Abstract
The link between mechanics and biology in generation and adaptation of bone has been studied for more than a century in the context of skeletal development and fracture healing. However, the interplay between mechanics and biology in de novo generation of bone in postnatal defects as well as healing of morcellized bone graft or massive cortical bone autografts is less well understood. To address this, here we integrate insights from our previously published studies describing the mechanobiology on both de novo bone generation and graft healing in a common ovine femoral defect model. Studying these effects in a common experimental model provides a unique opportunity to elucidate factors conducive to harnessing the regenerative power of the periosteum and ultimately to provide mechanistic insights into the multiscale mechanobiology of bone generation, remodeling and adaptation. Taken together, the studies indicate that, as long as adequate, directional transport of cells and molecules can be insured (e.g. with peristeum in situ or a delivery device), biological factors intrinsic to the periosteum suffice to bridge critical sized bone defects, even in the absence of a patent blood supply. Furthermore, mechanical stimuli are crucial for the success of periosteal bone generation and bone graft healing. Interestingly, areas of highest periosteal strain around defects correlate with highest areas albeit less mineralized areas of new bone. This may indicate a role for convection enhanced transport of cells and molecules in modulation of tissue generation by pluripotent cells that ingress into the defect center, away from the peristeum and toward the surface of the intramedullary nail that fills the medullary cavity. These insights bring us much closer to understanding the mechanobiological environment and stimuli that stimulate the proliferation and differentiation of periosteum derived progenitor cells and ultimately drive the generation of new bone tissue. Furthermore, these insights provide a foundation to create virtual predictive computational models of bone mechanophysiology, to develop cell seeding protocols for scale up and manufacture of engineered tissues, to optimize surgical procedures, and to develop post-surgical therapies with the ultimate goal of achieving the best possible healing outcomes for treatment and/or reconstruction of postnatal bone defects.
1. Background
A newly described one stage bone transport procedure for segmental defect repair provides a unique healing scenario to study the multiscale (cell → tissue length scales and days → weeks → months time scales) mechanobiology of bone generation, as well as remodeling and adaptation of autograft in a common in vivo ovine femur model exposed to similar loading patterns among and between experimental groups (Fig. 1,2) (Knothe and Springfield, 2005; Knothe Tate et al., 2007). The experimental model is designed to mimic a clinical scenario in which a critical sized bone defect arises from tumor resection, traumatic injury, debridement after infection, or congenital malformation. One permuation of the model involves a single stage bone transport procedure to treat a critical sized defect (Fig. 2A-D) (after Knothe and Springfield, 2005); after creation of a critical sized defect (2.54 cm) in the middiaphysis of the ovine femur, periosteum proximal to the defect is peeled back and the underlying bone is osteotomized and transferred distally along the previously placed intramedullary nail to fill the defect zone. The transported bone segment is thereby denuded of periosteum and cut off from the blood supply, acting essentially as a massive bone autograft. It is anchored in place with ligament sutures and intramedullary nailing for mechanical stabilization. In transferring the denuded massive autograft bone distally, a new defect is created and the periosteum that was peeled back is now sutured in situ to envelop the new defect zone. A second permutation of the experimental model (Fig. 2E) involves the simple creation of a middiaphyseal, critical sized defect that is treated with a newly developed surgical reconstruction membrane in the adult ovine femurwith intramedullary nailing for mechanical stabilization (Knothe Tate et al., 2010B).
Figure 1.
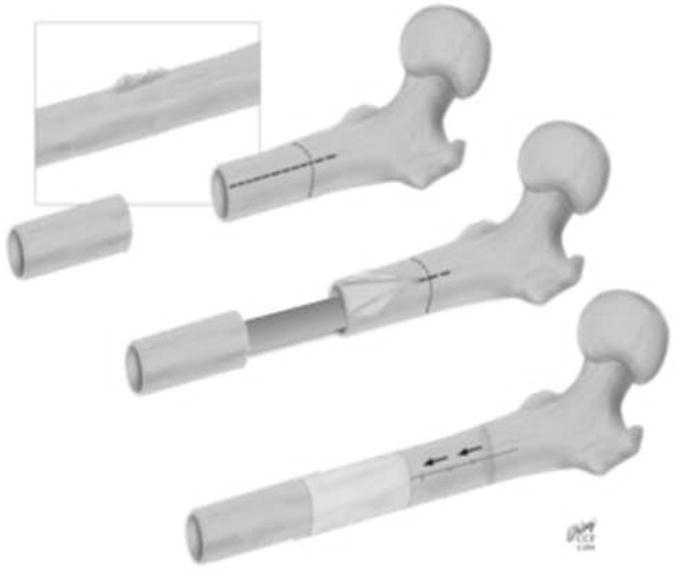
Schematic diagram of the one stage bone transport procedure after Knothe (Knothe and Springfield, 2005; Knothe Tate et al., 2007). Proximal to the defect, the surgeon gently peels back the periosteum, exposing the cortical bone beneath. The denuded section of bone is osteotomized and translocated distally to fill the original defect zone. The periosteum is sutured around the newly created defect zone. The entire construct is stabilized by an intramedullary nail.
Figure 2.
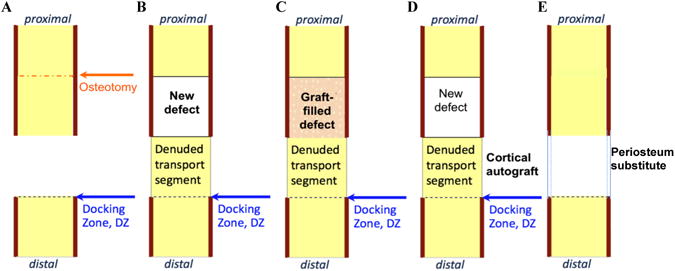
The ovine critical sized defect model (implemented in the femur for this study) provides multiple healing scenarios using a common surgical technique, enabling study of healing in the original critical sized defect (A), the newly created defect surrounded by periosteum (B), healing of a defect packed with morcellized autograft (C), and healing of a massive cortical autograft denuded of periosteum (D). This paper integrates and reviews these approaches to understanding de novo bone generation in defects (A,B), as well as bone remodeling and adaptation (C-D) under different conditions in a common surgical model. Figure adapted after (Knothe Tate et al., 2010A).
Although the first permutation of the in vivo model was developed primarily to provide a new method to treat critical sized long bone defects in a single index procedure, the model provides a unique means to study different aspects of bone healing after surgical reconstruction, including periosteum-derived bone regeneration in a critical sized defect (Knothe Tate et al., 2007; Knothe et al., 2010), autograft healing within the defect (Knothe Tate et al., 2007; Knothe et al., 2010A), massive cortical autograft healing at the docking zone (Knothe Tate et al., 2010A), periosteal regeneration in the denuded massive autograft segment (Yu et al., 2011). The second permuation of the in vivo model allows for periosteal substitution to elucidate effects of specific periosteal factors on de novo bone generation (Knothe Tate et al., 2010B). Study of cell and tissue scale generation, adaptation and remodeling of bone within the defect zone surrounded by periosteum (Fig. 2B) allows for elucidation of mechanobiological effects on de novo bone generation by progenitor cells that reside within the periosteum. When the defect zone is filled with morcellized cancellous bone graft from the iliac crest (Fig. 2C), mechanobiology of autograft healing can be studied within the defect zone. Furthermore, in all groups treated using the one stage bone transport procedure, healing of a massive cortical bone autograft devoid of periosteum and vasculature (Fig. 2D) can be elucidated. Finally, efficacy of a novel periosteum substitute implant cum delivery device can be tested if the substitute periosteum is used in place of the natural periosteum to envelop the defect; using the modular periosteum substitute, the role of individual and combinations of periosteal factors on defect healing can be elucidated.
Here we review the results of previously published studies reporting data after the so-called one stage bone transport procedure and periosteum substitution procedure; specifically, we integrate the results regarding de novo bone generation in the defect zone via periosteum-derived mesenchymal stem cells, defect healing in presence of morcellized autograft, healing of the massive, denuded transport segment, optical measurement of tissue and cell scale strains in the periosteum (Knothe and Springfield, 2005; Knothe Tate et al., 2007; Knothe et al., 2010; Knothe Tate et al., 2010A,B; McBride et al. 2010A,B), in light of the current state of the art in bone mechanobiology. Our goal is to integrate insights related to the interplay between biology and mechanics in context of a common ovine model, which provides a unique opportunity to elucidate factors conducive to harnessing the regenerative power of the periosteum to generate tissue where there is none, ultimately providing mechanistic insights into the multiscale mechanobiology of bone generation, remodeling and adaptation. Our overarching hypothesis is that both proximity to factors inherent to periosteum as well as prevailing mechanical loading patterns modulate the generation of new bone as well as the remodeling and adaptation of autograft in the different healing scenarios presented by the common ovine femur surgical model.
2. Biology of Periosteum Derived De Novo Bone Generation
The periosteum is a specialized tissue that contributes to new bone formation by providing a population of osteoprogenitor cells and an abundant plexus of blood vessels, both of which are essential to new bone formation and fracture healing (Thomson et al., 1999; Knothe Tate et al., 2007). The periosteum derives its vascularity from the neighboring muscles and soft tissue, ringing and perforating the cortical bone and anastomosing with the intramedullary blood supply. In healthy cortical bone, approximately 3/4 of the cortex's blood supply derives from the intramedullary cavity and the remaining 1/4 of the cortical blood supply from the periosteum (Brookes, 1998). In the current studies using the ovine femoral defect model (Knothe Tate et al. 2007), the intramedullary cavity is filled completely by the intramedullary nail after reaming, rendering the periosteum and surrounding soft tissues as the predominant source of vascularity for the defect and massive autograft docking zones. The current and several previous studies have shown that the periosteal blood supply is capable of sustaining the diaphyseal bone when the medullary nutrient artery has been obliterated by trauma, disease, or surgical instrumentation (Eyre-Brooke, 1984; Brookes and Revel, 1998; Ueno et al., 2001). Furthermore, previous studies indicate that the periosteal blood supply is an essential component for periosteally derived osteogenesis (Burstein and Canalis, 1985; Jupiter et al., 1997; Liu et al., 1994A,B; Ueno et al., 2001A,B), although, as indicated below, our recent study using a periosteum substitute with vectorial transport properties as a delivery vehicle indicates otherwise.
The periosteum is a membrane or sleeve that provides a microenvironment both for the progenitor cells that inhabit it as well as for the cells residing in bone enveloped by it. The periosteal membrane exhibits anisotropic architecture, both in elastic modulus (higher along the length of the ovine femur than along its circumference) and in shrinkage upon release from the underlying bone (more shrinkage in the longitudinal than in the circumferential direction) of the ovine femur (McBride et al. 2010A,B; 2011). The periosteum is organized as a two-layered membrane characterized by matrix proteins i.a. collagen and elastin in the outer layer and the cell rich cambium layer within (Allen et al. 2004, Allen and Burr 2005). As such, the periosteal membrane per se exhibits putative vectorial barrier function, controlling the magnitude and direction of transport for inductive factors inherent to muscle overlying the periosteum, the periosteum itself, and bone tissue (Knothe Tate et al. 2010B).
Although the relative contribution of muscle-derived, periosteum-derived, and bone-derived factors to bone healing after trauma and surgical reconstruction has yet to be elucidated, recent studies using a periosteum substitute membrane cum delivery device provide a unique means to address the research question (Knothe Tate et al., 2010B). The newly developed surgical reconstruction membrane was developed as a substitute for the periosteum to treat a critical sized (2.54 cm) defect using the adult ovine femur model with intramedullary nailing for mechanical stabilization. Acting as a delivery device cum implant, the periosteum substitute exhibits a modular design with pockets to allow for spatial and temporal control of periosteal factor delivery and integrating a specially designed architecture to enable vectorial transport of molecules and cells longitudinally, from edges of the defect to the midline, and radially, from the implant to the surface of the intramedullary nail, through the entire defect zone. The outer layer of the implant is essentially impermeable along its length except along the suture seams that create the pockets. The inner layer of the implant is permeable, with a pore gradient decreasing longitudinally from the midline to the edges of the defect zone.
Using the periosteum substitute implant cum delivery device, it was shown that the addition of increasing numbers of periosteal factors results in increasing bone regeneration within the defect zone (Fig. 7B). Specifically, periosteal strips tucked into the pockets showed superior defect bridging to periosteum-derived cells seeded on collagen sponges tucked into the pockets and collagen sponges alone, respectively. The superior healing observed with periosteal strips tucked into the implant indicate that vectorial delivery of periosteal factors alone may be sufficient to ensure bony bridging of critical sized bone defects, even in absence of a patent blood supply. Of note, the superior healing response was observed in absence of transport from the muscle tissue to the defect zone; although the muscle would normally surround the periosteum, virtually all transport from that direction was blocked by the outer impermeable membrane in this study. Furthermore, the superior healing response to the implant with periosteal strips was observed in absence of a patent blood supply, although the periosteum implant was designed to facilitate longitudinal transport from the distal and proximal edges of the defect toward the midline of the defect, as well as radial transport from the implant surface to the outer surface of the intramedullary nail. Although placing collagen sheets within the modular implant did not appear to facilitate long term (16 weeks after surgery) healing compared to isotropic elastic membranes alone, seeding of the collagen sheets with autologous cells isolated from periosteal tissue (resected to form the defect) prior to implantation did show improved defect bridging. Interestingly, short term healing (woven bone observed at 3 weeks after surgery) of the substitute periosteum group with collagen sheets alone was superior to the isotropic membrane control; studies are underway to understand why this early bone was resorbed rather than remodeled at later timepoints (Moore et al., 2011). In sum, based on this novel study, periosteal factors including periosteum derived cells and factors intrinsic to periosteal transplants, not including collagen alone, are sufficient to bridge critical sized defects in ovine femoral defect model, even in the absence of a patent blood supply. (Knothe Tate et al., 2010B).
Figure 7.
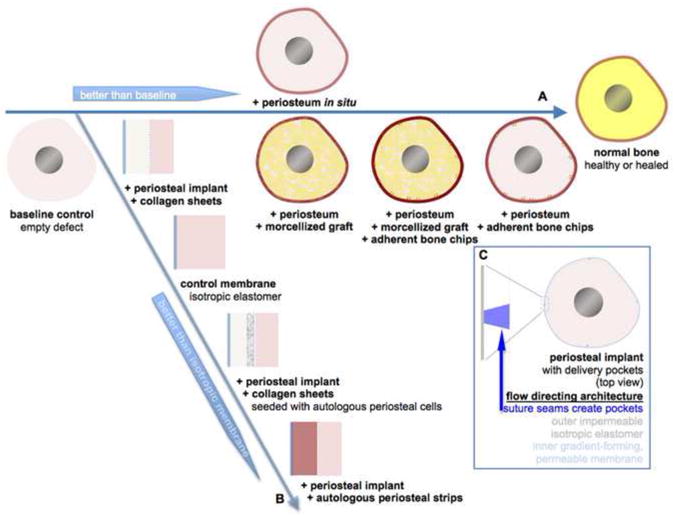
Biological factors conducive to de novo bone generation in a critical sized defect after the one stage bone transport procedure (horizontal axis) and in a critical sized defect surrounded by a periosteum substitute implant cum delivery device with modular design to control the vectorial delivery of specific periosteal factors.
In addition to the previously described study as well as clinical case descriptions (Camilli and Penteado, 1984; Liu et al., 1994A,B; Eyre-Brook et al., 1994; Knothe and Springfield, 2005), significant experimental work documents the osteogenic capacity of the periosteum. Periosteal and osteoperiosteal grafts have been used experimentally in the treatment of fractures, osseous defects, and pseudarthroses (Camilli and Penteado, 1984; Liu et al., 1994A,B; Hertel et al., 1997; Klaue et al., 1998; Klaue et al., 2009; Knothe Tate et al., 2010B). Uddstromer demonstrated that in situ tibial periosteum restores cortical defects in rabbits with “mature appearing woven bone” (Uddstromer, 1978; Uddstromer and Ritsila, 1978). Takato et al. corroborated the osteogenic capacity of isolated periosteum in a rabbit rib model where bone was consistently regenerated in 5 cm defects (Takato et al., 1998). Nishimura et al. further confirmed the osteogenic potential of periosteum using a free periosteal flap in a rabbit model (Nishimura et al., 1997); Nishimura et al.'s work demonstrated not only that osteoprogenitor cells, arising from the inner cambial layer of the periosteum, differentiate into osteoblasts, but also that the periosteum is a source of BMP-2 which has been shown to play a significant role in osteoinduction. More recently, cells derived from bovine, chick and ovine periosteum have shown osteogenic capacity in culture (Takushima et al. 1998, Nakahara et al. 1990) and in vivo (Knothe Tate et al., 2010B).
3. Use of Periosteum in Conjunction with Bone Graft
Implementation of adjuvant bone graft has shown contradictory effects on the osteogenic capacity of the periosteum. Several studies have demonstrated superior bone formation from grafted periosteum in association with autogenous morcellized cancellous bone graft compared to grafted perosteum alone (Camilli and Penteado, 1994; Romana and Masquelet, 1990; Vogelin et al., 2002). Thomson et al. reported new bone formation only in areas containing morcellized bone graft directly in contact with periosteum. Morcellized bone graft is a putative promoter for “healing of osseous defects because it provides a porous degradable scaffold to conduct new tissue and an array of bone induction factors that are stored in the non-calcified portion of the extracellular matrix” (Thomson et al., 1999). Contrary to these studies, we observed the presence of bone graft in the periosteum-enveloped defect to retard de novo bone formation in studies using the one stage procedure (Fig. 3) (Knothe Tate et al., 2007; Knothe et al., 2010), likely because graft must first be resorbed before new bone can be laid down by osteoblasts. Although we observed more new woven bone formation in areas of the defect in closest proximity to the periosteum, as suggested by Thomson et al., it appeared that cells from the periosteum did not ingress into the graft, rather that the cells were able to generate small amounts of new bone in the space between the periosteum and the outer edge of the pack graft in the defect zone (Fig. 4) (Knothe et al., 2010).
Figure 3.
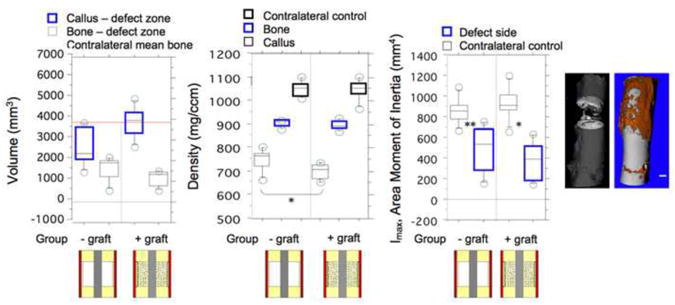
Microcomputed tomographic measures of bone density, volume and area moment of inertia in the defect zone at 16 weeks after surgery, after (Knothe Tate et al., 2007), for the experimental groups in which small cortical bone chips were left adherent to the inner surface of the periosteum and the defect zone was either filled with morcellized bone autograft from the iliac crest or left empty. These two experimental groups were clinically superior to all other groups studied. All experimental groups bridged the defect zone within the 16 week study period (far right microCT), and the defect persisted in the control group which was not treated, proving that the defect was indeed critically sized (left microCT).
Figure 4.
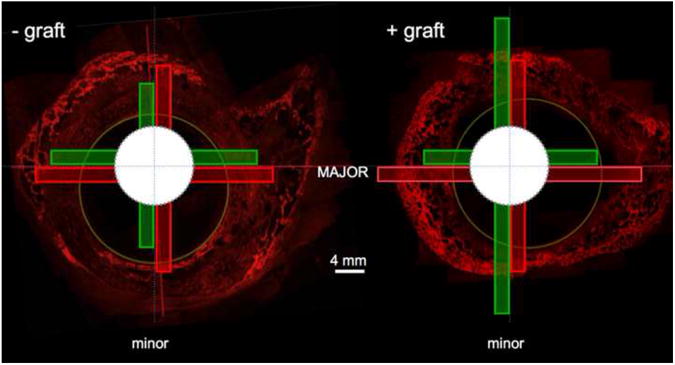
Confocal micrographs showing early bone formation (green) in empty defects surrounded by periosteum (A) and defects packed with morcellized cancellous autograft (B). In the presence of autograft, new bone formation predominates between the outer edge of the autograft and the inner surface of the periosteum. In the absence of autograft, a rapid proliferative woven bone response is observed to fill the defect within the first weeks after surgery.
Interestingly, retention of sparse, mm-sized cortical bone chips on the inner surface of the periosteum greatly enhanced periosteum-derived de novo bone formation in the defect zone. Our working hypothesis is that the bone chips provided inductive factors, not present or not efficiently transported from the periosteum devoid of bone chips, but did not impede ingression of periosteum derived cells into the defect zone. Romana and Masquelet first reported that small pieces of cortical bone could be raised with the periosteum and allowed to remain attached to the periosteum (Romana and Masquelet, 1990). In testing our one stage bone transport procedure using the previously described ovine critical sized defect model, bone and callus volume, density and area moments of inertia were measured for each group, at a tissue length scale, using a high resolution micro-computed tomograph (μCT, Scanco AG, Fig. 3). When implemented in conjunction with our one stage bone transport procedure, we showed that retention of cortical bone chips to the periosteum significantly increased the density of bone generated within the defect zone, compared to defects surrounded by periosteum alone (not shown) as well as those periosteum enveloped defects that were packed with cancellous bone graft (Fig. 3) (Knothe Tate et al., 2007). However, no significant differences were observed between groups with respect to the volume and area moment of inertia (a surrogate for mechanical testing) of bone generated within the defect zone.
Taken together, the presence of some vascularized bone appears to increase the osteogenic capacity of the periosteum, but filling defects with bone graft may delay de novo bone formation due to the volume of devitalized bone that must first be resorbed prior to laying down new bone (Knothe Tate et al., 2007; Knothe et al., 2010). This result can be explained mechanistically in terms of maturity of the regenerate tissue. Direct ingression of bone building cells from the periosteum into the defect allow for more rapid extracellular matrix (osteoid) deposition, with the potential to fill the entire defect zone with proliferative woven bone within two weeks of surgery. The earlier formation of osteoid provides more time for mineralization and a subsequent increase in density at later time points compared to experimental groups with delayed initial bone formation. At greater length and time scales, the earlier bone is laid down, the early the initiation of bone remodeling and progress of mechanoadaptation.
4. Role of Mechanical Loading in De Novo Bone Generation by Periosteal Cells
Several studies have documented the importance of dynamic internal fixation of bone to facilitate not only a rapid return to original physiological function but also to enhance biological healing processes (Matter, 1998; Ruedi et al., 2000). However, although a plethora of studies describe the role of mechanical loading in healing of fractures, the role of mechanical loading in the osteogenic capacity of the periosteum is less well understood. In canine studies, Puckett et al. discovered a 62% decrease in the capacity of grafted periosteum to regenerate bone in diaphyseal defects when transferred to the non-weight bearing fibula compared to the tibia (Puckett et al., 1979). Puckett et al. concluded that mechanical stress transfer through weight bearing is a necessary stimulus for the periosteum to form new bone. This is further supported by data showing that, when grafted to a defect in a weight-bearing site, calvarial periosteum forms bone with five times the mechanical strength of that resulting from in situ grafting in the calvarium (Uddstromer and Ritsila, 1978). Finally, experimental studies in which controlled three and four point bending loads are applied to long bones in animal models of various species further demonstrate the osteogenic capacity of the periosteum in conjuction with mechanical loading (Raab-Cullen 1994; Cullen et al., 2000) although some of these models discount bone generated underneath the periosteum as an artifact of direct pressure placed on the periosteum to induce four point bending (Turner, 1994; Forwood, 1998).
Finally, in consideration of our recent studies demonstrating the exquisite mechanosensitivity of mesenchymal stem cells compared to terminally differentiated cells, such as osteoblasts and chondrocytes, (Knothe Tate et al., 2008; McBride and Knothe Tate, 2008; McBride et al., 2008), we proposed that mechanical loads prevailing in the postoperative period modulate the amount and time course of bone regeneration within the defect after implementation of the one stage bone transport procedure. To determine whether de novo bone generation in critical sized defects surrounded by periosteum in situ correlated to mechanical loading patterns in the first two weeks after surgery, we calculated the second moment of areas for cross sections of bone prepared from the middiaphysis. We then measured the amount of bone formed between one and two weeks after the surgical procedure, along the major and minor centroidal axes, which have been validated recently (Knothe et al., 2010; Knothe Tate et al., 2010A) as a good basis for comparison of predominant mechanical loading modes among bones from experimental cohorts (Lieberman et al., 2004). Finally, we measured the degree of perfusion at 16 weeks after surgery.
Testing of mechanical modulation was carried out by determining significant correlations between early bone apposition (first two weeks after surgery) and late measures of healing (perfusion at 16 weeks after surgery), and prevailing loading patterns, as defined by the major and minor centroidal axes of bending. The centroidal axes of bending are the axes of the long bone about which the bone is most (major CA) and least able (minor CA) to resist bending, respectively (Lieberman et al., 2004); measurement along the major and minor CA provides a means to compare areas exposed to similar loading patterns in the absence of experimental measures of strain and determination of neutral axes. Quantitative measures of bone resorption area and infilling resorption spaces are assessed to determine relative levels of remodeling compared to baseline controls.
If one considers a beam in bending, the major centroidal axis would correspond to the strongest plane in bending and the minor centroidal axis would correspond to the weakest plane, in which buckling first occurs. We quantified the amount of bone and the amount of perfusion along the minor and major centroidal axes of cross sections from the defect zone in samples from experimental Groups 4 and 5 (Fig. 5). Bone apposition could be assessed with respect to time and location, as fluorescent-tagged mineral chelating agents (fluorochromes) were administeredintravitally at specific time intervals during the experiment. Bone perfusion was evaluated quantitatively by measuring the distribution of alizarin red, a fluorescent marker that was administered immediately prior to euthanasia.
Figure 5.
Area of early bone apposition and late bone perfusion along the minor and major centroidal axes in the experimental groups without graft and with graft filling the defect zone. Figure adapted after (Knothe et al., 2010).
Based on quantitative measurement of the spatial distribution and intensity of fluorochromes marking early bone apposition and late measures of bone perfusion, proximity to the periosteum and the lack of graft within the defect zone enhance the amount and rate of bone generation and remodeling within the critical sized long bone defect. Areas of the cross section least able to resist bending loads show a higher degree of mineralization albeit less volume of bone in the first two weeks of healing compared to areas most able to resist bending loads. However, for areas of bone subjected to similar loading patterns, the amount and the quality of new bone produced in the first weeks after surgery is not significantly different between groups treated with or without bone graft. In contrast, both the radial distribution of bone apposition in the first two weeks after surgery, as well as the perfusion of bone 16 weeks after surgery, are significantly affected by both the presence of bone graft as well as by prevailing loading patterns. The presence of bone graft retards initial bone formation in the defect zone, as measured by a steeper average slope in bone distribution between the periosteum and the IM nail. The presence of bone graft is also associated with less evenly distributed tissue perfusion at 16 weeks after surgery. Finally, although baseline levels of remodeling are similar, the maturity of the regenerated bone within the defect zone is higher in bones not treated with bone graft, compared to the healing defect zones which are packed with morcellized bone graft. (Knothe Tate et al., 2010A)
5. Optical Measurement of Periosteum's Mechanical Load-Induced Strain In Situ
Mechanical loading is a known promoter of bone regeneration and adaptation; mechanotransduction is thought to occur both directly through matrix deformation and load-induced fluid flow that is transduced to the cellular level as well as indirectly through augmentation of molecular transport via fluid flow (load-induced fluid flow results in convective transport that significantly enhances molecular transport through bone) (refer to Knothe Tate et al., 2008; Knothe Tate, 2003 for recent reviews of these subjects). However, the degree to which mechanical loads are transduced via direct deformation (strain) of tissue (matrix) and indirect flow of tissue fluid is unknown due to the complexities of measuring spatiotemporally varying strains in real time, in the living animal.
Due to its natural curvature, the sheep femur is exposed to bending loads during gait, and the anterior, medial, lateral and posterior aspects of the middiaphysis are exposed to varying magnitudes of compression and/or tension. Although strain gauges can be applied to the outer surface of the femoral cortex, the periosteum must be removed and the surface of the bone defatted in order to insure adequate adhesion of the gauge for accurate strain measurement (Steck et al., 2003). Furthermore, strains occurring within the cortex cannot be measured directly but rather can be deduced from finite element models using boundary conditions that can be validated using experimental strain gauge measurements. Nonetheless, it is impossible to measure strain in situ in the periosteum using experimental mechanics tools such as strain gauges and the prevailing mechanical environment within a defect zone is completely unknown.
Hence, we adapted high resolution, optical strain measurement methods to measure in situ strains in an ex vivo preparation of the femur designed to mimic the physiologic environment of the defect and surrounding periosteum during stance shift loading in the first two weeks after the one stage bone transport procedure. We hypothesized that mechanical strain of the periosteum correlates to areas in which highest bone formation was observed in histological sections of the defect zone. Sheep femora were treated surgically (IACUC approved in the Canton of Grisons, Switzerland), identical to previous studies, and then subjected to compression via the femoral head, while strains were measured optically using high definition digital TV camera (PDW-7000, Sony Co., NY) and the image correlation function of MatLab (Mathworks, Natick, MA). Strains from all views for all bones (n=4) were evaluated and compiled to estimate the strain distribution around the circumference of the central periosteal flap at maximum load. Then, histological samples from a parallel in vivo study were analyzed to relate areas of new bone formation to the strain distribution measurements of the current study. Cross sections (4 sections per bone, n = 5 bones) from the center of the defect zone were imaged at high resolution (Leica DMIRE2, Mannheim, Germany) and analyzed using MatLab to determine the spatial distribution of the calcein green label, the intravitally administered fluorochrome label that chelated to mineralizing bone at 1-2 weeks, with respect to the anterior, posterior, medial and lateral axes. Image analysis allowed us to determine relationships between strain distribution and initial bone apposition in the one-stage bone transport technique.
Interestingly, using these optical imaging methods, we found that treatment with the one stage bone procedure is associated with a complete shift in predominant loading modes of the anterior and posterior aspects of the femoral periosteum in the area of the defect zone (McBride et al., 2010). Whereas the normal femur is subject to tension along the anterior aspect and tension along the posterior aspect during stance shift loading, our optical imaging methods show that the periosteum surrounding the defect zone in the anterior aspect of the femur is subjected to compression and the posterior aspect of the femur is subjected to tension under stance shift loading after surgical treatment. In both the untreated and treated case, cells within the periosteum surrounding the area corresponding to the critical sized defect are exposed to a spatially and temporally varying strain field as the sheep bears weight, the change in the local strain environment experienced by cells during stance loading was shown to correlate significantly to areas exhibiting greatest amounts of new bone apposition after treatment (Fig. 4, 5). These studies indicate that the change in the local strain environment, attributable to treatment with the surgical procedure, provides a potent stimulus for the cells to adapt. (McBride et al., 2010)
6. Role of Mechanics in Healing of Massive, Bone Autografts Denuded of Periosteum
Finally, we were interested in determining the role of mechanical loading on healing of the massive bone allograft segment that was transported after denudation of the periosteum. To our knowledge, no published experimental studies had addressed this issue since it was first raised by Wheeler and Thomas, respectively, in 1919 (Wheeler, 1919; Thomas, 1919; Knothe Tate et al., 2010A). We hypothesized that proximity to healthy periosteum and prevailing mechanical loads influence the healing of the transported bone segment, much like they significantly influence the generation of new bone and healing of autograft in the defect zone of different experimental groups tested with the common ovine model. We carried out high resolution histomorphometric measures of bone apposition and perfusion in serial bone cross sections, taking particular note of the location of each specimen with respect to the docking zone (which could be ascertained precisely based on high resolution micro-computed tomograph and faxitron [radiographic] data). In these first studies, sections were obtained from a subset (n = 3) of the experimental group in which the defect was surrounded by healthy periosteum with adherent vascularized bone chips, as described above.
With regard to the massive, denuded cortical bone autograft, at 16 weeks after surgery, no significant differences in bone perfusion or early bone apposition were observed between sections proximal to, within, and distal to the transport segment docking zone. Qualitatively, bone in each of these respective areas was well perfused, both vascularly as well as pericellularly, and was highly remodeled. When all cross sections were pooled (from distal to proximal through the docking zone) and normalized for total bone area, significant differences in the amount of early proliferative woven bone could be related to prevailing mechanical loading patterns (minor versus major CA). In contrast no such significant differences in normalized perfusion area could be attributed to prevailing loads. Finally, significant differences in perfusion and early bone apposition were observed in relation to increasing radial distance from the outer bone surface, with a decrease in both perfusion and areas of early proliferative woven bone persisting at 16 weeks after surgery. Based on these quantitative and qualitative data, bone healing was roughly equivalent in the transported bone segment, the docking zone, and the distal femur, which is a key factor in translating the procedure to the clinic. (Knothe Tate et al., 2010A)
7. Discussion
Study of de novo bone generation and bone healing in segmental defects, in the absence and presence of the periosteum, morcellized cancellous bone graft, and/or vascularized adherent bone chips, as well as the healing of massive cortical bone grafts denuded of periosteum and devoid of a patent blood supply, in a common ovine femur model, provides a unique opportunity to unravel the roles of specific endogenous biological and biophysical factors on bone health, healing and generation. Here we reviewed and integrated quantitative measures of early bone apposition and late measures of bone perfusion in bone generated de novo within the defect zone surrounded by periosteum or a periosteum substitute, bone healing with morcellized autograft in the defect zone, and healing of a massive bone autograft to unravel biological and mechanical factors conducive to bone generation, healing and adaptation in space and time (Fig. 7A).
The role of biologic factors
As one might expect, biological factors factors play a key role in generation, healing and adaptation of bone, whether it is newly generated or grafted or injured. However, based on integration of results from the studies presented in this manuscript, the factors determined to be neccessary and/or conducive to bone healing and health in the one stage bone transport procedure and the critical sized defect model testing the periosteum substitute membrane are somewhat surprising given the current state of the art in clinical treatment and bone research (Fig. 7). Taken together, the studies presented here indicate that packed, morcellized bone graft retards the ingression of osteochondroprogenitor cells from the periosteum as well as healing (manifested as remodeling and adaptation) of the defect zone. Interestingly, the presence of small, vascularized adherent bone chips to the inner surface of the periosteum enveloping an “empty” defect (the defect actually fills with haematoma during surgery), provides sufficient inductive factors while maintaining ample space for unimpeded cell ingression, to enable filling of the entire defect with proliferative woven bone within two weeks of surgery. We know that pluripotent cells ingress from the periosteum and not the bone marrow since the medullary cavity was reamed and filled with an intramedullary rod at the time of surgery. Furthermore, given the successful healing of the experimental group with the defect surrounded by periosteum alone, the fibrin network of the haematoma that fills the defect zone during surgery appears to provide adequate scaffolding and biochemical gradients for pluirpotent cell navigation into the defect zone, away from the nearest blood supply, even in absence of inductive factors provided by adherent, vascularized bone chips.
Interestingly, if molecular transport and the formation of biochemical gradients can be maintained, e.g. through use of a periosteum substitute implant incorporating flow directing technology, bone defects heal even in the absence of a patent blood supply normally insured by presence of the periosteum and the intramedullary niche (Fig. 7B). In combination with the possibility for vectorial delivery of factors (controlling magnitude and direction of transport), factors intrinsic to the periosteum itself appear to play the greatest role in bridging of critical sized defects presented in these studies. Even when cut off from the patent blood supply as well as in absence of mechanical signals intrinsic to the native periosteum's attachment to underlying bone via Sharpey's fibers, directional delivery of factors intrinsic to periosteal transplants results in superior defect bridging compared to all other groups studied; we speculate that both the cells as well as factors intrinsic to the extracellular matrix of periosteum promote bone generation and healing in this experimental group. Even in absence of their natural environment, directed delivery of cells derived from the periosteum promotes defect bridging. Studies are underway to further elucidate mechanisms underlying success of this novel periosteum substitute implant cum delivery device (Moore et al., 2011).
The role of mechanics
In addition to the importance of biological factors noted above, mechanics plays an important role in harnessing the regenerative potential of the periosteum to generate and heal bone in bone defects and massive bone autografts. Interestingly, the distribution and quality of bone generated within empty defects as well as defects filled with morcellized autograft appears to depend on biophysical signals experienced by cells within. For example, early proliferative woven bone formation emanating from the periosteum of an empty critical sized defect is higher along the major centroidal axis than along the minor centroidal axis, and early bone quality (density, as measured by concentration of fluorochrome) is higher along the minor centroidal axis than along the major centroidal axis. These contrary effects may provide mechanistic clues regarding cellular bone generation and remodeling and indicate a significant role for mechanical modulation of de novo bone formation by resident mesenchymal stem cells in the periosteum (Knothe et al., 2010). Similarly, ex vivo high resolution optical imaging methods show that areas of greatest change in mechanical strain correlate to areas of the defect cross section in which early areas bone generation is greatest (McBride et al., 2010A,B).
Taken together, our current working hypothesis is that both direct and indirect effects of strain result in spatial and temporal transport of bone building cells that result in higher distribution of bone building cells in areas experiencing higher strains and in higher concentration bone mineral (degree of ossification) in areas experiencing lower strains where bone buildingcells are less distributed. Given that the defect zone is filled by hematoma and bounded by the periosteum, comprising a poroelastic system, oscillatory strain will exert direct and indirect mechanical effects at both tissue and cellular length scales. On a tissue length scale, oscillatory strain creates a convective flow similar to that seen in whole bone four point bending experiments (Knothe Tate et al., 2000) or in fluid-filled, elastic cylinders under cyclic loading. (Munro, 1976; Piekarski and Munro, 1977). Compressive strain (or relaxation of tension) creates a high-pressure area effectively “pushing” fluids (plasma, blood, etc., that carry cells and molecules) out of that area. Similarly, tensile strain (or relaxation of compression) creates a low-pressure area effectively “pulling” biological fluids into that area. In both situations the fibrous layer of the periosteum acts as a barrier, forcing fluidic movement through the less resistant hematoma towards the center of the defect. Resultant convective fluid flow then in turn affects early bone formation on the cellular level. Furthermore, recent studies show that mechanical strain enhances persistence of collagen micronetworks in the presence of collagenase (Bhole et al., 2009), the changes in strain fields resulting from the one stage bone transport procedure may also directly modulate remodeling of the collagen network in the first weeks after surgery.
Oscillatory strain of the poroelastic defect zone will also exert direct and indirect effects on cells. First, the actual strain or change in strain of the periosteum could be transmitted to adherent cells which adhere via cell-ECM junctions to proteins within the periosteal sheath. Cells subjected directly to strain via the periosteum may then be stimulated to generate matrix or biochemical factors that promote bone formation. Previous studies have proven the release of osteogenic factors by isolated explanted periosteal cells exposed to tensile substrate strain in the absence of fluidic shear strain (Jones et al., 1991; Kanno et al., 2005). Convective flow, an indirect consequence of oscillatory strain on the periosteum, exerts fluid drag induced shear stress on membranes of adherent cells and may physically displace nonadherent cells. Fluidic shear stress has been shown to significantly affect early osteogenic genetic markers (McBride et al. 2008) and cytoskeleton organization (Chang et al., 2010; Song et al., 2010) in mesenchymal stem cell lines and primary cells derived from the murine mesoderm at the time of condensation. (Knothe Tate et al., 2008; Knothe et al., 2010). In our current studies, we hypothesize that periosteal cells exposed to shear stress upregulate genes similar to embryonic cells derived from the mesoderm during mesenchymal condensation, the first step of skeletogenesis (Knothe Tate et al., 2008).
These data underscore the importance of cells, perfusion, and mechanical loading to harness the periosteum's remarkable tissue building capacity as well as to heal morcellized cancellous and massive cortical bone autografts. It appears that promotion of endogenous bone generation and autograft remodeling at early time points after surgery enables faster maturation and adaptation of the tissue.
8. Conclusions
As a whole, these data indicate a key role for mechanobiologic factors, modulated through mechanical loading, in the efficacy of the one stage bone transport procedure (Knothe and Springfield, 2005) to generate new bone in defects, to heal morcellized autograft packed defects, and to heal massive cortical autografts denuded of periosteum and devoid of a patent blood supply. An understanding of the mechanobiological factors underlying the efficacy of the surgical procedure may help to enable standardized implementation of the procedure in clinical situations where the current standard typically involves bone grafting and distraction osteogenesis. These unprecedented studies may provide a basis to develop clinical guidelines to exploit bone's endogeneous healing potential. In general the current standard of care allows for full weight bearing after clinical and radiological healing of bone defects (Miric et al., 2005), but there are currently no clear guidelines for augmenting the speed and quality of healing, in particular in association with treatment of bone defects.
Figure 6.
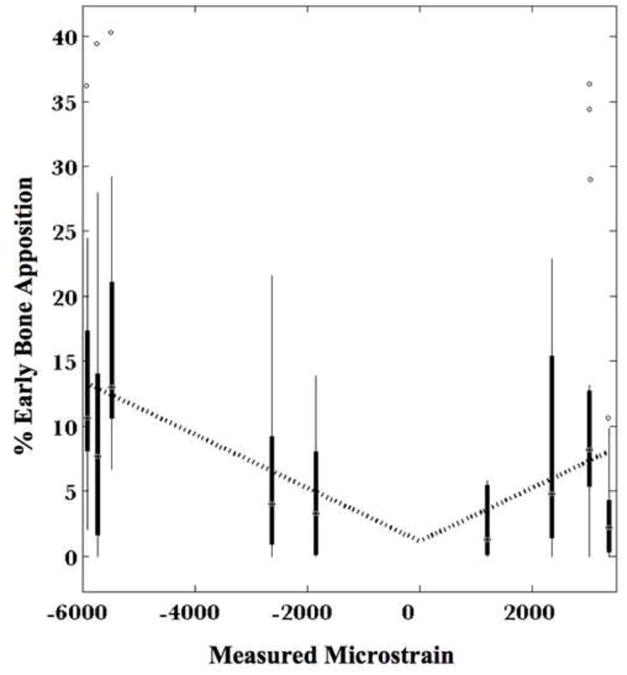
Relationship of absolute mechanical strain to percent of bone apposition occuring in the first two weeks after surgery, in the defect zone, measured in periostea in situ in ex vivo preparations of the one stage bone transport procedure.
Acknowledgments
The study was funded through grants from the AO Foundation. Specifically, the histological studies reported in the current study were supported through grants numbered F-07-99K and 04-K3. The original surgical study, approved through the animal review board of the Canton of Grisons, Switzerland, was funded through AO Grant No. 02-K83 and a grant from the Research Programs Council of The Cleveland Clinic (Grant No. 07316). SD was supported in part by an REU grant from the National Science Foundation (EEC-0552804), as was Shannon Moore, who carried out much of the sectioning. RMM was supported in part through the Center for Stem Cell and Regenerative Medicine's program for undergraduate student research, ENGAGE.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen MR, Hock JM, Burr DB. Periosteum: biology, regulation, and response to osteoporosis therapies. Bone. 2004;35:1003–12. doi: 10.1016/j.bone.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Allen MR, Burr DB. Human femoral neck has less cellular periosteum, and more mineralized periosteum, than femoral diaphyseal bone. Bone. 36:311–6. doi: 10.1016/j.bone.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Bhole AP, Flynn BP, Liles M, Saeidi N, Dimarzio CA, Ruberti JW. Mechanical strain enhances survivability of collagen micronetworks in the presence of collagenase: implications for load-bearing matrix growth and stability. Philos Trans R Soc A. 2009;367:3339–3362. doi: 10.1098/rsta.2009.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes M, Revell W. Blood supply of bone Scientific Aspects. New York: Springer-Verlag; 1998. pp. 109–141. [Google Scholar]
- Burstein F, Canalis R. Studies on the osteogenic potential of vascularized periosteum: behavior of periosteal flaps transferred onto soft tissues. Otolaryngol Head Neck Surg. 1985;93:731–735. doi: 10.1177/019459988509300606. [DOI] [PubMed] [Google Scholar]
- Camilli J, Penteado C. Bone formation by vascularized periosteal and osteoperiosteal grafts. An experimental study in rats. Arch Orthop Trauma Surg. 1994;114:18–24. doi: 10.1007/BF00454730. [DOI] [PubMed] [Google Scholar]
- Chang H, Zimmermann J, Knothe Tate ML. Mechanical Adaptation of Embryonic Stem Cells. Proc 56th Ann Mtg of the Orthop Res Soc. 2010:0722. [Google Scholar]
- Cullen DM, Smith RT, Akhter MP. Time course for bone formation with long-term external mechanical loading. J Appl Physiol. 2000;88:1943–8. doi: 10.1152/jappl.2000.88.6.1943. [DOI] [PubMed] [Google Scholar]
- Eyre-Brook A. The Periosteum: Its function reassessed. Clin Orthop Rel Res. 1984;189:300–307. [PubMed] [Google Scholar]
- Forwood MR, Bennett MB, Blowers AR, Nadorfi RL. Modification of the in vivo four-point loading model for studying mechanically induced bone adaptation. Bone. 1998;23:307–10. doi: 10.1016/s8756-3282(98)00090-8. [DOI] [PubMed] [Google Scholar]
- Hertel R, Knothe U, Gerber A, Cordey J, Rahn R. The osteogenic potential of vascularized periosteum and cancellous bone graft in sheep. Trans Orthopaedic Research Society 1997 [Google Scholar]
- Jones DB, Nolte H, Scholübbers JG, Turner E, Veltel D. Biochemical signal transduction of mechanical strain in osteoblast-like cells. Biomaterials. 1991;12:101–110. doi: 10.1016/0142-9612(91)90186-e. [DOI] [PubMed] [Google Scholar]
- Jupiter JB, Gerhard HJ, Guerrero J, Nunley JA, Levin LS. Treatment of segmental defects of the radius with use of the vascularized osteoseptocutaneous fibular autogenous graft. J Bone Jt Surg. 1997;79:542–550. doi: 10.2106/00004623-199704000-00009. [DOI] [PubMed] [Google Scholar]
- Kanno T, Takahashi T, Ariyoshi W, Tsujisawa T, Haga M, Nishihara T. Tensile mechanical strain up-regulates Runx2 and osteogenic factor expression in human periosteal cells: implications for distraction osteogenesis. J Oral Maxillo Surg. 2005;63:499–504. doi: 10.1016/j.joms.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Klaue K, Knothe U, Anton C, Masquelet AC, Perren SM. Biological implementation of autologous foreign body membranes in corticalization of massive cancellous bone grafts. Trans Orthopaedic Trauma Association 1998 [Google Scholar]
- Klaue K, Knothe U, Anton C, Pfluger DH, Stoddart M, Masquelet AC, Perren SM. Bone regeneration in long-bone defects: tissue compartmentalisation? In vivo study on bone defects in sheep. Injury. 2009;40(4):S95–102. doi: 10.1016/j.injury.2009.10.043. [DOI] [PubMed] [Google Scholar]
- Knothe U, Springfield DSS. A novel surgical procedure for bridging of massive bone defects. World J Surg Oncol. 2005;3(1):7. doi: 10.1186/1477-7819-3-7. http://www.wjso.com/content/3/1/7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knothe UR, Dolejs S, Miller RM, Knothe Tate ML. Effects of mechanical loading patterns, bone graft, and proximity to periosteum on bone defect. J Biomechanics. 2010 doi: 10.1016/j.jbiomech.2010.06.026. [DOI] [PubMed] [Google Scholar]
- Knothe Tate ML, Steck R, Forwood MR, Niederer P. In vivo demonstration of load-induced fluid flow in the rat tibia and its potential implications for processes associated with functional adaptation. J Exp Bio. 2000;203:2737–45. doi: 10.1242/jeb.203.18.2737. [DOI] [PubMed] [Google Scholar]
- Knothe Tate ML. “Whither flows the fluid in bone?”: An Osteocyte's Perspective. J Biomechanics. 2003;36(10):1409–1424. doi: 10.1016/s0021-9290(03)00123-4. [DOI] [PubMed] [Google Scholar]
- Knothe Tate ML, Ritzman T, Schneider E, Knothe U. Testing of a New One-Stage Bone-Transport Surgical Procedure Exploiting the Periosteum and Bone Transport for Repair of Long Bone Defects. J Bone Jt Surg Am. 2007;89:307–316. doi: 10.2106/JBJS.E.00512. [DOI] [PubMed] [Google Scholar]
- Knothe Tate ML, Falls T, McBride SH, Atit R, Knothe UR. Mechanical Modulation of Osteochondroprogenitor Cell Fate. Int J Biochem Cell Bio. 2008;40:2720–2738. doi: 10.1016/j.biocel.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knothe Tate ML, Dolejs S, Miller RM, Knothe UR. Role of mechanical loading in healing of massive bone autografts. J Orth Res. 2010A doi: 10.1002/jor.21190. [DOI] [PubMed] [Google Scholar]
- Knothe Tate ML, Chang H, Knothe UR. Periosteal Bone Regeneration in a Critical Sized Long Bone Defect: Role of Vectorial Transport, Collagen, Periosteal Cells and Blood Supply. Transactions of the Orthopaedic Research Society 2010B [Google Scholar]
- Lieberman DE, Polk JD, Demes B. Predicting Long Bone Loading From Cross-sectional Geometry. Am J Physical Anthropology. 2004;123:156–171. doi: 10.1002/ajpa.10316. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang D, Cheng H. Experimental study of the osteogenic capacity of periosteal allografts: a preliminary report. Microsurgery. 1994A;15:87–92. doi: 10.1002/micr.1920150202. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang D, Cheng H. Use of revascularized periosteal allografts for repairing bony defects: an experimental study. Microsurgery. 1994B;15:93–97. doi: 10.1002/micr.1920150203. [DOI] [PubMed] [Google Scholar]
- Matter P. History of the AO and its global effect on operative fracture treatment. Clin Orthop Relat Res. 1998;347:11–8. [PubMed] [Google Scholar]
- McBride SH, Knothe Tate ML. Modulation of Stem Cell Shape and Fate, A: Role of Density and Seeding Protocol on Nucleus Shape and Gene Expression. Tissue Engineering. 2008;14:1561–1572. doi: 10.1089/ten.tea.2008.0112. [DOI] [PubMed] [Google Scholar]
- McBride SH, Knothe Tate ML. Modulation of Stem Cell Shape and Fate, B: Mechanical Modulation of Cell Shape and Gene Expression. Tissue Engineering. 2008;14:1573–1580. doi: 10.1089/ten.tea.2008.0113. [DOI] [PubMed] [Google Scholar]
- McBride SH, Knothe U, Brianza S, Dolejs S, Knothe Tate ML. Periosteal mechanics from the macro to nano scales; Proceedings of the United States National Applied and Theoretical Mechanics Conference; Pittsburgh. 2010A. [Google Scholar]
- McBride SH. PhD Dissertation. Case Western Reserve University; 2010B. Multiscale mechanobiology of periosteal bone generation: cell scale studies to translational models. [Google Scholar]
- Miric D, Bumbasirevic M, Radulovic N, Lesic A. External fixation of war injuries of the proximal femur. Acta Chir Jugosl. 2005;52:101–5. doi: 10.2298/aci0502101m. [DOI] [PubMed] [Google Scholar]
- Moore S, Knothe U, Knothe Tate ML. Elucidation of cellular mechanisms underlying efficacy of a periosteal replacement implant. Transactions of the Orthopaedic Research Society 2011 [Google Scholar]
- Munro M, Piekarski K. A solid/liquid composite. Composites. 1976;7:195–199. [Google Scholar]
- Nakahara H, Bruder SP, Goldberg VM, Caplan AI. In vivo osteochondrogenic potential of cultured cells derived from the periosteum. Clin Orthopaedics Rel Res. 1990;259:223–232. [PubMed] [Google Scholar]
- Nishimura T, Simmons D, Mainous E. The origin of bone formed by heterotopic periosteal autografts. J Oral Maxillofac Surg. 1997;55:1265–1268. doi: 10.1016/s0278-2391(97)90182-8. [DOI] [PubMed] [Google Scholar]
- Piekarski K, Munro M. Transport mechanism operating between blood supply and osteocytes in long bones. Nature. 1977;269:80–82. doi: 10.1038/269080a0. [DOI] [PubMed] [Google Scholar]
- Puckett CL, Hurvitz JS, Metzler MH, Silver D. Bone formation by revascularized periosteal and bone grafts, compared with traditional bone grafts. Plast Reconstr Surg. 1979;64:361–5. doi: 10.1097/00006534-197909000-00013. [DOI] [PubMed] [Google Scholar]
- Raab-Cullen DM, Akhter MP, Kimmel DB, Recker RR. Periosteal bone formation stimulated by externally induced bending strains. J Bone Miner Res. 1994;9:1143–52. doi: 10.1002/jbmr.5650090803. [DOI] [PubMed] [Google Scholar]
- Romana M, Masquelet A. Vascularized periosteum associated with cancellous bone graft: an experimental study. Plastic and Reconstructive Surgery. 1990;85:587–592. doi: 10.1097/00006534-199004000-00014. [DOI] [PubMed] [Google Scholar]
- Ruedi TP, Buckley RE, Moran CG. AO Principles of Fracture Management. AO Publishing; 2000. [Google Scholar]
- Song MJ, Dean D, Knothe Tate ML. In situ spatiotemporal mapping of flow fields around seeded stem cells at the subcellular length scale. PLoS ONE. 2010;5:e12796. doi: 10.1371/journal.pone.0012796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck R, Gatzka C, Schneider E, Niederer P, Knothe Tate ML. Measurement of bone surface strains on the sheep metacarpus in vivo and ex vivo et al. Vet Comp Orthop Traumatol. 2003;1/2003:1–6. [Google Scholar]
- Takato T, Harii K, Nakatsuka T. Osteogenic capacity of vascularized periosteum: experimental study using rib periosteum in rabbits. British Journal of Plastic Surgery. 1988;41:528–532. doi: 10.1016/0007-1226(88)90012-4. [DOI] [PubMed] [Google Scholar]
- Takushima A, Kitano Y, Harii K. Osteogenic potentioal of cultured periosteal cells in a distracted bone gap in rabbits. Journal of Surgical Research. 1998;78:68–77. doi: 10.1006/jsre.1998.5378. [DOI] [PubMed] [Google Scholar]
- Thomas TT. Progress of Medical Science. Surgery. Regarding some points about bone grafts by Wheeler. Am J Med Sci. 1919;158:887–888. [Google Scholar]
- Thomson RC, Mikos AG, Beahm E, Lemon JC, Satterfield WC, Aufdemorte TB, Miller MJ. Guided tissue fabrication from periosteum using preformed biodegradable polymer scaffolds. Biomaterials. 1999;20:2007–2018. doi: 10.1016/s0142-9612(99)00103-9. [DOI] [PubMed] [Google Scholar]
- Turner CH, Forwood MR. On animal models for studying bone adaptation. Calcif Tissue Int. 1994;55:316–7. [PubMed] [Google Scholar]
- Uddstromer L. The osteogenic capacity of tubular and membranous bone periosteum. Scand J Plast Reconstr Surg. 1978;12:195–205. doi: 10.3109/02844317809012995. [DOI] [PubMed] [Google Scholar]
- Uddstromer L, Ritsila V. Osteogenic capacity of periosteal grafts. Scand J Plast Reconstr Surg. 1978;12:207–214. doi: 10.3109/02844317809012996. [DOI] [PubMed] [Google Scholar]
- Ueno T, Kagawa T, Mizukawa N, Nakamura H, Sugahara T, Yamamoto T. Cellular origin of endochondral ossification from grafted periosteum. Anat Rec. 2001A;264:348–357. doi: 10.1002/ar.10024. [DOI] [PubMed] [Google Scholar]
- Ueno T, Kagawa T, Ishida N, Fukunaga J, Mizukawa N, Sugahara T, Yamamoto T. Prefabricated bone graft induced from grafted periosteum for the repair of jaw defects:and experimental study in rabbits. Surg. 2001B;29:219–223. doi: 10.1054/jcms.2001.0226. [DOI] [PubMed] [Google Scholar]
- Vögelin E, Jones NF, Rao UN. Long-term viability of articular cartilage after microsurgical whole-joint transplantation and immunosuppression with rapamycin, mycophenolate mofetil, and tacrolimus. J Hand Surg Am. 2002;27:307–15. doi: 10.1053/jhsu.2002.32078. [DOI] [PubMed] [Google Scholar]
- Wheeler WIDC. Some points about bone grafts. Br Med J. 1919;1(3031):119–120. [PMC free article] [PubMed] [Google Scholar]
- Yu M, Knothe U, Knothe Tate ML. Regeneration of periosteum in denuded bone. Transactions of the Orthopaedic Research Society 2011 [Google Scholar]


