Abstract
Primary restless leg syndrome (RLS) is a common sensory-motor disorder that is characterized by an irresistible urge to move the limbs and unpleasant sensations in the legs, which affects 1.9%–4.6% adults. Pramipexole, a potent dopamine D2/3 agonist, is recommended as “effective” in the short-term and “possibly effective” in the long-term treatment of primary RLS in the European guidelines on management of RLS. In this meta-analysis, we summarized the efficacy and tolerability of pramipexole in treatment for primary RLS. Results of this meta-analysis showed a favorable effect of pramipexole versus placebo on RLS symptoms (mean change on International RLS Study Group Rating Scale [IRLS] score: mean difference [MD] = −5.96; 95% confidence interval [CI]: −7.79 to −4.41, P < 0.00001) and sleep quality (pooled standard mean difference [SMD] = −0.48, 95% CI: −0.61 to −0.35, P < 0.00001). Nausea (relative risk [RR] = 2.68, 95% CI: 1.82 to 3.95, P < 0.001) and fatigue (RR = 1.82, 95% CI: 1.14 to 2.93, P = 0.013) were the most common adverse events, but, by and large, pramipexole was well-tolerated in patients with primary RLS. Nevertheless, long-term studies and more evidence of head-to-head comparisons of pramipexole with other dopamine agonists, anticonvulsants, and levodopa are needed.
Keywords: restless legs syndrome, pramipexole, meta-analysis
Background
Restless leg syndrome (RLS) is characterized by an irresistible urge to move the limbs that is usually associated with uncomfortable sensations in the legs, like creeping, burning, or itching, especially at night. RLS is classified into primary (idiopathic) form and secondary form. In primary RLS no definite pathogenic factors have been found. Secondary RLS is associated with pregnancy, uremia, iron deficiency, anemia, or Parkinson’s disease.1 The frequency of RLS in adults ranges between 2.2% to 7.9%, and primary RLS accounts for 1.9%–4.6%.2 Pramipexole (PPX), a potent dopamine D2/3 agonist, has been proven to be a first-line drug for the treatment of movement disorders such as Parkinson’s disease and RLS worldwide. In the European guidelines on management of RLS, pramipexole was recommended as “effective” in the short-term and “possibly effective” in the long-term for treatment of primary RLS (Class I evidence).3 We conducted a meta-analysis in order to summarize the efficacy and tolerability of pramipexole for the treatment of primary RLS.
We searched PUBMED, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) to identify the randomized controlled-trials (RCTs) without language or year of publication restrictions. Medical subject headings (MeSH) terms and free texts consisted of “pramipexole”, “mirapex”, “sifrol”, “restless legs syndrome”, “restless leg syndrome”, “Ekbom syndrome”, “Ekbom’s syndrome”, “Ekboms syndrome”, “randomized controlled trial”, “controlled clinical trial”, “randomized”, “randomly”, and “placebo”.
Literature selection
We conducted this study according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement.4 Studies were included if they met the following criteria: (1) they were double-blind, randomized, placebo-controlled trials; (2) the participants were .18 years old, fulfilled the essential diagnosis criteria of International Restless Legs Syndrome Study Group (IRLSSG),5 with baseline scores at least 15 on the International RLS Study Group Rating Scale (IRLS). Conditions such as pregnancy, uremia, severe insomnia, Parkinson’s disease, or peripheral nerve disease were ruled out; (3) pramipexole medication use lasted for at least 3 weeks; and (4) change in the IRLS was used as the primary endpoint.
Outcome measures
Effect on RLS symptoms
The IRLS is a ten item questionnaire scale to measure disease severity of RLS over the previous week, developed by the International RLS Study Group. Besides IRLS, other subjective outcome assessments have been adopted to evaluate the improvement or severity of RLS symptoms, including a proportion of IRLS responders, clinical global impressions-improvement (CGI-I) responders, and patient global impressions (PGI) responders. IRLS responders refer to patients whose IRLS total scores reduced ≥50% from baseline. CGI-I scale is a clinician administrated scale of 7 points, ranging from “very much improved” to “very much worse”, to assess how much the patient’s illness has improved or worsened relative to baseline. CGI-I responders are defined as patients rated “much” or “very much improved” on the CGI-I scale. Likewise, PGI responders are defined as participants who have “much” or “very much” improvement in self-rated overall condition over the preceding week.
Effect on sleep
Almost all RLS patients suffer from sleep disturbances and sleep initiation and maintenance are the most frequently reported problems.6 As such, self-rated satisfaction of sleep is an important factor of outcome. Effect on sleep was rated by a variety of scales in different studies as following: visual analog scale (VAS), medical outcome study (MOS) sleep scale, subjective sleep quality (SSQ), Pittsburgh Sleep Quality Index (PSQI), and RLS-6 (a six item scale to assess the severity of RLS symptoms).
Safety and tolerability
The most common (>5%) adverse events (AEs) and withdrawals due to AEs were included in this meta-analysis. The most frequently reported AEs included nausea, fatigue, headache, dizziness, somnolence, and nasopharyngitis. Serious AEs were defined as sudden onset of sleep (SOOS), life-threatening hazards, or death.
Data extraction
Two reviewers independently extracted data from each study including study design, patient baseline characteristics (age, gender, and IRLS score), efficacy outcomes (change in IRLS score and self-rated sleep quality, number of IRLS responders, CGI-I responders, and PGI responders) and AEs. Discrepancies were resolved by mutual consensus.
Assessment of study quality
The Cochrane Collaboration’s tool for assessing risk of bias was used for quality assessment of included studies. The grade assessment consists of random sequence generation, allocation concealment, incomplete outcome data, selective outcome reporting, blinding of patients and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases.7 Each domain was graded into “low risk of bias”, “high risk of bias”, or “unclear risk of bias”.
Assessment of heterogeneity
Heterogeneity between studies was assessed using Chi-squared test and I2 statistics. Fixed-effect model was used if the heterogeneity was considered “small” or “moderate” (I2 < 0.5). Random-effect model was applied if heterogeneity was considered “substantial” (I2 > 0.5). Subgroup analyses based on race (Caucasian versus Asian), medication period (≤6 weeks versus >6 weeks), dose regimen (fixed dose versus flexible dose) were performed in order to explore sources of heterogeneity.
Sensitivity analysis was performed to test the robustness of the results when substantial heterogeneity was detected. Publication bias was assessed by visual inspection of Begg’s funnel plot.
Efficacy and safety outcome statistics
There were two continuous outcomes included in this meta-analysis. Based on the data obtained from the studies, mean change in IRLS score was presented as mean difference ± standard error (SE) while change in self-rated quality of sleep was reported using mean ± standard deviation (SD).
Dichotomous outcomes such as number of IRLS responders, CGI-I responders, and PGI responders were calculated using odds ratio (OR). Safety and tolerability outcomes including incidence of AEs and withdrawals due to AEs were measured by relative risk (RR). Two-sided P-values less than 0.05 were considered statistically significant.
Statistics analysis was performed using Revman 5.2 software (Cochrane, Oxford, UK, available at http://www.cochrane.org) and Stata software, version 11.0 (StataCorp LP, College Station, TX, USA).
Results
Of 147 citations found by initial search, six parallel RCTs were finally included in the meta-analysis (Figure 1). The study design and the basic characteristics are shown in Table 1. The included studies were published between 2006 and 2012. The participants in four studies were mostly Caucasian and in the other two they were Asian. Trials lasted for 3 to 12 weeks using flexible dose (0.125, 0.25, 0.5, 0.75 mg/day, four studies) or fixed dose (0.125, 0.25, 0.5, 0.75 mg/day, two studies).
Figure 1.
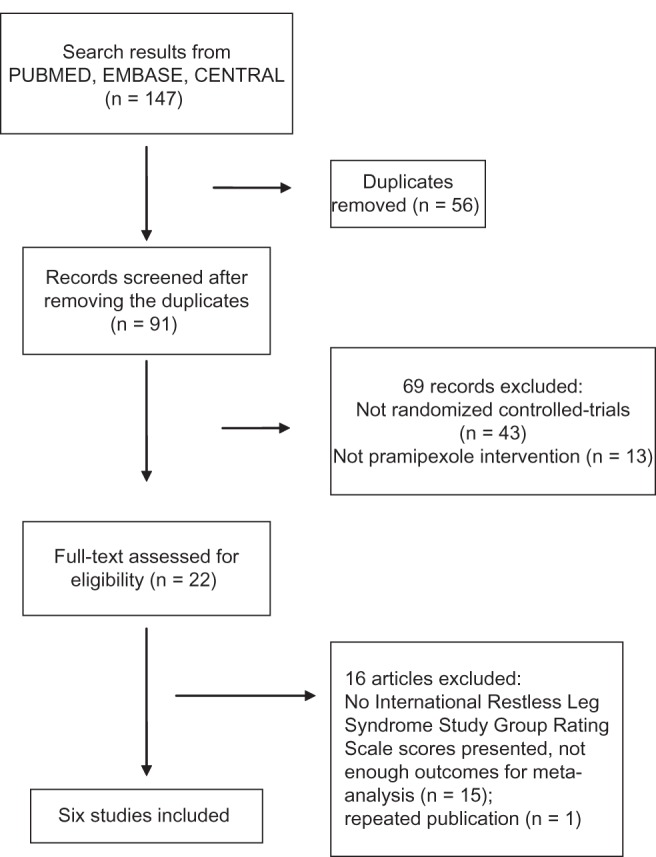
Flow diagram of study selection.
Table 1.
Baseline characteristics and overview of the included studies
| Study | Follow-up (weeks) | ITT population (n)
|
Race | Age (years) (mean ± SD)
|
Sex (female%)
|
Baseline IRLS score (mean ± SD)
|
PPX administration | Scale of self-rated sleep quality | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PPX | PBO | PPX | PBO | PPX | PBO | PPX | PBO | |||||
| Winkelman et al8 | 12 | 254 | 85 | White (97.3%) | 51.4 ± 12.7 | 51.5 ± 14.0 | 61.8 | 63.5 | 23.4 ± 5.1 | 23.5 ± 5.2 | Starting dose = 0.125 mg/day forced titrated up to 0.25, 0.5, 0.75 mg/day 3 weeks after | VAS |
| Partinen et al9 | 3 | 86 | 21 | White | 56.9 ± 10.8 | 53.3 ± 11.1 | 72.1 | 81 | 22.7 ± 4.1 | 22.9 ± 4.2 | Fixed dose: 0.125, 0.25, 0.5, 0.75 mg/day | SSQ* |
| Oertel et al10 | 6 | 224 | 144 | White (98.8%) | 55.4 ± 11.6 | 55.8 ± 10.9 | 64.3 | 68.4 | 24.7 ± 5.2 | 24.9 ± 5. 4 | Starting dose = 0.125 mg/day Stepwise increased to optimum dose: 0.25, 0.5, 0.75 mg/day | VAS |
| Ferini-Strambi et al11 | 12 | 182 | 182 | White (99.5%) | 56.3 ± 12.4 | 56.9 ± 13.0 | 72.5 | 63.6** | 24.2 ± 5.2 | 24.6 ± 5.7 | As above | MOS |
| Inoue et al12 | 6 | 20 | 21 | Asian | 48.7 ± 16.1 | 62.3 ± 11.9 | 55 | 47.6 | 23.4 ± 6.4 | 25.1 ± 5.8 | As above | PSQI |
| Ma et al13 | 6 | 195 | 92 | Asian | 56.46 ± 11.88 | 56.86 ± 11.89 | 60.5** | 80.6** | Not available | Not available | As above | RLS-6* |
Notes:
Data from these two studies cannot be combined for meta-analysis because it is not feasible to merge the scores of diverse items
calculation was based on safety population.
Abbreviations: IRLS, International Resdess Leg Syndrome Study Group Rating Scale; ITT, intent-to-treat; MOS, medical outcome study; PBO, placebo; PPX, Pramipexole; PSQI, Pittsburgh Sleep Quality Index; RLS, restless leg syndrome; RLS-6, a six item scale to assess the severity of RLS symptoms; SD, standard deviation; SSQ, subjective sleep quality; VAS, visual analog scale.
The overall bias of included studies is low (displayed in Figure 2). Generation of random codes was not adequately subscribed in three studies (unclear bias). No information about how the randomized allocation was concealed is given in three studies (unclear bias).
Figure 2.
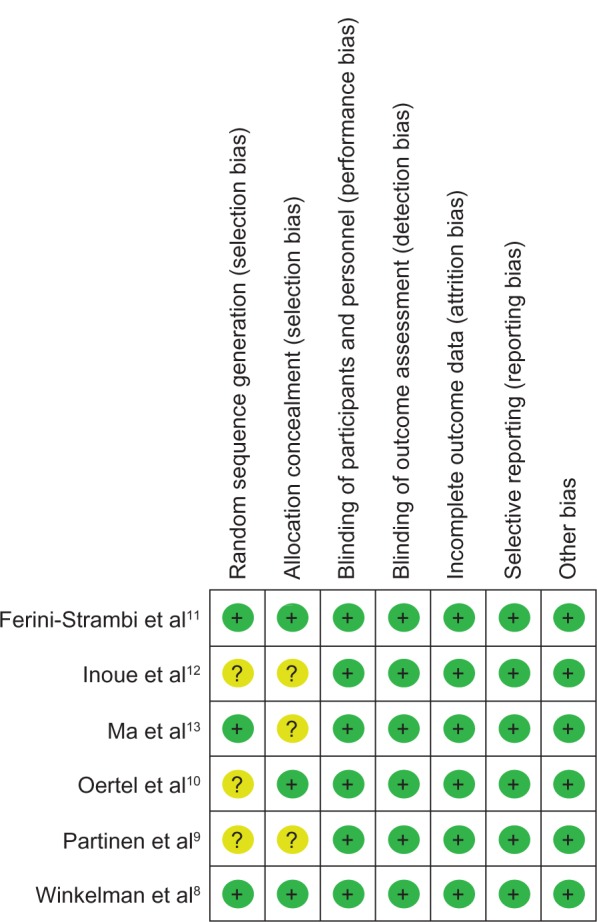
Author’s judgment on risk of bias across all included studies.
Notes: ?, unclear risk of bias; +, low risk of bias.
Efficacy outcomes
Compared to placebo, the overall mean change in the IRLS score of PPX was significantly larger (mean difference [MD] = −5.96; 95% confidence interval [CI]: −7.79 to −4.41, I2 = 67%; P < 0.00001; Figure 3). The treatment effect of PPX versus placebo on self-rated quality of sleep was significantly larger with a pooled standard mean difference (SMD) of −0.48 (95% CI: −0.61 to −0.35; I2 = 20%; P < 0.00001; Figure 4). Additionally, PPX therapy produced statistically higher ORs of IRLS responder rate (OR = 2.51; 95% CI: 2.00 to 3.16; I2 = 0; P < 0.00001; Figure 5), CGI-I responder rate (OR = 3.13; 95% CI: 2.48 to 3.95; I2 = 0; P < 0.00001; Figure 6), and PGI responder rate (OR = 2.80; 95% CI: 1.9 to 4.1; I2 = 56%; P = 0.05; Figure 7) than that of placebo, further confirming its positive therapeutic effect. Four studies provided available data of self-rated quality of sleep for meta-analysis. Due to the presence of substantial between-study heterogeneity of mean change on IRLS score (I2 = 67%) and PGI responder rate (I2 = 57%), subgroup analyses were performed. Outcomes of subgroup analyses showed race, medication period, or dose regimen were no source of heterogeneity because heterogeneity was still substantial within subgroups (data not shown). The between-study heterogeneity of efficacy outcomes was found to be low in change in self-rated quality of sleep (I2 = 20%), IRLS responder rate (I2 = 0), and CGI responder rate (I2 = 0).
Figure 3.
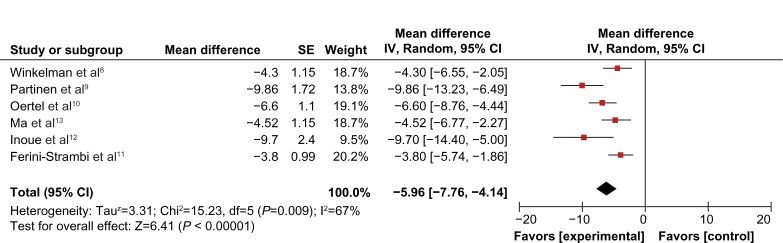
Forest plot of adjusted mean change on IRLS score.
Abbreviations: CI, confidence interval; IRLS, International Restless Leg Syndrome Study Group Rating Scale; SE, standard error; IV, inverse variance; df, degree of freedom.
Figure 4.
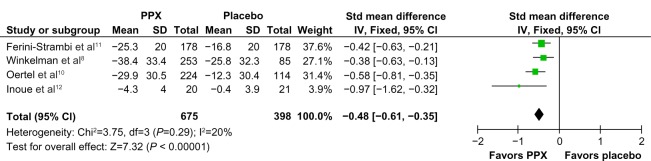
Forest plot: comparison of change in self-rated quality of sleep.
Abbreviations: CI, confidence interval; PPX, Pramipexole; SD, standard deviation; Std, standard; IV, inverse variance; df, degree of freedom.
Figure 5.
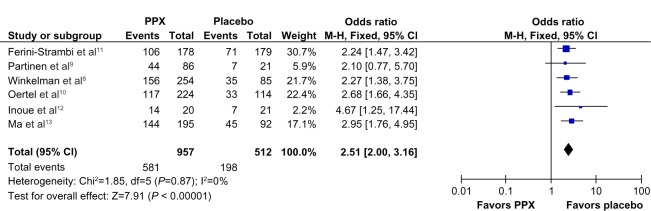
Forest plot: comparison of IRLS responder rate.
Abbreviations: CI, confidence interval; PPX, Pramipexole; IRLS, International Restless Leg Syndrome Study Group Rating Scale; M-H, Mantel-Haenszel method; df, degree of freedom.
Figure 6.
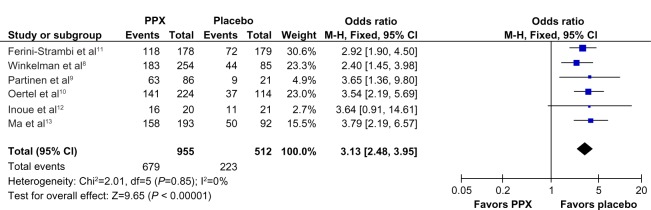
Forest plot: comparison of CGI-I responder rate.
Abbreviations: CGI-I, clinical global impressions – improvement; CI, confidence interval; PPX, Pramipexole; M-H, Mantel-Haenszel method; df, degree of freedom.
Figure 7.
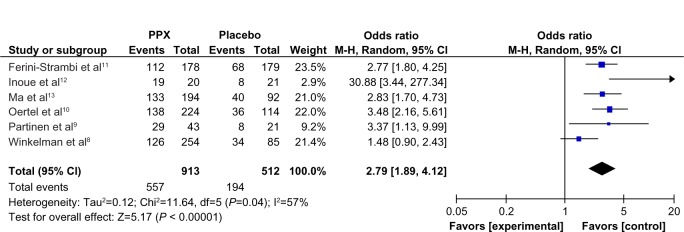
Forest plot: comparison of PGI responder rate.
Abbreviations: CI, confidence interval; PPX, Pramipexole; M-H, Mantel-Haenszel method; PGI, patient global impressions; df, degree of freedom.
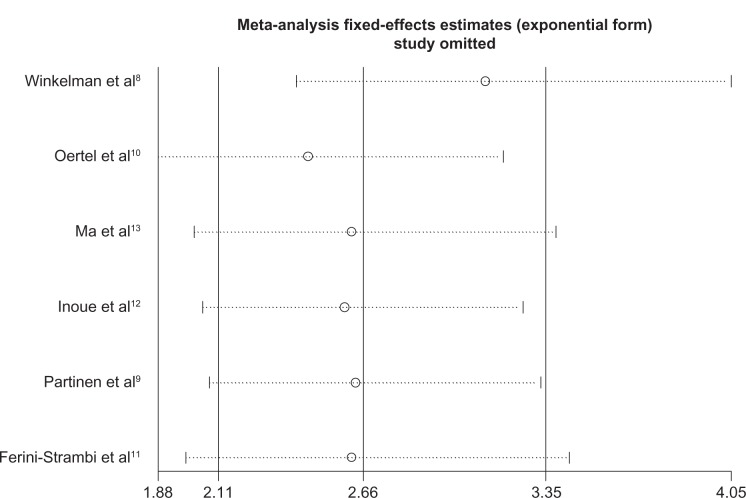
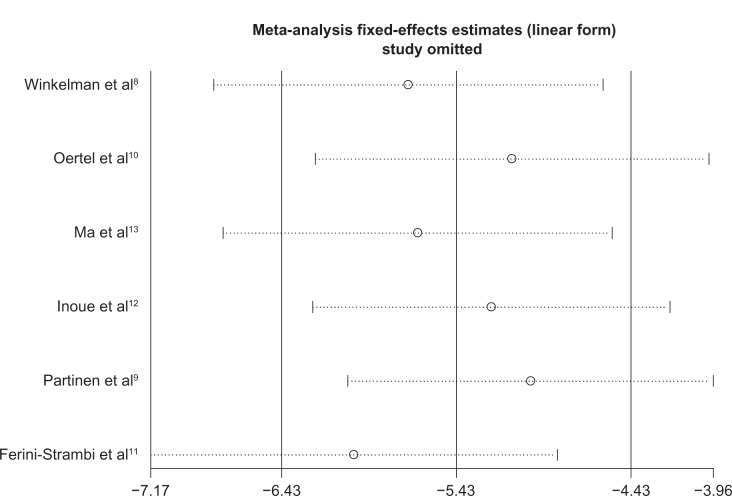
Sensitivity analyses did not show any significant variation in mean change on the IRLS score or OR of PGI responder rate when any single study was omitted (Figures 8 and 9). There was no obvious publication bias detected by Begg’s funnel plot (Figure 10).
Figure 8.
Sensitivity analysis of mean change on IRLS score: results did not alter significantly when one study was removed from the analysis.
Abbreviation: IRLS, international Restless Leg Syndrome Study Group Rating Scale.
Figure 9.
Sensitivity analysis of PGI responder rate.
Abbreviation: PGI, patient global impressions.
Figure 10.
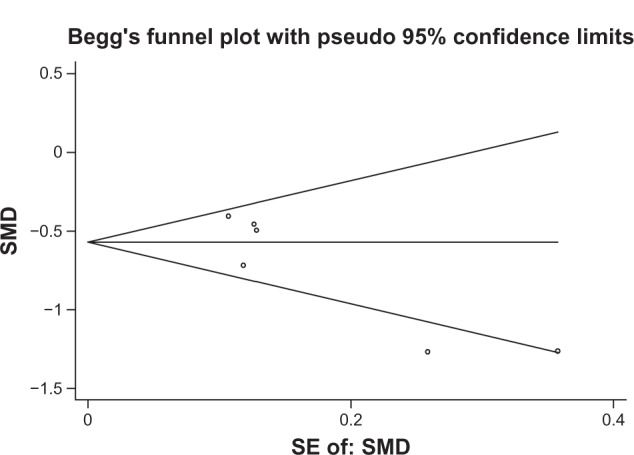
Begg’s test for publication bias of mean change in IRLS score.
Abbreviations: IRLS, international Restless Leg Syndrome Study Group Rating Scale; SMD, standard mean difference; SE; standard error.
Safety outcomes
As shown in Table 2, a statistically higher incidence of nausea (RR = 2.68, 95% CI: 1.82 to 3.95, P < 0.001) and fatigue (RR = 1.82, 95% CI: 1.14 to 2.93, P = 0.013) was found in patients receiving PPX compared to placebo. Incidence of other usual AEs including headache (RR = 1.15, 95% CI: 0.85, 1.55), dizziness (RR = 1.40, 95% CI: 0.85, 2.30), somnolence (RR = 1.45, 95% CI: 0.87, 2.41), nasopharyngitis (RR = 1.10, 95% CI: 0.67, 1.78), and withdrawals due to AEs (RR = 1.20, 95% CI: 0.77, 1.85) was not significantly different between both groups.
Table 2.
Safety and tolerability outcomes of pramipexole compared to placebo
| Winkelman et al8
|
Partinen et al9
|
Oertel et al10
|
Ferini-Strambi et al11
|
Inoue et al12
|
Ma et al13
|
Total
|
Pooled RR 95% CI (M-H) | I2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PPX (n = 258) | PBO (n = 86) | PPX (n = 87) | PBO (n = 22) | PPX (n = 230) | PBO (n = 115) | PPX (n = 182) | PBO (n = 187) | PPX (n = 20) | PBO (n = 21) | PPX (n = 202) | PBO (n = 103) | PPX (n = 1123) | PBO (n = 534) | |||
| Nausea | 49 | 4 | 13 | 1 | 22 | 6 | 32 | 11 | 5 | 2 | 23 | 6 | 144 | 40 | 2.68 (1.82, 3.95)** | 0.0% |
| Fatigue | 13 | 4 | 16 | 5 | 21 | 5 | 16 | 4 | 2 | 0 | – | – | 68 | 18 | 1.82 (1.14, 2.93)* | 41.5% |
| Headache | 46 | 15 | 17 | 7 | 16 | 7 | 27 | 24 | 3 | 2 | 13 | 1 | 122 | 56 | 1.15 (0.85, 1.55) | 14.6% |
| Dizziness | 25 | 6 | – | – | 8 | 4 | – | – | – | – | 28 | 9 | 61 | 19 | 1.40 (0.85, 2.30) | 0.0% |
| Somnolence | 26 | 4 | – | – | 6 | 3 | – | – | 2 | 3 | 25 | 9 | 59 | 19 | 1.45 (0.87, 2.41) | 0.0% |
| Nasopharyngitis | 17 | 4 | 6 | 0 | 10 | 9 | 13 | 9 | 5 | 1 | – | – | 51 | 23 | 1.10 (0.67, 1.78) | 0.0% |
| Withdrawal due to AEs | 29 | 5 | 1 | 0 | 6 | 5 | 17 | 16 | 0 | 0 | 10 | 4 | 63 | 30 | 1.20 (0.77, 1.85) | 0.0% |
Notes:
P < 0.001
P = 0.013.
Abbreviations: AEs, adverse events; CI, confidence interval; M-H, Mantel-Haenszel method; PBO, placebo; PPX, pramipexole; RR, relative risk.
Conclusion
Results of this meta-analysis showed a favorable effect of PPX versus placebo on RLS symptoms and sleep quality. Nausea and fatigue were the most common adverse events in patients receiving PPX compared to placebo. Incidence of headache, dizziness, somnolence, nasopharyngitis, and withdrawals due to AEs was not significantly different between patients receiving PPX or placebo, thus PPX was well-tolerated in patients with primary RLS.
Discussion
All studies included in the meta-analysis were high-quality RCTs that might minimize selection and measurement bias. When dealing with missing data, all included trials performed ITT (intention-to-treat) analyses to avoid overoptimistic estimates of the efficacy.14 But in safety analyses, ITT principles may make it difficult to detect adverse effects due to a dilution effect.15 Therefore, safety outcomes might be “overoptimistic” because of the possibly underestimated incidence of AEs. In another published meta-analysis including four trials, nausea was found to be the only statistically significant adverse event of PPX compared to placebo in treatment of RLS.16 However, our meta-analysis showed a statistically higher incidence of nausea and fatigue in patients receiving PPX because two more trials were included.
By far, polysomography is the only objective way to assess treatment efficacy on RLS, in which parameters such as periodical leg movements and sleep latency can be recorded.17 However, there were only two trials conducting polysomography,12,18 and all efficacy outcomes in the meta-analysis were subjective. Results of these subjective rating scales are less accurate and tend to lead to bias. To some degree, this might be an explanation for the substantial heterogeneity of mean change on IRLS score and PGI responder rate.
Only six published studies were included in this meta-analysis. The power of test for funnel plot asymmetry seemed to be lower.7 Because unpublished studies were not included, publication bias could not be completely excluded even though no obvious evidence of such bias was detected.
It is noteworthy that all included studies had a dosage period ranging from 3 to 12 weeks. At present, evidence for long-term efficacy and safety of PPX on RLS is still absent. Augmentation, the most serious side-effect of dopaminergic medication, needs to be thoroughly evaluated in future studies. Therefore, long-term studies and observations should be carried out.
Other meta-analyses,16,19 have demonstrated that dopamine agonists, including cabergoline, lisuride, pergolide, pramipexole, ropinirole, rotigotine, and sumanirole, are effective for the treatment of primary RLS, but the overall treatment effect is moderate. Indirect placebo comparison showed a superior reduction in the mean IRLS score, higher CGI-I responder rate, and significantly lower incidence of nausea, vomiting, and dizziness for PPX compared to ropinirole in one meta-analysis.20 Evidence of head-to-head comparisons of PPX with other dopamine agonists, anticonvulsants, and levodopa is needed.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Schattschneider J, Bode A, Wasner G, Binder A, Deuschl G, Baron R. Idiopathic restless legs syndrome: abnormalities in central somatosensory processing. J Neurol. 2004;251(8):977–982. doi: 10.1007/s00415-004-0475-3. [DOI] [PubMed] [Google Scholar]
- 2.Ohayon MM, O’Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16(4):283–295. doi: 10.1016/j.smrv.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Borreguero D, Ferini-Strambi L, Kohnen R, et al. European Federation of Neurological Societies. European Neurological Society; European Sleep Research Society European guidelines on management of restless legs syndrome: report of a joint task force by the European Federation of Neurological Societies, the European Neurological Society and the European Sleep Research Society. Eur J Neurol. 2012;19(11):1385–1396. doi: 10.1111/j.1468-1331.2012.03853.x. [DOI] [PubMed] [Google Scholar]
- 4.Swartz MK. The PRISMA statement: a guideline for systematic reviews and meta-analyses. J Pediatr Health Care. 2011;25(1):1–2. doi: 10.1016/j.pedhc.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J, Restless Legs Syndrome Diagnosis and Epidemiology workshop at the National Institutes of Health. International Restless Legs Syndrome Study Group Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4(2):101–119. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 6.Trenkwalder C, Paulus W. Restless legs syndrome: pathophysiology, clinical presentation and management. Nat Rev Neurol. 2010;6(6):337–346. doi: 10.1038/nrneurol.2010.55. [DOI] [PubMed] [Google Scholar]
- 7.The Cochrane Collaboration [homepage on the Internet] Higgins JPT, Green S.Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available from: http://www.cochrane-handbook.org2011. Accessed March 20, 2011
- 8.Winkelman JW, Sethi KD, Kushida CA, et al. Efficacy and safety of pramipexole in restless legs syndrome. Neurology. 2006;67(6):1034–1039. doi: 10.1212/01.wnl.0000231513.23919.a1. [DOI] [PubMed] [Google Scholar]
- 9.Partinen M, Hirvonen K, Jama L, et al. Efficacy and safety of pramipexole in idiopathic restless legs syndrome: a polysomnographic dose-finding study – the PRELUDE study. Sleep Med. 2006;7(5):407–417. doi: 10.1016/j.sleep.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Oertel WH, Stiasny-Kolster K, Bergtholdt B, et al. Pramipexole RLS Study Group Efficacy of pramipexole in restless legs syndrome: a six-week, multicenter, randomized, double-blind study (effect-RLS study) Mov Disord. 2007;22(2):213–219. doi: 10.1002/mds.21261. [DOI] [PubMed] [Google Scholar]
- 11.Ferini-Strambi L, Aarskog D, Partinen M, et al. Effect of pramipexole on RLS symptoms and sleep: a randomized, double-blind, placebo-controlled trial. Sleep Med. 2008;9(8):874–881. doi: 10.1016/j.sleep.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Inoue Y, Hirata K, Kuroda K, et al. Efficacy and safety of pramipexole in Japanese patients with primary restless legs syndrome: A polysomnographic randomized, double-blind, placebo-controlled study. Sleep Med. 2010;11(1):11–16. doi: 10.1016/j.sleep.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Ma JF, Wan Q, Hu XY, et al. Efficacy and safety of pramipexole in chinese patients with restless legs syndrome: results from a multicenter, randomized, double-blind, placebo-controlled trial. Sleep Med. 2012;13(1):58–63. doi: 10.1016/j.sleep.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Gupta SK. Intention-to-treat concept: A review. Perspect Clin Res. 2011;2(3):109–112. doi: 10.4103/2229-3485.83221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lange S. The all randomized/full analysis set (ICH E9) – May patients be excluded from the analysis? Drug Inf J. 2001;35(3):881–891. [Google Scholar]
- 16.Hornyak M, Trenkwalder C, Kohnen R, Scholz H. Efficacy and safety of dopamine agonists in restless legs syndrome. Sleep Med. 2012;13(3):228–236. doi: 10.1016/j.sleep.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Zucconi M, Ferri R, Allen R, et al. International Restless Legs Syndrome Study Group (IRLSSG) The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7(2):175–183. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Partinen M, Hirvonen K, Jama L, et al. Efficacy and safety of pramipexole in idiopathic restless legs syndrome: a polysomnographic dose-finding study – the PRELUDE study. Sleep Med. 2006;7(5):407–417. doi: 10.1016/j.sleep.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Scholz H, Trenkwalder C, Kohnen R, Riemann D, Kriston L, Hornyak M. Dopamine agonists for restless legs syndrome [review] Cochrane Database Syst Rev. 2011;3:CD006009. doi: 10.1002/14651858.CD006009.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quilici S, Abrams KR, Nicolas A, et al. Meta-analysis of the efficacy and tolerability of pramipexole versus ropinirole in the treatment of restless legs syndrome. Sleep Med. 2008;9(7):715–726. doi: 10.1016/j.sleep.2007.11.020. [DOI] [PubMed] [Google Scholar]