SUMMARY
Among human natural killer (NK) cell intermediates in secondary lymphoid tissue (SLT), stage 3 CD34− CD117+CD161+CD94− immature NK (iNK) cells uniquely express aryl hydrocarbon receptor (AHR) and interleukin-22 (IL-22), supporting a role in mucosal immunity. The mechanisms controlling proliferation and differentiation of these cells are unknown. Here we demonstrate that the IL-1 receptor IL-1R1 was selectively expressed by a subpopulation of iNK cells that localized proximal to IL-1β-producing conventional dendritic cells (cDCs) within SLT. IL-1R1hi iNK cells required continuous exposure to IL-1β to retain AHR and IL-22 expression, and proliferate in direct response to cDC-derived IL-15 and IL-1β. In the absence of IL-1β, a substantially greater fraction of IL-1R1hi iNK cells differentiated to stage 4 NK cells and acquired the ability to kill and secrete IFN-γ. Thus, cDC-derived IL-1β preserves and expands IL-1R1hiIL-22+AHR+ iNK cells, potentially influencing human mucosal innate immunity during infection.
INTRODUCTION
Natural killer (NK) cells comprise a unique, diverse set of lymphocytes with emerging roles in the innate and adaptive immune response (Sun and Lanier, 2009). Within secondary lymphoid tissue (SLT), human NK cells appear to proceed through five discrete states of developmental maturity, starting as multipotent CD34+ pro-NK cells, and eventually progressing to functionally competent mature NK cells (Freud et al., 2005; Freud et al., 2006). Our laboratory initially characterized four stages of NK cell development in SLT in terms of lineage commitment, response to cytokines, and NK cell effector function (Freud et al., 2006). CD34+CD117− stage 1 pro-NK cells and CD34+CD117+ stage 2 pre-NK cells retain potential for differentiation into T cells and dendritic cells (DCs), whereas CD34− CD117+CD94− stage 3 immature NK (iNK) cells and CD34−CD117+/−CD94+ stage 4 mature NK cells appear exclusively committed to the NK cell lineage. In addition, a fifth and seemingly final stage of NK cell development has recently been described in SLT, characterized by acquisition of CD16 and killer cell immunoglobulin (Ig)-like receptors, with a surface antigen repertoire highly similar to that of peripheral blood CD56dim NK cells (Freud and Caligiuri, 2006; Romagnani et al., 2007). Stage 3 cells have been classified as immature because they lack certain NK cell surface receptors, the capacity for IFN-γ production, and the cytolytic activity that are characteristic of mature NK cells including the more differentiated stage 4 NK cells (Freud et al., 2006).
Resting stage 3 iNK cells found in human tonsil constitutively express interleukin-22 (IL-22) (Cupedo et al., 2009; Hughes et al., 2009), a cytokine implicated in mucosal immunity (Wolk et al., 2004). “NK-22” cells found in mucosa-associated lymphoid tissue (MALT) express the aryl hydrocarbon receptor (AHR) (Cella et al., 2009). These data suggest that stage 3 iNK cells within SLT may have an important role in mucosal immunity. IL-22+ iNK cells from human fetal lymph node possess lymphoid tissue inducer (LTi) activity and a phenotype identical to that of LTi cells (Cupedo et al., 2009). More recently, Crellin et al demonstrated LTi-like function within the CD127+ subset of this Lin−CD117+CD161+ population (Crellin et al.).
Indeed, stage 3 iNK cells represent the most abundant NK cell developmental intermediate in human SLT (Figure S1). Conceivably, microbial invasion of MALT requires an expansion of the IL-22+ stage 3 iNK cell for effective early mucosal defense (Cupedo et al., 2009; Hughes et al., 2009). Under such circumstances, expansion of the IL-22+ stage 3 population would need to occur with minimal differentiation to IFN-γ-producing stage 4 mature NK cells. Likewise, the architecture of SLT can be drastically altered by excessive inflammation caused by acute microbial infections, and needs to be regenerated in order to restore the SLT microenvironment. During this process, LTi cells must proliferate and upregulate their expression of molecules such as lymphotoxin (LT) α1β2 in order to initiate tissue reorganization (Junt et al., 2008). The signal(s) which regulate expansion and differentiation of the stage 3 iNK cell population are unknown and were investigated in this report. Here we show that the IL-1 receptor IL-1R1 was selectively expressed by a subpopulation of stage 3 iNK cells. In vitro, IL-1β was required to retain AHR and IL-22 expression in IL-1R1hi iNK cells. In the absence of IL-1β, a substantially greater fraction of IL-1R1hi iNK cells differentiated to stage 4 NK cells capable of cytotoxicity and IFN-γ secretion. These stage 3 iNK cells localized proximal to IL-1β-producing conventional dendritic cells (cDCs) within SLT, and proliferated in direct response to cDC-derived IL-15 and IL-1β. Together, our findings demonstrate a role for IL-1β as a factor which influences the homeostasis of human IL-22+ stage 3 iNK cells, and suggest a potential physiological role for CD11chiIL-1β+ cDCs in regulating expansion of this IL-1R1hi subpopulation of stage 3 iNK cells in SLT.
RESULTS
Robust expression of IL-R1 surface protein is restricted to stage 3 iNK cells in SLT
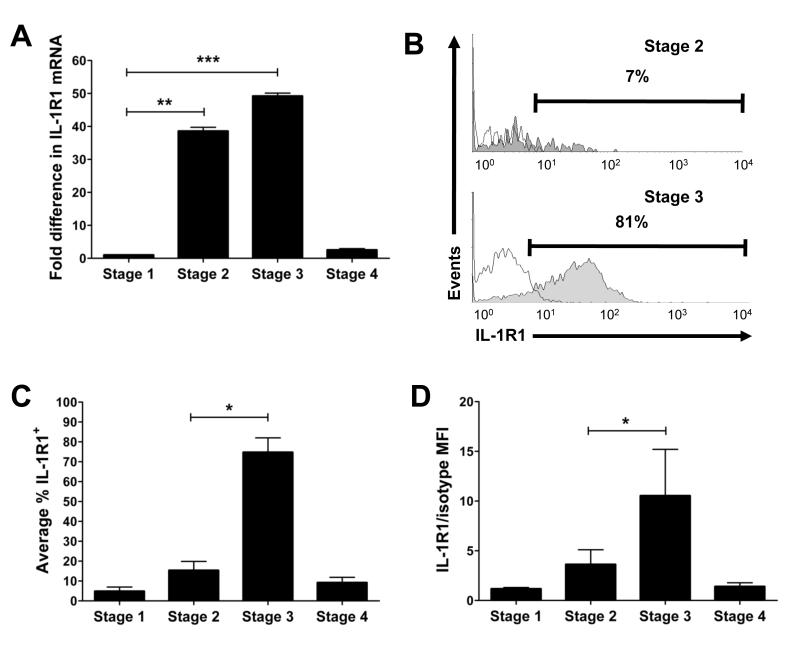
IL-1β is released by monocytes, macrophages, and DCs in direct response to microbial invasion (Ikejima et al., 1984), and promotes T helper 17 (Th17) cell homeostasis (Chung et al., 2009). We postulated that IL-1β may serve a similar role in stage 3 iNK cell homeostasis within SLT. We therefore assayed flow cytometry purified stage 1-4 NK developmental intermediates from human tonsil for expression of IL-1R1 mRNA using real-time RT-PCR. As shown in Figure 1A, significant IL-1R1 mRNA expression was confined to stage 2 pre-NK cells and stage 3 iNK cells. In particular, IL-1R1 mRNA increased at least 38 ± 2.6-fold between stages 1 and 2, and increased 49.2 ± 2.46-fold in stage 3 iNK cells over that seen in stages 1 or 4, where expression was negligible. Thus, IL-1R1 mRNA is selectively and abundantly expressed within stages 2 and 3 of human NK cell development.
Figure 1. IL-1R1 expression during NK cell development.
(A) IL-1R1 mRNA expression ex vivo in flow cytometry purified human stage 1-4 SLT NK developmental intermediates assessed via Real-Time PCR. Fold difference in IL-1R1 mRNA is relative to that in stage 1, arbitrarily normalized to 1 (n = 4). (B-D) Total CD3−CD19− tonsillar mononuclear cells were stained for surface expression of CD34, CD117, CD94, and IL-1R1, and gated for each stage as described (Freud et al., 2006). (B) Histograms show IL-1R1 (filled) and isotype (empty) surface staining in stage 2 (top) or stage 3 (bottom) cells from a representative donor (n = 4). (C,D) Shown within each gated SLT NK developmental intermediate populations are average: (C) % of cells with IL-1R1 surface expression (n = 4), and (D) density of IL-1R1 surface expression, as represented by the ratio of IL-1R1 mean fluorescence intensity (MFI) to isotype MFI (n = 6). Data in A, C, D presented as mean ± SEM (*, P ≤ 0.05; **, P ≤ 0.005; ***, P ≤ 0.0005).
Using flow cytometry, we next determined whether stage 2 pre-NK cells and stage 3 iNK cells from SLT display IL-1R1 cell surface protein. Whereas ≤ 20% of stage 2 pre-NK cells IL-1R1 surface protein, ≥ 70% of stage 3 iNK cells expressed IL-1R1 (Figure 1B,C). Notably, < 10% of stage 4 mature NK cells expressed IL-1R1, data consistent with the restricted manner in which IL-1R1 mRNA was expressed. The ratio of IL-1R1 mean fluorescence intensity (MFI) to isotype control MFI, which was used to gauge the relative density of IL-1R1 expression on IL-1R1hi cells, was more than 3.01 ± 0.31-fold higher on stage 3 iNK cells when compared to stage 2 pre-NK cells (Figure 1D). Collectively, these findings indicate that among human SLT NK developmental intermediates, high density surface expression of IL-1R1 protein is only found on a majority of stage 3 iNK cells.
IL-22 and AHR are restricted to the IL-1R1hi subpopulation of stage 3 iNK cells in SLT
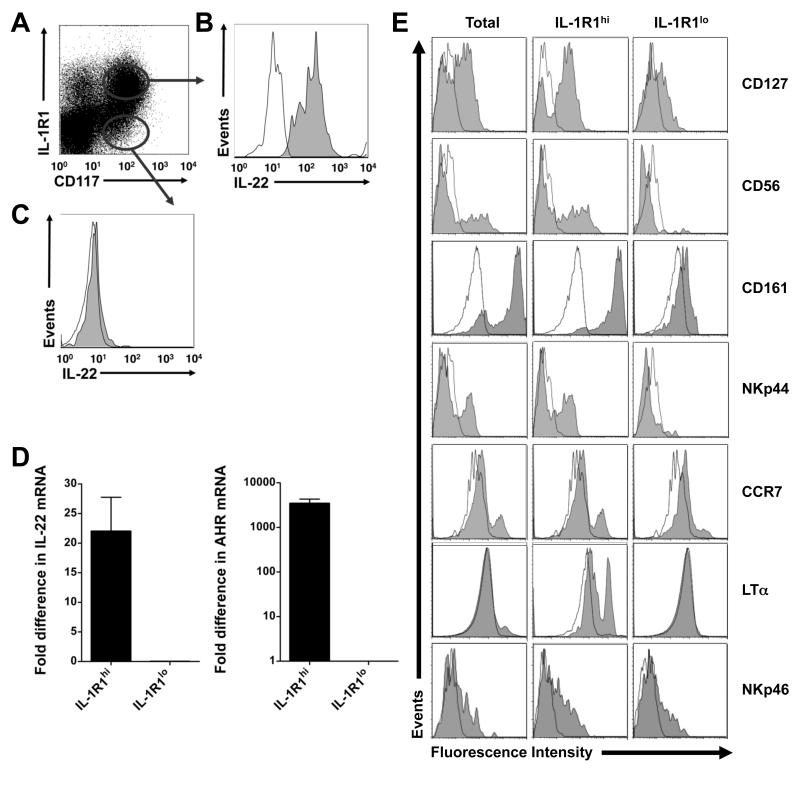
It has been shown previously that freshly isolated, unstimulated stage 3 iNK cells may have LTi-like properties, and constitutively express IL-22, RAR-related orphan receptor C (RORC), and AHR (Cella et al., 2009; Cupedo et al., 2009; Hughes et al., 2009). Flow cytometry performed on unstimulated SLT mononuclear cells indicated that IL-1R1 surface expression identifies two distinct subpopulations of stage 3 iNK cells: an IL-1R1hi subpopulation and an IL-1R1lo subpopulation. We discovered that expression of IL-22 protein was restricted to IL-1R1hi stage 3 iNK cells, which were uniformly IL-22+. In contrast, the minor population of IL-1R1lo stage 3 iNK cells was IL-22−. A donor representative of 5 such analyses is shown in Figure 2A-C. With this in mind, we measured IL-22, AHR, and RORC mRNA expression within sorted resting IL-1R1hi and IL-1R1lo subpopulations of stage 3 iNK cells. Expression of IL-22 and AHR transcripts were restricted to the IL-1R1hi subpopulation of stage 3 iNK cells, where they were present at amounts at least 22 ± 5.75-fold and 3439 ± 860-fold higher, respectively, than those seen in the IL-1R1lo subpopulation of stage 3 iNK cells (Figure 2D). In contrast, expression of the LTi-associated transcription factor RORC, which is restricted to the stage 3 iNK cells in SLT (Cupedo et al., 2009), could be detected in both the IL-1R1hi and IL-1R1lo subpopulations of stage 3 iNK cells, as were the NK lineage-associated transcription factors, E4BP4 and Ets-1 (Figure S2A-C). Flow cytometry revealed that expression of CD127, CD56, CD161, NKp44, CCR7, and LT-α – associated with the LTi-like phenotype (Cupedo et al., 2009) – are also relatively restricted to the IL-1R1hi subpopulation of stage 3 iNK cells (Figure 2E). Together, our data suggest that IL-22 and AHR are specific to the IL-1R1hi subpopulation of resting human stage 3 iNK cells from SLT, and that these cells also share the phenotype of LTi-like cells.
Figure 2. IL-1R1hi and IL-1R1lo subpopulations of stage 3 iNK cells ex vivo.
(A-C) Tonsillar mononuclear cells were stained ex vivo for surface expression of CD117, IL-1R1, and (B,C) intracellular IL-22. (A) Gated on Lin− (CD3−CD14−CD16−CD19−CD34−CD94−) lymphocytes, dot plot indicates gates for IL-1R1hi (upper) or IL-1R1lo (lower) subpopulations of CD117+ stage 3 iNK cells as sorted in a representative donor (n = 18). (B,C) Gating as indicated in (A), histograms depict staining with isotype (empty) or α-IL-22 (filled) antibody in a representative donor (n = 5). (D) IL-22 and AHR mRNA measured via Real-Time PCR ex vivo in flow cytometry sorted IL-1R1hi and IL-1R1lo subpopulations of stage 3 iNK cells. “Fold difference” was quantified relative to expression in the IL-1R1lo subpopulation, arbitrarily normalized to 1 (P ≤ 0.05). Data in D presented as mean ± SEM (n = 6). (E) Using gating as indicated in (A), histograms depict a representative donor stained ex vivo with isotype (empty) or antibody specific for the indicated antigen (filled), within total stage 3 iNK cells or IL-1R1hi and IL-1R1lo subpopulations of stage 3 iNK cells (n ≥ 4). The IL-1R1−CD117− and IL-1R1+CD117− subsets noted in (A) were both non-reactive for intracellular IL-22 protein staining.
Human IL-1R1hi stage 3 iNK cells selectively display a functional response to IL-1β
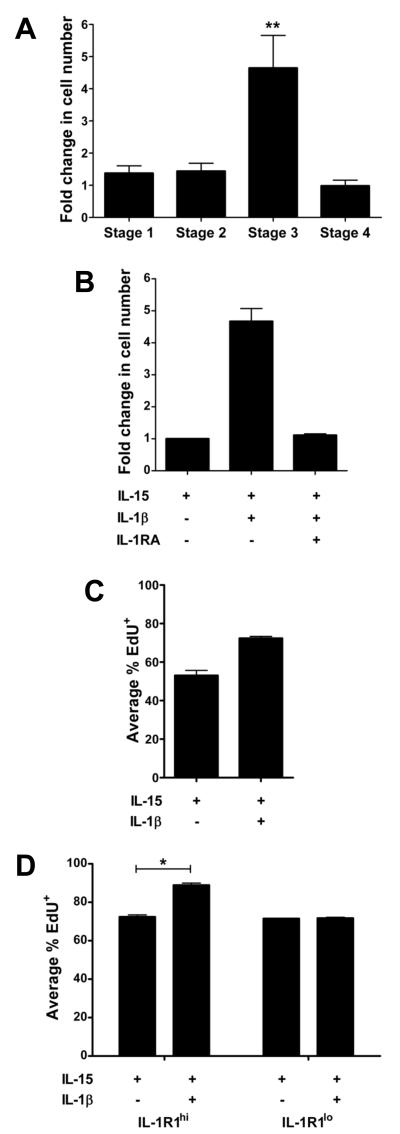
To explore the functional relevance of differential IL-1R1 expression in human NK cell development, we isolated stage 1-4 NK developmental intermediates from SLT and treated each with exogenous IL-1β, a physiologic ligand for this receptor. Not surprisingly, given that IL-15 has been demonstrated to serve as a survival factor during NK cell development (Huntington et al., 2008), cells treated with IL-1β alone were uniformly dead after 48 h, regardless of stage. However, when compared to cells from the same stage and donor cultured with IL-15 alone, the combination of IL-15 and IL-1β led to an expansion in the number of viable stage 3 iNK cells when examined after 2, 7, or 14 d. Notably, IL-1β expanded stage 3 iNK cells to 4.7 ± 0.7-fold relative to the quantity of cells generated after 14 d with IL-15 alone (Figure 3A). The expansion in the presence of IL-15 and IL-1β was diminished after withdrawal of IL-1β starting at d 7 of culture (not depicted). Likewise, culture of stage 3 iNK cells in the continuous presence of a 100-fold molar excess of IL-1 receptor antagonist (IL-1RA), a naturally occurring specific competitive inhibitor of IL-1β binding to IL-1R1 (Arend et al., 1991), completely abrogated the IL-1β-mediated expansion observed earlier (Figure 3B).
Figure 3. Treatment with IL-15 and IL-1β promotes the selective expansion of stage 3 iNK cells.
(A,B) “Fold change” was calculated and averaged from 11 donors as: absolute number of cells enumerated (by Trypan blue exclusion) after 14 d of culture in IL-15 and IL-1β divided by the absolute number of cells enumerated after 14 d of culture in IL-15 alone. For example, starting with 5,000 stage 3 iNK cells/well, four representative donors cultured in IL-15 and IL-1β expanded to 90,000, 285,000, 162,000, and 40,000, while the same four donors’ stage 3 iNK cells cultured in IL-15 alone expanded to 20,000, 55,0000, 45,000 and 10,000 cells, respectively. Results revealed a selective growth advantage among stage 3 iNK cells in response to culture with IL-15 and IL-1β (**, P < 0.005; n = 11), (B) which was completely abrogated in the presence of IL-1RA (P < 0.05; n = 4). (C,D) Proliferation assessed via EdU incorporation in (C) total stage 3 iNK cells (P < 0.05; n = 8) or (D) IL-1R1hi and IL-1R1lo subpopulations of stage 3 iNK cells (*, P = 0.01; n = 3) after culture for 14 d in the presence of IL-15 or IL-15 and IL-1β. Data in A-D depicted as mean ± SEM.
We determined this IL-1β-induced expansion of stage 3 iNK cells resulted from enhanced proliferation rather than survival (Figure 3C). Notably, despite modest yet measurable amounts of IL-1R1 surface expression, the addition of IL-1β did not substantially effect proliferation of stages 1, 2 or 4. Likewise, parallel cultures comparing IL-1R1hi and IL-1R1lo subpopulations of stage 3 iNK cells revealed that, whereas IL-15 alone maintained survival of both IL-1R1hi and IL-1R1lo subpopulations of stage 3 iNK cells in vitro, the addition of IL-1β enhanced proliferation of the IL-1R1hi, but not the IL-1R1lo, subpopulation of stage 3 iNK cells (Figure 3D).
IL-1β sustains expression of IL-22 and AHR in IL-1R1hi stage 3 iNK cells
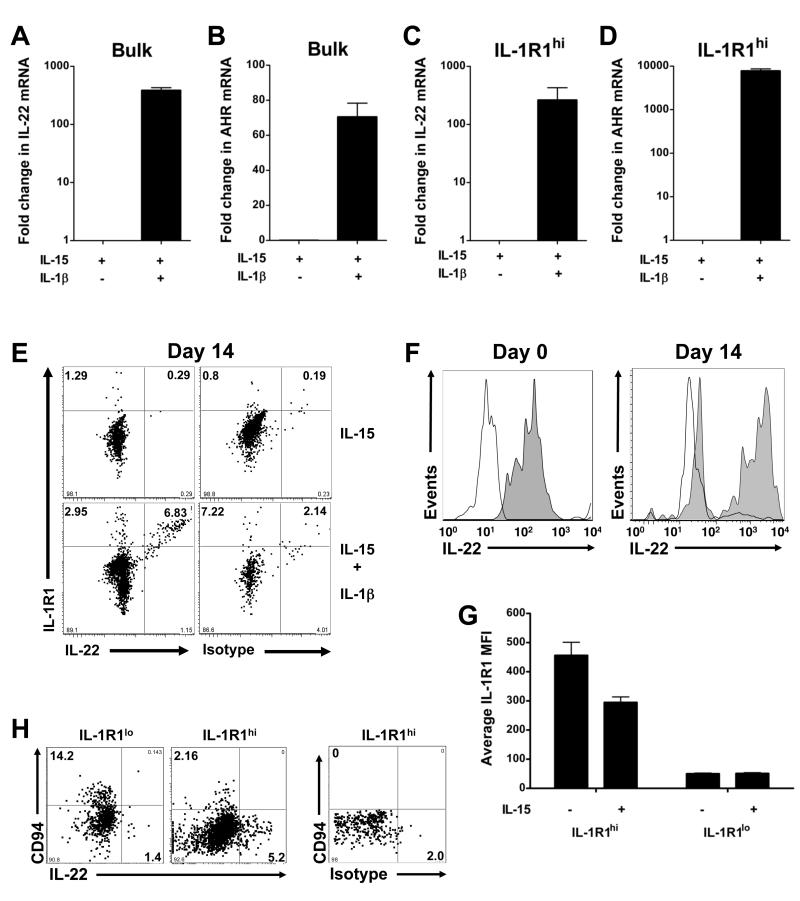
To explore the potential role of IL-1β in regulating expression of IL-22 and AHR, which are restricted to IL-1R1hi stage 3 iNK cells ex vivo, we measured expression of each transcript after in vitro exposure to IL-15 or IL-15 and IL-1β. Whereas IL-22 and AHR mRNA all but disappeared in the presence of IL-15 alone, exposure to IL-1β preserved IL-22 and AHR expression in total stage 3 iNK cells (Figure 4A,B). Furthermore, IL-22 and AHR remained restricted to the IL-1R1hi stage 3 iNK subpopulation, which also required exposure to IL-1β for continued expression in vitro (Figure 4C,D). In contrast, culture in the presence or absence of IL-1β resulted in similar expression of RORC mRNA, which was sustained in IL-1R1lo and IL-1R1hi subpopulations of stage 3 iNK cells, and induced in stage 4 mature NK cells (Figure S3A-C). These correlative data suggest that human stage 3 iNK cell production of IL-22 may be more dependent on the expression of AHR than RORC, as has recently reported for IL-17−IL-22+ human T cells (Trifari et al., 2009).
Figure 4. IL-1β sustains IL-22 and AHR expression in IL-1R1hi stage 3 iNK cells.
(A-D) IL-22 and AHR transcript expression following 14 d in the presence of IL-15 or IL-15 and IL-1β, shown in (A,B) total (“bulk”) stage 3 iNK cells, or (C,D) the IL-1R1hi subpopulation of stage 3 iNK cells. Results depicted as the mean ± SEM, and P ≤ 0.05 in each (n ≥ 4). (E) Dot plots depict surface IL-1R1 and intracellular staining with IL-22 or isotype antibody in IL-1R1hi stage 3 iNK cells from a representative donor cultured for 14 d in the presence of IL-15 or IL-15 and IL-1β (n = 6). All IL-22+ cells co-expressed IL-1R1. (F) Histograms from a representative donor (n = 4) depict staining with IL-22 (filled) or isotype (empty) antibody in the IL-1R1hi subpopulation of stage 3 iNK cells d 0, and within IL-1R1+ cells remaining after culture for 14 d with IL-15 and IL-1β (G) IL-1R1 MFI assessed via flow cytometry after a 6 h culture in the presence of IL-15 or media alone (n = 3). (H) Representative staining for surface CD94 and intracellular IL-22 protein at d 14 in cells cloned from either a single IL-1R1lo stage 3 iNK cell or an IL-1R1hi stage 3 iNK cell. Isotype staining on far right.
Although culture of IL-1R1hi stage 3 iNK cells for 14 d in IL-15 and IL-1β resulted in a decrease in the overall number of cells expressing IL-1R1 and overall expression of IL-22, IL-22 remained restricted to progeny which were also IL-1R1+ (Figure 4E,F). Declining numbers of IL-1R1+ progeny during culture in IL-15 and IL-1β was not due to overgrowth of the minor IL-1R1lo subpopulation of stage 3 iNK cells (Figure 3D), but rather resulted from an IL-15-mediated decrease in IL-1R1 on the surface of IL-1R1hi stage 3 iNK cells (Figure 4G). Furthermore, we assessed the clonal outgrowth from single IL-1R1hi and IL-1R1lo stage 3 iNK cells and found that the capacity for sustained expression of IL-22 remained restricted to the progeny of IL-1R1hi stage 3 iNK cells and exclusive of CD94 expression (Figure 4H; Table S1). Thus, IL-22+ cells derived in vitro appear to have been generated exclusively from the IL-1R1hi subpopulation. Together, our data suggest that continued expression of IL-22 by stage 3 iNK cells in vitro is dependent on exposure to IL-1β, and that IL-22 expression remains restricted to the IL-1-responsive subpopulation of IL-1R1hi stage 3 iNK cells.
IL-1β inhibits differentiation of human IL-1R1hi stage 3 iNK cells
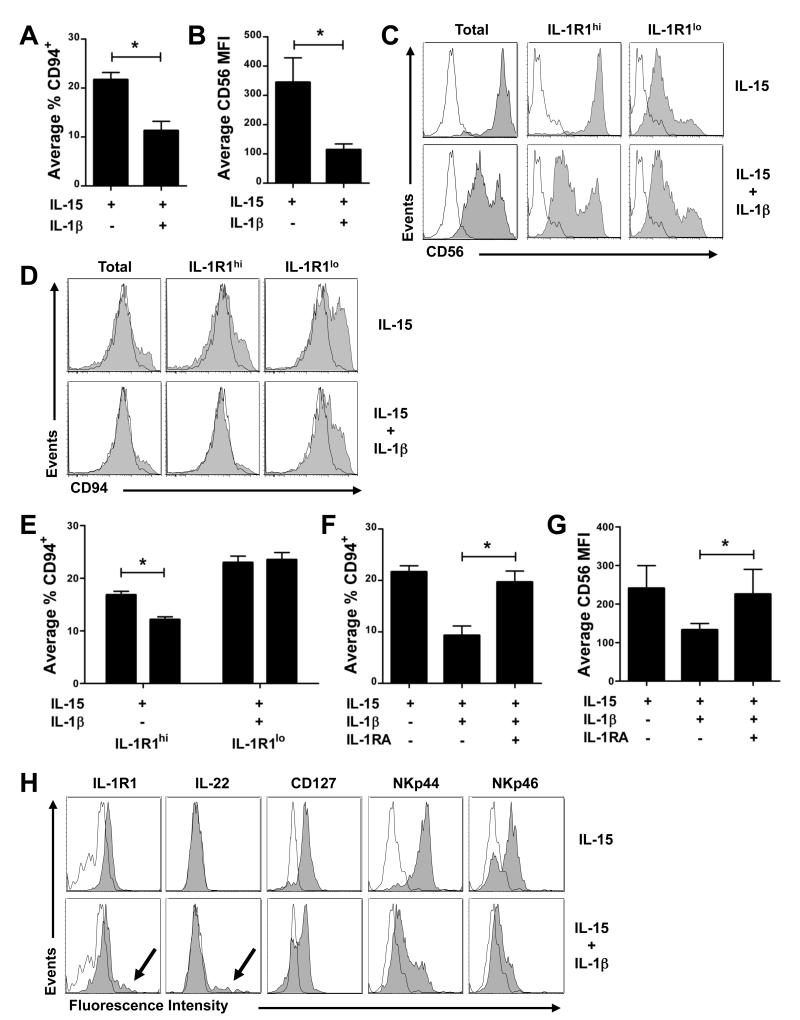
IL-15 promotes the differentiation of stage 3 iNK cells to stage 4 mature NK cells, which are characterized by co-expression of CD94 and high density CD56, as well as the potential for IFN-γ production and cytolytic activity (Freud et al., 2006; Huntington et al., 2008; Sanos et al., 2009). If IL-1β expands stage 3 iNK cells for the purposes of sustaining IL-22 expression and mucosal immunity, it should also inhibit differentiation to stage 4 mature NK cells. With this in mind, we assessed expression of CD94 and CD56 via flow cytometry after culture of stage 3 iNK cells, either in bulk, or as IL-1R1hi and IL-1R1lo subpopulations, with IL-15 alone or IL-15 and IL-1β. The presence of IL-1β conferred a 50% decrease in the proportion of stage 3 iNK cells that progressed to stage 4 mature NK cells as measured by CD94 acquisition (Figure 5A; 11.3 ± 2.7% with IL-1β vs. 21.7 ± 2.1% with IL-15 alone), and a concomitant 3.02 ± 0.4-fold decrease in MFI of CD56 surface expression (Figure 5B). These effects, which could be observed within 2 d of in vitro culture, were restricted to IL-1R1hi stage 3 iNK cells and were not seen in cultures of the IL-1R1lo subset (Figure 5C-E) or cells from stages 2 or 4 (not depicted). Inclusion of a molar excess of IL-1RA in cultures containing IL-15 and IL-1β fully restored acquisition of CD94 (Figure 5F) and density of CD56 surface expression (Figure 5G). Figure 5H shows additional maturation markers assessed in IL-1R1hi stage 3 iNK cells cultured for 14 d in IL-15 or IL-15 and IL-1β.
Figure 5. IL-1β inhibits differentiation of IL-1R1hi stage 3 iNK cells.
Surface expression of (A,D,E) CD94 and (B,C) CD56 assessed after 14 d of in vitro culture of sorted (A,B) total, or (C,D,E) IL-1R1hi and IL-1R1lo subpopulations of stage 3 iNK cells with IL-15 or IL-15 and IL-1β. (F,G) CD94 and CD56 were also assessed after bulk stage 3 iNK cells were cultured for 14 d in the presence of IL-15, IL-15 and IL-1β, or IL-15 with IL-1β and IL-1RA. (H) Histograms depict staining with isotype (empty) or antibody specific for the indicated antigen (filled) after culture of IL-1R1hi stage 3 iNK cells for 14 d in the presence of IL-15 or IL-15 and IL-1β. Arrows indicate high density expression for IL-1R1 and IL-22 (also see Figure 4F). Data in A, B, E, F, and G presented as the mean ± SEM (*, P ≤ 0.05; n ≥ 4). Histograms in C, D, and H depict staining in a representative donor (n ≥ 4).
IL-1β inhibits the functional maturation of human IL-1R1hi stage 3 iNK cells
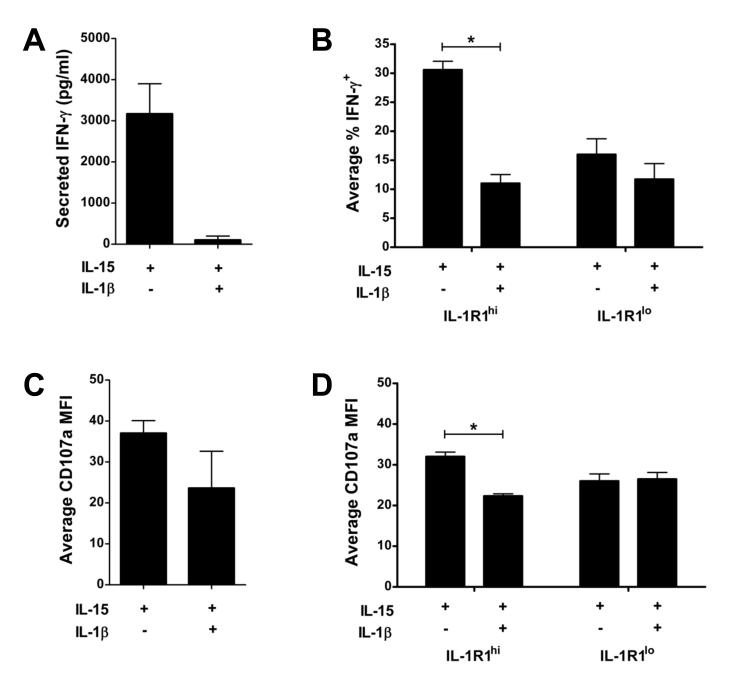
We next assessed whether treatment of human stage 3 iNK cells with IL-15 and IL-1β also inhibited the acquisition IFN-γ production and cytotoxic activity seen in stage 4 mature NK cells. Freshly isolated human stage 3 iNK cells do not produce IFN-γ or display natural cytotoxicity against K562 target cells (Freud et al., 2006). Indeed, stage 3 iNK cells incubated in vitro with IL-15 and IL-1β for 14 d produced negligible amounts of IFN-γ (~100 pg/ml) after co-activation with IL-12, IL-15, and IL-18. In contrast, stage 3 iNK cells incubated in vitro with IL-15 alone for 14 d produced substantial IFN-γ, averaging 3,167 ± 734 pg/ml, following co-activation with IL-12, IL-15, and IL-18 (Figure 6A). As shown in Figure 6B, this IL-1β-mediated reduction in IFN-γ production was restricted to the IL-1R1hi subpopulation of stage 3 iNK cells. As judged by effector cytokine secretion, exposure to IL-1β inhibited the functional maturation of stage 3 iNK cells.
Figure 6. IL-1β impedes the IL-1R1hi subpopulation of stage 3 iNK cells from acquiring IFN-γ production and degranulation.
(A,C) Total or (B,D) IL-1R1hi and IL-1R1lo subpopulations of stage 3 iNK cells sorted from SLT were cultured for 14 d in vitro with IL-15 or IL-15 and IL-1β. Cells were replated in equal numbers and either: (A,B) stimulated for 12 h with IL-15 + IL-12 + IL-18 to assess IFN-γ secretion via (A) ELISA (P < 0.005; n = 7) or (B) intracellular flow (n = 8); or (C,D) incubated overnight with K562 targets (4:1 E/T ratio) and assessed for surface expression of the degranulation marker CD107a by flow cytometry (n = 3; for C, P = 0.14). Data in B depicts IFN-γ expression after gating on the population which had acquired CD94 surface expression. Data presented as mean ± SEM (*, P ≤ 0.01).
Although the capacity to secrete IFN-γ was restricted to the population which had acquired surface expression of CD94 in vitro (Figure S4A), surface expression of the degranulation marker CD107a, which can be used as a functional marker to identify cytotoxic NK cells (Alter et al., 2004), was observed in both CD94+ and CD94− cells generated from culture of stage 3 iNK cells in vitro (Figure S4B). Surface expression of the degranulation marker CD107a indicated that, compared to bulk stage 3 iNK cells cultured with IL-15 alone, bulk culture of stage 3 iNK cells in the presence of IL-15 and IL-1β for 14 d did not significantly influence CD107a surface expression (Figure 6C; P = 0.14), however, exposure to IL-15 and IL-1β significantly reduced degranulation within the IL-1R1hi subpopulation of stage 3 iNK cells (Figure 6D; P = 0.01).
Human stage 3 iNK cells proliferate in direct response to proximate cDC-derived IL-15 and IL-1β
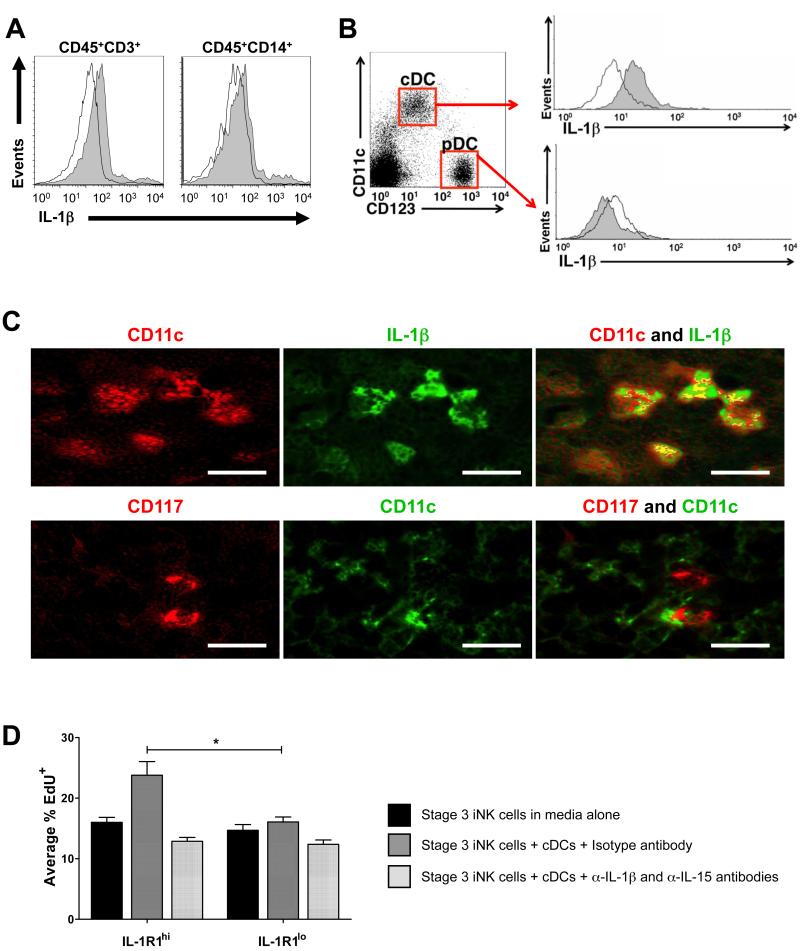
To identify potential physiological sources of IL-1β which may interact with stage 3 iNK cells, we used flow cytometry to analyze fresh SLT mononuclear cells for intracellular IL-1β. Although we detected low intracellular IL-1β in a modest fraction of CD3+ T cells and CD14+ monocytes and macrophages (Figure 7A), IL-1β expression occurred in a large portion of tonsillar CD11chiCD123lo cDCs (Figure 7B and C, top row). In contrast, CD11cloCD123hi plasmacytoid DCs (pDCs) and the population of CD11cloCD123lo cells did not contain intracellular IL-1β (Figure 7B and not depicted, respectively).
Figure 7. Stage 3 iNK cells reside in the vicinity of IL-1β+ CD11chi cDCs within SLT.
(A,B) Intracellular staining of unstimulated SLT mononuclear cells from a representative donor (n = 3) with isotype (empty) or α-IL-1β (filled) antibody shown after gating on (A) CD45+CD3+ T cells and CD45+CD14+ monocytes and macrophages, or (B) CD123hiCD11clo pDCs and CD123loCD11chi cDCs subsets. (C) Immunohistochemical staining depicted in serial sections of human tonsil from a representative donor (n = 4). Yellow areas indicate co-expression, as shown for CD11chi and IL-1β (top). CD117+ cells did not co-express CD11c, but were located in proximity of CD11chi cDCs (bottom). Magnification, 400x. Bar, 30 μM. (D) EdU incorporation in sorted IL-1R1hi and IL-1R1lo subpopulations of stage 3 iNK cells after 6 h with α-MEM medium alone, or in the presence of autologous BDCA-1+ cDCs (4:1 iNK:cDC ratio) with either isotype or α-IL-15 and α-IL-1β blocking antibodies. Depicted is % of CD117+ lymphocytes which were also EdU+ (*, P = 0.05; n = 3). Data in D presented as mean ± SEM.
Stage 3 iNK cells were previously identified in situ as CD117+ cells with lymphoid morphology, and localized to the lamina propria or the parafollicular T-cell-rich region of the tonsil (Hughes et al., 2009). These CD117+ stage 3 iNK cells, which are primarily CD161+IL-1R1hiIL-22+ (Figure 2; Figure S5), were found to be located in close proximity to IL-1β-producing CD11chi cDCs in the parafollicular T-cell rich region of human tonsil (Figure 7C, bottom row). Thus, tonsillar cDCs appear to be a plausible cellular source of IL-1β for human stage 3 iNK cells in vivo.
Indeed, compared to parallel cultures performed in the absence of cDCs, short term co-culture of IL-1R1hi stage 3 iNK cells with autologous BDCA-1+CD11chiCD123lo cDCs enhanced proliferation (measured via 5-ethynyl-2′-deoxyuridine (EdU) incorporation assay) of IL-1R1hi stage 3 iNK cells, but not of IL-1R1lo stage 3 iNK cells (Figure 7D). Although neither α-IL-15 nor α-IL-1β antibody altered EdU incorporation as a single blocking reagent (not depicted), addition of α-IL-15 and α-IL-1β antibodies together reduced EdU incorporation to amounts seen in the absence of cDCs, implicating specific roles for cDC-derived IL-15 and IL-1β in the proliferative response observed in IL-1R1hi stage 3 iNK cells (Figure 7D). Thus, cDC-derived IL-15 and IL-1β are capable of directly stimulating proliferation of IL-1R1hi stage 3 iNK cells. Similar co-stimulatory effects may also be possible between IL-1R1hi stage 3 iNK cells and IL-1β-producing CD14+ monocytes or macrophages which also produce IL-15.
DISCUSSION
Here we identify a role for IL-1R1 and its cognate ligand, IL-1β, in the homeostasis of stage 3 iNK cells, which selectively expressed IL-1R1 compared to other NK developmental intermediates residing in human tonsil. In the presence of IL-15, binding of IL-1β to IL-1R1 expanded IL-1R1hi stage 3 iNK cells and preserved expression of IL-22 and AHR. In contrast, culture of IL-1R1hi stage 3 iNK cells in IL-15 alone resulted in a loss of IL-22 and AHR expression and enhanced differentiation toward IFN-γ producing stage 4 mature NK cells. Furthermore, CD117+ cells, which co-express CD161 and IL-22, and thus correspond to the IL-1R1hi subpopulation of stage 3 iNK cells, reside in the lamina propria and parafollicular T cell-rich area of human tonsil, and co-localized with a cellular source of IL-1β found in CD11chi cDCs, attesting to the physiologic relevance of the IL-1β-IL-1R1 interaction in vivo.
RORC is a hallmark of mature Th17 cells (Ivanov et al., 2006), but has also been detected in murine FOXP3+ regulatory T (Treg) cells (Lochner et al., 2008) and human FOXP3+ Treg cells (Ayyoub et al., 2009). Indeed, unstimulated human stage 3 iNK cells in SLT express RORC and AHR mRNA (Cupedo et al., 2009), IL-22 protein (Hughes et al., 2009), and as we establish here, IL-1R1. Although IL-1β appears to be required by human stage 3 iNK cells for continued expression IL-22 and AHR in vitro, other LTi-associated genes – IL-26 and RORC – were maintained in stage 3 iNK cells by IL-15 alone. Furthermore, culture of stage 4 mature NK cells in the presence of IL-15 induced expression of RORC in vitro, suggesting that RORC may not be among the subset of LTi-associated genes which are dependent upon IL-1β for continued expression, especially within human stage 3 iNK cells. This is consistent with our observation that unlike IL-22 and AHR, constitutive expression of RORC is not restricted to the IL-1R1hi subpopulation of stage 3 iNK cells, as well as RORC-independent development of MALT reported in murine tear duct (Nagatake et al., 2009).
Murine studies indicate that RORC+CD127+NKp46+NK1.1+ cells are decreased in the setting of IL-15 gene deficiency as well as in animals lacking the IL-2 receptor beta chain (IL-2Rβ, CD122) (Satoh-Takayama et al., 2008), which is required for IL-2 and IL-15 signaling. However, this population is absent in IL-2Rγ–deficient animals that cannot respond to any of the so-called γc cytokines (Vosshenrich et al., 2005). Situations have been reported in which IL-2 or IL-7 may be substituted in place of IL-15 (Cupedo et al., 2009; Freud et al., 2006; Satoh-Takayama et al., ; Satoh-Takayama et al., 2008). Given that nearly all human stage 3 iNK cells – as well as the equivalent RORC+NKp46+NK1.1int murine population – express CD127 (Luci et al., 2009), the role of IL-7 deserves particular attention. Compared to IL-2 or IL-15, IL-7 alone was much less potent at maintaining stage 3 iNK cell survival in vitro. Furthermore, stage 3 iNK cells cultured with IL-2 or IL-15 did not significantly differ qualitatively or quantitatively compared to parallel cultures supplemented with IL-7. This is in agreement with murine studies indicating that IL-7 and IL-7Rα deficient mice do not exhibit NK developmental defects (Maki et al., 1996; Vosshenrich et al., 2005). It remains possible that this cytokine plays a role in regulating additional functions of this population in SLT, such as the LTi-like behavior that has been reported in human stage 3 iNK population (Cupedo et al., 2009).
Both human Th17 cells and stage 3 iNK cells produce IL-22 (Cella et al., 2009) and express the transcription factors RORC and AHR (Cupedo et al., 2009). Murine Th17 cells have higher expression of IL-1R1 mRNA than in seen in Th1 or Th2 cells, and exposure to IL-1β promotes Th17 cell proliferation, differentiation, and expression of IL-17 and RORC (Chung et al., 2009). Like Th17 cells (Chung et al., 2009; Rao et al., 2007; Veldhoen et al., 2008), the IL-1R1hi subpopulation of stage 3 iNK cells selectively expresses AHR, expands upon in vitro treatment with IL-1β, and depends on IL-1β for expression of AHR and IL-22. We also noted that expression of IL-1R1 on stage 3 iNK cells diminishes over time during in vitro culture with a proportional decrease in IL-22 expression, and we show that this down regulation of IL-1R1 is mediated by IL-15. This observation lends further support to our notion that in vivo, constitutive expression of IL-22 in stage 3 iNK cells likely requires co-expression of IL-1R1 and proximity to cells expressing its cognate ligand, IL-1β.
In addition to IL-1β, we investigated the role of IL-23, which did not markedly alter the quantity of cells generated in vitro from stage 3 iNK cells, whether cultured in the presence of IL-15 or IL-15 and IL-1β. IL-23 acts on human Th17 cells to promote expression of Th17 cell-associated genes, such as IL-22 (Zheng et al., 2007), RORC and IL-26 (Manel et al., 2008). We did not observe any marked effect on IL-22, IL-26, or RORC expression when stage 3 iNK cells were cultured with IL-23, regardless of the presence of IL-15 or IL-15 and IL-1β. Together, these findings suggest that distinct repertoires of cytokines regulate the development of human Th17 cells and stage 3 iNK cells found in SLT.
Unabated expression of pro-inflammatory cytokines can lead to chronic inflammatory states, autoimmune disease, and cancer. IL-1β production is subject to intense regulation at the level of its synthesis and excretion. Its interaction with IL-1R1 is further regulated by competitive inhibition by IL-1RA (Carter et al., 1990), as well as expression of the IL-1R2 decoy receptor (Colotta et al., 1993). We found that IL-1RA is capable of fully neutralizing the impact of IL-1β on IL-1R1hi stage 3 iNK cell proliferation and on in vitro differentiation toward CD94+ stage 4 NK cells. Similarly, IL-22 signaling triggers expression of a naturally occurring antagonist, IL-22 binding protein (IL-22BP), which binds IL-22 to prevent signaling through IL-22R (Dumoutier et al., 2001). These regulatory pathways serve to quickly and precisely adjust biological responses to IL-1β and IL-22. Indeed, the assessment of anti-IL-1β therapy might be considered in illnesses such as Crohn’s disease and psoriasis where excessive IL-22 production is felt to be pathological (Kleinschek et al., 2009; Wilson et al., 2007). Ablation of IL-1R1 signaling should enhance NK cell differentiation and diminish IL-22 production from the IL-1R1hi stage 3 iNK cell population.
Excessive inflammation can disrupt SLT architecture, necessitating local tissue regeneration for restoration of the SLT microenvironment (Kratz et al., 1996). LTi cells have a proposed role in re-establishing the integrity of lymphoid tissues following chronic infections (Scandella et al., 2008). Cupedo et al first identified RORC+ LTi-like cells in human fetal lymph node as committed iNK cells expressing IL-22, CD117, CD127, LT-α and CCR7 (Cupedo et al., 2009). A study by Crellin et al provides strong evidence that LTi-like activity is most robust within a subset of cells co-expressing CD127 and CD161 (Crellin et al.). We found that the IL-1R1hi subpopulation of iNK cells possesses a strikingly similar phenotype. Among total stage 3 iNK cells, the IL-1R1hi subset is enriched for cells co-expressing CD127, CD161, LT-α, CCR7, and IL-22. As we observed in the IL-1R1hi subpopulation of stage 3 iNK cells, Crellin et al reported that CD127+ LTi-like cells varied in expression of CD56 and NKp44, and contained cells capable of giving rise to cytotoxic mature NK cells in the presence of IL-15 in vitro. They reported that, compared to the CD127− subpopulation, the CD127+ subpopulation is reduced in its propensity to generate mature NK cells in IL-15. Our study shows a reduced propensity to generate mature NK cells when IL-1β was added to culture of IL-1R1hi stage 3 iNK cells in the presence of IL-15. These data suggest that the developmental relationship between the CD127+IL-1R1hi, CD127+IL-1R1lo, CD127−IL-1R1hi and CD127−IL-1R1lo populations will need to be explored in future studies. They also serve to emphasize the heterogeneity within the CD117+CD161+IL-1R1hi stage 3 iNK cell population, and the need for caution in assigning definitive functions and developmental potential.
We previously reported limited differentiation of stage 3 iNK cells to CD94+ stage 4 NK cells with IL-15 in vitro [5-20% (Freud et al., 2006)], but the developmental relationship between stage 3 iNK cells and CD94+ stage 4 NK cells remains unclear, and was pursued in this study. The current report describes two subpopulations of stage 3 iNK cells distinguished by surface expression of functional IL-1R1. Predictably, the presence of IL-1β in culture had no appreciable effect on differentiation of IL-1R1lo stage 3 iNK cells, but during culture of IL-1R1hi stage 3 iNK cells, IL-1β limited maturation to stage 4 NK cells and sustained expression of AHR and IL-22 mRNA, as well as IL-22 protein in the remaining IL-1R1hi stage 3 cells. In the absence of IL-1β, culture of IL-1R1hi stage 3 iNK cells with IL-15 had a significantly greater propensity to mature into CD94+CD56bright stage 4 NK cells.
These findings support the hypothesis that the human stage 3 iNK cell population (CD3−CD34− CD117+CD161+CD94−) consists of a minor IL-1R1lo subset that does not express IL-22, as well as an IL-1R1hi subset which constitutively expresses IL-22, yet can also give rise to CD94+ mature NK cells. The CD117+IL-1R1lo stage 3 iNK cell may acquire IL-1R1 and then under some circumstances proceed to a CD94+CD56bright stage 4 mature NK cell, or may bypass the acquisition of IL-1R1. The proportion of IL-1R1hi stage 3 iNK cells which differentiate to CD94+CD56bright stage 4 mature NK cells relative to those that continue to express IL-22 appears dependent on their continued expression of IL-1R1, as well as exposure to IL-1β. The regulation of the IL-1R1 by IL-15, and the regulation of exposure to its ligand IL-1β by cell-cell interactions and microbial invasion, may dictate the relative abundance of the CD117+IL-1R1hi stage 3 iNK and the CD94+CD56bright stage 4 mature NK cell in SLT. Finally, this study identifies a stage 3 iNK subpopulation in human tonsil as an innate immune target of IL-1β, and provides a mechanistic connection between IL-1β and IL-22 production, with implications for mucosal innate immunity.
EXPERIMENTAL PROCEDURES
Isolation of human NK precursors from SLT
All protocols were approved by The Ohio State University (OSU) Institutional Review Board. Normal human tonsils were obtained within 24 h of elective surgery through the NCI-Sponsored Cooperative Human Tissue Network and the Biopathology Center at Nationwide Children’s Hospital. NK cell developmental intermediates were isolated as described (Freud et al., 2006). Briefly, mononuclear fractions were depleted of CD3+ and CD19+ cells via magnetic negative selection (Miltenyi). For certain experiments, T and B cell-depleted preparations were stained immediately for phenotypic analyses. Alternatively, CD34+ cells were enriched from CD3−CD19− cells using a CD34 progenitor isolation kit (Miltenyi) for flow cytometric sorting of stage 1 and 2 cells, while total stage 3 iNK cells and stage 4 NK cells were sorted from the CD34− fraction as previously described (Freud et al., 2006). IL-1R1hi and IL-1R1lo stage 3 iNK subpopulations were isolated from the CD34− fraction using surface staining with goat α-IL-1R1 and α-goat APC (R&D Systems) in addition to the previously described stage 3 cell surface antigens (Freud et al., 2006). Populations were sorted to purity ≥ 95% with a FACS Aria II cell sorter (BD Biosciences).
Flow cytometry
All antibodies used for flow cytometry were purchased from BD Biosciences, except BDCA-1 (Miltenyi), CD56, CD127, CD161, NKp44, NKp46 (Beckman Coulter), goat IgG (Santa Cruz), CD94 (clone 131412), IL-22 (clone 142928), IL-1R1 (MAB269), and α-goat APC (R&D Systems). Unless otherwise indicated, antibodies were used according to manufacturer’s instructions. Non-specific staining was detected through the use of an appropriately labeled isotype antibody. IL-1R1 staining was performed on ice for 15 min with α-IL-1R1 or goat IgG (1 μg/105 cells) in parallel with any additional directly conjugated antibodies indicated. Samples were washed, then incubated on ice for 30 min with α-goat IgG APC (0.25 μg/105 cells). Intracellular staining was performed after surface staining using the BD Cytofix/Cytoperm Plus Fixation/Permeabilization Kit (BD Biosciences). Ex vivo staining was performed without additional stimulation, and in the absence of brefeldin A, immediately following CD3- and CD19-depletion. Intracellular staining after culture was performed following 4 h incubation with 2 μM GolgiPlug (BD Biosciences). Intracellular staining for IL-1β was performed according to manufacturer’s instructions, and IL-22 staining was as previously described (Hughes et al., 2009). Cells were analyzed immediately as previously described (Freud et al., 2005) using a FACS Calibur or BD LSR II cytometer (BD Biosciences).
Real-Time PCR
RNA was obtained by lysing a portion of each sorted population in approximately equal quantity (≥ 1×104 cells). For some experiments, RNA was also obtained after 14 d in vitro culture. Samples from ≤ 5×104 cells were processed using Absolutely RNA Nanoprep Kit (Stratagene); samples with ≥ 5×104 cells were processed using RNeasy (Qiagen). cDNA was synthesized according to manufacturer’s instructions using MMLV reverse transcriptase kit (Invitrogen). Real-Time PCR was performed on an ABI Prism 7900HT (Applied Biosystems) using Taqman primer/probe sets for IL-22, AHR, and IL-1R1 purchased from Applied Biosystems. Expression levels were normalized to an 18S internal control, then analyzed by the ΔΔCt method (Fehniger et al., 1999).
Cell culture
Flow cytometry purified NK developmental intermediates were cultured in a round-bottom 96-well plate (Costar) at a starting density of 25,000 cells/ml in α-MEM medium containing 10% FBS, penicillin G (100 μg/ml), and streptomycin (100 μg/ml) (Invitrogen). Cells from stages 2-4 were cultured in the indicated recombinant human cytokines, including IL-15 (1 nM; Amgen), IL-1β (10 ng/ml; Peprotech), and IL-1RA (100 ng/ml; R&D Systems). Medium for stage 1 cells was also supplemented with IL-7 (10 ng/ml), IL-3 (10 ng/ml; R&D Systems), and FL (100 ng/ml; Peprotech). For cultures lasting more than 24 h, half the medium was removed every 2-3 d and replaced with media containing 2x cytokines.
Clonal assays
FACS Aria II was used to deposit single IL-1R1hi or IL-1R1lo stage 3 iNK cells directly into each well of a 96-well flat bottom plate containing GFP+ murine OP9 stroma in α-MEM medium containing IL-15 plus IL-1β. After 14 d, each of 60 replicate wells was individually assessed via flow cytometry for human CD45 and CD94 surface expression in addition to intracellular IL-22. OP9 cells were excluded by gating on GFP− events expressing human CD45.
Proliferation assays
Click-iT EdU kit (Invitrogen) was used according to manufacturer’s instructions to assess proliferation of stage 3 iNK cells purified via flow cytometry, either in bulk, or as IL-1R1hi and IL-1R1lo subpopulations, in response to cDC-derived or recombinant IL-15 or IL-15 and IL-1β. After CD3- and CD19-depletion, SLT cDCs were enriched via magnetic selection using BDCA-1-biotin antibody and α-biotin microbeads (Miltenyi). Purity was ≥ 87% via flow cytometric staining. Autologous IL-1R1hi and IL-1R1lo stage 3 iNK subpopulations were sorted from BDCA-1-depleted fractions. Co-cultures were performed at 4:1 iNK:cDC ratio. After 6 h in the presence of 5 mM EdU, the proportion of CD117+ cells positive for EdU uptake (corresponding to DNA replication, and S phase of the cell cycle) was assessed via flow cytometry. Controls included cells cultured in the absence of EdU and samples without AlexaFluor 488 azide (used to fluorescently label EdU). Additional controls included for cDCs co-cultures included: iNK cell monocultures performed in media alone or media recombinant IL-15 and IL-1β. For neutralization experiments, iNK/cDC co-cultures were performed in the presence of α-IL-15 (1 μg/ml) and/or α-IL-1β (0.5 μg/ml) blocking antibodies (R&D Systems) and/or an equal concentration of isotype IgG (Santa Cruz).
Effector function assays
After 14 d of culture with IL-15 or IL-15 and IL-1β, cells were counted and replated in equal numbers for each assay. Surface staining for CD107a, a degranulation marker used to identify cytotoxic NK cells (Alter et al., 2004), was assayed via flow cytometry as previously described (Betts et al., 2003) in response to 8 h incubation with K562 target cells (4:1 E/T ratio). IFN-γ secretion, in response to 12 h stimulation with recombinant monokines [IL-15 with IL-12 (10 ng/ml; Genetics Institute) and IL-18 (50 ng/ml; BASF)], was measured as previously described via intracellular flow cytometry (Cooper et al., 2001) or ELISA (detection limit 10-30 pg/ml) using commercial antibody pairs (Endogen) (Trotta et al., 2005). Controls for effector function assays included staining with isotype control antibody, and parallel cultures performed in the absence of K562 or monokines.
Immunohistochemical staining
Immunohistochemistry was performed on human tonsils as previously described (Fehniger et al., 2003). The ultraView Universal system (Ventana Medical) was employed to assess paraffin-embedded tonsillar tissue in serial sections (0.5 μM) stained with the indicated antibodies, including: α-CD117 (1:500; DakoCytomation), α-IL-1β (1:50), and α-CD11c (Abcam, 1:100). Images were digitally acquired using: DP 12 camera, BX50 microscope, and UPLANF1 objectives (Olympus) and analyzed using Photoshop CS3 software (Adobe). The Nuance FX system (Cambridge Research & Instrumentation) digitally converted staining with DAB or fast red to fluorescent green or red, respectively. Using the Nuance System’s multispectral image analysis, we were able to identify both CD11chi and CD11clo cells. Subsequently, the threshold of signal strength, corresponding specifically to the CD11c chromagen, was increased to display only the CD11chi cells within tissue sections.
Statistical analysis
For comparison of two conditions, data were analyzed using paired t-tests. Linear mixed models were used for analysis when more than two treatment conditions were used. All tests were two sided. Holm’s method was used to correct for multiple comparisons when needed. P < 0.05 was considered significant for single comparisons, and after adjustment for multiple comparisons.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by NCI grants (CA95426 and CA68458). The authors thank Ventana Medical Systems and Kathleen Sergott for providing reagents for the Immunohistochemistry.
Footnotes
The authors have no conflicting financial interest.
REFERENCES
- Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Arend WP, Malyak M, Bigler CF, Smith MF, Jr., Janson RW. The biological role of naturally-occurring cytokine inhibitors. Br J Rheumatol. 1991;30(Suppl 2):49–52. [PubMed] [Google Scholar]
- Ayyoub M, Deknuydt F, Raimbaud I, Dousset C, Leveque L, Bioley G, Valmori D. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc Natl Acad Sci U S A. 2009;106:8635–8640. doi: 10.1073/pnas.0900621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- Carter DB, Deibel MR, Jr., Dunn CJ, Tomich CS, Laborde AL, Slightom JL, Berger AE, Bienkowski MJ, Sun FF, McEwan RN, et al. Purification, cloning, expression and biological characterization of an interleukin-1 receptor antagonist protein. Nature. 1990;344:633–638. doi: 10.1038/344633a0. [DOI] [PubMed] [Google Scholar]
- Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colotta F, Re F, Muzio M, Bertini R, Polentarutti N, Sironi M, Giri JG, Dower SK, Sims JE, Mantovani A. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science. 1993;261:472–475. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Fehniger TA, Ponnappan A, Mehta V, Wewers MD, Caligiuri MA. Interleukin-1beta costimulates interferon-gamma production by human natural killer cells. Eur J Immunol. 2001;31:792–801. doi: 10.1002/1521-4141(200103)31:3<792::aid-immu792>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Crellin NK, Trifari S, Kaplan CD, Cupedo T, Spits H. Human NKp44+IL-22+ cells and LTi-like cells constitute a stable RORC+ lineage distinct from conventional natural killer cells. J Exp Med. 207:281–290. doi: 10.1084/jem.20091509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, Fibbe WE, Cornelissen JJ, Spits H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC(+) CD127(+) natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- Dumoutier L, Lejeune D, Colau D, Renauld JC. Cloning and characterization of IL-22 binding protein, a natural antagonist of IL-10-related T cell-derived inducible factor/IL-22. J Immunol. 2001;166:7090–7095. doi: 10.4049/jimmunol.166.12.7090. [DOI] [PubMed] [Google Scholar]
- Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–3057. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- Fehniger TA, Shah MH, Turner MJ, VanDeusen JB, Whitman SP, Cooper MA, Suzuki K, Wechser M, Goodsaid F, Caligiuri MA. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol. 1999;162:4511–4520. [PubMed] [Google Scholar]
- Freud AG, Becknell B, Roychowdhury S, Mao HC, Ferketich AK, Nuovo GJ, Hughes TL, Marburger TB, Sung J, Baiocchi RA, et al. A human CD34(+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity. 2005;22:295–304. doi: 10.1016/j.immuni.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Freud AG, Caligiuri MA. Human natural killer cell development. Immunol Rev. 2006;214:56–72. doi: 10.1111/j.1600-065X.2006.00451.x. [DOI] [PubMed] [Google Scholar]
- Freud AG, Yokohama A, Becknell B, Lee MT, Mao HC, Ferketich AK, Caligiuri MA. Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med. 2006;203:1033–1043. doi: 10.1084/jem.20052507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes T, Becknell B, McClory S, Briercheck E, Freud AG, Zhang X, Mao H, Nuovo G, Yu J, Caligiuri MA. Stage 3 immature human natural killer cells found in secondary lymphoid tissue constitutively and selectively express the T H 17 cytokine interleukin-22. Blood. 2009;113:4008–4010. doi: 10.1182/blood-2008-12-192443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, Corcuff E, Mortier E, Jacques Y, Spits H, Di Santo JP. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med. 2008 doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikejima T, Dinarello CA, Gill DM, Wolff SM. Induction of human interleukin-1 by a product of Staphylococcus aureus associated with toxic shock syndrome. J Clin Invest. 1984;73:1312–1320. doi: 10.1172/JCI111334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Junt T, Scandella E, Ludewig B. Form follows function: lymphoid tissue microarchitecture in antimicrobial immune defence. Nat Rev Immunol. 2008;8:764–775. doi: 10.1038/nri2414. [DOI] [PubMed] [Google Scholar]
- Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP, Raskin L, Desai B, Faubion WA, de Waal Malefyt R, et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med. 2009;206:525–534. doi: 10.1084/jem.20081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz A, Campos-Neto A, Hanson MS, Ruddle NH. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J Exp Med. 1996;183:1461–1472. doi: 10.1084/jem.183.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, Riethmacher D, Si-Tahar M, Di Santo JP, Eberl G. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J Exp Med. 2008;205:1381–1393. doi: 10.1084/jem.20080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luci C, Reynders A, Ivanov II, Cognet C, Chiche L, Chasson L, Hardwigsen J, Anguiano E, Banchereau J, Chaussabel D, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- Maki K, Sunaga S, Komagata Y, Kodaira Y, Mabuchi A, Karasuyama H, Yokomuro K, Miyazaki JI, Ikuta K. Interleukin 7 receptor-deficient mice lack gammadelta T cells. Proc Natl Acad Sci U S A. 1996;93:7172–7177. doi: 10.1073/pnas.93.14.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatake T, Fukuyama S, Kim DY, Goda K, Igarashi O, Sato S, Nochi T, Sagara H, Yokota Y, Jetten AM, et al. Id2-, ROR{gamma}t-, and LT{beta}R-independent initiation of lymphoid organogenesis in ocular immunity. J Exp Med. 2009 doi: 10.1084/jem.20091436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao DA, Tracey KJ, Pober JS. IL-1alpha and IL-1beta are endogenous mediators linking cell injury to the adaptive alloimmune response. J Immunol. 2007;179:6536–6546. doi: 10.4049/jimmunol.179.10.6536. [DOI] [PubMed] [Google Scholar]
- Romagnani C, Juelke K, Falco M, Morandi B, D’Agostino A, Costa R, Ratto G, Forte G, Carrega P, Lui G, et al. CD56brightCD16- killer Ig-like receptor- NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J Immunol. 2007;178:4947–4955. doi: 10.4049/jimmunol.178.8.4947. [DOI] [PubMed] [Google Scholar]
- Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh-Takayama N, Lesjean-Pottier S, Vieira P, Sawa S, Eberl G, Vosshenrich CA, Di Santo JP. IL-7 and IL-15 independently program the differentiation of intestinal CD3-NKp46+ cell subsets from Id2-dependent precursors. J Exp Med. 207:273–280. doi: 10.1084/jem.20092029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Scandella E, Bolinger B, Lattmann E, Miller S, Favre S, Littman DR, Finke D, Luther SA, Junt T, Ludewig B. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat Immunol. 2008;9:667–675. doi: 10.1038/ni.1605. [DOI] [PubMed] [Google Scholar]
- Sun JC, Lanier LL. Natural killer cells remember: an evolutionary bridge between innate and adaptive immunity? Eur J Immunol. 2009;39:2059–2064. doi: 10.1002/eji.200939435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- Trotta R, Parihar R, Yu J, Becknell B, Allard J, 2nd, Wen J, Ding W, Mao H, Tridandapani S, Carson WE, Caligiuri MA. Differential expression of SHIP1 in CD56bright and CD56dim NK cells provides a molecular basis for distinct functional responses to monokine costimulation. Blood. 2005;105:3011–3018. doi: 10.1182/blood-2004-10-4072. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- Vosshenrich CA, Ranson T, Samson SI, Corcuff E, Colucci F, Rosmaraki EE, Di Santo JP. Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J Immunol. 2005;174:1213–1221. doi: 10.4049/jimmunol.174.3.1213. [DOI] [PubMed] [Google Scholar]
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.