Abstract
Rationale
Methamphetamine (METH) is a highly potent and addictive psychostimulant with severe detrimental effects to the health of users. Currently, METH addiction is treated with a combination of cognitive and behavioral therapies, but these traditional approaches suffer from high relapse rates. Furthermore, there are currently no pharmacological treatment interventions approved by the FDA specifically for the treatment of METH addiction.
Objectives
Metabotropic glutamate receptor 5 (mGluR5) negative allosteric modulators (NAMs) have shown promise in significantly attenuating drug self-administration and drug-seeking in reinstatement paradigms. However, studies assessing the potential efficacy of mGluR5 NAMs that have been tested in human subjects are lacking. The current study sought to assess the effect of the mGluR5 NAM fenobam on METH-seeking behavior.
Methods
Rats were trained to self-administer METH (0.05 mg/kg i.v.), and following extinction, tested for effects of fenobam (5, 10 or 15 mg/kg i.p.) on cue- and drug-induced reinstatement of METH-seeking. To determine if fenobam also alters reinstatement of seeking of natural reinforcers, separate groups of rats were trained to self-administer sucrose or food pellets and were tested for the effects of fenobam on cue-induced reinstatement of sucrose- and food-seeking.
Results
Fenobam attenuated drug- and cue-induced reinstatement of METH-seeking behavior at doses of 10 and 15 mg/kg. Fenobam also attenuated cue-induced reinstatement of sucrose- and food-seeking at all doses tested.
Conclusions
The mGluR5 NAM fenobam attenuates the reinstatement of METH-seeking behavior, but these effects may be due to non-specific suppression of general appetitive behaviors.
Keywords: methamphetamine, sucrose, food, cue, drug prime, reinstatement, mGluR5, negative allosteric modulator
Methamphetamine (METH) is a highly addictive psychostimulant with potent effects on the central nervous system (Shrem and Halkitis 2008). METH use is highly correlated with various medical and neuropsychiatric complications and has numerous adverse neurological and other health effects (Darke et al. 2008; Rusyniak 2011; Scott et al. 2007). In many regions of the United States, METH use has recently reached epidemic levels, and recent epidemiological data suggest that METH use is again on the rise despite decreasing trends in use in the mid- to late-2000’s (Anglin et al. 2000; Maxwell and Brecht 2011; Maxwell and Rutkowski 2008).
METH’s reinforcing effects are generally attributed to its actions as a potent releaser of monoamines (Cruickshank and Dyer 2009; Kish 2008; Sulzer 2011). These effects are caused by a displacement of monoamines from vesicular stores by METH, which acts as a substrate for vesicular monoamine transporters (VMAT). This results in increased cytoplasmic monoamine levels and a subsequent reversal of plasma membrane monoamine transporter direction. Attempts at developing pharmacological treatments for METH addiction have historically focused on compounds which modulate the actions of METH on VMAT, plasma membrane monoamine transporters, or GABAergic functioning within mesolimbic brain circuits (Ciccarone 2011; Karila et al. 2010; Vocci and Appel 2007). However, as of yet there are currently no medications approved by the U.S. Food and Drug Administration for METH addiction.
Various studies have shown that METH increases extracellular levels of glutamate in forebrain regions such as the striatum, hippocampus, and prefrontal cortex (Mark et al. 2007; Rocher and Gardier 2001; Shoblock et al. 2003; Stephans and Yamamoto 1995). However, in other regions of the brain such as the nucleus accumbens and ventral midbrain, it has been shown that METH can increase, have no effect, or even decrease extracellular levels of glutamate (Ito et al. 2006; Shoblock et al. 2003; Zhang et al. 2001). Thus, the effects of METH on extracellular glutamate appear to be complex, and likely dependent on brain region, dose, and frequency of drug administration. While most research on METH–induced changes in extracellular glutamate has focused on its role in excitotoxicity, more recent research has revealed a primary role for glutamatergic neurotransmission in mediating the rewarding and reinforcing effects of METH (Gass and Olive 2008). Thus, glutamatergic transmission may be a novel therapeutic target for the treatment of METH addiction (Kalivas and Volkow 2011; Olive 2009; Olive et al. 2012).
Receptors for glutamate are broadly classified as ionotropic (iGluR) or metabotropic (mGluR) receptors. There are 8 mGluR receptor subtypes (mGluR1 – mGluR8) which are further subclassified into three distinct families (Group I, II, or III) based upon their pharmacology and signaling transduction mechanisms (Conn and Pin 1997; Pin and Duvoisin 1995). In a seminal study by Chiamulera and colleagues, it was shown that mice lacking the mGluR5 gene did not acquire cocaine self-administration and were unresponsive to its locomotor stimulant effects (Chiamulera et al. 2001). Since this study, numerous investigators have shown that mGluR5 antagonists reduce intravenous drug self-administration and reinstatement of drug-seeking in animal studies, as reviewed elsewhere (Duncan and Lawrence 2012; Kenny and Markou 2004; Olive 2009). We have previously demonstrated that the selective mGluR5 negative allosteric modulator (NAM) 3-((2-methyl-1,3-thiazol-4-yl)ethynyl)pyridine (MTEP) attenuates intravenous METH self-administration while exerting no effects on food self-administration (Gass et al. 2009; Osborne and Olive 2008). In addition, MTEP also attenuated reinstatement of METH-seeking behavior induced by METH-associated cues or acute METH exposure, but did not alter cue-induced reinstatement of food-seeking. Taken together, these studies indicate that mGluR5 receptors play a key role in METH reinforcement and METH-seeking behaviors, and justify further investigation into mGluR5 antagonists as potential anti-addiction therapeutics.
Fenobam was first developed in the 1970’s as a non-benzodiazepine anxiolytic for human use despite unknown pharmacological mechanisms of action (Itil et al. 1978; Pecknold et al. 1980, 1982). In 2005, it was revealed that fenobam is a selective mGluR5 NAM (Porter et al. 2005), renewing interest in fenobam as a potential therapeutic for the treatment of various dysfunction of the nervous system. Fenobam possesses antidepressant, analgesic, and anxiolytic effects in experimental animals (Jacob et al. 2009; Montana et al. 2009), symptoms that often accompany withdrawal from chronic METH use (Scott et al. 2007). Fenobam and several other mGluR5 NAMs have recently been tested in clinical trials for a number of medical disorders including Fragile X syndrome and L-dopa induced dyskinesias (Berry-Kravis et al. 2009; Hagerman et al. 2008; Jaeschke et al. 2008). While these clinical trials showed that fenobam was generally well tolerated with only moderate side effects, unfortunately, clinical testing of fenobam was recently discontinued due to somewhat limited efficacy and large variability in plasma levels of the drug following oral administration. Nevertheless, there is a great need to develop medications for the treatment of METH addiction, particularly with respect to compounds that have demonstrated safety and tolerability in human subjects. We therefore sought to determine the effects of fenobam on the reinstatement of METH-seeking behavior. To examine the potential generalization of effects on the reinstatement of seeking of natural reinforcers, we assessed the effects of fenobam on reinstatement of sucrose- and food-seeking behavior.
Material and Methods
Subjects
Fifty-four male Sprague-Dawley rats (Harlan Laboratories, Livermore, CA), weighing approximately 250–275 g, were individually housed upon arrival. Animals were maintained on a 12 hr light-dark cycle (lights off at 0700 hr) in a temperature and humidity controlled rodent colony. All experimentation was conducted during the dark phase of the light-dark cycle, with the exception of a 16 hr overnight operant training session for METH and sucrose self-administration groups which commenced near the end of the dark phase (at approximately 1600 hr) and continued through the light phase into the following morning (ending at approximately 0800 hr). Rats undergoing METH and sucrose self-administration procedures were given ad libitum access to food and water during all phases of the experiment except during drug self-administration and for 12 hr prior to the initial operant training session. Rats undergoing food self-administration procedures were maintained at approximately 85% of their free-feeding bodyweight and received food in their home cage for one hour each day approximately 2 hr after behavioral testing. Rats undergoing locomotor assessment procedures received ad libitum access to food and water during all experimental phases. Two rats were eliminated from the study due to catheter patency failure. All experimental procedures were conducted with the approval of an Institutional Animal Care and Use Committee at Arizona State University and in accordance with the Principles of Laboratory Animal Care and the 8th Edition of the Guide for the Care and the Use of Laboratory Animals (National Research Council, 2011).
Surgical Procedures
Prior to arrival, rats undergoing METH self-administration procedures were prepared with intravenous catheters into the jugular vein by Harlan Laboratories Surgical Services. Upon arrival, rats were allowed one day of acclimation before vascular port implantation. Rats were anesthetized and implanted with vascular access ports (Model 313000BM15, Plastics One, Roanoke, VA, USA) as described previously (Gass et al. 2009). Following surgical procedures, rats were given 5 days of post-operative care during which they received daily intravenous infusions of 70 U/ml heparin (0.2 ml volume) to maintain catheter patency and 100 mg/ml cefazolin (0.1 ml volume) to protect against infection. Rats also received daily subcutaneous injections of 2.5 mg/ml of meloxicam (0.15 ml volume) to relieve surgery-related discomfort. During post-operative care, observation of weight loss on any day resulted in a 5 ml subcutaneous injection of saline to combat dehydration. The surgery site was also treated with topical lidocaine and triple antibiotic ointments to facilitate healing of the wound. Rats undergoing locomotor testing, sucrose reinstatement, or food reinstatement procedures did not undergo catheter implantation.
Methamphetamine, Sucrose, and Food Self-Administration
Self-administration, extinction, and reinstatement tests were conducted in self-administration chambers (ENV-008, Med Associates, St. Albans, VT, USA) as described previously (Gass et al. 2009). To initiate operant responding for METH and sucrose self-administration, rats were underwent overnight sucrose pellet training according to a fixed-ratio 1 (FR1) schedule of reinforcement as described elsewhere (Gass et al. 2009). Approximately 24 hr following the initial overnight training session, 2 hr daily self-administration sessions were initiated, whereby presses on the active lever resulted in delivery of METH (0.05 mg/kg/infusion, delivered in a volume of 0.06 ml over a 2 sec period) on a FR1 schedule of reinforcement. Each active lever press was accompanied by a concurrent illumination of a stimulus light located above the active lever, and presentation of an auditory stimulus (~65 dB, 2900 Hz) for 2 sec. Animals trained to self-administer sucrose underwent the same training procedures, except each active lever press resulted in delivery of a single 45-mg sucrose pellet (TestDiet, Richmond, IN, USA) according to a FR1 schedule of reinforcement. For food self-administration procedures, rats did not undergo a 16 hr overnight training session, and began 2 hr self-administration through spontaneous acquisition where active lever presses resulted in delivery of a single 45-mg food pellet (Bio-Serv, Frenchtown, NJ) according to a FR1 schedule of reinforcement. Self-administration sessions were conducted 7 consecutive days per week. For METH self-administration procedures, each session was preceded by intravenous infusion of 0.1 ml of 70 U/ml heparin, and followed by infusion of 0.1 ml of 70 U/ml of heparin as well as 0.1 ml of 100 mg/ml cefazolin.
Stabilization of self-administration was considered to have been reached when the average number of active lever presses during each 2 hr session differed by less than 15% for 2 consecutive days, after a minimum of 8 days of self-administration. Self-administration data reported represent the average number of active lever presses during the final two self-administration sessions prior to extinction training.
Extinction Procedures
Extinction sessions were 2 hr in length and commenced following stabilization of self-administration. During extinction training, responding on the previously active lever no longer produced any programmed consequences, as described previously (Gass et al. 2009). Extinction sessions were conducted daily until the number of active lever presses per 2 hr session was less than 25% of the average number of active lever presses during the final two days of self-administration responding, and when this level of pressing was observed for 2 days.
Reinstatement Procedures
Reinstatement test sessions were 2 hr in length and commenced on the day immediately following the last extinction session. For all groups of rats (METH-prime, METH-cue, sucrose-cue, and food-cue), fenobam or vehicle was injected i.p. 20 min prior to the reinstatement sessions. For the METH-prime group, a single METH injection (0.5 mg/kg i.p.) was given 30 min prior to reinstatement testing and 10 min prior to fenobam administration. Following each reinstatement test session, animals were placed into additional daily 2 hr extinction sessions starting on the following day. These additional extinction sessions were carried out until extinction criteria were again met, at which point another reinstatement test was conducted on the following day. Each group of rats were subjected to either 3 or 4 reinstatement tests, and each rat received 3 or 4 of the different treatments (vehicle or 3 doses of fenobam) in a randomized counterbalanced design. After the final reinstatement test, the animals were euthanized by anesthesia with isoflurane followed by decapitation.
Locomotor Procedures
Locomotor activity was measured as rotational behavior and recorded by Rotorat version 1.2 software (Med Associates). Rats were placed into stainless steel bowls (40.6 cm diameter × 25.4 cm high; model ENV-500, Med Associates) surrounded by clear Plexiglas walls to prevent rats from escaping the apparatus. Rats were connected to spring tether secured to the top of the apparatus by a rotational sensor which recorded activity. A zip-tie collar was placed around the neck of the rat and connected to the spring tether via a stainless steel alligator clip. Measurements taken were full (360° turns) and quarter (90°) turns, in both clockwise and counter-clockwise directions.
Prior to locomotor assessment rats were placed into the locomotor apparatus for 90 min for two consecutive days to allow for habituation to the apparatus. Next, rats were randomly assigned to receive either fenobam (10 mg/kg, i.p.) or vehicle prior to the first locomotor test session. The next day, rats that previously received fenobam were administered vehicle, and vice versa. Locomotor test sessions were 90 min in length, and fenobam or vehicle injections were administered 20 min prior to placing rats in the locomotor apparatus.
Drugs
Fenobam (1-(3-chlorophenyl)-3-(3-methyl-5-oxo-4H-imidazol-2-yl)urea) was custom synthesized by Chemir Analytical Services (Maryland Heights, MO, USA) and suspended in a vehicle consisting of 0.3% v/v Tween 80 via sonication. For reinstatement procedures, fenobam was administered by the intraperitoneal (i.p.) route at doses of 5, 10, or 15 mg/kg in a volume of 1 ml/kg. These doses were chosen based on previous reports that they do not produce significant signs of sedation or anhedonia (Cleva et al. 2012; Porter et al. 2005). Fenobam (10 mg/kg) was also injected via the i.p. route prior to locomotor assessment. (+)Methamphetamine hydrochloride was obtained from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in a sterile saline for intravenous infusion. For METH-primed reinstatement procedures, METH was prepared at a concentration of 0.5 mg/ml in saline and administered i.p. in a volume of 1 ml/kg.
Statistical Analyses
For all groups (METH-prime, METH-cue, sucrose-cue, and food-cue), verification that extinction training produced significant decreases in the number of active lever presses was perfomed by a Student’s t-test, comparing the average number of active lever presses emitted during the final 2 days of extinction (Ext) training to the average of the final 2 days of self-administration (SA). The effects of fenobam on reinstatement was analyzed by a one-way repeated measures ANOVA, with the number of active or inactive lever presses with dose/experimental phase (extinction, vehicle, 5, 10, and 15 mg/kg) serving as the within subjects factor. Holm-Sidak post hoc tests were used to determine effects of fenobam dose on reinstatement as compared to vehicle. One-way repeated measures ANOVA were also used to analyze the number of inactive lever presses during extinction and reinstatement procedures. For assessment of locomotor activity, a one-way repeated measures ANOVA was conducted on the number of full and quarter turns with treatment (fenobam 10 mg/kg vs. vehicle) as the within-subjects factor. When tests of data normality failed, a Friedman ANOVA on ranks was utilized. Level of statistical significance was set to p<0.05 for all tests. Statistical tests were performed using SigmaPlot version 12.0 (Systat Software, San Jose, CA)
Results
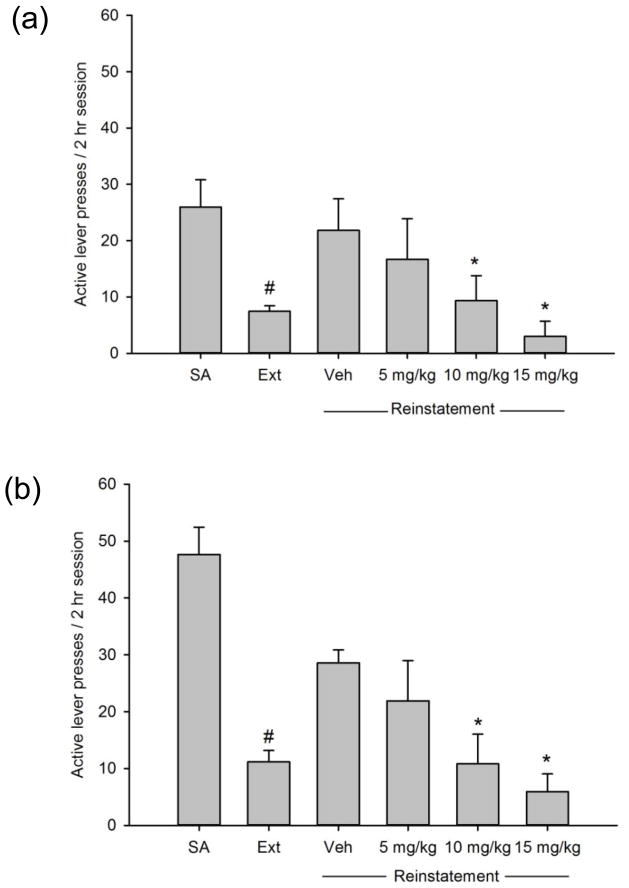
For all groups, extinction training produced a significant reduction in the number of active lever presses as assessed on the final 2 days of Ext as compared to the final 2 days of SA (all p’s <0.05). During METH-primed reinstatement (n=12), a significant main effect of fenobam dose/experimental phase on active lever presses was observed (F(4,32)=4.341, p<0.01; see Fig. 1a). Post-hoc comparisons revealed a reinstatement of METH-seeking following the METH-prime injection, as evidenced by a significant increase in the number of active lever presses following vehicle administration versus the average of the last 2 days of Ext (p<0.05). Fenobam at both the 10 and 15 mg/kg doses significantly attenuated reinstatement as compared to following vehicle treatment (p<0.05 and p<0.01, respectively). Analysis of inactive lever presses did not reveal any significant main effects of dose/experimental phase (p>0.05; Table 1).
Fig. 1.
Effects of fenobam on the reinstatement of METH-seeking induced by (a) acute administration of METH (0.5 mg/kg i.p., n=12) or (b) METH-associated cues (n=10). SA values represent the average of the last 2 days of METH self-administration. Extinction (Ext) values represent the average of the last 2 days of extinction training prior to the first reinstatement test. *p<0.05 vs. vehicle treatment, #p<0.05 vs. SA. Data are presented as mean±SEM.
Table 1.
Inactive lever presses per 2 hr session during extinction and reinstatement procedures.
| Group | Ext | ———Reinstatement——— | |||
|---|---|---|---|---|---|
| Vehicle | 5 mg/kg | 10 mg/kg | 15 mg/kg | ||
| Drug-METH | 3.35±0.71 | 3.36±0.92 | 3.67±1.45 | 1.66±0.73 | 1.14±0.77 |
| Cue-METH | 6.67±0.86 | 3.60±1.55 | 1.00±0.49 | 0.90±0.60 | 0.30±0.21 |
| Cue-Sucrose | 5.96±1.03 | 3.33±1.17 | 3.33±1.88 | 1.67±0.91 | 0.17±0.17 |
| Cue-Food | 6.92±2.26 | 6.00±2.02 | 3.42±1.28 | 3.00±0.86 | 4.92±2.28 |
Data are presented as mean ± SEM. Extinction (Ext) data represent the average of the last 2 days of extinction training prior to the first reinstatement test.
During cue-induced reinstatement of METH-seeking (n=10), a significant main effect of fenobam dose/experimental phase was observed for the number of active lever presses (F(4,36)=6.44, p<0.001; see Fig. 1b). Post-hoc tests revealed a significant increase in active lever presses following vehicle administration as compared to extinction responding, indicating that METH-associated cues induced a reinstatement of METH-seeking behavior. Fenobam at doses of 10 and 15 mg/kg significantly attenuated cue-induced reinstatement (p<0.01 and p<0.001, respectively). For inactive lever pressing, a repeated measures one-way ANOVA failed tests of data normality, and a Friedman’s repeated measures ANOVA on ranks revealed a significant main effect of fenobam dose/experimental phase on inactive lever presses (χ2=26.064, p<0.001). However, Dunn’s method of multiple comparisons did not reveal any significant differences in the number of inactive lever presses on any of the reinstatement tests (p>0.05; Table 1).
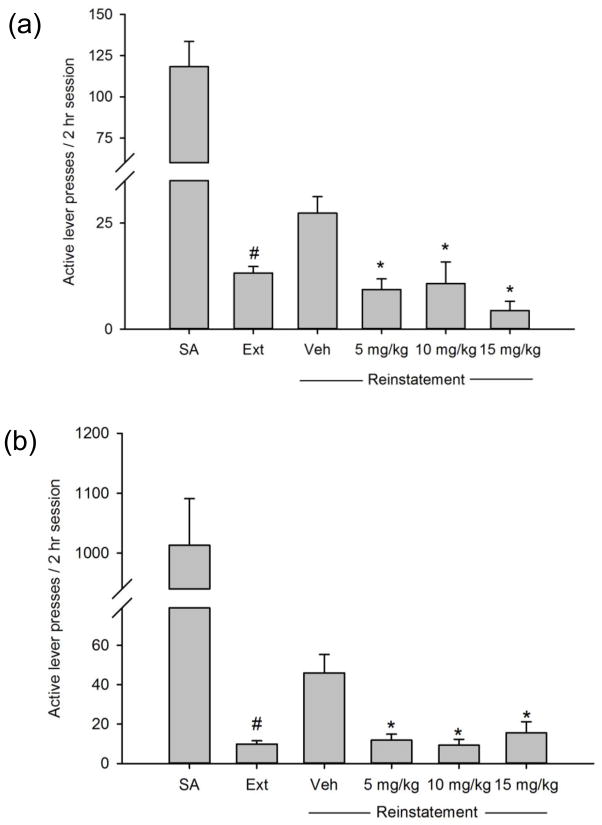
During cue-induced reinstatement of sucrose-seeking (n=12), a significant main effect of fenobam dose/experimental phase was observed for the number of active lever presses (F(4,59)=6.653, p=0.001; see Fig. 2a). Post-hoc tests revealed a significant increase in the number of active lever presses during reinstatement tests following vehicle treatment as compared to extinction values (p=0.005), demonstrating reinstatement of sucrose-seeking in response to sucrose-associated cues. Post-hoc tests revealed significant decreases in the number of active lever presses following the 5, 10 and 15 mg/kg doses of fenobam as compared with vehicle (p<0.01). A significant main effect of fenobam dose/experimental phase was also found on inactive lever presses (F(4,59)=4.369, p=0.005). However, post-hoc tests revealed no significant differences in the number of inactive lever presses across reinstatement tests (p>0.05; Table 1).
Fig. 2.
Effects of fenobam on the reinstatement of (a) sucrose-seeking induced by sucrose-associated cues (n=12) and (b) food-seeking induced by food-associated cues (n=12). SA values represent the average of the last 2 days of sucrose or food self-administration. Extinction (Ext) values represent the average of the last 2 days of extinction training prior to the first reinstatement test. *p<0.05 vs. vehicle treatment, #p<0.05 vs. SA. Data are presented as mean±SEM.
During cue-induced reinstatement of food-seeking (n=12), a significant main effect of fenobam dose/experimental phase was observed for the number of active lever presses (F(4,59)=8.589, p=0.001; see Fig. 2b). Post-hoc tests revealed a significant increase in the number of active lever presses during reinstatement following vehicle treatment as compared to extinction values (p=0.001), demonstrating reinstatement of food-seeking in response to food-associated cues. Post-hoc tests revealed significant decreases in the number of active lever presses following the 5, 10 and 15 mg/kg doses of fenobam as compared with vehicle (p<0.001). For inactive lever presses, no significant effects were observed (p>0.05; Table 1).
For locomotor behavior, repeated measures ANOVA did not reveal a significant main effect of fenobam dose on full or quarter turns (all p’s >0.05, Fig 3).
Fig. 3.
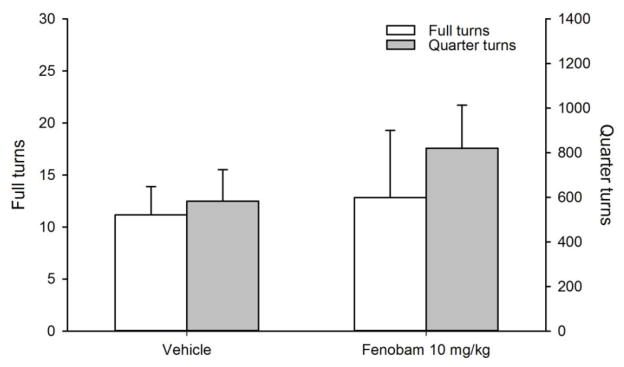
Lack of effects of vehicle or fenobam (10 mg/kg) on locomotor behavior (n=6). Data are presented as mean±SEM and represent the average number of full turns (open bars and left y-axis) or quarter turns (shaded bars and right y-axis) during 90 min locomotor test sessions.
Discussion
The present study demonstrated that fenobam, an mGluR5 NAM that has previously been tested in human subjects for treatment of other medical conditions, effectively reduced METH-seeking behavior elicited by either METH-paired cues or a METH priming injection. Administration of fenobam resulted in a significant attenuation of reinstatement of METH-seeking behavior elicited by both METH-associated cues and by a METH priming injection at doses of 10 and 15 mg/kg. However, an attenuation of cue-induced reinstatement of sucrose- and food-seeking behavior was also observed, and all doses tested (5, 10, and 15 mg/kg). These results are in agreement with our previous findings that the mGluR5 NAM 3-((2-methyl-4-thiazolyl)ethynyl)pyridine (MTEP) attenuated cue- and drug-primed reinstatement of METH-seeking (Gass et al. 2009). However, the reduction in cue-induced reinstatement of both sucrose- and food-seeking indicates that fenobam also affects seeking of natural reinforcers.
These findings extend those of previous studies showing that either genetic or pharmacological blockade of mGluR5 receptors leads to reductions in drug reward, reinforcement, and relapse-like behavior (reviewed in Duncan and Lawrence 2012; Olive 2009). While the exact mechanisms by which fenobam and other mGluR5 NAMs reduce drug-seeking or relapse-like behavior are not completely understood, a likely mechanism is by decreasing glutamatergic transmission in the nucleus accumbens (NAcc) and/or ventral tegmental area (VTA). Various studies have revealed that glutamatergic transmission in the NAcc mediates reinstatement of drug-seeking for numerous addictive drugs, including amphetamines (Cornish and Kalivas 2000; Di Ciano et al. 2001; Gass and Olive 2008; Knackstedt and Kalivas 2009; Lalumiere and Kalivas 2008). Furthermore, while drug-, cue-, and stress-primed reinstatement of drug-seeking initially engage distinct neural circuits, these circuits converge onto the regions of the prefrontal cortex which in turn send glutamatergic projections to the NAcc. This prefrontal-NAcc connection has been hypothesized as the final common pathway mediating the reinstatement of drug-seeking (Kalivas and McFarland 2003; Kalivas et al. 2005).
mGluR5 receptors are widely distributed in many regions of the brain, with the NAcc and VTA showing moderate to high levels of mGluR5 receptor expression (Mitrano and Smith 2007; Romano et al. 1995; Shigemoto et al. 1993). Bilateral microinfusions of the mGluR5 NAM 2-methyl-6-(phenylethynyl)pyridine (MPEP) or MTEP into the NAcc attenuates the reinstatement of cocaine-seeking elicited by drug priming and drug-associated cues (Backstrom and Hyytia 2007; Kumaresan et al. 2009) as well as cue-elicited alcohol-seeking behavior (Sinclair et al. 2012). Thus, it is likely that systemic administration of fenobam exerts its effects on the reinstatement of METH-seeking by modulating the glutamatergic transmission within this region. Further studies are needed to confirm this, as well as the role of the NAcc in mediating fenobam-induced suppression of cue-induced reinstatement of sucrose- and food-seeking behavior.
Another possible mechanism through which fenobam may attenuate METH-, sucrose-, and food-seeking is via its effects on brain reward function. Fenobam, along with the prototypic mGluR5 NAMs MPEP and MTEP, have been shown to decrease brain reward functioning as measured by intracranial self-stimulation (ICSS) (Cleva et al. 2012; Harrison et al. 2002; Kenny et al. 2003, 2005). Specifically, doses of MPEP (1–9 mg/kg) which significantly decrease cocaine self-administration, also elevate ICSS thresholds (Harrison et al. 2002; Kenny et al. 2003, 2005). However, others have found that MPEP does not alter ICSS thresholds, nor does MPEP decrease amphetamine-induced potentiation of brain stimulation reward (Gormley and Rompre 2011). A 3 mg/kg dose of the more selective mGluR5 NAM MTEP has been shown to decrease cue- and drug-primed reinstatement of METH-seeking (Gass et al. 2009) and also elevate ICSS thresholds (Cleva et al. 2012). However, only a high dose of fenobam (30 mg/kg, twice the highest dose tested in the current study) significantly elevated ICSS thresholds in this latter study, whereas a 10 mg/kg dose (which attenuated cue-induced METH-seeking in the current study) did not significantly increase ICSS thresholds (Cleva et al. 2012). Thus, the inhibitory effects of fenobam to reduce METH-, sucrose-, and food-seeking are not likely explained by an anhedonic state produced by this compound. In addition, the lack of effects of fenobam on locomotor activity or inactive lever presses during reinstatement tests, as demonstrated in the present study, suggest that motor impairing effects of fenobam did not likely contribute to its observed effects on reinstatement. However, the effects of fenobam on ICSS thresholds (Cleva et al. 2012) and locomotor activity have only been examined thus far in drug-naïve animals, and fenobam may have differential effects on brain reward function in animals with a history of drug self-administration. This possibility warrants further investigation.
While the 10 and 15 mg/kg doses of fenobam significantly attenuated the reinstatement of METH-seeking, all doses of fenobam tested attenuated sucrose- and food-seeking. Although mice lacking mGluR5 receptors do not show an attenuation of food self-administration (Chiamulera et al. 2001), recently it has been shown that mGluR5-deficient mice do show an attenuation of food-seeking under reinstatement conditions relative to wild-type controls (Eiler et al. 2011). This finding is not without precedent, as mGluR5 receptors have been implicated in playing a central role in regulating appetite (Bradbury et al. 2005) and pharmacological blockade of mGluR5 receptors has been shown to decrease responding for food (Paterson and Markou 2005). Although we have previously shown that the selective mGluR5 NAM MTEP does not affect cue-induced reinstatement of food-seeking (Gass et al. 2009), possible non-specific effects of fenobam that have yet to be characterized may account for these observations. For example, when compared to MPEP and MTEP, fenobam has been observed to exert more non-specific behavioral disruptions in animal models of anxiety, possibly due to active metabolites of fenobam (Porter et al. 2005). In addition, Jacob and colleagues (Jacob et al. 2009) revealed fenobam-induced learning impairments in both the Morris water maze and contextual fear learning paradigms at a dose as low as 10 mg/kg. Furthermore, it was previously shown that in humans, high doses of fenobam exerted some psychostimulant and psychotomimetic effects in a subset of individuals (Pecknold et al. 1982). Other side effects of fenobam that have been reported in humans include dizziness, nausea and sedation (Berry-Kravis et al. 2009). It is therefore possible that such effects may have led to the observed reductions in sucrose-, food-, and METH-seeking behavior produced by fenobam in the current study.
In summary, we observed that fenobam attenuates the reinstatement of METH-seeking behavior induced by acute METH exposure as well as METH-associated cues. These findings have important implications for the potential use of fenobam or fenobam-related compounds as novel treatments for METH addiction, since studies examining the effects of pharmacological agents that are safe and relatively well-tolerated in humans on METH-seeking behavior in preclinical studies are generally lacking. We also observed that fenobam suppressed cue-induced reinstatement of sucrose- and food-seeking. Therefore, fenobam may induce a suppression of general appetitive behaviors, and thus optimization of fenobam analogues (Jaeschke et al. 2007) may be warranted for further development of mGluR5 NAMs as treatments for METH addiction.
Acknowledgments
This work was supported by Public Health Service grant DA025606 from the National Institute on Drug Abuse. All experimental procedures conducted were performed in compliance with the current laws of the United States of America.
Footnotes
Conflict of Interest Disclosure
All authors have no conflicts of interest to declare.
References
- Anglin MD, Burke C, Perrochet B, Stamper E, Dawud-Noursi S. History of the methamphetamine problem. J Psychoact Drugs. 2000;32:137–41. doi: 10.1080/02791072.2000.10400221. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2007;192:571–580. doi: 10.1007/s00213-007-0753-8. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis EM, Hessl D, Coffey S, Hervey C, Schneider A, Yuhas J, Hutchison J, Snape M, Tranfaglia M, Nguyen DV, Hagerman R. A pilot open-label single-dose trial of fenobam in adults with fragile X syndrome. J Med Genet. 2009;46:266–271. doi: 10.1136/jmg.2008.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury MJ, Campbell U, Giracello D, Chapman D, King C, Tehrani L, Cosford ND, Anderson J, Varney MA, Strack AM. Metabotropic glutamate receptor mGlu5 is a mediator of appetite and energy balance in rats and mice. J Pharmacol Exp Ther. 2005;313:395–402. doi: 10.1124/jpet.104.076406. [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- Ciccarone D. Stimulant abuse: pharmacology, cocaine, methamphetamine, treatment, attempts at pharmacotherapy. Prim Care. 2011;38:41–58. doi: 10.1016/j.pop.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleva RM, Watterson LR, Johnson MA, Olive MF. Differential modulation of thresholds for intracranial self-stimulation by mGlu5 positive and negative allosteric modulators: implications for effects on drug self-administration. Front Pharmacol. 2012;2:93, 1–7. doi: 10.3389/fphar.2011.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Pin J-P. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104:1085–1099. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- Darke S, Kaye S, McKetin R, Duflou J. Major physical and psychological harms of methamphetamine use. Drug Alcohol Rev. 2008;27:253–62. doi: 10.1080/09595230801923702. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ. Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of pavlovian approach behavior. J Neurosci. 2001;21:9471–7. doi: 10.1523/JNEUROSCI.21-23-09471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JR, Lawrence AJ. The role of metabotropic glutamate receptors in addiction: Evidence from preclinical models. Pharmacol Biochem Behav. 2012;100:811–24. doi: 10.1016/j.pbb.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Eiler WJ, 2nd, Baez M, Yu J, Witkin JM. mGlu5 receptor deletion reduces relapse to food-seeking and prevents the anti-relapse effects of mGlu5 receptor blockade in mice. Life Sci. 2011;89:862–7. doi: 10.1016/j.lfs.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Osborne MP, Watson NL, Brown JL, Olive MF. mGluR5 antagonism attenuates methamphetamine reinforcement and prevents reinstatement of methamphetamine-seeking behavior in rats. Neuropsychopharmacology. 2009;34:820–33. doi: 10.1038/npp.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormley S, Rompre PP. Blockade of mGluR5 receptors differentially alters amphetamine-induced enhancement of locomotor activity and of brain stimulation reward. J Psychopharmacol. 2011;25:393–401. doi: 10.1177/0269881110367460. [DOI] [PubMed] [Google Scholar]
- Hagerman R, Berry-Kravis E, Hessl D, Coffey S, Schneider A, Nguyen D, Hervey C, Hutchison J, Snape M. Trial of fenobam, an mGluR5 antagonist, in adults with Fragile X Syndrome. J Intellect Disabil Res. 2008;52:814. doi: 10.1136/jmg.2008.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AA, Gasparini F, Markou A. Nicotine potentiation of brain stimulation reward reversed by DHbE and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology (Berl) 2002;160:56–66. doi: 10.1007/s00213-001-0953-6. [DOI] [PubMed] [Google Scholar]
- Itil TM, Seaman PA, Huque M, Mukhopadhyay S, Blasucci D, Tat K, Ciccone PE. The clinical and quantitative EEG effects and plasma levels of fenobam (McN-3377) in subjects with anxiety: an open rising dose tolerance and efficacy study. Curr Ther Res. 1978;24:708–724. [Google Scholar]
- Ito K, Abekawa T, Koyama T. Relationship between development of cross-sensitization to MK-801 and delayed increases in glutamate levels in the nucleus accumbens induced by a high dose of methamphetamine. Psychopharmacology (Berl) 2006;187:293–302. doi: 10.1007/s00213-006-0423-2. [DOI] [PubMed] [Google Scholar]
- Jacob W, Gravius A, Pietraszek M, Nagel J, Belozertseva I, Shekunova E, Malyshkin A, Greco S, Barberi C, Danysz W. The anxiolytic and analgesic properties of fenobam, a potent mGlu5 receptor antagonist, in relation to the impairment of learning. Neuropharmacology. 2009;57:97–108. doi: 10.1016/j.neuropharm.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Jaeschke G, Porter R, Buttelmann B, Ceccarelli SM, Guba W, Kuhn B, Kolczewski S, Huwyler J, Mutel V, Peters JU, Ballard T, Prinssen E, Vieira E, Wichmann J, Spooren W. Synthesis and biological evaluation of fenobam analogs as mGlu5 receptor antagonists. Bioorg Med Chem Lett. 2007;17:1307–11. doi: 10.1016/j.bmcl.2006.12.033. [DOI] [PubMed] [Google Scholar]
- Jaeschke G, Wettstein JG, Nordquist RE, Spooren W. mGlu5 receptor antagonists and their therapeutic potential. Exp Opin Ther Patents. 2008;18:123–142. [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–50. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16:974–86. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karila L, Weinstein A, Aubin HJ, Benyamina A, Reynaud M, Batki SL. Pharmacological approaches to methamphetamine dependence: a focused review. Br J Clin Pharmacol. 2010;69:578–92. doi: 10.1111/j.1365-2125.2010.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Boutrel B, Gasparini F, Koob GF, Markou A. Metabotropic glutamate 5 receptor blockade may attenuate cocaine self-administration by decreasing brain reward function in rats. Psychopharmacology (Berl) 2005;179:247–54. doi: 10.1007/s00213-004-2069-2. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Gasparini F, Markou A. Group II metabotropic and alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate glutamate receptors regulate the deficit in brain reward function associated with nicotine withdrawal in rats. J Pharmacol Exp Ther. 2003;306:1068–76. doi: 10.1124/jpet.103.052027. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol Sci. 2004;25:265–272. doi: 10.1016/j.tips.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Kish SJ. Pharmacologic mechanisms of crystal meth. Can Med Assn J. 2008;178:1679–82. doi: 10.1503/cmaj.071675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Glutamate and reinstatement. Curr Opin Pharmacol. 2009;9:59–64. doi: 10.1016/j.coph.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD, Pierce RC. Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming- and cue-induced reinstatement of cocaine seeking. Behav Brain Res. 2009;202:238–44. doi: 10.1016/j.bbr.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28:3170–7. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark KA, Quinton MS, Russek SJ, Yamamoto BK. Dynamic changes in vesicular glutamate transporter 1 function and expression related to methamphetamine-induced glutamate release. J Neurosci. 2007;27:6823–6831. doi: 10.1523/JNEUROSCI.0013-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell JC, Brecht ML. Methamphetamine: here we go again? Addict Behav. 2011;36:1168–73. doi: 10.1016/j.addbeh.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell JC, Rutkowski BA. The prevalence of methamphetamine and amphetamine abuse in North America: a review of the indicators, 1992–2007. Drug Alcohol Rev. 2008;27:229–35. doi: 10.1080/09595230801919460. [DOI] [PubMed] [Google Scholar]
- Mitrano DA, Smith Y. Comparative analysis of the subcellular and subsynaptic localization of mGluR1a and mGluR5 metabotropic glutamate receptors in the shell and core of the nucleus accumbens in rat and monkey. J Comp Neurol. 2007;500:788–806. doi: 10.1002/cne.21214. [DOI] [PubMed] [Google Scholar]
- Montana MC, Cavallone LF, Stubbert KK, Stefanescu AD, Kharasch ED, Gereau RW. The metabotropic glutamate receptor subtype 5 antagonist fenobam is analgesic and has improved in vivo selectivity as compared to the prototypical antagonist 2-methyl-6-(phenylethynyl)-pyridine. J Pharmacol Exp Ther. 2009;330:834–843. doi: 10.1124/jpet.109.154138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for drug addiction. Curr Drug Abuse Rev. 2009;2:83–98. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Cleva RM, Kalivas PW, Malcolm RJ. Glutamatergic medications for the treatment of drug and behavioral addictions. Pharmacol Biochem Behav. 2012;100:801–10. doi: 10.1016/j.pbb.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne MPH, Olive MF. A role for mGluR5 receptors in intravenous methamphetamine self-administration. Ann NY Acad Sci. 2008;1139:206–211. doi: 10.1196/annals.1432.034. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine, cocaine and food in rats. Psychopharmacology (Berl) 2005;179:255–61. doi: 10.1007/s00213-004-2070-9. [DOI] [PubMed] [Google Scholar]
- Pecknold JC, McClure DJ, Appeltauer L. Fenobam in anxious outpatients. Curr Ther Res. 1980;27:119–123. [Google Scholar]
- Pecknold JC, McClure DJ, Appeltauer L, Wrzesinski L, Allan T. Treatment of anxiety using fenobam (a nonbenzodiazepine) in a double-blind standard (diazepam) placebo-controlled study. J Clin Psychopharmacol. 1982;2:129–33. [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Porter RH, Jaeschke G, Spooren W, Ballard T, Buettelmann B, Kolczewski S, Peters JU, Prinssen E, Wichmann J, Vieira E, Muehlemann A, Gatti S, Mutel V, Malherbe P. Fenobam: a clinically validated non-benzodiazepine anxiolytic is a potent, selective and non-competitive mGlu5 receptor antagonist with inverse agonist activity. J Pharmacol Exp Ther. 2005;315:711–721. doi: 10.1124/jpet.105.089839. [DOI] [PubMed] [Google Scholar]
- Rocher C, Gardier AM. Effects of repeated systemic administration of d-Fenfluramine on serotonin and glutamate release in rat ventral hippocampus: comparison with methamphetamine using in vivo microdialysis. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:422–8. doi: 10.1007/s002100000381. [DOI] [PubMed] [Google Scholar]
- Romano C, Sesma MA, McDonald CT, O’Malley K, Van den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol. 1995;355:455–69. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- Rusyniak DE. Neurologic manifestations of chronic methamphetamine abuse. Neurol Clin. 2011;29:641–55. doi: 10.1016/j.ncl.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17:275–97. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett. 1993;163:53–7. doi: 10.1016/0304-3940(93)90227-c. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Sullivan EB, Maisonneuve IM, Glick SD. Neurochemical and behavioral differences between d-methamphetamine and d-amphetamine in rats. Psychopharmacology (Berl) 2003;165:359–369. doi: 10.1007/s00213-002-1288-7. [DOI] [PubMed] [Google Scholar]
- Shrem MT, Halkitis PN. Methamphetamine abuse in the United States: contextual, psychological and sociological considerations. J Health Psychol. 2008;13:669–79. doi: 10.1177/1359105307082461. [DOI] [PubMed] [Google Scholar]
- Sinclair CM, Cleva RM, Hood LE, Olive MF, Gass JT. mGluR5 receptors in the basolateral amygdala and nucleus accumbens regulate cue-induced reinstatement of ethanol-seeking behavior. Pharmacol Biochem Behav. 2012;101:329–35. doi: 10.1016/j.pbb.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephans SE, Yamamoto BY. Effect of repeated methamphetamine administrations on dopamine and glutamate efflux in rat prefrontal cortex. Brain Res. 1995;700:99–106. doi: 10.1016/0006-8993(95)00938-m. [DOI] [PubMed] [Google Scholar]
- Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–49. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocci FJ, Appel NM. Approaches to the development of medications for the treatment of methamphetamine dependence. Addiction. 2007;102 (Suppl 1):96–106. doi: 10.1111/j.1360-0443.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Loonam TM, Noailles PA, Angulo JA. Comparison of cocaine- and methamphetamine-evoked dopamine and glutamate overflow in somatodendritic and terminal field regions of the rat brain during acute, chronic, and early withdrawal conditions. Ann N Y Acad Sci. 2001;937:93–120. doi: 10.1111/j.1749-6632.2001.tb03560.x. [DOI] [PubMed] [Google Scholar]