Abstract
Background
Exercise training can improve endothelial function in patients with diabetes. We hypothesized that the favorable effect of exercise training on endothelial function in patients with diabetes is counteracted by cigarette smoking.
Purpose:
To assess whether there is a difference in the effect of exercise on endothelial function in smokers and non-smokers with type 2 diabetes.
Methods:
We performed a 3-month controlled trial in 27 never-smoking and 17 smoking individuals with type 2 diabetes who participated in a home-based walking program. The percentage decrease in post-exercise ankle-brachial pressure index (ABI), which is an index of endothelial function, was assessed at baseline and after 3 months.
Results:
Compared to the smoking group, the never-smoking group showed a more significant improvement in post exercise ABI during the 3 months of home-based training (interaction, P < 0.01).
Conclusions:
These results indicate that smoking may counteract the favorable effects of exercise training on endothelial function. Endothelial function plays an important role in the prevention of cardiovascular disease among patients with diabetes. Therefore, a Certified Diabetes Educator should strongly advise diabetic patients not to smoke.
Keywords: smoking, endothelial function, exercise
Introduction
The most common cause of mortality among patients with diabetes is cardiovascular disease (CVD).1 Therefore, CVD prevention has the potential to drastically limit the mortality.2 Recent studies have suggested that endothelial function and, specifically, the regulation of vasomotor tone through the release of molecules that mediate vasoconstriction play pivotal roles in the prevention of macrovascular complications in patients with type 2 diabetes.3
Physical activity and exercise promote longevity and ameliorate type 2 diabetes and insulin resistance. Moreover, exercise training can improve endothelial function in patients with diabetes.4–6 These results suggest that the improvement of endothelial function could be a surrogate therapeutic target for exercise interventions to reduce the development of CVD in patients with type 2 diabetes.
Cigarette smoking is a recognized risk factor for cardiovascular disease and is known to promote the development of endothelial dysfunction.7 In other words, the effects of exercise training and smoking exert opposing effects on vascular endothelial function. Therefore, we hypothesized that smoking counteracts the favorable effect of exercise training on endothelial function. To test this hypothesis, we assessed the effects of exercise on endothelial function in type 2 diabetics who have a history of smoking compared to those who have never smoked.
Materials and Methods
Study subjects and the intervention protocol
In our study, 44 patients with type 2 diabetes (18 women and 26 men aged 61.8 ± 9.2 years), who visited our outpatient department for an exercise prescription and self-management training, were divided into 2 groups: the never-smoking group (n = 27) and the past or current smoking group (smoking group: n = 17). Thirty-four patients had a medical history of hypertension, but none of the subjects had uncontrolled severe systemic hypertension. Hypercholesterolemia was present in 22 patients, and all of the patients were being administered statins at the time of the study. There was no evidence of autonomic cardiac neuropathy in any of the patients. The exclusion criteria included uncontrolled heart failure, severe or unstable angina pectoris, and age > 80 years. Patients who were not able to appear for check-up visits and patients refusing to provide written consent were also excluded.
A home-based walking program was adopted as the exercise intervention, because this type of program can foster long-term adherence owing to its convenience and flexibility. This program consisted of a 3 month of daily walking exercise (duration, 20–30 min) at home. Adherence of program was confirmed at the time of check-up visit.
Assessment of endothelial function
Endothelial function examinations were performed at baseline and after 3 months. In this study, endothelial function was as the percentage decrease in post-exercise ankle-brachial pressure index (ABI) relative to the baseline values. This simple exercise test can be a useful surrogate marker of endothelial dysfunction.8 In brief, resting ABI was measured using a Doppler flow meter (VaSera, VS-1000, Fukuda denshi, Japan), with the patient in a supine position, according to a standard protocol. ABI was also measured immediately (60 times/min) after 2.5 min of active pedal flexion exercises.
Statistical analysis
Data is expressed as means ± standard deviation (SD). To compare the baseline findings of the 2 groups, we used the unpaired t test for continuous variables. Analysis of variance with Bonferroni post-hoc test analysis revealed within time differences between the groups. P values below 0.05 were considered statistically significant.
Results
All of the enrolled patients completed the study. In the smoking group, 9 participants (53%) had quit smoking and 8 (47%) smoked at the time of the study. The average non-smoking period of patients who quit smoking was 6.4 ± 5.5 years.
During the study period, medical treatment was maintained effectively, and no severe clinical event was recorded. The basic clinical characteristics were presented in Table 1. The proportion of women in the smoking group was significantly lower than in the never-smoking group. Patients in the smoking group tended to have lower levels of HDL cholesterol than those in the never-smoking group.
Table 1.
Patient’s characteristics.
| Variables | Never smoking (n = 27) | Smokers (n = 17) | P value |
|---|---|---|---|
| Age (years) | 61 ± 9 | 62 ±10 | 0.74 |
| Gender (females, %) | 59 | 12 | 0.00 |
| Yers since diagonosis (years) | 6 ± 7 | 8 ± 10 | 0.57 |
| Oral hypoglycemic medication (%) | 52 | 65 | 0.30 |
| Insulin use (%) | 52 | 47 | 0.50 |
| Statins (%) | 26 | 41 | 0.23 |
| BMI | 27 ± 4 | 27 ± 4 | 0.80 |
| Hypertension (%) | 70 | 88 | 0.16 |
| Obesity (%) | 67 | 59 | 0.42 |
| History of hypercholesterolmia (%) | 52 | 47 | 0.50 |
| History of myocardial infarction (%) | 4 | 0 | 0.61 |
| HbA1c (%) | 9.0 ± 1.6 | 8.4 ± 1.4 | 0.14 |
| Systolic blood pressure (mmHg) | 130 ± 14 | 134 ± 19 | 0.47 |
| Triglycerides (mg/dL) | 122 ± 61 | 115 ± 48 | 0.70 |
| HDL cholesterol (mg/dL) | 55 ± 16 | 47 ± 10 | 0.06 |
| LDL cholesterol (mg/dLl) | 119 ± 35 | 105 ± 21 | 0.16 |
| Resting ABI | 1.16 ± 0.9 | 1.14 ± 0.8 | 0.24 |
After intervention, HDL cholesterol levels increased similarly in both groups (54.8 ± 17.2 mg/dL to 58.8 ± 18.8 mg/dL in the never-smoking group and 46.6 ± 10.6 mg/dL to 52.9 ± 8.4 mg/dL in the smoking group). Hba1c levels decreased more significantly in the never-smoking group compared to the smoking group during this period (9.1% ± 1.6% to 7.2% ± 1.0% and 8.4% ± 1.4% to 7.7% ± 1.4%, respectively; P < 0.05).
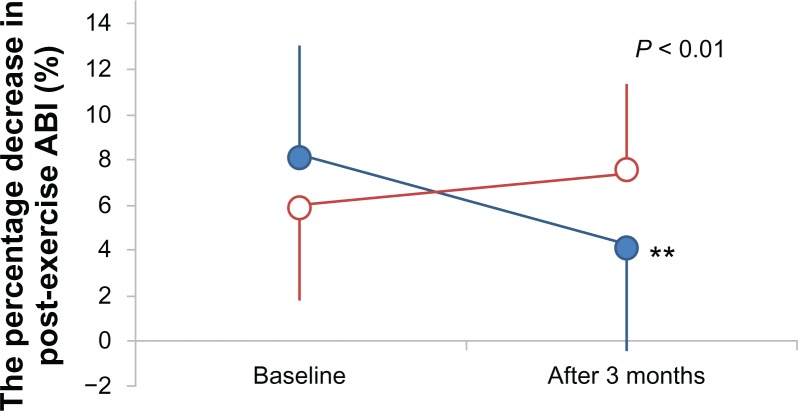
The change in the post-exercise ABI, which is an index of endothelial function, is shown in Figure 1. There was a greater improvement in the post-exercise ABI in the never-smoking group compared to the smoking group (P < 0.01). In the never-smoking group, the post-exercise ABI improved significantly (8.2 ± 5.2 to 4.2 ± 5.4; P < 0.01). No change was observed in the smoking group (6.0 ± 4.7 to 7.4 ± 4.9; not significant). These results remained unchanged after adjustment for age, gender, duration of diabetes, insulin use, HDL cholesterol level at baseline, and change in HbA1c (Table 2). In addition, no significant correlation was observed between smoking habits (quitter or continuing smoker, number of cigarettes smoked per day) and the improvement of endothelial function (Table 3).
Figure 1.
Change in the percentage decrease in post-exercise ABI from baseline in never smoking (•) and smoking (○) groups after 3 months.
Note: **P< 0.01 within-group difference for the changes from baseline.
Table 2.
Results of multivariate analysis within group.
| F vale | P value | |
|---|---|---|
| Time | 0.36 | 0.55 |
| Time × age | 0.02 | 0.90 |
| Time × gender | 0.03 | 0.86 |
| Time × duration of diabetes | 0.69 | 0.42 |
| Time × insulin use | 0.19 | 0.67 |
| Time × HDL cholesterol level at baseline | 0.52 | 0.48 |
| Time × change in HbA1c | 3.66 | 0.07 |
Table 3.
Mean change (as percentage from initial) in smoking habits.
| Change from initial (%) | 95% CI | P value | |
|---|---|---|---|
| Quitter or continuing smoker | |||
| Quitter | 2.5 ± 5 | −4.1–9.0 | 0.41 |
| Continuing smoker | 0.09 ± 7 | ||
| Number of cigarettes smoked per day | |||
| <20 | 0.7 ± 4 | −10–13 | 0.83 |
| ≧20 | −0.3 ± 9 | ||
Discussion
The present study shows that a home-based walking program can improve the post-exercise ABI, a marker of endothelial function, in never-smoking patients with type 2 diabetes; further, there was no such change in smokers. These results indicate that smoking may counteract the favorable effect of exercise training on endothelial function.
Cigarette smoking is a strong risk factor for CVD and increases overall mortality in patients with diabetes.9 The Nurses’ Health Study showed that mortality rates among women with diabetes are strongly related to their smoking habits, with current smokers who smoke 35 cigarettes/day having a twofold higher risk than never-smokers.10 A prospective trial of smoking cessation showed a reduction in total mortality rate, with a trend towards a reduction in CVD-related deaths.11 Our findings support the results of a prior research on the damage induced by cigarette smoke among patients with diabetes and provide new insight into the potential mechanisms by which smoking contributes to CVD.
In our study, even a history of smoking or current smoking of only a few cigarettes has potentially deleterious effects contributing to endothelial dysfunction. Barua et al have shown that light smoking is just as detrimental as heavy smoking with respect to effects on the NO biosynthetic pathway.12 Prior and present studies have shown that the endothelial dysfunction induced by smoking may not be reversible. These results highlight the importance of education to target diabetic patients who have not ever smoked.
Our study had several limitations. First, we used the post-exercise ABI for the assessment of endothelial function; this method is acceptable but is not perfect.8 Second, we had no information on the dietary regimen of the patients during the study period; we cannot exclude the possibility that smokers also had poor diets, which would have confounded our results. Third, the smoking assessment was based on self-reports and was not verified. Finally, the number of patients was small—only a large-scale clinical trial would be able to provide definitive evidence on this interesting clinical topic.
Conclusions
To summarize, our study shows that smoking counteracts the favorable effects of exercise training on endothelial function in patients with type 2 diabetes. These data may have important implications concerning the damage associated with smoking in patients with diabetes.
Footnotes
Author Contributions
Conceived and designed the experiments: SS, NN, SO, ST, HN, HK, RN. Analyzed the data: SS. Wrote the first draft of the manuscript: SS. Contributed to the writing of the manuscript: SS. Agree with manuscript results and conclusions: SS, NN, SO, ST, HN, HK, RN. Jointly developed the structure and arguments for the paper: SS, NN, SO, ST, HN, HK, RN. Made critical revisions and approved final version: SS, NN, SO, ST, HN, HK, RN. All authors reviewed and approved of the final manuscript.
Funding
Author(s) disclose no funding sources.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Stamler J, Vaccaro O, Neaton J, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16(2):434–4. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 2.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–7. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 3.Calver A, Collier J, Vallance P. Inhibition and stimulation of nitric oxide synthesis in the human forearm arterial bed of patients with insulin-dependent diabetes. J Clin Invest. 1992;90(6):2548–54. doi: 10.1172/JCI116149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuchsjager-Mayrl G, Pleiner J, Wiesinger G, et al. Exercise training improves vascular endothelial function in patients with type 1 diabetes. Diabetes Care. 2002;25(10):1795–801. doi: 10.2337/diacare.25.10.1795. [DOI] [PubMed] [Google Scholar]
- 5.Okakda S, Hiuge A, Makino H, et al. Effect of exercise intervention on endothelial function and incidence of cardiovascular disease in patients with type 2 diabetes. J Atheros Thrombosis. 2010;17(8):828–33. doi: 10.5551/jat.3798. [DOI] [PubMed] [Google Scholar]
- 6.Maiorana A, O’Driscoll G, Cheetham C, et al. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. J Am Coll Cardiol. 2001;38(3):860–6. doi: 10.1016/s0735-1097(01)01439-5. [DOI] [PubMed] [Google Scholar]
- 7.Pittilo RM. Cigarette smoking, endothelial injury and cardiovascular disease. Int J Exp Pathol. 2000;81(4):219–30. doi: 10.1046/j.1365-2613.2000.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato S, Masami K, Otsuki S, et al. Post-exercise ankle-brachial pressure index demonstrates altered endothelial function in the elderly. Jpn Clin Med. 2011;2:21–4. doi: 10.4137/JCM.S7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haire-Joshu D, Glasgow RE, Tibbs TL. Smoking and diabetes. Diabetes Care. 1999;22(11):1887–98. doi: 10.2337/diacare.22.11.1887. [DOI] [PubMed] [Google Scholar]
- 10.Al-Delaimy WK, Willet WC, Manson JE, Speizer FE, Hu FB. Smoking and mortality among women with type 2 diabetes. Diabetes Care. 2001;24(12):2043–8. doi: 10.2337/diacare.24.12.2043. [DOI] [PubMed] [Google Scholar]
- 11.Anthonisen NR, Skearns MA, Wise RA, et al. The effects of a smoking cessation intervention on 14.5-year mortality. Ann Intern Med. 2005;142(4):233–9. doi: 10.7326/0003-4819-142-4-200502150-00005. [DOI] [PubMed] [Google Scholar]
- 12.Barua RS, Ambrose JA, Eales-Reynolds L, DeVoe MC, Zervas JG, Saha DC. Heavy and light cigarette smokers have similar dysfunction of endothelial vasoregulatory activity. J Am Coll Cardiol. 2002;39(11):1758–63. doi: 10.1016/s0735-1097(02)01859-4. [DOI] [PubMed] [Google Scholar]