Abstract
The mechanical properties of periosteum are not well characterized. An understanding of these properties is critical to predict the environment of pluripotent and osteochondroprogenitor cells that reside within the periosteum and that have been shown recently to exhibit a remarkably rapid capacity to generate bone de novo. Furthermore, the effects of cryopreservation on periosteal mechanical properties are currently unknown. We hypothesized that the periosteum is pre-stressed in situ and that the periosteum exhibits anisotropic material properties, e.g. the elastic modulus of the periosteum depends significantly on the direction of loading. We measured the change in area, axial length, and circumferential length of anterior, posterior, medial, and lateral fresh periosteal samples removed from underlying bone (t = 0–16 hrs) as well as the average strain in axially and circumferentially oriented anterior periosteal samples subjected to tensile strain (0.004 mm/s) until failure. The elastic modulus was calculated from the resulting stress-strain curves. Tensile testing was repeated with axially aligned samples that had been slowly cryopreserved for comparison to fresh samples. Periosteal samples from all aspects immediate shrank 44–54%, 33–47%, and 9–19% in area, axial length, and circumferential length, respectively. At any given time, the periosteum shrank significantly more in the axial direction than the circumferential direction. Tensile testing showed that the periosteum is highly anisotropic. When loaded axially, a compliant toe region of the stress-strain curve (1.93±0.14 MPa) is followed by a stiffer region until failure (25.67±6.87 MPa). When loaded circumferentially, no toe region is observable and the periosteum remained compliant until failure (4.41±1.21 MPa). Cryopreservation had no significant effect on the elastic modulus of the periosteum. As the periosteum serves as the bounding envelope of the femur, anisotropy in periosteal properties may play a key role in modulating bone growth, healing and adaptation, in health, disease, and trauma.
Keywords: Periosteum, mechanical properties, cryopreservation
INTRODUCTION
The material properties of ovine femoral periosteum are not well characterized. An understanding of these properties is critical to predict the environment of pluripotent and osteochondroprogenitor cells that reside within the periosteum and that have been shown recently to exhibit a remarkably rapid capacity to generate bone de novo (Knothe Tate et al., 2007; Knothe & Springfield, 2005). Furthermore, as the periosteum serves as the bounding envelope of the femur, anisotropy in periosteal properties may play a key role in modulating bone growth, healing and adaptation, in health, disease and trauma.
Histological studies of periosteal architecture reveal an axially aligned collagen structure in the mid-diaphysis (Foolen et al., 2008) and a high density of elastin (Allen & Burr, 2005; Allen et al., 2004), suggesting that long bone periosteum is elastic and anisotropic. Previously published studies describe the mechanical testing and materials characterization of periosteum from long bones of the frozen pig metacarpus (Popowics et al., 2002), fresh chick tibiotarsus (Bertram et al., 1998), and frozen bovine tibia (Uchiyama et al., 1998). As a whole these studies show that long bone periosteum is pre-stressed (Bertram et al., 1998; Popowics et al., 2002) and exhibits non-linear behavior in response to tension (Bertram et al., 1998; Popowics et al., 2002; Uchiyama et al., 1998). Upon release from the underlying bone, a significant recoiling force or pre-stress is measured in the periosteum (Bertram et al., 1998). Studies on flat bones, e.g. the pig mandible and pig zygomatic arch, suggest that the pre-stress is anisotropic (i.e. more free shrinkage is observed along the length of the bone compared to the orthogonal direction) (Popowics et al., 2002). Under tensile loading, long bone periosteum shows two regions of compliance (Bertram et al., 1998; Popowics et al., 2002; Uchiyama et al., 1998). Initially, under small strains, the periosteum is very compliant. At a transition point, reported to be slightly longer than in situ length (Bertram et al., 1998), the periosteum becomes significantly stiffer. However, whether the periosteum exhibits anisotropy under tension is unclear. To our knowledge, no studies to date have evaluated the material properties of the long bone periosteum in the transverse or circumferential direction (Bertram et al., 1998) and the limited published data available is too variable to establish significant differences (Popowics et al., 2002; Uchiyama et al., 1998).
In addition to the dearth of knowledge regarding mechanical properties of freshly resected long bone periosteum, the mechanical properties of cryopreserved periosteum are even less well understood. Organs are often cryopreserved, frozen and stored at −80°C, for transplantation or preservation for later use, e.g. in experiments. Although, previous studies describing periosteal mechanics have implemented both fresh and frozen periosteal samples, no published study to date compares the Young’s modulus of fresh and frozen samples to determine whether cryopreservation affects mechanical properties of the periosteum. Cryopreservation of similar collagenous and elastic soft tissues such as arteries (Masson et al., 2009; Salvucci et al., 2008; Venkatasubramanian et al., 2006), veins (Brossollet & Vito, 1997), tendons (Ng et al., 2005), menisci (Arnoczky et al., 1988), and skin (Foutz et al., 1992) has been shown not to change the elastic modulus of the tissue if cryopreservation is gradual (slowing freezing to −80°C). Also, snap freezing collagen-based tissue engineered constructs has been shown to significantly decrease the elastic modulus while slow cooling does not change the modulus (Devireddy et al., 2003).
The pivotal role of the periosteum in providing a habitat for mechanosensitive pluripotent cells as well as the dearth of knowledge about the material properties of periosteal tissue provided the impetus for the current study. In a previous study, we elucidated the mechanical milieu of the periosteum locally, during stance shift loading of the femur with critical sized defect, to define the local mechanical milieu of periosteal cells that egress into the defect zone, filling it with woven bone within two weeks of surgery (McBride et al., 2011; Knothe et al., 2010). Here, we aim to measure the mechanical properties of the periosteum under tension and to determine effects of cryopreservation on those properties. Ultimately, we aim to understand periosteum mechanobiology across length and time scales, tying tissue level mechanobiology to cell scale events that underlie tissue building. Specifically, in the current study we hypothesize that (i) ovine femoral periosteum is pre-stressed in situ, (ii) ovine femoral periosteum exhibits anisotropic mechanical properties, and (iii) slow cryopreservation will not change the mechanical properties of periosteum compared to fresh, equivalent samples.
Our approach was to quantify shrinkage upon release from underlying bone as measure of pre-stress in the periosteum and to measure the modulus of elasticity in diaphyseal periosteum loaded in the axial and circumferential directions. Then modulus of elasticity in the axial direction was compared for fresh and cryopreserved diaphyseal periosteum.
MATERIALS AND METHODS
Animal Model
All studies were performed on periosteal samples obtained from the femora of skeletally mature sheep (2 year old ewes, ¾ hampshire and ¼ dorset, 62.2–79 kg, Ohio Agricultural Research and Development Center, Wooster, OH) in accordance with Case Western Reserve University’s IACUC approved protocol 2010–0014. Samples used in shrinkage and fresh tensile testing were harvested within five hours of euthanasia to maintain hydration and body temperature. Samples subjected to cryopreservation and tensile testing were procured from separate femora treated to the following protocol before tissue harvesting.
Within one hour of euthanasia the femora were disarticulated, and overlying skin was removed while preserving all overlying muscle. The femora were then sealed in a Ziploc bag and stored in a −80°C cryofreezer. After 10 days of cryopreservation, the sealed femora were left to thaw at room temperature for 24 hours. The soft tissue surrounding the bone remained cold after 24 hours of thawing. To raise the samples' temperature to ovine body temperature, while maintaining hydration, thick muscle tissue was trimmed back, preserving up to a thickness of one inch of tissue from the bone surface. Then femora were resealed in a Ziploc bag and placed in an incubator (5% CO2, 37°C).l
Shrinkage Studies
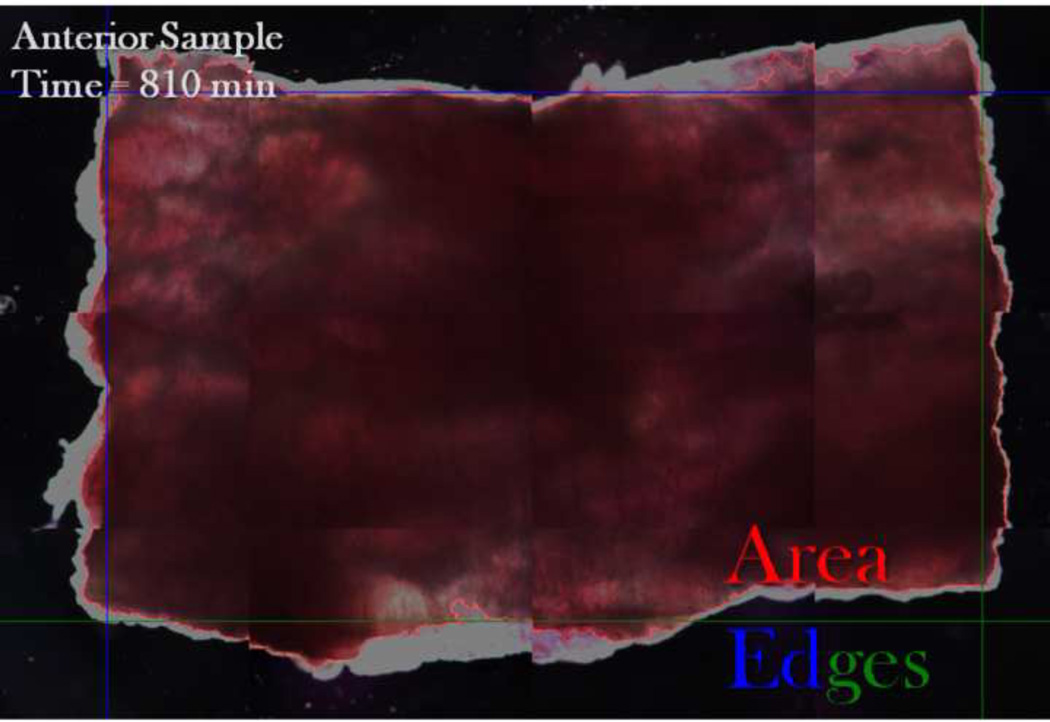
In situ evaluation of periosteal pre-stress necessitated development of protocols to prevent artifacts such as tissue swelling, which we had observed during pilot studies. First, squares of periosteum (n=3 bones), measuring circa 25% of the diaphyseal circumference, were removed from the anterior, medial, posterior, and lateral aspects of the femoral diaphysis. Samples were submerged in a thin layer of Dow Corning 200 fluid (3 mL per well in a 6-well plate, Univar USA, Strongsville, OH), a 100 centistoke silicon oil that minimizes friction and mitigates swelling and dehydration while allowing free shrinkage during testing. Then, the percent change in area, axial length, and circumferential length was measured over 16.5 hours, at 5 minute intervals for the first hour and 30 minute intervals for the remainder of testing, using high-resolution imaging (1.6x objective, 8.13 pixels/µm, Leica DM IRE2, Leica, Wetzlar, Germany) and processing (MatLab, Natick, MA) methods (Figure 1).
FIGURE 1.
Periosteal shrinkage measurements. A representative image of the periosteum shows reference points used for automated determination of change in area (red outline), axial length (difference between top and bottom edges), and circumferential length (difference between left and right edges). The left and top edges are shown in blue and the right and bottom edges of the sample are shown in green.
Mechanical Testing
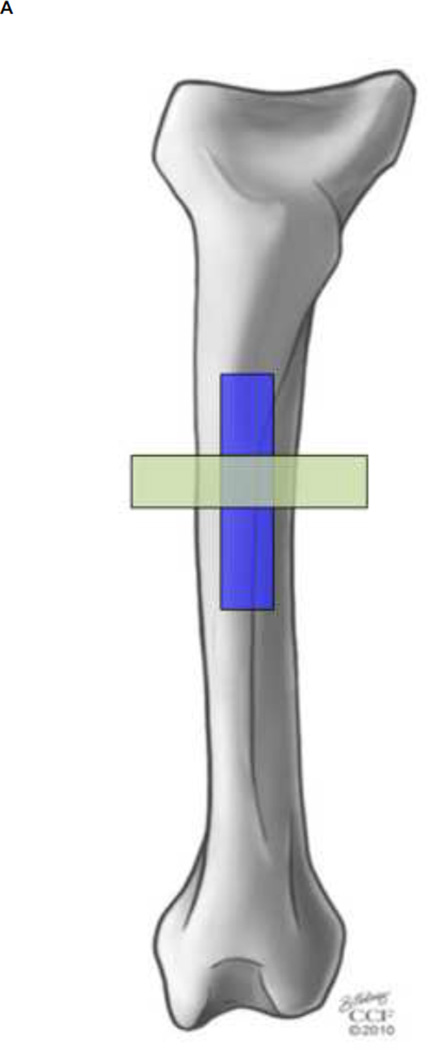
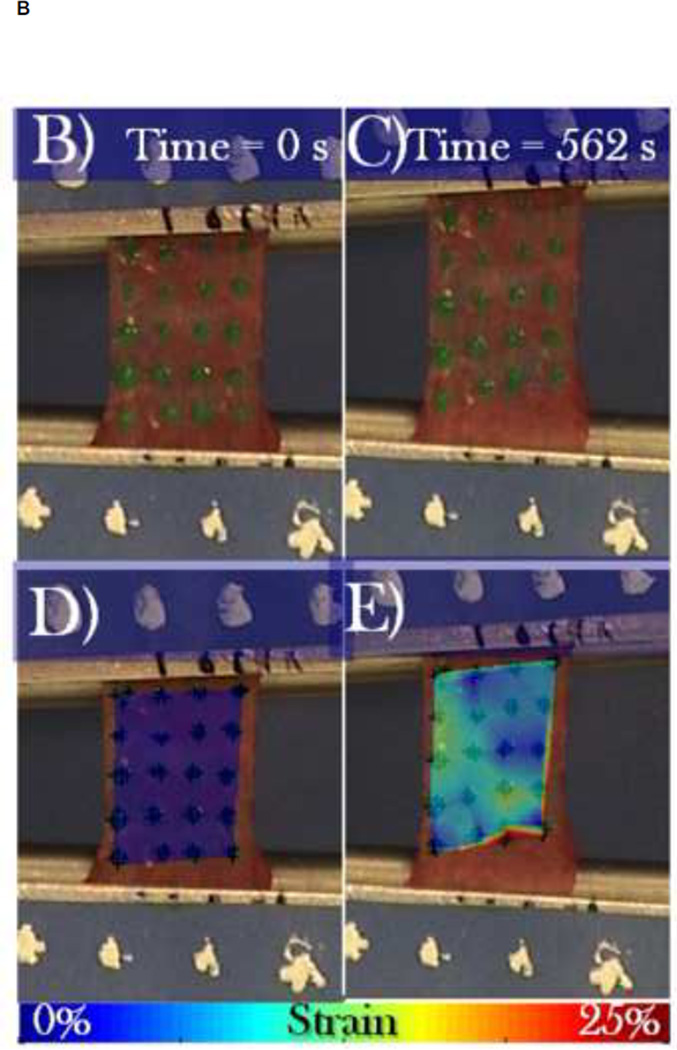
To measure the elastic modulus of the periosteum in the axial and circumferential directions, a circa 7×1 cm strip of diaphyseal periosteum, centered at the anterior aspect, was removed in either an axial (n = 6) or circumferential (n = 6) orientation (Figure 2A). The periosteum was fixed to a flexible frame during resection to maintain the in situ length (7 cm) while allowing limited width (1 cm) relaxation until the strip center was secured in the testing grips (Figure 2B–E). Once secured in the grips the tested gauge length was approximately 1 cm. The custom designed grips were adapted from a design used for skin testing developed by the lab of Professor Joseph Mansour (Mansour, Davis, Srour, & Theberge, 1993). After being secured in the rigid testing grips, a rectangular pattern of green dots was applied to provide contrasting landmarks for use in the tissue strain calculations (Figure 2B). Samples were stretched, at a rate of 0.004 mm/sec (Enduratec ELF 3200, Bose Co, Eden Prarie, MN), to failure (> 0.15 N drop in force) (Figure 2 C & E). To maximize relevance in the context of strain rates occurring physiological loading, a strain rate of 0.004 mm/sec was calculated for testing from peak strains measured in sheep femora during normal gait cycles (Lanyon et al., 1981) and accounting for mean time to reach peak load (Steck et al. 2003). We used similar extrapolations in a previous study to develop ex vivo loading protocols that mimic stance shift loading in an ovine femur defect model after treatment with the one stage bone transport procedure (McBride et al., 2011). In this previous study, we mapped strains optically in the periosteum, in situ and at high resolution albeit ex vivo, to elucidate the mechanical environment of the pluripotent cells residing therein; remarkably, we measured similar peak strains to those measured by Lanyon et al. in vivo on bone surfaces during gait (Lanyon et al., 1981).
FIGURE 2.
Mechanical testing of periosteum samples in the axial and circumferential directions. (A) Schematic of location and orientation of areas from which periosteum samples are taken (blue – axial, green – circumferential) and tested in tension. (B–E) A representative sample is shown during tensile testing, at zero load (B, D) and at failure (C, E). Digital image correlation was used to calculate the strain between green spots, as depicted in color overlays (D,E), averaging at each time point to create stress-strain curves.
Stress was calculated from the measured force and the cross sectional area. Cross sectional area was calculated from the initial width of the specimen in the grips just prior to testing (measured in the video footage) and the thickness of excess tissue not secured in the grip and cut away just prior to testing (measured with a micrometer). The associated tissue strain was measured optically by taking digital video during the test (Sony DC-HC52 video, Sony Co, New York, NY), segmenting the film footage into one still frame every two seconds (Final Cut Pro 7, Apple Inc, Cupertino, CA), using the digital image correlation function in MatLab to track the displacement of the applied green dots, and calculating the average strain for each dot over time (Figure 2 D & E). To create stress-strain curves, the strains for all locations were averaged at each time point to represent the whole tissue strain. The elastic moduli of the respective axial and circumferential toe and stiff regions were measured from the slope of the resulting stress-strain curves.
Cryopreservation Effects
To assess the effects of cryopreservation on the mechanical properties of periosteum, the identical mechanical testing protocol was carried out on the cryopreserved samples. Cryopreserved samples (n = 5) were tested in the axial direction to make best use of the tissues allotted within the IACUC approved protocol.
Statistics
All statistics were carried out using MatLab. To test hypotheses related to shrinkage studies, a one-way ANOVA test with pair-wise comparisons was used. Data from each aspect were treated as a group. To determine differences in length change for each aspect, we analyzed each time point using a paired t-test of the differences between change in circumferential and axial length. Statistical analysis of differences between elastic moduli of each region (axial and circumferential toe and stiff regions) of the fresh samples was carried out using the one-way ANOVA test with pair-wise comparisons. Each region was treated as a group. Statistical analysis of differences between elastic moduli of the axial toe and stiff regions of the fresh and cryopreserved samples was carried out using the one-way ANOVA test with pair-wise comparisons. Each region was treated as a group. P-values of less than 0.05 were considered significant.
RESULTS
Shrinkage Studies
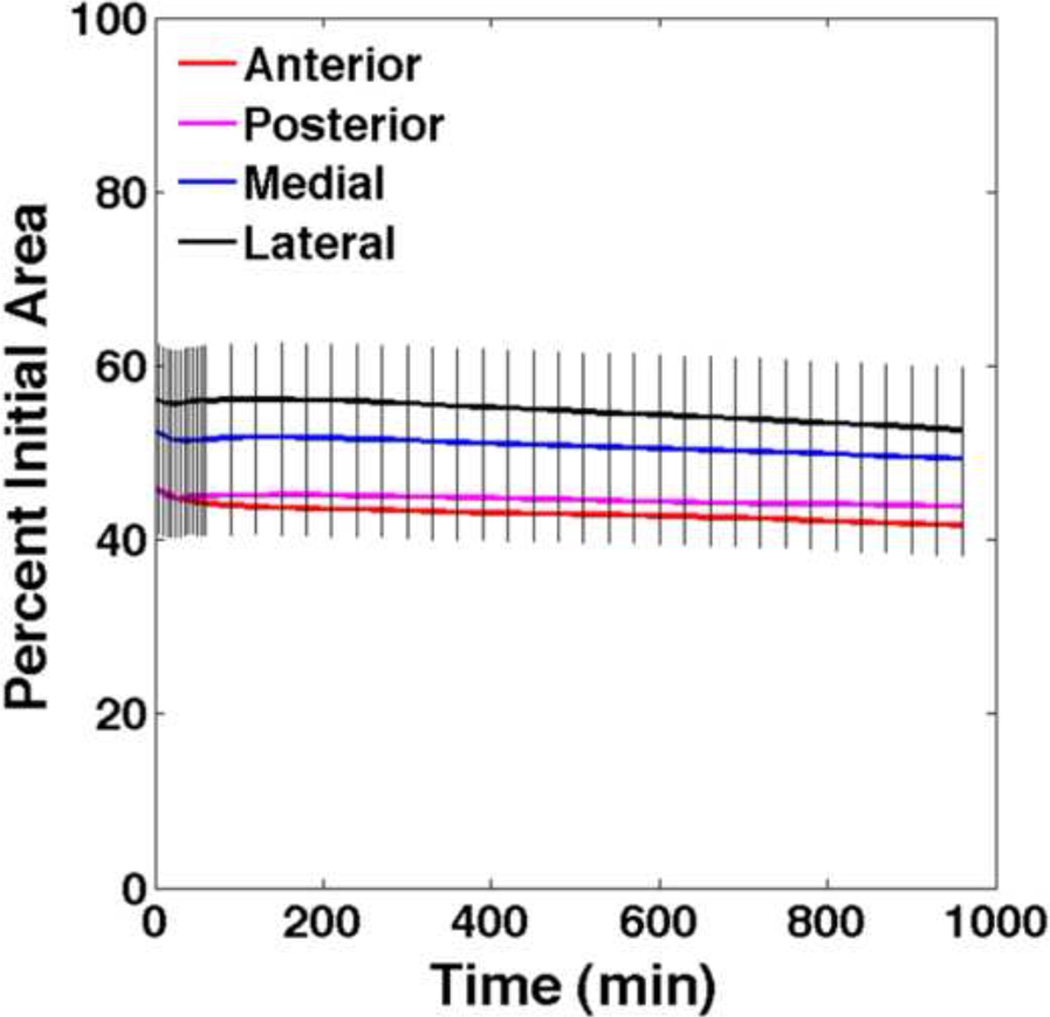
Periosteum from the diaphysis of the ovine femur experiences an average of 44–54% decrease in area immediately upon release from the underlying bone. Over the next 90 minutes there is a noticeable, but statistically insignificant, increase in area followed by a steady, but also statistically insignificant, decrease in area for the remainder of the test. At any time point, the change in area is not significantly different between aspects (Figure 3).
FIGURE 3.
Change in area for anterior, posterior, medial, and lateral periosteal samples. At any time point there are no significant differences between periosteum samples taken from the anterior, posterior, medial and lateral aspects of the femur.
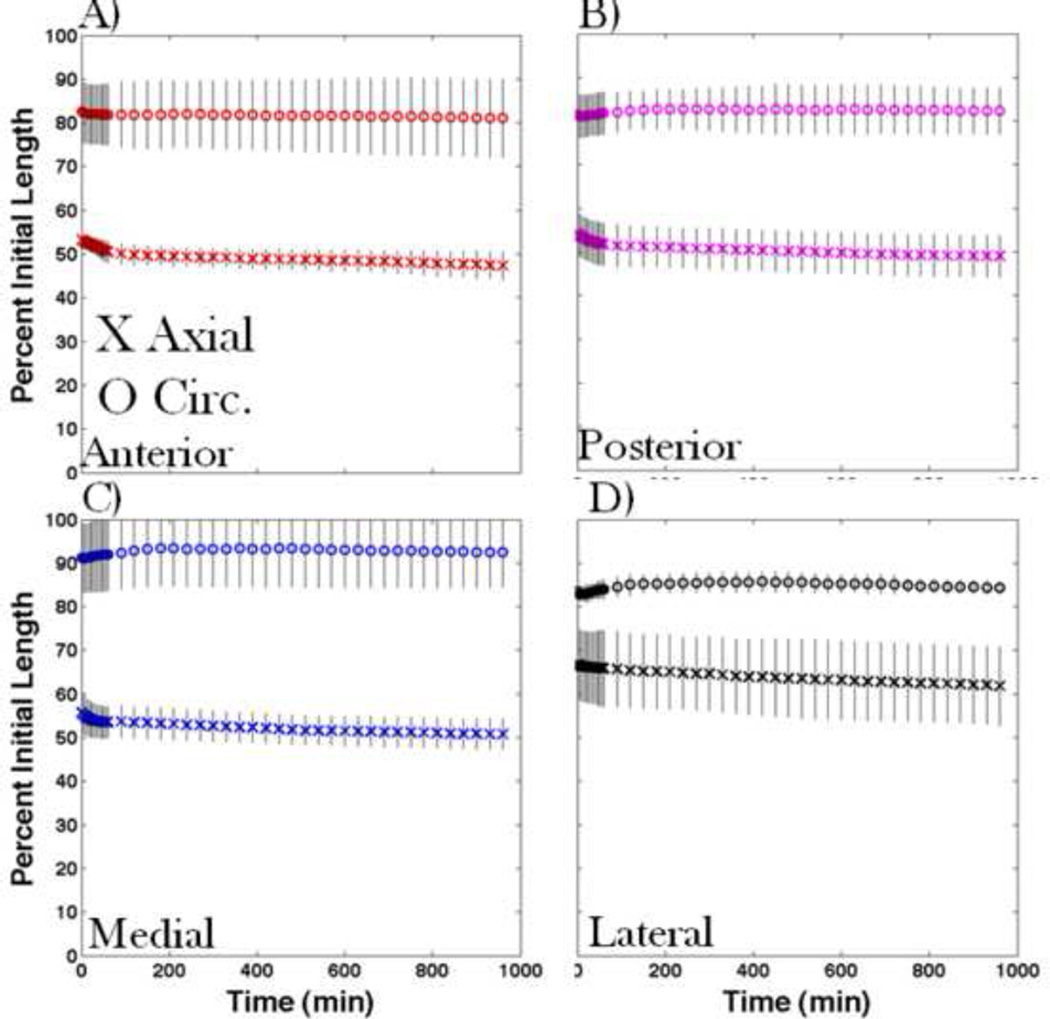
The overall length shrinks 33–47% in the axial-direction of samples and 9–19% in the circumferential-direction of samples. The change in length is significantly different in the axial and circumferential directions for all aspects of the femur (Figure 4).
FIGURE 4.
Change in axial (X) and circumferential (O) lengths of periosteum samples taken from the A) anterior, B) posterior, C) medial, D) lateral aspects of the femur, with 95% confidence intervals. Shrinkage is always significantly higher in the axial than in the circumferential direction for samples from all aspects.
Mechanical Testing
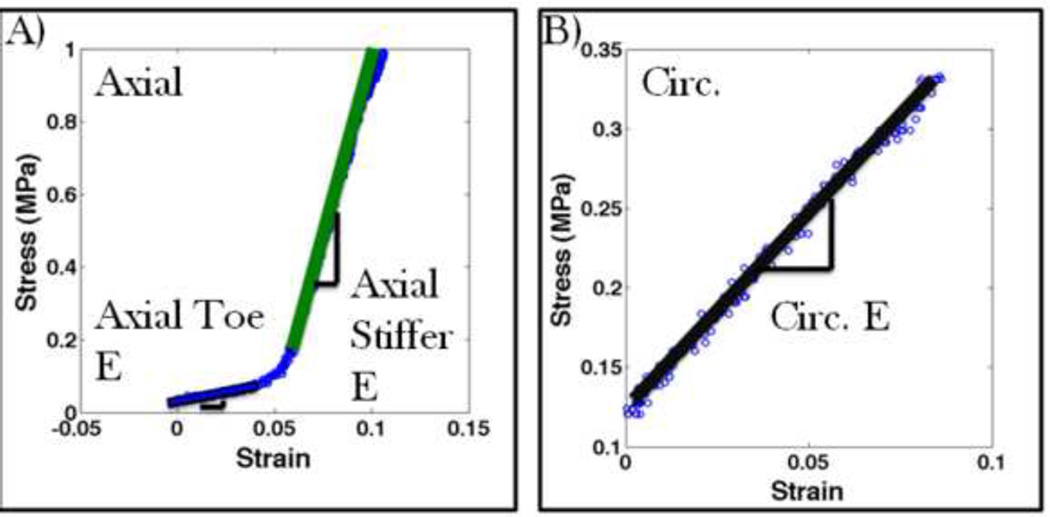
Periosteum from the diaphysis of the ovine femur exhibits a high degree of anisotropy. When strained in the axial direction, an initial compliant toe region is followed by a much stiffer linear elastic region (Figure 5A, Table 1). When strained in the circumferential direction there is no toe region and the tissue behaves approximately twice as stiff as the compliant axial toe region until failure (Figure 5B, Table 1). The point of transition from axial toe elasticity to stiffer elasticity differs greatly from axial sample to axial sample and appears to vary inversely with the measured pre-stress. This indicates that some samples relax slightly from in situ length even with the use of the template. Nonetheless, the toe elasticity and stiffer linear elasticity remain similar before and after transition.
FIGURE 5.
Examples of stress strain curves for the periosteum, showing best fit lines for (A) an axially oriented sample and (B) a oriented sample.
TABLE 1.
Average elastic moduli for fresh and cryopreserved periosteal samples in both the axial and circumferential directions. All regions are significantly different for fresh samples. There is no significant change after cryopreservation.
| Region | Mean E (MPa) & 95% CI | |
|---|---|---|
| Fresh | Cryopreserved | |
| Axial Toe | 1.93 (0.79, 3.06) | 2.16 (1.27, 3.04) |
| Axial Stiff | 25.67 (18.8, 32.5) | 23.3 (16.4, 30.2) |
| Circumferential | 4.41 (3.19, 5.62) | Not Measured |
Cryopreservation
Cryopreservation does not affect the elasticity of either the toe or stiffer region of axially aligned samples (Table 1).
DISCUSSION
The periosteum is a pre-stressed, anisotropic material. Based on the results of this study, the periosteum of the adult ovine femoral diaphysis is pre-stressed in the axial direction, resulting in three and a half times higher shrinkage upon release from underlying bone compared to the circumferential direction. Furthermore, the stiffer elastic modulus in the axial direction is more than five times greater than that in the circumferential direction. Cryopreservation does not change the axial elastic modulus. Interestingly, the mechanical properties of periosteum change with loading condition. Namely, the elastic modulus shows compliance in ranges of strain typical for peak physiological loads but becomes remarkably stiff in strain ranges typical for impact or high velocity loading situations. Thus, strong anisotropies in shrinkage and elastic modulus present themselves at high levels of strain (>5% shrinkage or 30,000µε stretch).
Qualitatively these results agree well with previous studies with regard to residual stress and non-linearity. For instance, Popowics et al. showed significant (17.2%) shrinkage of pig tibial periosteum upon release from the underlying bone (Popowics et al. 2002) while Bertram et al. showed a residual stress of approximately 1MPa when chick tibiotarsal periosteum was maintained at its in vivo length (Bertram et al. 1998). Also, several previous studies show non-linear behavior, i.e. transition from a compliant elasticity at low strains to stiffer elasticity at high strains, of periosteal tissue (Bertram et al., 1998; Uchiyama et al., 1998; Zeng et al., 2002). However, with regard to the magnitude of shrinkage observed and Young’s modulus measured, data from previous studies are inconsistent and differ from our measurements. These discrepancies likely relate to inherent differences in the bones studied and the age of the animals from which samples were obtained; for, not only does the elastic modulus of the periosteum vary from week to week within a given bone, e.g. during growth of the chick (Bertram et al. 1998), but also the elastic modulus shows significant site specificy, e.g. varying significantly along the length of given long bone (Uchiyama et al. 1998). Taking into consideration these inherent variations in elastic modulus, differences in anisotropy of the periosteum's mechanical properties might also be expected. Although some trends have been reported previously, variations ranging from ±32–50MPa have been reported previously for equivalent periosteal tissues (Uchiyama et al. 1998; Popowics et al. 2002). In comparison, the variation in our data is very small (±7MPa) and shows significant anisotropy in the axial versus circumferential directions. To our knowledge, no previously published studies addressed cryopreservation and its effect on mechanical properties of periosteum.
The normal physiological strain range for a sheep comprises −3000 to 3000µ£ (0.3% strain shrinkage or stretching) (Lanyon et al., 1981; McBride et al., 2011). In this range of very small magnitude strain, the mechanical properties of the periosteum are almost isotropic (as evident in Fig. 5B); hence, under these ranges of strain one would expect shrinkage of the periosteum to be identical in both the axial and circumferential directions. Furthermore, in these ranges of strain, the compliant elastic modulus in the circumferential direction (circa 4 MPa) is only twice the elastic modulus in the axial direction (circa 2 MPa) (Fig. 5).
The data reported in the current study have important implications with respect to multiscale elucidation and modeling of periosteum mechanobiology. Recent studies show a significant effect of mechanical loading on the regenerative capacity of stem cells, e.g. those residing in the periosteum. Hence, depending on the (patho)physiological condition being modeled or managed clinically (trauma versus physiological loading or etiology), the sensitivity of periosteal osteochondroprogenitor cells, (Knothe Tate et al., 2008; McBride et al., 2008; McBride and Knothe Tate, 2008) as well as anticipated range of loading and desired safety factors, the anisotropy of periosteum may have a significant impact on the mechanobiology of bone in health and healing. Hence, the anisotropies in mechanical properties of periosteum may be important factors to be incorporated in predictive multiscale computational modeling of bone mechanobiology and in the design parameters for synthetic replacement periosteum (Knothe Tate et al., 2010), depending on conditions to be modeled (where loading scenarios can be precisely controlled) and/or anticipated clinical management scenarios (where loading conditions can be prescribed and depend on patient compliance).
If periosteal cells are sensitive to a 2 MPa substrate modulus difference within the physiologic range (−3000 to 3000 µ£) then the anisotropic mechanical properties will need to be incorporated when modeling or managing physiologic conditions. To date, there are only two in vitro studies that investigate the response of periosteal cells to substrate strain (Jones et al., 1991; Kanno et al., 2005). While these pioneering studies demonstrate that periosteal progenitor cells are more sensitive to mechanical strain than mesenchymal stem cells (Jones et al., 1991), the cells' sensitivity has been assessed in a limited range of substrate strains, including zero (Jones et al., 1991; Kanno et al., 2005), 0.3% (Jones et al., 1991), and 12% (Kanno et al., 2005) strain. Our lab is currently carrying out follow on in vitro studies to ascertain the effect of substrate elastic modulus, substrate strain, and shear stress on periosteal cell mechanobiology.
The limitations of our current study relate mainly to inherent limitations of the mechanical testing system and availability of sample material. For example, tensile strains are applied uniaxially because the testing machine is designed to apply loads in a single direction over a total displacement of 6 mm. As shown in the shrinkage study results, if samples are allowed to shrink freely when removed from the bone, 6 mm of displacement would be sufficient to return axially aligned samples only to their in situ length and would not allow us to test the tissue to failure. In order to capture the full range of elastic moduli described in the current studies, the samples were tested starting at in situ length. Furthermore, due to limitations in sample availability (related both to cost and availability of a controlled cohort), the cohort of animals used for this study provided for 3 testing groups (i.e. fresh axial, fresh circumferential, and one cryopreserved), thus the elastic modulus of cryopreserved samples was examined in the axial direction only. The rationale behind this prioritization was based on pilot studies as well as the data from fresh sample, i.e. if cryopreservation does significantly alter tissue mechanics, it would be clearly evident in the axial direction. The data measured in the axial direction in fresh and cryopreserved samples are so similar that we would hypothesize similar effects in the circumferential direction.
The results of this study demonstrate the anisotropic mechanical properties of ovine femoral periosteum and, for the first time to our knowledge, the effects of cryopreservation on the same. These disparate material properties are expected to exert a profound influence on femur mechanobiology during development, growth, healing and aging, in health and disease (Knothe et al., 2010). These studies may have important implications with regard to the development of predictive computational models and of implants to replace periosteum when the amount of healthy periosteum is a limiting factor, e.g. after tumor resection as well as battlefield or other high impact, high energy injuries (Knothe Tate et al., 2010). However, at present very little is known about the response of periosteal cells to their mechanical environment or mechanical stimulation. From a mechanics point of view, cryopreserved periosteum is equivalent to fresh periosteum and can be used to study these topics in the future.
ACKNOWLEDGMENTS
This study was supported in part by the AO Research Fund (F-07-99K) and the NIH Musculoskeletal T32 Training Grant (NIH/ NIAMS 2T32AR007505). We would also like to acknowledge and thank Dr. Joseph Mansour, Chris Roberts, Nick LaFrenz, and Matt Kirsch for their advice and assistance in the development of the mechanical testing protocols (Mansour) and image processing (Roberts, LaFrenz, and Kirsch). Finally, we would like to thank Foster’s Meats (Westside Market, Cleveland, OH) for their generous donations of sample tissue that was needed for pilot testing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
None declared.
None of the authors have any conflicts of interest.
REFERENCES
- Allen MR, Burr DB. Human femoral neck has less cellular periosteum, and more mineralized periosteum, than femoral diaphyseal bone. Bone. 2005;36:311–316. doi: 10.1016/j.bone.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Allen MR, Hock JM, Burr DB. Periosteum: biology, regulation, and response to osteoporosis therapies. Bone. 2004;35:1003–1012. doi: 10.1016/j.bone.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Arnoczky SP, McDevitt CA, Schmidt MB, Mow VC, Warren RF. The effect of cryopreservation on canine menisci: a biochemical, morphologic, and biomechanical evaluation. Journal of Orthopaedic Research. 1988;6:1–12. doi: 10.1002/jor.1100060102. [DOI] [PubMed] [Google Scholar]
- Bertram JE, Polevoy Y, Cullinane DM. Mechanics of avian fibrous periosteum: tensile and adhesion properties during growth. Bone. 1998;22:669–675. doi: 10.1016/s8756-3282(98)00035-0. [DOI] [PubMed] [Google Scholar]
- Brossollet LJ, Vito RP. The effects of cryopreservation on the biaxial mechanical properties of canine saphenous veins. Journal of Biomechanical Engineering. 1997;119:1–5. doi: 10.1115/1.2796059. [DOI] [PubMed] [Google Scholar]
- Devireddy RV, Neidert MR, Bischof JC, Tranquillo RT. Cryopreservation of collagen-based tissue equivalents. I. Effect of freezing in the absence of cryoprotective agents. Tissue engineering. 2003;9:1089–1100. doi: 10.1089/10763270360728008. [DOI] [PubMed] [Google Scholar]
- Foolen J, Donkelaar Cvan, Nowlan N, Murphy P, Huiskes R, Ito K. Collagen orientation in periosteum and perichondrium is aligned with preferential directions of tissue growth. Journal of Orthopaedic Research. 2008;26:1263–1268. doi: 10.1002/jor.20586. [DOI] [PubMed] [Google Scholar]
- Foutz TL, Stone EA, Abrams CF. Effects of freezing on mechanical properties of rat skin. American Journal of Veterinary Research. 1992;53:788–792. [PubMed] [Google Scholar]
- Jones DB, Nolte H, Scholübbers JG, Turner E, Veltel D. Biochemical signal transduction of mechanical strain in osteoblast-like cells. Biomaterials. 1991;12:101–110. doi: 10.1016/0142-9612(91)90186-e. [DOI] [PubMed] [Google Scholar]
- Kanno T, Takahashi T, Ariyoshi W, Tsujisawa T, Haga M, Nishihara T. Tensile mechanical strain up-regulates Runx2 and osteogenic factor expression in human periosteal cells: implications for distraction osteogenesis. Journal of Oral and Maxillofacial Surgery. 2005;63:499–504. doi: 10.1016/j.joms.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Knothe Tate ML, Falls TD, McBride Sarah H, Atit R, Knothe UR. Mechanical modulation of osteochondroprogenitor cell fate. The International Journal of Biochemistry & Cell Biology. 2008;40:2720–2738. doi: 10.1016/j.biocel.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knothe Tate ML, Ritzman TF, Schneider E, Knothe UR. Testing of a new one-stage bone-transport surgical procedure exploiting the periosteum for the repair of long-bone defects. The Journal of Bone and Joint Surgery. American Volume. 2007;89:307–316. doi: 10.2106/JBJS.E.00512. [DOI] [PubMed] [Google Scholar]
- Knothe Tate M, Chang H, Knothe U. Role of periosteal factors in long bone defect healing. Transactions Orthopaedic Research Society. 2010;56:0384. [Google Scholar]
- Knothe UR, Springfield DS. A novel surgical procedure for bridging of massive bone defects. World Journal of Surgical Oncology. 2005;3:7. doi: 10.1186/1477-7819-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knothe UR, Dolejs S, Matthew Miller R, Knothe Tate ML. Effects of mechanical loading patterns, bone graft, and proximity to periosteum on bone defect healing. Journal of Biomechanics. 2010;43:2728–2737. doi: 10.1016/j.jbiomech.2010.06.026. [DOI] [PubMed] [Google Scholar]
- Lanyon LE, Paul IL, Rubin CT, Thrasher EL, DeLaura R, Rose RM, et al. In vivo strain measurements from bone and prosthesis following total hip replacement. An experimental study in sheep. The Journal of Bone and Joint Surgery. American Volume. 1981;63:989–1001. [PubMed] [Google Scholar]
- Mansour JM, Davis BR, Srour M, Theberge R. A method for obtaining repeatable measurements of the tensile properties of skin at low strain. Journal of Biomechanics. 1993;26:211–216. doi: 10.1016/0021-9290(93)90050-o. [DOI] [PubMed] [Google Scholar]
- Masson I, Fialaire-Legendre A, Godin C, Boutouyrie P, Bierling P, Zidi M. Mechanical properties of arteries cryopreserved at −80 degrees C and −150 degrees C. Medical Engineering & Physics. 2009;31:825–832. doi: 10.1016/j.medengphy.2009.03.009. [DOI] [PubMed] [Google Scholar]
- McBride SH, Falls T, Knothe Tate ML. Modulation of stem cell shape and fate B: mechanical modulation of cell shape and gene expression. Tissue Engineering. Part A. 2008;14:1573–1580. doi: 10.1089/ten.tea.2008.0113. [DOI] [PubMed] [Google Scholar]
- McBride SH, Dolejs S, Brianza S, Knothe UR, Knothe Tate ML. Net change in periosteal strain correlataes to rapid de novo bone generation in critical sized defects. Annals of Biomedical Engineering. 2011;39(5):1570–1581. doi: 10.1007/s10439-010-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng BH, Chou SM, Lim BH, Chong A. The changes in the tensile properties of tendons after freeze storage in saline solution. Proceedings of the Institution of Mechanical Engineers. Part H, Journal of Engineering in Medicine. 2005;219:387–392. doi: 10.1243/095441105X63309. [DOI] [PubMed] [Google Scholar]
- Popowics TE, Zhu Z, Herring SW. Mechanical properties of the periosteum in the pig, Sus scrofa. Archives of Oral Biology. 2002;47:733–741. doi: 10.1016/s0003-9969(02)00065-1. [DOI] [PubMed] [Google Scholar]
- Salvucci F, Armentano RL, Atienza JM, Bia D, Perez H, Barra JG, et al. Arterial complex elastic modulus was preserved after an intercontinental cryoconserved exchange. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society; IEEE Engineering in Medicine and Biology Society. Conference; 2008. pp. 3598–3601. [DOI] [PubMed] [Google Scholar]
- Steck R, Gatzka C, Schneider E, Niederer P, Knothe Tate ML. Measurement of bone surface strains on the sheep metacarpus in vivo and ex vivo. Veterinary and Comparative Orthopaedics and Traumatology. 2003;16:38–43. [Google Scholar]
- Uchiyama E, Yamakoshi K, Sasaki T. Measurement of mechanical characteristics of tibial periosteum and evaluation of local differences. Journal of Biomechanical Engineering. 1998;120:85–91. doi: 10.1115/1.2834311. [DOI] [PubMed] [Google Scholar]
- Venkatasubramanian RT, Grassl ED, Barocas VH, Lafontaine D, Bischof JC. Effects of freezing and cryopreservation on the mechanical properties of arteries. Annals of Biomedical Engineering. 2006;34:823–832. doi: 10.1007/s10439-005-9044-x. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Wu W, Yang J, Li Z, Yu H. Comparison of biomechanical properties between human nasal periosteum and fascia] Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2002;19(3):389–391. [PubMed] [Google Scholar]