteria were included in the meta-analysis. The change in ejection fraction was reported in 11 studies, and the change in NYHA functional class was reported in 3 studies. MEDLINE: http://www.ncbi.nlm.nih.gov/pubmed; EMBASE: http://www.embase.com/home; Cochrane Collaboration: http://www.thecochranelibrary.com/view/0/AboutTheCochraneLibrary.html. CHF, congestive heart failure; NYHA, New York Heart Association.
Data abstraction
Data were independently abstracted from each of the studies by 2 investigators (ADF and AMT-P) by using a standardized data-abstraction form. Trial characteristics recorded included study design (ie, crossover or parallel), blinding (single or double blind), duration of the intervention and entire trial, type of control, description of inclusion and exclusion criteria, and demographic characteristics of study participants. Outcomes recorded included changes in EF, NYHA classification, and blood CoQ10. Because there was variability between articles used when referring to concentrations of CoQ10, plasma and serum were used only when specifically mentioned as such, whereas blood was used when referring to multiple citations that used different terms or when already used as such in the article. None of the studies reported on changes in the number or rate of hospitalizations for heart failure; therefore, this outcome was omitted from the meta-analysis.
A total of 13 unique studies (12–24) were included; however, 2 of these studies (14, 16) did not aggregate the results of 2 groups within the same trial. Because these are nonoverlapping groups of participants, data from each group were included separately in the meta-analysis. Letters A and B were used to distinguish 2 groups reported separately within one publication. Finally, the change in EF was estimated from figures for one study (16).
Quality assessment
The quality of studies was independently assessed by 2 investigators (ADF and AMT-P) by using an established tool (25). Discrepancies were discussed, and a consensus was achieved for each study included. See Table 1 under “Supplemental data” in the online issue for the quality score for each study.
Statistical analysis
Net changes in EF and NYHA classification were used to estimate effect sizes. For crossover trials, the net change was calculated as the difference between results after treatment with CoQ10 supplementation and after the control condition. For parallel trials, the change within groups was calculated (follow-up minus baseline for each condition), and net changes were calculated as the difference between CoQ10 treatment and the control condition. Variances for the net change were calculated by using reported SDs, SEs, CIs, or P values. We calculated the overall pooled-effect estimates by using inverse variance weighting to calculate both fixed-effects and DerSimonian and Laird random-effects models. The Q test was used to assess the presence of heterogeneity, and the I2 index was used to quantify the extent of heterogeneity (26, 27).
Results from the random-effects models are presented because of the heterogeneity identified in trials. A sensitivity analysis was conducted to assess the influence of individual studies on the overall results. Outliers were defined on the basis of the IQR and defined as a net change less than the first quartile minus 1.5 times the IQR or greater than the third quartile plus 1.5 times the IQR (STATA, version 10; StataCorp LP). The presence of publication bias was assessed by using a funnel plot in which the SE of each study was plotted against its corresponding effect sizes. Begg's rank correlation test was used to examine the asymmetry of the funnel plot, and Egger's linear regression test was used to examine the association between the mean effect estimate and its variance (28, 29). A cumulative meta-analysis was performed to determine whether there had been a change in the effect of CoQ10 on the EF with the addition of each subsequent trial.
Prespecified subgroup analyses were performed by study design (parallel compared with crossover), median quality scores (≤6 compared with >6 points by using the Delphi List), median treatment duration (≤12 compared with >12 wk), median year of publication (≤1993 compared with >1993), median daily dose of CoQ10 (≤100 compared with >100 mg/d), median age of study participants (≤60.6 compared with >60.6 y), and median proportion of male participants (≤82.5% compared with >82.5%). Statistical significance for each subgroup was tested by using ANOVA. A meta-regression analysis was performed to examine the association between the net change in EF and year of publication, dose, treatment duration, mean age of study participants, and proportion of male study participants as continuous variables. One post hoc subgroup analysis was performed to examine the effect of CoQ10 on the change in EF by baseline EF (<30% compared with ≥30%).
P < 0.05 was considered significant, and all tests were 2 sided. All analyses were conducted with STATA software (version 10; StataCorp LP). We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for this report (30).
RESULTS
From a total of 120 potentially relevant studies, 13 studies were included in the meta-analysis, of which the EF and NYHA classification were included as outcomes in 11 and 3 studies, respectively (Figure 1) (12–24). Baseline characteristics of studies included in the meta-analysis are described in Table 1. Studies were conducted in 7 different countries, and reports were published between 1985 and 2005. There were a total of 7 crossover and 6 parallel-arm studies, with 12 double-blinded studies and 1 single-blind study. Study duration ranged from 4 to 28 wk, and the daily dosage of CoQ10 supplement ranged from 60 to 300 mg. Compared with crossover studies, most of the parallel trials were longer in duration and included a larger number of participants. As expected, the clinical history of participants included in each trial differed slightly among trials, but all studies included participants with diagnosed CHF (Table 1).
TABLE 1.
Characteristics of 13 randomized controlled trials of CoQ10 supplementation included in the meta-analysis1
| Participant inclusion criteria |
||||||||
| First author (reference) | Year | Country | Study design | Treatment duration | Dosage of CoQ10 | Clinical indications | NYHA class | LVEF cutoff or range |
| wk | mg/d | % | ||||||
| Langsjoen (14) | 1985 | United States | X | 12 | 100 | Chronic and moderately advanced but relatively stable myocardial disease | III, IV | NA |
| Pogessi (15) | 1991 | Italy | X | 82 | 100 | Primitive or secondary dilative cardiomyopathy | II, III | 30–50 |
| Permanetter (16) | 1992 | Germany | X | 16 | 100 | Left cardiac catheterization, idiopathic dilated cardiomyopathy, and medication stable ≥2 mo | I, II, III | NA |
| Judy (17) | 1993 | United States | P | 2 | 100 | High-risk surgery candidates, low resting cardiac pumping, high LV preload, low LVEF, and low blood CoQ10 concentrations | NA | NA |
| Rengo (18) | 1993 | Italy | P | 28 | 100 | Constant dose of diuretics | III | <45 |
| Morisco (19) | 1994 | Italy | X | 4 | 150 | Clinically documented CHF, no evidence of primary pulmonary disease, and normal sinus rhythm | NA | NA |
| Hoffman-Bang (20) | 1995 | Sweden | X | 12 | 100 | Symptomatic stable chronic CHF treated ≥2 mo | NA | NA |
| Munkholm (21) | 1999 | Denmark | P | 12 | 200 | Aged 43–75 y, in sinus rhythm, IHD or DCMP, and LV diameter in diastole >60 mm by echocardiography | II, III | <45 |
| Watson (22) | 1999 | Australia | X | 12 | 99 | Aged 18–75 y, ischemic or idiopathic DCMP, chronic CHF, cared for in HF and transplant unit, and clinically stable on maximum tolerated doses of ACE inhibitor | NA | <353 |
| Khatta (23) | 2000 | United States | P | 24 | 200 | Maximal O2 consumption <17.0 mL · kg−1 · min−1 or <50% of predicted value, and medication stable ≥1 mo | III, IV | <40 |
| Keogh (24) | 2003 | Australia | P | 12 | 150 | Aged 18–80 y, baseline CHF medications stable for 2 mo, and not taking β blockers | II, III | <40 |
| Berman (12) | 2004 | Israel | P | 12 | 60 | End-stage CHF, candidates for heart transplant, and CARPET with maximal O2 consumption <14.0 mL · kg−1 · h−1 | III, IV | <25 |
| Belardinelli (13) | 2005 | Italy | X | 4 | 300 | Documented CAD, coronary angiography in past 6 mo, and clinically stable chronic HF in previous 3 mo | II, III | NA |
All studies were double-blinded, except Rengo et al (18), which was a single-blinded study. ACE, angiotensin-converting enzyme; CAD, coronary artery disease; CARPET, cardiopulmonary exercise test; CHF, congestive heart failure; CoQ10, coenzyme Q10; DCMP, dilated cardiomyopathy; HF, heart failure; IHD, ischemic heart disease; LV, left ventricular; LVEF, left ventricular ejection fraction; NA, not applicable; NYHA, New York Heart Association; P, parallel; X, crossover.
Each treatment arm was 60 d in length.
Duration ≥3 mo.
Characteristics of study participants included in the trials are described in Table 2. In a total of 395 participants included in the trials, 79% of all study participants were men, and the mean age ranged from 49.8 to 68.0 y. The baseline blood CoQ10 concentration ranged from 0.61 to 1.01 μg/mL. The EF at baseline ranged from 22% to 46%. The baseline NYHA functional class ranged from 2.3 to 3.4. See Table 2 under “Supplemental data” in the online issue for a summary of CoQ10 formulations, when included in trials, and funding sources.
TABLE 2.
Baseline characteristics of the participants included in randomized controlled trials of CoQ10 supplementation1
| First author (reference) | Year | Sample size2 | Age3 | Men4 | Blood CoQ10 | Ejection fraction | NYHA class |
| y | % | μg/mL | % | ||||
| Langsjoen – A (14) | 1985 | 8 | 60.6 | 75.0 | 0.8 ± NR5 | 43.0 | NR |
| Langsjoen – B (14) | 1985 | 11 | 64.2 | 45.0 | 0.8 ± NR | 44.0 | NR |
| Poggesi (15) | 1991 | 18 | 66.9 | 95.0 | NR | 46.6 | NR |
| Permanetter – A (16) | 1992 | 10 | 54.0 | 100.0 | NR | 39.5 | 2.3 |
| Permanetter – B (16) | 1992 | 15 | 51.0 | 69.0 | NR | 37.6 | 2.3 |
| Judy (17) | 1993 | 20 | 66.0 | 80.0 | 0.61 ± 0.09 | 31.0 | NR |
| Rengo (18) | 1993 | 60 | 68.0 | 65.0 | NR | NR | NR |
| Morisco (19) | 1994 | 6 | 49.8 | NR | NR | 29.0 | NR |
| Hoffman-Bang (20) | 1995 | 69 | 61.0 | 87.3 | 1.01 ± 0.48 | 22.0 | 2.9 |
| Munkholm (21) | 1999 | 22 | 57.0 | 68.2 | 1.05 ± 0.38 | 28.5 | NR |
| Watson (22) | 1999 | 27 | 55.0 | 87.0 | 0.8 ± 0.316 | 26.0 | NR |
| Khatta (23) | 2000 | 46 | 64.0 | 85.0 | 0.94 ± 0.5 | 28.5 | NR |
| Keogh (24) | 2003 | 35 | 61.5 | 77.1 | 0.7 ± 0.05 | NR | 2.8 |
| Berman (12) | 2004 | 27 | 54.6 | 87.5 | 0.20 ± NR | NR | 3.4 |
| Belardinelli (13) | 2005 | 21 | 59.0 | 85.7 | 0.82 ± 0.5 | 37.0 | NR |
Letters A and B were used to distinguish 2 groups reported separately within one publication. CoQ10, coenzyme Q10; NR, not reported; NYHA, New York Heart Association.
No. of participants included in the analysis.
All values are means.
For the purpose of the meta-analysis, the proportion of men was assumed to be the same for the analysis dataset as it was in randomly assigned subjects.
Mean ± SD (all such values).
Baseline concentration was reported as 903 ± 345 nmol/L.
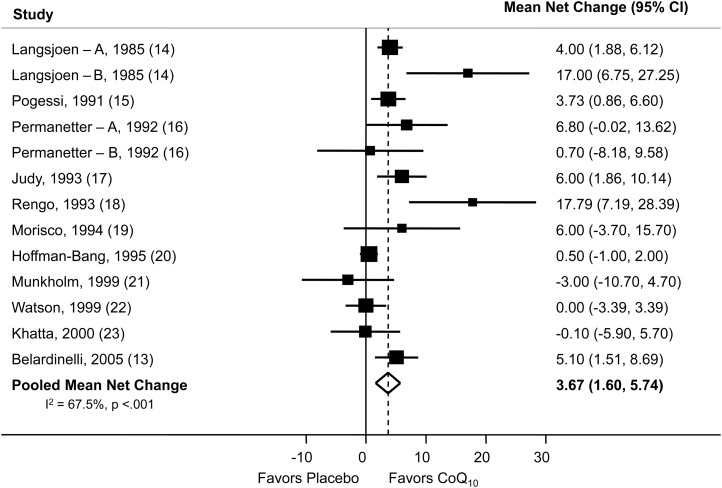
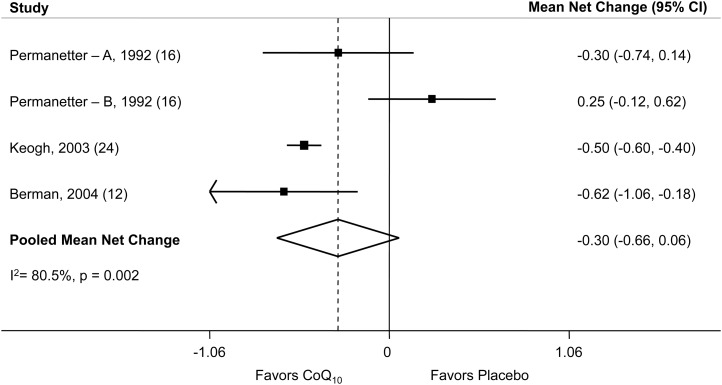
Eight studies reported changes in blood CoQ10 concentration (13, 14, 17, 20, 21, 23, 24) after supplementation, and as expected, all of these studies showed a net increase. The pooled mean net increase in blood CoQ10 concentration was 1.4 μg/mL (95% CI: 1.1, 1.7 μg/mL). CoQ10 supplementation also resulted in a pooled mean net increase in EF of 3.67% (95% CI: 1.60%, 5.74%) (Figure 2). Study-specific changes in EF, comparing CoQ10 supplementation to a placebo, ranged from −3.0% (95% CI: −10.7%, 4.7%) to 17.8% (95% CI: 7.2%, 28.4%). The pooled mean net change in NYHA classification was −0.30 (95% CI: −0.66, 0.06) (Figure 3), which indicated a slight improvement in NYHA classification after treatment with CoQ10, although not significant. Because of the small number of studies that included the NYHA heart failure classification as an outcome, these results should be interpreted cautiously. The main side effect encountered was gastrointestinal upset.
FIGURE 2.
Net percentage change in ejection fraction. The Forest plot shows the effect of CoQ10 supplementation on the ejection fraction. With the use of a random-effects model, CoQ10 supplementation resulted in a pooled mean net increase of 3.67% (95% CI: 1.60%, 5.74%) in the ejection fraction. The point estimate and the 95% CI for each study are denoted by filled squares and lines, respectively. The relative weight of the study is indicated by the size of the square. The open diamond indicates the point estimate for the pooled mean net change. Letters A and B were used to distinguish 2 groups reported separately within one publication. CoQ10, coenzyme Q10.
FIGURE 3.
Net change in NYHA classification. With the use of a random-effects model, CoQ10 supplementation resulted in a pooled mean net decrease of −0.30 (95% CI: −0.66, 0.06) for the NYHA functional class, although this change was not significant. The point estimate and the 95% CI for each study are denoted by filled squares and lines, respectively. The relative weight of the study is indicated by the size of the square. The open diamond indicates the point estimate for the pooled mean net change. Letters A and B were used to distinguish 2 groups reported separately within one publication. CoQ10, coenzyme Q10; NYHA, New York Heart Association.
There was significant heterogeneity between studies for changes in outcomes, EF (I2 = 67.5%, P < 0.001; Q test) and NYHA classification (I2 = 80.5%, P = 0.002; Q test). We showed no evidence of publication bias for either outcome as indicated by Egger's linear regression test or Begg's rank correlation test. The exclusion of any single study did not change the significance of pooled estimates for either outcome. The cumulative meta-analysis showed that the pooled net effect of CoQ10 on the EF and variance declined with the addition of subsequent studies from 9.56% (97% CI: −3.04%, 22.17%) to 3.48% (97% CI: 1.51%, 5.46%).
Analyses were conducted to evaluate the net change in EF in prespecified subgroups (Table 3). Quality scores ranged from 4 to 7 of 9 possible points, but the net improvement in the EF was significant regardless of the quality score. Similarly, the net improvement in the EF was significant in studies including both higher and lower proportions of men and in studies with an older or younger mean age. The net change in EF for CoQ10 supplementation compared with a placebo was significant for crossover trials, interventions ≤12 wk in length, and trials published before 1994 and at a daily dose ≤100 mg. The net change was not significant in parallel trials, trials published after 1993, interventions >12 wk in length, or trials that used >100 mg CoQ10/d. Subgroup analyses were not conducted for the outcome of NYHA classification because of the small number of studies included in the outcome.
TABLE 3.
Subgroup analyses of coenzyme Q10 supplementation on net change in ejection fraction
| Effect Size |
Heterogeneity |
|||||
| Subgroup | No. of studies | Pooled net change1 | P | I2 | X2 | P |
| % | ||||||
| Overall | 13 | 3.48 (1.51, 5.46) | 0.001 | 71.2 | 41.69 | <0.001 |
| Study design | ||||||
| Crossover | 9 | 3.44 (1.30, 5.58) | 0.002 | 65.7 | 23.32 | 0.003 |
| Parallel | 4 | 4.38 (−2.32, 11.07) | 0.20 | 76.0 | 12.49 | 0.006 |
| Median year of publication | ||||||
| ≤1993 | 7 | 5.97 (3.14, 8.80) | <0.001 | 56.4 | 13.76 | 0.03 |
| >1993 | 6 | 1.25 (−0.80, 3.30) | 0.23 | 37.2 | 7.97 | 0.16 |
| Median quality score | ||||||
| <6 | 4 | 6.86 (3.13, 10.59) | <0.001 | 39.5 | 4.96 | 0.18 |
| ≥6 | 9 | 2.42 (0.24, 4.59) | 0.03 | 64.1 | 22.29 | 0.004 |
| Median duration of treatment | ||||||
| ≤12 wk | 9 | 3.32 (1.17, 5.48) | 0.003 | 69.7 | 26.40 | 0.001 |
| >12 wk | 4 | 5.54 (−1.39, 12.47) | 0.12 | 68.7 | 9.57 | 0.02 |
| Median daily dose of coenzyme Q10 | ||||||
| ≤100 mg | 9 | 4.25 (1.73, 6.78) | 0.001 | 74.7 | 31.64 | <0.001 |
| >100 mg | 4 | 2.31 (−1.70, 6.31) | 0.26 | 41.3 | 5.11 | 0.16 |
| Median age of study participants | ||||||
| ≤60.6 y | 7 | 3.02 (0.82, 5.22) | 0.01 | 34.9 | 9.22 | 0.162 |
| >60.6 y | 6 | 5.22 (1.33, 9.11) | 0.01 | 80.8 | 26.08 | <0.001 |
| Median proportion of male participants | ||||||
| ≤82.5% | 6 | 5.92 (1.45, 10.38) | 0.01 | 70.2 | 16.80 | 0.005 |
| >82.5% | 7 | 2.37 (0.38, 4.37) | 0.02 | 50.2 | 12.05 | 0.061 |
| Baseline ejection fraction | ||||||
| <30% | 5 | 0.40 (−0.91, 1.70) | 0.55 | 0.0 | 2.13 | <0.001 |
| ≥30% | 7 | 4.82 (3.01, 6.59) | <0.001 | 24.4 | 7.94 | 0.24 |
All values are means; 95% CIs in parentheses. Pooled net change was calculated for each subgroup by using a random-effects model.
A post hoc subgroup analysis was performed to examine the effect of CoQ10 on the change in EF by baseline EF (<30% compared with ≥30%) and showed that the net improvement in the EF was significant for subjects with a baseline EF ≥30% (net change: 4.82; 95% CI: 3.01, 6.59), but the change was not significant for subjects with a baseline EF <30% (net change: 0.40; 95% CI: −0.91, 1.70).
DISCUSSION
In this meta-analysis, we focused on the effects of CoQ10 supplementation on clinical outcomes that directly reflected CHF, namely the EF and NYHA classification. We showed that there was a 3.7% improvement in the EF for subjects who received CoQ10 supplementation compared with a control. In addition, we showed a decrease in the NYHA functional class, although it did not reach significance, possibly because of the small number of studies that reported the NYHA classification.
Our findings were consistent with those of previous studies, which reported a net increase in EF after supplementation with CoQ10 (9–11). To our knowledge, of the meta-analyses performed to date (9–11), our meta-analysis was the most comprehensive with 13 studies. However, we were unable to include one of the largest studies that addressed CoQ10 supplementation and heart failure conducted in 1993 by Morisco et al (31), which showed a significant decrease in hospitalizations, because a measure of variance was not reported. In comparison, a 1997 meta-analysis included 6 studies and showed that CoQ10 supplementation resulted in an increase of 1.37% (P < 0.0001) in EF (9). Soja et al (9) included far fewer studies of EF and may have underestimated the effect of CoQ10 supplementation. In contrast, a meta-analysis from 2003 (10) with 7 studies reported that supplementation with CoQ10 resulted in a nonsignificant mean net increase of 1.9% (95% CI: −0.13%, 3.9%) in EF. In 2006, a meta-analysis that included 10 studies reported a mean net increase in EF of 3.68% (1.59%, 5.77%) after supplementation with CoQ10 (11).
To our knowledge, only one previous meta-analysis has examined the effect of CoQ10 supplementation on the NYHA classification, and it reported a change of −0.09 (−0.037, 0.18) in the NYHA classification, which correlated to an improvement (a decrease in severity) that was similar to our findings (10).
We showed differences in the effect of supplementation with CoQ10 by subgroups. Supplementation resulted in a significant improvement in the EF in crossover trials, trials conducted before 1994, trials ≤12 wk in length, trials that used doses ≤100 mg CoQ10/d, and trials that included participants with a baseline EF ≥30%. Although these subgroup analyses indicated possible differential effects in these groups, the results should be interpreted with caution because of the small number of studies included in each subgroup, the small number of participants in each study, and the large number of statistical tests involved in subgroup analyses. In addition, there may be other explanations for these subgroup findings. Concomitant medication usage was not reported in all studies and was inconsistently reported in studies that included the information. The earlier studies also included patients with an EF ≥30%, whereas the majority of later studies included patients with an EF <30%, which indicated that a sicker patient population may have been examined in the later studies. One additional consideration was that we were not able to examine the change in NYHA classification by subgroups.
Finally, most of the earlier studies relied on doses ≤100 mg CoQ10/d, whereas the daily dose of CoQ10 used in more recent studies ranged from 60 to 300 mg/d The dose required to produce a significant improvement in the EF is not known, and whether the dose required is dependent on the severity of CHF or baseline EF is also not known. Higher doses of CoQ10 may be required to adjust for the increasing severity of metabolic dysfunction and energy depletion that occurs at more severe levels of heart failure (5). It has been suggested that blood CoQ10 concentrations should be >2 μg/mL to improve the EF in subjects with more severe heart failure (32). Of the studies included in this meta-analysis, only 3 studied achieved this concentration of blood CoQ10 after the intervention (13, 21, 24). Belardinelli et al (13) included patients with an EF ≥30% and showed a significant improvement in the EF after 4 wk of supplementation. Munkholm et al (21) included patients with an EF <30% and failed to find a significant improvement in the EF after 12 wk of supplementation. Keogh et al (24) did not report the baseline EF in their patient population; however, they did report a significant improvement in the NYHA classification after 12 wk of supplementation.
CoQ10 has 2 important roles as an integral component of the mitochondrial respiratory chain used for ATP production (33–35) and the only lipid-soluble antioxidant that slows lipid peroxidation in the circulation (7, 36). The biosynthesis of CoQ10 has several steps in common with the synthesis of cholesterol (35), and studies have indicated that statin use has also been associated with decreased blood CoQ10 concentrations (37–41). Furthermore, some studies have shown a correlation between blood and myocardial concentrations of CoQ10 (42, 43). Also, there is some evidence that has suggested that decreased myocardial function is associated with decreased CoQ10 myocardial tissue concentrations (5, 17, 44).
Our meta-analysis had several strengths. We only selected randomized controlled trials that had both CoQ10 and placebo control groups, which made our meta-analysis less subject to bias. In addition, all articles reported a measure of variance, and CHF outcomes of interest were based on useful and clinically practical outcomes, such as the change in EF and NYHA class.
Our study also had several limitations. First, there was limited information available on study participant characteristics such as race, blood pressure, weight, comorbid conditions, or medication regimens. Furthermore, women were markedly underrepresented and constituted ∼15% of participants across studies. Included studies spanned 2 decades, and over this time period, the standard of care for CHF has changed considerably. It is difficult to determine the effect that changes in the standard of care may have had and the effect of CoQ10 supplementation. An additional limitation was the size of these trials, which ranged between 6 and 69 participants. Therefore, our meta-analysis may have been underpowered to detect a true effect. Finally, ≥6 different formulations of CoQ10 were used, which may have affected absorption.
Our meta-analysis suggested that CoQ10 supplementation may be beneficial for patients with CHF; however, additional, well-designed studies that include more diverse populations are needed. Currently, there is a large multicenter, randomized, controlled trial under way that is investigating the impact of CoQ10 on cardiovascular morbidity and mortality over a 2-y period (45). To our knowledge, this study is the largest and longest study of CoQ10 on cardiovascular disease, and its results will provide additional information.
In conclusion, CHF is a leading cause of morbidity and mortality in the United States and worldwide. The findings of this meta-analysis suggest that supplementation with CoQ10 may be of benefit in patients. However, our findings also suggest that the benefit may be limited to patients with less severe stages of CHF, such as patients with an EF ≥30% or those with an NYHA class of II or III. Because of the small number of studies and patients included in this meta-analysis, the results should be interpreted with caution. Although there is a possible benefit of supplementation with CoQ10, additional larger studies are warranted and should examine whether there is an effect when this supplementation is added to the current standard of therapy for CHF or whether there is a dose-response effect between the stage of CHF at baseline and the dose of CoQ10 required for an improvement to be seen.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—ADF and AMT-P: had full access to all study data, took responsibility for the integrity of data and accuracy of the data analysis, and drafted and revised the manuscript; and LAB: provided critical oversight of the project and revisions to the manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: CHF, congestive heart failure; CoQ10, coenzyme Q10; EF, ejection fraction; NYHA, New York Heart Association.
REFERENCES
- 1.World Health Organization. The top 10 causes of death. 2008. 1 p. Report No.: Fact Sheet No. 310. Available from: www.who.int/mediacentre/factsheets/fs310/en/index.html.
- 2.Writing Group Members Lloyd-Jones D, Adams RJ, Brown TM, Carpethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2010 update: a report from the American Heart Asssociation. Circulation 2010;121:e46–215 [DOI] [PubMed] [Google Scholar]
- 3.Levy D, Kenchaiah S, Larson MG, Benjamin E, Kupka M, Ho Kalon K, Murabito J, Vasan R. Long-term trends in the incidence of and survival with heart failure. N Engl J Med 2002;347:1397–402 [DOI] [PubMed] [Google Scholar]
- 4.Roger VL, Weston SA, Redfield MM, Hellemann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA 2004;292:344–50 [DOI] [PubMed] [Google Scholar]
- 5.Folkers K, Vadhanavikit S, Mortensen SA. Biochemical rationale and myocardial tissue data on the effective therapy of cardiomyopathy with coenzyme Q10. Proc Natl Acad Sci USA 1985;82:901–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langsjoen PH, Langsjoen AM. Overview of the use of CoQ10 in cardiovascular disease. Biofactors 1999;9:273–84 [DOI] [PubMed] [Google Scholar]
- 7.Belardinelli R, Tiano L, Littarru GP. Oxidative stress, endothelial function and coenzyme Q10. Biofactors 2008;32:129–33 [DOI] [PubMed] [Google Scholar]
- 8.Molyneux SL, Florkowski CM, George PM, Pilbrow AP, Frampton CM, Lever M, Richards AM. Coenzyme Q10: an independent predictor of mortality in chronic heart failure. J Am Coll Cardiol 2008;52:1435–41 [DOI] [PubMed] [Google Scholar]
- 9.Soja AM, Mortensen SA. Treatment of congestive heart failure with coenzyme Q10 illuminated by meta-analyses of clinical trials. Mol Aspects Med 1997;18(suppl):S159–68 [DOI] [PubMed] [Google Scholar]
- 10.Rosenfeldt F, Hilton D, Pepe S, Krum H. Systematic review of effect of coenzyme Q10 in physical exercise, hypertension and heart failure. Biofactors 2003;18:91–100 [DOI] [PubMed] [Google Scholar]
- 11.Sander S, Coleman CI, Patel AA, Kluger J, White CM. The impact of coenzyme Q10 on systolic function in patients with chronic heart failure. J Card Fail 2006;12:464–72 [DOI] [PubMed] [Google Scholar]
- 12.Berman M, Erman A, Ben-Gal T, Dvir D, Georghiou GP, Stamler A, Vered Y, Yidpe BA, Aravot D. Coenzyme Q10 in patients with end-stage heart failure awaiting cardiac transplantation: a randomized, placebo-controlled study. Clin Cardiol 2004;27:295–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belardinelli R, Mucaj A, Lacalaprice F, Solenghi M, Tiano L, Littarru GP. Coenzyme Q10 improves contractility of dysfunctional myocardium in chronic heart failure. Biofactors 2005;25:137–45 [DOI] [PubMed] [Google Scholar]
- 14.Langsjoen PH, Vadhanavikit S, Folkers K. Effective treatment with coenzyme Q10 of patients with chronic myocardial disease. Drugs Exp Clin Res 1985;11:577–9 [PubMed] [Google Scholar]
- 15.Pogessi L, Galanti G, Comeglio M, Toncelli L, Vinci M. Effect of coenzyme Q10 on left ventricular function in patients with dilative cardiomyopathy. Curr Therapeutic Res 1991;49:878–86 [Google Scholar]
- 16.Permanetter B, Rossy W, Klein G, Weingartner F, Seidl KF, Blomer H. Ubiquinone (coenzyme Q10) in the long-term treatment of idiopathic dilated cardiomyopathy. Eur Heart J 1992;13:1528–33 [DOI] [PubMed] [Google Scholar]
- 17.Judy WV, Stogsdill WW, Folkers K. Myocardial preservation by therapy with coenzyme Q10 during heart surgery. Clin Investig 1993;71(suppl):S155–61 [DOI] [PubMed] [Google Scholar]
- 18.Rengo F, Abete P, Landino P, Leosco D, Covelluzzi F, Vitale D, Fedi V, Ferrara N. Role of metabolic therapy in cardiovascular disease. Clin Investig 1993;71(suppl):S124–8 [DOI] [PubMed] [Google Scholar]
- 19.Morisco C, Nappi A, Argenziano L, Sarno D, Fonatana D, Imbriaco M, Nicolai E, Romano M, Rosiello G, Cupcolo A. Noninvasive evaluation of cardiac hemodynamics during exercise in patients with chronic heart failure: effects of short-term coenzyme Q10 treatment. Mol Aspects Med 1994;15(suppl):s155–63 [DOI] [PubMed] [Google Scholar]
- 20.Hofman-Bang C, Rehnqvist N, Swedberg K, Wiklund I, Astrom H. Coenzyme Q10 as an adjunctive in the treatment of chronic congestive heart failure. the Q10 study group. J Card Fail 1995;1:101–7 [DOI] [PubMed] [Google Scholar]
- 21.Munkholm H, Hansen HH, Rasmussen K. Coenzyme Q10 treatment in serious heart failure. Biofactors 1999;9:285–9 [DOI] [PubMed] [Google Scholar]
- 22.Watson PS, Scalia GM, Galbraith A, Burstow DJ, Bett N, Aroney CN. Lack of effect of coenzyme Q on left ventricular function in patients with congestive heart failure. J Am Coll Cardiol 1999;33:1549–52 [DOI] [PubMed] [Google Scholar]
- 23.Khatta M, Alexander BS, Krichten CM, Fisher MI, Freudenberger R, Robinson SW, Gottlieb SS. The effect of coenzyme Q10 in patients with congestive heart failure. Ann Intern Med 2000;132:636–40 [DOI] [PubMed] [Google Scholar]
- 24.Keogh A, Fenton S, Leslie C, Aboyoun C, Macdonald P, Zhao YC, Bailey M, Rosenfeldt F. Randomised double-blind, placebo-controlled trial of coenzyme Q, therapy in class II and III systolic heart failure. Heart Lung Circ 2003;12:135–41 [DOI] [PubMed] [Google Scholar]
- 25.Verhagen AP, de Vet HCW, de Bie RA, Kessels-Alphons GH, Boers M, Bouter LM, Knipschild PG. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 1998;51:1235–41 [DOI] [PubMed] [Google Scholar]
- 26.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58 [DOI] [PubMed] [Google Scholar]
- 27.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods 2006;11:193–206 [DOI] [PubMed] [Google Scholar]
- 28.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101 [PubMed] [Google Scholar]
- 29.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, Clark M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W65–94 [DOI] [PubMed] [Google Scholar]
- 31.Morisco C, Timarco B, Condorelli M. Effect of coenzyme Q10 therapy in patients with congestive heart failure: a long-term multicenter randomized trial. Clin Investig 1993;71:S134–6 [DOI] [PubMed] [Google Scholar]
- 32.Langsjoen PH. Lack of effect of coenzyme Q on left ventricular function in patients with congestive heart failure. J Am Coll Cardiol 2000;35:816–7 [DOI] [PubMed] [Google Scholar]
- 33.Littarru GP, Tiano L. Clinical aspects of coenzyme Q10: an update. Nutrition 2010;26:250–4 [DOI] [PubMed] [Google Scholar]
- 34.Crane FL, Navas P. The diversity of coenzyme Q function. Mol Aspects Med 1997;18(suppl):S1–6 [DOI] [PubMed] [Google Scholar]
- 35.Hargreaves IP. Ubiquinone: cholesterol's reclusive cousin. Ann Clin Biochem 2003;40:207–18 [DOI] [PubMed] [Google Scholar]
- 36.Littarru GP, Tiano L. Bioenergetic and antioxidant properties of coenzyme Q10: recent developments. Mol Biotechnol 2007;37:31–7 [DOI] [PubMed] [Google Scholar]
- 37.Berthold HK, Naini A, Di Mauro S, Hallikainen M, Gylling H, Krone W, Gouni-Berthold I. Effect of ezetimibe and/or simvastatin on coenzyme Q10 levels in plasma: a randomised trial. Drug Saf 2006;29:703–12 [DOI] [PubMed] [Google Scholar]
- 38.De Pinieux G, Chariot P, Ammi-Saïd M, Louarn F, Lejonc JL, Astier A, Jacotot B, Gherardi R. Lipid-lowering drugs and mitochondrial function: effects of HMG-CoA reductase inhibitors on serum ubiquinone and blood lactate/pyruvate ratio. Br J Clin Pharmacol 1996;42:333–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mortensen SA, Leth A, Agner E, Rohde M. Dose-related decrease of serum coenzyme Q10 during treatment with HMG-CoA reductase inhibitors. Mol Aspects Med 1997;18(suppl):S137–44 [DOI] [PubMed] [Google Scholar]
- 40.Ghirlanda G, Oradei A, Manto A, Lippa S, Uccioli L, Caputo S, Greco AV, Littarru GP. Evidence of plasma CoQ10-lowering effect by HMG-CoA reductase inhibitors: a double-blind, placebo-controlled study. J Clin Pharmacol 1993;33:226–9 [DOI] [PubMed] [Google Scholar]
- 41.Mabuchi H, Higashikata T, Kawashiri M, Katsuda S, Mizuno M, Nohara A, Inazu A, Koizumi J, Kobayashi J. Reduction of serum ubiquinol-10 and ubiquinone-10 levels by atorvastatin in hypercholesterolemic patients. J Atheroscler Thromb 2005;12:111–9 [DOI] [PubMed] [Google Scholar]
- 42.Keith M, Mazer CD, Mikhail P, Jeejeebhoy F, Briet F, Errett L. Coenzyme Q10 in patients undergoing CABG: effect of statins and nutritional supplementation. Nutr Metab Cardiovasc Dis 2008;18:105–11 [DOI] [PubMed] [Google Scholar]
- 43.Nobuyoshi M, Saito T, Takahira H, Yamano Y, Kanazawa T. Levels of coenzyme Q10 in biopsies of left ventricular muscle and influence of administration of coenzyme Q10. Biomedical and Clinical Aspects of Coenzyme Q. 1984;4:221–9 [Google Scholar]
- 44.Kitamura N, Yamaguchi A, Otaki M, Sawatani O, Minoji T, Tamura H, Atobe M. Myocardial tissue level of coenzyme Q10 in patients with cardiac failure. Biomedical and Clinical Aspects of Coenzyme Q. 1984;4:221–9 [Google Scholar]
- 45.Mortensen SA. Overview on coenzyme Q10 as adjunctive therapy in chronic heart failure. rationale, design and end-points of “Q-symbio”–a multinational trial. Biofactors 2003;18:79–89 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.