Abstract
Colorectal cancer is one of the commonest tumors in the Westernized world affecting mainly the elderly. This neoplasm in older individuals occurs more often in the right colon and grows more rapidly than in the young, often shows a mucinous histology and mismatch repair gene changes. Effective screening permits discovery of colorectal cancer at an early highly treatable stage and allows for detection and removal of premalignant colorectal adenomas. Screening methods that focus on cancer detection use fecal assays for the presence of blood or altered DNA, those for detection of adenomas (and early cancer) use endoscopic or computerized radiologic techniques. Broad use of screening methods has lowered colorectal cancer development by about 50%. In addition, prevention of the earliest stage of colon carcinogenesis has been shown to be effective in small prospective studies and epidemiologic surveys but have not been employed in the general population.
Since 1996 the chemotherapeutic armamentarium for metastatic colorectal cancer has grown beyond 5-fluorouracil to include an oral 5-fluorouracil prodrug, capecitabine as well as irinotecan and oxaliplatin. Three targeted monoclonal antibodies (Moabs), bevacizumab (an anti-vascular endothelial growth factor Moab) and cetuximab/panitumumab, both anti-epidermal growth factor receptor inhibitors, have also earned regulatory approval. Most stage IV patients are treated with all of these drugs over 2 or 3 sequential lines of palliative chemotherapy and attain median survivals approaching 24 months. Lastly, adjuvant oxaliplatin plus 5-fluorouracil for high risk resected stage II and stage III colon cancer patient has led to substantial improvement in cure rates. With appropriate care of age associated comorbidities these treatment modalities are feasible and effective in the geriatric population.
Keywords: Colorectal cancer, age and gender; Colorectal cancer screening strategies; Colorectal cancer prevention
Colorectal cancer the third commonest cancer and the second commonest cause of death in the Western World is increasing worldwide especially in less industrial nations of the world [1]. Approximately 5% of colorectal cancers are directly related to heredity or chronic inflammatory disease such as ulcerative colitis; although a family history may be found in another 25% [2]. Thus, most colorectal cancers in the Western world are sporadic and are believed to be caused by environmental factors. The incidence of colorectal cancer is uncommon under the age of 50 years predominantly in tumors induced by heredity and with a family history but increases exponentially to the age of about 85 years [3]. The incidence of colorectal adenomas also rises with age[4]. Indeed two thirds of all colorectal cancers occurred in patients over the age of 65 [5].
Colorectal cancer does not arise de novo but rather is preceded by histologic progression from a normal appearing mucosa at risk for colorectal neoplasia to benign neoplastic tubular and villous adenomas to carcinoma formation [6]. This process takes about a decade or more and is accompanied by a large number of abnormalities in the genes of the colonic epithelium [7]. Two major gene pathways precede overt cancer formation, chromosomal instability, characterized by chromosomal aberrations, occurring in 80% or more of sporadic colorectal cancers [8] and mismatch repair responsible for about 15% [9]. These two pathways reflect gene changes found in hereditary disorders, familial adenomatous polyposis (FAP) and hereditary non-polyposis colon cancer (HNPCC). Epidemiologic, histological and genetic data support separation of colorectal cancer into rectal and proximal (right) and distal (left) colon cancers. Advanced adenomas are more likely to develop into malignant tumors and are defined by size, multiplicity and degree of dysplasia.
The distribution of colon neoplasia between left and right colon (distinguished as proximal or distal to the splenic flexure) differs according to a subject's ethnic background, gender and age [10]. Before introduction of a western lifestyle for example, most colon cancers in Asians occurred in the proximal colon. Westernization of populations, as characterized by migration from Japan to Hawaii and to the United States, increased colon cancer incidence mostly in the distal colon [11]. One explanation is that micro-satellite instability the characteristic genetic change in HNPCC tumors is common in tumors from Asian countries and in advancing age [12].
Why should colorectal neoplasia occur more commonly as we age? The standard answer is that molecular and pathophysiologic changes occurring throughout life progressively modify molecular homeostasis of colonic epithelial cells leading to neoplasia [13]. DNA damage certainly increases in older rodents, suggesting frequent stochastic cellular insults [14-16]. Aging also increases (rather than decreases) epithelial proliferation in rodent [17] and human [18] colon. The mechanism is unclear but recent studies have focused on the importance of SIRT1 deacetylase in suppressing tumorogenesis and colon neoplastic growth [19] that deacetylases beta-catenin. An additional surprising epidemiologic observation is that the incidence of colorectal cancer and that of many other cancers actually decreases after age 85 years [20] (Figure 1). Data from the surveillance, epidemiology and end result registry [21] demonstrates an abrupt reduction in colorectal cancer after age 85 [22] which falls to zero after age 100. This is not due to an under-reporting bias [23] and should impact upon recommendations for colorectal cancer screening in older individuals. Furthermore the biochemical and molecular basis needs clarification since mechanistic understanding may give clues about increased carcinogenesis with age before age 85. In that regard, the concept, championed by Judith Campisi's group, that aging and cancer have opposing gene effects is of great interest [24].
Figure 1.
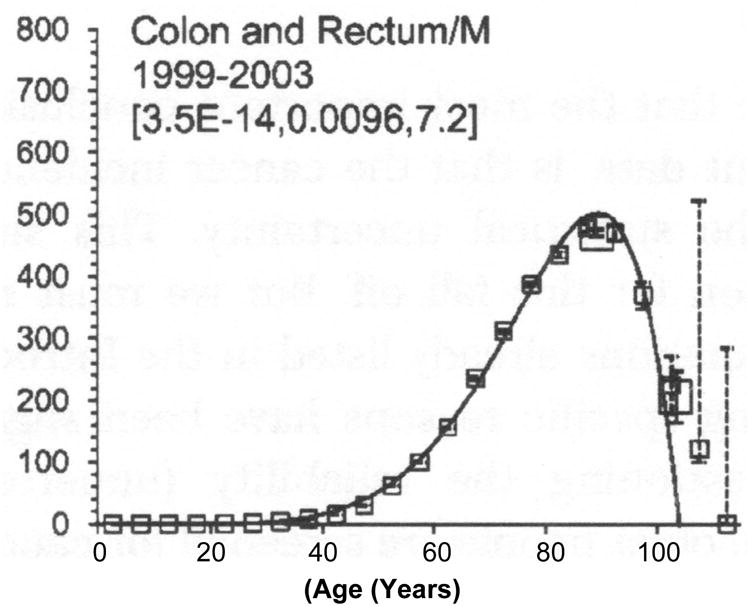
Age specific colorectal cancer incidence per 100,000. Best fit multifit model. The large squares are for ages 85 to 100 and 100+ [20].
There is a common misconception that the incidence and mortality from colorectal cancer in women is less than in men resulting in less screening in women. However, the overall incidence and mortality is similar in both sexes. The age specific incidence is greater in men and colorectal adenomas and cancer appear several years later in women probably due to protection by estrogen/progesterone. Several studies have shown that postmenopausal estrogen/progesterone hormone treatment in women can lower colorectal neoplasia risk by as much as 30% [25, 26].
The hypermethylated phenotype involving CPG island methylation (CIMP) has clinical, pathologic and histologic features fundamentally different from most colorectal cancers and increases with age to over 50% of patients over age 75 compared to the CIMP negative phenotype [12] suggesting that epigenetic alterations of candidate tumor suppressor genes occurs predominantly in the older population. Furthermore, hypermethylation in CPG islands in the absence of colorectal neoplasia also is more frequent in the older population [27] but the mechanism and the consequences have not been elucidated. As defined by promoter hypermethylation of 5 genes [28], the clinical features favor proximal colon location and female predominance[29]. Associated molecular changes include reduced nuclear p27 and Cox 2 expression [30, 31]. Since hypermethylated promoter silencing of gene action is potentially reversible with demethylating agents, these observations require follow-up.
As described above, sporadic colorectal cancer usually is preceded by benign neoplastic adenomas with risk for later cancer development. As many as 50% of Western populations develop adenomatous polyps in their lifetime but the lifetime risk for colon cancer is about 5%, only one in ten adenomas lead to cancer formation. To date, we cannot determine which individuals with adenomas (or a histologically normal colon at risk for adenoma and cancer formation) will develop a cancer. Therefore, preventive methods use detection and removal of benign neoplastic colorectal adenomas which lowers colon cancer formation and mortality [32]. Since colorectal cancer prognosis depends on the stage of diagnosis and disease localized to the bowel wall has a 90% chance of no recurrence in 5 years reducing colorectal cancer mortality include early detection of an established cancer.
Should screening be modified in the elderly patient and if so why and how? The question is best answered using epidemiologic data, what changes may occur in the biology of colorectal neoplasia with age and whether the clinical or histological features differ. Furthermore, the benefits of screening decrease with age and with comorbidities that are present in individual subjects.
Present screening guidelines recommend screening in average risk individuals beginning at age 50 based upon data on the frequency of adenomas and cancer of the colorectum at differing ages and evidence from randomized controlled studies [32, 33]. In the past 2 decades, colorectal cancer incidence has fallen by 23% accompanied by a shift of cancer staging downwards and redistribution of colon cancer from the distal to the proximal colon [34]. This trend, as documented in Olmstead County, Minnesota, was associated with fourfold more colorectal polyp resections. Colorectal cancer incidence rises to a peak at between 70 and 75 years but most colorectal screening studies usually have not examined effectiveness at all ages. Colorectal neoplasia differs in older patients over age 65 since the total number and more advanced lesions increase to age 75 [35]. Malignant colorectal polyps recurring after clearing colonoscopy are found three-fold more frequently after age 60 than earlier [36]. These cancers were evenly distributed between left and right colon and multivariate analysis showed that age was the sole determining factor [37].
Screening colonoscopy was examined in over 1000 patients aged 50-54; 147 subjects aged 75-79 and 63 subjects over the age of 80 [38]. Patients with bowel symptoms, family history or lower endoscopy in the previous five years were excluded. The prevalence of any neoplasm increased with age to a maximum of 28% in those over 80; advanced adenomas occurred in only 3.2% of screened subjects aged 50-54 but 14% in the over 80s (Table 1). However, life expectancy was extended by only 0.17 years at 75-79, 0.13 years over age 80, compared to 0.85 years in subjects aged 50 to 54. Sensitivity analysis, showed that larger polyp lag times (calculated time from polyp discovery to cancer development) in the elderly reduced the mean gain in life expectancy even more.
Table 1. Effect of age upon colorectal adenoma incidence.
| Age | Any Neoplasm | Adenoma 5-9 MM Percent | Advanced Adenoma |
|---|---|---|---|
| 50-54 | - | 4.3 | 3.2 |
| 75-59 | - | 11.0 | 4.7 |
| 80+ | - | 7.9 | 14.0 |
| Modified from Lin OS et al. JAMA 2006, 295;2357 | |||
| 40-49 | 8.7 | 1.1 | |
| 50-59 | 12.5 | 2.4 | 0.5 |
| 60-69 | 15.5 | 6.6 | 2.0 |
| 70-75 | 25.0 | 10.6 | 1.3 |
| 76-80 | 14.3 | 11.7 | 2.6 |
Modified from Strul H et al. Am. J. Gastro 2006, 101:255
Differing screening strategies for elderly populations were quantified in two studies based upon life expectancies. The impact of screening calculated from published data of populations from age 50 in five year groups to over 90 years [39] assumed that benefits accrued five or more years after the screening procedure, representing the mean time from polyp to advanced neoplasm development. The data were balanced by competing causes of mortality including poor health and complications from procedures. The authors concluded that screening over age 80 in poorer health individuals was of no benefit and that colonoscopy risks increased with age and reduced overall benefit. A study using a Markov model to assess impact on increasing life expectancy to age 75 upon screening strategy costs [40] suggested that cost effectiveness increased with rising life expectancy. These combined data led the United States Preventive Task Force on screening to recommend against routine screening between the ages of 75 and 85 except in individuals in excellent health and no screening after the age of 85 [41].
Screening and surveillance methods
Preventive methods to reduce the incidence and mortality from colorectal cancer distinguishes screening from surveillance. Screening includes testing of asymptomatic individuals at average risk for colorectal cancer or at higher risk because of a family history to diagnose colorectal cancer or precancerous neoplastic adenomas. Surveillance involves monitoring of individuals with premalignant conditions including inflammatory bowel disease, primary sclerosing cholangitis or with previous adenomatous colorectal polyps or cancer. Patients who are at risk for hereditary colorectal neoplasia require special surveillance programs, a description of which is beyond the scope of this review.
Screening methods to diagnose colorectal cancers and adenomatous polyps are divided into fecal, endoscopic and radiologic evaluation (Table 2).
Table 2. Colorectal neoplasia screening strategies for average-risk subjects.
| To diagnose colorectal cancer | |
|---|---|
|
| |
| 1. Fecal occult blood tests |
|
| 2. Fecal NDA tests |
|
| To diagnose colorectal cancer and premalignant colon neoplasia | |
|
| |
| |
Modified from (2008) – Screening for Colorectal Cancer. Ann. Int. Med. 149. [41]
Fecal detection testing focuses upon demonstration of fecal occult blood or aberrant human DNA.
1. Fecal occult blood testing
Fecal occult blood testing divides into guaiac-based (gFOBT) and immunochemical testing (FIT). Blood in the stool is a non-specific test deriving from colorectal cancer or large adenomas and many other conditions. Small adenomatous polyps usually do not bleed and even colorectal cancers may only bleed intermittently. Guaiac-based occult blood testing depends upon the pseudo-peroxidase activity of heme or hemoglobin requiring two fecal samples each from three consecutive stools. To minimize false positive tests, subjects are advised to avoid aspirin, red meat and raw vegetables, to minimize false negative tests they avoid taking vitamin C. This is still the commonest fecal test performed in the United States. Large randomized studies showed that cancers are detected at an earlier stage with fecal occult blood screening than in unscreened individuals and reduces mortality rates between 15 and 33% [42, 43]. Widespread testing originally employed low sensitivity tests with HEMOCULT II which shows higher specificity than high sensitivity Hemocult SENSA. One problem is that physicians have used single office stool testing for screening instead of standardized testing of three consecutive stools. The overall conclusions indicate that annual screening using high sensitivity guaiac-based occult blood testing detects colorectal cancers in an asymptomatic population. Although the sensitivity of screening with fecal occult blood tests is low, repeat annual testing may find up to 90% of colorectal cancers [44]
2. Fecal immunochemical testing
Immunochemical testing (FIT) for human globin or hemoglobin is more specific, particularly for lower gastrointestinal bleeding and requires simpler collection methods than qFOBT tests and has become the preferred fecal test in Europe and Japan. Several FIT are available whose sensitivity for detection of cancer and advanced adenomas depend upon the hemoglobin threshold used. Side to side testing of differing FIT's against standard gFOBT methods have regularly shown superiority for the FIT's tests in acceptance and in detection of advanced neoplasms [45, 46]. One Japanese study has shown that FIT reduced colorectal cancer mortality and incidence by 72% and 59% respectively [47]. FIT is less demanding and requires no restricted diet and can be performed in physician's offices whereas FIT tests to date utilizes automated specialized laboratory evaluation.
Stool DNA testing
Since colorectal cancer and precancerous adenomas often contain altered DNA which is shed into the gastrointestinal lumen and DNA is stable in the stool, fecal tests seeking altered DNA are being evaluated. Multiple DNA markers need to be tested usually including point mutations in K-ras, APC, P53, BAT26 (a probe for micro satellite instability), methylated promoter markers and several DNA integrity tests. One disadvantage of DNA testing is that at least 30 gms of stool need to be collected and rapidly sent to the testing laboratory. The specificity for the detection of colorectal cancer is stated to be about 95% with a sensitivity of 50% [48, 49] but the sensitivity for detection of advanced adenomas is only about 20%. Future research will develop further fecal DNA markers and may simplify collection procedures. Present United States recommendations do not include such DNA testing for average risk individuals.
The positive predictive value of fecal testing for detection of advanced neoplasms has not been evaluated.
Endoscopic screening methods
1. Flexible sigmoidoscopy typically is performed without sedation, with limited bowel preparation and can be performed in an office setting by general practitioners or non-physicians. Flexible sigmoidoscopy used for colorectal cancer screening is supported by high quality case control and cohort studies showing reduction in colorectal cancer mortality by 60-80% for the lower colon persisting 10 years [33, 50]. Sigmoidoscopy sensitivity for detecting colonic advanced adenomas and cancers is less after age 65 since proximal lesions are more common. Furthermore, screening flexible sigmoidoscopy in asymptomatic patients over age 75 showed that incomplete examinations, particularly in females, was common and the cancer detection rate was only about 0.5% [51].
Early recommendations suggested five year intervals for repeat procedures after normal sigmodoscopy examination (while recommending a ten year interval between colonoscopy examinations). More recent data may justify longer intervals between flexible sigmoidoscopy procedures for screening young individuals. Much of the benefit of sigmoidoscopy may be that patients who have small distal adenomas will undergo a colonoscopy resulting in discovery of proximal advanced adenomas and cancer. However, the complication rate of flexible sigmoidoscopy is small and perforation occurs in fewer than 1 in 20,000 examinations.
2. Flexible colonoscopy is one of the most commonly performed medical procedures in the United States allowing for mucosal inspection of the entire colon from the appendiceal orifice to the dentate line as well as biopsy sampling or definitive polypectomy if neoplastic polyps are found. Reductions in colorectal cancer incidence after colonoscopy and polypectomy is used to support this screening procedure. In the National Polyp Study colorectal cancer after clearing colonoscopy was reduced by 75 to 90% compared with non-current reference populations [52]. Subsequent studies have shown less protection. Combined data from three United States prevention trials showed only 50% reduced colorectal cancer after clearing colonoscopy [32]. A recent study from Canada emphasized reduced colorectal cancer rates only in distal and not in proximal cancers [53]. Although this study has been criticized, the message that detection of proximal lesions is less complete than distal lesions is probably correct.
Patients prefer colonoscopy to flexible sigmoidoscopy screening probably because they are sedated but the procedure is limited by requiring dietary preparation, bowel cleansing, time for examination and recovery and has a 0.2 % risk of perforation and post polypectomy bleeding. Furthermore, colonoscopy is not infallible since large adenomas may be found after clearing colonoscopy in 6-10% [54] and interval tumors may develop in 3 or so years in up to 5% [55]. Present recommendations are that appropriate intervals between negative colonoscopy screening examinations should be approximately 10 years.
3. Radiologic procedures. Double contrast barium enema testing was the standard colon neoplasia detection method before endoscopy but much of the literature on its efficacy is confusing and depend on the authors specialty. The procedure is limited by patient discomfort, the need for colonic preparation and biopsy or polypectomy is not done with a low sensitivity for finding adenomas compared to colonoscopy. Recent guidelines suggest substitution of CTC for barium enema examinations [56].
Computed tomographic colonography (CTC) is a minimally invasive imaging examination of the entire colon and rectum. The equipment and methods of performing CTC have rapidly improved including 3 dimensional and advanced 2 dimensional techniques. Full cathartic preparation and gaseous colonic distention presently are essential but tagging of residual solid stool with barium or other radiopaque contrast agents may be validated in the future.
The efficacy and test performance of CTC is an area of controversy although one metaanalysis suggests that CTC sensitivity and specificity for large (greater than 10 mm) polyps is between 85-93% [57]. Some institutions have organized back to back CTC examinations with colonoscopy for any lesions that are detected by the radiologic procedure. CTC is useful to detect proximal lesions following incomplete colonoscopic examinations and the presence of obstructing lesions preventing proximal passage of the colonoscope. One disadvantage is detection of lesions outside the colon that demands additional testing which may be unrevealing [57]. It is also unclear how effective CTC will be in the detection of flat colorectal neoplasias [58]. A prospective comparison of the efficacy of CT colonography, colonoscopy, sigmoidoscopy and fecal occult blood testing from Germany reported that CT colonography identified over 90% of adenomas larger than 6 mm. Addition of fecal blood testing to sigmoidoscopy did not increase detection rates [59].
A relatively recently recognized problem is that many colorectal neoplasms are flat or depressed [60]. These were initially described from Japan [61] but are increasingly found in the Western world [58], may be missed by colonoscopy, may be more aggressive and could be responsible for finding interval cancers after clearing colonoscopy. New colonoscopic techniques such as high magnification, chromo-colonoscopy may improve detection rates of such flat and depressed colorectal neoplasms [62, 63].
The United States does not have a homogeneous population but rather is a melting pot of differing ethnic groups so that risk and benefits should be based upon gender, age and race [3]. Questions regarding intensity and timing of colorectal neoplasia screening also has been raised in Europe [64]. Globally, the incidence of this cancer varies tenfold attributed to differing environmental and genetic factors [1]. Thus, screening recommendations vary in differing geographical areas.
Patients with a history of colorectal cancer are at increased risk of colorectal second primary tumors for which they usually receive surveillance [65]. The overall risks of colorectal second primary tumors from the National SEER Program Database totaling over 200,000 patients was 1.9% during a median follow up of about 4.2 years mainly in younger patients (<50 years of age) which decreased progressively with advancing age. These data would suggest that surveillance following the initial management of a colorectal cancer should be less intense in older patients [66].
Chemoprevention for reducing the risk of colorectal cancer risk
Presently, the sole accepted method of risk reduction (prevention) of colorectal cancer is detection and removal of preneoplastic adenomas. This represents secondary prevention – the elimination of existing lesions may progress to cancer. Primary prevention focuses upon risk reduction of the carcinogenic process from a normal colorectal mucosa to benign adenomatous neoplasia. Risk reduction methods have included life style changes, including increasing physical activity, dietary factors and pharmaceutical agents including non-steroidal anti-inflammatory drugs (NSAID's) and difluoromethylornithine (DFMO).
Increased physical activity reduces colorectal cancer risk by about 30% [67]. The dramatic increase in CRC incidence in Japanese migrants to Hawaii and the United States led to the hypothesis that a high calorie, high fat and meat and low fiber diet was principally responsible [68, 69]. However, repeated prospective studies of differing diets have failed to reduce adenomatous polyp recurrence in at risk individuals [70] and antioxidants also are ineffective [23]. However, there is interesting data that suggests that consumption of a Western style diet may be associated with a higher risk of recurrence and mortality in patients with stage III colon cancer treated with surgery and adjuvant chemotherapy [71].
Furthermore, numerous epidemiologic studies indicate that calcium or dairy products or/and increased vitamin D lowers the risk of colorectal neoplasia [72]. Prospective randomized studies showed that calcium supplementation of 1.2 gms per day reduced adenoma recurrence in patients with previous resected adenomas [73], that this effect may last for years after stopping the supplement [74], and the efficacy of calcium administration depends upon the levels of circulating vitamin D [75]. Studies of the effects of sunlight, of dietary vitamin D intake and of vitamin D status also show reduction in colorectal incidence [76]. The relative importance of the intake of calcium and vitamin D currently is under study. Epidemiologic [77] and experimental data in rodents [78] suggested that folic acid intake might be an important dietary factor in colorectal cancer development. This may hold for individuals who are folate depleted but not those who are folate replete. Preliminary data [79] and theoretical evidence suggest that excess folate intake may increase development of advanced adenomas [80].
The anti-neoplastic properties of aspirin and other non-steroidal anti-inflammatory drugs have been documented extensively [81]. Aspirin may lower colorectal cancer risk by 40-50% based on observational data [82]. One prospective study showed that 81 mg of aspirin (but not 325 mg) lowered adenoma recurrence [83] and another that 300 mgs was effective [84]. Both 3 year studies reduced advanced adenomas by about 40% and the first showed a continued effect of aspirin containing compounds taken for over 4 days per week for 4 years [85]. A metaanalysis has shown a pooled absolute risk ratio of adenoma recurrence of 0.83 and of advanced adenomas of 0.72 [86] with aspirin ingestion. However, the bleeding side effects of aspirin has raised questions about use for colorectal cancer prevention [87] and the US Preventive Services Task Force recommended against the routine use of aspirin and NSAID's for colorectal cancer prevention in at average risk individuals [88]. To mitigate side effects, cyclooxygenase 2 inhibitors were tested as chemopreventive agents and were found to be effective [89][90] but cardiac side effects [91] prevented these drugs from being used except for individuals at very high risk [92]. Research on other NSAID's-like compounds that could be anti-neoplastic but safe have focused upon nitric oxide donating agents [93] and studies in cancer cells [94] and rodents [95] have shown efficacy with little short term toxicity [96]. Combination chemotherapy has come of age with the demonstration that dosages of DFMO plus sulindac that do not cause short term side effects reduced colorectal adenoma recurrence by as much as 70% [97]. It is likely that primary preventive measures to drastically lower CRC risk will need lifelong effective agents so that little effect might be expected in elderly individuals. However, adenoma recurrence studies have shown effects in 3-5 years with safe, nutritional agents such as calcium and vitamin D supplements, which also have other beneficial effects in the elderly.
Surgery in elderly patients with colorectal cancer
Thirty eight percent of colorectal cancer cases present as stage I or II disease, 38% percent as stage III disease and 19% present as metastatic disease. Resection is a necessary step toward cure for stages 1-3 and appropriately selected stage 4 colorectal cancer patients. The question of whether geriatric patients, either by virtue of differences in physiologic reserve, comorbidities or previously unidentified differences in tumor biology benefit from curative intent resection has been addressed by several registry and data base reviews.
A review of the Rotterdam Cancer Registry evaluated the influence of age on resection rates and operative risk in 6457 patients with colorectal cancer diagnosed from 1985 through 1992. The post operative mortality of patients less than 60 years was 1% and continually increased with age to about 10% for those over 80. Despite these age associated surgical outcome disparities the authors concluded that surgery should not be denied to elderly patients with acceptable preoperative risk [98].
Thirty day post-operative mortality among octogenarians with colorectal cancer has been shown to be 10.6%. However, a significant difference was seen in those 80-84 years (8%) rising to 13% in those 85-89 and 20% in nonagenarians (p<0.001) [98]. A French review of over 8,000 cases of colon adenocarcinoma over a 20 year period defined operative mortality in those under 60 to be 1.7%, increasing to 5% in those 60-74 years and 12% in those patients over 75 (p<0.001.)[99]. A risk tool depicted in Figure 1 has identified additional risk factors of postoperative mortality including age older than 95 and ASA (American Society of Anesthesiologists) grade IV or V. Heriot et al (Figure 1) [100].
Not surprisingly, data also supports elective as opposed to emergency surgery as well as laparoscopic surgery as preferred procedures in elderly patients. Aggressive post operative measures to prevent pneumonias and other respiratory complications also improves survival outcomes. No differences in age associated cancer specific survival following curative intent surgery have been observed, i.e. no difference in tumor biology between older and younger colorectal cancer patients has been identified . Therefore resection should remain an option in fit elderly patient with stages 1-3 colorectal cancer [101, 102].
Rectal cancer surgery, while technically more demanding than partial colectomy, has demonstrated survival benefits in patients older than 80. A study reviewing patients in the SEER database between 1993-2002 analyzed survival in patients over 80 undergoing rectal cancer resection. The mean age of patients analyzed was 85 and stage distribution was balanced among stages I-III disease: 2.5% AJCC stage 0, 28.5% stage I, 27.3 % stage II, 23.3% stage III and 18.4 percent stage IV. Survival among patients undergoing surgery for stage 0-3 disease was 93-98.7 %. Comparison of patients who underwent resection versus those who did not demonstrated significant improvement in median survivals favoring patients undergoing resection: stage I disease median survival was 55 months compared with 11 months (p<0.0001); stage II 41 vs. 14 months (p<0.0001); stage III- 28 vs. 14 months (p<0.05). Even in stage 4 patients the survival difference was significant, 8 vs. 3 months (p<0.0001). This data shows at least a doubling of overall survival in patients undergoing surgery for all stages of disease and therefore supports radical resection of rectal cancer in octogenarians who are able to tolerate such surgery [103].
Multidisciplinary treatment of operable colorectal cancer patients should consist of efforts to optimize perioperative medical health and to optimize balance between acceptable surgical risk and the goals of surgery. The International Society of Geriatric Oncology recently published guidelines for surgical management of patients 70 years and older. Key consensus points were that emergency colorectal surgery due to unforeseen complications should be avoided whenever possible, colorectal stents should be used whenever possible to palliate complications such as obstruction and subsequent surgery should be planned one to two weeks after a patient has been stabilized. Specific studies have not been done evaluating success of colorectal stents in the elderly, but overall this is a less complicated procedure with significantly reduced operative risk. Elective surgery with prospective identification and management of perioperative risks and careful treatment planning should be the pathway of choice [104].
Adjuvant Chemotherapy in the elderly
The risk of metastatic relapse, typically within 3 years following resection, and subsequent cancer associated death following resection of high-risk stage II (T4 tumor, and tumor perforation, preoperative bowel obstruction, poorly differentiated tumor, venous invasion by tumor or less than 10 peritumoral lymph nodes identified in pathology specimen) and stage III (peritumoral lymph node metastases identified in pathology specimen) is substantial, ranging between 50 and 75% in the case of patients with 4 or more peritumoral lymph node metastases. Adjuvant therapy to improve cure rates should therefore be offered to all stage II with high-risk features and all stage III patients medically fit enough to tolerate chemotherapy. Several trials have shown a modest benefit in recurrence free and overall survival [Table 1]. In the 1990's adjuvant 5- fluorouracil (5-FU)/leucovorin based chemotherapy became the accepted standard of care on the basis of two randomized trials. The U.S. Intergroup 0089 trial and a French study by deGramont et al established optimal dosing of the 5-FU [105, 106]. Since then the MOSAIC trial established that the addition of oxaliplatin to 5 fluorouracil (FOLFOX) improved disease free survival at three years by about 5% (78.2 % for 5-FU/ oxaliplatin vs. 72.9% for 5-FU alone (p=0.002) [107]. The NSABP C-07 trial confirmed the modest benefit of oxaliplatin added to 5-FU but in a different dosing regimen (FLOX) in terms of 3 and 4 year disease free survival. The oral flouropyrimadine capecitabine, which converts in tissue to fluorouracil has been accepted as an alternative to bolus infusion of 5-FU in the adjuvant setting and equivalent to infusional 5FU in the metastatic setting [108, 109].
While several of these trials excluded patients over the age of 75, subsequent data supports the use of adjuvant chemotherapy for fit elderly patients with an excellent performance status. Surveillance, Epidemiology and End Results (SEER) database review of adjuvant chemotherapy use in elderly patients with stage III colon cancer revealed that use of adjuvant therapy substantially declined with increasing age: 78% of those aged 65-69 years, 74% of those aged 70-74 years, 58% of those aged 75-79 years, 34% of those aged 80-84 years and 11% of those 85-89 years received adjuvant chemotherapy. A correlation between increasing age and hospitalization rates during the 6 month interval following chemotherapy initiation was demonstrated. Seven percent aged 65-74 were hospitalized for side effects including mucositis, neutropenia, dehydration, bacteremia, sepsis, or diarrhea. The hospitalization rate increased to 9% in those 75-79, 12% (80-74), 13% (85-89) and 20% in the population over 90 years old. (Schrag)
Studies have established the safety, tolerability and disease control in elderly patients using 5-fluorouracil based adjuvant therapy [110, 111]. An 8 year follow-up of a pooled analysis of 7 phase III adjuvant 5-fluorouracil randomized trials established that patients over 70 years old had the same rate of death secondary to recurrent disease as younger age groups, 29% versus 31-33%. Older patients did have a significantly higher rate of death without recurrence of cancer than the other age groups at 13% (2-7 % in other groups). All age groups benefited similarly in terms of disease free and overall survival with adjuvant 5-FU. While patients over the age of 70 had a higher rate of significant leucopenia, age was not related to significantly higher rates of grade 3 or higher stomatitis, nausea/ vomiting, or diarrhea [111].
Multimodality curative intent therapy for metastatic colorectal cancer
One of the most significant advancements in the standard of care for liver only metastatic colorectal cancer (i.e., no visible extrahepatic metastases) is the broadening use of hepatic resection with curative intent. Reported 5-year survival rates for resection range from 28-58%, [112-115]. Resection of hepatic metastasis should be considered for patients without extra-hepatic disease detected by CT and/or PET scans and those with anticipated adequate post resection functional hepatic reserve. The safety and feasibility of hepatic resection in the elderly population has been evaluated and is thought to be a valid method of improving survival outcomes. A single institution study reviewed 191 patients from 1999 to 2005 and found 30 and 60 day operative mortality rates similar in patients younger and older than 70 years. Post operative mortality rates were low (0-4%) for both groups. Similar 1, 3 and 5-year disease free survival between the older and younger groups was demonstrated, 76% vs 62%, 35% vs 38%, and 29% vs. 32%, respectively [116]. Another single institution study also found similar results in the over 70 year-old population in terms of surgical resection of liver metastasis. This group detected similar hospital mortality between the age groups (5.7% vs. 2.1% p=0.19) and determined the 5 year survival in the older group to be 30%; 38% in the younger group [117].
In some cases neoadjuvant chemotherapy can be used to convert non-resectable disease to resectable disease. However, irrespective of whether liver only metastatic disease is initially resectable the rate of relapse either in the liver or other sites following metastectomy is significant. EORTC 40983, a randomized controlled trial is the only study to date to show that systemic chemotherapy can improve relapse free survival following resection of liver metastases. In this trial 6 cycles of preoperative followed by 6 cycles of postoperative 5-fluorouracil/oxaliplatin was compared with surgery alone. Perioperative chemotherapy was associated with improved 3 year disease free survival, 36.2% versus 28.1%, p= .041 [118].
Data regarding the feasibility and efficacy of perioperative chemotherapy specifically for patients over the age of 70 undergoing hepatic metastectomy is limited. One retrospective analysis evaluating 29 patients over 70 with liver only metastasis compared to 41 patients not receiving chemotherapy showed no difference in surgical mortality or morbidity in association with chemotherapy. A significant improvement in recurrence free survival (RFS) and overall survival in those receiving an oral 5-fluorouracil prodrug, capecitabine, plus oxaliplatin was observed (p=0.002) [119].
Chemotherapy in the Metastatic Setting
For patients with unresectable stage IV colorectal cancer systemic therapy is intended to prevent or reverse disease-related symptoms, the hallmarks of which are unintended weight loss, progressive fatigue leading to difficulty performing activities of daily living and tumor associated pain/discomfort. Also, palliative chemotherapy has repeatedly demonstrated overall survival benefits.
Between 1996 and today multiple landmark phase 3 randomized controlled trials have confirmed the clinical benefit of two new classes of cytotoxic chemotherapies and two classes new so-called biologically targeted monoclonal antibodies(moabs). Table 2 summarizes the clinical benefit associated with 5-fluorouracil and its associated biological modifier leucovorin as well as multidrug combinations that represent standard of care options for chemotherapy naïve palliative chemotherapy for unresectable stage 4 colorectal cancer. The most important message from these data are that median overall survivals have progressed from an average of 12 months in the 5-fluorouracil only era to median survivals approaching two years when patients are treated with sequences of all 3 cytotoxic agents plus appropriately selected monoclonal antibody-based therapy.
By way of introduction, medical oncologists typically choose from 2 chemotherapy “backbones”, either FOLFOX or FOLFIRI (5FU plus either oxaliplatin or irinotecan, respectively) plus the monoclonal antibody bevacizumab as initial therapy for patients with unresectable stage IV colorectal cancer. Bevacizumab neutralizes the vascular endothelial growth factor (VEGF) and has demonstrated clinical benefit and gained regulatory approval for the treatment of metastatic non-squamous nonsmall cell lung cancer, metastatic breast cancer and metastatic colorectal cancer. Objective response rates with these regimens range between 35-45%, median time to disease progression is 6-8 months and median overall survivals are 21-24+ months. Notably, for patients deemed to not be “fit” enough to receive FOLFIRI or FOLFOX the combination of infusional five-fluorouracil plus bevacizumab produces clinical benefit comparable with the chemotherapy doublet + bevacizumab regimens.
It is not unusual that dose adjustments to minimize myelosuppression and changes to antiemetic programs are required, typically during the initial 3-4 cycles of biweekly therapy. Patients are assessed for nonhematologic as well as hematologic toxicity as well as signs/symptoms of disease progression prior to each repeat cycle of therapy. While consistently rising CEA levels are concerning for disease progression this data should never alone be used to change to non-cross resistant therapy. Optimally, imaging studies are repeated every 8-12 weeks to ensure that disease control is being achieved. If new lesions develop or the existing metastases significantly increase ( standard international progression and response criteria are used) and the patient remains independent of activities of daily living, then second and in some cases third line non-cross resistant therapy is offered, Figure 1.
Given that colorectal cancer is usually a disease of the elderly and unfortunately a large proportion of patients with the disease present with nonoperable stage IV disease, whether from disease recurrence or denovo diagnosis, studies have been done to validate the safety and toxicity specifically in an older population [Table 3]
Table 3. Risk Score for Postoperative Mortality for Elderly Patients Undergoing Surgery for Colorectal Cancer.
| Conversion Table | |||
|---|---|---|---|
|
|
|||
| Risk Factor and Subcategory | Score | Total Score | Predicted Mortality (%) |
| Age group | 0 | 5.9 | |
| 80-84.9 | 0 | 3 | 7.B |
| 85-89.9 | 1 | 6 | 10.3 |
| 90-94.9 | 6 | 9 | 13.4 |
| ≥95 | 11 | 12 | 17.2 |
| ASA grade | 15 | 21.9 | |
| l-ll | 0 | 18 | 27.5 |
| III | 10 | 21 | 33.9 |
| IV-V | 18 | 24 | 40.9 |
| Metastases | 27 | 48.3 | |
| No | 0 | 30 | 55.7 |
| Yes | 6 | 33 | 63 |
| Urgency | 36 | 69.7 | |
| Elective | 0 | 39 | 75.6 |
| Emergency | 7 | 42 | 80.7 |
| Tumor resection | 45 | 85 | |
| Yes | 0 | ||
| No | 1 | ||
| Large-bowel obslrutition | |||
| No | 0 | ||
| Yes | 1 | ||
| Total score | |||
ASA = American Society of Anesthesiology.
A conversion chart is displayed for estimating the predicted mortality based on the total score.
5- Fluorouracil
Historically first line regimens have been 5-FU based and many trials have attempted to optimize the dose and the schedule of this drug. The practical conclusion of the multiple trials attempting to identify optimal ways to use the very schedule-dependent 5-fluorouracil is that, based on a favorable efficacy and toxicity profile, the so-called deGramont schedule has emerged as the de facto reference 5-fluorouracil regimen. The central difference between the deGramont regimen and the United States 5-FU regimens that it has popularly replaced is that the every other week bolus plus 46 hour infusion 5-fluorouracil deGramont schedule has a better side effect profile than the bolus-only Mayo Clinic and Roswell Park regimens particularly with regard to febrile neutropenia and gastrointestinal side effects [120, 121].
Two large retrospective trials have evaluated 5-FU use in the older patient. Folprecht et al reviewed 3825 patients receiving 5-FU in 22 European studies. This review included both infusional and bolus regimens. Median overall survival in patients over 70 years was not statistically different from patients younger than 70, 10.8 months versus 11.3 months, respectively. Even age above 80 years was not identified as a predictor of worse chemotherapy associated survival outcomes. In terms of bolus vs. infusional dosing, infusional 5-FU was associated with significantly higher survival in all age groups. Notably, in the population over 70 a median overall survival of 10.3 vs. 11.9 months favored infusional versus bolus 5-FU.
A similar pooled analysis was done by D'Andre et al reviewing 1748 patients from 4 North Central Cancer Treatment Group trials using 5-FU. This analysis determined increased rates of severe (≥ grade 3) toxicities in patients over 65 (53% vs. 46% p=0.01), specifically increased rates of diarrhea, stomatitis and infection. This study also found no significant difference in overall survival or time to tumor progression with regard to age.
A single institution retrospective analysis in 5-FU treated subjects over the age of 70 demonstrated chemotherapy associated improvement in performance status and weight occurred in 26% of the younger than 75 and 31% of the 75 and older patients. Such survival and palliative benefits are among the main goals of chemotherapy emphasized to patients [122].
Capecitabine
Capecitabine (Xeloda) is an oral 5-FU prodrug that is absorbed intact through the intestinal tract and converted to 5-FU by enzymatic reactions. Two randomized trials demonstrated that capecitabine is non-inferior to 5-fluorouracil. Importantly, capecitabine was associated with significantly less nausea, diarrhea and stomatitis. However, hand-foot syndrome which can range in intensity from minimally symptomatic palm/sole erythema to pain and desquamation was more frequent in patients receiving the oral drug. [123, 124] in order to improve capecitabine tolerability a 1000 mg/M2 b.i.d. dose has been shown to be effective and is now standard in the United States.
Effective strategies to minimize capecitabine associated side effects thereby permitting continued therapy have been developed. Hand-foot syndrome, also known as palmar-plantar erythrodysthesia, is classified as grade 3 when it interferes with activities of daily living, typically with skin blistering and desquamation. Withholding additional capecitabine usually effects a rapid reversal of symptoms with no long term sequela; in most cases capecitabine can be restarted at a lower dose. Patients are educated prior to initiation of capecitabine to preventively moisturize their hands and feet twice daily. Capecitabine associated diarrhea, a side effect which can complicate 5-FU and irinotecan therapy as well, is managed with over the counter anti-diarrheals such as loperimide [125].
Oxaliplatin
Oxaliplatin in combination with 5-FU (FOLFOX) has been validated in many studies as effective treatment of colorectal cancer in both the adjuvant and metastatic setting [106, 126, 127]. FOLFOX has also been determined to be non inferior to FOLFIRI, and using either sequence of these doublets has equivalent survival outcomes. However, the relative contribution of 5-fluorouracil to irinotecan once the patient's disease has progressed on FOLFOX is negligible at most. Therefore, if FOLFOX is used as first line therapy single agent irinotecan rather than FOLFIRI is usually selected as second line therapy [128, 129]. Capecitabine in combination with oxaliplatin (XELOX or CapeOX) has also been determined to be non-inferior as a 5-FU substitute in several large randomized clinical trials [130-132].
A pooled analysis of FOLFOX in older (>70 years) and younger (<70 years) patients in the adjuvant, metastatic first-line and second line setting determined that age was not associated with differences in disease free survival, and that addition of oxaliplatin was associated with improved disease free survival as adjuvant treatment of high risk stage II and stage III patients [133]. A phase II trial of capecitabine-oxaliplatin combinations in patients over 70 years old showed clinical outcomes consistent with those reported in non-age restricted trials [134]. A prospective randomized study comparing infusional 5-FU/leucovorin plus oxaliplatin vs. capecitabine plus oxaliplatin showed no difference in terms of overall response rate or survival outcomes when comparing patients younger and older than 70 years. Older patients derive benefits from first line oxaliplatin combinations similar to younger patients without increased toxicity[135].
Neuropathy, acute and chronic types, is the most common oxaliplatin specific side effect. The acute type affects 90% of patients and typically begins within a few hours of infusions, is precipitated by exposure to cold and is self limited. The chronic type is a cumulative effect not typically seen until after patients have received > 540mg/m2 - 850 mg/m2 lifetime oxaliplatin treatment. This corresponds to cycle 8 or 9 in patients receiving doses of 85 mg/m2 . One study revealed grade three peripheral neuropathy in 3.2% of patients receiving 7-9 cycles, 28% receiving 10 -12 cycles and 50 % receiving >12 cycles [136]. Oxaliplatin neuropathy can consist of paresthesia, hypoesthesia and changes in proprioception that can affect activities of daily living that require fine motor coordination, such as writing, buttoning and picking up small objects. Difficulties in proprioception may even cause gait abnormalities. It is because of this toxicity that oxaliplatin should be used with more care and frequent monitoring in the elderly. Gait abnormalities and fine motor functions should be assessed in all patients at baseline and oxaliplatin should not be used or used for a limited amount of cycles in patients with pre-existing neuropathy.
Several preventive measures have been tried with mixed results in order to reduce the risk of oxaliplatin induced neuropathy. Administration of intravenous calcium and magnesium on treatment days immediately before and after oxaliplatin infusions was shown to reduce all grade neuropathy from happening in 75% of patients to only 27% of patients. Due to waning concerns about calcium and magnesium abrogating oxaliplatin's efficacy most clinicians are restricting calcium and magnesium to patients receiving oxaliplatin in the palliative setting. Glutamine 15 grams b.i.d. 7 days on/7 days off has also been shown to minimize oxaliplatin associated chronic neuropathy [137-140] .
Irinotecan
Irinotecan gained initial regulatory approval in the United States as a single agent following metastatic disease progression on 5-FU based treatment. Using irinotecan as a single agent, as second line therapy, remains a reasonable option for patients whose disease has progressed on 5FU or 5FU-oxaliplatin [141]. A meta-analysis of three phase III trials as well as one phase II trial [142] evaluating the efficacy and toxicity of 5FU plus irinotecan in the elderly (≥70 years) noted no significant difference in progression free survival, overall survival or response rate when comparing the older and younger groups [143]. Irinotecan associated diarrhea should be aggressively managed in the elderly as dehydration and electrolyte imbalances in the setting of competing comorbitities can lead to severe consequences. Loperamide 4 mg at time of first loose stool continued every 4 hours until normalization of stools is standard. Lomotil can be added if diarrhea is refractory to loperamide. Clinicians should have a low threshold to consider hospitalization and iv hydration in elderly persons with significant diarrhea not responsive to outpatient therapy.
Targeted Agents
Bevacizumab is a monoclonal antibody with putative antitumor activity via neutralization of VEGF. VEGF controls angiogenesis and therefore tumor growth and has been approved in both the first and second line setting in combinations with 5-FU/L, oxaliplatin and irinotecan. FOLFIRI and bevacizumab has been associated with median overall survival of 28.0 months and full Fox + bevacizumab has demonstrated median overall survival's of 26 months [144, 145]. Bevacizumab with 5-FU alone has also been shown to prolong survival in two large phase II studies and is considered a valid regimen in patients who would be felt to not tolerate 5-FU combination therapy.
Bevacizumab side effects include hypertension, gastrointestinal perforation, thromboembolic events and bleeding, because of these toxicities several authors have attempted to define the safety of bevacizumab in an elderly population. Kozloff et al reviewed the 896 patients that were greater than 65 years [65–74 (533), ≥75 (363) and 161 were ≥80] enrolled in a registrational analysis, the BRiTE study [146]. While similar efficacy and safety was seen in patients over 65 when compared to their younger cohorts, further stratification within the 65 and older group did show decreasing overall survival with increasing age. Notably progression-free survival was similar among all over 65 year old cohorts. Patients greater than 75 had a two-fold increase risk of arterial thromboembolism. This paper concluded that age alone should not restrict bevacizumab use [147]. A review of patients over 65 receiving bevacizumab at a single institution confirmed the increase in arterial thrombosis in the elderly population, especially those over 75, as well as increasing incidence of hypertension [148].
Anti-EGFR Monoclonal Antibodies (moabs): Cetuximab and Panitumumab
Cetuximab is a mouse/human chimeric monoclonal antibody and panitumimab is a human monoclonal antibody. Both drugs bind to the EGFR of tumor and normal cells, competitively inhibiting ligand binding and inducing receptor dimerization and internalization. Both drugs are effective against only non mutated (wild type) KRAS tumors. Mutated KRAS tumors have constitutively activated intracellular signal transduction pathways independent of extracellular signaling. Therefore, evaluation of tumor KRAS status is now part of standard care for metastatic colorectal cancer.
The initial studies of cetuximab, prior to the validation of KRAS as a selection tool, demonstrated the benefit of this drug in chemotherapy refractory patients compared with supportive care alone [149, 150]. Cetuximab has at least additive benefit when combined with irinotecan, even in patients with irinotecan, 5FU and oxaliplatin resistant tumors [151]. While cetuximab has clinical benefit in patients with chemotherapy refractory disease, trials have failed to demonstrate its benefit in the first line setting [152].
Retrospective review of 56 elderly patients (median age 76) receiving cetuximab ± irinotecan, most with chemotherapy resistant disease reported an objective response rate of 21%, median progression-free survival of 4.4 months and a median overall survival was 16.0 months. Skin rash occurred in 75% of the patients (11% grade 3) and diarrhea in 80% (20% grades 3–4). These outcomes are akin to that established in younger groups [153]. Panitumimab has a toxicity and efficacy profile similar to cetuximab. While it has been less extensively evaluated, its perceived advantage is lack of infusion related hypersensitivity reactions and an every other week dosing schedule [154, 155].
The primary toxicities of cetuximab and panitumimab include acneiform rash, magnesium and potassium wasting, infusion hypersensitivity reactions (cetuximab) and weakness/malaise. Infusion reactions occur in 3-25% of patients and are thought to be partially related to the murine component of cetuximab. Therefore pretreatment with an antihistamine and glucocorticoid is given to all cetuximab patients [156]. The anti-EGFR moab associated rash can be mitigated by concurrent use of minocycline at treatment initiation [157]. Magnesium, potassium and calcium levels should be checked weekly in patients receiving cetuximab and repleted aggressively as needed. Special attention should be paid to this in the elderly who likely have a higher rate of cardiovascular comorbidity that could be severely compromised by electrolyte imbalance.
Summary
Colorectal cancer in the elderly is common, shows differing clinical and biologic features and can be detected and prevented using several screening strategies. Because progression of adenomas to cancer takes a decade, invasive screening colonoscopy has risks and comorbidities reduce life span in the elderly, screening should be modified after age 75 and avoided after 85. However, nutritional measures may have a place in prevention.
Treatment algorithms for all stages of colorectal cancer, established by evidence largely from clinical trial subjects younger than 75 years old, appears feasible in patients greater than 75 years old. Ongoing recruitment of elderly patients into clinical trials and clinical databases will help inform future treatment strategies. Increasingly, medical oncology will be moving towards individualized care which will factor not only patients' age, comorbidities and histopathologic diagnosis but also molecular and genetic, also known as non-anatomic tumor features.
Practice Points.
Colon cancer in the elderly is more often right-sided, mucinous, grows faster and differs in gene changes from those in the young
Colorectal screening reduces cancer incidence and mortality
Colorectal screening should be modified according to age, gender and race
Prevention includes calcium and vitamin D supplements
5-fluorouracil, capecitabine, irinotecan, oxaliplatin, bevacizumab and panitumumab/cetuximab have demonstrated clinical benefit and regulatory approval for the treatment of metastatic colorectal cancer.
5-fluorouracil or capecitabine plus oxaliplatin improved survival outcomes as adjuvant therapy for resected high risk stage II and stage III colon cancer.
All medically fit patients with metastatic colorectal cancer but only a single site of metastatic spread (particularly liver only) should undergo multidisciplinary evaluation for curative intent metastectomy.
Research Agenda.
The population wide efficacy of CT colonoscopy for screening needs to be defined
The relative importance of calcium and vitamin D in primary prevention needs clarification
Acknowledgments
Supported in part by NCI grant U54CA100926; UL1RR024143 from the NCRR and the NIH Roadmap for Medical Research.
Footnotes
Conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Peter R. Holt, Email: holtp@rockefeller.edu, The Rockefeller University, 1230 York Avenue, Box 179, New York, New York 10065, US, Tel: 212-327-7706, Fax: 212-327-7165.
Peter Kozuch, Email: pkozuch@chpnet.org, Beth Israel Medical Center, 10 Union Square East, Suite 4C, New York, New York 10003, Tel: 212-844-8070, Fax: 212-844-2027.
Seetal Mewar, Email: smewar@chpnet.org, Beth Israel Medical Center, 10 Union Square East, Suite 4C, New York, New York 10003, Tel : 212-844-8288, Fax: 212-844-8297.
References
- 1.Parkin DM, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Calvert PM, Frucht H. The genetics of colorectal cancer. Ann Intern Med. 2002;137(7):603–12. doi: 10.7326/0003-4819-137-7-200210010-00012. [DOI] [PubMed] [Google Scholar]
- 3.Rim SH, et al. Colorectal cancer incidence in the United States, 1999-2004 : an updated analysis of data from the National Program of Cancer Registries and the Surveillance, Epidemiology, and End Results Program. Cancer. 2009 doi: 10.1002/cncr.24216. [DOI] [PubMed] [Google Scholar]
- 4.Clark JC, et al. Prevalence of polyps in an autopsy series from areas with varying incidence of large-bowel cancer. Int J Cancer. 1985;36(2):179–86. doi: 10.1002/ijc.2910360209. [DOI] [PubMed] [Google Scholar]
- 5.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part II: lower gastrointestinal diseases. Gastroenterology. 2009;136(3):741–54. doi: 10.1053/j.gastro.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Kronborg O, Fenger C. Clinical evidence for the adenoma-carcinoma sequence. Eur J Cancer Prev. 1999;8(1):S73–86. [PubMed] [Google Scholar]
- 7.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 8.Fodde R, et al. Mutations in the APC tumour suppressor gene cause chromosomal instability. Nat Cell Biol. 2001;3(4):433–8. doi: 10.1038/35070129. [DOI] [PubMed] [Google Scholar]
- 9.Samowitz WS, et al. Evaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology. 2005;129(3):837–45. doi: 10.1053/j.gastro.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 10.Glebov OK, et al. Distinguishing right from left colon by the pattern of gene expression. Cancer Epidemiol Biomarkers Prev. 2003;12(8):755–62. [PubMed] [Google Scholar]
- 11.Wynder EL, Shigematsu T. Environmental factors of cancer of the colon and rectum. Cancer. 1967;20(9):1520–61. doi: 10.1002/1097-0142(196709)20:9<1520::aid-cncr2820200920>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Barault L, et al. Hypermethylator phenotype in sporadic colon cancer: study on a population-based series of 582 cases. Cancer Res. 2008;68(20):8541–6. doi: 10.1158/0008-5472.CAN-08-1171. [DOI] [PubMed] [Google Scholar]
- 13.Ono T, et al. Mutation theory of aging, assessed in transgenic mice and knockout mice. Mech Ageing Dev. 2002;123(12):1543–52. doi: 10.1016/s0047-6374(02)00090-8. [DOI] [PubMed] [Google Scholar]
- 14.Taylor RW, et al. Mitochondrial DNA mutations in human colonic crypt stem cells. J Clin Invest. 2003;112(9):1351–60. doi: 10.1172/JCI19435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ono T, et al. Spontaneous mutations in digestive tract of old mice show tissue-specific patterns of genomic instability. Cancer Res. 2004;64(19):6919–23. doi: 10.1158/0008-5472.CAN-04-1476. [DOI] [PubMed] [Google Scholar]
- 16.Dolle ME, et al. Distinct spectra of somatic mutations accumulated with age in mouse heart and small intestine. Proc Natl Acad Sci U S A. 2000;97(15):8403–8. doi: 10.1073/pnas.97.15.8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holt PR, Yeh KY. Colonic proliferation is increased in senescent rats. Gastroenterology. 1988;95(6):1556–63. doi: 10.1016/s0016-5085(88)80077-5. [DOI] [PubMed] [Google Scholar]
- 18.Roncucci L, et al. The influence of age on colonic epithelial cell proliferation. Cancer. 1988;62(11):2373–7. doi: 10.1002/1097-0142(19881201)62:11<2373::aid-cncr2820621120>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 19.Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev. 2005;126(9):987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Harding C, et al. Cancer suppression at old age. Cancer Res. 2008;68(11):4465–78. doi: 10.1158/0008-5472.CAN-07-1670. [DOI] [PubMed] [Google Scholar]
- 21.Surveillance, E., End Results (SEER) Program (internet) Cancer Statistics Branch c2006- [Database], Incidence - SEER 17 Regs Limited-Use, Attributes - Total US, 1969-2003. Bethesda (MD): National Cancer Institute, DCCPS, Surveillance Research Program; Apr, 2006. based on November 2005 submission [cited 2007 Jan]. Available from: http://seer.cancer.gov/seerstat/ by downloading SEER*Stat software. Version 6.2.4. [Google Scholar]
- 22.Pompei F, Lee EE, W R. Cancer turnover at old age. Nat Rev Cancer (internet) 2004 [Google Scholar]
- 23.Bjelakovic G, et al. Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet. 2004;364(9441):1219–28. doi: 10.1016/S0140-6736(04)17138-9. [DOI] [PubMed] [Google Scholar]
- 24.Campisi J. Suppressing cancer: the importance of being senescent. Science. 2005;309(5736):886–7. doi: 10.1126/science.1116801. [DOI] [PubMed] [Google Scholar]
- 25.Johnson JR, et al. Menopausal hormone therapy and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2009;18(1):196–203. doi: 10.1158/1055-9965.EPI-08-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chlebowski RT, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004;350(10):991–1004. doi: 10.1056/NEJMoa032071. [DOI] [PubMed] [Google Scholar]
- 27.Issa JP. CpG-island methylation in aging and cancer. Curr Top Microbiol Immunol. 2000;249:101–18. doi: 10.1007/978-3-642-59696-4_7. [DOI] [PubMed] [Google Scholar]
- 28.Weisenberger DJ, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38(7):787–93. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 29.Grady WM. Genomic instability and colon cancer. Cancer Metastasis Rev. 2004;23(1-2):11–27. doi: 10.1023/a:1025861527711. [DOI] [PubMed] [Google Scholar]
- 30.Ogino S, et al. Loss of nuclear p27 (CDKN1B/KIP1) in colorectal cancer is correlated with microsatellite instability and CIMP. Mod Pathol. 2007;20(1):15–22. doi: 10.1038/modpathol.3800709. [DOI] [PubMed] [Google Scholar]
- 31.Ogino S, et al. Cytoplasmic localization of p27 (cyclin-dependent kinase inhibitor 1B/KIP1) in colorectal cancer: inverse correlations with nuclear p27 loss, microsatellite instability, and CpG island methylator phenotype. Hum Pathol. 2007;38(4):585–92. doi: 10.1016/j.humpath.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller AD, Sonnenberg A. Protection by endoscopy against death from colorectal cancer. A case-control study among veterans. Arch Intern Med. 1995;155(16):1741–8. doi: 10.1001/archinte.1995.00430160065007. [DOI] [PubMed] [Google Scholar]
- 33.Newcomb PA, et al. Screening sigmoidoscopy and colorectal cancer mortality. J Natl Cancer Inst. 1992;84(20):1572–5. doi: 10.1093/jnci/84.20.1572. [DOI] [PubMed] [Google Scholar]
- 34.Gupta AK, et al. Changing trends in the incidence, stage, survival, and screen-detection of colorectal cancer: a population-based study. Clin Gastroenterol Hepatol. 2005;3(2):150–8. doi: 10.1016/s1542-3565(04)00664-0. [DOI] [PubMed] [Google Scholar]
- 35.Strul H, et al. The prevalence rate and anatomic location of colorectal adenoma and cancer detected by colonoscopy in average-risk individuals aged 40-80 years. Am J Gastroenterol. 2006;101(2):255–62. doi: 10.1111/j.1572-0241.2006.00430.x. [DOI] [PubMed] [Google Scholar]
- 36.Brenner H, et al. Risk of progression of advanced adenomas to colorectal cancer by age and sex: estimates based on 840,149 screening colonoscopies. Gut. 2007;56(11):1585–9. doi: 10.1136/gut.2007.122739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaji Y, et al. The malignant potential of freshly developed colorectal polyps according to age. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2418–21. doi: 10.1158/1055-9965.EPI-06-0136. [DOI] [PubMed] [Google Scholar]
- 38.Lin OS, et al. Screening colonoscopy in very elderly patients: prevalence of neoplasia and estimated impact on life expectancy. JAMA. 2006;295(20):2357–65. doi: 10.1001/jama.295.20.2357. [DOI] [PubMed] [Google Scholar]
- 39.Ko CW, Sonnenberg A. Comparing risks and benefits of colorectal cancer screening in elderly patients. Gastroenterology. 2005;129(4):1163–70. doi: 10.1053/j.gastro.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 40.Macafee DA, et al. Population screening for colorectal cancer: the implications of an ageing population. Br J Cancer. 2008;99(12):1991–2000. doi: 10.1038/sj.bjc.6604788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 42.Mandel JS, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328(19):1365–71. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 43.Hardcastle JD, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348(9040):1472–7. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 44.van Rossum LG, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology. 2008;135(1):82–90. doi: 10.1053/j.gastro.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 45.Hol L, et al. Screening For Colorectal Cancer; Randomised Trial Comparing Guaiac-Based And Immunochemical Faecal Occult Blood Testing And Flexible Sigmoidoscopy. Gut. 2009 doi: 10.1136/gut.2009.177089. [DOI] [PubMed] [Google Scholar]
- 46.Allison JE, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. J Natl Cancer Inst. 2007;99(19):1462–70. doi: 10.1093/jnci/djm150. [DOI] [PubMed] [Google Scholar]
- 47.Lee KJ, et al. Colorectal cancer screening using fecal occult blood test and subsequent risk of colorectal cancer: a prospective cohort study in Japan. Cancer Detect Prev. 2007;31(1):3–11. doi: 10.1016/j.cdp.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Parekh M, Fendrick AM, Ladabaum U. As tests evolve and costs of cancer care rise: reappraising stool-based screening for colorectal neoplasia. Aliment Pharmacol Ther. 2008;27(8):697–712. doi: 10.1111/j.1365-2036.2008.03632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Itzkowitz SH, et al. Improved fecal DNA test for colorectal cancer screening. Clin Gastroenterol Hepatol. 2007;5(1):111–7. doi: 10.1016/j.cgh.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Selby JV, et al. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326(10):653–7. doi: 10.1056/NEJM199203053261001. [DOI] [PubMed] [Google Scholar]
- 51.Pabby A, et al. Flexible sigmoidoscopy for colorectal cancer screening in the elderly. Dig Dis Sci. 2005;50(11):2147–52. doi: 10.1007/s10620-005-3022-x. [DOI] [PubMed] [Google Scholar]
- 52.Winawer SJ, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329(27):1977–81. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 53.Baxter NN, et al. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150(1):1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 54.Schoen RE, et al. Yield of advanced adenoma and cancer based on polyp size detected at screening flexible sigmoidoscopy. Gastroenterology. 2006;131(6):1683–9. doi: 10.1053/j.gastro.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 55.Robertson DJ, et al. Colorectal cancer in patients under close colonoscopic surveillance. Gastroenterology. 2005;129(1):34–41. doi: 10.1053/j.gastro.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 56.Rex DK, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2008. Am J Gastroenterol. 2009;104(3):739–50. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 57.Johnson CD, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359(12):1207–17. doi: 10.1056/NEJMoa0800996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soetikno RM, et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA. 2008;299(9):1027–35. doi: 10.1001/jama.299.9.1027. [DOI] [PubMed] [Google Scholar]
- 59.Graser A, et al. Comparison of CT colonography, colonoscopy, sigmoidoscopy and faecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut. 2009;58(2):241–8. doi: 10.1136/gut.2008.156448. [DOI] [PubMed] [Google Scholar]
- 60.Rembacken BJ, et al. Flat and depressed colonic neoplasms: a prospective study of 1000 colonoscopies in the UK. Lancet. 2000;355(9211):1211–4. doi: 10.1016/s0140-6736(00)02086-9. [DOI] [PubMed] [Google Scholar]
- 61.Kudo S, et al. Colonoscopic diagnosis and management of nonpolypoid early colorectal cancer. World J Surg. 2000;24(9):1081–90. doi: 10.1007/s002680010154. [DOI] [PubMed] [Google Scholar]
- 62.Hurlstone DP, et al. Efficacy of high magnification chromoscopic colonoscopy for the diagnosis of neoplasia in flat and depressed lesions of the colorectum: a prospective analysis. Gut. 2004;53(2):284–90. doi: 10.1136/gut.2003.027623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Uronis JM, et al. Flat colorectal cancers are genetically determined and progress to invasion without going through a polypoid stage. Cancer Res. 2007;67(24):11594–600. doi: 10.1158/0008-5472.CAN-07-3242. [DOI] [PubMed] [Google Scholar]
- 64.Brenner H, Hoffmeister M, Haug U. Should colorectal cancer screening start at the same age in European countries? Contributions from descriptive epidemiology. Br J Cancer. 2008;99(3):532–5. doi: 10.1038/sj.bjc.6604488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fajobi O, et al. Metachronous colorectal cancers. Br J Surg. 1998;85(7):897–901. doi: 10.1046/j.1365-2168.1998.00800.x. [DOI] [PubMed] [Google Scholar]
- 66.Shureiqi I, et al. Effect of age on risk of second primary colorectal cancer. J Natl Cancer Inst. 2001;93(16):1264–6. doi: 10.1093/jnci/93.16.1264. [DOI] [PubMed] [Google Scholar]
- 67.Wolin KY, et al. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer. 2009;100(4):611–6. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ghadirian P, et al. Nutritional factors and colon carcinoma: a case-control study involving French Canadians in Montreal, Quebec, Canada. Cancer. 1997;80(5):858–64. doi: 10.1002/(sici)1097-0142(19970901)80:5<858::aid-cncr5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 69.Flood A, et al. Dietary patterns as identified by factor analysis and colorectal cancer among middle-aged Americans. Am J Clin Nutr. 2008;88(1):176–84. doi: 10.1093/ajcn/88.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lanza E, et al. The polyp prevention trial continued follow-up study: no effect of a low-fat, high-fiber, high-fruit, and -vegetable diet on adenoma recurrence eight years after randomization. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1745–52. doi: 10.1158/1055-9965.EPI-07-0127. [DOI] [PubMed] [Google Scholar]
- 71.Meyerhardt JA, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007;298(7):754–64. doi: 10.1001/jama.298.7.754. [DOI] [PubMed] [Google Scholar]
- 72.Shaukat A, Scouras N, Schunemann HJ. Role of supplemental calcium in the recurrence of colorectal adenomas: a metaanalysis of randomized controlled trials. Am J Gastroenterol. 2005;100(2):390–4. doi: 10.1111/j.1572-0241.2005.41220.x. [DOI] [PubMed] [Google Scholar]
- 73.Baron JA, et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med. 1999;340(2):101–7. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- 74.Grau MV, et al. Prolonged effect of calcium supplementation on risk of colorectal adenomas in a randomized trial. J Natl Cancer Inst. 2007;99(2):129–36. doi: 10.1093/jnci/djk016. [DOI] [PubMed] [Google Scholar]
- 75.Grau MV, et al. Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. J Natl Cancer Inst. 2003;95(23):1765–71. doi: 10.1093/jnci/djg110. [DOI] [PubMed] [Google Scholar]
- 76.Holt PR. New insights into calcium, dairy and colon cancer. World J Gastroenterol. 2008;14(28):4429–33. doi: 10.3748/wjg.14.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim YI. Folate and carcinogenesis: evidence, mechanisms, and implications. J Nutr Biochem. 1999;10(2):66–88. doi: 10.1016/s0955-2863(98)00074-6. [DOI] [PubMed] [Google Scholar]
- 78.Choi SW, Mason JB. Folate status: effects on pathways of colorectal carcinogenesis. J Nutr. 2002;132(8 Suppl):2413S–2418S. doi: 10.1093/jn/132.8.2413S. [DOI] [PubMed] [Google Scholar]
- 79.Cole BF, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297(21):2351–9. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 80.Ulrich CM, Potter JD. Folate supplementation: too much of a good thing? Cancer Epidemiol Biomarkers Prev. 2006;15(2):189–93. doi: 10.1158/1055-9965.EPI-152CO. [DOI] [PubMed] [Google Scholar]
- 81.Baron JA. Epidemiology of non-steroidal anti-inflammatory drugs and cancer. Prog Exp Tumor Res. 2003;37:1–24. doi: 10.1159/000071364. [DOI] [PubMed] [Google Scholar]
- 82.Asano TK, McLeod RS. Nonsteroidal anti-inflammatory drugs and aspirin for the prevention of colorectal adenomas and cancer: a systematic review. Dis Colon Rectum. 2004;47(5):665–73. doi: 10.1007/s10350-003-0111-9. [DOI] [PubMed] [Google Scholar]
- 83.Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348(10):891–9. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 84.Logan RF, et al. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology. 2008;134(1):29–38. doi: 10.1053/j.gastro.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 85.Grau MV, et al. Nonsteroidal anti-inflammatory drug use after 3 years of aspirin use and colorectal adenoma risk: observational follow-up of a randomized study. J Natl Cancer Inst. 2009;101(4):267–76. doi: 10.1093/jnci/djn484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cole BF, et al. Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J Natl Cancer Inst. 2009;101(4):256–66. doi: 10.1093/jnci/djn485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Imperiale TF. Aspirin and the prevention of colorectal cancer. N Engl J Med. 2003;348(10):879–80. doi: 10.1056/NEJMp030005. [DOI] [PubMed] [Google Scholar]
- 88.Routine aspirin or nonsteroidal anti-inflammatory drugs for the primary prevention of colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2007;146(5):361–4. [PubMed] [Google Scholar]
- 89.Bertagnolli MM, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355(9):873–84. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 90.Arber N, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355(9):885–95. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 91.Solomon SD, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352(11):1071–80. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 92.Steinbach G, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342(26):1946–52. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 93.Rigas B, Kashfi K. Nitric-oxide-donating NSAIDs as agents for cancer prevention. Trends Mol Med. 2004;10(7):324–30. doi: 10.1016/j.molmed.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 94.Rigas B, Kashfi K. Cancer prevention: a new era beyond cyclooxygenase-2. J Pharmacol Exp Ther. 2005;314(1):1–8. doi: 10.1124/jpet.104.080564. [DOI] [PubMed] [Google Scholar]
- 95.Williams JL, et al. NO-donating aspirin inhibits intestinal carcinogenesis in Min (APC(Min/+)) mice. Biochem Biophys Res Commun. 2004;313(3):784–8. doi: 10.1016/j.bbrc.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 96.Fiorucci S, et al. Gastrointestinal safety of NO-aspirin (NCX-4016) in healthy human volunteers: a proof of concept endoscopic study. Gastroenterology. 2003;124(3):600–7. doi: 10.1053/gast.2003.50096. [DOI] [PubMed] [Google Scholar]
- 97.Meyskens FL, Jr, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila Pa) 2008;1(1):32–8. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Damhuis RA, Wereldsma JC, Wiggers T. The influence of age on resection rates and postoperative mortality in 6457 patients with colorectal cancer. Int J Colorectal Dis. 1996;11(1):45–8. doi: 10.1007/BF00418856. [DOI] [PubMed] [Google Scholar]
- 99.Mitry E, et al. Improvement in colorectal cancer survival: a population-based study. Eur J Cancer. 2005;41(15):2297–303. doi: 10.1016/j.ejca.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 100.Heriot AG, et al. Prediction of postoperative mortality in elderly patients with colorectal cancer. Dis Colon Rectum. 2006;49(6):816–24. doi: 10.1007/s10350-006-0523-4. [DOI] [PubMed] [Google Scholar]
- 101.Surgery for colorectal cancer in elderly patients: a systematic review. Colorectal Cancer Collaborative Group. Lancet. 2000;356(9234):968, 74. [PubMed] [Google Scholar]
- 102.McMillan DC, Hole DJ, McArdle CS. The impact of old age on cancer-specific and non-cancer-related survival following elective potentially curative surgery for Dukes A/B colorectal cancer. Br J Cancer. 2008;99(7):1046–9. doi: 10.1038/sj.bjc.6604669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kiran RP, Pokala N, Dudrick SJ. Long-term outcome after operative intervention for rectal cancer in patients aged over 80 years: analysis of 9,501 patients. Dis Colon Rectum. 2007;50(5):604–10. doi: 10.1007/s10350-006-0802-0. [DOI] [PubMed] [Google Scholar]
- 104.Papamichael D, et al. Treatment of the elderly colorectal cancer patient: SIOG expert recommendations. Ann Oncol. 2009;20(1):5–16. doi: 10.1093/annonc/mdn532. [DOI] [PubMed] [Google Scholar]
- 105.Haller DG, et al. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: final report of Intergroup 0089. J Clin Oncol. 2005;23(34):8671–8. doi: 10.1200/JCO.2004.00.5686. [DOI] [PubMed] [Google Scholar]
- 106.de Gramont A, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18(16):2938–47. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 107.Andre T, et al. Improved Overall Survival With Oxaliplatin, Fluorouracil, and Leucovorin As Adjuvant Treatment in Stage II or III Colon Cancer in the MOSAIC Trial. J Clin Oncol. 2009 doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 108.Twelves C, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352(26):2696–704. doi: 10.1056/NEJMoa043116. [DOI] [PubMed] [Google Scholar]
- 109.Cassidy J, et al. Capecitabine (X) vs bolus 5-FU/leucovorin (LV) as adjuvant therapy for colon cancer (the X-ACT study): positive efficacy results of a phase III trial. Journal of Clinical Oncology. 2004;22(14S):3509. [Google Scholar]
- 110.Fata F, et al. Efficacy and toxicity of adjuvant chemotherapy in elderly patients with colon carcinoma: a 10-year experience of the Geisinger Medical Center. Cancer. 2002;94(7):1931–8. doi: 10.1002/cncr.10430. [DOI] [PubMed] [Google Scholar]
- 111.Sargent DJ, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345(15):1091–7. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 112.Fernandez FG, et al. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET) Ann Surg. 2004;240(3):438–47. doi: 10.1097/01.sla.0000138076.72547.b1. discussion 447-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Abdalla EK, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239(6):818–25. doi: 10.1097/01.sla.0000128305.90650.71. discussion 825-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pawlik TM, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241(5):715–22. doi: 10.1097/01.sla.0000160703.75808.7d. discussion 722-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Choti MA, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235(6):759–66. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mann CD, et al. Major resection of hepatic colorectal liver metastases in elderly patients - an aggressive approach is justified. Eur J Surg Oncol. 2008;34(4):428–32. doi: 10.1016/j.ejso.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 117.Mazzoni G, et al. Surgical treatment of liver metastases from colorectal cancer in elderly patients. Int J Colorectal Dis. 2007;22(1):77–83. doi: 10.1007/s00384-006-0096-7. [DOI] [PubMed] [Google Scholar]
- 118.Nordlinger B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371(9617):1007–16. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tamandl D, et al. Surgery after neoadjuvant chemotherapy for colorectal liver metastases is safe and feasible in elderly patients. J Surg Oncol. 2009 doi: 10.1002/jso.21259. [DOI] [PubMed] [Google Scholar]
- 120.Poon MA, et al. Biochemical modulation of fluorouracil: evidence of significant improvement of survival and quality of life in patients with advanced colorectal carcinoma. J Clin Oncol. 1989;7(10):1407–18. doi: 10.1200/JCO.1989.7.10.1407. [DOI] [PubMed] [Google Scholar]
- 121.Petrelli N, et al. A prospective randomized trial of 5-fluorouracil versus 5-fluorouracil and high-dose leucovorin versus 5-fluorouracil and methotrexate in previously untreated patients with advanced colorectal carcinoma. J Clin Oncol. 1987;5(10):1559–65. doi: 10.1200/JCO.1987.5.10.1559. [DOI] [PubMed] [Google Scholar]
- 122.Magne N, et al. Palliative 5-fluorouracil-based chemotherapy for advanced colorectal cancer in the elderly: results of a 10-year experience. Am J Clin Oncol. 2002;25(2):126–30. doi: 10.1097/00000421-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 123.Van Cutsem E, et al. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol. 2001;19(21):4097–106. doi: 10.1200/JCO.2001.19.21.4097. [DOI] [PubMed] [Google Scholar]
- 124.Hoff PM, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol. 2001;19(8):2282–92. doi: 10.1200/JCO.2001.19.8.2282. [DOI] [PubMed] [Google Scholar]
- 125.Saif MW, Katirtzoglou NA, Syrigos KN. Capecitabine: an overview of the side effects and their management. Anticancer Drugs. 2008;19(5):447–64. doi: 10.1097/CAD.0b013e3282f945aa. [DOI] [PubMed] [Google Scholar]
- 126.Giacchetti S, et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18(1):136–47. doi: 10.1200/JCO.2000.18.1.136. [DOI] [PubMed] [Google Scholar]
- 127.Andre T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350(23):2343–51. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 128.Colucci G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell'Italia Meridionale. J Clin Oncol. 2005;23(22):4866–75. doi: 10.1200/JCO.2005.07.113. [DOI] [PubMed] [Google Scholar]
- 129.Tournigand C, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22(2):229–37. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 130.Porschen R, et al. Phase III study of capecitabine plus oxaliplatin compared with fluorouracil and leucovorin plus oxaliplatin in metastatic colorectal cancer: a final report of the AIO Colorectal Study Group. J Clin Oncol. 2007;25(27):4217–23. doi: 10.1200/JCO.2006.09.2684. [DOI] [PubMed] [Google Scholar]
- 131.Diaz-Rubio E, et al. Phase III study of capecitabine plus oxaliplatin compared with continuous-infusion fluorouracil plus oxaliplatin as first-line therapy in metastatic colorectal cancer: final report of the Spanish Cooperative Group for the Treatment of Digestive Tumors Trial. J Clin Oncol. 2007;25(27):4224–30. doi: 10.1200/JCO.2006.09.8467. [DOI] [PubMed] [Google Scholar]
- 132.Cassidy J, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26(12):2006–12. doi: 10.1200/JCO.2007.14.9898. [DOI] [PubMed] [Google Scholar]
- 133.Goldberg RM, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol. 2006;24(25):4085–91. doi: 10.1200/JCO.2006.06.9039. [DOI] [PubMed] [Google Scholar]
- 134.Feliu J, et al. XELOX (capecitabine plus oxaliplatin) as first-line treatment for elderly patients over 70 years of age with advanced colorectal cancer. Br J Cancer. 2006;94(7):969–75. doi: 10.1038/sj.bjc.6603047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sastre J, et al. Elderly patients with advanced colorectal cancer derive similar benefit without excessive toxicity after first-line chemotherapy with oxaliplatin-based combinations: comparative outcomes from the 03-TTD-01 phase III study. Crit Rev Oncol Hematol. 2009;70(2):134–44. doi: 10.1016/j.critrevonc.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 136.Souglakos J, et al. Triplet combination with irinotecan plus oxaliplatin plus continuous-infusion fluorouracil and leucovorin as first-line treatment in metastatic colorectal cancer: a multicenter phase II trial. J Clin Oncol. 2002;20(11):2651–7. doi: 10.1200/JCO.2002.08.015. [DOI] [PubMed] [Google Scholar]
- 137.Wang WS, et al. Oral glutamine is effective for preventing oxaliplatin-induced neuropathy in colorectal cancer patients. Oncologist. 2007;12(3):312–9. doi: 10.1634/theoncologist.12-3-312. [DOI] [PubMed] [Google Scholar]
- 138.Cascinu S, et al. Neuroprotective effect of reduced glutathione on oxaliplatin-based chemotherapy in advanced colorectal cancer: a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2002;20(16):3478–83. doi: 10.1200/JCO.2002.07.061. [DOI] [PubMed] [Google Scholar]
- 139.Cersosimo RJ. Oxaliplatin-associated neuropathy: a review. Ann Pharmacother. 2005;39(1):128–35. doi: 10.1345/aph.1E319. [DOI] [PubMed] [Google Scholar]
- 140.Mariani G, et al. Oxaliplatin Induced Neuropathy: Could Gabapentin be the Answer?. Proc Am Soc Clin Oncol; ASCO Annual Meeting; 2000; 2000. abstr 2397. [Google Scholar]
- 141.Cunningham D, et al. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet. 1998;352(9138):1413–8. doi: 10.1016/S0140-6736(98)02309-5. [DOI] [PubMed] [Google Scholar]
- 142.Sastre J, et al. Irinotecan in combination with fluorouracil in a 48-hour continuous infusion as first-line chemotherapy for elderly patients with metastatic colorectal cancer: a Spanish Cooperative Group for the Treatment of Digestive Tumors study. J Clin Oncol. 2005;23(15):3545–51. doi: 10.1200/JCO.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 143.Folprecht G, et al. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: combined analysis of 2,691 patients in randomized controlled trials. J Clin Oncol. 2008;26(9):1443–51. doi: 10.1200/JCO.2007.14.0509. [DOI] [PubMed] [Google Scholar]
- 144.Hochster HS, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol. 2008;26(21):3523–9. doi: 10.1200/JCO.2007.15.4138. [DOI] [PubMed] [Google Scholar]
- 145.Fuchs CS, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol. 2007;25(30):4779–86. doi: 10.1200/JCO.2007.11.3357. [DOI] [PubMed] [Google Scholar]
- 146.Grothey A, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE) J Clin Oncol. 2008;26(33):5326–34. doi: 10.1200/JCO.2008.16.3212. [DOI] [PubMed] [Google Scholar]
- 147.Kozloff MF, et al. Safety and effectiveness of bevacizumab (BV) and chemotherapy (CT) in elderly patients (pts) with metastatic colorectal cancer (mCRC): Results from the BRiTE observational cohort study. J Clin Oncol. 2008;26 Meeting Abstracts. [Google Scholar]
- 148.Raman AK, et al. Bevacizumab (BV) related adverse events among various age groups of elderly patients with advanced colorectal cancer. Journal of Clinical Oncology; ASCO Annual Meeting Proceedings Part I; 2007; [Google Scholar]
- 149.Saltz LB, et al. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22(7):1201–8. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 150.Jonker DJ, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357(20):2040–8. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 151.Sobrero AF, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(14):2311–9. doi: 10.1200/JCO.2007.13.1193. [DOI] [PubMed] [Google Scholar]
- 152.Van Cutsem E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–17. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 153.Bouchahda M, et al. Cetuximab efficacy and safety in a retrospective cohort of elderly patients with heavily pretreated metastatic colorectal cancer. Crit Rev Oncol Hematol. 2008;67(3):255–62. doi: 10.1016/j.critrevonc.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 154.Van Cutsem E, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25(13):1658–64. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 155.Saif MW, Peccerillo J, Potter V. Successful re-challenge with panitumumab in patients who developed hypersensitivity reactions to cetuximab: report of three cases and review of literature. Cancer Chemother Pharmacol. 2009;63(6):1017–22. doi: 10.1007/s00280-008-0831-6. [DOI] [PubMed] [Google Scholar]
- 156.O'Neil BH, et al. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol. 2007;25(24):3644–8. doi: 10.1200/JCO.2007.11.7812. [DOI] [PubMed] [Google Scholar]
- 157.Scope A, et al. Randomized double-blind trial of prophylactic oral minocycline and topical tazarotene for cetuximab-associated acne-like eruption. J Clin Oncol. 2007;25(34):5390–6. doi: 10.1200/JCO.2007.12.6987. [DOI] [PubMed] [Google Scholar]


