Abstract
Objective
To summarize promising areas of investigation into polycystic ovary syndrome (PCOS) and to stimulate further research in this area.
Summary
Potential areas of further research activity include the analysis of predisposing conditions that increase the risk of PCOS, particularly genetic background and environmental factors, such as endocrine disruptors and lifestyle. The concept that androgen excess may contribute to insulin resistance needs to be re-examined from a developmental perspective, since animal studies have supported the hypothesis that early exposure to modest androgen excess is associated with insulin resistance. Defining alterations of steroidogenesis in PCOS should quantify ovarian, adrenal and extraglandular contribution, as well as clearly define blood reference levels by some universal standard. Intraovarian regulation of follicle development and mechanisms of follicle arrest should be further elucidated. Finally, PCOS status is expected to have long-term consequences in women, specifically the development of type 2 diabetes, cardiovascular diseases and hormone dependent cancers. Identifying susceptible individuals through genomic and proteomic approaches would help to individualize therapy and prevention. A potential limitation of our review is that we focused selectively on areas we viewed as the most controversial.
Keywords: steroids, androgens, folliculogenesis, inflammation, anovulation
INTRODUCTION
The polycystic ovary syndrome (PCOS) is a hyperandrogenic disorder associated with chronic oligo-anovulation and polycystic ovarian morphology 1, 2. It is often associated with psychological impairments, including depression and other mood disorders and metabolic derangements, chiefly insulin resistance and compensatory hyperinsulinemia, which is recognized as a major factor responsible for altered androgen production and metabolism 3. Most women with PCOS are also overweight or obese, further enhancing androgen secretion while impairing metabolism and reproductive functions and possibly favoring the development of the PCOS phenotype. The definition of PCOS has led to an impressive increase of scientific interest in this disorder, which should be further directed to improve individualized clinical approaches and, consequently therapeutic strategies.
To further dialogue and exchange ideas on PCOS an international group of PCOS researchers, has gathered every other year to summarize the state of the field and stimulate further research. We have previously published our presentations in book form 4, but elected here to create a shorter summary of our presentations. We designed the meeting to focus on specific areas of uncertainty in the pathophysiology and treatment of women with PCOS.
Defining alterations of steroidogenesis in PCOS
In normal women, androgen production rate (PR) is the result of adrenal and ovarian secretion and conversion from precursors in peripheral tissues, particularly the adipose tissue and skin 5. Similarly, the metabolic clearance rate (MCR) of androgens may occur in both glandular and extraglandular tissues. Both PR and MCR of androgens in females depends on age and physiological status. All androgens exhibit a daily rhythm, less variable for androstenedione and testosterone than that of DHEA and cortisol. A few studies, all performed several decades ago, documented higher PRs for both androstenedione and testosterone in women with PCOS, associated with a less pronounced increase of their MCR 6. In addition, it was shown that testosterone MCR was higher in obese PCOS women and varied according to its PR, whereas MCR of androstenedione was marginally different with respect to normal weight affected women, suggesting that factors [peripheral conversion or possibly binding to sex hormone binding globulin (SHBG)] in addition to body size influenced testosterone MCR in PCOS women. Notably, there are no studies in PCOS women with different obesity phenotypes, although there is evidence that in women with simple obesity, those with abdominal fat distribution have higher testosterone PR, but not higher androstenedione, with respect to those with the peripheral phenotype 7. Similar studies should therefore be replicated in PCOS women with different obesity phenotypes. Estrogen and progesterone PRs in women with PCOS have been poorly investigated.
One of the main problems in the diagnosis of hyperandrogenic states such as PCOS is the accurate measurement of androgens and particularly testosterone. Many radioimmunoassays, especially platform assays, for androgens are decidedly unsatisfactory. Most of these intrisic methodological limitations are bypassed by the growing use of liquid chromatography-tandem mass spectrometry (LM/MS-MS), the modern gold standard for all steroid hormone measurement, particularly in women. By the use of LM/MS-MS it would be expected that additional kinetic studies in different phenotypes of this disorder may favour a better understanding of complex pathophysiological events leading to androgen excess in women with PCOS, as preliminary clinical studies seem to indicate.
Significance of adrenal androgen production
It has been estimated that 25% of androstenedione and testosterone production is of ovarian origin, 25% is of adrenal origin and 50% is produced in peripheral tissues, while the adrenal cortex accounts almost uniquely for the synthesis of dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) as well as that of androstenediol and 11β-hydroxy androstenedione 10. In women androgens serve as precursors of estrogen biosynthesis, which starts to decrease 3–4 years before menopause 11. At the same time basal serum levels of ovarian androgens decrease only slightly and remain relatively stable until menopause, while the decrease of adrenal androgens can already be observed after the age of 30 years 12. Compared with healthy subjects, women with previous PCOS have an increased adrenal capacity to secrete androgens that remains until after menopause. These results confirm the adrenals contribute significantly to hyperandrogenism in PCOS and similarly to ovarian androgen secretion capacity, women with PCOS exhibit enhanced adrenal androgen production until their late reproductive years 13. The pathophysiological mechanisms responsible for increased androgen production by the adrenals in women with PCOS remains, however, poorly elucidated and should require further investigation. Difficulty in obtaining viable and appropriate adrenal tissue has limited in vitro study of human tissue, but long term culture is possible, and the derivation of stem cell adrenal cortex tissue could significantly enhance studies of this important gland.
Specific steroidogenic enzyme defects in PCOS
The etiology of PCOS remains uncertain but intrinsic abnormalities in the synthesis and secretion of androgens are a plausible basis for the syndrome. There is clear evidence for constitutive hyper-secretion of androgen by ovarian theca cells 14 but abnormalities of adrenal androgen production have also been implicated in the etiology. It is therefore reasonable to pose the question “are specific primary enzyme abnormalities in the steroidogenic pathway an important cause of PCOS?”. On the basis of currently available evidence, the answer to this question is probably “no”. Amongst plausible candidate genes in genesis of hyperandrognemia are CYP17 (coding for P450c17, and the associated P450 reductase) and, because of evidence for a global increase in steroidogenic enzyme activity in PCO theca cells, CYP11a (P450scc) 15, 16. To date, case-control and family-based studies have shown no clear evidence that variants in these genes (or for that matter, many others involved in steroidogenesis) contribute to the pathogenesis of PCOS. Recent work has focused on metabolism of cortisol and adrenal androgens but, although specific enzyme defects may be associated with a PCOS phenotype (e.g. defects in cortisone reductase), the data from large association studies suggest that such defects are but a very minor contributor to the etiology of PCOS 16.
In addition, extraglandular synthesis of androgens, particularly in the adipose tissue, has been found to be involved in the pathophysiology of PCOS. They involve alteration in the activity of 11β-hydroxysteroid dehydrogenase 17 and both 5α-reductase and 5β-reductase 10, 18, 19. Alterations of these enzyme systems which are involved in peripheral cortisol metabolism may in turn activate the neuroendocrine drive to support adrenal steroidogenesis and may partly explain the increased androgen production in specific subsets of women with PCOS.
Sympathetic nerve activity and hyperandrogenism
Many factors associated with polycystic ovary syndrome (PCOS) are also associated with increased activity in the sympathetic nervous system 20. The involvement of sympathetic nervous system in PCOS pathology is supported by the greater density of catecholaminergic nerve fibres in polycystic ovaries (PCO) 21. Increased ovarian sympathetic nerve activity might contribute to PCOS by stimulating androgen secretion 22. Nerve growth factor (NGF) is a strong marker for sympathetic nerve activity and recently it was demonstrated that women with PCOS has enhanced ovarian NGF production 23. In a transgenic mouse model overexpressing NGF in the ovaries, they found that that a persistent elevation in plasma LH levels is required for the typical morphological abnormalities to appear 23. These results suggest that overproduction of ovarian NGF is a component of PCO morphology.
Studies using indirect markers of autonomic function – heart rate variability and heart rate recovery after exercise – have shown that women with PCOS have increased sympathetic and decreased parasympathetic components 24, 25, 26. Recently, for the first time it was demonstrated that women with PCOS have high general activity in the sympathetic nervous system which may be relevant to the pathophysiology of the syndrome 27. Interestingly, testosterone was the strongest independent factor explaining high sympathetic nerve activity in women with PCOS 27. As the degree of androgen concentration can reflect the severity of PCOS, the relationship between sympathetic nerve activity and testosterone concentration indicates that the degree of sympatho-excitation is related to the degree of PCOS severity.
Recently, a randomized controlled trial demonstrated that low-frequency electro-acupuncture (EA) and physical exercise (both known to modulate sympathetic nerve activity) decreases high levels of circulating sex steroid precursors, estrogens, androgens, and glucuronidate androgen metabolites and improve menstrual bleeding pattern in women with PCOS, and thus break the vicious circle of androgen excess 28. In a subset of these women, low-frequency EA and physical exercise was shown to decrease high sympathetic nerve activity in women with PCOS 29, which may at least in part explain the beneficial effects of these therapies. It may also be hypothesized that therapies such as ovarian wedge resection or laparoscopic laser cauterization 30, utilize its effect by temporary disruption of ovarian sympathetic innervation, and thus increase ovulatory function and decrease androgen synthesis in women with PCOS.
Mechanism of follicle arrest
The finding that granulosa cells from anovulatory polycystic ovaries responded well to FSH in culture directed initial investigations into follicular arrest towards discovery of raised levels of a locally produced inhibitor. It is difficult to deduce cause and effect, however if the factor is causing follicular arrest, or did the follicular arrest elicit the production of the inhibitor. Androgens are an obvious candidate, but production is raised in theca from ovulatory PCO also 31. Insulin causes premature acquisition of LH receptors possibly leading to early follicular luteinisation 32, but the insulin signalling defect in the polycystic ovary remains to be clarified. More comprehensive investigation of insulin/glucose interactions in these cells has been undertaken utilising a metabolomic approach. Anti-Mullerian Hormone (AMH) is raised in women with PCOS and granulosa cell production is considerably higher in anovulatory than ovulatory women with PCOS. AMH’s suppressive effects on folliculogenesis may make this the sought-after local inhibitor 33. Recent data indicated that it is those women in whom AMH levels fall who have the best response to methods to induce ovulation. Interestingly, the incubation of cells with metformin inhibited AMH production suggesting that this may be one mechanism of the action of this drug in PCOS 34.
Intra-ovarian regulation of ovarian morphology
Kit ligand (KL) is an intra-ovarian cytokine that promotes multiple aspects of folliculogenesis in animal models including primordial follicle activation, follicle growth and survival, stromal cell differentiation, and theca cell proliferation and androgen biosynthesis 35. Perturbation of these biological processes occur in PCO, particularly in anovulatory women with PCOS, in whom there is evidence for abnormal oocyte growth, increased follicle and stromal density, thecal hypertrophy, and increased thecal cell androgen biosynthesis. Therefore, KL may play a key role in the morphogenesis of PCO, particularly in women with PCOS. Androgen regulation of KL has been reported 36, but the role of KL signalling, its regulation in human ovaries and its relevance to PCOS are currently unknown.
Determinants of ovarian morphology – Influence of gonadotropins
Initiation of growth and early differentiation of follicles are thought to be regulated independently of gonadotropin stimulation. The later stages of growth and differentiation, selection of the cohort and cyclic recruitment are largely dependent on FSH activity. In the PCO there is loss of the selection process from an increased pool to a dominant follicle. Enhanced steroidogenesis, excess androgens, hyperinsulinemia and lack of growth differentiation factor (GDF9) have all been implicated but FSH refractoriness may be key. FSH concentrations in PCOS are generally in the lower normal range. Adding FSH (with clomiphene or exogenous FSH) restores normal follicular growth, suggesting an endogenous inhibition of FSH action in PCOS. The source of this inhibition is probably ovarian as loss of ovarian tissue (wedge resection or laparascopic ovarian diathermy, or age >40 years old) 37 is capable of restoring normal follicular development and ovulation. Following laparoscopic ovarian drilling (LOD), there is a rapid steep rise in FSH in those who respond. Thus, it seems that the size of the 2–5mm follicle pool is an independent, important contributor to the follicular arres. Candidates for the source of FSH refractoriness include transforming growth factor (TGF)-alpha, epidermal growth factor (EGF), follistatin and, particularly the high concentrations of AMH in PCOS 38. LH receptor over-expression in PCO granulosa cells leads to terminal differentiation and premature arrest of follicle growth 39. Finally, the lack of circulating progesterone encourages high LH levels, exacerbating androgen excess, multiple small follicles and the consequences. These data highlight the role of appropriate gonadotropin action within the ovary in restoring follicular development and ovulation in women and PCOS, and provide evidence for the ovary as the primary determinant of inappropriate gonadotropin secretion in PCOS, though this remains an area of debate among researchers.
Clinical significance of polycystic ovaries in normal women
Polycystic ovaries (PCO) are the morphological ovarian phenotype in women with the polycystic ovary syndrome (PCOS). Several studies have been performed to attempt to determine the prevalence of PCO as detected by ultrasound alone in the general population, and have found prevalence rates in the order of 17–33% 40. In 2003 a joint ESHRE/ASRM consensus meeting produced a refined definition of PCOS (1), and the morphology of the polycystic ovary, was defined as an ovary with 12 or more follicles measuring 2–9 mm in diameter and/or increased ovarian volume (>10 cm3) 38. It is interesting also to note that the presence of PCO is a marker for increased ovarian reserve and a reduced rate of ovarian aging 41. The question of whether PCO alone are pathological or a normal variant of ovarian morphology is debated. It has been found that some women with hypogonadotropic hypogonadism (HH) also have polycystic ovaries detected by pelvic ultrasound and when these women were treated with pulsatile GnRH to induce ovulation they had significantly higher serum LH concentrations than women with HH and normal ovaries 42. These results suggest that the cause of hypersecretion of LH involves a perturbation of ovarian-pituitary feedback, rather than a primary disturbance of hypothalamic pulse regulation. A consensus statement on defining the morphology of the PCO stated that “A woman having PCO in the absence of an ovulation disorder or hyperandrogenism (“asymptomatic PCO”) should not be considered as having PCOS, until more is known about this situation” 43. Whilst the spectrum of “normality” might include the presence of PCO in the absence of signs or symptoms of PCOS, there is evidence that women with PCO morphology alone show typical responses to stresses such as gonadotropin stimulation during IVF treatment or to weight gain, whether spontaneous or as stimulated by sodium valproate therapy 44. The difficulty in answering this question lies in the fact that to date there are no large scale, longitudinal prospective studies of women with polycystic ovaries.
Information about the prevalence of polycystic ovaries can be obtained from cross sectional studies of ovarian size and morphology in normal women without PCOS. For instance a large scale study of ovarian aging among women enrolled in the Kaiser Permanente Health Plan in California found a high prevalence of polycystic ovaries among younger women which resolved with aging.
However a better study design would be a prospective longitudinal study to examine via imaging changes in the size and morphology of the ovary over time to establish the permanence of the polycystic ovary in affected and unaffected women with PCOS. This would also address the important and understudied issue of the fate of the polycystic ovary in the perimenopause and menopause.
Hyperthecosis
Hyperthecosis is the development of nests of luteinized thecal cells, usually diffusely, in the ovary with the subsequent production of androgens and presentation with signs of androgen excess. Unlike PCOS there is not an abundance of antral follicles surrounded by theca, in fact it often develops in postmenopausal women devoid of follicles 46. The cause of hyperthecosis is unknown. The phenotype in hyperthecosis can be more severe than PCOS, as women can present with markedly elevated testosterone levels and may develop frank signs of virilization. Hyperinsulinemia is also frequently part of the phenotype 47. Though this condition responds to GnRH agonist suppression 48, the usual treatment is oophorectomy, especially in a postmenopausal woman. Because this condition is rare, most publications are case reports and case series, however it offers an intriguing clinical model for hyperandrogenism and insulin resistance solely due to an ovarian factor. Hyperthecosis, especially as an acquired condition of sudden onset lends itself to the possibility of an infectious and/or autoimmune response to an infection or some external antigen, a possibility discussed below in relation to PCOS.
Impact of metabolic abnormalities on development of PCOS in a nonhuman primate model
A fetal testosterone excess model for PCOS manifests metabolic defects in adult female as well as adult male rhesus monkeys 49. Testosterone treatment of monkey dams results in mild-to-moderate maternal glucose intolerance that adds a metabolic perturbation to in utero testosterone exposure and may explain why both female and male testosterone -exposed offspring exhibit metabolic defects in adulthood 50, 51. Testosterone -exposed female offspring also demonstrate subtle increases in fetal head growth and postnatal body weight, as well as indications of fetal hyperglycemia and neonatal hyperinsulinemia. Neonatal hyperinsulinemia may synergize with infant hyperandrogenism in testosterone -exposed females to increase lipogenesis and muscle protein synthesis 51, thus enhancing insulin-sensitive tissue mass that may contribute to increased adiposity and insulin resistance found in testosterone -exposed adults 49. Since insulin defects have been found in prepubertal daughters born to women with PCOS 52, metabolic abnormalities during gestation may provide an important developmental contribution to the expression of PCOS phenotype.
The fetal programming hypothesis, however, while well defined in animal models, has yet to be confirmed in humans, despite two recent studies attempting to define fetal testosterone exposure in PCOS women. A long-term prospective study investigating a large cohort of unselected adolescents found that blood levels of testosterone from their mothers at 18 and 34–36 wk gestation, and from an umbilical cord sample, were not related to the subsequent development of PCOS 53. A separate study examining umbilical cord blood levels in newborns found unchanged testosterone, but diminished androstenedione and estradiol levels, in girls born to women with PCOS 53. These negative data are not surprising since human fetuses are protected from maternal androgen excess of PCOS by placental aromatase, and umbilical cord blood testosterone and androstenedione levels do not reliably distinguish boys from girls 53, despite male fetal androgen excess earlier in gestation. This animal model offers a unique method to explore the effects of the intrauterine milieu on the development of future metabolic and reproductive abnormalities, and allows controlled manipulation and long term follow up of offspring in a manner that would be unethical and frankly impossibile in humans.
Dyslipidemia in PCOS
PCOS is frequently associated with various patterns of dyslipidemia including low high-density lipoprotein cholesterol (HDL-C), high levels of triglycerides, total cholesterol, and low-density lipoprotein cholesterol (LDL-C) 54–56. Although the data from large series suggest that the mean values for circulating lipids in women with PCOS are in normal limits, up to 70% of patients have at least one abnormal lipid level according to NCEP-ATPIII criteria 57. Body fat amount and distribution, presence and degree of insulin resistance, and androgen excess appear to have independent and interrelated effects on the type and extent of lipid abnormalities in PCOS 58. Prevalence rates of dyslipidemia show significant variability in different studies. Several factors including age, race, glucose intolerance, and diagnostic criteria used to define PCOS might have an influence on this variation. Nevertheless, most of the studies assessing dyslipidemia in PCOS have certain limitations, including (but not limited to) small sample size and lack of information on environmental modulators of serum lipid levels such as diet, physical activity, smoking and alcohol consumption.
Large scale follow up studies are warranted to investigate lipid alterations in PCOS as well as to determine the impact of commonly used long term therapeutic interventions in the syndrome. There is debate at what age to institute therapy for dyslipidemia, as the treatment, for example with statins does include slight risk of a serious adverse side effect including rhabdomyolysis, whereas events are unlikely in younger women with PCOS. Further there is concern that treatment with these agents will improved reproductive aspects and result in increased and unexpected ovulation and potential undesired fetal exposure. Many of these drugs are given a categorical teratogenic designation because they interfere with cholesterol synthesis or metabolism, and LDL-C remains the primary precursor for sex steroid synthesis in the placenta.
Predisposing risk factors for PCOS and risk reduction
Evidence suggests there are contributions from both heritable and non-heritable factors in the development of PCOS. The typical presentation of PCOS in adolescence suggests that the predisposition to the endocrine and metabolic abnormalities of PCOS originates prior to puberty. There is likely a genetic heritability that is enhanced by environmental factors notably increased dietary consumption and development of obesity. Studies demonstrate that peripubertal obesity is associated with hyperandrogenism 59, although prospective studies linking this to the development of PCOS are lacking. If indeed peripubertal obesity, acting either through increased insulin resistance or other adipocyte factors, increases the development of hyperandrogenism, reduction in adiposity should reduce this risk.
No long-term studies are available to demonstrate that reduction in body weight reduces the risk of PCOS development. Peripubertal weight reduction has been shown to be associated with a reduction in testosterone levels in the general population of obese pre-pubertal girls 60. There are limited studies that demonstrate the induction of modest weight reduction, with or without concomitant oral contraceptives, improves serum androgens in adolescents diagnosed with PCOS 61. Limited data have been reported on the use of insulin sensitizers in the management of PCOS in adolescence with mixed result 62, 63. Future research should focus on early identification of predisposing risk factors in PCOS development, and long-term studies that modify environmental factors to abrogate the risk.
The role of diet in the pathogenesis of PCOS: Focus on dietary Advanced Glycated End products (AGEs)
Lifestyle contributors to disease include not only calorie excess but also the dietary intake of specific nutrients. Advanced Glycated End products (AGEs) is a class of nutrients incriminated in the pathogenesis of diet-related diseases. AGEs are reactive derivatives of nonenzymatic glucose–protein reactions either produced endogenously or ingested from dietary sources. Cooking or processing at high temperatures such as broiling, grilling, frying, and roasting are the major sources of AGEs. By modulating the activity of protein kinases, AGEs promote oxidative stress and insulin resistance in peripheral tissues. PCOS women have increased serum AGEs levels and these have been positively correlated with serum androgen levels 64. In women with PCOS dietary modification or use of a gastric lipase inhibitor may reduce serum AGEs and oxidative stress markers as well as serum testosterone levels 65. PI3K mediates insulin signalling at the postreceptor level and also, mediates the clearance of AGEs via the Macrophage Scavenger Receptor (MSR) pathway. The inhibition of Phosphatidylinositol 3 kinase (PI3K) may play a dual role in the coexistence of AGE excess and insulin resistance in PCOS. By activating protein kinase C, AGEs may impair insulin action, thereby perpetuating insulin resistance, an intrinsic feature of PCOS 66. Furthermore, a potential direct action of AGEs on ovarian function is suggested by their increased immunohistochemical localization in polycystic ovaries 67. Overall, AGEs, both endogenously and exogenously derived, may play a part in the pathogenesis of PCOS. However, there are no data in comparing different ethnic populations with different diets regarding the impact of AGEs. The environmental source of AGEs can be reduced by dietary modifications.
PCOS: Inflammation and infection
Growing evidence supports the concept that PCOS is associated with increased oxidative stress and systemic inflammation. When compared to healthy control subjects, women with PCOS have increased markers of lipid peroxidation, elevated levels of c -reactive protein, inflammatory cytokines, as well as higher concentrations of blood lymphocytes and monocytes 68, 69. However, the cause/causes of these alterations have not yet been identified. This suggests a new hypothesis that chronic infections may be involved in the etiology of PCOS; such chronic infections may induce inflammation and oxidative stress, which in turn may contribute to insulin resistance, ovarian dysfunction and other alterations characteristic of PCOS. In support of this concept, there is evidence that PCOS is associated with greater risk of exposure to intracellular pathogens capable of inducing long-term inflammation including Chlamydia pneumonia and Chlamydia trachomatis 70. A correlation between Chlamydia pneumonia and insulin resistance has also been observed 71. Furthermore, Chlamydia pneumonia infection in mice resulted in increased ovarian size and a greater number of antral follicles 71.
Long Term Outcomes in PCOS: Vascular Disease
The higher sex-specific coronary mortality observed in women compared with men, combined with a greater proportion of women in the population, has resulted in relatively more women dying of cardiovascular disease (CVD) each year than men.72
Endogenous sex hormones including estrogen are hypothesized as the primary reason for the lower incidence of CVD among normal ovulatory premenopausal women compared with age-matched men and the subsequent age-related rise in women postmenopausally. 73 Moreover, there is a clear evidence from a large number of clinical studies that women with PCOS have a higher prevalence of classical and non classical risk factors, which are strictly related to the presence of insulin resistance, excess body fat, and low-grade inflammation, other than PCOS status per se. 74 However, despite risk factor clustering, studies published to date have failed to demonstrate a uniform association between PCOS and CV disease. 75, 76 An apparent lack of association between PCOS and CVD may be due to inadequate PCOS characterization, inadequate CVD measurement, insufficient duration of follow-up, or a true lack of association. A recent study tested the hypothesis that women with clinical features of PCOS more often had angiographic coronary artery disease and CVD events in a carefully characterized group of postmenopausal women in USA.77 This study confirmed that clinical features of PCOS were associated with more angiographic coronary heart disease (CHD) and worsening cardiovascular event-free servival, which suggests that the identification of postmenopausal women with clinical features of PCOS may provide an opportunity for risk factor intervention for the prevention of CAD and CVD events. However, this still require much more intensive reasearch and, possibly, longitudinal prospective studies.
Summary
Potential areas of further research activity include the analysis of predisposing conditions that increase the risk of PCOS, particularly genetic background and environmental factors, such as endocrine disruptors and diet 78. In addition, defining alterations of steroidogenesis in PCOS needs to be re-examined to quantify ovarian, adrenal and extraglandular contribution, as well as a change in the blood androgen reference values due to the expanding use of mass spectrometry. Clearly identifying premenarchal and postmenopausal phenotypes of androgen excess and PCOS would significantly enhance our epidemiologic studies of natural history and intervention studies. Intraovarian regulation of follicle development and mechanisms of follicle arrest and the impact of metabolic abnormalities on these processes, as well as molecular mechanisms by which insulin excess regulates androgen secretion and metabolism and disrupts follicle development 79 are other potential issues for investigation. Current information would suggest androgens alone may be necessary but not sufficient to cause follicular arrest, and it is likely that other inhibitors and non-steroid directed pathways are implicated in follicular arrest. Future studies should utilize both existing cell culture and animal models discussed above, but also utilize ovarian follicles grown and matured in 3-D matrices or created out of stem cells.
The concept that androgen excess may be responsible for the development of insulin resistance also needs to be re-examined, since studies performed in the last decade in experimental animals have supported the hypothesis that early exposure to modest androgen excess may favor the development of insulin resistance and enlarged visceral adiposity, although available data in humans are still sparse and controversial 80 and preliminary prospective data in humans seem to not support this hypothesis 81. There have been a number of recent well designed adequately powered trials examining infertility treatment in women with PCOS. While this is a positive development, it is only a start. PCOS status is expected to lead to many long-term consequences in women, specifically the development of type 2 diabetes, cardiovascular diseases and hormone dependent cancers. Identifying susceptible individuals would help to individualize therapeutic and, possibly, preventive strategies.
Is there a Broad Experimental Approach to PCOS?
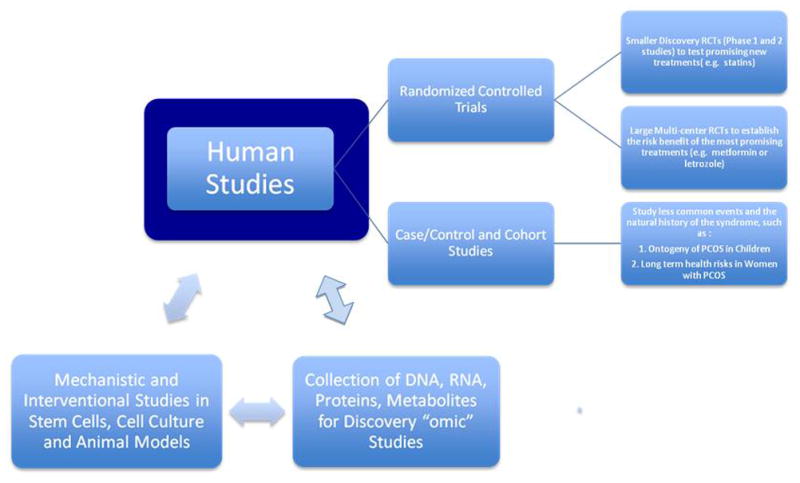
Though it was beyond the scope of our meeting to formulate a unifying experimental approach to better understanding and treating polycystic ovary syndrome, the close interaction of basic and clinical scientists allowed speculation on future directions for research. The utilization of discovery “Omic” technologies should identify new and unsuspected proteins and metabolic pathways that could lead to new treatments for the disorder. A recent mathematical review of microarray data in women with PCOS identified 504 protein nodes and 1048 interactions among them, and theorized that there was a cell cycle protein in this network yet to be identified. 82
Several Genome Wide Association Studies (GWAS) devoted soley to PCOS are ongoing in the U.S., Europe and Asia, and should yield new candidate genes and proteins as intriguing (or baffling) as those discovered in other complex disorders such as type 2 diabetes. As discussed above a number of existing cell culture and animal models exist to identify pathways, pathophysiological perturbances, and interventions that normalize signal transduction in these pathways. These in turn may eventually be tested in randomized trials large and small in affected women with PCOS. Thus there is a potential beneficial cycle of bed to bench to bed studies (Figure 1).
Figure 1.
Acknowledgments
Acknowledgement of Financial Support: Supported in part by U54 HD034449; U10 HD 38992, R01HD056510.
Participation in the PCOS Forum was self-funded without industry support. We would also like to acknowledge the contributions of members of the group who participated in the meeting and discussions: Andrea Dunaif, M.D., Anuja Dokras, M.D., R. Jeffrey Chang, M.D., John C. Marshall, M.D., Ph.D., John E. Nestler, M.D. Robert J. Norman, M.D., Eva Dahlgren, M.D. and Silva Arslanian, M.D.
Footnotes
Financial Disclosure: None
References
- 1.Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to Polycystic Ovary Syndrome (PCOS) Human Reproduction. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R, Carmina E, Dewailly, et al. Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an androgen excess society guideline. Journal of Clinical Endocrinology & Metabolism. 2006;91:4237–4245. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 3.Escobar-Morreale HF, Botella-Carretero JI, Alvarez-Blasco F, et al. The polycystic ovary syndrome associated with morbid obesity may resolve after weight loss induced by bariatric surgery. Journal of Clinical Endocrinology & Metabolism. 2005;90:6364–6369. doi: 10.1210/jc.2005-1490. [DOI] [PubMed] [Google Scholar]
- 4.Dunaif ACR, Franks S, Legro RS, editors. Polycystic Ovary Syndrome: Current Controversies, from the Ovary to the Pancreas. Humana Press; 2008. [Google Scholar]
- 5.Longcope C. Adrenal and gonadal androgen secretion in normal females. Clinical Endocrinology & Metabolism. 1986;15:213–228. doi: 10.1016/s0300-595x(86)80021-4. [DOI] [PubMed] [Google Scholar]
- 6.Bardin CW, Lipsett MB. Testosterone and androstenedione blood production rates in normal women and women with idiopathic hirsutism or polycystic ovaries. Journal of Clinical Investigation. 1967;46:891–902. doi: 10.1172/JCI105588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasquali R, Casimirri F. The impact of obesity on hyperandrogenism and polycystic ovary syndrome in premenopausal women. [Review] Clinical Endocrinology (Oxford) 1993;39:1–16. doi: 10.1111/j.1365-2265.1993.tb01744.x. [DOI] [PubMed] [Google Scholar]
- 8.Rosner W, Auchus RJ, Azziz R, et al. Utility, Limitations, and Pitfalls in Measuring Testosterone: An Endocrine Society Position Statement. Journal of Clinical Endocrinology and Metabolism. 2007;92:405–413. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- 9.Stener-Victorin E, Holm G, Labrie F, et al. Are there any sensitive and specific sex steroid markers for polycystic ovary syndrome? Journal of Clinical Endocrinology and Metabolism. 2010;95:810–819. doi: 10.1210/jc.2009-1908. [DOI] [PubMed] [Google Scholar]
- 10.Piltonen T, Koivunen R, Morin-Papunen L, et al. Ovarian and adrenal steroid production: regulatory role of LH/HCG. Human Reproduction. 2002;17:620–624. doi: 10.1093/humrep/17.3.620. [DOI] [PubMed] [Google Scholar]
- 11.Lasley BL, Santoro N, Randolf JF, et al. The relationship of circulating dehydroepiandrosterone, testosterone, and estradiol to stages of the menopausal transition and ethnicity. Journal of Clinical Endocrinology & Metabolism. 2002;87:3760–3767. doi: 10.1210/jcem.87.8.8741. [DOI] [PubMed] [Google Scholar]
- 12.Piltonen T, Koivunen R, Ruokonen A, et al. Ovarian age-related responsiveness to human chorionic gonadotropin. Journal of Clinical Endocrinology & Metabolism. 2003;88:3327–3332. doi: 10.1210/jc.2002-021549. [DOI] [PubMed] [Google Scholar]
- 13.Puurunen J, Piltonen T, Jaakkola P, et al. Adrenal androgen production capacity remains high up to menopause in women with polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism. 2009;94:1973–1978. doi: 10.1210/jc.2008-2583. [DOI] [PubMed] [Google Scholar]
- 14.Gilling-Smith C, Willis DS, Beard RW, et al. Hypersecretion of androstenedione by isolated thecal cells from polycystic ovaries. Journal of Clinical Endocrinology & Metabolism. 1994;79:1158–1165. doi: 10.1210/jcem.79.4.7962289. [DOI] [PubMed] [Google Scholar]
- 15.Tee MK, Dong Q, Miller WL. Pathways leading to phosphorylation of p450c17 and to the posttranslational regulation of androgen biosynthesis. Endocrinology. 2008;149:2667–2677. doi: 10.1210/en.2007-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Draper N, Powell BL, Franks S, et al. Variants implicated in cortisone reductase deficiency do not contribute to susceptibility to common forms of polycystic ovary syndrome. Clinical Endocrinology (Oxford) 2006;65:64–70. doi: 10.1111/j.1365-2265.2006.02547.x. [DOI] [PubMed] [Google Scholar]
- 17.Rodin A, Thakkar H, Taylor N, et al. Hyperandrogenism in polycystic ovary syndrome. Evidence of dysregulation of 11 beta-hydroxysteroid dehydrogenase [see comments] New England Journal of Medicine. 1994;330:460–465. doi: 10.1056/NEJM199402173300703. [DOI] [PubMed] [Google Scholar]
- 18.Stewart PM, Shackleton CH, Beastall GH, et al. 5 alpha-reductase activity in polycystic ovary syndrome [see comments] Lancet. 1990;335:431–433. doi: 10.1016/0140-6736(90)90664-q. [DOI] [PubMed] [Google Scholar]
- 19.Gambineri A, Forlani G, Munarini A, et al. Increased clearance of cortisol by 5beta-reductase in a subgroup of women with adrenal hyperandrogenism in polycystic ovary syndrome. Journal of Endocrinological Investigation. 2009;32:210–218. doi: 10.1007/BF03346454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fagius J. Sympathetic nerve activity in metabolic control--some basic concepts. Acta Physiologica Scandinavica. 2003;177:337–343. doi: 10.1046/j.1365-201X.2003.01086.x. [DOI] [PubMed] [Google Scholar]
- 21.Heider U, Pedal I, Spanel-Borowski K. Increase in nerve fibers and loss of mast cells in polycystic and postmenopausal ovaries. Fertility Sterility. 2001;75:1141–1147. doi: 10.1016/s0015-0282(01)01805-2. [DOI] [PubMed] [Google Scholar]
- 22.Dissen GA, Lara HE, Leyton V, et al. Intraovarian excess of nerve growth factor increases androgen secretion and disrupts estrous cyclicity in the rat. Endocrinology. 2000;141:1073–1082. doi: 10.1210/endo.141.3.7396. [DOI] [PubMed] [Google Scholar]
- 23.Dissen GA, Garcia-Ruda C, Paredes A, et al. Excessive ovarian production of nerve growth factor facilitates development of cystic ovarian morphology in mice and is a feature of polycystic ovarian syndrome in humans. Endocrinology. 2009;150:2906–2914. doi: 10.1210/en.2008-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giallauria F, Palomba S, Manguso F, et al. Abnormal heart rate recovery after maximal cardiopulmonary exercise stress testing in young overweight women with polycystic ovary syndrome. Clinical Endocrinology (Oxf) 2008;68:88–93. doi: 10.1111/j.1365-2265.2007.03004.x. [DOI] [PubMed] [Google Scholar]
- 25.Tekin G, Tekin A, Kilicarslan EB, et al. Altered autonomic neural control of the cardiovascular system in patients with polycystic ovary syndrome. International Journal of Cardiology. 2007 doi: 10.1016/j.ijcard.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 26.Yildirir A, Aybar F, Kabakci G, et al. Heart rate variability in young women with polycystic ovary syndrome. Annals of Noninvasive Electrocardiology. 2006;11:306–312. doi: 10.1111/j.1542-474X.2006.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sverrisdottir YB, Mogren T, Kataoka J, et al. Is polycystic ovary syndrome associated with high sympathetic nerve activity and size at birth? American Journal of Physiology Endocrinology and Metabolism. 2008;294:E576–581. doi: 10.1152/ajpendo.00725.2007. [DOI] [PubMed] [Google Scholar]
- 28.Jedel E, Labrie F, Odén A, et al. Impact of electroacupuncture and physical exercise on hyperandrogenism and oligo/amenorrhoea in women with polycystic ovary syndrome: A randomized controlled trial. American Journal of Physiology Endocrinology and Metabolism. 2010 doi: 10.1152/ajpendo.00495.2010. [DOI] [PubMed] [Google Scholar]
- 29.Stener-Victorin E, Jedel E, Janson PO, et al. Low-frequency electroacupuncture and physical exercise decrease high muscle sympathetic nerve activity in polycystic ovary syndrome. American Journal of Physiology Regulatory Integrative and Comparative Physiology. 2009;297:R387–395. doi: 10.1152/ajpregu.00197.2009. [DOI] [PubMed] [Google Scholar]
- 30.Balen A. Surgical treatment of polycystic ovary sundrome. Best Practice Research Clinical Endocrinology Metabolism. 2006;20:271–280. doi: 10.1016/j.beem.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Gilling-Smith C, Story H, Rogers V, et al. Evidence for a primary abnormality of thecal cell steroidogenesis in the polycystic ovary syndrome. Clinical Endocrinology (Oxford) 1997;47:93–99. doi: 10.1046/j.1365-2265.1997.2321049.x. [DOI] [PubMed] [Google Scholar]
- 32.Willis D, Mason H, Gilling-Smith C, et al. Modulation by insulin of follicle-stimulating hormone and luteinizing hormone actions in human granulosa cells of normal and polycystic ovaries. Journal of Clinical Endocrinology & Metabolism. 1996;81:302–309. doi: 10.1210/jcem.81.1.8550768. [DOI] [PubMed] [Google Scholar]
- 33.Pellatt L, Hanna L, Brincat M, et al. Granulosa cell production of anti-Mullerian hormone is increased in polycystic ovaries. Journal of Clinical Endocrinology & Metabolism. 2007;92:240–245. doi: 10.1210/jc.2006-1582. [DOI] [PubMed] [Google Scholar]
- 34.Rice S, Pellatt L, Ramanathan K, et al. Metformin inhibits aromatase via an extracellular signal-regulated kinase-mediated pathway. Endocrinology. 2009;150:4794–4801. doi: 10.1210/en.2009-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Driancourt MA, Reynaud K, Cortvrindt R, et al. Roles of KIT and KIT LIGAND in ovarian function. Reviews in Reproduction. 2000;5:143–152. doi: 10.1530/ror.0.0050143. [DOI] [PubMed] [Google Scholar]
- 36.Shiina H, Matsumoto T, Sato T, et al. Premature ovarian failure in androgen receptor-deficient mice. Proceeding of the National Academy of Science U S A. 2006;103:224–229. doi: 10.1073/pnas.0506736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elting MW, Kwee J, Korsen TJ, et al. May) Aging women with polycystic ovary syndrome who achieve regular menstrual cycles have a smaller follicle cohort than those who continue to have irregular cycles. Fertility & Sterility. 2003;79:1154–1160. doi: 10.1016/s0015-0282(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 38.Pigny P, Merlen E, Robert Y, et al. Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. Journal of Clinical Endocrinology & Metabolism. 2003;88:5957–5962. doi: 10.1210/jc.2003-030727. [DOI] [PubMed] [Google Scholar]
- 39.Willis DS, Watson H, Mason HD, et al. Nov) Premature response to luteinizing hormone of granulosa cells from anovulatory women with polycystic ovary syndrome: relevance to mechanism of anovulation. Journal of Clinical Endocrinology & Metabolism. 1998;83:3984–3991. doi: 10.1210/jcem.83.11.5232. [DOI] [PubMed] [Google Scholar]
- 40.Balen AH, Conway GS, Kaltsas G, et al. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Human Reproduction. 1995;10:2107–2111. doi: 10.1093/oxfordjournals.humrep.a136243. [DOI] [PubMed] [Google Scholar]
- 41.Mulders AG, Laven JS, Eijkemans MJ, et al. Changes in anti-Mullerian hormone serum concentrations over time suggest delayed ovarian ageing in normogonadotrophic anovulatory infertility. Human Reproduction. 2004;19:2036–2042. doi: 10.1093/humrep/deh373. [DOI] [PubMed] [Google Scholar]
- 42.Schachter M, Balen AH, Patel A, et al. Hypogonadotropic patients with ultrasonographically detected polycystic ovaries: endocrine response to pulsatile gonadotropin-releasing hormone. Gynecological Endocrinology. 1996;10:327–335. doi: 10.3109/09513599609012819. [DOI] [PubMed] [Google Scholar]
- 43.Balen AH, Laven JS, Tan SL, et al. Ultrasound assessment of the polycystic ovary: international consensus definitions. Human Reproduction Update. 2003;9:505–514. doi: 10.1093/humupd/dmg044. [DOI] [PubMed] [Google Scholar]
- 44.Isojarvi JI, Laatikainen TJ, Pakarinen AJ, et al. Polycystic ovaries and hyperandrogenism in women taking valproate for epilepsy. New England Journal of Medicine. 1993;329:1383–1388. doi: 10.1056/NEJM199311043291904. [DOI] [PubMed] [Google Scholar]
- 45.Johnstone EB, Rosen MP, Neril R, et al. The Polycystic Ovary Post-Rotterdam: A Common, Age-Dependent Finding in Ovulatory Women without Metabolic Significance. Journal of Clinical Endocrinology & Metabolism. 2010;95(11):4965–4972. doi: 10.1210/jc.2010-0202. Epub 2010 Aug 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barth JH, Jenkins M, Belchetz PE. Ovarian hyperthecosis, diabetes and hirsuties in post-menopausal women [see comments] Clinical Endocrinology (Oxford) 1997;46:123–128. doi: 10.1046/j.1365-2265.1997.1050916.x. [DOI] [PubMed] [Google Scholar]
- 47.Nagamani M, Hannigan EV, Dinh TV, et al. Jul) Hyperinsulinemia and stromal luteinization of the ovaries in postmenopausal women with endometrial cancer. Journal of Clinical Endocrinology & Metabolism. 1988;67:144–148. doi: 10.1210/jcem-67-1-144. [DOI] [PubMed] [Google Scholar]
- 48.Pascale MM, Pugeat M, Roberts M, et al. Androgen suppressive effect of GnRH agonist in ovarian hyperthecosis and virilizing tumours. Clinical Endocrinology (Oxford) 1994;41:571–576. doi: 10.1111/j.1365-2265.1994.tb01820.x. [DOI] [PubMed] [Google Scholar]
- 49.Abbott DH, Tarantal AF, Dumesic DA. Fetal, infant, adolescent and adult phenotypes of polycystic ovary syndrome in prenatally androgenized female rhesus monkeys. American Journal of Primatology. 2009;71:776–784. doi: 10.1002/ajp.20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eisner JR, Dumesic DA, Kemnitz JW, et al. Mar) Timing of prenatal androgen excess determines differential impairment in insulin secretion and action in adult female rhesus monkeys. Journal of Clinical Endocrinology & Metabolism. 2000;85:1206–1210. doi: 10.1210/jcem.85.3.6453. [DOI] [PubMed] [Google Scholar]
- 51.Abbott DH, Barnett DK, Levine JE, et al. Endocrine antecedents of polycystic ovary syndrome in fetal and infant prenatally androgenized female rhesus monkeys. Biology of Reproduction. 2008;79:154–163. doi: 10.1095/biolreprod.108.067702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kent SC, Gnatuk CL, Kunselman AR, Demers LM, Lee PA, Legro RS. Hyperandrogenism and Hyperinsulinism in Children of Women with PCOS: A Controlled Study. Journal of Clinical Endocrinology & Metabolism. 2008;93:1662–9. doi: 10.1210/jc.2007-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anderson H, Fogel N, Grebe SK, et al. Infants of women with polycystic ovary syndrome have lower cord blood androstenedione and estradiol levels. Journal of Clinical Endocrinology & Metabolism. 2010;95:2180–2186. doi: 10.1210/jc.2009-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wild RA, Painter PC, Coulson PB, et al. Lipoprotein lipid concentrations and cardiovascular risk in women with polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism. 1985;61:946–951. doi: 10.1210/jcem-61-5-946. [DOI] [PubMed] [Google Scholar]
- 55.Talbott E, Clerici A, Berga SL, et al. Adverse lipid and coronary heart disease risk profiles in young women with polycystic ovary syndrome: results of a case-control study. J of Clinical Epidemiology. 1998;51:415–422. doi: 10.1016/s0895-4356(98)00010-9. [DOI] [PubMed] [Google Scholar]
- 56.Ehrmann DA, Liljenquist DR, Kasza K, et al. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism. 2006;91:48–53. doi: 10.1210/jc.2005-1329. [DOI] [PubMed] [Google Scholar]
- 57.Legro RS, Kunselman AR, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. American Journal of Medicine. 2001;111:607–613. doi: 10.1016/s0002-9343(01)00948-2. [DOI] [PubMed] [Google Scholar]
- 58.Graf MJ, Richards CJ, Brown V, et al. The independent effects of hyperandrogenaemia, hyperinsulinaemia, and obesity on lipid and lipoprotein profiles in women. Clinical Endocrinology (Oxford) 1990;33:119–131. doi: 10.1111/j.1365-2265.1990.tb00472.x. [DOI] [PubMed] [Google Scholar]
- 59.McCartney CR, Blank SK, Prendergast KA, et al. Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. Journal of Clinical Endocrinology & Metabolism. 2007;92:430–436. doi: 10.1210/jc.2006-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reinehr T, de Sousa G, Roth CL, Andler W. Androgens before and after weight loss in obese children. Journal of Clinical Endocrinology & Metabolism. 2005;90:5588–5595. doi: 10.1210/jc.2005-0438. [DOI] [PubMed] [Google Scholar]
- 61.Hoeger K, Davidson K, Kochman L, et al. The impact of metformin, oral contraceptives, and lifestyle modification on polycystic ovary syndrome in obese adolescent women in two randomized, placebo-controlled clinical trials. Journal of Clinical Endocrinology & Metabolism. 2008;93:4299–4306. doi: 10.1210/jc.2008-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ibanez L, Valls C, Marcos MV, et al. Insulin sensitization for girls with precocious pubarche and with risk for polycystic ovary syndrome: effects of prepubertal initiation and postpubertal discontinuation of metformin treatment. Journal of Clinical Endocrinology & Metabolism. 2004;89:4331–4337. doi: 10.1210/jc.2004-0463. [DOI] [PubMed] [Google Scholar]
- 63.Allen HF, Mazzoni C, Heptulla RA, et al. Randomized controlled trial evaluating response to metformin versus standard therapy in the treatment of adolescents with polycystic ovary syndrome. Journal of Pediatric Endocrinology & Metabolism. 2005;18:761–768. doi: 10.1515/jpem.2005.18.8.761. [DOI] [PubMed] [Google Scholar]
- 64.Diamanti-Kandarakis E, Katsikis I, Piperi C, et al. Increased serum advanced glycation end-products is a distinct finding in lean women with polycystic ovary syndrome (PCOS) Clinical Endocrinology (Oxford) 2008;69:634–641. doi: 10.1111/j.1365-2265.2008.03247.x. [DOI] [PubMed] [Google Scholar]
- 65.Diamanti-Kandarakis E, Katsikis I, Piperi C, et al. Effect of long-term orlistat treatment on serum levels of advanced glycation end-products in women with polycystic ovary syndrome. Clinical Endocrinology (Oxford) 2007;66:103–109. doi: 10.1111/j.1365-2265.2006.02693.x. [DOI] [PubMed] [Google Scholar]
- 66.Diamanti-Kandarakis E, Piperi C, Korkolopoulou P, et al. Accumulation of dietary glycotoxins in the reproductive system of normal female rats. Jourrnal Molecular Medicine. 2007;85:1413–1420. doi: 10.1007/s00109-007-0246-6. [DOI] [PubMed] [Google Scholar]
- 67.Diamanti-Kandarakis E, Piperi C, Patsouris E, et al. Immunohistochemical localization of advanced glycation end-products (AGEs) and their receptor (RAGE) in polycystic and normal ovaries. Histochemistry and Cell Biology. 2007;127:581–589. doi: 10.1007/s00418-006-0265-3. [DOI] [PubMed] [Google Scholar]
- 68.Sabuncu T, Vural H, Harma M, et al. Jul) Oxidative stress in polycystic ovary syndrome and its contribution to the risk of cardiovascular disease. Clinical Biochemistry. 2001;34:407–413. doi: 10.1016/s0009-9120(01)00245-4. [DOI] [PubMed] [Google Scholar]
- 69.Orio F, Jr, Palomba S, Cascella T, et al. The increase of leukocytes as a new putative marker of low-grade chronic inflammation and early cardiovascular risk in polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism. 2005;90:2–5. doi: 10.1210/jc.2004-0628. [DOI] [PubMed] [Google Scholar]
- 70.Morin-Papunen LC, Duleba AJ, Bloigu A, et al. Chlamydia antibodies and self-reported symptoms of oligoamenorrhea and hirsutism: A new etiologic factor in polycystic ovary syndrome? Fertility & Sterility. 2009 doi: 10.1016/j.fertnstert.2009.10.021. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 71.Fernandez-Real JM, Lopez-Bermejo A, Vendrell J, et al. Burden of infection and insulin resistance in healthy middle-aged men. Diabetes Care. 2006;29:1058–1064. doi: 10.2337/diacare.2951058. [DOI] [PubMed] [Google Scholar]
- 72.Thorn T, Haase N, Rosamond W, et al. AHA Statistical Update. Heart disease and stroke statistics update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 73.Shaw LJ, Bairey Merz CN, Reis SE, et al. for the WISE Investigators. Ischemic heart disease in women: insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study. Part I: sex differences in traditional and novel risk factors, symptom evaluation and gender-optimized diagnostic strategies. Journal of the American College of Cardiology. 2006;47:S4–S20. doi: 10.1016/j.jacc.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 74.Wild RA, Carmina E, Diamanti-Kandarakis E, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. Journal Clinical Endocrinology Metabolism. 2010;95(5):2038–2049. doi: 10.1210/jc.2009-2724. [DOI] [PubMed] [Google Scholar]
- 75.Pierpoint T, McKeigue PM, Isaacs AJ, et al. Mortality of women with polycystic ovary syndrome at long-term follow-up. Journal Clinical Epidemiology. 1998;51:581–586. doi: 10.1016/s0895-4356(98)00035-3. [DOI] [PubMed] [Google Scholar]
- 76.Legro RS. Polycystic ovary syndrome and cardiovascular disease: a pre- mature association? Endocrine Review. 2003;24:302–312. doi: 10.1210/er.2003-0004. [DOI] [PubMed] [Google Scholar]
- 77.Shaw LJ, Bairey Merz CN, Azziz R, et al. Postmenopausal Women with a History of Irregular Menses and Elevated Androgen Measurements at High Risk for Worsening Cardiovascular Event-Free Survival: Results from the National Institutes of Health—National Heart, Lung, and Blood Institute Sponsored Women’s Ischemia Syndrome Evaluation. Journal Clinical Endocrinology and Metabolism. 2008;93:1276–1284. doi: 10.1210/jc.2007-0425. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocrine Reviews. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Human Reproduction Update. 2008;14:367–378. doi: 10.1093/humupd/dmn015. [DOI] [PubMed] [Google Scholar]
- 80.Abbott DH, Zhou R, Bird IM, et al. Fetal programming of adrenal androgen excess: lessons from a nonhuman primate model of polycystic ovary syndrome. Endocrine Devices. 2008;13:145–158. doi: 10.1159/000134831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hickey M, Sloboda DM, Atkinson HC, et al. The relationship between maternal and umbilical cord androgen levels and polycystic ovary syndrome in adolescence: a prospective cohort study. Journal of Clinical Endocrinology & Metabolism. 2009;94:3714–3720. doi: 10.1210/jc.2009-0544. [DOI] [PubMed] [Google Scholar]
- 82.Mohamed-Hussein ZA, Harun S. Construction of a polycystic ovarian syndrome (PCOS) pathway based on the interactions of PCOS-related proteins retrieved from bibliomic data. Theoretical Biology and Medical Modelling. 2009 Sep;1:6–18. doi: 10.1186/1742-4682-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]


