Introduction
Diagnostic applications for global health have exploded in the past 10 years. Numerous articles have been generated on global health priorities, constraints of resource-limited settings, and technological innovations for diagnostics development, including several comprehensive reviews [1-3]. In this article, we aim to provide (i) a focused summary of the most highly-needed diagnostics, (ii) a discussion of noteworthy recent developments of technologies in the field, and (iii) a perspective on the evolution, challenges, and future directions of diagnostics for global health applications.
Need for Early Detection and Treatment
Disease treatment in the absence of a diagnostic test is often based on syndromic management (i.e. observing clinical symptoms and factoring in local prevalence of the disease). This situation can result in incorrect treatment of patients manifesting symptoms common to multiple diseases with local prevalence. Unnecessary treatment may compromise the patient (from harmful side effects of a treatment) or the community (through accelerated drug resistance, as has been found in the case of malaria [4]). Further, the patient remains untreated for the relevant condition, potentially leading to higher mortality and morbidity. A diagnostic test that can provide an accurate and timely diagnosis enables (i) earlier interventions before the appearance of advanced symptoms, (ii) correct diagnosis and treatment for each patient, and (iii) effective use of limited resources [4]. Thus, appropriate point-of-care (POC) diagnostics development for high impact diseases could significantly reduce the global disease burden [4-6].
Unmet Needs for Diagnostic Tests
Health conditions can be divided into the two broad categories of infectious diseases and non-communicable diseases. Infectious disease is a major cause of mortality in the developing world, killing nearly 15 million people each year [7]. A more comprehensive measure of disease burden is disability adjusted life years (DALYs), and includes the effects of decreased quality of life due to disease [8]. An estimate from 2006 indicates that for a core set of infectious diseases, the disease burden is an astounding 325 million DALYs per year [4]. Priority infectious diseases, based on disease burden, are HIV/AIDs, tuberculosis, and malaria [7]. Some progress has been made in developing POC diagnostic tests for HIV and malaria. However, there are still many unmet diagnostic needs. For example, in the case of tuberculosis (TB), there is still no POC test appropriate for low-resource settings [9]. The gold-standard, culture, is highly sensitive and provides information on drug resistance, but requires laboratory facilities and a long time to result (i.e. 10 days for rapid culture) [9]. Traditional microscopy is still often used, though it requires trained personnel and has a poor sensitivity of 70% [9]. High performance nucleic acid amplification methods for the diagnosis of TB are now commercially available, but are too expensive for use in low-resource settings (i.e. Cepheid GeneXpert is ~$27,000 plus ~$30-$85 per cartridge). With respect to HIV, CD4 counts and viral load testing remain unmet needs that would have great value to inform treatment decisions (e.g. time to start antiviral therapy and monitoring the effectiveness of the therapy). In addition, a combined diagnostic for HIV and TB would be of value given the high rates of co-infection [9]. Within the set of neglected tropical diseases [10], there is an unmet need for an effective POC diagnostic for dengue/dengue haemorrhagic fever, human African trypanosomiasis, and Leishmaniasis [9, 11]. The ability to simultaneously test for multiple disease conditions could be useful for the differential diagnosis of conditions with similar symptoms. Specifically, studies suggest that in malaria-endemic regions, children presenting with fever, but who do not have malaria, often go without proper diagnosis and treatment, resulting in high mortality rates [12, 13]. Thus, a POC diagnostic that tested for a panel of fever-causing illnesses and could be adapted to geographic area would have value [9]. Additionally, a diagnostic for multiplexed detection of HIV, malaria, syphilis, and anemia, would have specific utility for the care of women during pregnancy in the developing world [9]. In their recent review, Peeling and Mabey [9] have also highlighted the need for diagnostic tests for acute lower respiratory infections to determine appropriateness of antibiotic treatment, and early diagnosis of the often asymptomatic sexually-transmitted infections gonorrhea and chlamydia for proper patient treatment and to reduce transmission.
Despite the disproportionate impact of infectious diseases in the developing world, noncommunicable diseases (NCDs) are the leading cause of death globally, with almost 80% of deaths occurring in low- and middle- income countries [15]. For example, in 2008, there were 36 million deaths, 63% of total deaths globally, due to NCDs; the predominant causes include cardiovascular disease (CVD), cancer, diabetes, and lung disease [15]. In the case of cancer, early detection of breast, cervical, colorectal, skin, and oral cancers, in particular, can lead to a reduction in mortality [15]. Additional NCDs of interest with respect to early diagnosis are gastrointestinal diseases and renal diseases, the latter being a related complication to both CVD and diabetes [15]. Early detection is especially compelling for certain NCDs (e.g., CVD and diabetes) because of the often simple behavioral interventions that can be implemented to improve the health condition [15].
The Continuing Challenge
Gold-standard diagnostic assays are often high performance laboratory-based tests that require multi-step protocols for complex sample processing. Trade-offs for the high performance include long sample processing times, long times for samples to be transported to the lab and for results to be transmitted back to the patient/caregiver, need for trained personnel to run the test and interpret the results, and need for specialized instrumentation for processing samples and detecting analytes. Also assumed is access to electricity to power the instrumentation, to maintain strict environmental conditions, and to refrigerate reagents until use in the assay. The requirements of laboratory-based tests are often incompatible with the constraints of resource-limited settings. Constraints in these settings include patients with limited access to clinics and limited contact time while there, limited training of test providers, lack of laboratory facilities and testing environments with uncontrolled temperatures and humidity levels, and limited local infrastructure, including a lack cold chain for refrigeration of reagents [1, 2, 4]. The World Health Organization has coined an acronym for the characteristics of POC diagnostics that are appropriate for even the lowest-resource global health settings: ASSURED (affordable, sensitive, specific, user-friendly, rapid and robust, equipment free, and deliverable to users) [16]. Thus, the overall challenge has been and continues to be to create high-performance assays that are appropriate for the various multi-constraint settings relevant for global health applications, including the lowest-resource settings.
Dedicated Global Health Lab-on-a-Chip (LOC)
While significant progress has been made recently in genomics, proteomics and other disciplines, few of the scientific discoveries have impacted clinical practice globally [17, 18]. There is strong potential to leverage these discoveries for broad impact in diagnostics for global heath applications using chip-based approaches. One important trend relevant here, is the miniaturization of designs afforded by the small dimension scales of microfluidic-based devices; this allows for portability, and the use of small sample and reagent volumes. These also enable rapid POC results at the bedside, in the ambulance, or at other remote locations [19]. The LOC technology is often configured with a permanent instrument and disposable “cards”. The instrumentation can often be battery powered, and the cards can incorporate reagents stored in dry form to remove the immediate need for the power grid. Automation of the processes results in ease of use for minimally-trained users. In many cases, the cards can be affordable for the specific setting when scaled up for high-volume manufacturing. Key steps have been realized by numerous LOC efforts, and important goals defined with the micro total analysis system (μTAS) paradigm; these have led to technologies suitable for dedicated global health applications at the POC, in genetic [20, 21], proteomic [22, 23], and cellular testing [24-26]. Yet, very few complete workable POC clinical devices have emerged despite tremendous progress in microelectromechanical systems (MEMS), microfabrication, microfluidics, and related areas [17, 18]. Indeed, while the core of typical LOC systems is substantially smaller than that of benchtop counterparts, they still often rely on a network of macroscopic laboratory-based infrastructure for sample processing, sample introduction, analyte detection, data processing, and reagent handling, thus limiting their utility for POC applications. The selected examples of LOC approaches described below highlight efforts to address the challenges of evolving from “chips-in-a-lab” to a true “lab-on-a-chip”.
The Yager group [27] has developed a microfluidic flow-through membrane immunoassay, featuring gold–antibody conjugates stored in dry form on a disposable laminate card, that works in conjunction with external pumping and imaging instrumentation. The system, demonstrated for the malarial antigen Plasmodium falciparum histidine-rich protein II (PfHRP2), retained high activity after 60-day storage at elevated temperatures, with a detection limit in the sub-nanomolar range in under nine minutes. Other approaches by Sia with MEMS and Singh using chip-based separation and quantitation have continued to increase the level of integration of diagnostic devices [28, 29]. Significant progress has been achieved in fluid handling through implementation of innovative LOC components and actuation strategies. Another example by the Sia group, demonstrated the actuation of multiple microfabricated microvalves with rapid response in an enzymatic assay, via liquid-filled control channels in a handheld instrument powered by a simple 9 V battery [30].
Madou and others have demonstrated the use of microfluidic compact disc (CD) formats [31] as a cost-effective approach to eliminate pumps, tubing, and valves, and drive fluids with centripetal force. Recently, the Liu group [32] has applied the approach to detect microparticles and cells. The device, compatible with the use of standard CD drives, was demonstrated with Chinese Hamster Ovarian (CHO) cells of various concentrations, and could eventually be employed to perform ELISAs. In a recent perspective article on optical biosensors, Ligler highlights innovations that may lead to faster, smaller, and more cost-effective optical biosensor systems. Among the most promising examples for facilitating integration, is a new generation of polymer components, e.g. organic photodiodes (OPDs), for use as optical detectors [33].
Work by Klapperich et al. has shown the suitability of plastic microfabrication methods, compatible with global diagnostics costs, to produce high performance parts for use in continuous flow polymerase chain reaction assays (CF-PCR) [34]. This approach may have significant impact in infectious diseases diagnosis in the developing world, and also provides a cost-effective approach to DNA testing that resonates with the promise of personalized medicine in developed countries to drive down the cost of and improve healthcare [20, 21].
Magnetic nanotechnology has recently been used to enable rapid, multiplex immunoassays for POC applications. The portable battery-powered platform, developed by the Wang lab [35], also has reduced requirements for operator training. Their approach has provided promising analytical results using p24 protein as model. It is envisioned that when demonstrated with clinical samples, this technology could be used to detect a number of infectious disease agents such as HIV, HCV, Mycobacterium tuberculosis, Salmonella typhi, toxigenic E. coli, as well as swine (H1N1) flu and avian (H5N1) flu.
A Fully-integrated Standards-based Systems Approach
Another viable strategy to reduce instrumentation costs for resource-limited settings, in development in the McDevitt laboratory, is to leverage the capabilities of a global network of diagnostic devices based on universal standards. This new approach is a significant departure from current diagnostic test systems that are fragmented in terms of specialized instruments dedicated to specific analytes, as well as specific geographic and demographic sectors. For example, today the five most active POC sectors globally are diabetes, acute coronary syndrome (ACS), coagulation, HIV, and platelet function [36]. For the area of cardiac heart disease alone, there are three major sectors involving risk, heart attack detection, and congestive heart failure with at least two qualitative and eight quantitative rapid whole blood devices deployed in POC and remote lab settings [36, 37].
The programmable bio-nano-chip (PBNC) system is inspired by the microelectronics industry, in which a standard operating system is used in conjunction with modular software programs specific to a variety of applications, to provide significant cost reductions and produce increasing performance. The PBNC system is a platform that enables new test configurations to be quickly adapted, developed, and applied for a variety of diagnostic indications through the insertion of reagent specific molecular level code [38, 39]. As such, the PBNC system has the capacity to serve cell counting, typing, and differentiating functions [40-42]. Alternatively, PBNCs can complete analysis of chemical, genomic and proteomic analytes using bead-based microreactors [43-52].
These two distinct PBNC assay platforms are packaged within a disposable, single-use injection-molded plastic “lab card”, comprised of a network of microfluidic components for the complete transfer and processing of biological samples. These sensors provide quick and accurate information on cellular, genomic or proteomic biomarkers of disease at the POC. All assay steps are conducted without human intervention within the lab card that sits within an analyzer equipped with a light emitting diode (LED)/charged coupled device (CCD)-based detection systems and mechanical actuators. This approach eliminates the need for external fluidics, such as pumps, tubing, and connectors. The assay is performed through a sequence programmed into the controller of the analyzer with control over the flow rate, incubation time, and reagent wash achieved by the actuation of stepping motors that direct the fluid flow through the depression of fluid pouches. The sample is directed to an on-chip waste reservoir, and the entire biochip can be discarded as solid waste after the assay, facilitating bio-hazard waste management.
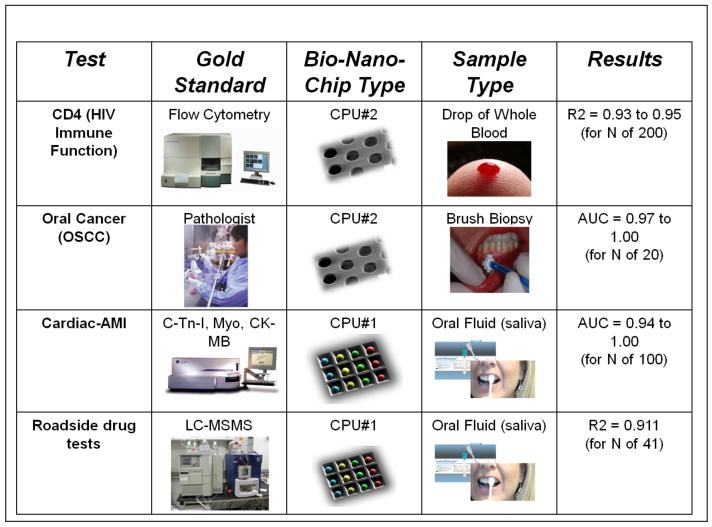
The bead-based PBNC is now moving through 6 major clinical trials and has been successfully applied to serve a variety of important health applications, including ovarian, prostate and oral cancer screening and monitoring [47, 48, 53], cardiac risk assessment [44, 45], and diagnosis of acute myocardial infarction (AMI) [51]. Compared to gold standard and laboratory confined methods, most of which are based on enzyme-linked immunoassay (ELISA) methodology completed in bulky and expensive instruments, the miniaturized bead-based PBNCs exhibit assay times in minutes instead of hours, limits of detection two or more orders of magnitude lower [38], and a proven capacity to multiplex [38, 39, 43-45]. Likewise, the PBNC sensor may be programmed to detect various panels of targets proteins, antibodies, toxins, and drugs of abuse in biological fluids.
The membrane-based PBNC serves as a miniaturized analysis system that mimics flow cytometry instrumentation in their capacity to complete important cell counting applications, such as HIV immune function testing using CD4 cell counts [42]. In addition to lymphocyte enumeration in resource-limited settings, the same membrane system is now being applied for oral cancer screens for the analysis of minimally invasive brush biopsies of oral mucosal lesions [47, 48]. Here, cyto-morphometric data and information about the relevant expression of molecular biomarkers of malignant potential are acquired in an automated manner using refined image analysis algorithms based on pattern recognition techniques and advanced statistical methods. This dedicated PNBC approach has the potential to turn around biopsy results in a matter of minutes as compared to days for traditional pathology methods.
Most importantly, results achieved with the PBNC system correlate well with those of high-performing but laboratory-confined methods, a feature that when considered along with the system’s modularity and advanced performance characteristics in multiplexed capacity mode, promises to remove one the main barriers for the ultimate acceptance and implementation of POC testing; POC tests no longer have to be associated with high cost and limited performance.
Towards Instrument-free Devices for the Lowest-resource Settings
An especially compelling need in the lowest-resource settings is for equipment-free diagnostics such that ongoing maintenance and repair are not required. A major challenge to creating a diagnostic device that is free of dedicated equipment is how to transport fluids within the device without the commonly used active pumping systems. The Delamarche group has developed a microfluidic capillary system with autonomous pumping capability [54]. Their silicon/PDMS microchip performs a conventional sandwich format assay on C-reactive protein (used as an indicator for myocardial infarction) using a single-step delivery of sample and conjugate (similar in operation to the chemical delivery steps in a conventional lateral flow strip test) with fluorescence-based detection [55, 56].
Capillary pumping is the method of fluid transport in the simple lateral flow tests that have been used in low-resource settings for decades. Though lateral flow tests fulfill many of the ASSURED criteria, they have been criticized for both their inability to multiplex (i.e., assay for multiple analytes from a single biosample) and their lack of sensitivity for many analytes of clinical importance [57, 58]. In 2008, the Whitesides group pioneered the use of microfluidic paper-based analytical devices (μPADS), two and three-dimensional paper-based structures that enable colorimetric assays (e.g. for detection of glucose and protein) with multiplexing capability [59, 60]. The original μPAD structures were created by photolithography [61], but since then, numerous alternative fabrication methods have been demonstrated, including wax printing [62, 63], cutting [64], and inkjet printing [65]. Additional work in the area of paper-based assay development has focused on implementing multiplexed assays for the detection of additional biomarkers using one-step colorimetric reactions (e.g. nitrite, uric acid, and lactate) [66, 67] or performing the simultaneous analysis of multiple controls for on-device calibration [68]. Alternative detection methods in paper-based assays have been investigated, including electrochemical detection from screen-printed electrodes for metabolites and heavy metal ions (Pb(II) and Zn(II)) [69-71].
The second limitation of lateral flow devices is their inability to perform the controlled manipulation of multiple reagent volumes in a timed sequence of sample processing steps characteristic of high-performance gold-standard assays. Recently, the collaboration of Yager, Lutz, and Fu, has addressed this issue, demonstrating two-dimensional paper networks (2DPNs) for autonomous multi-step sample processing. A key feature of the 2DPN assay is the configuration of the network, composed of multiple inlets per detection region, which functions as a program for the timed delivery of multiple reagent volumes within the network. 2DPNs that perform the processes of signal amplification [72], sample dilution and mixing [73], and small molecule extraction [73], have been demonstrated. Critical to the operation of multi-step paper-based assays is a set of paper fluidic tools, i.e. analogs to the pump controls and valves of conventional microfluidics, to manipulate fluids within the network for precise timing of reagent delivery and metering of reagent volumes [72, 74, 75]. Additional tools for controlling flow via modification of the wetting properties of the paper channel [76] and simple user-activated mechanical on-switches [77, 78] have been demonstrated by the Phillips, Whitesides, and Shen groups.
Also integral to the development of ASSURED diagnostic devices, is to have available robust methods for power-free temperature control. Recently, the Weigl group at PATH has demonstrated the use of chemical heating, e.g. hydration of CaO, and phase-change materials to perform loop-mediated nucleic acid amplification [79]. Their device achieved a controlled elevated temperature of 65 ±1.5°C for over an hour [79]. The specific combination of exothermic reactants and the composition of the phase change material can be used to tune the thermal properties of the instrument-free heater for numerous applications including other isothermal nucleic acid amplification methods [80], cell lysis protocols, and sample concentration methods based on temperature-responsive polymers [79].
A particularly challenging issue is how to achieve high-sensitivity or quantitative detection without dedicated instrumentation. The use of a dedicated reader in conjunction with non-visible labels, e.g. fluorescent or magnetic particles, has been a common strategy for improving the sensitivity of conventional lateral flow tests [81, 82]. Use of a dedicated reader has also been employed for the measurement of analyte levels and quantitative readout. For example, the Whitesides group has demonstrated the use of a transmission-based reader for measurements in index-matched paper devices [83]. Alternatively, there are several commercially-available readers for lateral flow tests that provide quantitative readout of fluorescence or colorimetric detection (e.g. from ESE GmbH/Qiagen) [81]. The ubiquity of cell phones (possessed by ~60% of people globally [84]), even in low resource settings, provides an opportunity for higher-capability assay readout without a dedicated instrument. The use of cell phones for the acquisition, analysis, and transmission of assay data is an area of active research and development. Challenges include the acquisition of high-quality image data given the expected wide-range of lighting conditions and user variability of camera positioning [85]. The Whitesides group has demonstrated the use of a cell-phone camera for direct acquisition of end-point intensity measurements from a colorimetric paper assay [86], while the Shen group has demonstrated quantitative detection of chemiluminescence [87]. A related approach has been to develop an adapter module to interface a standard cell phone. The Ozcan group has developed a compact adapter (28 g) consisting of LEDs, lens, and filter, that couples to a cell phone camera for wide-field fluorescent and dark-field imaging capability [88].
Challenges and Future Directions
Specifications Must Meet User Performance Requirements
Some technology developers have been arguing that having access to a poor performance POC diagnostic test is better than having no diagnostic test at all. This is demonstrably false in some cases: introduction of a diagnostic test with sub-standard performance specifications can have significant adverse consequences. For example, the case of poor sensitivity of rapid lateral flow tests for influenza has been highlighted recently. Those tests generally have an acceptable clinical specificity (the number of positive cases as measured by the diagnostic test divided by the total number of true positive cases as determined by a gold standard test) of >90%, but have poor clinical sensitivity (the number of negative cases as measured by the diagnostic test divided by the total number of true negatives as determined by a gold standard test) of 11% to 70% [90-93]. Low clinical sensitivity translates to false negatives and, thus, missed opportunities to appropriately treat patients suffering from influenza with anti-viral medication. The US Center for Disease Control issued a statement during the influenza pandemic of 2009 that recommended discontinuing use of those tests [94]. From the previous example, it is clear that an important factor in determining the required performance specifications for a given diagnostic test is consideration of the consequences of obtaining a false negative or false positive result with that test. The consequences of cases missed because of implementing a low-clinical-sensitivity diagnostic test can be severe in the context of an acute health condition with a high mortality rate. On the other hand, the consequences of implementing a low specificity diagnostic test (and the resulting high rate of false positives) can be equally problematic with respect to exposing the misdiagnosed patient to a treatment with potentially adverse side-effects, undue emotional stress, and the financial burden to the healthcare system of additional testing or unnecessary treatment. The latter case was highlighted recently in the context of prostate cancer screening. A false positive result for the serum PSA test impacted at least 1 in 8 men screened repeatedly [95], and has resulted in unnecessary procedures and expense to the healthcare system.
Also critical to understanding the required performance specifications of a potential diagnostic test is prevalence of the disease in the population targeted for screening. The utility of a diagnostic test in a population with a given disease prevalence can be quantified by positive and negative predictive values, the proportion of positives as measured by the diagnostic test that are true positives and the proportion of the negatives as measured by the diagnostic test that are true negatives, respectively. Thus, a diagnostic test with given clinical sensitivity and specificity will have a higher positive predictive value in settings with a higher disease prevalence, while the test will have a lower negative predictive value in settings with a higher disease prevalence. This makes clear the importance of targeted use of a diagnostic test in the most relevant populations.
Future Challenges
Rational diagnostics development for a particular health condition must begin with a thorough understanding of user requirements in the intended setting [4]; the next step is to apply a technology that can meet those user requirements of performance, ease-of-use, shelf life, tolerance for maintenance of instrumentation, and cost. Given the wide range of resource levels in global health applications [1], as well as the varied requirements for performance that are specific to a biomarker and health condition, it is very unlikely that any one technology will be appropriate for all global health applications. However, the use of numerous specialized tools, each tailored for a single diagnostic indication, also creates a challenge in terms of managing multiple tools, maintaining a supply chain and training end users to handle multiple diagnostic aids. This dichotomy will be a central challenge for the next decade.
There are additional factors that will contribute to the success or failure of particular diagnostic tests in getting to market and to the intended end-users. For example, there is generally a higher success rate for diagnostic tests that can be applied to large populations [33], such as tests with the added value of multiplexing for cost-effective testing. However, the regulatory processes for the approval of multiplexed diagnostic tests are even less understood than for diagnostic tests dedicated to single measurements [96, 97]. In addition, devices for detecting more than one biomarker may suffer from a limited understanding of their relevance to the particular disease, and a resulting lack of physician awareness and backing, and/or resistance from healthcare stakeholders to reimburse, especially in the cases of prevention and early diagnosis [33]. Further, diagnostics development activity in private industry has emphasized the developed rather than the developing world, resulting in the production of instrumentation that is complex and cost-prohibitive to use in resource-poor settings [5].
Recent efforts in diagnostics development for global health applications are beginning to produce solutions that could be used in the low-resource setting of developing countries. In particular, diagnostics devices that are free of dedicated instrumentation have the potential to be affordable (and maintenance-free) for even the lowest-resource settings. Another viable approach is to define and address the requirements of resource-limited settings in developing high-performance methods for a suite of disease applications on a common platform. With this standards-driven approach, inspired by the software and microelectronics industry, there is strong potential to be able to sustain the capital expansion that exploits the healthcare infrastructure of developed countries while bringing the same tools to end-users in the developing world.
The total LOC-based biochip market was $2.4 billion in 2009, and is projected to increase to $5.9 billion in 2014 [98] (part of the increasing POC market that is estimated to reach more than $18.7 billion by 2011 [99]). This should be a powerful incentive for commercial efforts to move towards true global health solutions. The recent US healthcare legislation debate and consensus for reform provides additional momentum with the recognition that POC, now representing most of the growth in the in-vitro diagnostics (IVD) sector [100], can deliver lower costs while improving the health of patients.
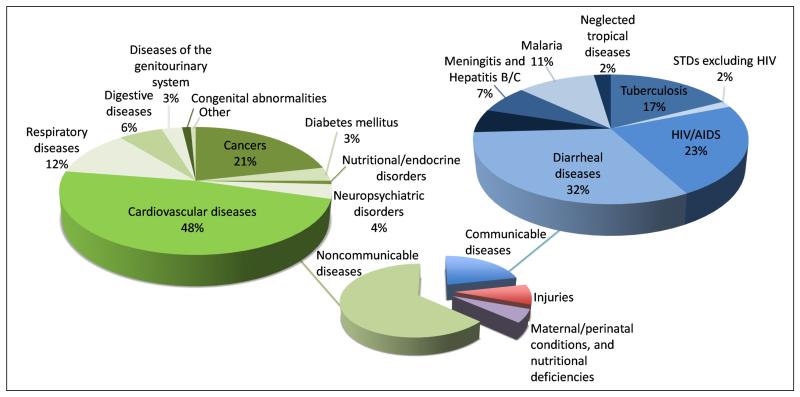
Figure 1.
Worldwide % mortality by health condition. The exploded pie chart shows the % mortality worldwide by health condition in the four main categories of (i) communicable diseases, (ii) non-communicable diseases, (iii) maternal and perinatal conditions and nutritional deficiencies, and (iv) injuries. A further breakdown of % mortality worldwide for the categories of communicable and non-communicable diseases is provided in the upper pie charts [14].
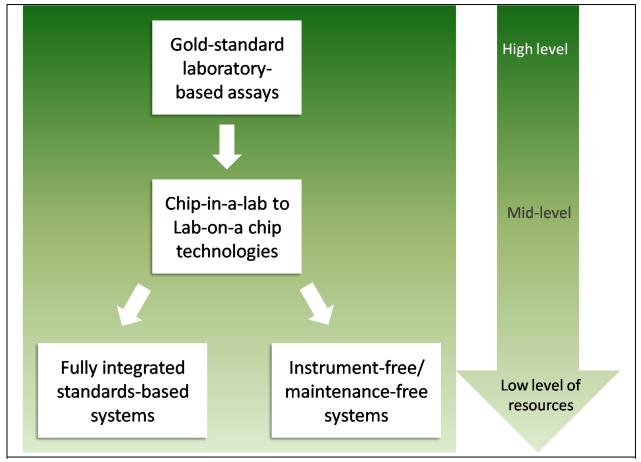
Figure 2.
Schematic of the evolution of POC diagnostics development. The gold-standard laboratory assays are appropriate for settings with a high level of resources. There has been much progress in the development of promising chip-in-a-lab technologies that have, in some cases, been converted to true lab-on-a chip systems for use at the POC. However, the costs of the systems are often a barrier to their use in settings with lower levels of resources. One viable strategy is to push towards fully integrated standards-based systems that leverage the microelectronics and software industries. Also underway, is a movement to create instrument-free diagnostics that will not only have a cost appropriate for the lowest-resource settings, but will also fulfill the equipment-free requirement that is so critical to those settings.
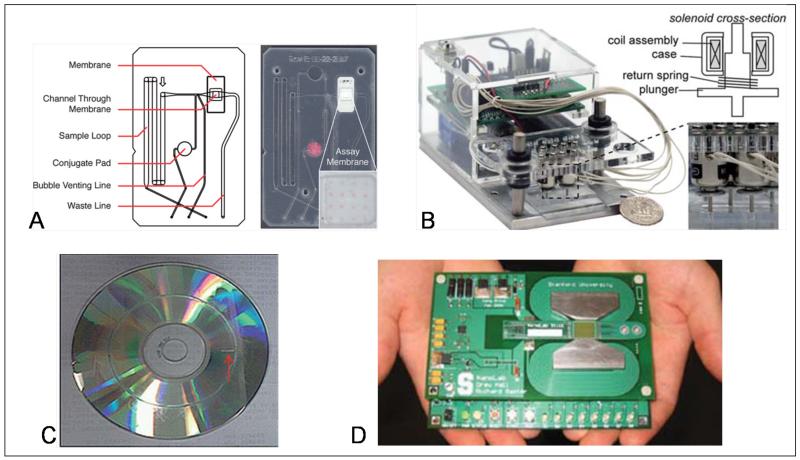
Figure 3.
Examples of promising LOC technologies. (A) Microfluidic flow-through membrane immunoassay developed in the Yager lab achieves rapid and sensitive detection using dry reagents stored on the disposable card [27]. (B) The Sia lab has demonstrated higher-level integration that is completely battery-powered [30]. (C) A CD-based approach for cell detection from the Liu lab reduces the requirements for pumps and valves [32]. (D) The Wang lab has developed a wash-free multiplexed immunoassay based on magnetic nanotechnology [35].
Figure 4.
The McDevitt group has developed a fully-integrated programmable bio-nano-chip (PBNC) platform that enables new test configurations to be quickly adapted, developed, and applied for a variety of diagnostic indications through the insertion of molecular level code (or disease-specific reagents).
Figure 5.
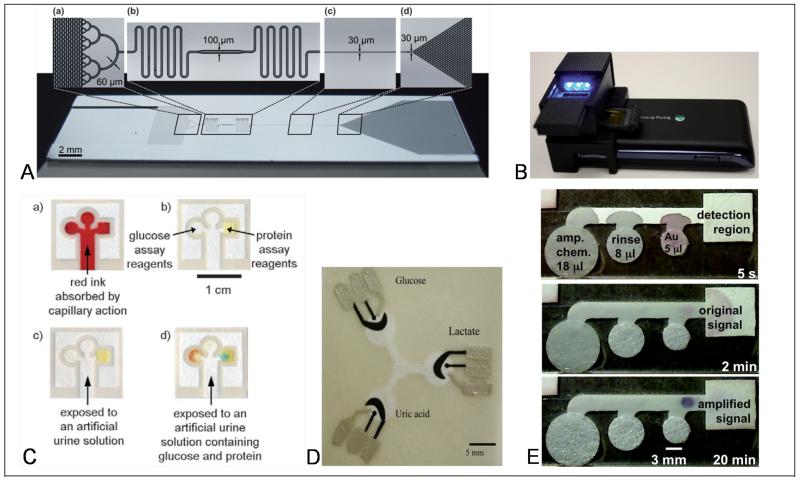
Examples of noteworthy technologies in the movement towards diagnostic devices that are free of dedicated instrumentation. (A) The Delamarche group has developed capillary-based microfluidics in hybrid silicon/PDMS device for the pump-free manipulation of fluids [56]. (B) The Ozcan lab has demonstrated the use of a compact adapter that couples to a cell phone for fluorescence and dark-field imaging of assay results [88]. (C) The Whitesides lab has developed μPADs for multiplexed detection in paper assays [59]. (D) The Henry lab has developed electrochemical detection in paper using screen-printed electrodes [69]. (E) Two-dimensional paper networks (2DPN) for autonomous multi-step sample processing, and thus higher performance assays have been demonstrated by the collaboration of Yager, Lutz, and Fu [89].
Footnotes
Competing Interests Statement
John T. McDevitt serves as the scientific founder for LabNow, Inc. The Rice authors have applied for patents in areas related to PNBC sensor systems.
Contributor Information
Elain Fu, Department of Bioengineering, University of Washington.
Paul Yager, Department of Bioengineering, University of Washington.
Pierre N. Floriano, Department of Bioengineering, Rice University
Nicolaos Christodoulides, Department of Bioengineering, Rice University.
John McDevitt, Department of Bioengineering, Rice University.
References
- 1.Chin CD, Linder V, Sia SK. Lab-on-a-chip devices for global health: past studies and future opportunities. Lab Chip. 2007;7(1):41–57. doi: 10.1039/b611455e. [DOI] [PubMed] [Google Scholar]
- 2.Yager P, Domingo GJ, Gerdes J. Point-of-care diagnostics for global health. Annu Rev Biomed Eng. 2008;10:107–44. doi: 10.1146/annurev.bioeng.10.061807.160524. [DOI] [PubMed] [Google Scholar]
- 3.Yager P, et al. Microfluidic diagnostic technologies for global public health. Nature Insight. 2006;442(7171):412–418. doi: 10.1038/nature05064. [DOI] [PubMed] [Google Scholar]
- 4.Urdea M, et al. Requirements for high impact diagnostics in the developing world. Nature. 2006;444(Suppl 1):73–9. doi: 10.1038/nature05448. [DOI] [PubMed] [Google Scholar]
- 5.Hay Burgess D, Wasserman J, Dahl C. Global Helath Diagnostics. Nature. 2006;444(Suppl 1):1–2. doi: 10.1038/nature05440. [DOI] [PubMed] [Google Scholar]
- 6.Girosi F, et al. Developing and interpreting models to improve diagnostics in developing countries. Nature. 2006;444(Suppl 1):3–8. doi: 10.1038/nature05441. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization WHO global burden of disease: 2004 update. 2008 www.who.int/healthinfo/global_burden_disease/2004_report_update/en/index.html.
- 8.Murray CJL, Lopez AD. The Global Burden of Disease. A comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Harvard University Press; Cambridge, MA: 1996. [Google Scholar]
- 9.Peeling RW, Mabey D. Point-of-care tests for diagnosing infections in the developing world. Clinical Microbiology and Infection. 2010;16(8):1062–1069. doi: 10.1111/j.1469-0691.2010.03279.x. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization 2011 http://www.who.int/neglected_diseases/diseases/en/
- 11.Guzman MG, et al. Dengue: a continuing global threat. Nature Reviews Microbiology. 2010:S7–S16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brent AJ, et al. Incidence of clinically significant bacteraemia in children who present to hospital in Kenya: community-based observational study. Lancet. 2006;367(9509):482–488. doi: 10.1016/S0140-6736(06)68180-4. [DOI] [PubMed] [Google Scholar]
- 13.Evans JA, et al. High mortality of infant bacteraemia clinically indistinguishable from severe malaria. Qjm-an International Journal of Medicine. 2004;97(9):591–597. doi: 10.1093/qjmed/hch093. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization Statistics for worldwide mortality. 2008 http://apps.who.int/ghodata/?vid=10012.
- 15.World Health Organization WHO global status report on noncommunicable diseases. 2010 whqlibdoc.who.int/publications/2011/9789240686458_eng.pdf.
- 16.Kettler H, White K, Hawkes S. WHO/TDR, Mapping the landscape of diagnostics for sexually transmitted infections. 2004 [Google Scholar]
- 17.Vilkner T, Janasek D, Manz A. Micro total analysis systems. Recent developments. Anal Chem. 2004;76(12):3373–85. doi: 10.1021/ac040063q. [DOI] [PubMed] [Google Scholar]
- 18.Whitesides GM. The origins and the future of microfluidics. Nature Insight. 2006;442(7101):368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 19.Ouellette AL, et al. Evolving Point-of-Care Diagnostics Using Up-Converting Phosphor Bioanalytical Systems. Analytical Chemistry. 2009;81(9):3216–3221. doi: 10.1021/ac900475u. [DOI] [PubMed] [Google Scholar]
- 20.Brennan D, et al. Emerging optofluidic technologies for point-of-care genetic analysis systems: a review. Analytical and Bioanalytical Chemistry. 2009;395(3):621–636. doi: 10.1007/s00216-009-2826-5. [DOI] [PubMed] [Google Scholar]
- 21.Dobson MG, Galvin P, Barton DE. Emerging technologies for point-of-care genetic testing. Expert Review of Molecular Diagnostics. 2007;7(4):359–370. doi: 10.1586/14737159.7.4.359. [DOI] [PubMed] [Google Scholar]
- 22.Sorger PK. Microfluidics closes in on point-of-care assays. Nature Biotechnology. 2008;26(12):1345–1346. doi: 10.1038/nbt1208-1345. [DOI] [PubMed] [Google Scholar]
- 23.Soper SA, et al. Point-of-care biosensor systems for cancer diagnostics/prognostics. Biosensors & Bioelectronics. 2006;21(10):1932–1942. doi: 10.1016/j.bios.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Dharmasiri U, et al. Microsystems for the Capture of Low-Abundance Cells. Annual Review of Analytical Chemistry. 2010;3:409–431. doi: 10.1146/annurev.anchem.111808.073610. [DOI] [PubMed] [Google Scholar]
- 25.Heikali D, Di Carlo D. A Niche for Microfluidics in Portable Hematology Analyzers. Jala. 2010;15(4):319–328. [Google Scholar]
- 26.Wlodkowic D, Cooper JM. Microfabricated analytical systems for integrated cancer cytomics. Analytical and Bioanalytical Chemistry. 2010;398(1):193–209. doi: 10.1007/s00216-010-3722-8. [DOI] [PubMed] [Google Scholar]
- 27.Stevens DY, et al. Enabling a microfluidic immunoassay for the developing world by integration of on-card dry-reagent storage. Lab on a Chip. 2008;8:2038–2045. doi: 10.1039/b811158h. [DOI] [PubMed] [Google Scholar]
- 28.Sia SK, et al. An integrated approach to a portable and low-cost immunoassay for resource-poor settings. Angewandte Chemie (International Edition) 2004;43(4):498–502. doi: 10.1002/anie.200353016. [DOI] [PubMed] [Google Scholar]
- 29.Herr AE, et al. Integrated microfluidic platform for oral diagnostics. Annals of the New York Academy of Sciences. 2007:1098. doi: 10.1196/annals.1384.004. (Oral-Based Diagnostics): p. 362-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Addae-Mensah KA, et al. Actuation of elastomeric microvalves in point-of-care settings using handheld, battery-powered instrumentation. Lab on a Chip. 2010;10(12):1618–1622. doi: 10.1039/c002349c. [DOI] [PubMed] [Google Scholar]
- 31.Madou M, et al. Lab on a CD. Annual Review of Biomedical Engineering. 2006;8:601–628. doi: 10.1146/annurev.bioeng.8.061505.095758. [DOI] [PubMed] [Google Scholar]
- 32.Imaad SM, et al. Microparticle and cell counting with digital microfluidic compact disc using standard CD drive. Lab on a Chip. 2011;11(8):1448–1456. doi: 10.1039/c0lc00451k. [DOI] [PubMed] [Google Scholar]
- 33.Ligler FS. Perspective on Optical Biosensors and Integrated Sensor Systems. Analytical Chemistry. 2009;81(2):519–526. doi: 10.1021/ac8016289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao QQ, Kim MC, Klapperich CM. Plastic microfluidic chip for continuous-flow polymerase chain reaction: Simulations and experiments. Biotechnology Journal. 2011;6(2):177–184. doi: 10.1002/biot.201000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaster RS, Hall DA, Wang SX. nanoLAB: An ultraportable, handheld diagnostic laboratory for global health. Lab on a Chip. 2011;11(5):950–956. doi: 10.1039/c0lc00534g. [DOI] [PubMed] [Google Scholar]
- 36.Melanson SF. What Is New in Point-of-Care Testing? Point of Care. 2009;8(4):166–170. [Google Scholar]
- 37.Friess U, Stark M. Cardiac markers: a clear cause for point-of-care testing. Analytical and Bioanalytical Chemistry. 2009;393(5):1453–1462. doi: 10.1007/s00216-008-2573-z. [DOI] [PubMed] [Google Scholar]
- 38.Jokerst JV, et al. Programmable Nano-Bio-Chip Sensors: Analytical Meets Clinical. Analytical Chemistry. 2010;82(5):1571–1579. doi: 10.1021/ac901743u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jokerst JV, et al. Location of Biomarkers and Reagents within Agarose Beads of a Programmable Bio-nano-chip. Small. 2011;7(5):613–624. doi: 10.1002/smll.201002089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Floriano PN, et al. Microchip-based enumeration of human white blood cells. In: Floriano PN, editor. Microchip-based Assay Systems, Methods and Applications. Humana Press; Totowa, NJ: 2007. pp. 53–64. [DOI] [PubMed] [Google Scholar]
- 41.Floriano PN, et al. Membrane-based on-line optical analysis system for rapid detection of bacteria and spores. Biosensors and Bioelectronics. 2005;20(10):2079–2088. doi: 10.1016/j.bios.2004.08.046. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez WR, et al. A microchip CD4 counting method for HIV monitoring in resource-poor settings. Plos Medicine. 2005;2(7):663–672. doi: 10.1371/journal.pmed.0020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christodoulides N, et al. Lab-on-a-chip methods for point-of-care measurements of salivary biomarkers of periodontitis. Oral-Based Diagnostics. 2007:411–428. doi: 10.1196/annals.1384.035. [DOI] [PubMed] [Google Scholar]
- 44.Christodoulides N, et al. Application of microchip assay system for the measurement of C-reactive protein in human saliva. Lab Chip. 2005;5(3):261–9. doi: 10.1039/b414194f. [DOI] [PubMed] [Google Scholar]
- 45.Christodoulides N, et al. A microchip-based multianalyte assay system for the assessment of cardiac risk. Analytical Chemistry. 2002;74(13):3030–3036. doi: 10.1021/ac011150a. [DOI] [PubMed] [Google Scholar]
- 46.Weigum SE, et al. Lab-on-a-chip sensor for analysis of cellular biomarkers in oral exfoliative cytology: A new diagnostic tool for early detection of oral cancer. Oral Oncology. 2009:111–111. [Google Scholar]
- 47.Weigum SE, et al. Nano-Bio-Chip Sensor Platform for Examination of Oral Exfoliative Cytology. Cancer Prevention Research. 2010;3(4):518–528. doi: 10.1158/1940-6207.CAPR-09-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weigum SE, et al. Cell-based sensor for analysis of EGFR biomarker expression in oral cancer. Lab on a Chip. 2007;7(8):995–1003. doi: 10.1039/b703918b. [DOI] [PubMed] [Google Scholar]
- 49.Li S, et al. A continuous flow polymerase chain reaction microchip with regional velocity control. Journal of Microelectromechanical Systems. 2006;15(1):223–236. doi: 10.1109/JMEMS.2005.859083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lavigne JJ, et al. Solution-based analysis of multiple analytes by a sensor array: Toward the development of an “electronic tongue”. Journal of the American Chemical Society. 1998;120(25):6429–6430. [Google Scholar]
- 51.Floriano PN, et al. Use of Saliva-Based Nano-Biochip Tests for Acute Myocardial Infarction at the Point of Care: A Feasibility Study. Clinical Chemistry. 2009;55(8):1530–1538. doi: 10.1373/clinchem.2008.117713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ali MF, et al. DNA hybridization and discrimination of single-nucleotide mismatches using chip-based microbead arrays. Analytical Chemistry. 2003;75(18):4732–4739. doi: 10.1021/ac034106z. [DOI] [PubMed] [Google Scholar]
- 53.Jokerst JV, et al. Nano-bio-chips for high performance multiplexed protein detection: Determinations of cancer biomarkers in serum and saliva using quantum dot bioconjugate labels. Biosensors & Bioelectronics. 2009;24(12):3622–3629. doi: 10.1016/j.bios.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Juncker D, et al. Autonomous microfluidic capillary system. Analytical Chemistry. 2002;74(24):6139–6144. doi: 10.1021/ac0261449. [DOI] [PubMed] [Google Scholar]
- 55.Zimmermann M, Hunziker P, Delamarche E. Autonomous capillary system for one-step immunoassays. Biomedical Microdevices. 2009;11(1):1–8. doi: 10.1007/s10544-008-9187-2. [DOI] [PubMed] [Google Scholar]
- 56.Gervais L, Delamarche E. Toward one-step point-of-care immunodiagnostics using capillary-driven microfluidics and PDMS substrates. Lab on a Chip. 2009;9(23):3330–3337. doi: 10.1039/b906523g. [DOI] [PubMed] [Google Scholar]
- 57.Posthuma-Trumpie GA, Korf J, van Amerongen A. Lateral flow (immuno) assay: its strengths, weaknesses, opportunities and threats. A literature survey. Analytical and Bioanalytical Chemistry. 2009;393(2):569–582. doi: 10.1007/s00216-008-2287-2. [DOI] [PubMed] [Google Scholar]
- 58.O’Farrell B. Evolution in lateral flow-based immunoassay systems. In: Wong R, Tse H, editors. Lateral Flow Immunoassay. Humana Press; New York: 2009. pp. 1–33. [Google Scholar]
- 59.Martinez AW, et al. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angewandte Chemie-International Edition. 2007;46(8):1318–1320. doi: 10.1002/anie.200603817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martinez AW, Phillips ST, Whitesides GM. Three-dimensional microfluidic devices fabricated in layered paper and tape. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(50):19606–19611. doi: 10.1073/pnas.0810903105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martinez AW, et al. FLASH: A rapid method for prototyping paper-based microfluidic devices. Lab on a Chip. 2008;8(12):2146–2150. doi: 10.1039/b811135a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu Y, et al. Fabrication and Characterization of Paper-Based Microfluidics Prepared in Nitrocellulose Membrane By Wax Printing. Analytical Chemistry. 2010;82(1):329–335. doi: 10.1021/ac9020193. [DOI] [PubMed] [Google Scholar]
- 63.Carrilho E, Martinez AW, Whitesides GM. Understanding Wax Printing: A Simple Micropatterning Process for Paper-Based Microfluidics. Analytical Chemistry. 2009;81(16):7091–7095. doi: 10.1021/ac901071p. [DOI] [PubMed] [Google Scholar]
- 64.Fenton EM, et al. Multiplex Lateral-Flow Test Strips Fabricated by Two-Dimensional Shaping. Acs Applied Materials & Interfaces. 2009;1(1):124–129. doi: 10.1021/am800043z. [DOI] [PubMed] [Google Scholar]
- 65.Abe K, et al. Inkjet-printed paperfluidic immuno-chemical sensing device. Analytical and Bioanalytical Chemistry. 2010;398(2):885–893. doi: 10.1007/s00216-010-4011-2. [DOI] [PubMed] [Google Scholar]
- 66.Li X, Tian JF, Shen W. Quantitative biomarker assay with microfluidic paper-based analytical devices. Analytical and Bioanalytical Chemistry. 2010;396(1):495–501. doi: 10.1007/s00216-009-3195-9. [DOI] [PubMed] [Google Scholar]
- 67.Dungchai W, Chailapakul O, Henry CS. Use of multiple colorimetric indicators for paper-based microfluidic devices. Analytica Chimica Acta. 2010;674(2):227–233. doi: 10.1016/j.aca.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 68.Wang W, et al. Tree-shaped paper strip for semiquantitative colorimetric detection of protein with self-calibration. Journal of Chromatography A. 2010;1217(24):3896–3899. doi: 10.1016/j.chroma.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 69.Dungchai W, Chailapakul O, Henry CS. Electrochemical Detection for Paper-Based Microfluidics. Analytical Chemistry. 2009;81(14):5821–5826. doi: 10.1021/ac9007573. [DOI] [PubMed] [Google Scholar]
- 70.Nie ZH, et al. Electrochemical sensing in paper-based microfluidic devices. Lab on a Chip. 2009;10(4):477–483. doi: 10.1039/b917150a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carvalhal RF, et al. Electrochemical Detection in a Paper-Based Separation Device. Analytical Chemistry. 2010;82(3):1162–1165. doi: 10.1021/ac902647r. [DOI] [PubMed] [Google Scholar]
- 72.Fu E, et al. Transport in two-dimensional paper networks. Microfluidics and Nanofluidics. 2011;10:29–35. doi: 10.1007/s10404-010-0643-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Osborn J, et al. Microfluidics without pumps: reinventing the T-sensor and H-filter in paper networks. Lab on a Chip. 2010 doi: 10.1039/c004821f. DOI: 10.1039/c004821f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fu E, et al. Controlled reagent transport in disposable 2D paper networks. Lab on a Chip. 2010;10(7):918–920. doi: 10.1039/b919614e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kauffman P, et al. Visualization and measurement of flow in two-dimensional paper networks. Lab on a Chip. 2010;10(19):2614–2617. doi: 10.1039/c004766j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Noh N, Phillips ST. Metering the Capillary-Driven Flow of Fluids in Paper-Based Microfluidic Devices. Analytical Chemistry. 2010;82(10):4181–4187. doi: 10.1021/ac100431y. [DOI] [PubMed] [Google Scholar]
- 77.Martinez AW, et al. Programmable diagnostic devices made from paper and tape (vol 10, pg 2499, 2010) Lab on a Chip. 2010;10(24):3428–3428. doi: 10.1039/c0lc00021c. [DOI] [PubMed] [Google Scholar]
- 78.Li X, Tian JF, Shen W. Progress in patterned paper sizing for fabrication of paper-based microfluidic sensors. Cellulose. 2010;17(3):649–659. [Google Scholar]
- 79.LaBarre P, et al. A Simple, Inexpensive Device for Nucleic Acid Amplification without Electricity—Toward Instrument- Free Molecular Diagnostics in Low-Resource Settings. Plos One. 2011;6(5):e19738. doi: 10.1371/journal.pone.0019738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Niemz A, Ferguson T, D B. Point-of-care nucleic acid testing for infectious diseases. Trends in Biotechnology. 2011 doi: 10.1016/j.tibtech.2011.01.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Faulstich K, et al. Handheld and portable reader devices for lateral flow immunoassays. In: Wong R, Tse H, editors. Lateral Flow Immunoassay. Humana Press; New York: 2009. pp. 75–94. [Google Scholar]
- 82.Chun P. Colloidal gold and other labels for lateral flow immunoassays. In: Wong R, Tse H, editors. Lateral Flow Immunoassay. Humana Press; New York: 2009. pp. 75–94. [Google Scholar]
- 83.Ellerbee A, et al. Quantifying colorimetric assays in paper-based microfluidic devices by measuring the transmission of light through paper. Analytical Chemistry. 2009;81:8447–8452. doi: 10.1021/ac901307q. [DOI] [PubMed] [Google Scholar]
- 84.International Telecommunication Union Market information and statistics. 2010 Avaialble at: http://www.itu.int/ITU-D/ict/statistics/index.html.
- 85.Stevens D. Bioengineering. University of Washington; Seattle: 2010. Development and Optical Analysis of a Microfluidic Point-of-Care Diagnostic Device; p. 230. [Google Scholar]
- 86.Martinez AW, et al. Simple telemedicine for developing regions: Camera phones and paper-based microfluidic devices for real-time, off-site diagnosis. Analytical Chemistry. 2008;80(10):3699–3707. doi: 10.1021/ac800112r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Delaney JL, et al. Electrogenerated Chemiluminescence Detection in Paper-Based Microfluidic Sensors. Analytical Chemistry. 2011;83(4):1300–1306. doi: 10.1021/ac102392t. [DOI] [PubMed] [Google Scholar]
- 88.Zhu H, et al. Cost-effective and compact wide-field fluorescent imaging on a cell-phone. Lab on a Chip. 2011;11:315–322. doi: 10.1039/c0lc00358a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fu E, et al. Chemical signal amplification in two-dimensional paper networks. Sensors and Actuators B-Chemical. 2010;149(1):325–328. doi: 10.1016/j.snb.2010.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Drexler JF, et al. Poor Clinical Sensitivity of Rapid Antigen Test for Influenza A Pandemic (H1N1) 2009 Virus. Emerging Infectious Diseases. 2009;15(10):1662–1664. doi: 10.3201/eid1510.091186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hurt AC, et al. Performance of six influenza rapid tests in detecting human influenza in clinical specimens. Journal of Clinical Virology. 2007;39(2):132–135. doi: 10.1016/j.jcv.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 92.Uyeki T. Influenza diagnosis and treatment in children: a review of studies on clinically useful tests and antiviral treatment for influenza. Pediatric Infectious Disease Journal. 2003;22(2):164–177. doi: 10.1097/01.inf.0000050458.35010.b6. [DOI] [PubMed] [Google Scholar]
- 93.Vasoo S, Stevens J, Singh K. Rapid Antigen Tests for Diagnosis of Pandemic (Swine) Influenza A/H1N1. Clinical Infectious Diseases. 2009;49(7):1090–1093. doi: 10.1086/644743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Center for Disease Control Interim guidance for detection of novel influenza A virus using rapid influenza testing. 2009 http://www.cdc.gov/h1n1flu/guidance/rapid_testing.htm.
- 95.Kilpeläinen T, et al. False-positive screening results in the Finnish prostate cancer screening trial. British journal of cancer. 2010;102:469–474. doi: 10.1038/sj.bjc.6605512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boja ES, et al. The Journey to Regulation of Protein-Based Multiplex Quantitative Assays. Clinical Chemistry. 2011;57(4):560–567. doi: 10.1373/clinchem.2010.156034. [DOI] [PubMed] [Google Scholar]
- 97.Regnier FE, et al. Protein-Based Multiplex Assays: Mock Presubmissions to the US Food and Drug Administration. Clinical Chemistry. 2010;56(2):165–171. doi: 10.1373/clinchem.2009.140087. [DOI] [PubMed] [Google Scholar]
- 98.bcc Research Market Forecasting . Global Biochip Markets: Microarrays and Lab-on-a-Chip. 2010. [Google Scholar]
- 99.American Association for Clinical Chemistry . CLIA Waivers Drive POCT Expansion. 2008. [Google Scholar]
- 100.Huckle D. Point-of-care diagnostics: an advancing sector with nontechnical issues. Expert Review of Molecular Diagnostics. 2008;8(6):679–688. doi: 10.1586/14737159.8.6.679. [DOI] [PubMed] [Google Scholar]