Abstract
Natural killer (NK) cells that populate the decidua are important regulators of normal placentation. In contrast to peripheral blood NK (pNK) cells, decidual NK cells (dNK) lack cytotoxicity, secrete pro-angiogenic factors and regulate trophoblast invasion. Here we show that exposure to a combination of hypoxia, transforming growth factor beta 1, and a demethylating agent, results in NK cells that express Killer cell Immunoglobulin like Receptors, the dNK cell markers CD9 and CD49a, and dNK pattern of chemokine receptors. These cells secrete vascular endothelial growth factor, a potent pro-angiogenic molecule, display reduced cytotoxicity and promote invasion of human trophoblast cell lines. These findings have potential therapeutic applications for placental disorders associated with altered NK cell biology.
Introduction
Natural killer cells are lymphocytes of the innate immune system characterized by high cytolytic potential against virus-infected and tumor-transformed cells(1-3). In addition to their role in host defense, NK cells are proposed to play an important role in reproduction. NK cells are present in the human cycling endometrium. Their numbers augment after ovulation and during early pregnancy, becoming a prominent population of the decidua(4). By the end of the first trimester of pregnancy decidual NK cells (dNK) account for 70% of the local lymphocytes, and 30-40% of all decidual cells(5-7).
Decidual NK cells are functionally and phenotypically different from peripheral blood NK cells (pNK)(8-10). pNK cells are commonly divided into two main subsets. CD56Dim CD16+ pNK cells form the major subset representing 90% of pNK cells. They are granular and express Killer cell Immunoglobulin like Receptors (KIRs), which regulate NK cell activity upon engagement with MHC class I molecules(1, 11, 12). The second and minor subset (∼10% of pNK cells) is composed of CD56Bright CD16− pNK cells, which are non-granular, do not express KIRs, and secrete cytokines in response to non-specific stimuli(12, 13). In general, cytotoxic activity is associated with CD56Dim pNKs and cytokine production with CD56Bright pNKs(12, 13). However, CD56Dim pNK cells are also capable of cytokine production and CD56Bright pNKs may acquire cytotoxic capacity upon cytokine induced activation(14-16).
Human dNK cells are a distinct NK cell subset(9). They are CD56Bright CD16−, express KIRs, are granular and have severely reduced cytotoxicity(17-19). They express markers that are not expressed by pNK cells such as CD9(9) and CD49a(20) and produce pro-angiogenic factors and trophoblast migratory factors (8, 21-25).
The abundance of NK cells in the decidua has suggested that these cells might play a role in pregnancy support and maintenance(10). Initial evidence in this direction has come from NK cell-deficient mice. NK null mice display placental vascular anomalies including thickening of the walls of spiral arteries with luminal narrowing(26, 27), a phenotype that is reverted upon restoration of uterine NK cell populations(26-28). In rats, NK cell depletion leads to altered trophoblast invasiveness, delayed spiral artery development and reduced placental oxygen tensions(29). In humans, spiral artery narrowing is associated with preeclampsia and intrauterine growth restriction. Certain combinations of maternal KIR haplotypes (KIR AA) and paternal HLA-C alleles (HLA-C2) have been associated with preeclampsia, intrauterine growth restriction and recurrent miscarriage, suggesting that dNK cells may play a role in the pathogenesis of these disorders(30, 31). Most importantly a recent report associates impaired decidual NK cell function with high uterine artery resistance in pregnancy(25).
The induction of a dNK-like phenotype on pNK cells would be of utmost importance. It may open new venues for research on NK cell based therapeutic approaches for preeclampsia and related disorders. Here we show that exposure of pNK cells to a combination of hypoxia, TGFβ1 and 5-aza-2′-deoxycytidine (Aza), a demethylating agent, yields NK cells that secrete VEGF-A, a potent pro-angiogenic molecule, display reduced cytotoxicity, and promote invasion of human trophoblasts. CD56Bright CD16- NK cells of these cultures express CD9, CD49a, KIRs and display a chemokine receptor profile similar to dNK cells.
Materials and Methods
Ethical approval
Human specimens were collected according to protocols approved by the institutional review board at the Beth Israel Deaconess Medical Center.
Human decidual NK cells and peripheral blood NK cells
Decidual NK cells were isolated from decidua basalis tissue from elective first trimester pregnancy terminations as previously described(9, 19). Specimens were washed with PBS, minced with scissors and digested 30 minutes at 37°C with 0.1% collagenase type IV and 0.01% DNAse I (both from Sigma-Aldrich, St Louis, MO) in plain RPMI 1640 media (Invitrogen, Carlsbad CA). The digestion was stopped by the addition of an excess of RPMI 1640 media containing heat inactivated 10% FCS. Cell suspensions were then sequentially passed through 100μm, 70μm and 40μm cell strainers (BD Falcon, San Diego CA) washing 3 times with RPMI 1640 media containing 10% FCS. Decidua stromal cells and macrophages were adhered to the surface of tissue culture plates by incubation at 37°C for 2 hours in humidified incubators. Decidual lymphocyte suspensions were prepared from the overlying cell suspension by density gradient (Ficoll-Hypaque PLUS from GE Healthcare,Pittsburgh, PA), and stained for analytical flow cytometry, preparative dNK cell FACS sorting or dNK cell isolation with magnetic beads. For the generation of conditioned media dNK cells were FACS sorted as CD3− CD56Bright CD16− cells. For cytotoxicity assays dNK cells were isolated with magnetic beads (Milteny Biotech,Bergisch Gladbach, Germany) by depletion of CD3+ cells and CD16+ cells and subsequent isolation of CD56+ cells.
Peripheral blood NK cells were enriched from discarded leukopacks using a negative enrichment antibody cocktail (NK Rosette-sep, Stem Cell Technologies, Vancouver, Canada) following manufacturer's instructions. Enriched pNK cell preparations were used for establishment of NK cell cultures or stained with monoclonal antibodies for analytical flow cytometry, for FACS sorting of CD3− CD56Bright CD16- and CD3− CD56Dim CD16+ pNK cells or incubated with magnetic beads (Milteny Biotech, Bergisch Gladbach, Germany) for NK cell isolation by depletion of CD3+ cells and subsequent isolation of CD56+ cells used in cytotoxicity assays.
Cell culture studies
All cell culture in this manuscript was done using IL-15 complete media (RPMI 1640 media containing 10% FCS, 1unit/mL penicillin, 100μg/mL sterptomycin, 2 mM L glutamine, 1 mM Sodium Pyruvate, Non-essential aminoacids, and 55nM 2-mercaptoethanol, all from Invitrogen, with the addition of 10 ng/ml recombinant human IL-15 (Peprotech, Rocky Hill, NJ).
Conversion of pNK cells to dNK-like cells
Enriched peripheral blood NK cells were seeded at 1 × 106 cells/ml, in IL-15 complete media in the presence or absence of recombinant human TGFβ1 (Peprotech, Rocky Hill, NJ) at 2 ng/ml or at the concentrations indicated in the figures, and in the presence or absence of Aza (Sigma Aldrich, St Louis, MO) 1 μM or at the concentrations indicated in the figures. Duplicate cultures were established and incubated at 37°C in humidified incubators in an atmosphere containing 5%CO2. One of the NK cell cultures was maintained under 21% O2, the other culture was maintained under hypoxia (1% O2). Cell culture supernatants were harvested after 7 days and used to determine VEGF-A content by ELISA or used in HUVEC tube formation assays. Cells were stained to evaluate CD9 and KIR expression by CD3- CD16+ CD56Bright and by CD3− CD16+ CD56DimNK cells by analytical flow cytometry, or harvested and used in cytotoxicity assays.
Reversion of dNK cells
FACS sorted CD3- CD56Bright CD16− dNK cells were cultured for 7 to 10 days in IL-15 complete media or seeded on the decidual adherent cell fraction containing decidual stromal cells to evaluate CD9 and KIR, CD49a, CD94 and CD62L expression by CD3− CD56Bright CD16- cells by flow cytometry. Magnetic bead sorted CD3− CD56+ dNK cells were cultured for 7 days in IL-15 complete media and used in 51Chromium release assays to evaluate their cytotoxic activity on K562 cells.
VEGF-A measurements
Enriched pNK cells, FACS-sorted CD3− CD16+ CD56Dim, CD3− CD16− CD56Bright pNK cells or CD3− CD16− CD56Bright dNK cells were seeded at 106 cells/ml, or at the cell densities indicated in the text, and incubated for 7 days, or 72hs when indicated in the text, at 37C in humidified incubators under 1% or 21% oxygen tensions. Cell culture supernatants were harvested and their VEGF-A content evaluated by ELISA using VEGF Quantikine ELISA Kit (R&D Systems) following manufacturer instructions.
IL-8 measurements
IL-8 content in cell culture supernatants of 1 week long i-dNK and control cells was evaluated by ELISA using IL-8 Quantikine ELISA Kit (R&D Systems) following manufacturer instructions. dNK cell media IL-8 content was measured from three-day-long cultures of freshly isolated dNK cells or dNK grown for 7 days. Cells were seeded at 1×106 cells/ml.
Cytotoxicity assays
Determination of NK cell cytotoxic activity on K562 cells was done by 4 hours 51Cr-release assays as previously described(32).
Flow cytometry
The following mouse anti-human mAbs conjugated with FITC, Alexa Fluor 488, PE, PE-Cy7, Prcp-Cy5.5, APC or APC-Cy7 were used for FACS analysis or FACS sorting: anti-CD56 (clone B159), anti-CD3 (clone SK7), anti-CD16 (clone 3G8), anti-CD9 (clone ML-13), anti-CD158a (clone HP-3E4), anti-CD158b (clone CH-L), anti-NKAT2 (clone DX27), anti-NKB1 (clone DX9), anti-CD151 (clone 14A2.H1), anti-CD49a (clone SR84), anti-CD94 (clone HP-3D9), anti-Granzyme B (clone GB11), anti-Granzyme A (clone CB9), anti-Perforin (clone δG9) and isotype controls were all from BD biosciences. Anti-2B4 mAb (clone PP35) was from eBiosciences. Cell surface molecules were stained with fluorescent dye conjugated antibodies in PBS supplemented with 2% FCS for 30 minutes on ice and washed three times with PBS containing 2% FCS. For chemokine receptor expression profiling cells were first stained with anti-CD3, anti-CD16 and anti-CD56 mAbs , washed, blocked with 30% heat inactivated human serum (Sigma Aldrich, St Louis, MO) in PBS 1 hour on ice and then stained with the following PE-conjugated mAbs or corresponding isotype controls: anti-CXCR1 (clone 42705), anti-CXCR3 (clone 49801), anti-CXCR4 (clone 12G5), anti-CCR6 (clone Clone 53103), anti-CCR7 (clone 150503), all from R&D Systems, Minneapolis, MN, and anti-CX3CR1 (clone 2A9-1, MBL International, Woburn, MA), For intracytoplasmic staining cells were first stained with anti-CD3, anti-CD16 and anti-CD56 mAbs, then fixed and permeabilized with Cytofix/cytoperm Plus Fixation Permeabilization Kit (BD Biosciences) following the manufacturers instructions and stained with anti-Granzyme A , anti-Granzyme B, anti-Perforin or the corresponding isotype control antibodies. Cell sorting was performed on a FACSAria II sorter (Becton Dickinson, San Jose, CA). Analytical measurements were done with a BD FACSCanto flow cytometer (Becton Dickinson, San Jose, CA). KIR expression evaluation was done using a mix of PE conjugated anti-CD158a, anti-CD158b, anti-NKAT2 and anti-NKB1 mAbs.
HUVEC tube formation
Growth Factor Reduced Matrigel (BD Biosciences, San Diego, CA) was placed in the wells (100 μl per well) of a pre-chilled 48-well cell-culture plate and incubated at 37°C for 30 minutes to allow polymerization. Twenty thousand human umbilical vein endothelial cells (HUVEC) were plated onto the Matrigel-coated wells, and incubated with 200μl NK cell conditioned or control media at 37°C for 6-8 hours. Tube formation was then assessed through an inverted phase-contrast microscope at ×40 and ×100 (Nikon TE 300, Nikon Corporation). Images were acquired using a Leica DFC350FX camera controlled with Leica Firecam version 1.5 (Leica Microsystems Imaging Solutions). The percentage surface per field covered by HUVECs was calculated from the average of 3 fields per well using Image J software (Image J, image processing and analysis in java, National Institutes of Health).
Matrigel Invasion Assay
Matrigel invasion assay was performed using extravillous cytotrophoblast immortalized HTR-8-svNeo cells (a kind gift of Dr Charles Graham) and BD Biocoat Matrigel Invasion Chambers (BD Biosciences) following manufacturer's instructions. HTR cells (1×104 or 2×104 in 200 ul serum-free RPMI 1640 medium) were placed on the upper chamber. pNK cells, from the same donor, that were grown either under standard tissue culture conditions in IL-15 complete media (control) or under 1% O2 in IL-15 complete media in the presence of 2ng/mL TGFβ1 and 1μM Aza (i-dNK cells) for a week, were washed with PBS and seeded in the lower chamber (6.5×105 to 7.5×105 cells) in 750 μl of BIO-MPM-1 serum-free media (Biological Industries, Israel) containing 10 ng/ml IL-15, 1unit/mL penicillin, 100μg/mL sterptomycin, 2 mM L glutamine, 1 mM Sodium Pyruvate, Non-essential aminoacids, and 55 nM 2-mercaptoethanol, all from Invitrogen. In addition fibronectin (1 μg/ml) was added to the lower chamber to aid adherence of HTR cells to the membrane after migration. After 48 hrs the wells were removed and stained with DiffQuick staining kit (Thermofisher, Waltham, MA). The membranes were mounted on microscopic slides, images were captured, and cells were counted using Image J software (Image J, image processing and analysis in java, National Institutes of Health). Invasion was calculated normalizing the number of migrated HTR-8-svNeo cells to that of the control for each of the donors or media alone.
Immunoblot analysis for HIF-1α protein expression
Total proteins from NK cells were extracted and western blot performed using published protocols (33). For detection of HIF-1a, monoclonal antibody against HIF-1α (1:500 dilution) (clone 54/HIF-α BD-Transduction Laboratories) was used. The membranes were stripped and re-probed with a monoclonal anti-β-actin antibody (Clone AC-15, Sigma, St. Louis, MO; 1:1000 dilution) to assess for protein loading.
Statistical Analyses
Data were analyzed by student t test and p<0.05 was considered significant. Wilcoxon rank sum test was used whenever data was not normally distributed.
Results
Phenotype of dNK and pNK cells
Figure S1 displays characteristics that distinguish pNK cells from dNK cells. In peripheral blood, the number of CD56Dim CD16+ NK cells is 10 to 20 times the number of CD56Bright CD16− NK cells whereas in the human decidua NK cells are almost exclusively CD56Bright CD16−(10, 12). Representative CD56 and CD16 expression profiles of ex vivo CD3− pNK and dNK cells are shown in Figure S1a. CD9 and CD49a serve as markers for dNK cells as they are expressed by dNK cells but not by CD56Bright CD16− or CD56Dim CD16+ pNK cells (Refs. (9, 20) and Figure S1b and S1d). Decidual NK cells like CD56Dim CD16+ pNK cells express KIRs, contrasting with CD56Bright CD16- pNK cells, the vast majority of which are devoid of this type of receptor (Refs.(9, 12) and Figure S1c). dNK cells are functionally distinct from pNK cells. Ex-vivo dNK cells have reduced cytotoxicity on K562 target cells when compared to pNK cells (Ref. (19) and Figure S1e) and have been reported to secrete pro-angiogenic molecules(8, 21, 22, 25).
Reversibility of the dNK cell phenotype
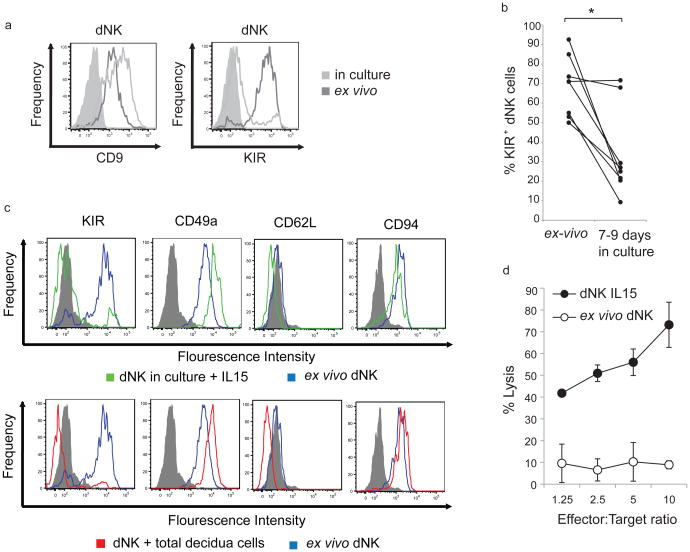
Is the phenotype of dNK cells conditioned by the decidual microenvironment? FACS sorted CD3− CD56Bright CD16− dNK cells maintained CD9 expression after 7 to 10 days in culture (Figure 1a left panel). Surprisingly the percentage of KIR+ dNK cells was substantially reduced (Figure 1a right panel and 1b) in these cultures. The expression of other molecules like CD49a, which is expressed by dNK cells but not by pNK cells(20), CD62L, which is only expressed by CD56Bright CD16− pNK cells(9), CD94, which is differentially expressed by CD56Bright and CD56Dim NK cells(34) and 2B4 did not change (Figure 1c and S2). Similar results were obtained when CD3- CD56Bright CD16− dNK cells were maintained in culture on total decidual cells (Figure 1c). Thus factors present in the decidual microenvironment, but not necessarily secreted by decidua stromal cells may be necessary to either induce or maintain KIR expression on dNK cells. IL-8 was secreted by freshly isolated dNK cells (55 to 739 pg/ml, n=4), but not by dNK cells previously grown for 7 days (below the ELISA detection level, n=4). Magnetic bead sorted dNK cells, cultured in IL-15 complete media for a week, were highly cytotoxic as opposed to freshly isolated dNK cells which displayed reduced cytotoxicity (Figure 1d). Thus, the reduction in the percentage of KIR+ cells and the acquisition of a cytotoxic phenotype by dNK cells in vitro are indicators of the importance of the decidual microenvironment in maintaining the phenotype of dNK cells.
Figure 1. Reversibility of the dNK cell phenotype.
a) CD9 (left panel), KIR (right panel) expression by ex vivo CD3−CD56Bright CD16− dNK cells (dark gray histograms) and CD3− CD56Bright CD16− NK cells from 7 to 9 days dNK cell cultures in IL-15 complete media (light gray histograms). Filled histograms, isotype control. b) Percentage of KIR+ cells among ex-vivo FACS sorted dNK cells and FACS sorted dNK cells cultured for 7 to 9 days in IL-15 complete media evaluated by flow cytometry. Each line represents an independent sample. * p-value=0.02. c) KIR, CD49a, CD62L and CD94 expression by ex vivo CD3−CD56Bright CD16− dNK cells (blue histograms) and CD3− CD56Bright CD16- NK cells from 7 to 9 days dNK cell cultures in which cells were seeded in IL-15 complete media (green histograms in top panels) or on total decidua cells (red histograms in bottom panels). Gray histograms: isotype control. d) Cytotoxic activity of ex vivo dNK cells (white circles) and dNK cells cultured for a week in IL-15 complete media (black circles) on K562 cells evaluated by 51Chromium release assays. Error bars represent standard deviation. Average of 3 experiments.
Hypoxia induces pro-angiogenic activity on pNK cells
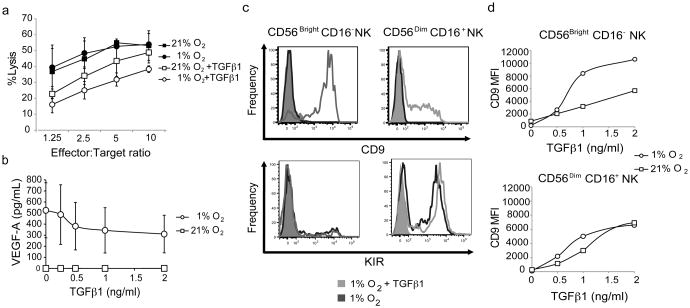
Hypoxia is a regulator of angiogenesis(35, 36) and immunity(37, 38). To test if hypoxia can induce a non-cytotoxic and pro-angiogenic phenotype on pNK cells, pNK cells were cultured for one week under normal tissue culture conditions (21% O2) or under hypoxia (1% O2). Cultures under hypoxia were enriched in CD56Bright CD16− NK cells relative to cultures under 21% O2 (Figure 2a). In all but one experiment the ratio of the percentage of CD56Bright CD16− NK cells to the percentage of CD56+ CD16+ NK cells was higher under 1% O2 than under 21% O2 (Figure 2b).
Figure 2. Hypoxia enriches pNK cell cultures in CD56BrightCD16-cells and turns pNKs into pro-angiogenic cells.
a) CD56 and CD16 expression profile of pNK cells cultured for one week under hypoxia (1% O2) or normal tissue culture conditions (21% O2) in the presence of IL-15. Squares delineate CD56Bright CD16− NK and CD56Dim CD16+ NK cell populations. Numbers indicate percentage of cells. b) CD56Bright CD16− NK cell to CD56Dim CD16+ NK cell ratio in hypoxia and 21% O2 cultures in experiments similar to the one shown in A. Each line represents an independent experiment. ** p-value=6×10−5 by paired t-test. c) VEGF-A levels in the supernatants of pNK cells, FACS sorted CD56Bright CD16− pNK cells and FACS sorted CD56Dim CD16+ pNK cells cultured under 21% or 1% O2 in the presence of IL-15 for one week. Cells were seeded at a density of 4×104 cells in 300 μl of media. Error bars represent standard deviation. Average of 3 experiments. * p-value=0.029, ** p-value=0.009 d) Tube formation by HUVECs in the presence of supernatants of pNK cells seeded at 1×106 cells per ml and cultured one week under 21% O2 or 1% O2. Representative fields (left) and tube formation quantitative evaluation (right) by the average percentage area per field covered by HUVECs. Results are average of 6 experiments. Error bars represent standard deviation. ** p-value=0.002. e) Cytotoxic activity of freshly isolated pNK and of pNK cells cultured in the presence of IL-15 under 21% O2 or 1% O2 on K562 target cells. Average of 2 51Chromium release assays. f) CD9 (top panel) and KIR (bottom panel) expression by CD3−CD56Bright CD16− NK and CD3-CD56Dim CD16+ NK cells from pNK cell cultures maintained for a week under 1% O2 (black histogram) or 21% O2 (gray histogram) in the presence of 10 ng/mL IL-15. In the top panels histograms are not visualized due to overlap with isotyope control..Filled histograms, isotype controls. Histograms are representative of 3 experiments.
Decidual NK and uterine NK cells but not pNK cells have been reported to secrete VEGF when cultured under different conditions (8, 21, 24, 25). The levels of VEGF-A secretion by dNK cells were not detectable in 3 day long cultures under 21% O2, in accordance with what others have reported(23). Independent dNK samples produced low levels of VEGF-A in 7 to 8 days long cultures (Figure S3 a). VEGF-A secretion was enhanced significantly when dNK cells were cultured under hypoxia (Figure S3 a). To evaluate if hypoxia could induce angiogenic function on pNK cells, pNK cells were cultured under hypoxia and cell culture supernatants were assayed for VEGF-A content by ELISA and for their capacity to induce tube formation by human umbilical vein endothelial cells (HUVECs) on matrigel. pNK cells did not secrete VEGF-A when cultured under 21% O2, however, they secreted considerable amounts of VEGF-A when cultured under hypoxia (Figure 2c). Peripheral blood NK cells cultured under hypoxia but not under 21% O2 expressed HIF-1α, a transcription factor that mediates responses to hypoxia and induces VEGF expression(39) (Figure S3b). Furthermore HUVECs formed more tubes when cultured with pNK supernatants generated under hypoxia than with supernatants generated under 21% O2 as evaluated by cell morphology and percentage area per field covered by HUVECs (Figure 2d). pNK cell preparations enriched by negative selection antibody cocktails may contain contaminating cells that could be the source of VEGF-A. To confirm that indeed pNK cells secrete VEGF-A under hypoxia, 99% pure FACS sorted CD56Bright CD16− pNK and CD56Dim CD16+ pNK cells were cultured under 1% O2 or 21% O2. VEGF-A was found in CD56Bright CD16− pNK cultures under 1% O2 but not under 21% O2 (Figure 2c). CD56Dim CD16+ pNK cells secreted low amounts of VEGF-A (Figure 2c).
Peripheral blood NK cells cultured under hypoxia or 21% O2 showed similar cytotoxic activity on K562 target cells (Figure 2e). The percentage of target cells lysed by NK cells cultured under hypoxia or 21% O2 was significantly higher than the percentage of cells lysed by freshly isolated pNK cells, due to the presence of IL-15 in the culture media in which they were maintained. The expression of cell surface markers such as CD9 or KIRs was not affected by hypoxia in one week long cultures (Figure 2f). Thus hypoxia alone does not affect pNK cell cytotoxicity nor does it induce the expression of CD9 or KIRs on CD56Bright CD16− pNK cells, but it confers pNK cells pro-angiogenic properties and enriches pNK cell cultures in CD56Bright CD16− cells.
TGFβ1 treatment combined with hypoxia results in VEGF-A secreting CD9+ NK cells with reduced cytotoxicity
TGFβ1 is a pleiotropic immunosuppressive molecule expressed by the decidual stroma(40) and trophoblasts(41) that is involved in NK cell differentiation and induces the expression of CD9 and to some extent of KIRs on NK cells(40, 42). TGFβ1 effects are often dependent on the environmental setting. We therefore tested if the combined effects of hypoxia and TGFβ1 on pNK cells can generate non-cytotoxic pro-angiogenic NK cells with similarities to dNK cells. As expected TGFβ1 reduced the cytotoxic activity of NK cells on K562 targets, but interestingly the inhibition of cytotoxicity was augmented by hypoxia (Figure 3a). VEGF-A secretion by NK cells under hypoxia was only marginally inhibited by TGFβ1 in one week long cultures (Figure 3b), while NK cells cultured under 21% O2 did not secrete VEGF-A at any of the TGFβ1 concentrations tested. As expected TGFβ1 induced CD9 expression on NK cells (Figure 3c top panel), but the level of CD9 expression on CD56Bright CD16− NK cells was enhanced under hypoxia (Figure 3d and Figure S4a). TGFβ1 exposure resulted in CD9 expression in a high proportion of CD56Bright CD16− pNK cells (Figure S4b). TGFβ1 however failed to induce significant KIR expression on CD56Bright CD16− NK cells present in one week long cultures under hypoxia (Figure 4c bottom panel).
Figure 3. NK cell incubation under hypoxia in the presence of TGFβ1 results in CD9+NK cells that secrete VEGF-A and have reduced cytotoxicity.
a) Cytotoxic activity of pNK cells cultured one week under 21% O2or 1% O2 in the presence or absence of 2ng/ml TGFβ1, on K562 target cells. Average of 2 51Chromium release assays. b) VEGF-A secretion by pNK cells cultured for a week under 21% O2 (squares) or 1% O2 (circles) in the presence of different concentrations of TGFβ1. Average of 4 experiments. Error bars represent standard deviation. Error bars of cultures under 21% O2 are not visualized due to the reduced standard deviation c) CD9 (top panels) and KIR (bottom panels) expression by CD56Bright CD16− (left) and CD56Dim CD16+ (right) pNK cells from one week-long pNK cell cultures under 1% O2in the presence (light gray histograms) or absence (dark gray histograms) of 2ng/mL TGFβ1 evaluated by flow cytometry. Filled histograms, isotype control. CD9 histograms are representative of 10 experiments. KIR histograms are representative of 5 experiments. d) Mean Florescence Intensity (MFI) of CD9 expression by CD56Bright CD16−NK cells (top panel) and CD56Dim CD16+ (bottom panel) from one week long pNK cell cultures under hypoxia (1% O2, circles) or 21% O2 (squares) in the presence of different concentrations of TGFβ1. Results from one representative experiment. Similar results were obtained in seven out of ten experiments.
Figure 4. Aza, hypoxia and TGFβ1 combined induce CD9+KIR+dNK-like cells that secrete VEGF-A and have reduced cytotoxicity.
a) KIR (top panels) and CD9 (bottom panels) expression by CD56Bright CD16− (left) and CD56Dim CD16+ (right) pNK cells from one week-long pNK cell cultures under 1% O2 in the presence of 2ng/mL TGFβ1 with (dark gray histograms) or without (light gray histogram) the addition of 1μM Aza, evaluated by flow cytometry. Tinted histograms, isotype control. Bars and numbers indicate the percentage of KIR+ cells in the corresponding NK cell subset in cultures performed under 1% O2 in the presence of Aza and TGFβ1. One experiment representative of 3. b) Percentage of KIR+ cells among CD56Bright CD16− pNK cells from a one week long culture under 1% O2 (circles) or 21% O2 (squares), in the presence (white symbols) or absence (black symbols) of 2ng/mL TGFβ1, at different concentrations of Aza. One experiment representative of two. c) VEGF-A content in the supernatants of one week long cultures of pNK cells, seeded at 1×106 cells /mL, in the presence of TGFβ1 under hypoxia (circles) or 21% O2 (squares) at different concentrations of Aza. Average of 2 experiments d) Cytotoxic activity of effector pNK cells cultured for a week under hypoxia in the presence (triangles) or absence (circles) of 1μM Aza, in the presence (white symbols) or absence (black symbols) of 2 ng/mL TGFβ1, on K562 target cells. Results are average of 2 independent experiments.
Thus the combination of hypoxia with TGFβ1 exposure resulted in NK cells that expressed CD9, had reduced cytotoxicity and secreted pro-angiogenic VEGF-A but lacked KIR expression. We then focused our attention on other synergistic factors that could induce KIR expression to generate KIR+ CD56Bright CD16− NK cells with similarities to dNK cells.
Induction of KIR expression on pNK cells by demethylating agents
Epigenetic mechanisms are important regulators of KIR expression by NK cells i.e. promoter demethylation of CpG islands induces KIR expression(43, 44). Promoter demethylation may be one of the underlying mechanisms of KIR expression in dNK cells. Interestingly, T cells in the decidua have higher levels of KIR expression than their peripheral blood counterparts(45). Although the transcriptional control of KIRs in T cells and NK cells may differ(46) DNA CpG demethylation was shown to increase KIR expression in T cells(47).
We therefore used Aza, a demethylating agent, to induce KIR expression on CD56Bright CD16- pNK cells (Figure 4a). Aza induced KIR expression by CD56Bright CD16− pNK cells, the percentage of KIR+ CD56Bright CD16− cells being higher under hypoxia than under 21% O2 (Figure 4a and b). TGFβ1 reduced to some extent the percentage of KIR+ cells induced by Aza (Figure 4b) under hypoxia, and Aza reduced to some extent the number of CD56Bright CD16− NK cells in the cultures (data not shown). Nevertheless, the percentage of KIR+ cells induced by 1uM Aza under hypoxia in the presence of TGFβ1 was still high, about 50-60% of CD56Bright CD16− NK cells (Figure 4b). Aza did not affect expression of CD9 on cells grown under hypoxia in the presence of TGFβ1 (Figure 4a bottom panel), and only inhibited marginally the secretion of VEGF-A in one week long cultures (Figure 4c). The cytotoxicity of pNK cells cultured under hypoxia with TGFβ1 was not affected by Aza and remained significantly reduced compared to cells grown under hypoxia alone (Figure 4d). Thus the exposure of pNK cells to a combination of hypoxia, TGFβ1 and Aza resulted in NK cells with reduced cytotoxicity that secrete pro-angiogenic VEGF-A, including CD56Bright CD16− NK cells that like dNK cells express CD9 and KIRs. We refer to these cells as induced dNK-like cells (i-dNK) cells.
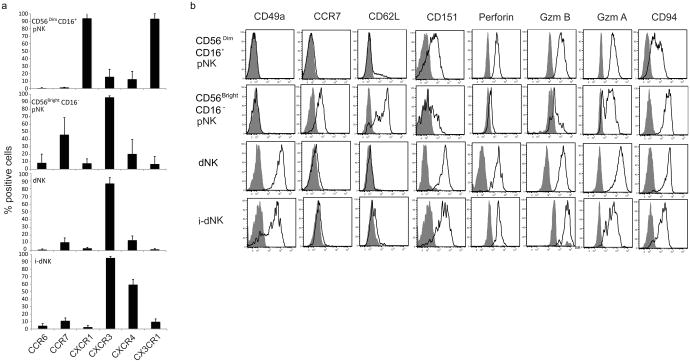
I-dNK cells phenotipically resemble dNK cells
To assess the phenotypic similarity of i-dNK cells to dNK cells, i-dNK cells were more fully characterized by flow cytometry. CD56Bright CD16− i-dNK cells presented a chemokine receptor expression profile similar to dNK cells and distinct from that of CD56Bright CD16− pNK and CD56Dim CD16+ pNK cells as evaluated by the percentage of cells expressing CCR6, CCR7, CXCR1, CXCR3, CXCR4 and CX3CR1 (Figure 5a). Furthermore, i-dNK cells expressed a panel of molecules that are differentially expressed by the three NK cell subsets (dNK, CD56Bright pNK and CD56Dim pNK) in a dNK-like pattern (Figure 5b). These included CD49a, which is expressed by dNK but not by pNK cells (20), CCR7 and CD62L ,overexpressed by CD56Bright CD16− pNK cells ((9) and Figure 5a), CD151, overexpresed by dNK cells(9), Perforin, Granzyme A, Granzyme B and CD94, all expressed by dNK cells. i-dNK cells like dNK cells expressed the receptor 2B4 (Figure S2) but did not secrete IL-8 as evaluated by ELISA (n=3).
Figure 5. i-dNK cells express chemokine receptors and molecules differentially expressed by dNK and pNK cells in a pattern similar to dNK cells.
A) Chemokine receptor expression profile of fresh CD56Dim CD16+ pNK , fresh CD56Bright CD16− pNK, fresh dNK and i-dNK cells (gated on CD56Bright CD16− cells) evaluated by flow cytometry. The percentage of positive cells for each chemokine receptor is shown. Average of 4 independent donors. Error bars represent standard deviation. B) CD49a, CCR7, CD62L, CD151, Perforin, Granzyme B, Granzyme A and CD94 expression by CD56Dim CD16+pNK , CD56Bright CD16− pNK, dNK and CD56Bright CD16− i-dNK cells . Gray histograms isotype control. Histogram plots were gated on CD3− cells and are representative of 3 or 4 independent donors.
Reversibility of the i-dNK cell phenotype
To evaluate if the phenotype induced in CD56Bright CD16− pNK cells by the combined treatment with hypoxia, TGFβ1 and Aza reflected terminal differentiation of the cells or was reversible upon removal of the inducers, pNK cells were treated for a week with these stimuli, were then washed and put in culture for a second week either under i-dNK inducing conditions or under basal conditions (21% O2). Cell culture supernatants were then tested for VEGF-A content and cells stained to evaluate expression of CD9 and KIRs by CD56Bright CD16- cells. VEGF-A secretion diminished dramatically if cells were not maintained under i-dNK cell inducing conditions (Figure 6a) indicating that hypoxia was necessary to maintain VEGF-A secretion. Under non-inducing conditions cells still expressed CD9 (Figure 6b top panel). The percentage of KIR+ cells among CD56Bright CD16− cells was markedly reduced in cultures kept the second week under 21% O2 when compared to cultures maintained under i-dNK-like cells inducing conditions (Figure 6b lower panel), or when compared to the first week hypoxia, TGFβ1 and Aza culture from which the cells were derived (data not shown). Thus i-dNK cell inducing stimuli were necessary to maintain a high percentage of KIR+ CD56Bright CD16− cells, but not for maintenance of CD9 expression in one week long cultures. This pattern of phenotype “reversion” is similar to the one observed when dNK cells are cultured for a week under 21% O2, removed from the decidual microenvironment (Figure 1a).
Figure 6. Reversibility of the phenotype of i-dNK cells.
a) VEGF-A content in the supernatant of pNK cells that were cultured for a week under i-dNK inducing conditions (1% O2 in the presence of 2 ng/ml TGFβ-1 and 1 μM Aza) and then replated for a second week either under i-dNK inducing conditions (1% O2 + TGFβ-1 + Aza) or under basal conditions (21% O2). Average of 4 independent experiments. ** p-value=0.0099 by two-tailed Student t-test. b) CD9 (top panel) and KIR (lower panel) expression by CD56Bright CD16− (left) and CD56Dim CD16+ (right) cells from pNK cultures similar to those described in a. dark gray histograms: i-dNK inducing conditions (1% O2 + TGFβ-1 + Aza). light gray histograms: standard tissue culture basal conditions (21% O2). One representative experiment out of 3 total is shown.
Regulation of trophoblast invasion by i-dNK cells
Trophoblast cells invade the maternal decidua and replace the endothelial lining transforming spiral arteries into low-resistance, high-flow vessels capable of providing adequate placental perfusion (48) . Decidual NK cells have been proposed to be involved in the regulation of trophoblast invasiveness and uterine spiral artery remodeling (8, 29, 49, 50).
To evaluate if i-dNK cells can modulate trophoblast invasion, NK cells treated under i-dNK inducing conditions for seven days were tested for their capacity to affect invasion of HTR-8-svNeo cells, an immortalized human extravillous trophoblast cell line that has been previously used in invasion studies(51), in matrigel invasion assays. Treated NK cells promoted invasion of HTR-8-svNeo cells as compared to control NK cells from the same donor maintained in culture under 21% O2. (Figure 7a and 7b).
Figure 7. NK cells cultured under i-dNK inducing conditions promote HTR trophoblast cell invasion.
a) Representative photomicropgraphs of immortalized extravillous trophoblast HTR-8-svNeo cells in a Matrigel invasion assay. HTR cells were seeded in the upper chamber of matrigel coated migration wells. pNK cells that have been cultured for a week under 1% O2 in the presence of 2 ng/ml TGFβ1 and 1μM Aza (i-dNK, right panel), or under 21% O2 and IL-15 as control (left panel) were seeded in the lower chamber. b) i-dNK and control cell data expressed as invasion normalized to control cells (pNK cells from the same donor maintained under 21%O2 and IL-15 ). Each dot represents one donor. N = 10. * p-value= 0.039. c) Invasion data normalized to media alone in the absence of NK cells for dNK cells from 3 donors , gestational ages 7, 9 and 10 weeks, or i-dNK cells from 4 separate donors (black bars), and reverted i-dNK cells, from the same 4 donors, that were maintained a second week under standard tissue culture basal conditions (21% O2 +IL-15) (white bars).
Figure 7c presents the HTR-8-svNeo migration inducing capacity of dNK cells from 3 separate donors and NK cells treated under i-dNK cell inducing conditions from 4 independent donors. While definitive conclusions can not be made, it appears that dNK cells obtained at later gestational age promote migration when compared to dNK cells obtained at earlier gestational age, in agreement with prior work by Lash et al (49). The HTR invasion inducing capacity of NK cells treated under i-dNK cell inducing conditions was reduced when the cells were maintained in culture under reversal conditions (Figure 7c, white bars). In additional studies, reverted cells promoted less invasion than did control cells maintained in culture under i-dNK cell inducing conditions for the same duration of the experiment (reverted cells, 0.84; control cells, 1.69; average of 2 experiments, normalized to media alone).
Discussion
Decidual NK cells as opposed to pNK cells are characterized by their reduced cytotoxicity and their capacity to secrete angiogenic molecules. Here, human pNK cells cultured with a combination of hypoxia, TGFβ1 and the demethylating agent Aza displayed reduced cytotoxicity, secretion of a potent pro-angiogenic molecule, VEGF-A and acquired capacity to promote trophoblast invasion in cell culture studies. Furthermore, CD56Bright CD16− cells from these cultures express CD9 and KIRs, CD49a, chemokine receptors and molecules such as and CD151, CD62L, CD94, Perforin Granzyme B and Granzyme A in a pattern that is unique to dNK cells (Figures 5).
While different possible origins have been proposed for human dNK cells, namely being derived from pNK cells homing to the decidua or from hematopoietic precursor present in decidual tissue(40, 52, 53), it is evident that the decidual microenvironment is necessary to induce or maintain dNK cell's phenotype. When dNK cells, the vast majority of which express KIRs, are removed from the decidua and placed in culture with IL-15 they become cytotoxic and harbor a high proportion of KIR− CD56Bright CD16− dNK cells (Figure 1). The reduction in the percentage of KIR+ dNK cells in the cultures may be due to loss of KIR expression by dNK cells, to a higher cell death rate of KIR+ cells or to an increased proliferation of KIR− cells that fail to acquire KIR expression in the absence of factors present in the decidua. The presence of other dNK markers such as CD94, CD49a and absence of CD62L makes it less likely that these KIR- cells are arising from contaminating KIR− CD56Bright pNK cells. It should be noted however that exogenous IL-15 may have contributed to the cytotoxicity phenotype.
Hypoxia is a regulator of angiogenesis(35, 36) and immunity(37, 38). Hypoxia enriched pNK cells cultures in CD56Bright CD16− cells and induced the secretion of VEGF by pNK cells (Figure 2). Furthermore hypoxia enhanced the effects of TGFβ1 on cytotoxicity inhibition and induction of CD9 expression (Figure 3). It is possible that the enhancement of TGFβ1 effects on NK cells by hypoxia be due to added TGFβ1 secreted by pNK cells when cultured under low oxygen tensions. It remains to be determined if the effects of hypoxia on NK cells are regulated directly through Hypoxia Inducible Factor 1α or through other downstream metabolites of hypoxia.
The i-dNK cells we have generated secrete the pro-angiogenic molecule VEGF-A. Previous reports have shown that dNK cells but not pNK cells secrete VEGF under different culture conditions.(8, 21, 23, 24). Controversy exists as to the type of VEGF produced. Some support the secretion of VEGF-A but not VEGF-C (25), others VEGF-C but not VEGF-A (23) and others don't specify the VEGF type secreted (8, 24). This may reflect differences in culture conditions, cell isolation procedures, gestational age of the samples or protein detection assays used. In our short term (3 days) cultures under 21% O2 dNK cells did not secrete detectable levels of VEGF-A, but did produce it when exposed to low oxygen tensions similarly to pNK cells treated under i-dNK inducing conditions. Interestingly a recent study in mice has shown the presence of VEGF-A+ decidual NK derived from blood (54).
The combined effects of hypoxia and TGFβ1 yielded cells that express CD9, have reduced cytotoxicity and secrete VEGF. However CD56Bright CD16− cells did not express KIRs like dNK cells. TGFβ1 has been reported to induce KIR expression on about 20% of CD56Bright CD16− cells in 2 weeks long or longer pNK cultures(40, 42). The absence of KIR induction by TGFβ1 on CD56Bright CD16− in this study may be due to the shorter length of the cultures, to a confounding effect introduced by hypoxia, or to differences in media composition. The induction of KIR expression on a higher proportion of CD56Bright CD16− pNK cells was achieved with the addition Aza to the culture media. Most interestingly hypoxia augmented the percentage of KIR+ cells induced by Aza among CD56Bright CD16− cells suggesting that hypoxia may be a modulator of KIR expression or alternatively may favor the proliferation of KIR+ CD56Bright CD16− NK cells. It should be noted however that the addition of Aza reduced the number of CD56Bright CD16− cells (data not shown).
Demethylating agents induce KIR expression on NK cells and to a lesser degree on T cells, but not on other tested cell types, which suggests a specific effect of demethylation on regulation of KIRs on NK cells and T cells (44) .The endogenous factor responsible for KIR expression in dNK cells is unknown. It is possible that the endocrine and cytokine milieu as well as cell-cell interactions may contribute to KIR induction. In this setting hormones are particularly interesting since they are known to regulate epigenetic mechanisms(55).
Thus, in addition to the phenotypic similarities to dNK cells, NK cells exposed to i-dNK inducing conditions secrete VEGF-A, a potent pro-angiogenic molecule, have reduced cytotoxicity and promote trophoblast invasion. Future research should establish if i-dNK cells can mimic dNK cell function in vivo. Manipulation of ex vivo expanded autologous peripheral NK cells to yield i-dNK-like cells may open the door to research on new therapeutic venues for the treatment of reproductive disorders associated with NK cell biology such as preeclampsia and intrauterine growth restriction.
Supplementary Material
Acknowledgments
HTR-8-svNeo cells were a kind gift of Dr Charles Graham.
We thank Neil Nadkarni for help with the trophoblast invasion studies.
Funding: This work was funded by the Harvard K12 HD051959 Building Interdisciplinary Research Careers in Women's Health (BIRCWH) Program supported by the NIH Office of Research in Women's Health to HDK and by the Howard Hughes Medical Institute to SAK and the Burroughs Welcome Fund. The Gulbenkian Programme for Advanced Medical Education is sponsored by Fundação Calouste Gulbenkian, Fundação Champalimaud, Ministério da Saúde e Fundação para a Ciência e Tecnologia, Portugal.
Abbreviations
- Aza
5-aza-2′-deoxycytidine
- dNK cell
decidual Natural Killer cell
- HUVEC
Human Umbilical Vein Endothelial Cell
- i-dNK cell
induced dNK-like cell
- KIR
Killer cell Immunoglobulin like Receptor
- pNK cell
peripheral blood Natural Killer cell
- VEGF
Vascular Endothelial Growth Factor
Footnotes
Editorial and Production offices should communicate with Hernan D. Kopcow.
R.I.T, V.P.S., and S.A.K. are coinventors on patents related to angiogenic markers in preeclampsia and have financial interest in Aggamin LLC. The other authors have no financial conflict of interest.
References
- 1.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orange JS, Fassett MS, Koopman LA, Boyson JE, Strominger JL. Viral evasion of natural killer cells. Nat Immunol. 2002;3:1006–1012. doi: 10.1038/ni1102-1006. [DOI] [PubMed] [Google Scholar]
- 3.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 4.Bulmer JN, Morrison L, Longfellow M, Ritson A, Pace D. Granulated lymphocytes in human endometrium: histochemical and immunohistochemical studies. Hum Reprod. 1991;6:791–798. doi: 10.1093/oxfordjournals.humrep.a137430. [DOI] [PubMed] [Google Scholar]
- 5.Bulmer JN, Williams PJ, Lash GE. Immune cells in the placental bed. Int J Dev Biol. 2010;54:281–294. doi: 10.1387/ijdb.082763jb. [DOI] [PubMed] [Google Scholar]
- 6.King A, Balendran N, Wooding P, Carter NP, Loke YW. CD3- leukocytes present in the human uterus during early placentation: phenotypic and morphologic characterization of the CD56++ population. Dev Immunol. 1991;1:169–190. doi: 10.1155/1991/83493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loke YW, King A. Human Implantation: Cell Biology and Immunology. Cambridge University Press; Cambridge: 1995. [Google Scholar]
- 8.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 9.Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F, Masch R, Lockwood CJ, Schachter AD, Park PJ, Strominger JL. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198:1201–1212. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 11.De Maria A, Bozzano F, Cantoni C, Moretta L. Revisiting human natural killer cell subset function revealed cytolytic CD56(dim)CD16+ NK cells as rapid producers of abundant IFN-gamma on activation. Proc Natl Acad Sci U S A. 2011;108:728–732. doi: 10.1073/pnas.1012356108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 13.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97:3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 14.Fauriat C, Long EO, Ljunggren HG, Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115:2167–2176. doi: 10.1182/blood-2009-08-238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vivier E. What is natural in natural killer cells? Immunol Lett. 2006;107:1–7. doi: 10.1016/j.imlet.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Cooper MA, Caligiuri MA. Isolation and characterization of human natural killer cell subsets. Curr Protoc Immunol. 2004;Chapter 7 doi: 10.1002/0471142735.im0734s60. Unit 7 34. [DOI] [PubMed] [Google Scholar]
- 17.Sharkey AM, Gardner L, Hiby S, Farrell L, Apps R, Masters L, Goodridge J, Lathbury L, Stewart CA, Verma S, Moffett A. Killer Ig-like receptor expression in uterine NK cells is biased toward recognition of HLA-C and alters with gestational age. J Immunol. 2008;181:39–46. doi: 10.4049/jimmunol.181.1.39. [DOI] [PubMed] [Google Scholar]
- 18.Verma S, King A, Loke YW. Expression of killer cell inhibitory receptors on human uterine natural killer cells. Eur J Immunol. 1997;27:979–983. doi: 10.1002/eji.1830270426. [DOI] [PubMed] [Google Scholar]
- 19.Kopcow HD, Allan DS, Chen X, Rybalov B, Andzelm MM, Ge B, Strominger JL. Human decidual NK cells form immature activating synapses and are not cytotoxic. Proc Natl Acad Sci U S A. 2005;102:15563–15568. doi: 10.1073/pnas.0507835102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geiselhart A, Dietl J, Marzusch K, Ruck P, Ruck M, Horny HP, Kaiserling E, Handgretinger R. Comparative analysis of the immunophenotypes of decidual and peripheral blood large granular lymphocytes and T cells during early human pregnancy. Am J Reprod Immunol. 1995;33:315–322. doi: 10.1111/j.1600-0897.1995.tb00900.x. [DOI] [PubMed] [Google Scholar]
- 21.Kalkunte SS, Mselle TF, Norris WE, Wira CR, Sentman CL, Sharma S. Vascular endothelial growth factor C facilitates immune tolerance and endovascular activity of human uterine NK cells at the maternal-fetal interface. J Immunol. 2009;182:4085–4092. doi: 10.4049/jimmunol.0803769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lash GE, Robson SC, Bulmer JN. Review: Functional role of uterine natural killer (uNK) cells in human early pregnancy decidua. Placenta. 2010;(31 Suppl):S87–91. doi: 10.1016/j.placenta.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 23.Lash GE, Schiessl B, Kirkley M, Innes BA, Cooper A, Searle RF, Robson SC, Bulmer JN. Expression of angiogenic growth factors by uterine natural killer cells during early pregnancy. Journal of leukocyte biology. 2006;80:572–580. doi: 10.1189/jlb.0406250. [DOI] [PubMed] [Google Scholar]
- 24.Vacca P, Cantoni C, Prato C, Fulcheri E, Moretta A, Moretta L, Mingari MC. Regulatory role of NKp44, NKp46, DNAM-1 and NKG2D receptors in the interaction between NK cells and trophoblast cells. Evidence for divergent functional profiles of decidual versus peripheral NK cells. Int Immunol. 2008;20:1395–1405. doi: 10.1093/intimm/dxn105. [DOI] [PubMed] [Google Scholar]
- 25.Fraser R, Whitley GS, Johnstone AP, Host AJ, Sebire NJ, Thilaganathan B, Cartwright JE. Impaired decidual natural killer cell regulation of vascular remodelling in early human pregnancies with high uterine artery resistance. J Pathol. 2012 doi: 10.1002/path.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashkar AA, Di Santo JP, Croy BA. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. 2000;192:259–270. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guimond MJ, Wang B, Croy BA. Engraftment of bone marrow from severe combined immunodeficient (SCID) mice reverses the reproductive deficits in natural killer cell-deficient tg epsilon 26 mice. J Exp Med. 1998;187:217–223. doi: 10.1084/jem.187.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashkar AA, Black GP, Wei Q, He H, Liang L, Head JR, Croy BA. Assessment of requirements for IL-15 and IFN regulatory factors in uterine NK cell differentiation and function during pregnancy. J Immunol. 2003;171:2937–2944. doi: 10.4049/jimmunol.171.6.2937. [DOI] [PubMed] [Google Scholar]
- 29.Chakraborty D, Rumi MA, Konno T, Soares MJ. Natural killer cells direct hemochorial placentation by regulating hypoxia-inducible factor dependent trophoblast lineage decisions. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1109478108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiby SE, Walker JJ, O'Shaughnessy K M, Redman CW, Carrington M, Trowsdale J, Moffett A. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004;200:957–965. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hiby SE, Apps R, Sharkey AM, Farrell LE, Gardner L, Mulder A, Claas FH, Walker JJ, Redman CW, Morgan L, Tower C, Regan L, Moore GE, Carrington M, Moffett A. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J Clin Invest. 2010;120:4102–4110. doi: 10.1172/JCI43998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orange JS, Ramesh N, Remold-O'Donnell E, Sasahara Y, Koopman L, Byrne M, Bonilla FA, Rosen FS, Geha RS, Strominger JL. Wiskott-Aldrich syndrome protein is required for NK cell cytotoxicity and colocalizes with actin to NK cell-activating immunologic synapses. Proc Natl Acad Sci U S A. 2002;99:11351–11356. doi: 10.1073/pnas.162376099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajakumar A, Jeyabalan A, Markovic N, Ness R, Gilmour C, Conrad KP. Placental HIF-1 alpha, HIF-2 alpha, membrane and soluble VEGF receptor-1 proteins are not increased in normotensive pregnancies complicated by late-onset intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol. 2007;293:R766–774. doi: 10.1152/ajpregu.00097.2007. [DOI] [PubMed] [Google Scholar]
- 34.Voss SD, Daley J, Ritz J, Robertson MJ. Participation of the CD94 receptor complex in costimulation of human natural killer cells. J Immunol. 1998;160:1618–1626. [PubMed] [Google Scholar]
- 35.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 36.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 37.Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, Luo W, Zeller K, Shimoda L, Topalian SL, Semenza GL, Dang CV, Pardoll DM, Pan F. Control of T(H)17/T(reg) Balance by Hypoxia-Inducible Factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. The New England journal of medicine. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keskin DB, Allan DS, Rybalov B, Andzelm MM, Stern JN, Kopcow HD, Koopman LA, Strominger JL. TGFbeta promotes conversion of CD16+ peripheral blood NK cells into CD16- NK cells with similarities to decidual NK cells. Proc Natl Acad Sci U S A. 2007;104:3378–3383. doi: 10.1073/pnas.0611098104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graham CH, Lysiak JJ, McCrae KR, Lala PK. Localization of transforming growth factor-beta at the human fetal-maternal interface: role in trophoblast growth and differentiation. Biol Reprod. 1992;46:561–572. doi: 10.1095/biolreprod46.4.561. [DOI] [PubMed] [Google Scholar]
- 42.Allan DS, Rybalov B, Awong G, Zuniga-Pucker JC, Kopcow HD, Carlyle JR, Strominger JL. TGF-beta affects development and differentiation of human natural killer cell subsets. Eur J Immunol. 2010;40:2289–2295. doi: 10.1002/eji.200939910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan HW, Kurago ZB, Stewart CA, Wilson MJ, Martin MP, Mace BE, Carrington M, Trowsdale J, Lutz CT. DNA methylation maintains allele-specific KIR gene expression in human natural killer cells. J Exp Med. 2003;197:245–255. doi: 10.1084/jem.20021127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santourlidis S, Trompeter HI, Weinhold S, Eisermann B, Meyer KL, Wernet P, Uhrberg M. Crucial role of DNA methylation in determination of clonally distributed killer cell Ig-like receptor expression patterns in NK cells. Journal of immunology. 2002;169:4253–4261. doi: 10.4049/jimmunol.169.8.4253. [DOI] [PubMed] [Google Scholar]
- 45.Tilburgs T, van der Mast BJ, Nagtzaam NM, Roelen DL, Scherjon SA, Claas FH. Expression of NK cell receptors on decidual T cells in human pregnancy. Journal of reproductive immunology. 2009;80:22–32. doi: 10.1016/j.jri.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Xu J, Vallejo AN, Jiang Y, Weyand CM, Goronzy JJ. Distinct transcriptional control mechanisms of killer immunoglobulin-like receptors in natural killer (NK) and in T cells. The Journal of biological chemistry. 2005;280:24277–24285. doi: 10.1074/jbc.M500727200. [DOI] [PubMed] [Google Scholar]
- 47.Li G, Yu M, Weyand CM, Goronzy JJ. Epigenetic regulation of killer immunoglobulin-like receptor expression in T cells. Blood. 2009;114:3422–3430. doi: 10.1182/blood-2009-01-200170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Red-Horse K, Zhou Y, Genbacev O, Prakobphol A, Foulk R, McMaster M, Fisher SJ. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest. 2004;114:744–754. doi: 10.1172/JCI22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lash GE, Otun HA, Innes BA, Percival K, Searle RF, Robson SC, Bulmer JN. Regulation of extravillous trophoblast invasion by uterine natural killer cells is dependent on gestational age. Hum Reprod. 2010;25:1137–1145. doi: 10.1093/humrep/deq050. [DOI] [PubMed] [Google Scholar]
- 50.Robson A, Harris LK, Innes BA, Lash GE, Aljunaidy MM, Aplin JD, Baker PN, Robson SC, Bulmer JN. Uterine natural killer cells initiate spiral artery remodeling in human pregnancy. Faseb J. 2012;26:4876–4885. doi: 10.1096/fj.12-210310. [DOI] [PubMed] [Google Scholar]
- 51.Kolundzic N, Bojic-Trbojevic Z, Kovacevic T, Stefanoska I, Kadoya T, Vicovac L. Galectin-1 is part of human trophoblast invasion machinery--a functional study in vitro. PLoS One. 2011;6:e28514. doi: 10.1371/journal.pone.0028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vacca P, Vitale C, Montaldo E, Conte R, Cantoni C, Fulcheri E, Darretta V, Moretta L, Mingari MC. CD34+ hematopoietic precursors are present in human decidua and differentiate into natural killer cells upon interaction with stromal cells. Proc Natl Acad Sci U S A. 2011;108:2402–2407. doi: 10.1073/pnas.1016257108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carlino C, Stabile H, Morrone S, Bulla R, Soriani A, Agostinis C, Bossi F, Mocci C, Sarazani F, Tedesco F, Santoni A, Gismondi A. Recruitment of circulating NK cells through decidual tissues: a possible mechanism controlling NK cell accumulation in the uterus during early pregnancy. Blood. 2008;111:3108–3115. doi: 10.1182/blood-2007-08-105965. [DOI] [PubMed] [Google Scholar]
- 54.Chen Z, Zhang J, Hatta K, Lima PD, Yadi H, Colucci F, Yamada AT, Croy BA. DBA-Lectin Reactivity Defines Mouse Uterine Natural Killer Cell Subsets with Biased Gene Expression. Biol Reprod. 2012 doi: 10.1095/biolreprod.112.102293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang X, Ho SM. Epigenetics meets endocrinology. Journal of molecular endocrinology. 2011;46:R11–32. doi: 10.1677/jme-10-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.