Abstract
HIV-1-associated neurocognitive disorders (HAND) afflict up to 50% of HIV-1-positive individuals, despite the effectiveness of combination antiretroviral therapy (CART) in reducing the prevalence of more severe neurocognitive impairment. Alterations in brainstem auditory evoked potentials (BAEP), a measure of temporal processing, are one of the earliest neurological abnormalities of HIV-1-positive individuals. Prepulse inhibition (PPI) of the auditory startle response (ASR), a measure of sensorimotor gating, was studied in HIV-1 transgenic (Tg) rats, which express 7 of the 9 HIV-1 genes. Ovariectomized female Fischer HIV-1 Tg and control rats (ns=41–42) were tested for PPI at three test periods, with at least 2 months separating each test period, using auditory and visual prepulses, an auditory startle stimulus, and interstimulus intervals (ISI) ranging from 0–4000 msec. Auditory and visual prepulse trial blocks were presented in counterbalanced order. For both auditory and visual prepulses, HIV-1 Tg animals exhibited a flatter ISI function, which did not sharpen with age, as it did in controls. Over time, auditory prepulses precipitated a temporal shift in peak inhibition in HIV-1 Tg animals relative to controls, whereas with visual prepulses, both groups displayed peak inhibition at the 40 msec ISI. A lack of perceptual sharpening with age and a relative insensitivity to the temporal dimension of sensorimotor gating are evident in the HIV-1 Tg rat prior to clinical signs of wasting. Deficits in sensorimotor gating may not only provide an early subtle diagnostic marker of HAND, but may also afford a key target for development of potential therapeutics.
Keywords: HIV-1 transgenic rat, HIV-1-associated neurocognitive disorders, sensorimotor gating, perceptual sharpening, prepulse inhibition, AIDS
Introduction
The incidence of HIV-1 associated dementia (HAD) has been significantly reduced with the advent of combination antiretroviral therapy (CART) (Heaton et al. 2010; Sacktor et al. 2002). However, HIV-1-associated neurocognitive disorders (HAND), ranging from mild deficits in memory and attention to the more severe and debilitating HAD, continue to afflict up to 50% of patients on CART (Ances and Ellis 2007; Heaton et al. 2011). Given the increased life expectancy provided by CART, as well as the current estimate of 34 million people worldwide living with HIV-1 (UNAIDS 2011), HAND is a significant health issue for millions, disrupting normal functioning of daily activities and posing a risk for early mortality (Ellis et al. 1997).
Before higher level cognitive symptoms manifest, the early stages of HIV-1 may be marked by alterations in temporal processing, as assessed with amplitude and latency measures of auditory evoked potentials (AEPs), both without (Castello et al. 1998; Fein et al. 1995; Gil et al. 1992; Koralnik et al. 1990; Pagano et al. 1992; Schroeder et al. 1996; Vigliano et al. 2000), and after, CART initiation (Chao et al. 2004; Matas et al. 2010). Simian and feline immunodeficiency virus models have also demonstrated alterations in evoked potentials (Phipps et al. 2000; Prospero-Garcia et al. 1999; Raymond et al. 1998; Riazi et al. 2009). Alterations in AEPs become more apparent with disease progression in HIV-1+ patients (Goodwin et al. 1996; Lalwani et al. 1992; Ollo et al. 1991; Schroeder et al. 1994), as is more generally observed in the progression from mild cognitive impairment to dementia (Golob et al. 2007). Given that HIV-1+ patients clearly exhibit basic abnormalities in auditory processing (both early and late components of AEP), it is surprising that few studies have addressed more specific aspects of temporal processing, such as the response to an increased rate of stimulus presentation, which may provide more information on sensory gating in HIV-1+ patients (Bankaitis 1995; Frank et al. 1992; Frank and Pahwa 1993).
Another experimental paradigm used to study auditory processing in both clinical and preclinical studies is prepulse inhibition (PPI) of the auditory startle response (ASR) (Braff et al. 1978; Hoffman and Searle 1965; Ison and Hammond 1971). PPI is an operational measure of sensorimotor gating, a preattentive process in which the startle reflex is reduced when the startle stimulus is preceded 30–500 msec by a weak prepulse (Hoffman and Ison 1980). Within the context of the ASR, we have observed alterations in PPI in HIV-1 transgenic rats as well as in rats stereotaxically injected with the HIV-1 viral proteins Tat and gp120. Specifically, female Sprague-Dawley HIV-1 transgenic (Tg) rats have peak inhibition to an auditory prepulse at the 40 msec interstimulus interval (ISI) when tested at 5, 6, and 7 months of age, which was a leftward shift from the control group’s peak inhibition at the 80 msec ISI (Moran et al. 2013). We have also reported a leftward shift in the inhibition function in 30- and 60-day old male Sprague-Dawley rats following neonatal Tat injection (Fitting et al. 2006a) and in 9-month old male and female Sprague-Dawley rats given neonatal gp120 injections (Fitting et al. 2006b). Assessment of PPI in HIV-1 Tg rats prior to adulthood, which may provide information regarding subtle early-onset neurological alterations analogous to those reported in HIV-1+ humans, has not been conducted to our knowledge. Such information would be especially valuable in its potential for diagnostic and therapeutic applications in HAND.
The utilization of a visual prepulse in the PPI paradigm affords the opportunity to determine the generality of any alterations in sensorimotor gating independent of prepulse stimulus modality. Comparable alterations in auditory and visual PPI would be consistent with a deficit in sensorimotor gating. Differential alterations in auditory versus visual PPI would, alternatively, be consistent with a more restricted sensory system impairment. The measurement of PPI in the HIV-1 Tg rat also provides the opportunity to assess general sensory system alterations, which are commonly observed in transgenic animals, but not always appropriately addressed in experimental designs. One of the readily apparent phenotypes of the HIV-1 Tg rat (and mouse, see Mozes et al. 2002) is cataracts, which present potential limitations in testing models of HAND with the HIV-1 Tg rat via its visual system. It is therefore critical to assess the basic ability to detect visual stimuli in order to design experiments appropriately. If the HIV-1 Tg rat can show inhibition of the ASR to a visual prepulse, it may be logically inferred that, despite the presence of cataracts, the rat can detect visual stimuli of the particular intensity and duration used. A functional assessment of stimulus detectability demonstrates that punctate visual stimuli may be utilized in other experimental paradigms with HIV-1 Tg rats, including measures of executive function and other cognitive domains relevant to HAND.
The purpose of the present study was to characterize PPI of the ASR in the HIV-1 Tg rat, which expresses 7 of the 9 HIV-1 genes and displays brain proinflammatory immune responses (Royal et al. 2012). The assessments were planned to be conducted from 2 to 8 months of age, antecedent to the documented neurological symptoms or clinical signs of wasting (Peng et al. 2010). It was hypothesized that subtle alterations in PPI would be detected early in the progression of the expression of the HIV-1 transgene, as are detected in HIV-1+ individuals. In addition to PPI with an auditory prepulse stimulus, PPI with a visual prepulse stimulus was measured to determine the generality of any alterations in sensorimotor gating.
Methods and Materials
Animals
Ovariectomized female Fischer (F344/N; Harlan Laboratories) rats (HIV-1 Tg, n=41; control, n=42) were tested for PPI starting at 2 months of age. Groups of 3–14 animals were delivered to the facility every week for 10 weeks and tested one week after arrival. All animals were group-or pair-housed throughout the experiments. Rodent food (2020X Teklad Global Extruded Rodent Diet (Soy Protein-Free)) and water were available ad libitum throughout the first test period. The animals were under food restriction (85% body weight) during the two later test periods for the purpose of contemporaneously conducted operant experiments. The animals were maintained according to the National Institute of Health (NIH) guidelines in AAALAC-accredited facilities. The animal facility was maintained at 21° ± 2°C, 50% ± 10% relative humidity and had a 12-h light:12-h dark cycle with lights on at 0700 h (EST). Rats were handled for one week prior to any behavioral testing procedures. The Institutional Animal Care and Use Committee (IACUC) of the University of South Carolina approved the project protocol.
Apparatus
The startle platform (SR-Lab Startle Reflex System, San Diego Instruments, Inc., San Diego, CA) was enclosed in a 10 cm-thick double-walled, 81×81x116-cm isolation cabinet (external dimensions) (Industrial Acoustic Company, INC., Bronx, NY), rather than the 1.9 cm thick ABS plastic or laminate cabinets offered with this system. This double-walled isolation chamber provides over 30dB(A) of sound attenuation relative to the external environment. The ambient sound level in the chamber without any stimuli presented is 22dB(A). The high-frequency loudspeaker of the SR-Lab system (Radio Shack model#40–1278B) that was used to deliver all auditory stimuli (frequency range of 5k-16k Hz) was mounted inside the chamber 30 cm above the Plexiglas animal test cylinder. Three white LED lights were mounted inside the chamber, one on the wall behind the test cylinder (22 lux) presented as the visual prepulse at each of the three test periods, and two on opposite walls on either side of the cylinder (100 lux combined) presented together as the visual prepulse during a session in the first test period. The animal’s whole body startle response to the auditory stimulus produced deflection of the test cylinder, which was converted into analog signals by a piezoelectric accelerometer integral to the bottom of the cylinder. The response signals were digitized (12 bit A to D) and saved to a hard disk. Response sensitivities were calibrated using a SR-LAB Startle Calibration System. Sound levels were measured and calibrated with a sound level meter (Bruel & Kjaer 2203) with the microphone placed inside the Plexiglas cylinder.
Procedure
ASR Habituation
At 2 months of age, the animals were administered a 36-trial auditory startle test session to habituate them to the auditory stimulus and test procedures. Each session began with a 5-min acclimation period of 70dB background white noise, followed by 36 trials of a 100dB(A) white noise stimulus with a 20 msec duration and a 10 sec intertrial interval (ITI). All test sessions were conducted in the dark.
Prepulse Inhibition Test
One day after ASR habituation, animals were tested for PPI of the ASR with both visual and auditory prepulse stimuli. All animals were administered a 30-min test session, which began with a 5-min acclimation period in the dark with 70dB(A) background white noise, followed by 6 pulse-only ASR trials with a 10 sec ITI. An equal number of visual and auditory prepulse trials [72 trials total; Interstimulus intervals (ISI) of 0, 8, 40, 80, 120, and 4000 msec, 6-trial blocks, Latin square design] were interdigitated in an ABBA order of presentation. The 0 and 4000 msec ISI trials were control trials used to calculate percent inhibition on PPI trials. The pulse stimulus intensity was 100dB(A) (20 msec duration) measured inside the test cylinder. A variable 20 sec ITI was used (range=15–25 sec). All animals were tested on two consecutive days. On the first day, one LED light (22 lux at the level of the test cylinder) was presented as the visual prepulse; on the second day, two LED lights (100 lux at the level of the test cylinder) were presented. On both test days, the auditory prepulse was an 85dB(A) white noise stimulus (20 msec duration) measured inside the test cylinder. Mean peak ASR amplitude values were collected for analysis. Percent PPI was derived from mean peak ASR amplitude data as the difference between average peak amplitude at 0 and 4000 msec ISIs and at the ISI at which peak inhibition was observed, divided by average peak amplitude at 0 and 4000 msec ISIs, multiplied by 100.
Animals were tested similarly a second and third time with a minimum of 2 months of age separating each of the 3 tests for each animal. Only the 22 lux visual prepulse was used in the second and third test sessions, as it was determined after the first test that this stimulus was sufficient to cause robust inhibition of the ASR.
Statistics
All data were analyzed using analysis of variance (ANOVA) techniques (IBM SPSS Statistics 20). For each prepulse type (auditory and visual), a four-way mixed-factor ANOVA was performed on mean peak ASR amplitude for the 0–4000 msec ISIs, with treatment condition (HIV-1 Tg vs. control) as the between-subjects factor, and age, ISI, and trial as the within-subjects factors. A four-way mixed-factor ANOVA was also performed on mean peak ASR amplitude during visual prepulse trials at 2 months of age, with treatment condition as the between-subjects factor, and light intensity, ISI, and trial as the within-subjects factors. A similar analysis was conducted on mean peak ASR amplitude during auditory prepulse trials at 2 months of age, to determine if the intensity of the light stimulus used in the interdigitated visual prepulse trials differentially affected auditory PPI. Mean peak ASR amplitude during the habituation session and during 0 msec ISI trials within each PPI session was also analyzed with linear and nonlinear regression analyses. An alpha level of p≤.05 was considered significant for all statistical tests. Sample sizes were chosen with the goal of sufficient statistical power (> 0.80) to maximize the likelihood of detecting subtle early alterations of expression of the HIV-1 transgene.
Results
ASR Intrasession Habituation
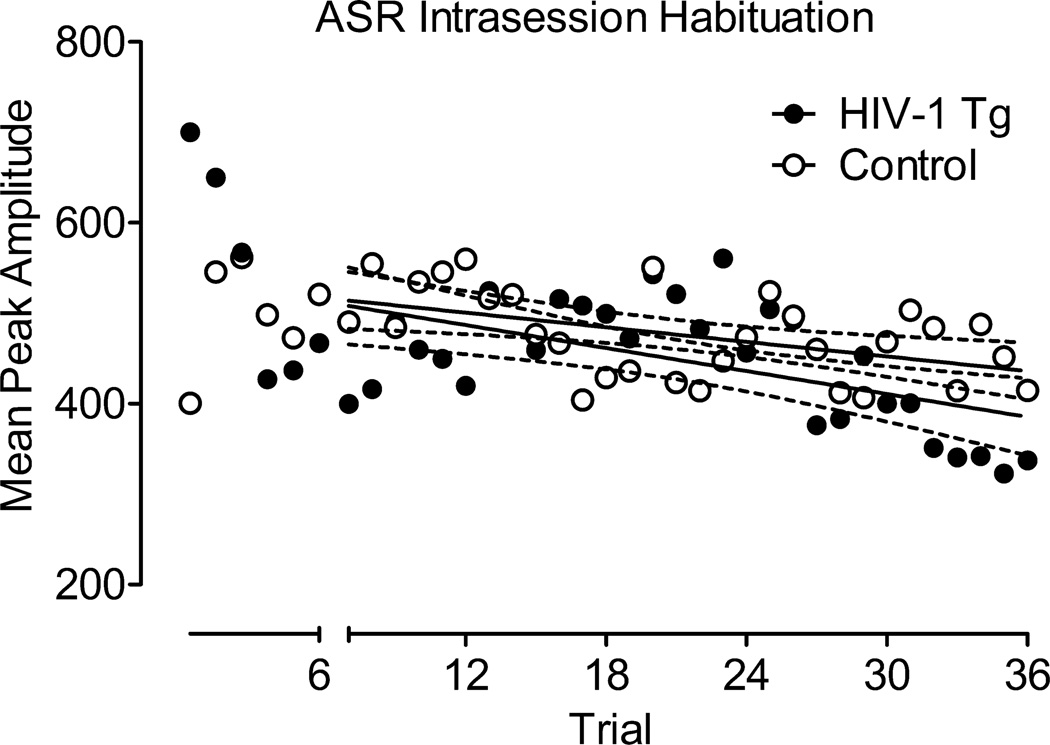
Mean peak ASR amplitude data from the habituation session are illustrated in Figure 1. With the exception of the first 6 trials (during which the HIV-1 Tg group showed a sharp decrease in the ASR), linear regression analysis revealed that there was no difference in the rate of habituation across the test session for the HIV-1 Tg and control groups (Regression line slopes: HIV-1 Tg, −4.24+/−1.24; Control: −2.7+/−0.91), with no difference in overall ASR between groups, F=0.17.
Fig. 1.
Mean peak ASR amplitude data from the habituation session (±95% CI). After the initial 6 trials, the HIV-1 Tg and control groups showed no difference in overall ASR (F=0.17), and did not differ in rate of habituation to the auditory startle stimulus. Linear regression line slopes: HIV-1 Tg, −4.24+/−1.24; Control, −2.7+/−0.91.
ASR Intersession Habituation
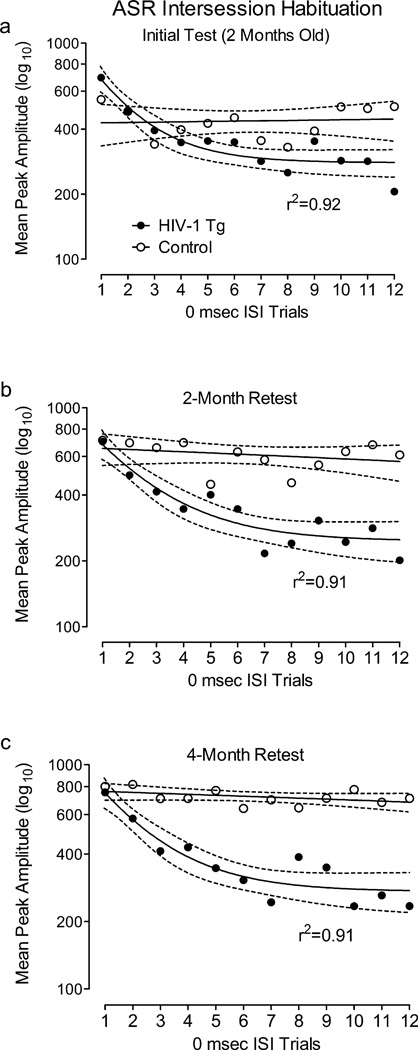
Mean peak ASR amplitude during 0 msec ISI control trials across the PPI test session at each age is illustrated in Figure 2. Nonlinear regression analysis revealed that the ASR of the HIV-1 Tg group decreased across 0 msec ISI trials within each test session, with a single phase decay function accounting for over 90% of the variance. In contrast, the ASR of the control group did not significantly change across 0 msec ISI trials during any test session.
Fig. 2.
Mean peak ASR amplitude during 0 msec ISI control trials across the PPI test session at each age (±95% CI). Trials are numbered 1 through 12 for simplicity, although they were presented across the 72-trial session in a Latin square. The ASR of the HIV-1 Tg group decreased across 0 msec ISI trials within each test session, with a single phase decay function accounting for over 90% of the variance in each session. In contrast, the ASR of the control group did not significantly change across 0 msec ISI trials during any test session.
HIV-1 Tg Rats Exhibit Significant PPI with an Auditory Prepulse (in Both 22 and 100 lux Visual Prepulse Contexts)
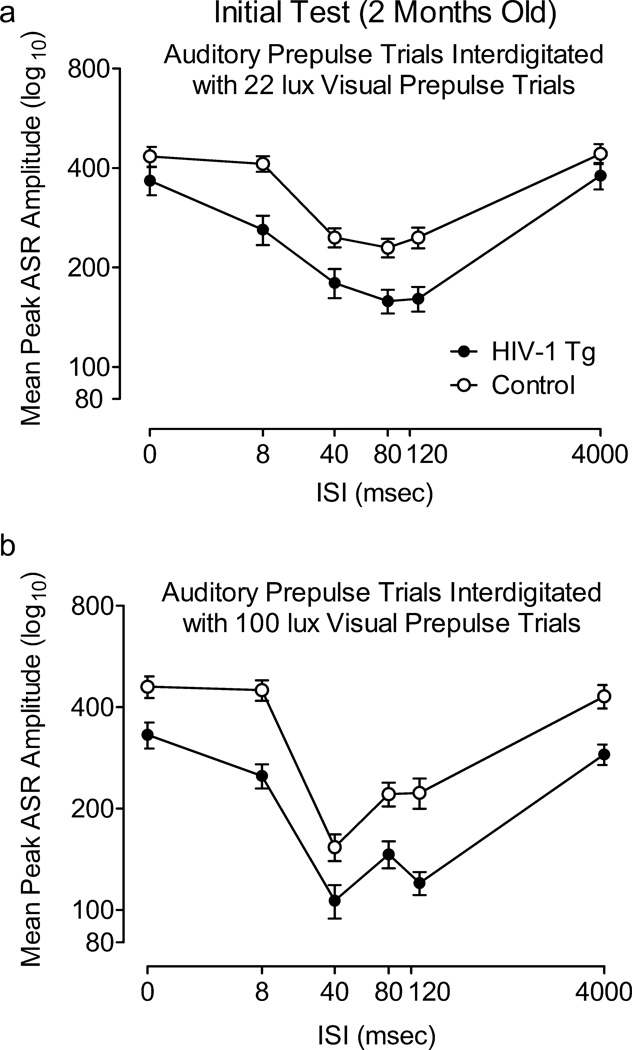
Mean peak ASR amplitude during auditory prepulse trials assessed at 2 months of age is illustrated in Figure 3. This first test period included a comparison of auditory prepulse trials that were interdigitated with 22 lux visual prepulse trials, and auditory prepulse trials that were interdigitated with 100 lux visual prepulse trials. Most importantly, there was no 3-way interaction between treatment condition, light intensity, and ISI, nor an interaction between treatment condition and light intensity, suggesting that the brightness of the visual prepulse did not differentially affect PPI of each group during auditory prepulse trials. In fact, the ISI functions of the HIV-1 Tg and control groups changed in a similar manner with the increased light intensity of the prepulse, reflected by a significant light intensity × ISI interaction in each group [Control: F(5,205)=5.0, p≤0.001, Power =0.98; HIV-1 Tg: F(5,200)=3.1, p≤0.05, Power =0.87]; with both groups, the ISI functions sharpened and the point of peak inhibition shifted from the 80 msec ISI to the 40 msec ISI. The HIV-1 Tg and control groups also both displayed quadratic trends for ISI during auditory prepulse trials in each visual prepulse context, characteristic of the fundamental temporal domain of PPI [22 lux visual prepulse: Control, F(1,41)=76.3, p≤0.002, Power =1.0; HIV-1 Tg, F(1,40)=69.6, p≤0.001, Power =1.0; 100 lux visual prepulse: Control: F(1,41)=122.0, p≤0.001, Power =1.0; HIV-1 Tg: F(1,40)=110.7, p≤0.001, Power =1.0]. However, there was a treatment condition × ISI interaction for mean peak ASR amplitude during auditory prepulse trials that were interdigitated with the 100 lux visual prepulse trials (Figure 3B), F(5,405)=6.3, p≤0.001 (Power =1.0), indicating a relative insensitivity to manipulation of ISI duration in the HIV-1 Tg group. There was no treatment condition × ISI interaction during trials interdigitated with 22 lux visual prepulse trials.
Fig. 3.
Prepulse inhibition (PPI) with an auditory prepulse during sessions interdigitated with 22 lux (A) and 100 lux (B) visual prepulse trials conducted at 2 months of age. A significant condition × interstimulus interval (ISI) interaction was detected, indicating that the HIV-1 Tg rats were less sensitive to the manipulation of ISI, as illustrated by their flatter ISI functions. Both groups’ ISI functions changed in a similar manner with the increased visual prepulse intensity; i.e., there was a leftward shift in peak inhibition to the 40 msec ISI, and the ISI functions sharpened. Percent PPI at point of peak inhibition: 1 light: HIV-Tg, 30.0%±5.5, Control, 15.2%±7.8; 2 lights: HIV-1 Tg, 46.3%±5.2; Control, 44.0%±4.5.
HIV-1 Tg Rats Exhibit Altered Development of PPI with an Auditory Prepulse
The ANOVA conducted on mean peak amplitude during auditory prepulse trials interdigitated with 22 lux visual prepulse trials across all three test periods revealed a significant treatment condition × age × ISI interaction, F(10, 790)=8.1, p≤0.001 (Power =1.0), as well as a treatment condition × ISI interaction, F(5,395)=23.3, p≤0.001 (Power =1.0), an age × ISI interaction, F(10, 790)=14.3, p≤0.001 (Power =1.0), and a treatment condition × age interaction, F(2,158)=16.6, p≤0.001 (Power =1.0). Additional analyses were conducted to identify the locus of these interactions.
Separate analyses of each group revealed a main effect of age, F(2,82)=21.3, p≤0.001 (Power =1.0), and an age × ISI interaction, F(10,410)=18.4, p≤0.001 (Power =1.0), in the control group, illustrated in figure 4A. These effects were not observed in the HIV-1 Tg group, suggesting that the expression of the HIV-1 transgene interfered with the age-dependent development of perceptual sharpening.
Fig. 4.
Prepulse inhibition (PPI) with an auditory prepulse interdigitated with 22 lux visual prepulse trials across all three test ages. A significant main effect of age and an age × interstimulus interval (ISI) interaction were found in the control group (A), but not confirmed in the HIV-1 Tg group (B).The control group increased in percent PPI across test ages, from 15.2%±7.8 at 2 months to 64.0%±3.9 at 6–8 months, whereas the HIV-1 Tg group showed a much smaller increase, from 30.0%±5.5 at 2 months of age to 52.8%±4.4 at 6–8 months.
Complementary results were obtained after separate analyses at each age, which revealed significant treatment condition × ISI interactions during the 2-month retest [F(5,395)=10.5, p≤0.001, Power =1.0] and the 4-month retest [F(5,395)=26.1, p≤0.001, Power =1.0], but not during the initial test (2 months old). The treatment condition × ISI interactions at the later ages indicate, as does the age × ISI interaction in the control group but not the HIV-1 Tg group, altered development of the ISI function in the HIV-1 Tg group; they did not exhibit the same sharpening of the ISI function with age that is apparent with the control group. Changes in percent PPI across age also reflect the differential development of the ISI function. The HIV-1 Tg group increased in percent PPI from 30.0%±5.5 during the initial test to 52.8%±4.4 during the 4-month retest, whereas the control group showed a much greater relative increase, from 15.2%±7.8 to 64.0%±3.9.
The overall treatment condition × ISI interaction reflects not only the relative insensitivity of the HIV-1 Tg group to manipulation of ISI duration, but also the differential peak inhibition of the two groups, observed at the 80 msec ISI during the initial test and the 120 msec ISI during the 2-month and 4-month retests for the HIV-1 Tg animals, and at the 80 msec ISI during the initial test and the 40 msec ISI during the 2-month and 4-month retests for the control animals. Both groups showed significant quadratic trends for ISI, characteristic of the fundamental temporal domain of PPI [Control: F(1,41)=261.9, p≤0.001, Power =1.0; HIV-1 Tg: F(1,38)=108.6, p≤0.001, Power =1.0].
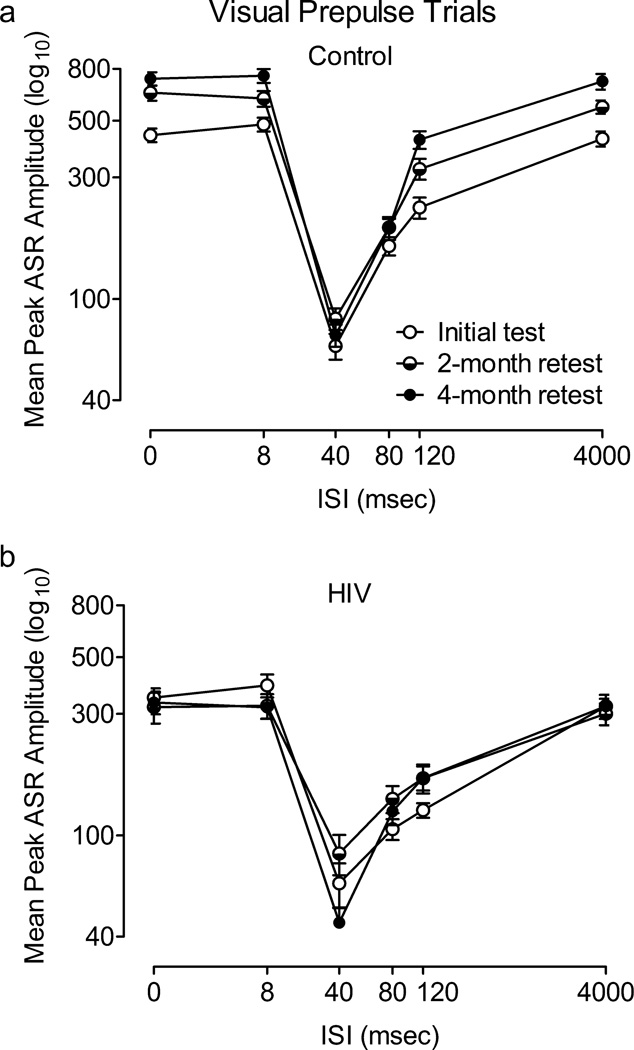
HIV-1 Tg Rats Exhibit Significant PPI with a Visual Prepulse
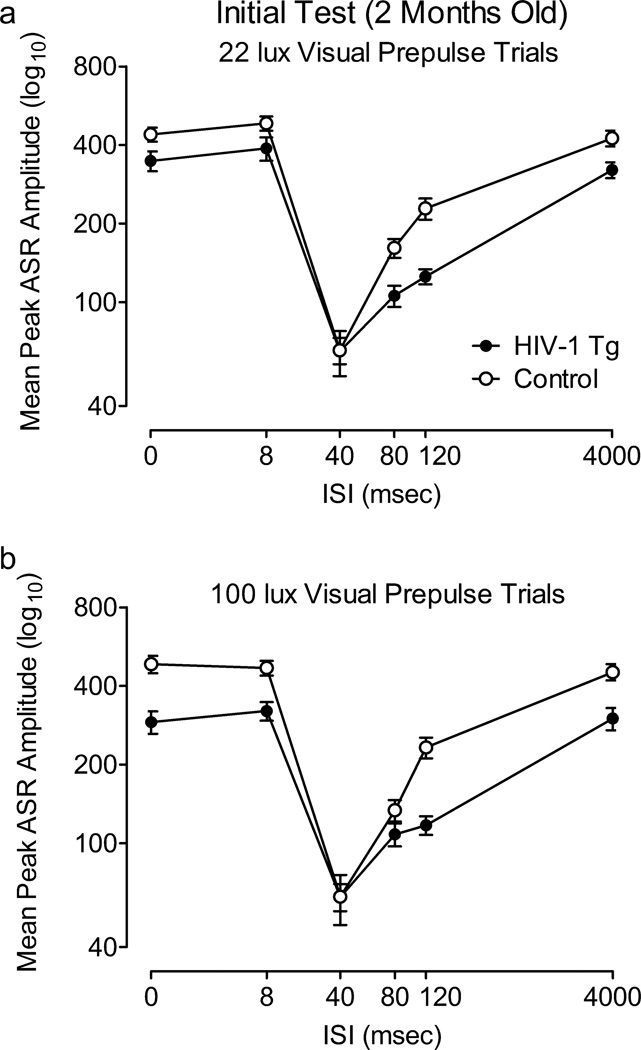
The ANOVA conducted on mean peak ASR amplitude during 22 and 100 lux visual prepulse trials at 2 months of age revealed that there was no interaction between treatment condition, light intensity, and ISI. There was also no treatment condition × light intensity or light intensity × ISI interaction, nor a significant main effect of light intensity. The effect of the 22 lux light on the ASR was robust and sufficient to demonstrate visual PPI in the HIV-1 Tg as well as the controls (percent PPI at the 40 msec ISI: Control, 72.2±2.6%; HIV-1-Tg, 74.9%±2.5); therefore, only the 22 lux visual prepulse was used for the subsequent test periods. Both groups displayed quadratic trends for ISI in each visual prepulse condition [22 lux: Control, F(1,41)=148.3, p≤0.001, Power =1.0; HIV-1 Tg, F(1,40)=110.7, p≤0.001, Power =1.0; 100 lux: Control, F(1, 41)=138.4, p≤0.001, Power =1.0, HIV-1-Tg: F(1,40)=54.9, p≤0.001, Power =1.0], with comparable peak inhibition at the 40 msec ISI (100 lux visual prepulse trials, percent PPI: Control, 73.9%±3.1; HIV-1-Tg, 66.2%±3.8). However, there was a significant treatment condition × ISI interaction during 22 lux visual prepulse trials, F(5,405)=2.4, p≤0.05 (Power =0.76) (Figure 5A), as well as during 100 lux visual prepulse trials, F(5,405)=9.0, p≤0.001 (Power =1.0) (Figure 5B), reflecting the relative insensitivity of the HIV-1 Tg group to manipulation of ISI duration.
Fig. 5.
Prepulse inhibition (PPI) with 22 lux (A) and 100 lux (B) visual prepulses conducted at 2 months of age. A significant condition × interstimulus interval (ISI) interaction was detected, indicating that the HIV-1 Tg group was less sensitive to the manipulation of ISI, as illustrated by their flatter ISI functions. Percent PPI at the point of peak inhibition: 1 light: HIV-1-Tg, 74.9%±2.5; Control, 72.2%±2.6; 2 lights: HIV-1-Tg, 66.3%±3.8; Control, 73.9%±3.1.
HIV-1 Tg Rats Exhibit Altered Development of PPI with a Visual Prepulse
Mean peak amplitude during 22 lux visual prepulse trials at each test period is illustrated in figure 6. There was a significant treatment condition × age × ISI interaction, F(10, 790)=7.7, p≤0.001 (Power =1.0), as well as a treatment condition × ISI interaction, F(5,395)=31.1, p≤0.001 (Power =1.0), an age × ISI interaction, F(10, 790)=5.5, p≤0.001 (Power =1.0), and a treatment condition × age interaction, F(2,158)=15.7, p≤0.001 (Power =1.0). Additional analyses were conducted to identify the locus of these interactions.
Fig. 6.
Prepulse inhibition (PPI) with a 22 lux visual prepulse across all three test sessions. A main effect of age and an age × interstimulus interval (ISI) interaction were found in the control group (A), but not confirmed in the HIV-1 Tg group (B). The HIV-1 Tg and control groups each displayed peak inhibition at the 40 msec ISI at each age tested. Percent PPI at the 40 msec ISI: 2 months:HIV-1-Tg, 74.9%±2.5, Control, 72.2%±2.6; 4–6 months:HIV-1 Tg = 58.2%±4.2; Control = 78.6%±1.9; 6–8 months: HIV-1 Tg: 72.1%±2.7; Control: 77.9%±3.6.
Separate analyses of each group revealed a main effect of age, F(2, 82)=24.2, p≤0.001(Power =1.0), and an age × ISI interaction in the control group, F(10,410)=10.4, p≤0.001 (Power =1.0), but neither effect was present in the HIV-1 Tg group, suggesting that the expression of the HIV-1 transgene interfered with the age-dependent development of perceptual sharpening.
Significant treatment condition × ISI interactions were detected at each test period [initial test: F(5,405)=2.4, p≤0.05, Power =0.76; 2-month retest: F(5,400)=18.7, p≤0.001, Power =1.0; 4-month retest: F(5,400)=29.3, p≤0.001, Power =1.0]. Each group showed a significant quadratic trend for ISI [Control: F(1,41)=381.3, p≤0.001, Power =1.0; HIV-1 Tg: F(1,38)=184.7, p≤0.001, Power =1.0], but the treatment condition × ISI interactions indicated that the HIV-1 Tg group was relatively insensitive to manipulation of ISI duration at each test period, providing further evidence that the HIV-1 Tg group did not develop normal perceptual sharpening.
The HIV-1 Tg and control groups each displayed peak inhibition at the 40 msec ISI at each test period. The HIV-1 Tg group showed a quadratic trend for percent PPI across age, F(1,38)=13.0, p≤0.005, whereas the control animals displayed similar percent PPI at each age. The quadratic trend reflects the reduction in percent PPI during the 2-month retest in the HIV-1 Tg group (58.2%±4.2, compared to 74.9%±2.5 during the initial test period and 72.1%±2.7 during the 4-month retest), which can be attributed to their lower ASR amplitude at the 0 and 4000 msec ISIs; amplitude at the point of peak inhibition, 40 msec, was almost identical for the two groups (HIV-1 Tg = 85.0±15.3; Control = 83.7±8.2).
Discussion
The present experiments demonstrate alterations in sensorimotor gating in the HIV-1 Tg rat early in the expression of the HIV-1 transgene and prior to any documented neurological symptoms or signs of wasting. The most apparent difference was that the HIV-1 Tg group exhibited a flatter ISI function, which did not sharpen with age, as it did with control animals. Furthermore, the flatter ISI function was observed in both auditory and visual prepulse conditions, demonstrating the generality of sensorimotor gating deficits across prepulse modality. Over time, auditory prepulses precipitated a temporal shift in peak inhibition in HIV-1 Tg animals relative to controls, whereas with visual prepulses, both groups displayed peak inhibition at the 40 msec ISI. The observed alterations in PPI indicate a lack of perceptual sharpening with age and a relative insensitivity to the temporal dimension of sensorimotor gating in the HIV-1 Tg rat, resembling the temporal processing deficits reported in HIV-1+ individuals early in the disease course.
Perceptual sharpening is a developmental process in which responses are evoked by more specific stimuli, i.e., stimulus discrimination (Ganz 1968; Gibson 1969; Tees 1976; Werner 1948). Younger subjects (Rubel and Rosenthal 1975) and sensory-deprived subjects (Kerr et al. 1979) exhibit significantly flatter stimulus generalization gradients. Thus, experience, often a function of age, is a crucial element in normal perceptual sharpening. In rats, the heart rate orienting response has been used to measure the ontogeny of perceptual sharpening. Rats at 16–17 days of age that have been habituated to an auditory stimulus will generalize the habituated response to a wide range of auditory stimuli; by day 20, stimulus discrimination is apparent with a much sharper generalization gradient (Campbell and Haroutunian 1983). We have previously observed perceptual sharpening of PPI with auditory, visual, and tactile prepulses in Long-Evans rats, at PD 18, 35, and 90 (Hord et al. 2008). These animals showed gradually sharper ISI functions for PPI with an auditory or tactile prepulse across age. As the visual system is the last system to develop in the rat (Gottlieb 1971), PPI with a visual prepulse was not apparent until PD 90, when peak inhibition was exhibited at the 40 msec ISI. At PD 18 and 35, the ISI functions were flat, reflecting the immaturity of the visual sensory system. The HIV-1 Tg and control rats in the present study exhibited robust PPI with a visual prepulse at two months of age, which suggests that the visual system and its afferents to PPI circuitry are well-developed at this age. However, the point of peak inhibition in the ISI functions of the HIV-1 Tg group under both prepulse conditions did not become clearly defined across age as was observed in the control group. Although the HIV-1 Tg rats appear to have functional auditory and visual systems, a more specific deficit in the development, or perceptual sharpening, of temporal sensitivity in the context of PPI was exhibited.
We have previously demonstrated that 1-month old male Fischer HIV-1 Tg and control rats display peak inhibition to an auditory prepulse at the 40 msec ISI, with overall similar ISI functions (Moran et al. 2012). In the present study, the ISI functions of the HIV-1 Tg and control groups were most similar at two months of age, and then changed in different ways across age. For PPI with an auditory prepulse, both groups had peak inhibition at the 80 msec ISI at two months of age. At the later ages, however, the HIV-1 Tg group displayed peak inhibition at the 120 msec ISI, representing a rightward shift from the control group’s peak of inhibition at the 40 msec ISI. We have previously observed differences in the point of peak inhibition in female Sprague-Dawley HIV-1 Tg and control rats as well, between 5–7 months of age (Moran et al. 2013). Shifts in peak inhibition have also been demonstrated in rats administered HIV-1 viral protein injections. Leftward shifts were observed in 30- and 60-day old male Sprague-Dawley rats following neonatal Tat injection (Fitting et al. 2006a) and in 9-month old male and female Sprague-Dawley rats given neonatal gp120 injections (Fitting et al. 2006b).
Despite the differences in sensorimotor gating observed between the HIV-1 Tg and control groups, they displayed similar inhibition to a visual prepulse, which is particularly notable given the presence of cataracts in the HIV-1 Tg animals. It is clear from this finding that the HIV-1 Tg group could detect the 20 msec visual stimulus despite their cataracts, and thus, a visual stimulus greater than or equal to the intensity and duration used in this experiment would have utility in other experimental paradigms with HIV-1 Tg rats, especially measures of executive function and other cognitive domains relevant to the study of HAND. The use of visual stimuli permits utilization of a variety of methods to test cognition in HIV-1 Tg rats.
Disruptions in the dopamine (DA) system have been implicated as an important factor in the progression of HAND, in both clinical and preclinical studies. Significant reductions in DA markers, including DA levels in the substantia nigra (Kumar et al. 2011), homovanillic acid (di Rocco et al. 2000), and DA transporter (DAT) levels (Chang et al. 2008; Wang et al. 2004) have been correlated with poor performance on measures of learning, memory, and executive function in HIV-1-positive individuals. HIV-1 Tg rats also display alterations in the DA system, with significantly lower MAO-A levels than control animals, as well as lower levels of tyrosine hydroxylase after treatment with methamphetamine (Moran et al. 2012). In vitro studies have shown that DAT is targeted by HIV-1 proteins Tat and gp120, resulting in transporter impairment (Aksenov et al. 2008; Ferris et al. 2009; Midde et al. 2013; Zhu et al. 2009; Zhu et al. 2011), due to direct protein-protein interactions (Zhu et al. 2009) involving an allosteric modulation of DAT by the Tat protein (Zhu et al. 2011). In addition, DA-dependent signaling has been identified as a mechanism of HIV-1 protein neurotoxicity (Aksenova et al. 2006; Silvers et al. 2007; Wallace et al. 2006).
The alterations in PPI observed in HIV-1 Tg rats may be explained by the disruptions in the DA system that are consequent to HIV-1 infection. Pharmacological studies have shown reductions in PPI after administration of direct and indirect DA agonists, such as apomorphine and amphetamine (Geyer et al. 2001). Apomorphine-induced PPI deficits have been used as a preclinical model of schizophrenia, capturing both the dysfunction of the DA system and preattentive sensory gating deficits as measured with event-evoked potentials (Adler et al. 1982) and the eyeblink response (Braff et al. 1978) in individuals with schizophrenia. The aforementioned early studies on sensory gating in schizophrenic patients revealed that they have flatter ISI functions than the healthy controls, indicating an insensitivity to manipulation of the duration of the ISI. We have observed a “flattening” of the ISI function in rats administered apomorphine (Moran et al., 2009), comparable to the ISI functions exhibited by the HIV-1 Tg rats in the present study. Although other neural systems may be involved, central DA system dysfunction often results from HIV-1 infection and is associated with subsequent cognitive deficits (Kumar et al. 2011; diRocco et al. 2000; Chang et al. 2008; Wang et al. 2004; for review, see Purohit et al. 2011). The use of behavioral measures such as the ASR and PPI that can detect early neurological alterations, especially those of the DA system, may be instrumental in predicting the development of HAND and thus determining an appropriate course of treatment.
Measuring the ASR and PPI also permitted the assessment of potential changes in sensitization and/or habituation to the startle stimulus across age. The HIV-1 Tg group’s robust response to the startle stimulus (during 0 msec ISI trials) at the beginning of a test session was followed by a response decrement, in accordance with a single phase decay function. Across repeated two month assessments, however, the HIV-1 Tg rats displayed no retention of their prior habituation. The failure to retain information about the testing context is consistent with an impairment in episodic memory. The control group, in contrast, displayed stable responding across 0 msec ISI trials. Across repeated two month assessments, the control group displayed response sensitization, as evident by an average increase in responding; an outcome indicative of retention of information about the testing context. We have previously reported an impairment in episodic memory in HIV-1 Tg rats that displayed deficient habituation in locomotor activity testing paradigm (Moran et al. 2013). In that study, the HIV-1 Tg group showed decreased intrasession habituation of motor activity across 3-day assessment periods that emerged across the at least monthly-spaced locomotor activity sessions; a pattern also consistent with impaired long-term episodic memory. As nearly half of HIV-1 positive individuals on CART show deficits in long-term episodic memory (Heaton et al. 2011), assessment of this cognitive domain in the HIV-1 Tg rat is particularly important.
In summary, the present study demonstrates that HIV-1 Tg rats exhibit neurological deficits early in the expression of the HIV-1 transgene, prior to clinical signs of wasting, which progress with age, bearing a marked resemblance to the temporal processing deficits observed in individuals with HIV-1. Both the relative insensitivity to the temporal dimension of sensorimotor gating and the lack of development of perceptual sharpening with age, suggest, time and time again, clear evidence of temporal processing deficits in the HIV-1 Tg rat. These functional consequences of chronic low level of exposure to the HIV-1 proteins are apparent under conditions which resemble the suppression of infection in HIV-1+ individuals under CART (Peng et al., 2010). Deficits in sensorimotor gating may not only serve as an early subtle diagnostic marker of HAND, but may also afford a key target for development of potential therapeutics for HAND.
Acknowledgments
This research was supported by the National Institute on Drug Abuse [RMB, Grant DA013137; CFM, Grant DA031604] and by the National Institute of Child Health and Human Development [CFM, Grant HD043680].
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry. 1982;17:639–654. [PubMed] [Google Scholar]
- Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol. 2007;27:86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Aksenova MV, Silvers JM, Mactutus CF, Booze RM. Different effects of selective dopamine uptake inhibitors, GBR 12909 and WIN 35428, on HIV-1 Tat toxicity in rat fetal midbrain neurons. Neurotoxicology. 2008;29:971–977. doi: 10.1016/j.neuro.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksenova MV, Silvers JM, Aksenov MY, Nath A, Ray PD, Mactutus CF, Booze RM. HIV- 1 Tat neurotoxicity in primary cultures of rat midbrain fetal neurons: changes in dopamine transporter binding and immunoreactivity. Neurosci Lett. 2006;395:235–239. doi: 10.1016/j.neulet.2005.10.095. [DOI] [PubMed] [Google Scholar]
- Bankaitis AE. The effects of click rate on the auditory brain stem response (ABR) in patients with varying degrees of HIV-infection: a pilot study. Ear Hear. 1995;16:321–324. doi: 10.1097/00003446-199506000-00009. [DOI] [PubMed] [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Campbell BA, Haroutunian V. Perceptual sharpening in the developing rat. J Comp Psychol. 1983;97:3–11. [PubMed] [Google Scholar]
- Castello E, Baroni N, Pallestrini E. Neurotological and auditory brain stem response findings in human immunodeficiency virus-positive patients without neurologic manifestations. Ann Otol Rhinol Laryngol. 1998;107:1054–1060. doi: 10.1177/000348949810701210. [DOI] [PubMed] [Google Scholar]
- Chao LL, Lindgren JA, Flenniken DL, Weiner MW. ERP evidence of impaired central nervous system function in virally suppressed HIV patients on antiretroviral therapy. Clin Neurophysiol. 2004;115:1583–1591. doi: 10.1016/j.clinph.2004.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, Fowler JS. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. Neuroimage. 2008;42:869–878. doi: 10.1016/j.neuroimage.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- diRocco A, Bottiglieri T, Dorfman D, Werner P, Morrison C, Simpson D. Decreased homovanilic acid in cerebrospinal fluid correlates with impaired neuropsychologic function in HIV-1-infected patients. Clin Neuropharmacol. 2000;23:190–194. doi: 10.1097/00002826-200007000-00004. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Deutsch R, Heaton RK, Marcotte TD, McCutchan JA, Nelson JA, Abramson I, Thal LJ, Atkinson JH, Wallace MR, Grant I. Neurocognitive impairment is an independent risk factor for death in HIV infection. Arch Neurol. 1997;54:416–424. doi: 10.1001/archneur.1997.00550160054016. [DOI] [PubMed] [Google Scholar]
- Fein G, Biggins CA, Mackay S. Delayed latency of the event-related brain potential P3A component in HIV disease: progressive effects with increasing cognitive impairment. Arch Neurol. 1995;52:1109–1118. doi: 10.1001/archneur.1995.00540350103022. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM. The human immunodeficiency virus-1-associated protein, Tat(1–86), impairs dopamine transporters and interacts with cocaine to reduce nerve terminal function: a no-net-flux microdialysis study. Neuroscience. 2009;159:1292–1299. doi: 10.1016/j.neuroscience.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal hippocampal Tat injections: developmental effects on prepulse inhibition (PPI) of the auditory startle response. Int J Dev Neurosci. 2006a;24:275–283. doi: 10.1016/j.ijdevneu.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal intrahippocampal glycoprotein 120 injection: The role of dopaminergic alterations in prepulse inhibition in adult rats. J Pharmacol Exp Ther. 2006b;318:1352–1358. doi: 10.1124/jpet.106.105742. [DOI] [PubMed] [Google Scholar]
- Frank Y, Pahwa S. Serial Brain-Stem Auditory-Evoked Responses in Infants and Children with Aids. Clin Electroencephalogr. 1993;24:160–165. doi: 10.1177/155005949302400406. [DOI] [PubMed] [Google Scholar]
- Frank Y, Vishnubhakat SM, Pahwa S. Brain stem auditory evoked-responses in infants and children with AIDS. Pediatr Neurol. 1992;8:262–266. doi: 10.1016/0887-8994(92)90362-3. [DOI] [PubMed] [Google Scholar]
- Ganz L. An analysis of generalization behavior in the stimulus deprived organism. In: Newton G, Levine S, editors. Early experience and behavior: The psychobiology of development. Springfield, IL: Charles C. Thomas; 1968. pp. 365–411. [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Gibson EJ. Principles of perceptual learning and development. New York: Appleton-Century-Crofts; 1969. [Google Scholar]
- Gil R, Breux JP, Neu JP, Becq-Giraudon B. Cognitive evoked potentials and HIV infection. Neurophysiol Clin. 1992;22:385–391. doi: 10.1016/s0987-7053(05)80096-6. [DOI] [PubMed] [Google Scholar]
- Golob EJ, Irimajiri R, Starr A. Auditory cortical activity in amnestic mild cognitive impairment: relationship to subtype and conversion to dementia. Brain. 2007;130:740–752. doi: 10.1093/brain/awl375. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, Pretsell DO, Chiswick A, Egan V, Brettle RP. The Edinburgh cohort of HIV- positive injecting drug users at 10 years after infection: A case-control study of the evolution of dementia. AIDS. 1996;10:431–440. doi: 10.1097/00002030-199604000-00012. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Ontogenesis of sensory function in birds and mammals. In: Tobach E, Aronson LR, Shaw E, editors. The Biopsychology of Development. New York: Academic Press; 1971. pp. 67–128. [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, LeBlanc S, et al. HIV- associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol Rev. 1980;87:175–189. [PubMed] [Google Scholar]
- Hoffman HS, Searle JL. Acoustic variables in modification of startle reaction in rat. J Comp Physiol Psychol. 1965;60:53–58. doi: 10.1037/h0022325. [DOI] [PubMed] [Google Scholar]
- Hord LL, Booze RM, Mactutus CF. Ontogeny of prepulse inhibition of the acoustic startle response across modality. Abstracts: International Society for Developmental Psychobiology, 41st Annual Meeting, November 12–15, 2008 Washington D.C. Dev Psychobiol. 2008;50:720–750. [Google Scholar]
- Ison JR, Hammond GR. Modification of startle reflex in rat by changes in auditory and visual environments. J Comp Physiol Psychol. 1971;75:435–452. doi: 10.1037/h0030934. [DOI] [PubMed] [Google Scholar]
- Kerr LM, Ostapoff EM, Rubel EW. Influence of acoustic experience on the ontogeny of frequency generalization gradients in the chicken. J Exp Psychol Anim Behav Process. 1979;5:97–115. doi: 10.1037//0097-7403.5.2.97. [DOI] [PubMed] [Google Scholar]
- Koralnik IJ, Beaumanoir A, Hausler R, Kohler A, Safran AB, Delacoux R, et al. A controlled-study of early neurologic abnormalities in men with asymptomatic human- immunodeficiency-virus infection. N Engl J Med. 1990;323:864–870. doi: 10.1056/NEJM199009273231303. [DOI] [PubMed] [Google Scholar]
- Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, Kumar M. Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. J Neurovirol. 2011;17:26–40. doi: 10.1007/s13365-010-0003-4. [DOI] [PubMed] [Google Scholar]
- Lalwani AK, Sooy CD. Otologic and neurotologic manifestations of acquired- immunodeficiency-syndrome. Otolaryngol Clin North Am. 1992;25:1183–1197. [Google Scholar]
- Matas CG, Silva SM, Marcon Bde A, Goncalves IC. Electrophysiological manifestations in adults with HIV/AIDS submitted and not submitted to antiretroviral therapy. Pro Fono. 2010;22:107–113. doi: 10.1590/s0104-56872010000200007. [DOI] [PubMed] [Google Scholar]
- Midde NM, Huang X, Gomez AM, Booze RM, Zhan CG, Zhu J. Mutation of tyrosine 470 of human dopamine transporter is critical for HIV-1 Tat-induced inhibition of dopamine transport and transporter conformational transitions. J Neuroimmune Pharmacol. 2013 doi: 10.1007/s11481-013-9464-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Aksenov MY, Booze RM, Webb KM, Mactutus CF. Adolescent HIV-1 transgenic rats: evidence for dopaminergic alterations in behavior and neurochemistry revealed by methamphetamine challenge. Curr HIV Res. 2012;10:415–424. doi: 10.2174/157016212802138788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Booze RM, Webb KM, Mactutus CF. Neurobehavioral alterations in HIV-1 transgenic rats: Evidence for dopaminergic dysfunction. Exp Neurol. 2013;239:139–147. doi: 10.1016/j.expneurol.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Mactutus CF, Booze RM. [Accessed 01 May 2013];Generality of disruption of prepulse inhibition by the dopamine agonist apomorphine. 2009 http://www.cpdd.vcu.edu/Pages/Meetings/CPDD09AbstractBook.pdf.
- Mozes MM, Bryant JL, Franks R, Chan CC, Kopp JB. Congenital nuclear cataracts and uveitis in HIV-transgenic mice. Eye. 2002;16:177–184. doi: 10.1038/sj.eye.6700101. [DOI] [PubMed] [Google Scholar]
- Ollo C, Johnson R, Grafman J. Signs of cognitive change in HIV disease: An event- related brain potential study. Neurology. 1991;41:209–215. doi: 10.1212/wnl.41.2_part_1.209. [DOI] [PubMed] [Google Scholar]
- Peng JS, Vigorito M, Liu XQ, Zhou DJ, Wu XW, Chang SL. The HIV-1 transgenic rat as a model for HIV-1 infected individuals on HAART. J Neuroimmunol. 2010;218:94–101. doi: 10.1016/j.jneuroim.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Pagano MA, Cahn PE, Garau ML, Mangone CA, Figini HA, Yorio AA, Dellepiane MC, Amores MG, Perez HM, Casiro AD. Brain stem auditory evoked-potentials in human immunodeficiency virus-seropositive patients with and without acquired-immunodeficiency- syndrome. Arch Neurol. 1992;49:166–169. doi: 10.1001/archneur.1992.00530260068022. [DOI] [PubMed] [Google Scholar]
- Phipps AJ, Hayes KA, Buck WR, Podell M, Mathes LE. Neurophysiologic and immunologic abnormalities associated with feline immunodeficiency virus molecular clone FIV-PPR DNA inoculation. J Acquir Immune Defic Syndr. 2000;23:8–16. doi: 10.1097/00126334-200001010-00002. [DOI] [PubMed] [Google Scholar]
- Prospero-Garcia O, Huitron-Resendiz S, Casalman SC, Sanchez-Alavez M, Diaz-Ruiz O, Navarro L, Lerner DL, Phillips TR, Elder JH, Henriksen SJ. Feline immunodeficiency virus envelope protein (FIVgp120) causes electrophysiological alterations in rats. Brain Res. 1999;836:203–209. doi: 10.1016/s0006-8993(99)01572-3. [DOI] [PubMed] [Google Scholar]
- Purohit V, Rapaka R, Shurtleff D. Drugs of abuse, dopamine, and HIV-1 associated neurocognitive disorders/HIV-associated dementia. Mol Neurobiol. 2011;44:102–110. doi: 10.1007/s12035-011-8195-z. [DOI] [PubMed] [Google Scholar]
- Raymond LA, Wallace D, Berman NEJ, Marcario J, Foresman L, Joag SV, Raghavan R, Narayan O, Cheney PD. Auditory brainstem responses in a Rhesus Macaque model of neuro-AIDS. J Neurovirol. 1998;4:512–520. doi: 10.3109/13550289809113495. [DOI] [PubMed] [Google Scholar]
- Riazi M, Marcario JK, Samson FK, Kenjale H, Adany I, Staggs V, Ledford E, Marquis J, Narayan O, Cheney PD. Rhesus macaque model of chronic opiate dependence and neuro-AIDS: Longitudinal assessment of auditory brainstem responses and visual evoked potentials. J Neuroimmune Pharmacol. 2009;4:260–275. doi: 10.1007/s11481-009-9149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal W, Zhang L, Guo M, Jones O, Davis H, Bryant JL. Immune activation, viral gene product expression and neurotoxicity in the HIV-1 transgenic rat. J Neuroimmunol. 2012;247:16–24. doi: 10.1016/j.jneuroim.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel EW, Rosenthal MH. Ontogeny of auditory frequency generalization in the chicken. J Exp Psychol Anim Behav Process. 1975;1:287–297. doi: 10.1037//0097-7403.1.4.287. [DOI] [PubMed] [Google Scholar]
- Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- Schroeder MM, Handelsman L, Torres L, Jacobson J, Ritter W. Consistency of repeated event-related potentials in clinically stable HIV-infected drug users. J Neuropsychiatry Clin Neurosci. 1996;8:305–310. doi: 10.1176/jnp.8.3.305. [DOI] [PubMed] [Google Scholar]
- Schroeder MM, Handelsman L, Torres L, Dorfman D, Rinaldi P, Jacobson J, Wiener J, Ritter W. Early and late cognitive event-related potentials mark stages of HIV-1 infection in the drug-user risk group. Biol Psychiatry. 1994;35:54–69. doi: 10.1016/0006-3223(94)91168-1. [DOI] [PubMed] [Google Scholar]
- Silvers JM, Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. Neurotoxicity of HIV-1 tat protein: involvement of D1 dopamine receptor. Neurotoxicology. 2007;28:1184–1190. doi: 10.1016/j.neuro.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tees RC. Perceptual development in mammals. In: Gottlieb G, editor. Studies on the development of behavior and the nervous system: (Vol. 3), Neural and behavioral specificity. New York: Academic Press; 1976. pp. 281–326. [Google Scholar]
- UNAIDS. UNAIDS World AIDS Day Report 2011. 2011 http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2011/JC2216_WorldAIDSday_report_2011_en.pdf.
- Vigliano P, Boffi P, Bonassi E, Gandione M, Marotta C, Raino E, Russo R, Rigardetto R. Neurophysiologic exploration: A reliable tool in HIV-1 encephalopathy diagnosis in children. Panminerva Med. 2000;42:267–272. [PubMed] [Google Scholar]
- Wallace DR, Dodson S, Nath A, Booze RM. Estrogen attenuates gp120-and tat(1–72)-induced oxidative stress and prevents loss of dopamine transporter function. Synapse. 2006;59:51–60. doi: 10.1002/syn.20214. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Chang L, Volkow ND, Telang F, Logan J, Ernst T, Fowler JS. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004;127:2452–2458. doi: 10.1093/brain/awh269. [DOI] [PubMed] [Google Scholar]
- Werner H. Comparative psychology of mental development. Chicago: Follett; 1948. [Google Scholar]
- Zhu J, Ananthan S, Mactutus CF, Booze RM. Recombinant human immunodeficiency virus-1 transactivator of transcription(1–86) allosterically modulates dopamine transporter activity. Synapse. 2011;65:1251–1254. doi: 10.1002/syn.20949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Mactutus CF, Wallace DR, Booze RM. HIV-1 Tat protein-induced rapid and reversible decrease in [3H]dopamine uptake: dissociation of [3H]dopamine uptake and [3H]2beta-carbomethoxy-3-beta-(4-fluorophenyl)tropane (WIN 35,428) binding in rat striatal synaptosomes. J Pharmacol Exp Ther. 2009;329:1071–1083. doi: 10.1124/jpet.108.150144. [DOI] [PMC free article] [PubMed] [Google Scholar]