Abstract
Enhancement of hematopoietic recovery after radiation, chemotherapy, or hematopoietic stem cell (HSC) transplantation is clinically relevant. Dipeptidylpeptidase (DPP4) cleaves a wide variety of substrates, including the chemokine stromal cell-derived factor-1 (SDF-1). In the course of experiments showing that inhibition of DPP4 enhances SDF-1–mediated progenitor cell survival, ex vivo cytokine expansion and replating frequency, we unexpectedly found that DPP4 has a more general role in regulating colony-stimulating factor (CSF) activity. DPP4 cleaved within the N-termini of the CSFs granulocyte-macrophage (GM)-CSF, G-CSF, interleukin-3 (IL-3) and erythropoietin and decreased their activity. Dpp4 knockout or DPP4 inhibition enhanced CSF activities both in vitro and in vivo. The reduced activity of DPP4-truncated versus full-length human GM-CSF was mechanistically linked to effects on receptor-binding affinity, induction of GM-CSF receptor oligomerization and signaling capacity. Hematopoiesis in mice after radiation or chemotherapy was enhanced in Dpp4−/− mice or mice receiving an orally active DPP4 inhibitor. DPP4 inhibition enhanced engraftment in mice without compromising HSC function, suggesting the potential clinical utility of this approach.
HSC and hematopoietic progenitor cell (HPC) transplantation saves lives. Cord blood is an accepted source of transplantable HSCs1; however, the number of HSCs in cord blood and bone marrow of young children can be limiting. Enhanced engraftment of limiting numbers of donor cells could change the paradigm of cord blood transplantation, but requires greater insight into HSC, HPC and cytokine biology2. The enzyme DPP4 (ref. 3, also known as CD26) is expressed on HSCs and HPCs; a non–membrane-bound, soluble form of DPP4 is also produced4. Genetic deletion of Dpp4 or inhibition of DPP4 activity with diprotin A (Ile-Pro-Ile) or Val-Pyr greatly enhances chemotaxis of HPCs toward SDF-1 (CXCL12)4 and engraftment of mouse5–8 and human9–11 HSCs, in part through increasing homing5 toward endogenous bone marrow SDF-1 (ref. 12). While investigating the role of DPP4 in regulating other functions of SDF-1, we unexpectedly found that inhibition of DPP4 enhances in vitro GM-CSF induction of GM-progenitor cell (CFU-GM) proliferation, independently of effects on SDF-1. Although it had not been previously reported that cytokines other than selected chemokines contain DPP4 truncation sites13, or that these sites regulate the potency of multiple CSFs, our serendipitous observations suggested the possibility that such sites are involved in regulating cytokine potency. We show here that DPP4 truncation decreases the potency of CSFs and that truncated CSFs partially block full-length CSF activity by receptor-binding competition and decreasing intracellular signaling. Moreover, deficiency or inhibition of DPP4 in vivo enhances recovery of hematopoiesis after stress, suggesting the clinical utility of DPP4 inhibition.
RESULTS
DPP4 regulates survival and ex vivo expansion capacity of SDF-1
SDF-1 is a chemotactic2,4, homing12 and survival14–17 molecule for HSCs and HPCs. We previously showed that knockout of Dpp4 enhanced chemotaxis of HPCs4 and homing of HSCs5 toward SDF-1. To determine the breadth of the effects of Dpp4 knockout on SDF-1 activity, we assessed additional SDF-1 functions. We first evaluated effects on SDF-1–enhanced HPC survival. Delaying addition of growth factors to bone marrow cells from Dpp4−/− and wild-type (WT) mice decreases HPC colony formation, owing to apoptotic death2,15,16. We found that colony formation by CFU-GM, erythroid (BFU-E) and multipotential (CFU-GEMM) progenitors was similar between WT and Dpp4−/− bone marrow with or without SDF-1 when growth factors were added at time 0 (Fig. 1a), whereas delaying growth factor addition for 24 h significantly reduced colony formation. Survival of HPCs was significantly enhanced by addition of SDF-1 to the culture at time 0, an effect further enhanced in Dpp4−/− cells (Fig. 1a). Dpp4−/− bone marrow cells did not show enhanced survival without SDF-1 addition, suggesting that the enhancing effects of DPP4 deletion were due to the absence of SDF-1 truncation by DPP4. Diprotin A (a DPP4 inhibitor) completely, but reversibly, inhibits DPP4 activity on cells within 15 min4,5. Whether or not human cord blood cells were pulse-treated with diprotin A 1 h before SDF-1 addition or left in culture with diprotin A, SDF-1 potency was increased 100-fold (Fig. 1b). These findings support a role for cell surface DPP4 in regulating SDF-1–enhanced HPC survival, and demonstrate that modulation of DPP4 has positive effects on multiple SDF-1 functions.
Figure 1.
Deletion or inhibition of Dpp4 enhances SDF-1’s effects on survival and ex vivo expansion of HPCs. (a) Colony formation assays in WT and Dpp4−/− bone marrow after 1 d delayed addition of growth factors. SDF-1 was added to plates at day 0, and growth factors were added either on day 0 or on day 1 (one of two reproducible experiments with 3 plates per point scored for each bar; each experiment used bone marrow pooled from three mice). *P < 0.05 compared to day 0 WT; **P < 0.05 compared to WT on day 1; ***P < 0.05 compared to WT + SDF-1 on day 1. (b) Effect of diprotin A on survival of human cord blood CFU-GEMM after 1 d delayed addition of growth factors (GF) as in a for mouse cells (one of three reproducible experiments scoring three plates per point; each experiment used bone marrow pooled from three mice). Human cord blood was pretreated with 5 mM diprotin A, and the cells were either washed or not prior to plating in semisolid culture medium in the absence or presence of SDF-1 at day 0 with GFs added on day 0 or day 1. Only the day 1 results are shown, as there was no effect of SDF-1 or of diprotin treatment on cells plated with growth factors at day 0. Control refers to PBS (no SDF-1) in culture plates at day 0. *P < 0.05 compared to day 0 without SDF-1; **P < 0.05 compared to day 1 control. (c) Ex vivo expansion of HPCs (average results of eight experiments, each using a different cord blood collection, with three plates scored per point for each experiment). CD34+ cells isolated from fresh cord blood were pretreated with 5 mM diprotin A or PBS, and the cells were then plated in suspension culture with SCF, FL and TPO and either PBS (control medium) or SDF-1. After 7 d, the cells were washed and plated in semisolid culture medium and scored for colonies. Results are expressed as fold change from the diprotin A and PBS group cultured with SCF, FL and TPO. *P < 0.05 compared to control; **P < 0.05 compared to SDF-1 without diprotin A pretreatment. FL, full-length; TPO, thrombopoietin. (d) Same experiment as in c except that CD34+ cells were purified from an unseparated cord blood that had been thawed after 21 years in a frozen cryopreserved state and the actual numbers of progenitors for input colony-forming cells (prior to suspension culture) were compared to colony-forming cells generated after 7-d culture with PBS, SDF-1 or SDF-1 with diprotin A. The numbers in parentheses above the bars refer to the fold increase compared to input colony-forming cells (n = 1 experiment with three plates scored per point). *P < 0.05 compared to input; **P < 0.05 compared to PBS control; ***P < 0.05 compared to SDF-1 without diprotin A. All results are mean ± s.e.m.
Ex vivo HPC expansion has potential clinical utility1; however, whether SDF-1 can promote HPC expansion has not previously been determined. The cytokine combination SFT (stem cell factor (SCF), Flt3-ligand (Flt3-L) and thrombopoietin)18 increased CFU-GM and CFU-GEMM plus BFU-E output of CD34+ cord blood cells 21.0 ± 10.0-fold (range 4.3–33.5) and 4.0 ± 2.0-fold (range 1.2–7.1), respectively, after 7 d of culture. SDF-1 further enhanced expansion by 1.7- and 2.0-fold, and pretreatment of cord blood with diprotin A resulted in a 2.4- to 2.7-fold enhancement (Fig. 1c). Notably, diprotin A in the absence of SDF-1 did not enhance SFT’s effects. Enhanced expansion also occurred with CFU-GM and CFU-GEMM treatment of CD34+ cells isolated from cord blood cells stored frozen for 21 years19 (Fig. 1d). Thus, SDF-1 enhances ex vivo expansion of HPCs, an effect made more potent with DPP4 inhibition.
Replating assays using single CFU-GEMM colonies measures their limited self-renewal capacity20. SDF-1 enhances replating21. We treated low-density cord blood cells with or without diprotin A before addition of growth factors (erythropoietin, SCF, GM-CSF and IL-3) added together with or without SDF-1. Treatment of these primary cultures with SDF-1 in the absence of diprotin A pretreatment significantly (P < 0.05) increased secondary CFU-GEMM replating frequency from 2.1-fold (76 secondary colonies from 36 replated primary colonies) to 4.2-fold (142 from 34). Diprotin A enhanced the SDF-1–induced increase in replating frequency 5.4-fold (195 from 36) (P < 0.05) but did not enhance replating frequency in the absence of SDF-1. Thus, diprotin A pretreatment also enhances the ability of SDF-1 to increase CFU-GEMM colony replating frequency, and our results indicate that deficiency or inhibition of DPP4 enhances multiple properties of SDF-1.
DPP4 Inhibition enhances in vitro activity of CSFs
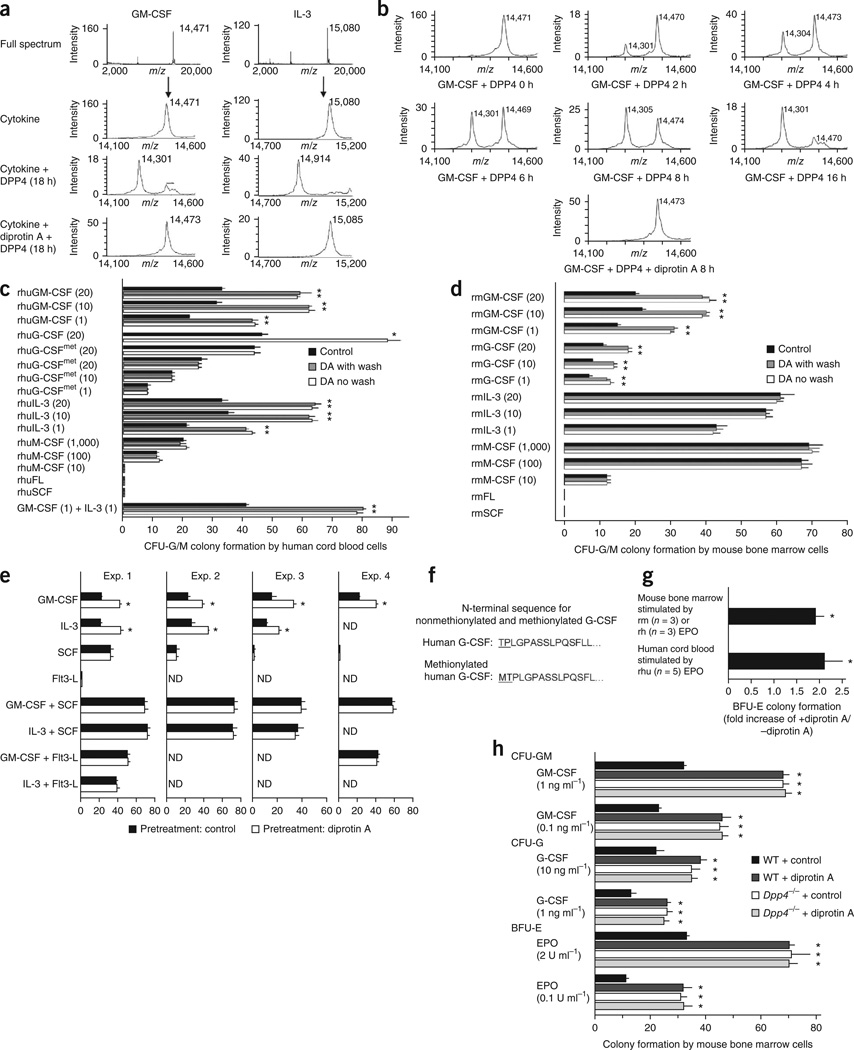
While controlling for GM-CSF activity in these experiments, we unexpectedly noticed that pretreatment with diprotin A resulted in a twofold increase in CFU-GM colonies from mouse bone marrow and human cord blood when stimulated with mouse and human GM-CSF, respectively (data not shown). We therefore searched protein databases for N-terminal amino acid sequences corresponding to alanine and proline DPP4 recognition sites4,13 in GM-CSF and other cytokines (Supplementary Table 1). We found such sites for mouse and human GM-CSF, G-CSF and erythropoietin and for human IL-3, but not for mouse IL-3 or mouse or human M-CSF, SCF or Flt3-L. Mass spectrometric analysis demonstrated that human GM-CSF and human IL-3 could be enzymatically truncated by purified soluble DPP4, and that this truncation was blocked by diprotin A (Fig. 2a,b).
Figure 2.
Inhibition of DPP4 enhances the in vitro activity of selected CSFs with DPP4 truncation sites. (a) Mass spectrometry analysis of recombinant human GM-CSF and recombinant human IL-3 before and after exposure of the CSFs to soluble DPP4. The shift in molecular weight after DPP4 action correlates with the presence of an N-terminal alanine or proline DPP4 truncation site. (b) Mass spectrometry analysis of recombinant human GM-CSF. Same as in a, with additional time points after DPP4 addition shown. (c) Influence of diprotin A (DA) inhibition of DPP4 on human cord blood cell CFU-GM colony formation with and without washing the cells prior to addition of different concentrations (ng ml−1 in parentheses) of recombinant human (rhu) human growth factors to semisolid culture medium (one of three reproducible experiments). Control refers to no DA treatment of cells. (d) Influence of diprotin A inhibition of DPP4 on unseparated mouse bone marrow cells with and without washing the cells prior to addition of different concentrations (ng ml−1 in parentheses) of recombinant mouse (rm) growth factors to semisolid culture medium (one of three reproducible experiments). For c and d, *P < 0.05 compared to control without diprotin A for that growth factor. (e) Lack of effect of diprotin A pretreatment of target cells on activity of potent co-stimulating cytokines SCF or Flt3-L, when used alone or in combination with a CSF. Mouse bone marrow cells were pretreated with PBS (control) or diprotin A and then plated with the cytokines shown. *P < 0.05 compared to control of that group. Exp., experiment; ND, not done. (f) N-terminal sequences for G-CSF. (g) Influence of diprotin A treatment on mouse bone marrow BFU-E colony formation with washing of cells prior to plating of cells with recombinant mouse or recombinant human erythropoietin (EPO), and on human cord blood cells with washing of cells prior to plating of cells with recombinant human erythropoietin. *P < 0.05 compared to without diprotin A treatment of cells. (h) Influence of diprotin A or PBS (control) pretreatment of cells on colony formation by bone marrow cells from Dpp4−/− and WT mice. Three plates per point were scored in one experiment, for which cells were pooled from three different mice. Shown is one of two reproducible experiments. *P < 0.05 compared to control without diprotin A for that growth factor. All results are mean ± s.e.m.
We first evaluated the effects of diprotin A on the activity of myeloid CSFs. Pulse or long-term treatment of human cord blood (Fig. 2c) or mouse bone marrow (Fig. 2d) with diprotin A enhanced colony formation induced by human or mouse GM-CSF, mouse G-CSF or human IL-3 by approximately twofold. Diprotin A did not enhance the effects of mouse IL-3 or human or mouse M-CSF, Flt3-L or SCF (Fig. 2c–e), consistent with their lack of DPP4 truncation sites. Treatment with human GM-CSF and human IL-3 had additive effects (Fig. 2c). Stimulation of diprotin A–pretreated cells with a CSF that has a DPP4 truncation site had a twofold greater effect on colony formation compared to non–diprotin A–treated cells (Fig. 2c). Initial studies with recombinant human G-CSF did not show enhanced activity on human cord blood HPCs (Fig. 2c); however, recombinant human G-CSF (Neupogen) is methionylated at the N-terminus, thereby shifting the predicted cleavage site (Fig. 2f). We therefore assessed the effects of nonmethionylated recombinant human G-CSF and found it was twice as stimulatory when cord blood cells were pretreated with diprotin A (Fig. 2c).
We next assessed the effects of diprotin A on the erythroid CSF erythropoietin. Diprotin A increased the number of erythropoietin-stimulated erythroid colonies originating from BFU-E in mouse bone marrow cells and human cord blood cells (Fig. 2g) by twofold. However, diprotin A treatment of Dpp4−/− mouse bone marrow cells did not further increase the activity of mouse GM-CSF or mouse G-CSF for CFU-G(M) colony formation, or the activity of human erythropoietin for BFU-E colony formation (Fig. 2h), confirming the selectivity of diprotin A for DPP4. Treatment with the GM-CSF together with SCF or Flt3-L had additive to synergistic effects, and colony formation by these cytokine combinations was unaffected by diprotin A pretreatment (Fig. 2e). Thus, the ability of DPP4 inhibition to enhance the activity of CSFs containing a DPP4 truncation site is not apparent when the CSFs are used in combination with the potent co-stimulating cytokines SCF or Flt3-L.
Truncation decreases CSF activity
As diprotin A enhanced the activity of different CSFs, we tested the effects of truncated CSFs on colony formation. Pretreatment of mouse GM-CSF or human erythropoietin with soluble, but not heat inactivated, DPP4 significantly decreased their potency (Fig. 3a, top). Mouse IL-3 (no DPP4 truncation site) potency was not affected. Enhancement was much more apparent when DPP4 on target HPCs was inhibited (Fig. 3a, bottom). We next pretreated GM-CSF or erythropoietin with DPP4 and then inactivated the DPP4 with diprotin A. The addition of this truncated GM-CSF or erythropoietin to the respective nontruncated cytokine resulted in an activity equivalent to that of the truncated cytokine (Fig. 3a, bottom). Thus, truncated CSFs block the optimal activity of full-length CSFs, a result that may be of potential physiological relevance.
Figure 3.
Influence of soluble DPP4 on activities of recombinant mouse CSFs in vitro, and effects of full-length and truncated CSFs alone and in combination on hematopoiesis in vivo in mice. (a) Influence of soluble DPP4 on activity of CSFs. Top, mouse bone marrow cells were treated with recombinant mouse GM-CSF, IL-3 or EPO which had been pretreated with PBS, soluble DPP4 or DPP4 first heat-treated at 56 °C for 1 h (DPP4-Δ*) to inactivate DPP4. Bottom, bone marrow was pretreated with diprotin A. Then, as indicated in the sequences shown on the y axis, cells were added to culture dishes with cytokines that had been first treated with PBS, DPP4 or DPP4-Δ* (as indicated in the first column in the sequence) and then with either PBS or diprotin A (second column); these treated cytokines were added with either PBS or the corresponding full-length cytokine (third column). *P < 0.05 compared to control medium (PBS, PBS, PBS) in that group. (b) Influence of full-length (FL) and truncated (T) recombinant mouse GM-CSF in vivo in WT and Dpp4−/− mice on absolute numbers and cycling status of bone marrow HPCs. n = 4 mice per group. (c) Influence of full-length and truncated recombinant human erythropoietin in vivo on reticulocyte release to the blood of WT and Dpp4−/− mice 18 h after a single (experiment 1, blood assessed 24 h later; n = 4 mice per group) or multiple injections of erythropoietin (experiments 2 and 3; n = 4 mice per group for each experiment). Experiment 3 used blood obtained from tail bleeds. The control treatment was PBS. (d) Influence of erythropoietin on absolute numbers of bone marrow HPCs in WT and Dpp4−/− mice (n = 4 mice per group). The control treatment was PBS. For experiments 2 and 3 in c and for d, erythropoietin was given s.c. twice per day for 3 d at 10 U per injection, and mice were assessed 24 h after the last injection. For b–d, × = fold change from the indicated comparisons; *P < 0.05. All results are mean ± s.e.m.
To determine whether the effects of CSF truncation noted in vitro were also apparent in vivo, we first assessed the effects of a single subcutaneous (s.c.) dose of 0.5 µg per mouse of full-length or truncated recombinant mouse GM-CSF or their combined effects when each was injected at a different site. Dosing with either form of GM-CSF or their combination did not influence HPC numbers in WT control mice 24 h after treatment (Fig. 3b, top). However, treatment with full-length GM-CSF, but not truncated GM-CSF, enhanced HPC numbers in Dpp4−/− mice, and truncated GM-CSF blocked the effects of full-length GM-CSF in Dpp4−/− mice. HPC cycling induced by low concentrations of CSFs in vivo is a more sensitive indicator for the effects of CSFs than is HPC number22,23. Full-length, but not truncated, GM-CSF enhanced HPC cycling, with greater effects in Dpp4−/− than in wild-type mice. Truncated GM-CSF significantly blocked the effects of full-length GM-CSF on HPC cell cycle increase in both wild-type and Dpp4−/− mice (Fig. 3b, bottom). These in vivo effects are consistent with those in vitro (Fig. 2c–e,h).
We next determined the effects of full-length and truncated erythropoietin in vivo. Full-length human erythropoietin administered as either single or multiple s.c. injections24 enhanced reticulocyte release into blood, and these effects were greater in Dpp4−/− mice (Fig. 3c, exps. 1 and 2). Truncated erythropoietin had low reticulocyte release activity and inhibited the effects of full-length erythropoietin (Fig. 3c). Baseline BFU-E and CFU-GEMM colony numbers without erythropoietin administration were similar in wild-type and Dpp4−/− mice. Injection of full-length erythropoietin enhanced bone marrow BFU-E and CFU-GEMM numbers, and these effects were greater in Dpp4−/− mice (Fig. 3c, exp. 3). Erythropoietin enhanced CFU-GM numbers in Dpp4−/− but not wild-type mice (Fig. 3d). Thus, the effects of full-length and truncated erythropoietin noted in vitro are also apparent in vivo.
Mechanisms of truncated versus full-length CSF actions
To gain mechanistic insight into the ability of truncated CSFs to block the activity of their full-length counterparts, we searched for a factor-dependent cell line that responds to CSFs and also expresses DPP4 on the cell surface. TF-1 cells proliferate in response to human GM-CSF, IL-3 and erythropoietin25 and we found that these cells express DPP4 on the cell surface (data not shown). Colony formation in response to full-length recombinant human GM-CSF, IL-3 or erythropoietin was greatly enhanced when we pretreated TF-1 cells with diprotin A (Fig. 4a). Truncated CSFs were significantly less active in promoting TF-1 colony formation than their respective full-length CSFs, and these effects were more apparent in cells pretreated with diprotin A. Truncated CSFs decreased the colony-stimulating activity of their respective full-length CSFs to the levels achieved by truncated CSFs (Fig. 4a). Thus, TF-1 cells behave similarly to primary HPCs.
Figure 4.
Influence of DPP4 inhibition on colony formation, receptor binding and signaling in the TF-1 factor-dependent human cell line and in CD34+ cord blood cells. (a) Influence of the full length and truncated forms of GM-CSF, IL-3 and erythropoietin on colony formation by TF-1 cells pretreated with or without diprotin A (DA). *P < 0.05 compared to full-length GM-CSF, IL-3 or EPO in the absence of diprotin A; **P < 0.001, compared to full-length GM-CSF, IL-3 or EPO in the absence of diprotin A; ***P < 0.001, compared to GM-CSF, IL-3 or EPO in the presence of diprotin A. (b) GM-CSF receptor binding analysis by Scatchard plot, shown for TF-1 cells. Results of full-length ligand (FL) and truncated ligand (T) are shown from a representative experiment. High-affinity and low- affinity binding sites are readily observed. Inset, statistical analysis of Kds from results of three experiments shown with error bars; *P < 0.05 for both affinity classes. (c) Scatchard analysis of CD34+ cord blood cells (one of two reproducible experiments). (d) Cold competition binding experiment. Concentration of cold (unlabeled) full-length or truncated ligand that was used to compete, or block, binding of the same amount (7 pM) of ‘hot’ ([125I]-full-length GM-CSF) ligand per point is shown. IC50, concentration of the cold ligand required to produce 50% inhibition of binding. Inset, full range of concentrations of cold competitor are shown, demonstrating that high concentrations of both FL and T ligands produce nearly 100% inhibition of binding of the labeled ligand. Diprotin A was added to all truncated samples to quench the DPP4 reaction before use. The arrows pointing to the left and right respectively refer to the IC50 for truncated and full-length GM-CSF. (e,f) Phosphorylation of JAK2 (e) and STAT5 (f) in TF-1 cells. Influence of varying concentrations of truncated GM-CSF and/or full-length GM-CSF. The flow analysis at the left of e and f are one representative of three experiments; at right is shown quantitative data (mean ± s.e.m.) for all three experiments. The bottom graphs in e and f show the results of one experiment assessing effects of different ratios of T to FL cytokine. *P < 0.004; values for full-length GM-CSF are compared to no stimulation, and P values for T or T plus FL are compared to full-length GM-CSF. (g) Influence of full-length versus truncated recombinant human GM-CSF on phosphorylation of JAK2 and STAT5 in CD34+ cord blood cells (n = 6 experiments for pJAK2 and 3 experiments for pSTAT5). *P < 0.05 compared to truncated GM-CSF. (h) Influence of full-length and truncated recombinant human GM-CSF (shown as ng ml−1) alone and in combination on colony formation by TF-1 cells in the presence and absence of diprotin A. *P < 0.002 for truncated CSF without diprotin A, or the combination of full-length plus truncated CSF without diprotin A, compared to the corresponding full-length CSF without diprotin A; **P < 0.001 for full-length CSF with diprotin A compared to the corresponding full-length CSF without diprotin A; ***P < 0.001 for truncated CSF or full-length plus truncated CSF with diprotin A compared to the corresponding full-length CSF with diprotin A. All results are mean ± s.e.m.
We performed equilibrium binding kinetics of full-length and truncated recombinant human [I125]GM-CSF by Scatchard analysis using TF-1 cells (Fig. 4b). We detected high- and low-affinity binding sites with Kds for full-length GM-CSF similar to those reported in the literature26–30. The Kds for high- and low-affinity receptor sites were significantly (P < 0.05) lower, by 92% and 83%, respectively, for truncated compared to full-length CSF, indicating that truncation by DPP4 increases the stability of GM-CSF binding under equilibrium conditions. We verified these TF-1 cell studies using primary cells. We also observed high- and low-affinity binding sites for human [I125]GM-CSF on human CD34+ cord blood cells (Fig. 4c). The Kd for high-affinity binding was greater on cord blood compared to TF-1 cells; however, we observed the same trend in primary cells as in TF-1 cells, with truncated GM-CSF having a greater affinity (lower Kd) than full-length GM-CSF (1,469 pM compared to 734 pM). In dose-response ‘cold-competition’ experiments with TF-1 cells (Fig. 4d), both ligand forms cold-competed [I125]GM-CSF binding by nearly 100% when cold-competitor concentrations were very high, but the concentration required to produce 50% maximum inhibition of binding (IC50) was 88% less for truncated compared to full-length GM-CSF. These results indicate that truncated GM-CSF is a better competitor for binding than is full-length GM-CSF, which may explain the ability of truncated CSFs to downmodulate the potency of full-length CSFs.
As GM-CSF activates the JAK2-STAT5 pathway2, we compared full-length and truncated recombinant human GM-CSF in varying ratios for their ability to induce phosphorylation of JAK2 and STAT5 in TF-1 cells. Full-length GM-CSF significantly induced phosphorylation of both JAK2 (maximally at 15 min) (Fig. 4e) and STAT5 (maximally at 30 min) (Fig. 4f). No significant induction of phosphorylation was detected at 45–60 minutes for GM-CSF (data not shown). Truncated GM-CSF elicited little or no JAK2 or STAT5 phosphorylation (Fig. 4e,f). We observed similar induction of JAK2 and STAT5 phosphorylation by full-length but not truncated recombinant human GM-CSF in CD34+ cord blood cells (Fig. 4g). When truncated and full-length GM-CSF were used at a ratio of 1:8, truncated GM-CSF blocked full-length GM-CSF–induced phosphorylation of JAK2 and STAT5 in TF-1 cells (Fig. 4e,f). This result is consistent with the ability of truncated GM-CSF at similarly low ratios to block full-length GM-CSF receptor binding (Fig. 4d) and stimulation of colony formation (Fig. 4h).
For full signaling by GM-CSF, the GM-CSF receptor (GM-CSFR) must form a dodecamer complex from the interaction of two hexamer units, an interaction mediated by βc site 4 on GM-CSFR (Fig. 5a)31–34. To gain further mechanistic insight, we treated cord blood cells with GM-CSF with or without an antibody, anti-Rβc (anti-RSite4), specific to the human GM-CSFR βc site 4, an antibody that blocks dodecamer formation33. Antibody treatment resulted in reduced colony formation, consistent with inhibition of dodecamer complex formation and full GM-CSF signaling (Fig. 5b). In contrast, anti-RSite4 had no effect on G-CSF–stimulated colony formation, demonstrating that anti-RSite4 specifically reduces GM-CSFR– but not G-CSFR–mediated activity. Similar numbers of colonies were generated by GM-CSF plus anti-RSite4, truncated GM-CSF or truncated GM-CSF plus anti-RSite4 treatment (Fig. 5b, with GM-CSF). Taken together with the results in Figure 4e–g and considering that only the dodecamer complex is capable of full JAK2-STAT5 signaling33, these results suggest that truncated GM-CSF may be able to signal through hexamer, but not dodecamer, GM-CSFR.
Figure 5.
Modeling of the GM-CSF GM-CSFR interaction, and effects of DPP4 deficiency or inhibition on hematopoietic recovery. (a) Model for the GM-CSF–GM-CSFR interaction, indicating the inhibitory effect of the βc site 4 GM-CSFR–specific antibody on dodecamer complex formation. (b) Influence of βc site 4 GM-CSFR antibody (anti-R) on actions of full length versus truncated CSFs. Cord blood cells were pretreated in sequence in the order shown with PBS, diprotin A (DA) or antibody specific to the human GM-CSFR βc site 4 (anti-R) prior to plating cells with GM-CSF or G-CSF that was either not treated (no pretreatment) or treated with DPP4 and 1 h later with DA before adding the CSF to cells (one of two reproducible experiments in which three plates were scored per point). *P < 0.05 compared to PBS, no treatment. (c) Time course of the effects of radiation on DPP4 activity in plasma and lysates of unseparated bone marrow cells from WT mice. DPP4 activity assays were done in duplicate, and data are expressed as mean ± s.d. for plasma or cell lysates from three mice per group at each day. DPP4 enzyme activity is shown in relative light units. *P < 0.05 compared to time 0. (d,e) Effects of sitagliptin treatment of WT mice or Dpp4−/− on in vivo recovery of nucleated cellularity and HPC numbers from treatment of mice with 400 cGy radiation (d) or 5-FU (e). For d and e, × = fold change compared to WT numbers of that day. *P < 0.05; a = one mouse only for these groups, all others n = 3. ND, not done. All results are mean ± s.e.m.
DPP4 in vivo dampens recovery of hematopoiesis after stress
Hematopoietic recovery after stress depends on cytokine activity2. We reasoned that if cytokine activity is limited by DPP4, deficiency or inhibition of DPP4 might accelerate hematopoietic recovery after radiation or cytotoxic drug treatment. Radiation induced transient increases in bone marrow DPP4 activity, with little effect on plasma activity (Fig. 5c). The numbers of functional bone marrow HPCs were equivalent in wild-type and Dpp4−/− mice (Supplementary Fig. 1a–c), as previously reported5. However, after treatment with either 400 cGy or 650 cGy (Supplementary Fig. 1a), recovery of HPCs was faster and reached higher levels in Dpp4−/− than in wild-type mice. We noted similar effects in mice treated with cyto-toxic drugs, with enhanced HPC recovery in Dpp4−/− mice 7 d after 5-fluorouracil (5-FU) treatment (Supplementary Fig. 1b) and 5 and 7d after Cytoxan treatment (Supplementary Fig. 1c). In Dpp4−/− mice recovering from 5-FU and Cytoxan treatments, accelerated recovery was associated with an increased percentage of HPCs in the S phase of the cell cycle (Supplementary Fig. 1d).
We next evaluated the effects of DPP4 inhibition using the DPP4 inhibitor sitagliptin (Januvia), which has been approved for clinical use by the US Food and Drug Administration. Sitagliptin was administered orally at 10 mg per kg body weight 1 d and 2 h before treatment of wild-type mice with 400 cGy radiation or 5-FU. Recovery of bone marrow cellularity was significantly increased 7 d after 400 cGy radiation in sitagliptin-treated mice compared to vehicle-treated control mice (Fig. 5d). There was a trend toward improved recovery of cellularity 7 d after radiation in Dpp4−/− as compared to wild-type mice (Fig. 5d). There was also a trend toward improved recovery of cellularity 7 d after 5-FU administration in sitagliptin-treated compared to vehicle-treated control mice and in Dpp4−/− compared to wild-type mice, as well as 10 d after 5-FU administration in Dpp4−/− compared to wild-type mice (Fig. 5e). We also observed greatly accelerated HPC recovery, as assessed by bone marrow colony-formation assays, in sitagliptin-treated or Dpp4−/− mice recovering from 400 cGy radiation (Fig. 5d) or 5-FU administration (Fig. 5e). We also evaluated ‘endogenous’ CFU-GM colony growth in the absence of added CSFs. Colonies form from bone marrow cells in the absence of exogenously added CSFs when cell plating numbers are increased, owing to endogenous release of CSFs35. This type of assay allows for an assessment of colony-stimulating activity released by bone marrow cells. Endogenous colony growth was significantly greater for sitagliptin-treated and Dpp4−/− mice 7 d after 400 cGy radiation (Fig. 5d) and 5-FU administration (Fig. 5e). That these differences reflected an increase in endogenous colony-stimulating activity, rather than increased cytokine responsiveness of the bone marrow cells, was especially apparent for day 7 recovery from 400 cGy, when CFU-GM numbers of exogenously stimulated growth were 6.9- and 9.4-fold higher, respectively, in sitagliptin-treated and Dpp4−/− mice, whereas endogenous colony growth was increased by 38.4- and 43.4-fold, respectively (Fig. 5d).
HSCs and HPCs can be identified by immunophenotypic markers2,36, although such markers are not always an indicator of function, especially after stress36. We detected increased numbers of long-term and short-term HSCs cells in marrow of Dpp4−/− compared to wild-type mice (2.7-to 3.1-fold increase for long-term HSCs and 1.8- to 2.1-fold increase for short-term HSCs) and to a lesser degree in marrow of sitagliptin-treated compared to vehicle control–treated mice (1.5- to 1.7-fold increase for long-term HSCs and 1.3- to 1.4-fold increase for short-term HSCs) (Supplementary Fig. 2a,b). We also detected a significant (P < 0.05) 1.5-fold increase in multipotential progenitors (MPPs) in sitagliptin-treated or Dpp4−/− bone marrow prior to stress, but not in common lymphoid progenitors (CLPs), myeloid-erythroid progenitors (MEPs) or granulocyte-macrophage progenitors (GMPs) (Supplementary Fig. 2a). The lack of an effect on MEP and GMP numbers is consistent with the findings that HPC function is not affected in Dpp4−/− or sitagliptin-treated mice prior to stress. After radiation, and to a greater degree after 5-FU treatment, the numbers of immunophenotypically defined long-term and short-term HSCs were greater in marrow of sitagliptin-treated compared to vehicle control–reated mice and in Dpp4−/− mice (compared to wild-type mice), and these numbers were also greater in the sitagliptin-treated and Dpp4−/− mice after radiation or 5-FU treatment compared to their no-treatment controls (Supplementary Fig. 2a,b). Thus, DPP4 deficiency or inhibition results in a faster and enhanced recovery of immunophenotypically defined long-term and short-term HSCs after stress.
Whereas DPP4 deficiency or inhibition led to greatly enhanced recovery of the numbers of functional myeloid and erythroid progenitors after stress (Fig. 5d,e and Supplementary Fig. 1a,b), immunophenotypically defined MPP, MEP and GMP numbers were not as consistently enhanced (Supplementary Fig. 2a). Thus, accelerated and enhanced recovery of functional HPCs by Dpp4−/− or Dpp4 inhibition exceeds that detected by immunophenotypic characterization of these cells. In fact, we detected few or no immunophenotypic GMPs after stress, suggesting that immunophenotype after stress for these progenitors is not a reliable marker. After radiation or 5-FU, recovery of morphologically recognizable dividing and nondividing myeloid cells was enhanced in sitagliptin-treated or Dpp4−/− mice (Supplementary Fig. 2c,d).
We next assessed recovery of white blood cells, neutrophils, lymphocytes, monocytes, red blood cells and platelets (Supplementary Fig. 3b,c) after 5-FU or 400 cGy using the same experimental design (Supplementary Fig. 3a) as for HPC and HSC recovery. Recovery of these lineages in peripheral blood was accelerated and maintained for longer periods in Dpp4−/− mice compared to wild-type mice; these effects were observed but to a lesser extent in sitagliptin-treated compared to vehicle-treated control WT mice. Enhanced peripheral blood cell recovery was also apparent in Dpp4−/− mice subjected to 5-FU treatment in a second experiment (Fig. 4b). However, whereas we observed a modest enhancement of accelerated recovery of blood cells in mice given sitagliptin prior to and shortly after 5-FU administration (Supplementary Fig. 3a), we did not observe this effect in mice given sitagliptin only shortly after 5-FU administration (Supplementary Fig. 4b).
To assess whether or not DPP4 inhibition in vivo has an effect on functional HSCs, we carried out competitive repopulation assays. WT B6.BoyJ CD45.1+ recipient mice were pretreated with diprotin A s.c. (Fig. 6a) or F1 (CD45.1+ CD45.2+) recipient mice were pretreated with sitagliptin by oral gavage (Fig. 6b) and lethally irradiated (950 cGy). We determined engraftment of congenic marrow cells from WT mice in a competitive transplantation experiment using C57BL/6 donor (CD45.2+) and B6.BoyJ competitor (CD45.1+) cells (C57BL/6 cells are known to out-compete B6.BoyJ cells37). Engraftment was significantly greater in primary lethally irradiated recipient mice in which DPP4 had been inhibited (Fig. 6a,b). Self-renewal activity of the engrafted cells was not compromised, as assessed by serial transplantation into lethally irradiated secondary recipients without DPP4 inhibition (Fig. 6a), or by long-term engraftment in primary mice (Fig. 6b). Thus, the enhanced recovery after stress caused by DPP4 inhibition is not at the expense of HSC function (that is, engraftment and self-renewal capability).
Figure 6.
Influence of DPP4 inhibition in vivo on engraftment of HSCs and of CXCR4 deficiency on sitagliptin-enhanced hematopoietic recovery. (a,b) Effects of pretreating mice with diprotin A (DA) (a) or sitagliptin (b) on engraftment of C57BL/6 (CD45.2+) cells in a competitive assay with B6.BoyJ mice (CD45.1). Number of mice used is given in the Online Methods section. For a and b, *P < 0.05. (c) Inducible Cxcr4 knockout. Wild-type mice are represented by a single 430-bp PCR product and knockout mice are represented by a single 510-bp PCR product due to deletion of the exon 2 region of the Cxcr4 gene, as diagrammed. (d) Nucleated cellularity and progenitors per femur in WT control mice and mice with induced Cxcr4−/− in the absence or presence of oral administration of sitagliptin before (top) and 7 d after receiving 5-FU treatment (bottom). Results are based on an analysis of four mice per group. *P < 0.05. NS, not significant (P > 0.05), and × = fold change from cells from the indicated mice. All results are mean ± s.e.m.
Given that SDF-1 has been implicated in migration homing and survival of HSCs and HPCs2,5,12,14–17, we determined whether SDF-1 has a role in enhanced recovery after DPP4 inhibition in vivo by studying mice with a tamoxifen-inducible global knockout of Cxcr4, which encodes the major receptor for SDF-1; this mouse strain has been described by others43 (Fig. 6c). At baseline, the absolute numbers of bone marrow HPCs in Cxcr4−/− mice were significantly decreased compared to control mice, as were endogenously stimulated CFU-GM numbers (Fig. 6d). In the absence of sitagliptin treatment, HPCs in Cxcr4−/− mice recovered more effectively than did those in control mice 7 d after 5-FU administration. However, sitagliptin-mediated enhancement of HPC recovery 7 d after 5-FU administration was similar for control and Cxcr4−/− mice, as was enhancement of endogenous CFU-GM numbers. These results suggest that the SDF-1–CXCR4 interaction may have a dampening effect on recovery after 5-FU treatment but is not crucial to the effect of DPP4 inhibition on enhancing hematopoietic recovery.
DISCUSSION
Our results demonstrate a previously unknown hematopoietic feedback loop. G-CSF and GM-CSF induce upregulation of DPP4, which downregulates SDF-1–induced chemotaxis38 and, as we show here, negatively regulates GM-CSF, G-CSF, IL-3 and erythropoietin potency in stress conditions. Truncated CSFs have much less activity than full-length CSFs and dampen or decrease the activity of full-length CSFs. Mechanistically, truncated GM-CSF binds with greater affinity than full-length GM-CSF to high- and low-affinity GM-CSF receptor sites and effectively competes at low concentrations with higher concentrations of full-length GM-CSF for binding. Also, compared to full-length GM-CSF, truncated GM-CSF elicits a lower level of JAK2 and STAT5 phosphorylation, which is crucially involved in GM-CSF–mediated cell proliferation2,31. Moreover, truncated GM-CSF decreases the effects of full-length GM-CSF on JAK2 and STAT5 phosphorylation. On the basis of these results and on our data using an antibody specific to βc site 4 in GM-CSFR, which is required for dodecamer formation of GM-CSFR upon binding of full-length GM-CSF and for optimal signaling33s, it appears that truncated GM-CSF is not sufficient to induce GM-CSFR dodecamer formation or is not capable of optimal signaling through the full dodecamer complex. Given that truncated GM-CSF at approximately one-eighth of the amount of full-length GM-CSF is able to block JAK2-STAT5 signaling, and that the dodecamer complex has six ligands bound in its final form33, we surmise that if one or more ligands is in a truncated form, signaling through the whole complex may be compromised, perhaps through lack of proper dodecamer formation. Definitive evidence for the effects of truncated GM-CSF on GM-CSFR dodecamer complex formation awaits X-ray crystallography studies on GM-CSFR complexed with full-length and truncated GM-CSF.
Our view of DPP4 action as a negative regulator of CSF potency potentially has key clinical implications. Accelerating hematopoiesis in patients after radiation and chemotherapy treatment, or after conditioning for HSC transplantation, especially when limiting numbers of donor HSCs and HPCs are used (such as those found in cord blood), could decrease hospital stays and improve treatment outcomes. Our preclinical mouse studies demonstrating that the orally active DPP4 inhibitor sitagliptin enhances recovery of HPCs and HSCs after radiation or 5-FU treatment of mice suggest the potential of such treatment for hematopoietic recovery in humans. Treatment of mice with a very short course of sitagliptin is almost as effective in enhancing HSC and HPC recovery as genetic deficiency of DPP4 but was not as effective in enhancing peripheral blood cell recovery. Thus, optimization of the amount, timing and duration of sitagliptin treatment may be required to enhance peripheral blood cell recovery to the level achieved with Dpp4 gene deletion. On the basis of our previous reports5,9 and the data presented here, we have initiated an ongoing pilot clinical study evaluating DPP4 inhibition by oral administration of sitagliptin for enhancement of single-unit red cell–depleted cord blood transplantation in adult patients with high risk hematological malignancies (S.S. Farag, H.E. Broxmeyer, S. Srivastava, S. Messina-Graham, J. Schwartz et al., unpublished data). Although it is not yet clear which cytokines in vivo are involved in the DPP4 inhibition–induced enhancement of hematopoiesis, it may involve the CSFs noted here, alone or in combination, or other as yet unidentified cytokines or growth factors. When antibodies are available that can distinguish truncated from full-length cytokines, we may be able to better address this question. However, SDF-1 does not seem to be involved in the enhanced recovery of hematopoiesis caused by DPP4 inhibition. On the basis of our studies in which truncated cytokines dampen the activity of their respective full-length cytokines, another implication of our work is that it may be possible in the future to downmodulate excessive hematopoiesis in patients with hematopoietic abnormalities, including leukemia, as leukemic clonogenic cells respond to the same cytokines as do normal HPCs2.
Our results have broad implications for CSF ligand-receptor biology. CSFs have multiple functional activities on immature and mature cell populations2. The enhancement of multiple functional activities of SDF-1 by DPP4 deficiency or inhibition suggests that DPP4 may influence multiple functions of other CSFs in vitro and in vivo. The finding that the activity of methionylated G-CSF is not affected by DPP4, as predicted by the shift in putative truncation site, demonstrates that recombinant growth factors can have profound differences in function compared to their endogenous counterparts. Neupogen, a widely used recombinant human G-CSF, is methionylated, presumably eliminating endogenous truncation by DPP4 in vivo and fortuitously increasing its potency. To our knowledge this is the first report suggesting biological differences between methionylated and unmethionylated recombinant human G-CSF. Several growth factors (IL-3 and IL-5) share a similar receptor subunit and receptor-mediated signaling complex to GM-CSF, and it remains to be determined whether the truncated form of GM-CSF interferes with the activity of full-length IL-3 or IL-5 or vice versa.
The role of DPP4 will probably extend beyond that of hematopoiesis. Other proteins are predicted to have DPP4 truncation sites. Although not an exhaustive list, mouse and human thrombopoietin, leukemia inhibitory factor, IL-1α, IL-6 and a number of splice variants of vascular endothelial growth factor A have putative N-terminal alanine or proline DPP4 truncation sites; such sites are not found in IL-4, transforming growth factor-α, transforming growth factor-β, tumor necrosis factor-α, tumor necrosis factor-β, IL-12α or IL-12β. This suggests, among other possible effects, that DPP4 may affect the growth of mouse embryonic stem cells (via leukemia inhibitory factor), inflammation (via IL-1α), angiogenesis (via vascular endothelial growth factor A) and myeloma cell growth (via IL-6), as well as effects of thrombopoietin and IL-6 on the enhanced recovery of platelets after stress. The putative DPP4 truncation site in thrombopoietin may in part be responsible for expanded megakaryocytes and megakaryocyte progenitors in Dpp4−/− mouse marrow39.
That diprotin A inhibition of DPP4 does not enhance ex vivo expansion or replating frequency of HPCs in the absence of SDF-1, but does so in the presence of SCF, Flt3-L and thrombopoietin, may be telling. As inhibition of DPP4 does not enhance colony formation more than that by a CSF plus SCF in the presence of active Dpp4, this may be the reason that pretreating target cells with diprotin A did not enhance the activity of erythropoietin, GM-CSF, IL-3 and SCF for replating CFU-GEMM colonies in the absence of SDF-1. Given the lack of further enhancing effects of DPP4 inhibition on SCF and Flt3-L activity when administered with a CSF in vitro, it would seem that neither SCF nor Flt3-L has a major role in the enhanced recovery of HPCs in Dpp4−/− or sitagliptin-treated mice subjected to radiation, 5-FU or Cytoxan.
Synergy between DPP4 inhibition and exogenously added G-CSF in improving cardiac function in mice with acute myocardial infarction40, which has been believed to be due to effects on SDF-1, may be due in part to preventing the truncation of G-CSF or other relevant proteins. Our results may also have important implications for cell migration in myeloproliferative neoplasms41 and the metastatic capacity of certain cancers42, as SDF-1 and possibly other cytokines with DPP4 truncation sites are involved in cell movement and metastasis. A search for other proteins with functional DPP4 truncation sites is warranted, as are efforts to identify other soluble or cell-membrane enzymes that control the activity of biomolecules affecting hematopoiesis or other organ systems.
ONLINE METHODS
Mice, mouse cells, and human cord blood and TF-1 cells
C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). Dpp4−/− mice, on a C57BL/6 mouse strain background, were as described5. Cre+Cxcr4fl/fl mice were obtained from Y.-R. Zou and bred at Indiana University. As previously described43, the mice were created by crossing Cxcr4-floxed mice (Cxcr4fl/fl) with tamoxifen-inducible Cre transgenic mice (ROSACRE-ERT2). Cre+Cxcr4fl/fl mice were treated with tamoxifen as previously reported43. Briefly, mice were injected with 1mg tamoxifen for three consecutive days. After a 3-d resting period, three more consecutive injections were performed and gene deletion was confirmed 14 d later. The following primers were used to confirm Cxcr4 gene deletion: forward primer (5′-cactacgcatgactcgaaatg-3′), reverse 1 primer (5′-gtgtgcggtggtatccagc-3′) and reverse 2 primer (5′-ggaagccataaatgtgtgcatta-3′). Controls were C57BL/6 mice. Mouse bone marrow and human cord blood cells (from the Wishard Hospital, Indiana University School of Medicine, Indianapolis, Indiana, USA, and from Cord:Use Cord Blood Bank, Orlando, Florida, USA) were as described5,44. Cryopreservation and thawing of cord blood cells were performed as reported19. The Indiana University Committee on Use and Care of Animals, and the Indiana University Institutional Review Board respectively approved mouse and human cord blood studies (IACUC study: 10342; IRB study: 9402-10). Cord blood received from Wishard Hospital was from to be discarded material. TF-1 cells were originally obtained from T. Kitamura, Japan25 and are available from American Type Culture Collection (CRL-2003) and were as described by others25 and ourselves45. All experiments in this paper except for mass spectrometry, as noted below, were performed at the Indiana University School of Medicine.
HPC assays
C57BL/6 mouse bone marrow cells were usually plated at 5 × 104 cells/ml in 1% methylcellulose culture medium in the presence of hemin 0.1 mM, 30% FBS (Hyclone, Utah) and the following growth factors, unless otherwise noted: 1 U per ml recombinant human erythropoietin (Amgen Corporation, South San Francisco, CA), 50 ng per ml recombinant mouse SCF (R&D Systems, Minneapolis, MN), and 5% vol/vol pokeweed mitogen mouse spleen cell–conditioned medium (PWMSCM)5,44. The percent HPCs in S phase of the cell cycle was estimated by the high-specific-activity tritiated thymidine kill technique44. Colonies were scored after a 7-d incubation, and CFU-GM, BFU-E and CFU-GEMM progenitors were distinguished44. Human cord blood cells were separated into a low-density fraction and usually plated at 2.5 × 104 cells/ml in 1% methylcellulose culture medium with 30% FBS and recombinant human cytokines (erythropoietin, 1 U per ml; IL-3, 10 ng per ml; GM-CSF, 10 ng per ml; SCF, 50 ng per ml). CD34+ cord blood cells (>95% CD34+) were purified as described19. Recombinant mouse and recombinant human GM-CSF, IL-3, SCF and Flt3-L were from R&D Systems5,44. Times evaluated for HPC recovery after radiation (H.E.B., S.C. and G.H., unpublished observations), 5-FU (H.E.B., S.C. and G.H., unpublished observations) or Cytoxan35 were based on previous studies in our laboratory. TF-1 colony formation (250–1,000 cells plated per ml) was assessed in 0.3% semisolid agar medium (Difco). For cell survival studies shown in Figure 1a,b, cytokines were added at either day 0 or day 1 to plates without or with SDF-1 (200 ng per ml) added at time 0. For ex vivo expansion studies shown in Figure 1c,d, 1,000 CD34+ cord blood cells were initiated in suspension culture medium with 10% FBS, 100 ng per ml recombinant human Flt3-L, 50 ng per ml recombinant human SCF and 10 ng per ml recombinant human thrombopoietin in the absence or presence of 200 ng per ml recombinant human SDF-1 after the CD34+ cells had been exposed to control medium or 5 mM diprotin A for 1 h, and the cells were then washed. Before initiation of culture, and after 7 d of culture, cells were plated in colony assays for enumeration of absolute numbers of HPCs. Experiments shown in Figure 2e were done as follows: Experiments 1–3 used 2.5 × 104 low-density human cord blood cells per ml. Experiment 4 used 5.0 × 104 unseparated C57BL/6 mouse bone marrow cells per ml. SCF and Flt3-L were respectively used at 50 and 100 ng per ml with recombinant human SCF for experiments 1–3 and recombinant mouse SCF for experiment 4. Recombinant human Flt3-L was used for experiments 1–4. Recombinant human GM-CSF and IL-3 were used at 1 ng per ml for experiment 1 and at 10 ng for experiments 2 and 3, and recombinant mouse GM-CSF and IL-3 were used at 10 ng per ml for experiment 4. All cell cultures were incubated at lowered (5%) 02 in 5% CO2 in a humidified chamber.
Immunophenotyping of stem and progenitor cells
Low-density bone marrow cells were stained as previously described46 to detect and enumerate different classes of stem and progenitor cells47–53. Cells were stained with a PE-conjugated cocktail of lineage (Lin) markers including CD3, CD4, CD45R, Ter1 19 and Gr1. To this cocktail, PE-conjugated anti-CD48 (eBiosciences, San Diego, CA; 12048183) was added so that in all subsequent analyses cells were selected as Lin−CD48−. Lineage antibodies (BD Biosciences, San Diego, CA) that were used include antibodies to CD3 (55064), CD4 (553049), CD45R (553090), Ter1 19 (553673) and Gr1 (553128). Other antibodies used included PeCy7-conjugated Sca1 (BD Biosciences 558162), Alexa Fluor 700-conjugated c-Kit (CD117; eBiosciences 56117282), FITC-conjugated CD34 (BD Biosciences 553733), APC-conjugated Flk2 (CD135; eBiosciences 17135182), PerCpCy5.5-conjugated CD 150 (BioLegend, San Diego, CA 115922) and APC-Cy7-conjugated FcyRII (CD16/32; Biolegend 101328). One microgram antibody was used per 1 million cells for each antibody. Boolean gating was used to identify cells based on SLAM markers50, with Lin-Sca1+CD117+(LSK)CD48-CD 1 50+ the phenotype for long-term HSCs and LSKCD48-CD150- the phenotype for short-term HSCs. With slight modifications to previously published reports47,49,52, long-term HSCs and short-term HSCs were also identified on the basis of CD34 and CD135 expression as (LSK)CD48−CD34−CD135−and LSKCD48-CD34+CD135+, respectively. MPPs were identified as (LSK)CD48−CD34+CD135+, CMPs were identified as Lin-CD48-Sca1−CD117+CD16/32loCD34+, MEPs were identified as Lin-CD48-Sca1−CD117+CD16/32loCD34-, and GMPs were identified as Lin-CD48-Sca1−CD117+CD16/32hiCD34+. Percentages were used to calculate the absolute numbers of all these classes of phenotyped stem and progenitor cells per femur.
HSC engrafting studies
For results shown in Figure 6a, recipient B6.BoyJ (CD45.1+) mice were pretreated s.c. with diprotin A on days −2 (5 µM, twice per day) and days −1 and 0 (5 µM, once per day) (n = 3 mice per group) or pyrogen-free PBS (n = 5 mice per group) as a control, lethally irradiated with 950 cGy on day −1 and transplanted on day 0 with 5 × 105 unseparated and untreated C57BL/6 (CD45.2+) bone marrow cells plus 5 × 105 (B6.BoyJ, CD45.1+) competitor marrow cells. At 6 months, marrow from primary engrafted mice were transplanted intravenously into secondary lethally irradiated B6.BoyJ mice without treatment with diprotin A or competitor cells (n = 9 or 10 mice per group). For results shown in Figure 6b, recipient C57BL/6/B6.BoyJ F1 (CD45.2/ CD45.1+) mice were given sitagliptin (200 µg per mouse per day) or control medium orally at days −1 and 0 (n = 5 mice per group), lethally irradiated with 950 cGy 3 h before the first dose of sitagliptin and injected intravenously with 2 × 105 C57BL/6 (CD45.2+) donor cells plus 2 × 105 B6.BoyJ (CD45.1+) competitor cells 4 h after irradiation and 1 h after the second dose of sitagliptin.
Other reagents
Recombinant human G-CSF was purchased from Amgen Corp. (Thousand Oaks, South San Francisco, CA), and nonmethionylated recombinant human G-CSF was purchased from ProSpec (Tanv TechnoGene, Ltd. Israel). Soluble human DPP4, prepared from human placental tissue, was purchased from MP Biomedicals, LLC (Solon, OH) and used at 1 mU (=76.9 ng) per digestion. Diprotin A was purchased from Peptides International (Louisville, KY) and used at 5 mM for in vitro studies. For mass spectrometry analysis, soluble DPP4 was first incubated with or without diprotin A before the DPP4 was added to the purified GM-CSF or IL-3. Sitagliptin was purchased from Merck (Rahway, NJ) and used at 10 mg per kg body weight, administered by oral gavage. To reduce possibilities of additional stress, oral gavages were limited to no more than three per mouse. GM-CSFR βc site 4 affinity-purified rabbit antibody was made and verified by Enzym Antibodies, LCC (South San Francisco, CA).
Mass spectrometry
The matrix-assisted laser desorption ionization (MALDI) mass spectrometric results were obtained using an Applied Biosystems (Framingham, MA) Voyager DE PRO mass spectrometer at Purdue University, West Lafayette, Indiana. This instrument uses a nitrogen laser (337 nm ultraviolet laser) for ionization with a time-of-flight mass analyzer. The positive ion mass spectra were obtained in the linear mode. The accelerating voltage was set at 25 kV and the grid voltage at 94%, with an extraction delay time at 98 ns. The acquisition mass range for this study was 1,000 to 20,000 Da, with 100 laser shots per spectrum. The sample and matrix (sinapinic acid) were mixed in a ratio of 1 microliter to 1 microliter on the sample plate and allowed to air dry prior to insertion into the mass spectrometer for analysis.
Equilibrium GM-CSF receptor binding analysis
Receptor binding analysis of full-length and truncated recombinant human GM-CSF was done under equilibrating conditions, and data analysis was done by Scatchard plotting essentially as previously described26,30 using the TF-1 leukemic cell line and cord blood CD34+ cells. Carrier-free recombinant human GM-CSF (R&D Systems; Minneapolis, MN) was radioiodinated by the chloramine-T method by Phoenix Pharmaceuticals, Inc. (Burlingame, CA). The [125I]-GM-CSF product was repurified to a concentration of 1.28 µg/ml and had a specific radioactivity of 109.68 Ci/mmole. The chloramine-T method iodinates on tyrosine residues in proteins; therefore, truncation by DPP4 does not reduce the specific radioactivity of the protein by the enzyme’s removal of the N-terminal Ala-Pro. Nonspecific binding was assessed by competing recombinant human [125I]-GM-CSF with a 1,000-fold excess of unlabeled recombinant human GM-CSF. Using TF-1 cells, this results in greater than 95% reduction in noncompeted c.p.m. of bound ligand, which was near background radiation, so specific binding under these conditions was considered to be 100% of measured 125I, which was measured with a Beckman Coulter Gamma 5500B gamma counter (Brea, CA). Linear-regression coefficient of correlation (r2) was considered acceptable at a value of 0.90 or greater.
Phosphorylation
Phosphorylation of JAK2 at tyrosines 1007/1008 and STAT5 at tyrosines 694/699 were assessed using antibodies to phosphorylated JAK2 (3771; 1:20 dilution) and STAT5 (FCMAB/05P; 1:50 dilution) (Cell Signaling Technology, Beverly, MA, and Millipore, Billerica, MA), and multiparameter intracellular flow cytometry54. Both TF-1 and CD34+ cord blood cells were factor starved for 24 h and subsequently stimulated with 10 ng/ml full-length or truncated GM-CSF, or in the ratios of full-length and truncated GM-CSF shown.
DPP4 activity assay
To assess DPP4 enzyme activity, we assayed plasma and whole bone marrow cell lysates obtained from C57BL/6 mice. Plasma was obtained from whole blood and red cell–depleted whole bone marrow cell lysates were made using GLO Lysis Buffer (Promega, Madison, WI, USA). Briefly, 5 × 105 unseparated bone marrow cells were resuspended in 50 µl of GLO Lysis Buffer and incubated at room temperature for 10 min with gentle shaking. Plasma and bone marrow cell lysates were then placed into a 96-well flat-bottom microtiter plate55. Cell lysates and plasma were incubated with 50 µl of the DPP4 substrate Gly-Pro-aminoluciferin and assay buffer optimized for this assay (DPP4-Glo Protease Assay; Promega, Madison, WI, USA). Plates were incubated at 37 °C for 30 min, and DPP4 enzyme activity was measured using an LMAX luminometer (Molecular Devices, Sunnyvale, CA, USA).
Statistical analyses
Results of colony assays and immunophenotyping were assessed by Student’s t-test. P values <0.05 were considered significant. For all colony assays, three plates per experimental point were scored. Numbers of experiments per group are noted in the figure legends or above in the Online Methods section. For recovery of peripheral blood cells after stress (5-FU or 400 cGy), results are provided as a percentage of baseline (steady-state, prestress) for each mouse type, as in some but not all experiments there was variability in pretreatment white blood cell, neutrophils and lymphocyte counts, but not in monocyte, red blood cell and platelet counts, between WT and Dpp4−/− mice. Expressing results as a baseline number in these experiments allowed us to monitor comparative recoveries of each animal type. Analysis of variance was used to assess recovery of blood cell counts for data shown in Supplementary Figures 3 and 4.
Supplementary Material
ACKNOWLEDGMENTS
These studies were supported by US Health Service National Institutes of Health (NIH) grants R01 HL056416, R01 HL067284 and R01 HL112669 and a Center of Excellence in Hematology grant P01 DK090948 to H.E.B., and HL69669 and HL96305 to L.M.P. J.H. was supported as a doctoral candidate on NIH T32 DK07519 (to H.E.B.) and as a postdoc on NIH T32 HL07910 (to H.E.B.); H.A.O. was supported as a postdoc on NIH T32 DK07519; S.M.-G. was supported as a doctoral candidate on NIH R25 GM079657 (to H.E.B.); and T.B.C. was supported as a doctoral candidate on NIH T32 DK07519. S.L.R. was supported by NIH T32 CA111198 for parts of this study. We thank Y.-R. Zhou, The Feinstein Insitute for Medical Research, for the Cre+Cxcr4Fl/Fl mice.
Footnotes
Note: Supplementary information is available in the online version of the paper.
AUTHOR CONTRIBUTIONS
H.E.B. initiated the study, supervised experiments, contributed to concepts and experimental design, scored experiments, interpreted experimental data, did data analysis and wrote the manuscript. J.H. supervised experiments, contributed to concepts and experimental design, conducted and scored experiments, interpreted experimental data, did data analysis and helped in the writing of the manuscript. H.A.O. supervised experiments, contributed to concepts and experimental design, conducted and scored experiments, interpreted experimental data, did data analysis and helped in the writing of the manuscript. C.M. contributed to concepts and experimental design, conducted and scored experiments, interpreted experimental data, did data analysis and helped in the writing of the manuscript. B.R.C. conducted and scored experiments. S.C. conducted experiments and did data analysis. S.M.-G. conducted and scored experiments and did data analysis. G.H. contributed to concepts and experimental design, conducted and scored experiments and did data analysis. S.F. contributed to concepts and experimental design. S.L.R. and X.O. conducted and scored experiments. J.S. contributed to concepts and experimental design. L.M.P. contributed to concepts and experimental design, interpreted experimental data and helped in the writing of the manuscript. E.F.S. contributed to concepts and experimental design and interpreted experimental data. T.B.C. contributed to concepts and experimental design.
COMPETING FINANCIAL INTERESTS
The authors declare competing financial interests: details are available in the online version of the paper.
References
- 1.Broxmeyer HE, Smith FO. Cord blood hematopoietic cell transplantation. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas’ Hematopoietic Cell Transplantation. 4th edn. West Sussex, UK: Wiley-Blackwell; 2009. pp. 559–576. [Google Scholar]
- 2.Shaheen M, Broxmeyer HE. The humoral regulation of hematopoiesis. In: Hoffman R, et al., editors. Hematology: Basic Principles and Practice. 5th edn. Philadelphia: Elsevier Churchill Livingston; 2009. pp. 253–275. [Google Scholar]
- 3.Rasmussen HB, Branner S, Wiberg FC, Wagtmann N. Crystal structure of human dipeptidyl peptidase IV/CD26 in complex with a substrate analog. Nat. Struct. Biol. 2003;10:19–25. doi: 10.1038/nsb882. [DOI] [PubMed] [Google Scholar]
- 4.Christopherson KW, Hangoc G, Broxmeyer HE. Cell surface peptidase CD26/ dipeptidylpeptidase IV regulates CXCL12/stromal cell-derived factor-1 alphamediated chemotaxis of human cord blood CD34+ progenitor cells. J. Immunol. 2002;169:7000–7008. doi: 10.4049/jimmunol.169.12.7000. [DOI] [PubMed] [Google Scholar]
- 5.Christopherson KW, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 6.Tian C, Bagley J, Forman D, Iacomini J. Inhibition of CD26 peptidase activity signifcantly improves engraftment of retrovirally transduced hematopoietic progenitors. Gene Ther. 2006;13:652–658. doi: 10.1038/sj.gt.3302695. [DOI] [PubMed] [Google Scholar]
- 7.Peranteau WH, et al. CD26 inhibition enhances allogeneic donor-cell homing and engraftment after in utero hematopoietic-cell transplantation. Blood. 2006;108:4268–4274. doi: 10.1182/blood-2006-04-018986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wyss BK, et al. Enhanced homing and engraftment of fresh but not ex vivo cultured murine marrow cells in submyeloablated hosts following CD26 inhibition by Diprotin A. Exp. Hematol. 2009;37:814–823. doi: 10.1016/j.exphem.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell TB, Hangoc G, Liu Y, Pollok K, Broxmeyer HE. Inhibition of CD26 in human cord blood CD34+ cells enhances their engraftment of nonobese diabetic/severe combined immunodefciency mice. Stem Cells Dev. 2007;16:347–354. doi: 10.1089/scd.2007.9995. [DOI] [PubMed] [Google Scholar]
- 10.Christopherson KW, Paganessi LA, Napier S, Porecha NK. CD26 inhibition on CD34+ or lineage human umbilical cord blood donor hematopoietic stem cells/ hematopoietic progenitor cells improves long-term engraftment into NOD/SCID/ β2null immunodefcient mice. Stem Cells Dev. 2007;16:355–360. doi: 10.1089/scd.2007.9996. [DOI] [PubMed] [Google Scholar]
- 11.Kawai T, et al. Diprotin A infusion into nonobese diabetic/severe combined immunodefciency mice markedly enhances engraftment of human mobilized CD34+ peripheral blood cells. Stem Cells Dev. 2007;16:361–370. doi: 10.1089/scd.2007.9997. [DOI] [PubMed] [Google Scholar]
- 12.Peled A, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 13.Campbell TB, Broxmeyer HE. CD26 inhibition and hematopoiesis: a novel approach to enhance transplantation. Front. Biosci. 2008;13:1795–1805. doi: 10.2741/2800. [DOI] [PubMed] [Google Scholar]
- 14.Lataillade JJ, et al. Stromal cell-derived factor 1 regulates primitive hematopoiesis by suppressing apoptosis and by promoting G0/G1 transition in CD34+ cells: evidence for an autocrine/paracrine mechanism. Blood. 2002;99:1117–1129. doi: 10.1182/blood.v99.4.1117. [DOI] [PubMed] [Google Scholar]
- 15.Broxmeyer HE, et al. Transgenic expression of stromal cell-derived factor-1/CXC chemokine ligand 12 enhances myeloid progenitor cell survival/antiapoptosis in vitro in response to growth factor withdrawal and enhances myelopoiesis in vivo . J. Immunol. 2003;170:421–429. doi: 10.4049/jimmunol.170.1.421. [DOI] [PubMed] [Google Scholar]
- 16.Broxmeyer HE, et al. Stromal cell-derived factor-1/CXCL12 directly enhances survival/ antiapoptosis of myeloid progenitor cells through CXCR4 and Gαi proteins and enhances engraftment of competitive, repopulating stem cells. J. Leukoc. Biol. 2003;73:630–638. doi: 10.1189/jlb.1002495. [DOI] [PubMed] [Google Scholar]
- 17.Lee Y, et al. Enhancement of intracellular signaling associated with hematopoietic progenitor cell survival in response to SDF-1/CXCL12 in synergy with other cytokines. Blood. 2002;99:4307–4317. doi: 10.1182/blood.v99.12.4307. [DOI] [PubMed] [Google Scholar]
- 18.Piacibello W, et al. Lentiviral gene transfer and ex vivo expansion of human primitive stem cells capable of primary, secondary, and tertiary multilineage repopulation in NOD/SCID mice. Blood. 2002;100:4391–4400. doi: 10.1182/blood.V100.13.4391. [DOI] [PubMed] [Google Scholar]
- 19.Broxmeyer HE, et al. Hematopoietic stem/progenitor cells, generation of induced pluripotent stem cells, and isolation of endothelial progenitors from 21- to 23.5-year cryopreserved cord blood. Blood. 2011;117:4773–4777. doi: 10.1182/blood-2011-01-330514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carow CE, et al. Human multipotential progenitor cells (CFU-GEMM) have extensive replating capacity for secondary CFU-GEMM: An effect enhanced by cord blood plasma. Blood. 1993;81:942–949. [PubMed] [Google Scholar]
- 21.Broxmeyer HE, et al. SDF-1/CXCL12 enhances in vitro replating capacity of murine and human multipotential and macrophage progenitor cells. Stem Cells Dev. 2007;16:589–596. doi: 10.1089/scd.2007.0044. [DOI] [PubMed] [Google Scholar]
- 22.Broxmeyer HE. The comparative effects in vivo of recombinant murine interleukin-3, natural murine colony stimulating factor-1 and recombinant murine granulocyte-macrophage colony stimulating factor on myelopoiesis in mice. J. Clin. Invest. 1987;79:721–730. doi: 10.1172/JCI112877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Broxmeyer HE, et al. Synergistic myelopoietic action in vivo of combinations of purifed natural murine colony stimulating factor-1, recombinant murine interleukin-3, and recombinant murine granulocyte-macrophage colony stimulating factor administered to mice. Proc. Natl. Acad. Sci. USA. 1987;84:3871–3875. doi: 10.1073/pnas.84.11.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broxmeyer HE, et al. Effects in vivo of purifed recombinant human activin and erythropoietin, alone and in combination, in mice. Int. J. Hematol. 1991;54:447–454. [PubMed] [Google Scholar]
- 25.Kitamura T, et al. Establishment and characterization of a unique human cell line that proliferates dependently on GM-CSF, IL-3, or erythropoietin. J. Cell. Physiol. 1989;140:323–334. doi: 10.1002/jcp.1041400219. [DOI] [PubMed] [Google Scholar]
- 26.Woodcock JM, et al. A single tyrosine residue in the membrane-proximal domain of the granulocyte-macrophage colony-stimulating factor, interleukin (IL)-3, and IL-5 receptor common β-chain is necessary and suffcient for high affnity binding and signaling by all three ligands. J. Biol. Chem. 1996;271:25999–26006. doi: 10.1074/jbc.271.42.25999. [DOI] [PubMed] [Google Scholar]
- 27.Woodcock JM, et al. Receptors of the cytokine superfamily: mechanisms of activation and involvement in disease. Baillieres Clin. Haematol. 1997;10:507–524. doi: 10.1016/s0950-3536(97)80023-6. [DOI] [PubMed] [Google Scholar]
- 28.Woodcock JM, et al. The functional basis of granulocyte-macrophage colony stimulating factor, interleukin-3 and interleukin-5 receptor activation, basic and clinical implications. Int. J. Biochem. Cell Biol. 1999;31:1017–1025. doi: 10.1016/s1357-2725(99)00084-9. [DOI] [PubMed] [Google Scholar]
- 29.Haman A, et al. Molecular determinants of the granulocyte-macrophage colony- stimulating factor receptor complex assembly. J. Biol. Chem. 1999;274:34155–34163. doi: 10.1074/jbc.274.48.34155. [DOI] [PubMed] [Google Scholar]
- 30.Niu L, et al. Kinetic resolution of two mechanisms for high-affnity granulocyte-macrophage colony-stimulating factor binding to its receptor. Blood. 1999;94:3748–3753. [PubMed] [Google Scholar]
- 31.Lopez AF, et al. Molecular basis of cytokine receptor activation. IUBMB Life. 2010;62:509–518. doi: 10.1002/iub.350. [DOI] [PubMed] [Google Scholar]
- 32.Guthridge MA, et al. The phosphoserine-585-dependent pathway of the GM-CSF/ IL-3/IL-5 receptors mediates hematopoietic cell survival through activation of NF-κB and induction of bcl-2. Blood. 2004;103:820–827. doi: 10.1182/blood-2003-06-1999. [DOI] [PubMed] [Google Scholar]
- 33.Hansen G, et al. The structure of the GM-CSF receptor complex reveals a distinct mode of cytokine receptor activation. Cell. 2008;134:496–507. doi: 10.1016/j.cell.2008.05.053. [DOI] [PubMed] [Google Scholar]
- 34.Hercus TR, et al. The granulocyte-macrophage colony-stimulating factor receptor: linking its structure to cell signaling and its role in disease. Blood. 2009;114:1289–1298. doi: 10.1182/blood-2008-12-164004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Broxmeyer HE. Inhibition in vivo of mouse granulopoiesis by cell-free activity derived from human polymorphonuclear neutrophils. Blood. 1978;51:889–901. [PubMed] [Google Scholar]
- 36.Chitteti BR, et al. Phenotypic characterization of hematopoietic stem cells. In: Broxmeyer HE, editor. Cord Blood: Biology, Transplantation, Banking, and Regulation. Bethesda, Maryland: AABB Press; 2011. pp. 75–86. [Google Scholar]
- 37.Waterstrat A, et al. Congenic interval of CD45/Ly-5 congenic mice contains multiple genes that may infuence hematopoietic stem cell engraftment. Blood. 2010;115:408–417. doi: 10.1182/blood-2008-03-143370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christopherson KW, et al. G-CSF- and GM-CSF-induced upregulation of CD26 peptidase downregulates the functional chemotactic response of CD34+ . Exp. Hematol. 2006;34:1060–1068. doi: 10.1016/j.exphem.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 39.Kidd S, et al. In vivo expansion of the megakaryocyte progenitor cell population in adult CD26-defcient mice. Exp. Hematol. 2011;39:580–590. doi: 10.1016/j.exphem.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 40.Zaruba MM, et al. Synergy between CD26/DPP-IV inhibition and G-CSF improves cardiac function after acute myocardial infarction. Cell Stem Cell. 2009;4:313–323. doi: 10.1016/j.stem.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Cho SY, et al. The effect of CXCL12 processing on CD34+ cell migration in myeloproliferative neoplasms. Cancer Res. 2010;70:3402–3410. doi: 10.1158/0008-5472.CAN-09-3977. [DOI] [PubMed] [Google Scholar]
- 42.Pang R, et al. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell. 2010;6:603–615. doi: 10.1016/j.stem.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Nie Y, Han Y-C, Zou Y-R. CXCR4 is required for the quiescence of primitive hematopoietic cells. J. Exp. Med. 2008;205:777–783. doi: 10.1084/jem.20072513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Broxmeyer HE, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J. Exp. Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gotoh A, et al. Steel factor induces serine phosphorylation of Stat3 in human growth factor-dependent myeloid cell lines. Blood. 1996;88:138–145. [PubMed] [Google Scholar]
- 46.Adolfsson J, et al. Identifcation of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 47.Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specifc stem cell. Am. J. Pathol. 2006;169:338–346. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chitteti BR, et al. Impact of interactions of cellular components of the bone marrow microenvironment on hematopoietic stem and progenitor cell function. Blood. 2010;115:3239–3248. doi: 10.1182/blood-2009-09-246173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: A simple method to isolate long-term stem cells. Proc. Natl. Acad. Sci. USA. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 51.Kondo M, et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu. Rev. Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- 52.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 53.Sitnicka E, et al. Key role of ft3 ligand in regulation of the common lymphoid progenitor but not in maintenance of the hematopoietic stem cell pool. Immunity. 2002;17:463–472. doi: 10.1016/s1074-7613(02)00419-3. [DOI] [PubMed] [Google Scholar]
- 54.Mantel C, et al. Cells enter a unique intermediate 4N stage, not 4N-G1, after aborted mitosis. Cell Cycle. 2008;7:484–492. doi: 10.4161/cc.7.4.5316. [DOI] [PubMed] [Google Scholar]
- 55.Kajiyama H, et al. Prolonged survival and decreased invasive activity attributable to dipeptidylpeptidase IV overexpression in ovarian carcinoma. Cancer Res. 2002;62:2753–2757. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.