Abstract
Saliva can be easily obtained in medical and non-medical settings, and contains numerous bio-molecules, including those typically found in serum for disease detection and monitoring. In the past two decades, the achievements of high-throughput approaches afforded by biotechnology and nanotechnology allow for disease-specific salivary biomarker discovery and establishment of rapid, multiplex, and miniaturized analytical assays. These developments have dramatically advanced saliva-based diagnostics. In this review, we discuss the current consensus on development of saliva/oral fluid-based diagnostics and provide a summary of recent research advancements of the Texas-Kentucky Saliva Diagnostics Consortium. In the foreseeable future, current research on saliva based diagnostic methods could revolutionize health care.
Introduction
Saliva, or oral fluid, has long been of interest as a substitute for blood and other body fluids for disease diagnosis and disease/drug monitoring because it is readily accessible, as it can be obtained non-invasively. However, saliva diagnostics are not widely used due to the lack of well-defined salivary biomarkers for specific diseases, appropriate technologies for low sample volume analysis, and social and medical professional acceptance 1;2.
To fully realize the potential of saliva as a diagnostic fluid, the National Institute of Dental and Craniofacial Research (NIDCR) of the National Institutes of Health (NIH) has recently invested in research efforts aimed at discovering and validating salivary biomarkers of disease, as well as in the development of dedicated technologies for their measurement. In the past few years, these efforts have fostered interdisciplinary research projects that allow clinicians, biologists, chemists, physicists, engineers, and commercialization partners to collaborate, investigate, discover and translate the potential of saliva to diagnose systemic disorders, such as neoplastic, cardiovascular, metabolic, infectious and neurological diseases.
Based on the reported results of these initial efforts, it may be envisioned that in the foreseeable future, saliva-based diagnostic testing can become a component in routine medical practice in doctors’ offices and/or in the field for disease diagnosis, prevention, screening and monitoring. Dental professionals who encounter saliva/oral fluid in their daily professional life, are perceived as saliva experts in the medical field, and can thus play an important role in the future of salivary diagnostics for dental and systemic diseases.
This brief review provides a description of salivary physiology and provides an update on current advances in salivary biomarker discovery and validation derived from the combined efforts of the Texas/Kentucky Saliva Diagnostic Consortium including the University of Texas Health Science Center at San Antonio, the University of Kentucky, and Rice University. Further, herein described is the development and application of a powerful point of care nano-bio-chip (NBC) technology that hosts saliva-based tests for the measurement of biomarkers for local and systemic diseases.
Saliva/oral fluid physiology
Oral fluid is usually referred to as whole saliva that includes secretions from salivary glands, upper gastrointestinal and respiratory tracks, and the gingival sulcus (crevicular fluid). Human salivary glands produce about 500–1000 mL of saliva per day by three distinct major salivary gland pairs, i.e., parotid, submandibular and sublingual glands, and numerous minor salivary glands in oral palatal, buccal, and labial mucosa. Glandular, parotid, and submandibular/sublingual saliva can be collected non-invasively. Microscopically, a salivary gland secretory unit consists of acina and a ductal system. There are two types of acinar cells, i.e., serous and mucous, depending on protein produced. The ductal system consists of intercalated, striated, and excretory ducts. Salivary secretion is tightly controlled by the autonomic nervous system through a two stage secretion – primary saliva produced by acinar cells, followed by ductal system modification - resulting in a hypotonic solution when it reaches the mouth. Saliva is known to play essential roles in lubrication, digestion, and host defense since it contains electrolytes (e.g., Na+, K+, Cl−, Ca2+, HCO3−, PO43−), digestive enzymes (e.g., amylase, lipases, proteases and DNAse/RNAse, etc.), antimicrobial proteins (e,g. lysozyme, IgA, lactoferin, defensin, peroxidase, histatins, etc.) and other major proteins (e.g., mucins, proline-rich proteins, statherin, etc.).
While salivary gland cells synthesize and secret many salivary components, serum contents such as cytokines, antibodies, hormones, and drugs can also be transferred to saliva by passing through capillary walls in salivary gland tissues. These molecules travel though the basement membrane and salivary cell barriers to enter saliva involving possible mechanisms of passive transcellular diffusion, paracellular ultrafiltration, energy dependent active transport, and/or pinocytosis 3–5. The relationship of salivary molecule concentration to blood (or saliva/plasma ratio; S/P) is influenced by serum/saliva pH, molecular pKa, molecular weight, lipophilicity, and protein binding. Serum components can also be transported to whole saliva via gingival fluids or mucosal cells. Additionally, normal human whole saliva contains numerous normal and pathogenic microorganisms (e.g., bacteria, fungi, or viruses) and their metabolites, as well as multiple cell types shed or migrated from oral mucosa or gingival crevices. Therefore, saliva provides a large number of analytes that are comparable to blood for disease diagnosis and monitoring (Table 1).
Table 1.
Biomarker Discovery and Strategies for Saliva/Oral Fluid Diagnostics
| Potential Biomarkers | Strategies of Saliva Diagnostics |
|---|---|
|
| |
| Electrolytes: Na+, K+, HCO3−, Ca2+, P043− Proteins (Proteome) Salivary proteins Salivary serum components Transcriptome mRNA and miRNA profiles DNA: mammalian cells (epithelial and inflammatory cells and microorganisms (e.g., bacteria, fungi, viruses) |
Multiplex: Multiple samples Multiple analyses High Sensitivity and Specificity Miniaturization: Portable Nanotechnologies: Lab-on-a-Chip (LOC) Automated and Self-powered Point of Care (POC) |
Saliva/oral fluid as diagnostic fluid
Saliva provides biological materials, e.g., mammalian and microorganism proteins, DNAs, and cells for potential medical and law enforcement use. Dentists and oral biologists have utilized the culture counts of Streptococcus mutans and lactobacillus from saliva to predict caries risk 6. It is well known that saliva samples have been used for forensic DNA testing. The development of salivary/oral fluid-based diagnostics has focused on testing hormones, drugs and antibodies with some success in the past few decades. For example, commercialized saliva based testing systems have been used for detection of HIV antibodies with high specificity and sensitivity similar to blood testing 7. Antibodies to hepatitis B, C, and several other infectious pathogens (e.g., rubeola and dengue) can also be detected in saliva 8. Further direct detection of local or systemic infectious pathogens in saliva such as bacteria, viruses, and fungi is also possible by using salivary culture and/or polymerase chain reaction (PCR).
The salivary/oral fluid based home testing system of estradiol has been used to predict premature birth. Changes in salivary levels of estrogen, testosterone, progesterone and electrolytes have been used for monitoring or assessing female reproductive cycles and overall health 3;4. It is also well accepted to assess a subjects’ stress level by measuring salivary cortisol level in psychological studies 4;9. The salivary steroid hormone levels are preferred by many investigators because hormones in saliva are in free form (active form) in contrast to in serum where most hormones are protein bound which complicates the estimation of true activity. Saliva has been widely studied as a medium for pharmacokinetic and therapeutic drug monitoring 2;3. The usefulness of saliva for drug monitoring is dependent on the saliva/plasma (S/P) ratio that has already been established for numerous drugs, a list which is continually expanding. In recent years, there has been vast interest from law enforcement agencies to develop oral fluid based point of detection methods for illegal drugs and/or legal intoxication limits, resulting in an international cooperative study for roadside testing [e.g., European Commission on Roadside testing assessment (Rosita)] 3;10;11. In this study, a large number of recreational and illicit drugs (e.g., amphetamine, opium, alcohol, lysergic acid diethylamide, marijuana, and phencyclidine, etc.) and their metabolites have been evaluated in saliva samples in comparison to their serum counterparts. In addition, the various commercial oral testing prototypes for drug detection will be evaluated with laboratory validation. Salivary cotinine and thiocyanate contents are also commonly used for documenting tobacco use and second-hand smoke exposure. A summary of current and potential saliva/oral fluid diagnostics is listed in Table 2. The future application of saliva/oral fluid diagnostics for medical use, epidemiological studies, and law enforcement is dependent on the availability of reliable point-of-care systems.
Table 2.
Current and Potential use of Saliva/Oral fluid based Diagnostics
| Current existing assays with active development of new detection systems | Potential use in near future |
|---|---|
|
| |
| Pharmacological monitoring 11 Therapeutic drugs Law enforcement applications Drug intoxication Illicit drugs Forensics Smoking exposure cotinine and thiocyanate Steroid Hormones 4 Cortisol, estrogen, testosterone, and progesterone Infectious Diseases 35 Antibody testing: HIV, HCV and HBV Antigen detection: Bacterial, Viral, Fungal DNA/RNA/Protein Microorganism recovery: Bacterial, Viral, Fungal cultures |
Autoimmune diseases 22;23 Autoantibodies: Sjögren’s syndrome Allergic markers Cardiovascular diseases13 Acute myocardial infarction Cardiac risk Cancer Screening and Diagnosis19;36–38 Oral cancer Breast cancer Cancer-specific markers Periodontal Diseases 20;39 |
Current challenges and advances in salivary/oral fluid diagnostics
The challenge for successful use of saliva for medical diagnostics resides in maximizing the advantages and overcoming the disadvantages of using saliva/oral fluids. Compared to serum samples, the volume of saliva that can be obtained is relatively limited and disease-specific salivary biomarkers are still largely unknown. However, saliva can be obtained by patients themselves or by personnel with little medical training. Furthermore, saliva collection is associated with less stress and discomfort to the patient/donor. Therefore, saliva-based diagnostics can be applied in medically disadvantaged areas or non-conventional medical settings, such as in developing or under-developed countries, remote rural areas, patients’ homes, as well as in the dentist’s office or neighborhood pharmacy.
Recent studies have demonstrated improvement of sensitivity and specificity using a combination of multiple biomarkers instead of a single biomarker in disease detection 12;13. Therefore, a successful saliva/oral based diagnostic should provide accurate, non-invasive, disease-specific, multi-analyte and rapid outcome measurements, as well as be portable and cost-effective. Current efforts emphasize the discovery and validation of disease biomarkers in saliva, the development of multiplexed nanotechnologies (lab-on-a-chip) for point-of-care, and their ultimate translation into the real world through an industrial partner (Table 1).
Development of analytical technologies in the post genomic era has allowed for large scale identification of proteins/peptides (proteome) and ribonucleic acids (RNA; transcriptome), and their functions/structures in cells and fluids. The high throughput proteomic studies have catalogued at least 1166 proteins in the major salivary gland secretions, of which 914 are recovered from parotid and 917 from submandibular/sublingual ductal saliva, with 57% of these proteins present in both glandular saliva 14. The proteome of human minor salivary gland secretion showed 56 proteins, 12 of these proteins have never been indentified in the glandular saliva 15. Analysis of human whole saliva and plasma has identified a total of 1939 proteins in whole saliva, with 740 proteins in glandular saliva proteomes and 597 saliva proteins in plasma 16. More surprisingly, the salivary transcriptome (RNAs) has been discovered using microarray profiling in recent years. It is estimated that approximately 3000 messenger RNAs (mRNAs) have been identified in cell-free whole saliva. Most recently, the presence of microRNA (miRNA; ~50) was also discovered in whole saliva. Unlike mRNA, miRNA consists of 18–24 nucleotides transcribed from non-protein coding genes and regulates protein translation through an RNA-induced silencing complex (RIST) 17. These advances have provided a large number of salivary molecular targets, e.g., proteins and RNAs, for disease biomarker discovery. Several investigators have already attempted to use high throughput technologies and current salivary proteomic and transcriptomic knowledge for biomarker discovery in the areas of oral cancer 17;18, breast cancer 19, periodontal diseases 20;21, cardiovascular disease 13 and Sjögren’s syndrome 22;23.
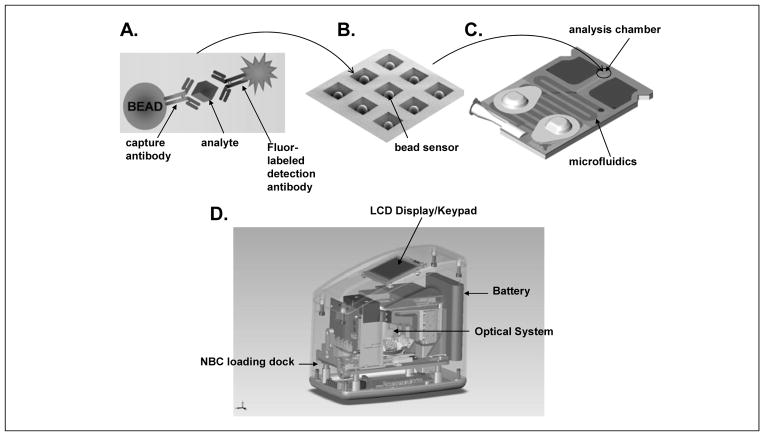
In the past few years, multiplex biomarker detection systems have emerged through remarkable progress in the development of lab-on-a-chip (LOC) and point-of-care (POC) technologies 24. The goal of these efforts is to build automated, miniaturized, and multiplexed platforms for rapid assays and readout. In general, the principles of conventional enzyme-liked immunosorbent assay (ELISA) and/or nucleic acid hybridization are applied often with either electrochemical sensors 12 or a microbead reactor 13;25. The electrochemical approach uses gold electrode arrays (multiplex chips) in which one set of electrodes (i.e., working, counter and reference electrodes) is used for one analyte measurement applied with the cyclic square wave electrical field to facilitate chemical reaction, followed by amperometric readout 12. The UCLA School of Dentistry “UCLA Collaborative Oral Fluid Diagnostic Research Center” is the leading institute for this nano/micro-electrical-mechanical development. Alternatively, the microbead reactor system developed by the Texas-Kentucky Saliva Diagnostic Consortium consists of porous bead sensors consisting of a nano-net of agarose fibers serving as a chemical reaction matrix sequestering and concentrating analytes. The beads are placed in a microchip holder with each bead serving as a 3-dimensional reactor. Multiple beads can be placed in the holder with modulation of their analyte specificity through the capturing antibody they are conjugated to, providing a multi-analyte testing platform. The reaction reagents are delivered through a self-contained microfluidic infrastructure and the measurement is reported by nano-particle fluorescent particles or dyes that are conjugated to detecting antibodies. This approach results in increased signal to noise ratios and amplification several orders above conventional assays 24 (Figure 1).
Figure 1. Microbead-based reactor systems.
Agarose microbeads that serve as single enzyme-linked immunosorbent assay (ELISA) reactor sensors (a) are arrayed in microchips (b) assembled into a disposable microfludic cassette that can be inserted into an analyzer (d) for automated assay execution and processing of image data acquired within this optical sensor (Modified from Jokerst et al. Nanomedicine, 2010 24).
The Texas/Kentucky Saliva Diagnostics Consortium is in the forefront of developing 3-D bead saliva/oral fluid diagnostics for cardiovascular, cancer, and periodontal diseases 13;26;27. As compared to other systems, this approach is cost-effective and more flexible than any other LOC system reported in the literature. For example, the bead reactor can be replaced with a thin-polymeric membrane for analyzing cell isolation/trapping from saliva or serum or oral brush biopsy samples, e.g., oral cancer cell studies.
Current efforts to develop a saliva-based nano-biochip test for acute myocardial infarction (AMI) at the-point-of-care, particularly in the emergency settings, and for cytological diagnosis of oral cancers are briefly described below.
Oral Fluid-based Lab-on-a-chip testing for detection of acute myocardial infraction (AMI) in pre-hospital settings
Cardiovascular disease remains the leading cause of death in developed countries, including the United States. Coronary artery disease (CAD), a major component of cardiovascular diseases, caused 1 of every 5 deaths in the United States in 2004, while CAD mortality was at 451,326. In 2010, an estimated 785,000 Americans will have a new coronary attack, and about 470,000 will have a recurrent attack. It is estimated that an additional 195,000 silent first myocardial infarctions occur each year. Every 26 seconds, an American will have a coronary event, and about every minute someone will die from it 28. The survival of AMI is dependent on how soon intervention can be initiated. Early diagnosis and early intervention is the key for a good patient prognosis.
Currently, electrocardiogram (EKG or ECG) is standard equipment in the emergency medical services (EMS) ambulance setting and is used as a diagnostic standard for emergency triage of patients with chest pain and/or unconsciousness. A typical EKG abnormality for an AMI is a ST segment elevation (STEMI). Unfortunately, EKG alone only identifies ~35 % of all AMI cases admitted to the emergency department (ED) and misses the remaining 65% (NSTEMI) that do not exhibit the characteristic EKG changes. The triage of potential AMI cases in the ED depends on supplemental blood testing that often includes cardiac troponins T and I (cTnT, cTnI), creatine kinases-MB (CK-MB), total CK and myoglobin (MYO) 13. However, these tests are, for the most part, limited to the clinical laboratory setting and the few that have been developed for point of care testing lack the analytical and clinical sensitivity and specificity to efficiently diagnose AMI 29. Furthermore, the invasive nature of blood testing and the absence of a sensitive enough test on a point of care instrument that could perform such a test preclude use of a blood test in an ambulance setting. There is indeed a need to have a non-invasive test with the required analytical and clinical performance that could be used in an ambulance setting to minimize the time from diagnosis to treatment of AMI patients. Saliva presents itself as an ideal fluid in this situation (Figure 2).
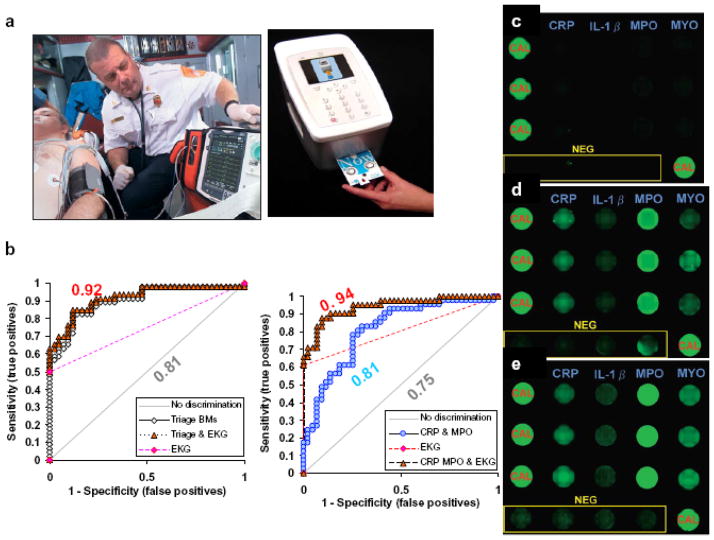
Figure 2. Saliva AMI testing in ambulance.
(a) 12 lead EKG used by paramedics to transmit initial findings to emergency room physicians (left). The portable saliva-based diagnostics NBC platform can complement EKG for the identification of AMI cases. (b) Logistic regression and ROC analysis using serum and salivary biomarkers in conjunction with EKG exhibited improvement of diagnosis of AMI. The EKG and AMI biomarkers of 42 healthy controls, 46 AMI (23 NSTEMI and 23 STEMI) are measured and compared. In serum, the ROC curve was improved from 0.81 to 0.92 in triage biomarkers (cTnI, myoglobin and CK-MB) were used as diagnostic indexes (left). However, the combined use of salivary CRP and MPO in conjunction with EKG (right), produced an excellent ROC 0.94 (i.e., >90% specificity and sensitivity of AMI diagnosis). (c) Multiplex lab-on-a-chip (LOC) for AMI biomarker antigens screening. Examples of fluorescence micrographs of a LOC multiplex assay for CRP, IL-1_, MYO and MPO are shown for non-AMI control, (d) NSTEMI and (e) STEMI patients. NEG, negative; CAL, calibrator (Modified from Floriano et al. Clin Chem, 2009 40).
To achieve this goal, our collaborative research group has first evaluated the potential use of AMI biomarkers in saliva. Unstimulated whole saliva was collected within 48 hours from more than 80 patients with a definitive diagnosis of AMI and from more than 80 healthy controls. Samples were assayed for 21 cardiac related proteins using conventional methodologies, such as LUMINEX, ELISA and Beckman Access instrumentation. Data gathered demonstrated cardiac biomarkers/proteins such as C-reactive protein (CRP), myeloperoxidase (MPO), interleukins, matrix metallo-perteinase-9 (MMP-9), and cellular adhesion molecule-1 (sICAM-1), can be detected in saliva samples but, most importantly, demonstrated a capacity to differentiate healthy controls from AMI patients. Strikingly, the logistic regression and receiver operating characteristics (ROC) analysis shows that AMI diagnosis was greatly improved with combination of EKG and the AMI proteins in saliva 13. For example one model has shown that the area under the ROC curve (AUC) was improved from 0.75 to 0.94 if the EKG readout was combined with salivary markers CRP and MPO 13 (Figure 2).
In parallel to discovering salivary AMI biomarkers, the critical steps for salivary marker measurement using NBC technology include ambulance sample collection, temperature, humidity, reagent stability, mechanical disturbance of the instrument, compromised light source, and sample contamination are being developed and standardized. Here, the NBC-based sensor system is in development as a portable, modular device (Figure 1) dedicated to saliva-based diagnosis of AMI 24. In Figure 1, an example of a fluorescence micrograph of a LOC multiplex assay for salivary CRP, IL-1β, MYO, and MPO in healthy control, NSTEMI, and STEMI patients is shown 13. The results demonstrate the increased expression of AMI biomarkers in saliva from NSTEMI and STEMI cardiac subjects as compared to a healthy control. This promising evidence suggests that saliva-based tests using the NBC system could provide a more convenient rapid screening method for initial and subsequent cardiac events in pre-hospital stage AMI patients.
Exfoliative cytology based on nano-bio-chip sensor platform for oral cancer detection
Oral cancer is a global health problem afflicting over 300,000 people worldwide each year. In the United States, greater than 35,000 new cases and nearly 7,600 deaths were estimated in 2009 30. Despite surgical and therapeutic advances in the treatment of oral cancer, the five-year survival rate (approximately 50%) remains among the lowest for all major cancers. At the present time, early diagnosis and intervention is the key for a better prognosis underscoring the value of advanced screening and diagnostic techniques for oral cancer and, more importantly, pre-cancerous lesions.
As compared to the conventional surgical biopsy procedure, an approach that is rapid and less invasive is desirable for early detection andscreening. Recently, non-invasive exfoliative cytology using OralCDx® Brush Test (OralScan Laboratories, Suffern, NY) has been widely promoted for oral cancer screening. This technique is based on quantitative cytomorphometry and DNA aneuploidy with computer-assisted analysis 31. However, the limited specificity of current cytology-based analysis is still a major hindrance for early oral cancer detection and intervention 32;33. Since exfoliative cytology also gathers cellular DNA, RNA, and protein biomarkers, new diagnostic techniques targeting early tumor biomarkers and molecular transformation could enhance the role and utility of oral cytology in clinical diagnostics.
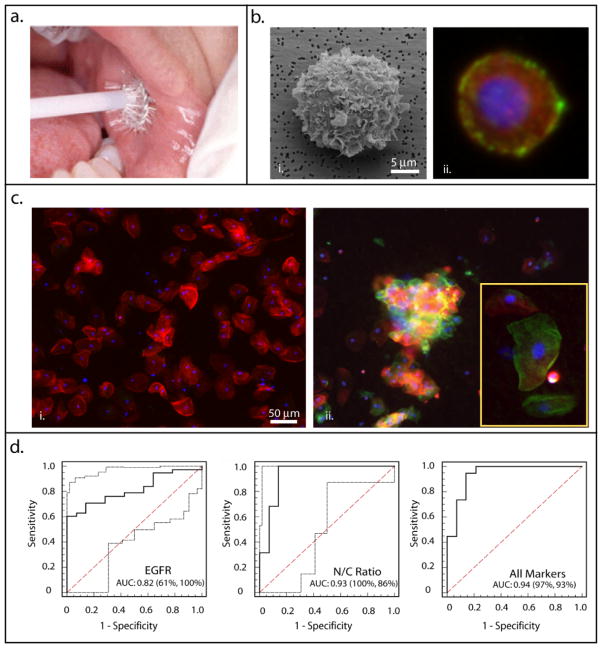
Addressing this clinical need, research groups at Rice and the University of Texas Health Science Centers at San Antonio and Houston have adapted the bead-based NBC sensor system to establish a platform for whole cell analysis of tumor biomarkers in oral exfoliative cytological specimens 34. The cellular-NBC sensor replaces the microbead array, found in the saliva-based NBC design, with a porous membrane that functions as a micro-sieve to capture and screen cells from a cytology suspension (Figure 3). Once captured, “on membrane” immunofluorescent assays reveal the presence and isotype of interrogated cells via automated microscopic imaging and analysis. This technique and its potential in oral diagnostics were recently described in a pilot study examining both molecular and morphological biomarkers associated with oral dysplasia and malignancy 34. Here, the oral epithelial cells (<10μm) were captured on a membrane filter (0.4μm pore size) followed by immunofluorescent labeling for the well-known epidermal growth factor receptor (EGFR) biomarker. Concurrently, the cytoplasm and nuclei were stained with fluorescent dyes Phalloidin and DAPI, respectively, for cytomorphometric measurements (Figure 3). The nuclear area, nuclear diameter, N/C ratio, and EGFR expression were found to be significantly altered in malignant and dysplastic oral lesions as compared to normal control epithelial cells. Logistic regression and ROC curve analysis further identified the morphological features as the best predictors of disease individually, [AUC ≤ 0.93 (97–100% sensitivity and 86% specificity)], while a combination of morphometric and EGFR biomarker expression further enhanced discrimination power between cancerous/precancerous and healthy conditions to an AUC 0.94 (97% sensitivity and 93% specificity; Figure 3). These results suggest that the combined cytomorphometry and EGFR panel likely holds the greatest potential for cancer detection and diagnosis. Yet, the true diagnostic need lies not in the identification of oral cancer but in the identification of dysplastic, premalignant lesions. An 850-patient clinical study targeting oral dysplasia using an expanded biomarker panel is currently underway and aims to further validate the clinical utility of the NBC system for early detection of high-risk premalignant oral lesions. With continued advances in cancer biomarker discovery and sensor technology, rapid and POC screening for cancer is likely achievable.
Figure 3. Application of the cell-based NBC sensor system for cytological assessment of healthy and cancerous oral mucosa.
(a) Exfoliative cytology specimens were obtained using the OralCDx® cytobrush (http://www.sopreventable.com/How2Use.htm); (b) Next, cells were captured on the membrane filter (panel i) followed by EGFR immunolabeling (panel ii, green) and staining of the cell cytoplasm (red) and nuclei (blue) for morphometric measurement; (c) Representative images of healthy epithelia (panel i) and a cancerous lesion (panel ii) examined using the NBC sensor illustrate the increase in EGFR expression and nuclear-to-cytoplasm ratio associated with disease progression; and (d) Logistic regression and ROC analysis of individual and combined biomarkers (adapted from Weigum et al. Cancer Detect. Prevent, 2010 34).
Conclusion
Saliva and oral fluids contain multiple biomarker materials that can be readily obtained in conventional and non-conventional medical or medical laboratory settings. With current advancements in the development of nano-bio-technology, saliva is now closer to meet its full capability to be used as a diagnostic fluid at the point of care. While oral fluid/saliva-based diagnosis of AMI is demonstrated, use of a saliva-based test is not intended to replace current serum based diagnosis, but simply to complement it. Current major challenges are discovery of disease specific markers, determinations of specificity and sensitivity of the specific tests, and standardization of saliva collection methods and holding solutions. Once these challenges are met, saliva-based diagnostics can be validated within the context of large clinical studies en route to final approval by the Food and Drug Administration (FDA) for ultimate clinical/field application. While still several years away from achieving this goal, practicing dentists, as a part of the health care team, should be kept updated about developments in the field of saliva/oral fluid diagnostics for oral and systemic diseases.
Acknowledgments
This review was supported by NIDCR/NIH U01 DE017793 grant which funded the program entitled “Development of a Lab-on-a-chip System for Saliva-Based Diagnostics”
References
- 1.Tabak LA. A revolution in biomedical assessment: the development of salivary diagnostics. J Dent Educ. 2001;65(12):1335–1339. [PubMed] [Google Scholar]
- 2.Mandel ID. The diagnostic uses of saliva. J Oral Pathol Med. 1990;19(3):119–125. doi: 10.1111/j.1600-0714.1990.tb00809.x. [DOI] [PubMed] [Google Scholar]
- 3.Choo RE, Huestis MA. Oral fluid as a diagnostic tool. Clin Chem Lab Med. 2004;42(11):1273–1287. doi: 10.1515/CCLM.2004.248. [DOI] [PubMed] [Google Scholar]
- 4.Groschl M. Current status of salivary hormone analysis. Clin Chem. 2008;54(11):1759–1769. doi: 10.1373/clinchem.2008.108910. [DOI] [PubMed] [Google Scholar]
- 5.Lee YH, Wong DT. Saliva: an emerging biofluid for early detection of diseases. Am J Dent. 2009;22(4):241–248. [PMC free article] [PubMed] [Google Scholar]
- 6.Arellano M, Jiang J, Zhou X, et al. Current advances in identification of cancer biomarkers in saliva. Front Biosci (Schol Ed) 2009;1:296–303. doi: 10.2741/S27. [DOI] [PubMed] [Google Scholar]
- 7.Roberts KJ, Grusky O, Swanson AN. Outcomes of blood and oral fluid rapid HIV testing: a literature review, 2000–2006. AIDS Patient Care STDS. 2007;21(9):621–637. doi: 10.1089/apc.2006.0196. [DOI] [PubMed] [Google Scholar]
- 8.Lima DP, Diniz DG, Moimaz SA, Sumida DH, Okamoto AC. Saliva: reflection of the body. Int J Infect Dis. 2009 doi: 10.1016/j.ijid.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Vreeburg SA, Hoogendijk WJ, van PJ, et al. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry. 2009;66(6):617–626. doi: 10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- 10.Samyn N, Laloup M, De BG. Bioanalytical procedures for determination of drugs of abuse in oral fluid. Anal Bioanal Chem. 2007;388(7):1437–1453. doi: 10.1007/s00216-007-1245-8. [DOI] [PubMed] [Google Scholar]
- 11.Lillsunde P. Analytical techniques for drug detection in oral fluid. Ther Drug Monit. 2008;30(2):181–187. doi: 10.1097/FTD.0b013e3181685088. [DOI] [PubMed] [Google Scholar]
- 12.Wei F, Patel P, Liao W, et al. Electrochemical sensor for multiplex biomarkers detection. Clin Cancer Res. 2009;15(13):4446–4452. doi: 10.1158/1078-0432.CCR-09-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Floriano PN, Christodoulides N, Miller CS, et al. Use of saliva-based nano-biochip tests for acute myocardial infarction at the point of care: a feasibility study. Clin Chem. 2009;55(8):1530–1538. doi: 10.1373/clinchem.2008.117713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denny P, Hagen FK, Hardt M, et al. The proteomes of human parotid and submandibular/sublingual gland salivas collected as the ductal secretions. J Proteome Res. 2008;7(5):1994–2006. doi: 10.1021/pr700764j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siqueira WL, Salih E, Wan DL, Helmerhorst EJ, Oppenheim FG. Proteome of human minor salivary gland secretion. J Dent Res. 2008;87(5):445–450. doi: 10.1177/154405910808700508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun X, Salih E, Oppenheim FG, Helmerhorst EJ. Activity-based mass spectrometric characterization of proteases and inhibitors in human saliva. Proteomics Clin Appl. 2009;3(7):810–820. doi: 10.1002/prca.200800242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park NJ, Zhou H, Elashoff D, et al. Salivary microRNA: discovery, characterization, and clinical utility for oral cancer detection. Clin Cancer Res. 2009;15(17):5473–5477. doi: 10.1158/1078-0432.CCR-09-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JM, Garon E, Wong DT. Salivary diagnostics. Orthod Craniofac Res. 2009;12(3):206–211. doi: 10.1111/j.1601-6343.2009.01454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Streckfus CF, Storthz KA, Bigler L, Dubinsky WP. A Comparison of the Proteomic Expression in Pooled Saliva Specimens from Individuals Diagnosed with Ductal Carcinoma of the Breast with and without Lymph Node Involvement. J Oncol. 2009;2009:Article ID 737619. doi: 10.1155/2009/737619. http://www.ncbi.nlm.nih.gov.libproxy.uthscsa.edu/pmc/articles/PMC2801014/pdf/JO2009-737619.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giannobile WV, Beikler T, Kinney JS, et al. Saliva as a diagnostic tool for periodontal disease: current state and future directions. Periodontol 2000. 2009;50:52–64. doi: 10.1111/j.1600-0757.2008.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christodoulides N, Floriano PN, Miller CS, et al. Lab-on-a-chip methods for point-of-care measurements of salivary biomarkers of periodontitis. Ann N Y Acad Sci. 2007;1098:411–428. doi: 10.1196/annals.1384.035. [DOI] [PubMed] [Google Scholar]
- 22.Baldini C, Giusti L, Bazzichi L, Lucacchini A, Bombardieri S. Proteomic analysis of the saliva: a clue for understanding primary from secondary Sjogren’s syndrome? Autoimmun Rev. 2008;7(3):185–191. doi: 10.1016/j.autrev.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Ryu OH, Atkinson JC, Hoehn GT, Illei GG, Hart TC. Identification of parotid salivary biomarkers in Sjogren’s syndrome by surface-enhanced laser desorption/ionization time-of-flight mass spectrometry and two-dimensional difference gel electrophoresis. Rheumatology (Oxford) 2006;45(9):1077–1086. doi: 10.1093/rheumatology/kei212. [DOI] [PubMed] [Google Scholar]
- 24.Jokerst JV, McDevitt JT. Programmable nano-bio-chips: multifunctional clinical tools for use at the point-of-care. Nanomed. 2010;5(1):143–155. doi: 10.2217/nnm.09.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu C, Qiu X, Ongagna S, et al. A timer-actuated immunoassay cassette for detecting molecular markers in oral fluids. Lab Chip. 2009;9(6):768–776. doi: 10.1039/b814322f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jokerst JV, Raamanathan A, Christodoulides N, et al. Nano-bio-chips for high performance multiplexed protein detection: determinations of cancer biomarkers in serum and saliva using quantum dot bioconjugate labels. Biosens Bioelectron. 2009;24(12):3622–3629. doi: 10.1016/j.bios.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christodoulides N, Floriano PN, Miller CS, et al. Lab-on-a-chip methods for point-of-care measurements of salivary biomarkers of periodontitis. Ann N Y Acad Sci. 2007;1098:411–428. doi: 10.1196/annals.1384.035. [DOI] [PubMed] [Google Scholar]
- 28.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart Disease and Stroke Statistics--2010 Update. A Report From the American Heart Association. Circulation. 2009 doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 29.Fermann GJ, Suyama J. Point of care testing in the emergency department. J Emerg Med. 2002;22(4):393–404. doi: 10.1016/s0736-4679(02)00429-8. [DOI] [PubMed] [Google Scholar]
- 30.American Cancer Society. Cancer Facts and Figures 2009. American Cancer Society; 2009. [Google Scholar]
- 31.Sciubba JJ. Improving detection of precancerous and cancerous oral lesions. Computer-assisted analysis of the oral brush biopsy. U.S. Collaborative OralCDx Study Group. J Am Dent Assoc. 1999;130(10):1445–1457. doi: 10.14219/jada.archive.1999.0055. [DOI] [PubMed] [Google Scholar]
- 32.Poate TW, Buchanan JA, Hodgson TA, et al. An audit of the efficacy of the oral brush biopsy technique in a specialist Oral Medicine unit. Oral Oncol. 2004;40(8):829–834. doi: 10.1016/j.oraloncology.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Scheifele C, Schmidt-Westhausen AM, Dietrich T, Reichart PA. The sensitivity and specificity of the OralCDx technique: evaluation of 103 cases. Oral Oncol. 2004;40(8):824–828. doi: 10.1016/j.oraloncology.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Weigum SE, Floriano PN, Redding SW, et al. Nano-bio-chip sensor platform for examination of oral exfoliative cytology. Cancer Detect Prevent. 2010;3(4):518–528. doi: 10.1158/1940-6207.CAPR-09-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malamud D, Abrams WR, Bau H, et al. Oral-based techniques for the diagnosis of infectious diseases. J Calif Dent Assoc. 2006;34(4):297–301. [PubMed] [Google Scholar]
- 36.Hu S, Arellano M, Boontheung P, et al. Salivary proteomics for oral cancer biomarker discovery. Clin Cancer Res. 2008;14(19):6246–6252. doi: 10.1158/1078-0432.CCR-07-5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weigum SE, Floriano PN, Christodoulides N, McDevitt JT. Cell-based sensor for analysis of EGFR biomarker expression in oral cancer. Lab Chip. 2007;7(8):995–1003. doi: 10.1039/b703918b. [DOI] [PubMed] [Google Scholar]
- 38.Jokerst JV, Raamanathan A, Christodoulides N, et al. Nano-bio-chips for high performance multiplexed protein detection: determinations of cancer biomarkers in serum and saliva using quantum dot bioconjugate labels. Biosens Bioelectron. 2009;24(12):3622–3629. doi: 10.1016/j.bios.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christodoulides N, Floriano PN, Miller CS, et al. Lab-on-a-chip methods for point-of-care measurements of salivary biomarkers of periodontitis. Ann N Y Acad Sci. 2007;1098:411–428. doi: 10.1196/annals.1384.035. [DOI] [PubMed] [Google Scholar]
- 40.Floriano PN, Christodoulides N, Miller CS, et al. Use of saliva-based nano-biochip tests for acute myocardial infarction at the point of care: a feasibility study. Clin Chem. 2009;55(8):1530–1538. doi: 10.1373/clinchem.2008.117713. [DOI] [PMC free article] [PubMed] [Google Scholar]