Abstract
Background: The relative contributions of fat and protein to the incretin effect are still largely unknown.
Objective: This study assessed the incretin effects elicited by a mixed meal, and by its fat and protein components alone, with the use of a hyperglycemic clamp combined with oral nutrients.
Design: Eight healthy volunteers were studied over 6 h after ingestion of a sandwich containing 1) dried meat, butter, and white bread; 2) dried meat alone; 3) butter alone; or 4) no meal (fasting control). Meals were ingested during a hyperglycemic clamp, and the incretin effect was calculated as the increment in plasma insulin after food intake relative to the concentrations observed during the control study.
Results: A significant augmentation of postprandial insulin secretion, independent of plasma glycemia, occurred after ingestion of the mixed nutrients and the lipid component of the mixed meal (203 ± 20.7% and 167.4 ± 22.9% of control, respectively; both P < 0.05), whereas the protein component did not induce a significant incretin effect (129.0 ± 7.9% of control; P = 0.6)
Conclusions: Fat ingestion, in an amount typical of a standard meal, increases insulin secretion during physiologic hyperglycemia and thus contributes to the incretin effect. In contrast, ingestion of protein typical of normal meals does not contribute to the augmentation of postprandial insulin secretion. This trial was registered at clinicaltrials.gov as NCT00869453.
INTRODUCTION
Orally administered glucose elicits a stronger insulin response than does an isoglycemic intravenous glucose infusion (1). The potentiation of glucose-induced insulin secretion by enteral glucose is called the “incretin effect” and has been attributed to the insulinotropic actions of 2 peptides secreted from the intestine: GLP-14 and GIP. These 2 incretins are also secreted during mixed nutrient meals when insulin secretion is augmented beyond the effects of hyperglycemia alone (2). Thus, a broad view of the incretin effect is that it represents the sum of the extraglycemic regulatory factors stimulating postprandial insulin secretion (3).
The regulation of GLP-1 and GIP release has been the object of many studies. Both are rapidly secreted into the hepatic portal venous system by specific enteroendocrine cells located in intestinal mucosa in response to food ingestion (4). The magnitude of GLP-1 and GIP secretion is proportionate to the caloric content of meals (5). Although carbohydrates seem to be the strongest activator of GLP-1 and GIP secretion, ingested fat also reliably stimulates their release (6, 7), whereas dietary proteins have had a more variable effect on incretin release (8, 9).
Most previous studies of the incretin effect have focused on the role of glucose to stimulate GIP and GLP-1 and enhance insulin secretion (1, 10–12); very few have assessed the effects of protein or fat (4–6, 11). Of these latter studies, plasma concentrations of GLP-1 and GIP were the primary outcome measurements, and the nutrient stimuli used were greater than what is typically consumed in normal meals. The incretin effect of separate nutrients consumed in amounts typical of normal eating has not been evaluated. In this study, we measured postprandial insulin secretion in response to a complete mixed meal, and to its fat and protein constituents ingested alone, by using a hyperglycemic clamp method that allows assessment of separate nutrient effects under isoglycemic conditions (13, 14). This approach allowed us to document that the fat, but not the protein, content of a mixed nutrient meal elicited a significant incretin effect.
SUBJECTS AND METHODS
Subjects
Eight healthy men with a mean (±SEM) age of 23.3 ± 0.7 y participated in the study. All subjects were in apparent good health on the basis of medical history and a brief physical examination, and none of them was taking any medication during the study. They had a BMI (in kg/m2) between 19 and 25 (22.4 ± 0.54), were nonsmokers, and were moderately physically active (<3 strenuous physical activity sessions/wk). The study protocol was approved by the ethics committee of the University of Lausanne, and each volunteer gave a written informed consent.
Study design
At inclusion, subjects underwent a brief medical examination, and body composition was estimated by skinfold thickness measurements (15). Each volunteer was then studied on 4 separate days, each separated by an interval of ≥1 mo. On 3 of these 4 d, subjects consumed 1of the following test meals: 1) a dried meat and butter sandwich on white bread; 2) butter alone, with a fat content corresponding to that of the sandwich; and 3) dried meat alone, with a protein content corresponding to that of the sandwich. Details of the meal composition are summarized in Table 1. On day 4, the subjects remained fasting and insulin secretion was measured in response to hyperglycemia over the full 4 h of the study.
TABLE 1.
Composition of the different test meals
| Meal content | Proteins | Lipids | Carbohydrates |
| g | g | g | |
| Sandwich | |||
| 120 g White bread | 10.9 | 1.1 | 63.2 |
| 20 g Butter | 0.1 | 16.4 | 0.1 |
| 10 g Dried meat | 3.9 | 0.5 | 0.1 |
| Total | 14.9 | 18.0 | 63.4 |
| Dried meat alone | |||
| 40 g Dried meat | 15.4 | 2.0 | 0.2 |
| Butter alone | |||
| 20 g Butter | 0.1 | 16.4 | 0.1 |
Metabolic investigation
After a 10-h overnight fast, the subjects arrived in the Cardiomet Clinical Investigation Center of Lausanne University Hospital at 0700. On arrival, the subjects were asked to void, and then were weighed and had their body composition assessed by bioimpedance plethysmography (Bioimpedance Analyzer BIA 101; Akern Srl). Thereafter, they rested in a semirecumbent position in bed until completion of the test. An intravenous catheter was inserted into a forearm vein to allow repeated blood sampling. The hand corresponding to this catheter was maintained in a thermostabilized box heated at 50°C to achieve partial arterialization of venous blood. A second catheter was inserted into a vein of the contralateral forearm for a variable 20% glucose (G 20; B. Braun Medical AG) infusion. After removal of the fasting blood samples, the glucose infusion was started to raise and maintain plasma concentrations at 8 mmol/L. At time −120 min, a bolus of 6,6-2H2-glucose (3.3 mg/kg over 10 min) was given, which was followed by a continuous infusion (33 μg · kg−1 · min−1) for the remainder of the study. After 30 min for tracer equilibration, a variable 20% (vol:vol) glucose infusion was started (time −90 min). Blood glucose was measured every 5 min at the subject's bedside by using a Beckmann glucose analyzer (Beckmann Instruments) and the glucose infusion rate adjusted to maintain the glycemic target. After 90 min of the hyperglycemic clamp (time 0 min), subjects consumed one of the 3 test meals, except on the day of the control experiment where they did not eat. Thereafter, the glucose infusion was varied to maintain the glucose clamp for a further 240 min. Blood was sampled for hormone measurements every 30 min during the first 90 min, every 20 min for the 2 h after the meal, and every 60 min during the final 2 h.
Energy expenditure and substrate oxidation were continuously monitored during the test by open-circuit indirect calorimetry (Deltatrac II; Datex Instruments). At the end of the test, urine was collected for determination of the urea nitrogen excretion rate.
Analytic procedures
Blood samples were collected in EDTA (insulin), trasylol-EDTA (GIP), or EDTA and dipeptidyl peptidase-4 inhibitor (GLP-1). Blood was immediately centrifuged and stored at −20 or −80°C (GLP-1 and GIP) until analyzed. Insulin concentrations were measured in plasma, and GLP-1 concentrations were measured in ethanol extracts of plasma by using radioimmunoassay kits (Millipore Corporation) according to the manufacturer's instructions. GIP was measured by ELISA (Millipore Corporation). Plasma 6,6-2H2-glucose enrichment was measured by gas chromatography–mass spectroscopy (GC/MS; Agilent Technologies), as previously described (16).
Calculations
Determination of peak values
For all variables tested, a postmeal peak was defined as a value that was ≥10% higher than the preceding and subsequent time points. The peak value and the time of peak occurrence were recorded and averaged.
Incretin effect
The incretin effect (expressed as %) was computed in each study as follows:
where AUCmeal is the area under the curve of insulin, calculated from 0 to 240 min for each test meal, and AUCfast is the area under the curve for insulin calculated during the same period in the fasting condition. This procedure allows the progressive increase in plasma insulin concentration observed during hyperglycemic clamps to be accounted for.
Glucose turnover
Total GRd was calculated from plasma 6,6-2H2-glucose enrichment by using Steele's non–steady state equations as modified by DeBodo et al using a volume of distribution for glucose of 0.2 × body weight and a pool fraction of 0.75 (17, 18).
Substrate oxidation and energy expenditure
Net carbohydrate and lipid oxidation rates and total energy expenditure were calculated from respiratory gas exchanges by using the equation of Livesey and Elia (19).
Statistical analysis
All results are expressed as means ± SEMs. Postprandial hormone secretion is expressed as incremental areas under the curve calculated by using the trapezoidal rule. Final data were plotted and assessed visually; skewed distributions (GIP) were log transformed before statistical analysis. For plasma glucose, insulin, GLP-1, and GIP and energy expenditure/substrate oxidation over time, between-test comparisons were done by using 2-factor ANOVA with interaction. For the incretin effect, 1-factor ANOVA followed by Tukey's post hoc test was used. All statistical calculations were made by using “R” version 2.9.1—open source statistical software (20). P < 0.05 was considered significant.
RESULTS
Hyperglycemic clamps
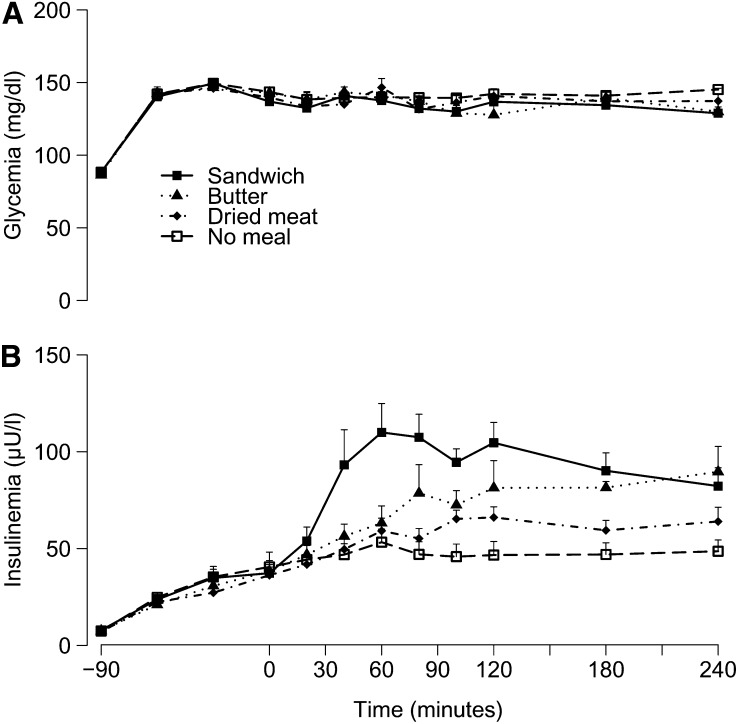
Plasma glucose was maintained between 136 and 141 mg/dL (7.5 and 7.8 mmol/L) in all tests and did not differ significantly (P = 0.3) between the 4 conditions tested: sandwich, 136.7 ± 1.3 mg/dL (7.6 ± 0.1 mmol/L); butter, 138.3 ± 1.5 (7.7 ± 0.1 mmol/L); dried meat, 138.8 ± 1.3 (7.7 ± 0.1 mmol/L); and fasting: 141.8 ± 0.9 mg/dL (7.9 ± 0.1 mmol/L) (Figure 1A). No interaction effect was found between meal and time (P = 0.4).
FIGURE 1.
Mean (±SEM) glycemia (A) and insulinemia (B) over time after meal ingestion (0 min). Two-factor ANOVA was used for comparison between the 4 conditions. Mean glycemia was not different between the 4 conditions (P = 0.3). There was a significant effect of time (P < 0.001) and no significant interaction between meal and time (P = 0.4). The insulin response, expressed as AUC, was significantly different from the “no meal” condition after ingestion of the sandwich meal (P < 0.001) and the butter meal (P < 0.05). Ingestion of dried meat alone did not induce a significant insulin response (P = 0.6). There was a significant effect of meal (P < 0.001) and time (P < 0.001) and a significant interaction between meal and time (P < 0.001) on insulinemia.
Plasma insulin
During the initial 90 min of the hyperglycemic clamp before meal ingestion, plasma insulin increased from a mean basal concentration of 0.36 ± 0.01 pg/L (7.47 ± 0.30 μU/L) (range for the 4 studies) to an average concentration of 1.70 ± 0.1 pg/L (38.07 ± 2.68 μU/L) at time 0. After meal ingestion, a rapid increase in insulin secretion occurred in the studies with the sandwich [5.88 ± 0.63 pg/L (129.3 ± 13.9 μU/L) at 80 ± 12 min]. After the butter and the dried meat meals, and during the hyperglycemic clamp with no meal, insulin concentrations increased progressively until the end of the test [butter: 4.99 ± 0.66 pg/L (109.7 ± 14.4 μU/L); dried meat: 3.61 ± 0.19 pg/L (79.3 ± 4.2 μU/L); and fasting: 2.61 ± 0.30 pg/L (57.4 ± 6.6 μU/L)] (Figure 1B). A statistically significant effect of meal (P < 0.001), time (P < 0.001), and interaction between meal and time were found (P < 0.001).
When insulin responses were expressed as area under the curve, the sandwich elicited the largest insulin secretion (21,614 ± 2062), followed by the butter (17,561 ± 1627), the dried meat (13,907 ± 1097), and the fasted (11,288 ± 1465 μU/mL · 240 min) conditions. The insulin response was significantly different from that of the fasting condition after ingestion of the whole sandwich (P < 0.001) and butter alone (P < 0.05). Ingestion of dried meat alone did not induce any significant insulin secretion relative to the fasting condition (P = 0.6).
Incretin effect
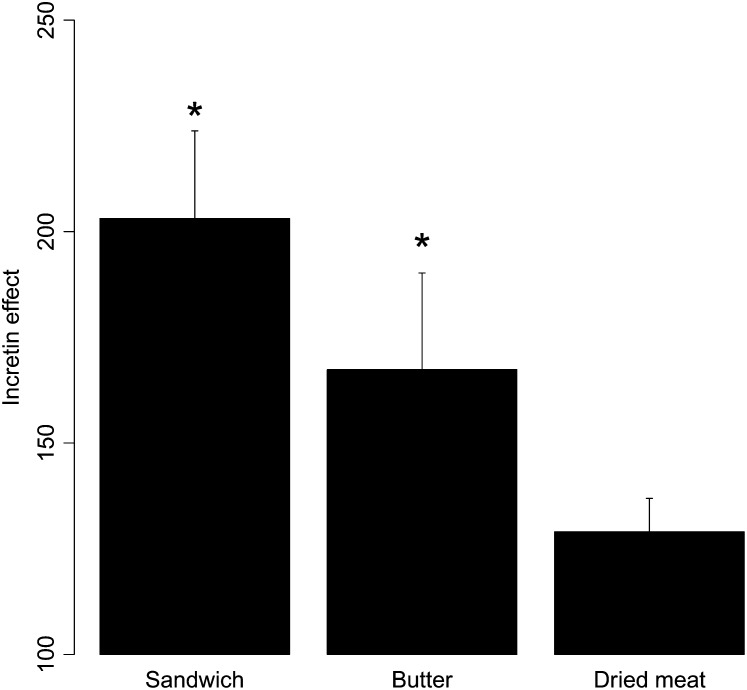
A significant incretin effect was measured after ingestion of the whole sandwich and of butter alone, (203.1 ± 20.7 and 167.4 ± 22.9% of the fasting insulin secretion, respectively; both P < 0.05 compared with no meal). Ingestion of dried meat alone did not induce any significant incretin effect (129.0 ±7.9%; P = 0.7) (Figure 2).
FIGURE 2.
Mean (±SEM) incretin effect observed after ingestion of the sandwich, butter, or dried meat meal. The incretin effect is expressed as the percentage of insulin concentrations observed with no meal. ANOVA with Tukey's post hoc test was used for comparison between the 3 conditions. A significant incretin effect was observed after sandwich and butter ingestion. *Significantly different from no meal, P < 0.05.
Glucose utilization
GRd was calculated over the 240 min after ingestion of the meal as a global index of insulin's action. Total GRd was higher after the sandwich (2281.5 ± 238.6 mg/kg · 240 min), followed by butter alone (2236.6 ± 84.6 mg/kg · 240 min), dried meat (2284.6 ± 123.6 mg/kg · 240 min), and fasting (1737.8 ± 146.9 mg/kg · 240 min). GRd was significantly higher with the 3 fed conditions than with the fast (after sandwich: P < 0.001; after butter: P = 0.009; and after dried meat: P = 0.02). Grd was higher after sandwich ingestion than after both butter (P = 0.02) and dried meat (P = 0.007) ingestion.
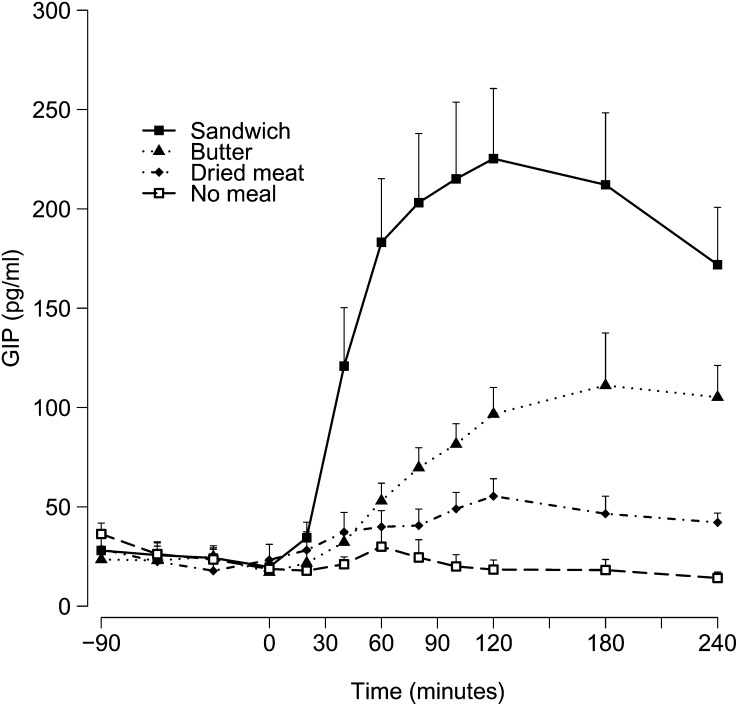
GIP concentrations
The total increase in GIP concentrations was greatest after ingestion of the whole sandwich (42,227 ± 6226 pg/mL · 240 min; P < 0.001 compared with the fasted conditions), followed by that elicited by ingestion of butter alone (19,029 ± 1942 pg/mL · 240 min; P < 0.001) and of dried meat alone (10,406 ± 1687 pg/mL · 240 min; P = 0.4). The time course of GIP concentrations was similar to that of insulin and GRd (Figure 3), with clear peaks in concentrations after ingestion of the whole sandwich [256.2 ± 32.5 pg/mL (51.42 ± 6.52 pmol/L) at 130 ± 20 min]. After consumption of butter and dried meat, GIP concentrations increased gradually to culminate at the end of the test [butter: 152.1 ± 16.9 pg/mL (30.53 ± 33.39 pmol/L) at 175 min; dried meat: 62.5 ± 7.4 pg/mL (12.54 ± 1.49 pmol/L) at 130 ± 20 min]. Over time, a significant effect of meal (P < 0.0001) and time (P < 0.0001) and a significant interaction effect (P < 0.0001) on GIP concentrations were found.
FIGURE 3.
Mean (±SEM) GIP concentrations over time after meal ingestion (0 min). Two-factor ANOVA was used for comparison between the 4 conditions. There was a significant effect of meal (P < 0.0001) and time (P < 0.0001) and a significant interaction between meal and time (P < 0.0001). GIP, glucose-dependent insulinotropic polypeptide.
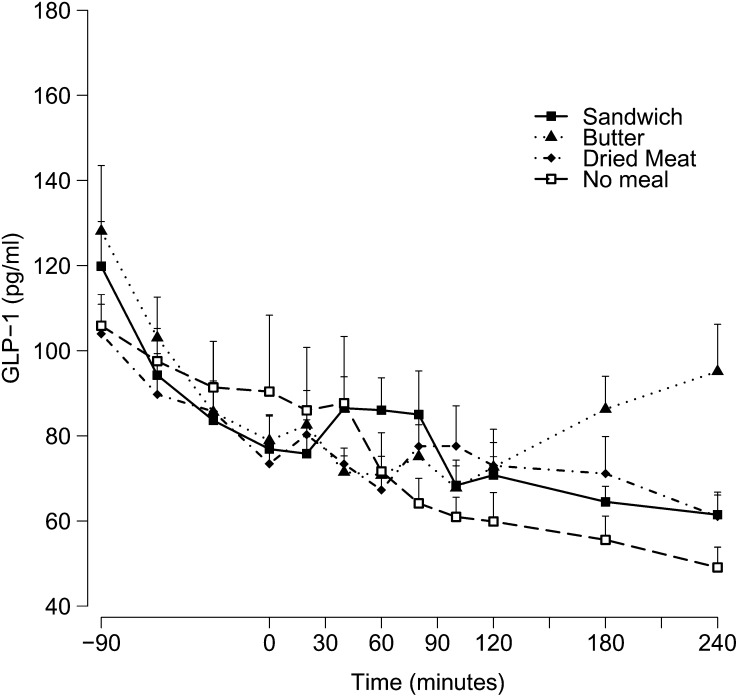
GLP-1 concentrations
In all tests, GLP-1 concentrations decreased from fasting concentrations during the hyperglycemic clamp. During the control study when no meal was ingested, GLP-1 concentrations decreased continuously, by ∼50% at time 240 min, ie, after 5.5 h of hyperglycemic clamp. Concentrations of GLP-1 during the 3 meal studies did not decrease after time 0, and there was a trend toward an increase with ingestion of the sandwich and butter (Figure 4). No statistically significant effect of meal was found (P = 0.07), but a significant effect of time (P < 0.0001) and a significant interaction between meal and time (P = 0.02) were found. The highest GLP-1 area under the curve was observed after ingestion of butter alone (19,081 ± 1122 pg/mL · 240 min), followed by ingestion of the whole sandwich (17,346 ± 855 pg/mL · 240 min) and the dried meat (17,272 ± 1254 pg/mL· 240 min). These values, however, were not significantly different from those observed in fasted conditions (15,512 ± 1356 pg/mL · 240 min).
FIGURE 4.
Mean (±SEM) GLP-1 concentrations after meal ingestion (0 min). Two-factor ANOVA was used for comparison between the 4 conditions. There was no significant difference between the 4 conditions (P = 0.07), but there was a significant effect of time (P < 0.001) and a significant interaction between meal and time (P = 0.02). GLP-1, glucagon-like peptide 1.
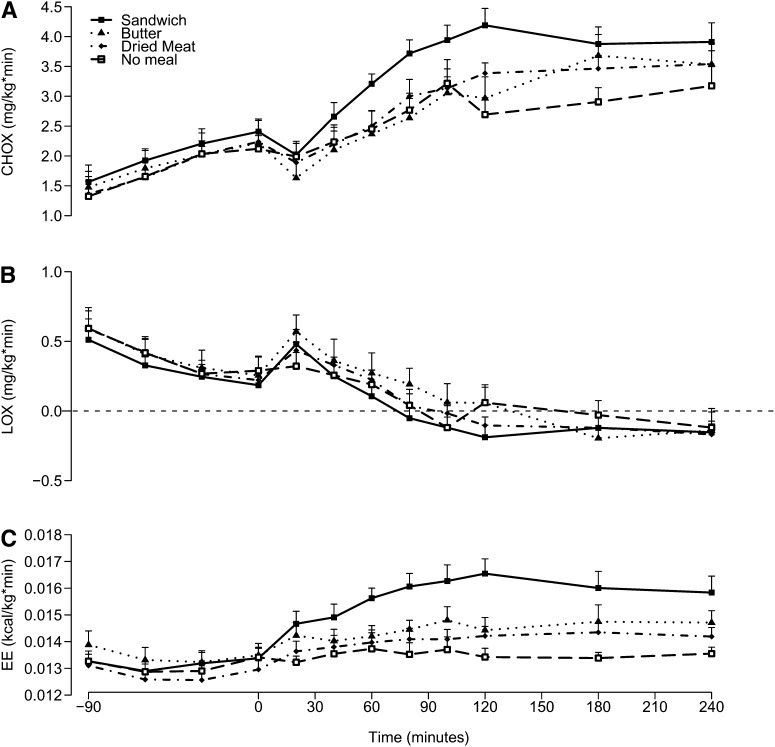
Net carbohydrate and lipid oxidation
during the initial 90 min of hyperglycemic clamp (ie, before meal ingestion), net glucose oxidation increased (time effect: P < 0.001) and lipid oxidation decreased (time effect: P < 0.001) and were similar with all 4 tests (meal effect: P = 0.4 for carbohydrates oxidation and P = 0.6 for lipids oxidation). No interaction effect was found between meal and time for either carbohydrate or lipid oxidation (P = 0.08 and 0.9, respectively). After the meal, a significant effect of meal was found for carbohydrate oxidation (P < 0.001) but not for lipid oxidation (P = 0.13). No significant interaction between time and meal was found for carbohydrate oxidation or for lipid oxidation (P = 0.3 and 0.2, respectively).
FIGURE 5.
Mean (±SEM) CHOX (A), LOX (B), and EE (C) over time after meal ingestion (0 min). Two-factor ANOVA was used for comparison between the 4 conditions. A: There was a significant effect of meal (P < 0.0001) and time (P < 0.0001) but no significant interaction between meal and time (P = 0.08). B: There was no significant effect of meal (P = 0.08), there was a significant effect of time (P < 0.0001), and there was no significant interaction between meal and time (P = 0.9). C: There was a significant effect of meal (P < 0.0001) and time (P < 0.0001) and a significant interaction between meal and time (P < 0.0001). CHOX, carbohydrate oxidation; EE, energy expenditure; LOX, lipid oxidation.
DISCUSSION
This study aimed to assess the relative contribution of lipids and of protein constituents to the incretin effect elicited by a mixed meal. For this purpose, insulin secretion was measured at a fixed level of glycemia before and after ingestion of a sandwich made with white bread (mostly carbohydrate), butter (lipid), and dried meat (mostly protein) or after ingestion of butter alone or of dried meat alone. The results of these experiments indicate that the ingestion of the whole sandwich and of butter alone, but of not dried meat alone, significantly enhanced glucose-induced insulin secretion. These effects on insulin secretion paralleled the plasma concentrations of GIP. Our findings indicate that the lipid component makes a significant contribution to the incretin effect of a mixed meal, whereas protein makes little contribution.
Many previous studies have assessed the effects of specific macronutrients on the secretion of the incretin hormones by the gut (5, 6, 8, 21, 22). However, in these studies, the overall effect of protein and fat at enhancing glucose-induced insulin secretion could not be accurately assessed because of the low glucose concentrations observed after ingestion of fat or protein alone or because of the significantly different plasma glucose responses to glucose alone or to glucose + fat or protein (21). Furthermore, the amounts of fat (≤67 g; 6) and protein (≤2 g/kg; 8) were far in excess of what is usually consumed with a mixed meal. To our knowledge, no previous study has addressed the respective contribution of fat and protein when administered in amounts corresponding to those ingested with a usual meal.
To accurately compare the enhancement of glucose-induced insulin secretion elicited by a mixed meal (dried meat, butter, and white bread sandwich) and of its fat (butter alone) or protein (dried meat alone) constituent, we used our previously described combined hyperglycemic clamp–oral nutrient administration protocol (14). This technique allows control of the glycemic stimulus to insulin secretion among the different test meals. Because insulin concentrations are known to increase continuously over time during a hyperglycemic clamp (13, 23), a control clamp with no meal ingestion was used as the measure of insulin secretion in response to IV hyperglycemia alone. We previously used this hyperglycemic-meal approach to assess the incretin effect on postprandial insulin responses to glucose (14, 24) and mixed meal ingestions (2).
Not surprisingly, our results confirm the importance of the incretin effect after ingestion of a mixed meal, with a >2-fold increase in postmeal plasma insulin concentration, despite constant hyperglycemia. GRd also increased by 130%, most likely as a consequence of the stimulation of insulin secretion. The sandwich also elicited a strong GIP response, which peaked at the same time as the peak insulin response; however, surprisingly, only a small nonsignificant increase in GLP-1 secretion.
Compared with the whole sandwich, ingestion of butter alone induced weaker but still significant incretin and GRd responses. Whereas it has been well documented that dietary lipids increase both the release of GIP and GLP-1 Principles and practices of kinetics analysis (7, 8, 21, 22, 25, 26) and glucose-stimulated insulin secretion (27), the relative contribution of fat to the overall incretin effect, have not been previously reported. In our study, plasma insulin, GIP, and GRd followed a similar time course, with a gradual increase that seemed to be ongoing 4 h after the meal. It seems likely that stomach emptying of the butter and mixed meals was delayed through the known effect of ingested fat on gastric motility (28). Given that plasma insulin and GIP during the lipid-containing meals were still near their maximal concentration at the conclusion of the test, it seems likely that the incretin effect in these studies was underestimated because of ongoing nutrient absorption at the end of our experiments.
Ingestion of dried meat did not increase plasma insulin relative to the hyperglycemic clamp alone, but produced a modest but significant increase in GRd. Moreover, there was very little stimulation of GIP or GLP-1 release in this study. This finding is consistent with that of most (9, 29, 30), but not all (21), previous studies on the secretion of incretins after protein meals. This finding, however, appears to be at odds with reports showing that peptones (31) or meat hydrolysate (32) stimulate GLP-1 secretion in human or murine enterocyte cell lines. This may indicate that GLP-1 secretion can be activated by high concentrations of protein hydrolysate, which are not attained with the ingestion of physiologic amounts of protein. Alternatively, this discrepancy may be due to differences in the regulation of GLP-1 secretion between enterocytes in vivo and enterocyte cell lines in vitro. Regardless of the explanation, ingestion of protein alone produced no significant increase in gluco-incretin hormone concentrations, did not potentiate glucose-induced insulin secretion, and did not enhance total glucose disposal, which indicates that it had little contribution to the incretin effect of the sandwich meal. The possibility that protein, when ingested with other nutrients, may have modulated the effects of carbohydrate or fat cannot be ruled out.
We were unable to detect significant stimulation of GLP-1 secretion after ingestion of our 3 test meals. This was surprising given the fact that meals with comparable carbohydrate contents have repeatedly been observed to produce significant and robust GLP-1 responses. It is unclear why the fasting GLP-1 concentrations in this study were as high as we found them to be. However, the decrease during the hyperglycemic clamp is consistent with recent findings by others that elevated glucose reduces incretin secretion (33). Such an effect of hyperglycemia, if present, was an important limitation of our study, because it may have led to an underestimation of the global incretin effect. However, the lack of GLP-1 response after the sandwich meal was in marked contrast with the robust incretin effect seen with this stimulus. One possible explanation is that the incretin effect of GLP-1 was not solely a function of plasma peptide concentrations, but also involved paracrine (34) or neurocrine actions (35). Indeed, recent studies indicate that infusion of a GLP-1 receptor antagonist to fasting humans with minimal plasma GLP-1 concentrations suppresses insulin secretion in response to intravenous glucose (2, 36).
Our study had another limitation that should be noted. On the basis of plasma GIP concentrations, even a 4-h study period was insufficient for complete absorption of the nutrients in the sandwich and butter meals, and this may have led to an underestimation of the incretin effect. Previous isotopic studies, however, have shown that the bulk of a mixed meal is absorbed over the 4 h after ingestion; hence, underestimation as a result of a too short observation time is likely to be minor (37). Moreover, it seems likely from the trends in the insulin curves that the estimates of postprandial insulin release were mostly captured in the time frame of the experiments. Hence, whereas the full effects of the lipid-containing meals may have been underestimated, this source of imprecision was unlikely to drastically affect our conclusions.
In conclusion, the findings of the current study indicate that ingestion of a mixed meal made of white bread, butter, and dried meat elicits an increase in plasma GIP concentrations and in glucose-induced insulin secretion. Ingestion of butter alone, but not of dried meal alone, significantly increased the secretion of GIP and glucose-induced insulin, which indicated that the fat component of a mixed meal makes a significant contribution to the overall incretin effect. In contrast, protein, when ingested in an amount comparable with that typically in a mixed meal, does not appear to contribute substantially to the incretin effect.
Acknowledgments
We thank the staff of the Department of Physiology of Lausanne and of the Cardiomet Clinical Investigation Center for their outstanding assistance and all the volunteers for their participation.
The authors’ responsibilities were as follows—GC, PS, VG, and LT: designed the study; GC, LE, and CT: recruited participants and performed the tests; GC and LE: analyzed the data; and GC, DD, and LT: drafted the manuscript. All of the authors revised the manuscript. None of the authors reported a conflict of interest.
Footnotes
GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide 1; GRd, glucose rate of disappearance.
REFERENCES
- 1.Elrick H, Stimmler L, Hlad CJ, Jr, Arai Y. Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab 1964;24:1076–82 [DOI] [PubMed] [Google Scholar]
- 2.Salehi M, Aulinger B, Prigeon RL, D'Alessio DA. Effect of endogenous GLP-1 on insulin secretion in type 2 diabetes. Diabetes 2010;59:1330–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Creutzfeldt W, Ebert R. New developments in the incretin concept. Diabetologia 1985;28:565–73 [DOI] [PubMed] [Google Scholar]
- 4.Orskov C, Wettergren A, Holst JJ. Secretion of the incretin hormones glucagon-like peptide-1 and gastric inhibitory polypeptide correlates with insulin secretion in normal man throughout the day. Scand J Gastroenterol 1996;31:665–70 [DOI] [PubMed] [Google Scholar]
- 5.Rijkelijkhuizen JM, McQuarrie K, Girman CJ, Stein PP, Mari A, Holst JJ, Nijpels G, Dekker JM.Effects of meal size and composition on incretin, alpha-cell, and beta-cell responses. Metabolism 2010;59:502–11 [DOI] [PubMed] [Google Scholar]
- 6.Herrmann C, Goke R, Richter G, Fehmann HC, Arnold R, Goke B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion 1995;56:117–26 [DOI] [PubMed] [Google Scholar]
- 7.Falko JM, Crockett SE, Cataland S, Mazzaferri EL. Gastric inhibitory polypeptide (GIP) stimulated by fat ingestion in man. J Clin Endocrinol Metab 1975;41:260–5 [DOI] [PubMed] [Google Scholar]
- 8.Carr RD, Larsen MO, Winzell MS, Jelic K, Lindgren O, Deacon CF, Ahrén B. Incretin and islet hormonal responses to fat and protein ingestion in healthy men. Am J Physiol Endocrinol Metab 2008;295:E779–84 [DOI] [PubMed] [Google Scholar]
- 9.Blom WA, Lluch A, Stafleu A, Vinoy S, Holst JJ, Schaafsma G, Hendriks HF. Effect of a high-protein breakfast on the postprandial ghrelin response. Am J Clin Nutr 2006;83:211–20 [DOI] [PubMed] [Google Scholar]
- 10.Cataland S, Crockett SE, Brown JC, Mazzaferri EL. Gastric inhibitory polypeptide (GIP) stimulation by oral glucose in man. J Clin Endocrinol Metab 1974;39:223–8 [DOI] [PubMed] [Google Scholar]
- 11.Hansen L, Hartmann B, Mineo H, Holst JJ. Glucagon-like peptide-1 secretion is influenced by perfusate glucose concentration and by a feedback mechanism involving somatostatin in isolated perfused porcine ileum. Regul Pept 2004;118:11–8 [DOI] [PubMed] [Google Scholar]
- 12.Sugiyama K, Manaka H, Kato T, Yamatani K, Tominaga M, Sasaki H. Stimulation of truncated glucagon-like peptide-1 release from the isolated perfused canine ileum by glucose absorption. Digestion 1994;55:24–8 [DOI] [PubMed] [Google Scholar]
- 13.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–23 [DOI] [PubMed] [Google Scholar]
- 14.Henchoz E, D'Alessio DA, Gillet M, Halkic N, Matzinger O, Goy JJ, Chioléro R, Tappy L, Schneiter P. Impaired insulin response after oral but not intravenous glucose in heart- and liver-transplant recipients. Transplantation 2003;76:923–9 [DOI] [PubMed] [Google Scholar]
- 15.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr 1974;32:77–97 [DOI] [PubMed] [Google Scholar]
- 16.Wolfe RR. Radioactive and stables isotope tracers in biomedicine: principles and practice of kinetics analysis. New York, NY: Wiley-Liss, 1992;471 [Google Scholar]
- 17.DeBodo RC, Steele R, Altszuler N, Dunn A, Bishop JS. On the hormonal regulation of carbohydrate metabolism; studies with C14 glucose. Recent Prog Horm Res 1963;19:445–88 [PubMed] [Google Scholar]
- 18.Delarue J, Normand S, Pachiaudi C, Beylot M, Lamisse F, Riou J-P. The contribution of naturally labelled 13C fructose to glucose appearance in humans. Diabetologia 1993;36:338–45 [DOI] [PubMed] [Google Scholar]
- 19.Livesey G, Elia M. Estimation of energy expenditure, net carbohydrate utilization, and net fat oxidation and synthesis by indirect calorimetry: evaluation of errors with special reference to the detailed composition of fuels. Am J Clin Nutr 1988;47:608–28 [DOI] [PubMed] [Google Scholar]
- 20.Team RDCR. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. 2008 [Google Scholar]
- 21.Elliott RM, Morgan LM, Tredger JA, Deacon S, Wright J, Marks V. Glucagon-like peptide-1 (7-36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol 1993;138:159–66 [DOI] [PubMed] [Google Scholar]
- 22.Karhunen LJ, Juvonen KR, Huotari A, Purhonen AK, Herzig KH. Effect of protein, fat, carbohydrate and fibre on gastrointestinal peptide release in humans. Regul Pept 2008;149:70–8 [DOI] [PubMed] [Google Scholar]
- 23.Porte D, Jr, Pupo AA. Insulin responses to glucose: evidence for a two pool system in man. J Clin Invest 1969;48:2309–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salehi M, Vahl TP, D'Alessio DA. Regulation of islet hormone release and gastric emptying by endogenous glucagon-like peptide 1 after glucose ingestion. J Clin Endocrinol Metab 2008;93:4909–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 2007;87:1409–39 [DOI] [PubMed] [Google Scholar]
- 26.Feinle C, Christen M, Grundy D, Faas H, Meier O, Otto B, Fried M. Effects of duodenal fat, protein or mixed-nutrient infusions on epigastric sensations during sustained gastric distension in healthy humans. Neurogastroenterol Motil 2002;14:205–13 [DOI] [PubMed] [Google Scholar]
- 27.D'Alessio DA, Prigeon RL, Ensinck JW. Enteral enhancement of glucose disposition by both insulin-dependent and insulin-independent processes. A physiological role of glucagon-like peptide I. Diabetes 1995;44:1433–7 [DOI] [PubMed] [Google Scholar]
- 28.Kroop HS, Long WB, Alavi A, Hansell JR. Effect of water and fat on gastric emptying of solid meals. Gastroenterology 1979;77:997–1000 [PubMed] [Google Scholar]
- 29.Lejeune MP, Westerterp KR, Adam TC, Luscombe-Marsh ND, Westerterp-Plantenga MS. Ghrelin and glucagon-like peptide 1 concentrations, 24-h satiety, and energy and substrate metabolism during a high-protein diet and measured in a respiration chamber. Am J Clin Nutr 2006;83:89–94 [DOI] [PubMed] [Google Scholar]
- 30.Thomas FB, Mazzaferri EL, Crockett SE, Mekhjian HS, Gruemer HD, Cataland S. Stimulation of secretion of gastric inhibitory polypeptide and insulin by intraduodenal amino acid perfusion. Gastroenterology 1976;70:523–7 [PubMed] [Google Scholar]
- 31.Cordier-Bussat M, Bernard C, Levenez F, Klages N, Laser-Ritz B, Philippe J, Chayvialle JA, Cuber JC. Peptones stimulate both the secretion of the incretin hormone glucagon-like peptide 1 and the transcription of the proglucagon gene. Diabetes 1998;47:1038–45 [DOI] [PubMed] [Google Scholar]
- 32.Reimer RA, Darimont C, Gremlich S, Nicolas-Metral V, Ruegg UT, Mace K. A human cellular model for studying the regulation of glucagon-like peptide-1 secretion. Endocrinology 2001;142:4522–8 [DOI] [PubMed] [Google Scholar]
- 33.Vollmer K, Gardiwal H, Menge BA, Goetze O, Deacon CF, Schmidt WE, Holst JJ, Meier JJ. Hyperglycemia acutely lowers the postprandial excursions of glucagon-like peptide-1 and gastric inhibitory polypeptide in humans. J Clin Endocrinol Metab 2009;94:1379–85 [DOI] [PubMed] [Google Scholar]
- 34.Hansen L, Hartmann B, Bisgaard T, Mineo H, Jorgensen PN, Holst JJ. Somatostatin restrains the secretion of glucagon-like peptide-1 and -2 from isolated perfused porcine ileum. Am J Physiol Endocrinol Metab 2000;278:E1010–8 [DOI] [PubMed] [Google Scholar]
- 35.Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology 1999;140:1687–94 [DOI] [PubMed] [Google Scholar]
- 36.Schirra J, Sturm K, Leicht P, Arnold R, Goke B, Katschinski M. Exendin(9-39)amide is an antagonist of glucagon-like peptide-1(7-36)amide in humans. J Clin Invest 1998;101:1421–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Normand S, Pachiaudi C, Khalfallah Y, Guilluy R, Mornex R, Riou JP. 13C appearance in plasma glucose and breath CO2 during feeding with naturally 13C-enriched starchy food in normal humans. Am J Clin Nutr 1992;55:430–5 [DOI] [PubMed] [Google Scholar]