Abstract
X-linked hypophosphatemia (XLH) is caused by mutations in the PHEX gene, which increase circulating levels of the phosphaturic hormone, fibroblast growth factor 23 (FGF23). Since XLH is a dominant disease, one mutant allele is sufficient for manifestation of the disease. However, dosage effect of a PHEX mutation in XLH is not completely understood. To examine the effect of Phex genotypes, we compared serum biochemistries and skeletal measures between all five possible genotypes of a new murine model of XLH (PhexK496X or PhexJrt). Compared to sex-matched littermate controls, all Phex mutant mice had hypophosphatemia, mild hypocalcemia, and increased parathyroid hormone and alkaline phosphatase levels. Furthermore, mutant mice had markedly elevated serum Fgf23 levels due to increased Fgf23 expression and reduced cleavage of Fgf23. Although females with a homozygous Phex mutation were slightly more hypocalcemic and hypophosphatemic than heterozygous females, the two groups had comparable intact Fgf23 levels. Similarly, there was no difference in intact Fgf23 or phosphorus concentrations between hemizygous males and heterozygous females. Compared to heterozygous females, homozygous counterparts were significantly smaller and had shorter femurs with reduced bone mineral density, suggesting the existence of dosage effect in the skeletal phenotype of XLH. However, overall phenotypic trends in regards to mineral ion homeostasis were mostly unaffected by the presence of one or two mutant Phex allele(s). The lack of gene dosage effect on circulating Fgf23 (and thus, phosphorus) levels suggests that a Phex mutation may create the lower set point for extracellular phosphate concentrations.
Keywords: gene dosage effect, Fgf23, phosphate, Phex, X-linked hypophosphatemia
Introduction
X-linked hypophosphatemia (XLH) is characterized by hypophosphatemia and low or inappropriately normal serum 1,25-dihydroxyvitamin D [1,25(OH)2D, calcitriol] concentrations, resulting in rickets and osteomalacia. The primary cause of the hypophosphatemia is elevated or inappropriately normal levels of the circulating phosphaturic hormone, fibroblast growth factor 23 (FGF23) [1-3], which binds to Klotho (Kl):FGF receptor complex in the kidney [4, 5]. In the kidney, FGF23 enhances renal tubular phosphate excretion by reducing expression of sodium-phosphate co-transporters IIa and IIc (encoded by Slc34a1 and Slc34a3, respectively) [6, 7]. In addition, FGF23 suppresses 1,25(OH)2D production by inhibiting 25-hydroxyvitamin D-1α–hydroxylase (Cyp27b1), which converts 25-hydroxyvitamin D to 1,25(OH)2D, and stimulating 24-hydroxylase (Cyp24a1), which inactivates 1,25(OH)2D [6, 7].
XLH is caused by inactivating mutations in the PHEX gene located on the X chromosome, which has homology to a family of endopeptidase genes [8, 9]. Since the mode of transmission is X-linked dominant, only one mutant allele is sufficient to manifest the disease and thus, heterozygous females are affected. However, it remains unclear whether heterozygous females having one normal allele have a less severe phenotype than hemizygous males as there is supporting evidence for and against gene dosage effect [10-14].
There exist several murine models of XLH [15-18]. Many studies were done on Hyp mice, which have a large deletion spanning the 3′ end of the Phex gene [19, 20]. However, none of the previous studies examined dosage effect of Phex mutations on concentrations of intact Fgf23 and Fgf23 fragments. In this study, we used a new murine model of XLH [21] to investigate how the number of Phex mutant alleles affects the disease phenotype, particularly Fgf23 levels, between hemizygous males (−/Y), heterozygous females (+/−), and homozygous females (−/−).
Materials and Methods
Animals
A new murine model of XLH carries a nonsense mutation (K496X) in exon 14 of the Phex gene. The PhexK496X mouse line was created and kindly provided by Drs. Jane E. Aubin and Frieda Chen at the University of Toronto and Dr. Ann M. Flenniken, Celeste Owen and other members of the Centre for Modeling Human Disease, Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada. A detailed phenotype of this animal (reported as PhexJrt) is described elsewhere [21]. To eliminate the effect of background strain, Phex mutant mice were backcrossed to C57/BL6J at least 10 generations. All experimental mice were generated by mating either unaffected (+/Y) or affected males (−/Y) to heterozygous females (+/−). All animals were maintained on a regular rodent diet, which contained 1.01% calcium, 0.65% phosphorus, and 2.05 IU/g vitamin D3 (Teklad Global 18% Protein Extruded Rodent Diet, 2018SX, Harlan Teklad, Madison, WI). The animals had access to the diet and tap water ad libitum. All animal studies were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee.
Biochemical measurements
Six-week-old mice were anesthetized with ketamine/xylazine mix, and blood was drawn by cardiac puncture and stored at −80 °C until analysis. Serum alkaline phosphatase, calcium, creatinine, and phosphorus were measured, using Roche COBAS Mira S (Roche Diagnostics, Indianapolis, Indiana). Intact Fgf23 levels were measured using FGF-23 ELISA Kit, which detects only intact Fgf23 (Kainos Laboratories, Inc., Tokyo, Japan). Fgf23 levels were also measured using mouse FGF-23 (C-Term) ELISA kit, which detects both intact Fgf23 protein and C-terminal Fgf23 fragments (Immutopics International, San Clemente, CA). Parathyroid hormone (Pth) was measured, using mouse PTH 1-84 ELISA kit (Immutopics). 1,25(OH)2D was measured, using 1,25-Dihydroxy Vitamin D EIA kit (Immunodiagnostic Systems Inc., Scottsdale, AZ).
mRNA quantification
After the blood draw, femurs and kidneys were collected and stored in RNAlater RNA Stabilization Reagent (QIAGEN Inc., Valencia, CA). Total RNA was extracted from the whole femurs and kidneys, using TRIzol Plus RNA Purification System (Invitrogen, Carlsbad, CA) and used for first-strand cDNA synthesis, using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). The cDNA was subsequently used for quantification of Fgf23, Slc34a1, Slc34a3, Kl, Cyp27b1, and Cyp24a1 expression by probe-based quantitative PCR, using TaqMan® Gene Expression Master Mix in the 7900HT Fast Real-Time PCR System (Applied Biosystems). To identify stably expressed genes for normalization, eight different “housekeeping” genes (Actb, Gapdh, Gusb, Hmbs, Hprt, Sdha, Tbp, and Tfrc) were amplified in eight cDNA samples each of all five possible genotypes. One of the two most stable genes determined by NormFinder [22] was used to normalize gene expression -Gapdh for the kidney and Tbp for the femur. Relative gene expression was determined by analyzing the data using the relative standard curve method. Probe-based quantitative real-time PCR assays (Integrated DNA Technologies, Coralville, IA) are listed in Supplemental Table 1.
Dual-energy x-ray absorptiometry (DXA)
Femurs were harvested from mice and fixed in 10% neutral-buffered formalin for 2 days. Areal bone mineral density (BMD) and bone mineral content (BMC) were measured, using a PIXImus2 densitometer (LUNAR Corp, Madison, WI). Coefficient of variation from 11 measurements of a frozen mouse specimen was 0.57% for BMD.
Statistical analysis
A male mouse (+/Y) with only one kidney and a female mouse with unexpected genotype (based on her parental genotypes) were excluded from all analyses. Differences between five genotypic groups were tested using analysis of variance (ANOVA). When the ANOVA p-values were significant, differences between two groups were tested for significance using unpaired student’s t-test. P-values less than 0.05 were considered significant for all analyses.
Results
Comparison between wild-type and mutant mice
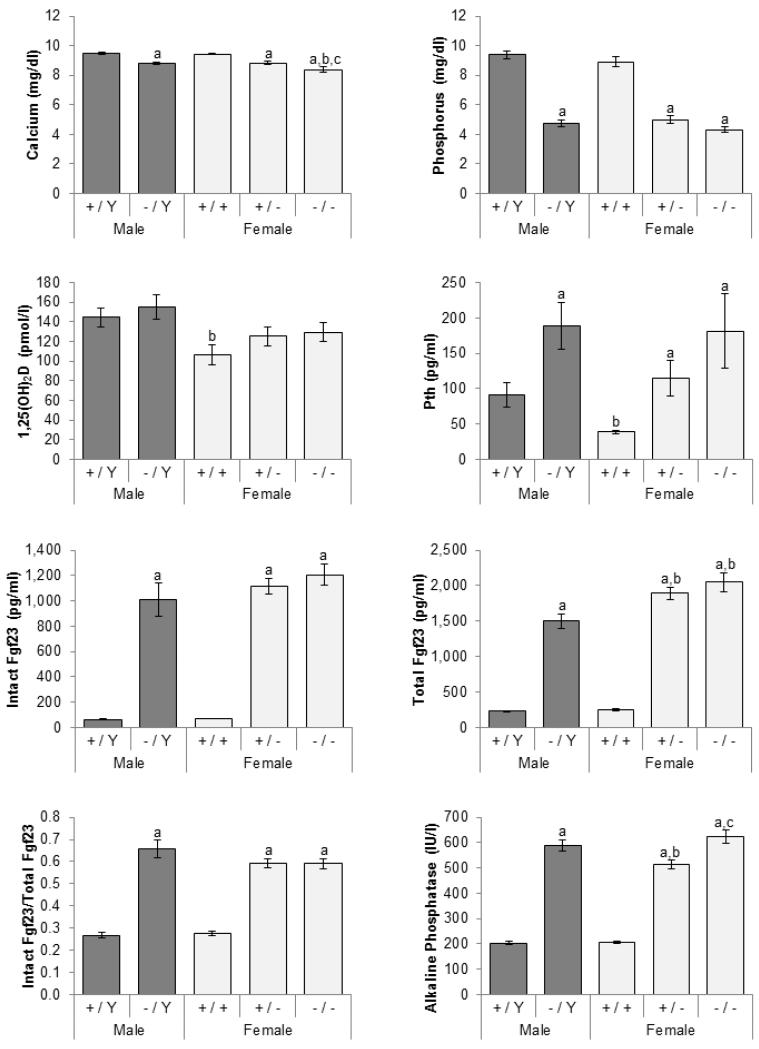
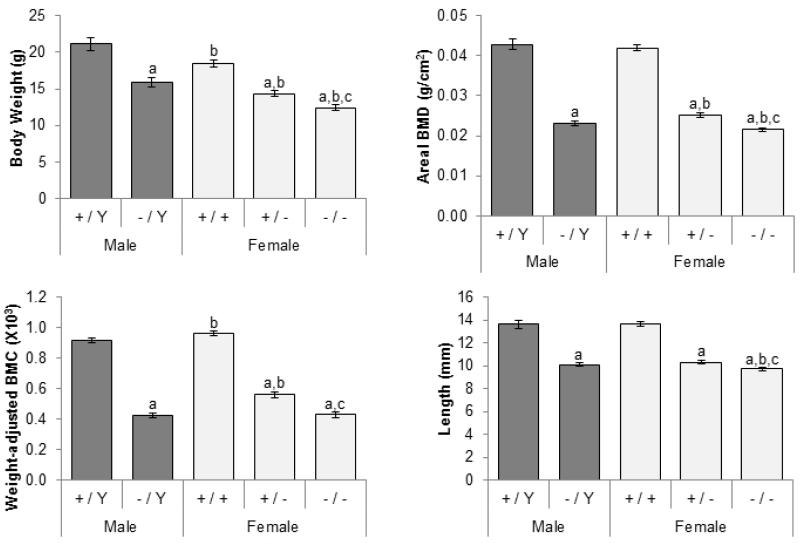
Compared to sex-matched littermate controls, carriers of at least one mutant allele invariably had low serum phosphorus, calcium, creatinine, as well as high alkaline phosphatase levels (Figure 1; creatinine data not shown). In the face of hypophosphatemia, 1,25(OH)2D concentrations were inappropriately normal, and Pth levels were elevated in mutant mice. Femurs in mutant mice were significantly shorter and had lower areal BMD (Figure 2). Since mutant mice were smaller, BMC was adjusted for body weight. Even after the adjustment, BMC was still lower in the mutant mice.
Figure 1.
Effects of Phex genotypes on serum biochemistries. Total Fgf23 represents intact Fgf23 protein and C-terminal fragments of Fgf23 measured by C-terminal ELISA. Dark gray bars, males; light gray, females. Number of animals = 11-13 per group. The differences between five genotypic groups were significant for all measured parameters (ANOVA p-values < 0.05). Differences between individual groups are indicated by superscript letters: Significant difference to same-sex littermate controls (+/Y vs. −/Y and +/+ vs. +/− or +/+)a, comparable male genotypes (+/Y vs. +/+ and −/Y vs. +/− or −/−)b, and heterozygous females (+/− vs. −/−)c by unpaired t-test (p-value < 0.05). All values are presented as mean ± SEM.
Figure 2.
Effects of Phex genotype on body weight and skeletal phenotype (femur). Dark gray bars, males; light gray, females. Number of animals = 6-13 per group. The differences between five genotypic groups were significant for all measured parameters (ANOVA p-values < 0.05). Differences between individual groups are indicated by superscript letters: Significant difference to same-sex littermate controls (+/Y vs. −/Y and +/+ vs. +/− or +/+)a, comparable male genotypes (+/Y vs. +/+ and −/Y vs. +/− or −/−)b, and heterozygous females (+/− vs. −/−)c by unpaired t-test (p-value < 0.05). All values are presented as mean ± SEM.
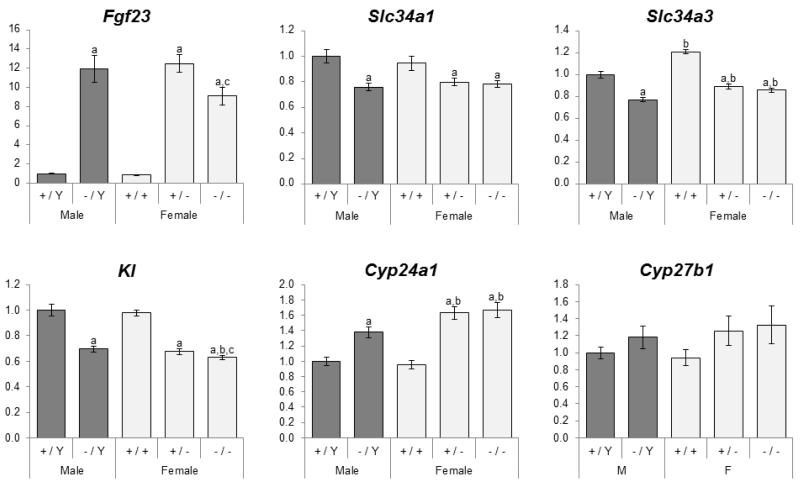
Circulating Fgf23 concentrations (measured by both intact and C-terminal ELISA) were significantly elevated in Phex mutant mice (Figure 1). Similarly, Fgf23 mRNA expression in mutant mice was 11-15 fold higher than that of wild-type mice (Figure 3). In addition to the increased Fgf23 expression, the proportion of intact Fgf23 in circulation was 32-39% higher in the mutant mice than in normal littermates (Figure 1).
Figure 3.
Effects of Phex genotype on expression of Fgf23 in the femur and its target genes in the kidney. Values are presented by arbitrarily setting wild-type males as 1.0. Dark gray bars, males; light gray, females. Number of animals = 9-13 per group. The differences between five genotypic groups were significant for all measured parameters except Cyp27b1 (ANOVA p-values < 0.05). Differences between individual groups are indicated by superscript letters: Significant difference to same-sex littermate controls (+/Y vs. −/Y and +/+ vs. +/− or +/+)a, comparable male genotypes (+/Y vs. +/+ and −/Y vs. +/− or −/−)b, and heterozygous females (+/− vs. −/−)c by unpaired t-test (p-value < 0.05). All values are presented as mean ± SEM.
As expected from the elevated Fgf23 levels, expression of sodium-phosphate co-transporters IIa and IIc (Slc34a1 and Slc34a3) was suppressed by 16-29% in all three mutant genotypes (Figure 3). Similarly, Kl mRNA level was on average 32% lower in the mutant mice. The mutant mice had markedly higher 24-hydroxylase (Cyp24a1) expression; however, there was no change in 1-α-hydroxylase (Cyp27b1) mRNA levels.
Comparison between male and female mice
Wild-type females had lower Pth, 1,25(OH)2D and creatinine levels than male counterparts, but other biochemical measurements were comparable (Figure 1). Similar comparisons between mutant males and females showed that heterozygous females have lower alkaline phosphatase activity than hemizygous males, whereas homozygous females have lower creatinine and calcium concentrations than the males. Although statistically not significant, there was a trend for Pth and 1,25(OH)2D to be lower in the mutant females. Interestingly, the mutant females, regardless of the number of Phex mutant alleles, produced more Fgf23 proteins (measured by the C-terminal ELISA) than mutant males. However, since intact Fgf23 levels were comparable between the three, there was no difference in serum phosphorus concentrations (Figure 1).
Renal expression of Slc34a1 and Cyp27b1 was similar between males and females of comparable genotypes (Figure 3). However, wild-type and mutant females had slightly higher Slc34a3 expression than male counterparts, and both affected female groups had significantly higher Cyp24a1 expression.
Females were smaller than males with comparable genotypes (Figure 2). Compared to hemizygous males, areal BMD was decreased in homozygous females, but increased in heterozygous females. Weight-adjusted BMC was higher in wild type and heterozygous females than male counterparts.
Comparison between mutant females
Compared to heterozygous females (i.e. mutants with one functional Phex allele), homozygous females had slightly lower calcium concentrations (Figure 1). There was a trend for phosphorus concentrations to be minimally lower in homozygotes than heterozygotes, but it did not reach statistical significance (p=0.062). There were no significant differences in intact and total Fgf23, Pth, and 1,25(OH)2D concentrations between the two affected female groups. In contrast to serum Fgf23 levels, Fgf23 mRNA in the femur was lower in homozygous females (Figure 3). Expression of the five genes in the kidney was similar between the groups although Kl expression was statistically lower in the homozygous females.
Homozygous females were significantly smaller than heterozygous females (Figure 2). The skeletal phenotype was also different between heterozygous and homozygous females, the latter having lower BMD and BMC and shorter limbs. In addition, females carrying two mutant alleles had the higher alkaline phosphatase than heterozygotes (Figure 1).
Discussion
Located on the X chromosome, the PHEX/Phex gene is under the control of dosage compensation by random X chromosome inactivation [23]. Therefore, approximately half of the cells in heterozygous females express normal PHEX allele, and the other half express mutant allele. However, loss of one PHEX allele in females is enough to manifest a full disease phenotype, which has been previously described as Phex haploinsufficiency [24]. In this study, we used a new murine model of XLH [21] to investigate dosage effect of a Phex mutation. All mutants had similar degrees of hypophosphatemia, hypocalcemia, decreased creatinine, and increased alkaline phosphatase and Fgf23 levels. Although female mutants (Phex heterozygotes and homozygotes) had higher total Fgf23 concentrations (measured by C-terminal ELISA) than male counterparts, there were no significant differences in intact Fgf23 concentrations. Consistent with intact Fgf23 concentrations, serum phosphorus concentrations were comparable between the three mutant groups. It should be noted that Pth and 1,25(OH)2D, which could mask the differences, were also similar between the three groups. In other murine models of XLH (Hyp and Ska1), serum phosphate concentrations also did not differ between three mutant groups [15, 25, 26]. In human patients with XLH, there was no evidence of gene dosage effect on serum biochemistries between affected males and females [10, 13] and even in the first known patient with a homozygous PHEX mutation [27]. Taken together, these data indicate that dosage of Phex mutation has no major effect on circulating intact Fgf23 levels and thus, phosphate homeostasis.
Despite half of the cells having normal Phex function, heterozygous females produced the same amounts of intact Fgf23 protein as hemizygous males and homozygous females. The apparent lack of dosage effect on Fgf23 concentrations suggests that the cells affected by the heterozygous Phex mutation are actively trying to maintain the same low phosphorus level as other two mutants. In other words, Phex mutation may create a lower set point for extracellular phosphate concentration. Although it remains unknown how the lower set point is determined, at least three potential mechanisms could explain how heterozygous females achieve the same Fgf23 level as in hemizygous and homozygous females:
In heterozygous females, cells expressing normal Phex allele retain normal function and shut down Fgf23 production in response to hypophosphatemia. However, cells expressing mutant Phex allele sense that phosphate being too high and produce twice as much Fgf23 as those in hemizygous males or homozygous females to maintain the same low level of extracellular phosphate.
Alternatively, cells expressing the mutant allele could, by an unknown mechanism, alter the function of surrounding normal cells even though these cells express normal Phex allele. Thus, all cells regardless of Phex mutation status express equal amounts of Fgf23. This scenario is most likely if Fgf23-producing cells such as osteoblasts and osteocytes work as one unit rather than individual cells.
Another potential mechanism involves circulating factors released from distant cells. These factors act on all bone cells equally, thereby keeping Fgf23 levels similar between heterozygotes and homozygotes.
Distinguishing these three mechanisms may shed light on how extracellular phosphate is sensed by Fgf23-producing cells and how Phex deficiency results in overproduction of Fgf23 protein.
In contrast to mineral ion homeostasis, the presence of one normal Phex allele significantly improved BMC, BMD, and alkaline phosphatase levels in heterozygous females (albeit still small effects). Alkaline phosphatase activity in Ska1 heterozygotes was also intermediate between wild-type and homozygous Ska1 females [15]. In addition, the presence of two mutant Phex alleles had significant dosage effect on some bone histomorphometric measures. Compared to heterozygous females, homozygous females had shorter vertebral length and increased cancellous osteoid thickness [25]. In humans, no sex-specific differences were found in prepubertal children [10, 12]; however, there is evidence for a more severe skeletal phenotype in adult males without any PHEX function than in females who have one normal PHEX gene [11, 13]. Similarly, postpubertal affected males have a more severe dental phenotype than affected females [12, 14]. These data suggest that although the degree of abnormal mineral metabolism is largely unchanged, the presence of one normal allele may reduce the severity of intrinsic bone defects in XLH likely because Phex plays a role in bone mineralization independent of phosphate sensing or the cells expressing a normal Phex allele can partially rescue the defects in those lacking Phex.
Interestingly, the females lacking a functional Phex allele were more severely affected, in terms of skeletal abnormalities and growth retardation, than the hemizygous males. Since these parameters were similar between wild-type males and females, these differences are specific to mutant mice. The underlying cause of these differences is unclear.
In addition to the increased Fgf23 expression, Phex mutant mice had higher proportion of intact Fgf23 protein than wild-type littermates. Post-translational processing of Fgf23 is mediated by subtilisin-like furin proprotein convertases [28] and UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 3 (GalNAc-T3) [29, 30]. Thus, lack of normal Phex function may affect activity of these enzymes, leading to reduced proteolytic cleavage of intact Fgf23 protein. It is also possible that Phex mutations directly increase the stability of Fgf23 protein by a yet unknown mechanism, which reduces intracellular processing or degradation in the circulation. However, the difference in the proportion between wild-type and mutant mice may simply reflect a function of the kinetics of the enzymes involved.
Together with observations made by other researchers [15, 25], our findings suggest that dosage of Phex mutation have small, if any, effects on mineral ion metabolism, but does have effects on bone that are independent of the biochemical environment. However, the differences between the skeletal parameters were not striking. Furthermore, consistent with the findings in our recent study in Galnt3/Phex double mutant mice [31], the Phex deficiency may lower the set point for extracellular phosphate concentrations, leading to increased intact Fgf23 production.
Supplementary Material
Acknowledgements
This study was supported by Indiana University-Purdue University Indianapolis Undergraduate Research Opportunities Program (UROP) Grant to (EB), National Institutes of Health Grant R01 AR042228 (to MJE), Indiana University School of Medicine Biomedical Research Grant (to SI), Showalter Research Trust Fund (to SI), and KL2 career development award (to SI) from the Indiana Clinical and Translational Sciences Institute funded in part by the National Institutes of Health grant RR025760.
Footnotes
Conflict of interest statement: Michael J. Econs receives royalties from and is a consultant for Kyowa Hakko Kirin Co. Ltd.
All other authors state that they have no conflicts of interest.
References
- 1.Endo I, Fukumoto S, Ozono K, Namba N, Tanaka H, Inoue D, Minagawa M, Sugimoto T, Yamauchi M, Michigami T, Matsumoto T. Clinical usefulness of measurement of fibroblast growth factor 23 (FGF23) in hypophosphatemic patients: proposal of diagnostic criteria using FGF23 measurement. Bone. 2008;42:1235–1239. doi: 10.1016/j.bone.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Juppner H. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348:1656–1663. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- 3.Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab. 2002;87:4957–4960. doi: 10.1210/jc.2002-021105. [DOI] [PubMed] [Google Scholar]
- 4.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 6.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 7.Larsson T, Marsell R, Schipani E, Ohlsson C, Ljunggren O, Tenenhouse HS, Juppner H, Jonsson KB. Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology. 2004;145:3087–3094. doi: 10.1210/en.2003-1768. [DOI] [PubMed] [Google Scholar]
- 8.The HYP Consortium. The HYP Consortium A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat Genet. 1995;11:130–136. doi: 10.1038/ng1095-130. [DOI] [PubMed] [Google Scholar]
- 9.Ichikawa S, Traxler EA, Estwick SA, Curry LR, Johnson ML, Sorenson AH, Imel EA, Econs MJ. Mutational survey of the PHEX gene in patients with X-linked hypophosphatemic rickets. Bone. 2008;43:663–666. doi: 10.1016/j.bone.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whyte MP, Schranck FW, Armamento-Villareal R. X-linked hypophosphatemia: a search for gender, race, anticipation, or parent of origin effects on disease expression in children. J Clin Endocrinol Metab. 1996;81:4075–4080. doi: 10.1210/jcem.81.11.8923863. [DOI] [PubMed] [Google Scholar]
- 11.Hardy DC, Murphy WA, Siegel BA, Reid IR, Whyte MP. X-linked hypophosphatemia in adults: prevalence of skeletal radiographic and scintigraphic features. Radiology. 1989;171:403–414. doi: 10.1148/radiology.171.2.2539609. [DOI] [PubMed] [Google Scholar]
- 12.Holm IA, Nelson AE, Robinson BG, Mason RS, Marsh DJ, Cowell CT, Carpenter TO. Mutational analysis and genotype-phenotype correlation of the PHEX gene in X-linked hypophosphatemic rickets. J Clin Endocrinol Metab. 2001;86:3889–3899. doi: 10.1210/jcem.86.8.7761. [DOI] [PubMed] [Google Scholar]
- 13.Reid IR, Hardy DC, Murphy WA, Teitelbaum SL, Bergfeld MA, Whyte MP. X-linked hypophosphatemia: a clinical, biochemical, and histopathologic assessment of morbidity in adults. Medicine (Baltimore) 1989;68:336–352. [PubMed] [Google Scholar]
- 14.Shields ED, Scriver CR, Reade T, Fujiwara TM, Morgan K, Ciampi A, Schwartz S. X-linked hypophosphatemia: the mutant gene is expressed in teeth as well as in kidney. Am J Hum Genet. 1990;46:434–442. [PMC free article] [PubMed] [Google Scholar]
- 15.Carpinelli MR, Wicks IP, Sims NA, O’Donnell K, Hanzinikolas K, Burt R, Foote SJ, Bahlo M, Alexander WS, Hilton DJ. An ethyl-nitrosourea-induced point mutation in phex causes exon skipping, x-linked hypophosphatemia, and rickets. Am J Pathol. 2002;161:1925–1933. doi: 10.1016/S0002-9440(10)64468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eicher EM, Southard JL, Scriver CR, Glorieux FH. Hypophosphatemia: mouse model for human familial hypophosphatemic (vitamin D-resistant) rickets. Proc Natl Acad Sci U S A. 1976;73:4667–4671. doi: 10.1073/pnas.73.12.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorenz-Depiereux B, Guido VE, Johnson KR, Zheng QY, Gagnon LH, Bauschatz JD, Davisson MT, Washburn LL, Donahue LR, Strom TM, Eicher EM. New intragenic deletions in the Phex gene clarify X-linked hypophosphatemia-related abnormalities in mice. Mamm Genome. 2004;15:151–161. doi: 10.1007/s00335-003-2310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyon MF, Scriver CR, Baker LR, Tenenhouse HS, Kronick J, Mandla S. The Gy mutation: another cause of X-linked hypophosphatemia in mouse. Proc Natl Acad Sci U S A. 1986;83:4899–4903. doi: 10.1073/pnas.83.13.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strom TM, Francis F, Lorenz B, Boddrich A, Econs MJ, Lehrach H, Meitinger T. Pex gene deletions in Gy and Hyp mice provide mouse models for X-linked hypophosphatemia. Hum Mol Genet. 1997;6:165–171. doi: 10.1093/hmg/6.2.165. [DOI] [PubMed] [Google Scholar]
- 20.Sabbagh Y, Gauthier C, Tenenhouse HS. The X chromosome deletion in HYP mice extends into the intergenic region but does not include the SAT gene downstream from Phex. Cytogenet Genome Res. 2002;99:344–349. doi: 10.1159/000071613. [DOI] [PubMed] [Google Scholar]
- 21.Owen C, Chen F, Flenniken AM, Osborne LR, Ichikawa S, Adamson SL, Rossant J, Aubin JE. A novel Phex mutation in a new mouse model of hypophosphatemic rickets. J Cell Biochem. 2012;113:2432–2441. doi: 10.1002/jcb.24115. [DOI] [PubMed] [Google Scholar]
- 22.Andersen CL, Jensen JL, Orntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 23.Lyon MF. X-chromosome inactivation. Curr Biol. 1999;9:R235–237. doi: 10.1016/s0960-9822(99)80151-1. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Du L, Ecarot B. Evidence for Phex haploinsufficiency in murine X-linked hypophosphatemia. Mamm Genome. 1999;10:385–389. doi: 10.1007/s003359901007. [DOI] [PubMed] [Google Scholar]
- 25.Qiu ZQ, Travers R, Rauch F, Glorieux FH, Scriver CR, Tenenhouse HS. Effect of gene dose and parental origin on bone histomorphometry in X-linked Hyp mice. Bone. 2004;34:134–139. doi: 10.1016/j.bone.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Qiu ZQ, Tenenhouse HS, Scriver CR. Parental origin of mutant allele does not explain absence of gene dose in X-linked Hyp mice. Genet Res. 1993;62:39–43. doi: 10.1017/s0016672300031542. [DOI] [PubMed] [Google Scholar]
- 27.Durmaz E, Zou M, Al-Rijjal RA, Baitei EY, Hammami S, Bircan I, Akcurin S, Meyer B, Shi Y. Novel and de novo PHEX mutations in patients with hypophosphatemic rickets. Bone. 2012;52:286–291. doi: 10.1016/j.bone.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Benet-Pages A, Lorenz-Depiereux B, Zischka H, White KE, Econs MJ, Strom TM. FGF23 is processed by proprotein convertases but not by PHEX. Bone. 2004;35:455–462. doi: 10.1016/j.bone.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Frishberg Y, Ito N, Rinat C, Yamazaki Y, Feinstein S, Urakawa I, Navon-Elkan P, Becker-Cohen R, Yamashita T, Araya K, Igarashi T, Fujita T, Fukumoto S. Hyperostosis-hyperphosphatemia syndrome: a congenital disorder of O-glycosylation associated with augmented processing of fibroblast growth factor 23. J Bone Miner Res. 2007;22:235–242. doi: 10.1359/jbmr.061105. [DOI] [PubMed] [Google Scholar]
- 30.Kato K, Jeanneau C, Tarp MA, Benet-Pages A, Lorenz-Depiereux B, Bennett EP, Mandel U, Strom TM, Clausen H. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem. 2006;281:18370–18377. doi: 10.1074/jbc.M602469200. [DOI] [PubMed] [Google Scholar]
- 31.Ichikawa S, Austin AM, Gray AK, Econs MJ. A Phex mutation in a murine model of X-linked hypophosphatemia alters phosphate responsiveness of bone cells. J Bone Miner Res. 2011 doi: 10.1002/jbmr.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.