Abstract
Background
Reactivity to smoking-related cues may play a role in the maintenance of smoking behavior and may change depending on smoking status. Whether smoking cue-related functional MRI (fMRI) reactivity differs between active smoking and extended smoking abstinence states currently is unknown.
Methods
We used fMRI to measure brain reactivity in response to smoking-related versus neutral images in 13 tobacco-dependent subjects prior to a smoking cessation attempt and again during extended smoking abstinence (52 ± 11 days) aided by nicotine replacement therapy.
Results
Pre-quit smoking cue induced fMRI activity patterns paralleled those reported in prior smoking cue reactivity fMRI studies. Greater fMRI activity was detected during extended smoking abstinence than during the pre-quit assessment subcortically in the caudate nucleus and cortically in prefrontal (BA 6, 9, 44, 46), primary somatosensory (BA 1,2,3), temporal (BA 22, 41, 42), parietal (BA 7, 40) anterior cingulate (BA 24, 32), and posterior cingulate (BA 31) cortex.
Conclusions
These data suggest that during extended smoking abstinence, fMRI reactivity to smoking versus neutral stimuli persists in brain areas involved in attention, somatosensory processing, motor planning, and conditioned cue responding. In some brain regions, fMRI smoking cue reactivity is increased during extended smoking abstinence in comparison to the pre-quit state, which may contribute to persisting relapse vulnerability.
Keywords: abstinence, addiction, caudate nucleus, fMRI, nicotine
Introduction
Smoking is a major public health problem of our time, leading to more than 440,000 deaths each year in the US alone (CDC, 2005). Despite smoking's well-known negative health consequences, only 3% of smokers trying to quit on their own are able to maintain abstinence for six months (Hughes et al., 1992). Failure to maintain abstinence appears to result from two main factors. Smoking is reinitiated in order to relieve nicotine withdrawal symptoms (for review see (Benowitz, 2008) and after exposure to smoking-related cues, which can elicit craving responses (Shiffman, Paty, Gnys, Kassel, & Hickcox, 1996).
While nicotine replacement therapy (NRT) is effective at reducing short-term withdrawal symptoms and doubles the short term abstinence rate compared with placebo (Silagy, Lancaster, Stead, Mant, & Fowler, 2004), NRT is not associated with effective maintenance of extended abstinence (Hughes, Shiffman, Callas, & Zhang, 2003), and does not effectively blunt subjective reports of cue-induced craving (Morissette, Palfai, Gulliver, Spiegel, & Barlow, 2005; Tiffany, Cox, & Elash, 2000). This suggests that brain responses to smoking-related cues during abstinence may persist and result in cue-induced craving or smoking urges that can trigger relapse.
Nicotine-related cues by themselves are able to maintain and reinstate nicotine-seeking behavior in rodents (Caggiula et al., 2001). In humans, functional magnetic resonance imaging (fMRI) studies have documented brain areas activated by smoking-related cues including regions known to be involved with reward, craving, emotional processing, memory, visual attention, and impulsivity (Brody et al., 2007; Due, Huettel, Hall, & Rubin, 2002; Franklin et al., 2007; McClernon, Kozink, Lutz, & Rose, 2009). To date, smoking cue-induced fMRI activity has not been evaluated during extended periods of NRT-facilitated smoking cessation. Since relapse potential is significant for prolonged periods even with ongoing NRT treatment (Hughes, Shiffman, Callas, & Zhang, 2003), examining smoking cue-induced brain activity patterns during extended smoking abstinence in smokers treated with NRT may be informative as to which brain areas contribute to relapse vulnerability.
Accordingly, we used blood oxygen level dependent (BOLD) fMRI to characterize brain reactivity to smoking-related versus neutral images. Tobacco-dependent subjects were scanned on two separate sessions, once prior to quitting smoking (scan 1) and again during extended smoking abstinence, which was aided by nicotine replacement therapy (NRT; scan 2). As noted above, NRT does not effectively blunt subjective reports of cue-induced craving (Morissette, Palfai, Gulliver, Spiegel, & Barlow, 2005; Tiffany, Cox, & Elash, 2000) and thus may not blunt brain responses to smoking-related cues during extended smoking abstinence. To determine whether brain activity changed as a function of smoking state, we compared fMRI activation patterns to smoking versus neutral stimuli between the two scan sessions.
Brain reactivity to smoking-related cues is increased following 24 hours of smoking abstinence (McClernon, Kozink, Lutz, & Rose, 2009) in a number of brain areas including the caudate nucleus. This is consistent with the incentive-sensitization theory of drug use (Robinson & Berridge, 1993), which suggests that the perceived reward value of a drug-associated cue increases during abstinence. This enhanced reactivity to smoking-related cues may then contribute to persisting relapse vulnerability. We hypothesized that fMRI reactivity (smoking-related > neutral images) also would be increased during extended smoking abstinence in comparison to the pre-quit state in brain regions including the caudate nucleus.
Methods
Research Participants
Subjects in this study were women aged 43.2 ± 11.5 years (mean ± standard deviation, n = 13) enrolled in a smoking cessation clinical trial at Massachusetts General Hospital (NCT00218465). The parent clinical trial involved a novel medication not yet approved for use in men. Subjects were referred to McLean Hospital to participate in this imaging study. Subjects met DSM-IV criteria for current nicotine dependence, reported smoking at least 10 cigarettes/day in the last 6 months, and had a minimum expired air carbon monoxide (CO) > 10 ppm at screening. Subjects were nicotine-dependent by DSM-IV criteria and had a mean Fagerstrom test for nicotine dependence (FTND) (Fagerstrom, 1978; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) score of 6.3 ± 1.5. Potential subjects were excluded if they had an unstable medical illness, lifetime diagnosis of organic mental disorder, schizophrenia, schizoaffective disorder, bipolar disorder, delusional disorder, or psychotic disorder not elsewhere classified. Potential subjects also were excluded if they had a history of alcohol abuse or had been unresponsive to an adequate course of NRT in the past month. All subjects had Hamilton Rating Scale for depression (HAM-D) (Hamilton, 1967) scores falling in the normal range and potential subjects were excluded if they had a diagnosis of major depressive disorder in the past 6 months. The HAM-D was administered again within one week of scan 2. The McLean Hospital and Massachusetts General Hospital Institutional Review Boards approved this study and subjects provided written informed consent. Subjects received $100.00 for completing both scanning visits.
Subject assessment during scan visits
A brief clinical assessment was conducted immediately before each scan, including vital sign measurements, urine testing to exclude pregnancy (QuPID One-Step Pregnancy Tests, Stanbio Laboratory, Boerne TX) and urine drug testing to exclude recent illicit drug use (QuickTox 11 Panel Drug Test Card, Branan Medical Corporation, Irvine CA). In addition, expired air CO (Bedfont Micro IV Smokerlyzer, Bedfont Scientific, Kent England) and breathalyzer tests (Alco-Sensor IV, Intoximeters Inc., St. Louis MO) were conducted to detect recent smoking and alcohol consumption.
Nicotine Replacement Therapy
After the pre-quit scan (scan 1), subjects quit smoking as part of the clinical trial and underwent an open label smoking cessation lead in phase of the clinical trial, consisting of a standard NRT taper schedule of transdermal nicotine patch, 21 mg per day or highest tolerated dose, for 4 weeks, 14 mg per day for 2 weeks then 7 mg per day for 2 weeks, or the lowest dose that effectively blunted nicotine withdrawal symptoms. To maximally reduce nicotine withdrawal symptoms, participants were allowed to supplement the patch with short acting NRT in the form of 2 mg lozenges or polacrilex gum, up to 18 mg per day. At scan 2, subjects had undergone extended NRT for 51.5 ± 11.3 days. Eight and 5 subjects were using 7 and 14 mg/day patches, respectively, and 8 of 13 subjects used gum or lozenges (2 – 8 mg/day).
We included subjects who reported smoking at least part of one cigarette while using NRT. These subjects were included since most smokers using NRT experience such minirelapses some refer to as slips (Glover et al., 2007). In our study cohort, 5 out of the 13 subjects (38%) slipped but all reestablished abstinence for a mean of 8.2 ± 6.3 days (range: 3 to 19 days) prior to scan 2. Smoking abstinence was assessed by weekly CO measurement and self-report.
Scanning Procedure
During scanning sessions, subjects viewed color photographs of smoking-related or neutral images (Gilbert & Rabinovich, 1999). Smoking-related images included photos of people smoking, hands holding cigarettes, or cigarettes alone. Neutral images were matched for general content (faces, hands, etc.) but were devoid of smoking cues. Target images (animals) were included to ensure that subjects attended to picture sets but were excluded from data analysis. When target images appeared, subjects were instructed to respond by pressing a button on a button box. Sixty smoking-related images, sixty neutral images, and fifteen target images were presented to each subject in a pseudorandom order, with no more than two of the same stimulus type appearing consecutively. Each image was displayed for 4 seconds and a fixation cross was shown for 14 seconds between each image presentation. To eliminate practice effects, different images were presented to subjects during the two scan sessions and were presented in a randomized order to each subject. The duration of each scan was equivalent.
Functional MRI was performed using a Siemens Trio 3 Tesla scanner (Erlangen, Germany) with a circularly polarized (CP) head coil. Structural images were acquired using a multiplanar rapidly acquired gradient-echo (MP-RAGE) sequence (TR = 2.1 sec, TE = 2.7 msec, slices = 128, matrix = 256 × 256, flip angle =12, resolution = 1mm × 1mm × 1.33 mm). Functional images were acquired using gradient echo echo-planar imaging (EPI) (TR = 2 sec, TE = 30 msec, matrix = 64 × 64, field of view = 224, flip angle = 75 slices = 30, resolution = 3.5 isotropic with a gap of 0).
Physiological, Subjective and Psychological Assessments
Mean (± standard deviation) scores were determined for CO, FTND and HAM-D rating scales. To detect between-scan session differences, Student's two-sided paired t-tests were performed.
Imaging Analysis
Image analysis was conducted using Brain Voyager QX 1.10.4 (Brain Innovation, Maastricht, The Netherlands). Images were slice-time corrected, motion corrected, spatially smoothed using a Gaussian kernel of 6mm, resampled to 3×3×3mm isotropic voxels and spatially normalized into Talairach space. To further reduce the effects of motion-related variability, an in-house program was used to model out data time points exhibiting absolute or relative motion exceeding threshold values (set to 1.75mm, equivalent to ½ the total voxel size). This procedure adjusted a total of 1.2% of data points for the scan 1 and 0.3% of data points for scan 2.
Data from each scan initially was analyzed independently. Voxels exhibiting fMRI activity were identified using a whole-brain random effects general linear model with three image predictors (smoking images, neutral images and target images), six motion confound predictors (x, y, z translation and rotation), and motion confound predictors identified by our in-house program. To conduct the within-subjects analysis, a fixed-effects GLM was run and contrast maps of smoking-related images verses neutral images were created for each of the subject's visits. These contrast maps then were used to run a random-effects ANOVA to compare fMRI activity between scans.
To determine the cluster extent necessary to correct for multiple comparisons, a Monte Carlo simulation was run (Forman et al., 1995; Ledberg, Akerman, & Roland, 1998; Poline & Mazoyer, 1993) with the Matlab script Cluster_threshold_beta (Slotnick, Moo, Segal, & Hart, 2003). In a single simulation the size of each contiguous cluster of voxels was determined by modeling the functional image matrix (64 × 64 × 30 voxels), by assuming an individual voxel type 1 error of p = 0.01 and by smoothing the activation map by a 3-dimensional 6mm FWHM Gaussian kernel. Following 10,000 simulations, the probability of each cluster size was determined and the cluster extent that yielded p < 0.005 was selected, which is more conservative than accepted threshold levels (Ross & Slotnick, 2008). The cluster extent used was defined as thirty-one 3mm resampled isotropic voxels equaling approximately 837 mm3.
Results
Physiological and Psychological Measurements
At scan 1, subjects had an expired CO level of 21.7 ± 9.2 ppm. At scan 2, CO levels were significantly reduced to 1.7 ± 1.5 ppm (t-test, t = -7.7, p < 0.001). Breath alcohol levels measured prior to both scan sessions were zero. FTND scores were measured only at screening (FTND score = 6.3 ± 1.5). At screening, HAM-D scores were within the normal range (2.3 ± 2.8). At scan 2, HAM-D scores remained in the normal range although there was a trend level increase versus scan 1 (5.8 ± 4.9, paired t-test, t = -2.0, p = 0.07). While hormonal status was not recorded, scan 2 took place 56.3 ± 8.7 days after the first scan indicating that subjects on average were at about the same point within the menstrual cycle at both scan sessions.
Imaging Results
At scan 1, smokers exhibited significantly greater fMRI responses to smoking-related versus neutral images in a number of brain areas (z ≥ 3.1, corrected p < 0.005; Figure 1; Table 1). These included the frontal (BA 6, 8, 9, 10), anterior cingulate (BA 32), posterior cingulate (BA 29, 30, 31), temporal (BA 20, 21, 22, 35), parietal (BA 1, 2, 3, 4, 7, 44), and occipital (BA 17, 18, 19) cortex, and cerebellum. Smoking-related images reduced fMRI responses versus neutral images in the middle occipital gyrus (BA 19) and inferior temporal gyrus (BA 20).
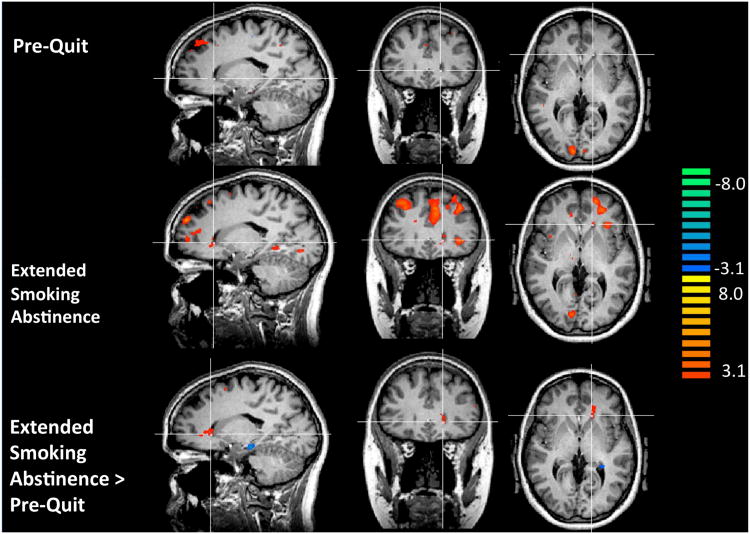
Figure 1.
Functional MRI Results. Top row: In the pre-quit (scan 1) state, the contrast smoking-related > netural images revealed activation in occipital, medial prefrontal, and posterior cingulate cortex, and in other regions (see Table 1). No activity was detected in the caudate nucleus (cross hair position in all images at Talairach coordinates: (x, y, z) -14, 25, 2). Middle row: During extended smoking abstinence (scan 2), the contrast smoking-related > neutral images revealed activation in the caudate nucleus, prefrontal, parietal, and cingulate cortex, and in other regions (see Table 2). Bottom row: The contrast scan 2 (smoking-related > neutral images) > scan 1 (smoking-related > neutral images) revealed increased reactivity in the caudate nucleus and in other brain regions (Table 3). For all tests: t ≥ 3.1, corrected p < 0.005, cluster sizes noted in tables.
Table 1. Pre-quit (Scan 1) fMRI Reactivity.
Brain areas, Brodmann areas and Talairach coordinates (Talairach & Tournoux, 1988) refer to the peak activation voxel within each cluster of continuous voxels. The t values refer to the maximum t statistic in each cluster. Voxel numbers refer to the total number of voxels per cluster. Gyrus (G).
| Smoking-Related > Neutral Images | Brodmann Area | Talairach Coordinates | t | Voxels | ||
|---|---|---|---|---|---|---|
|
|
||||||
| Brain Area | x | y | z | |||
|
| ||||||
| Frontal Cortex | ||||||
| Medial Frontal G. | 8, 9,10 | −18 | 35 | 43 | 5.8 | 7606 |
| 6 | 0 | −10 | 64 | 4.8 | 336 | |
| 6 | 6 | 32 | 56 | 4.2 | 75 | |
| Superior Frontal G. | 6 | −27 | −1 | 64 | 4.1 | 44 |
|
| ||||||
| Cingulate | ||||||
| Anterior | 32 | 9 | 20 | 31 | 4.6 | 79 |
| Posterior | 31 | 18 | −37 | 40 | 4.6 | 506 |
| 29, 30, 31 | 3 | −46 | 19 | 4.5 | 963 | |
| 31 | 0 | −16 | 34 | 5.6 | 1521 | |
|
| ||||||
| Temporal Cortex | ||||||
| Middle Temporal G | 21 | 45 | −34 | 7 | 3.8 | 153 |
| 22 | −48 | −40 | 7 | 3.7 | 58 | |
| Parahippocampal G. | 35 | −18 | −22 | −14 | 4.4 | 58 |
| Inferior Temporal G. | 20 | −39 | −4 | −23 | 4.9 | 358 |
|
| ||||||
| Parietal Cortex | ||||||
| Inferior Parietal Lobule | 44 | 54 | −31 | 31 | 3.6 | 44 |
| Precuneus | 7 | 12 | −52 | 61 | 4.0 | 133 |
| 7 | 6 | −49 | 49 | 5.1 | 1072 | |
| Paracentral Lobule | 4 | 3 | −37 | 64 | 4.2 | 90 |
| Postcentral G. | 1, 2, 3 | −30 | −34 | 52 | 4.5 | 155 |
|
| ||||||
| Occipital | ||||||
| Fusiform G. | 19 | 33 | −73 | −14 | 4.1 | 166 |
| 19 | −39 | −67 | −17 | 4.2 | 151 | |
| Cuneus | 17 | 24 | −82 | 10 | 5.3 | 327 |
| 17 | 12 | −91 | 7 | 7.4 | 5116 | |
| 19 | 15 | −82 | 31 | 3.4 | 48 | |
| 18 | 6 | −76 | 25 | 4.6 | 1625 | |
| Middle Occipital G. | 19 | −39 | −64 | 10 | 3.7 | 70 |
|
| ||||||
| Cerebellum | Right Hem. | 39 | −49 | −23 | 3.6 | 129 |
|
| ||||||
| Neutral > Smoking-related images | ||||||
|
| ||||||
| Inferior Temporal G. Occipital | 20 | −51 | −52 | −11 | 3.9 | 63 |
| Middle Occipital G. | 19 | −27 | −67 | 19 | 3.6 | 63 |
| 19 | −33 | −61 | 31 | 3.8 | 108 | |
At scan 2, subjects exhibited significantly greater fMRI responses to smoking-related versus neutral images in a number of brain areas (z ≥ 3.1, corrected p > 0.005; Figure 1; Table 2). These included the frontal (BA 6, 8, 9, 10, 43, 45, 47), anterior cingulate (BA 24 & 32), posterior cingulate (BA 31), temporal (BA 20, 21, 22, 36, 41), parietal (BA 1, 2, 3, 5, 7, 39, 40), occipital (BA 18, 19), and insular cortex (BA 13). Subcortically, increased fMRI responses to smoking-related versus neutral images were detected in the claustrum, thalamus, and caudate nucleus. Smoking-related images reduced fMRI responses versus neutral images in the subcallosal gyrus (BA 25), parahippocampal gyrus (BA 35, 36), fusiform gyrus (BA 37), and inferior occipital gyrus (BA 17).
Table 2. Extended Smoking Abstinence (scan 2) fMRI Reactivity.
Brain areas, Brodmann areas and Talairach coordinates (Talairach & Tournoux, 1988) refer to the peak activation voxel within each cluster of continuous voxels. The t values refer to the maximum t statistic in each cluster. Voxel numbers refer to the total number of voxels per cluster. Gyrus (G).
| Smoking-Related > Neutral Images | Brodmann Area | Talairach Coordinates | t | Voxels | |||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Brain Area | x | y | z | ||||
|
| |||||||
| Frontal Cortex | |||||||
| Precentral G. | 6 | 42 | −7 | 40 | 4.9 | 688 | |
| 43 | −51 | −7 | 13 | 3.9 | 55 | ||
| Middle Frontal G. | 8, 9, 10 | 27 | 35 | 37 | 6.2 | 6366 | |
| 10 | 21 | 38 | 16 | 4.6 | 415 | ||
| 6 | −6 | −22 | 52 | 4.3 | 156 | ||
| 6 | −36 | 5 | 52 | 4.5 | 414 | ||
| Superior Frontal G. | 10 | 15 | 65 | 7 | 3.7 | 44 | |
| 6 | −9 | 8 | 58 | 3.9 | 114 | ||
| 8 | −12 | 44 | 43 | 4.2 | 51 | ||
|
| |||||||
| Cingulate | |||||||
| Anterior | 32 | 12 | 35 | 4 | 3.8 | 81 | |
| 24 | 3 | 2 | 37 | 5.0 | 261 | ||
| 24 | 9 | −7 | 46 | 3.9 | 67 | ||
| 24 | 6 | 17 | 28 | 3.5 | 178 | ||
| Anterior, Caudate Nucleus | 24 | 18 | 23 | 22 | 4.5 | 332 | |
| Anterior, Medial Frontal G. | 8,9,10,24,32 | −30 | 35 | 37 | 7.0 | 18901 | |
| Caudate Nucleus | |||||||
| Posterior | 31 | 3 | −25 | 37 | 4.9 | 1109 | |
| 31 | −9 | −31 | 34 | 4.1 | 52 | ||
| 31 | −24 | −31 | 40 | 4.6 | 139 | ||
| Posterior, Paracentral G. | 31, 5 | 0 | −37 | 55 | 5.2 | 1332 | |
|
| |||||||
| Insula | 13 | 42 | 5 | 4 | 3.9 | 362 | |
| 13 | −27 | 14 | −5 | 4.0 | 185 | ||
| 13 | −30 | −19 | 13 | 4.4 | 76 | ||
| 13 | −33 | 8 | 10 | 4.5 | 483 | ||
| 13, 45 | 36 | 20 | 16 | 4.8 | 749 | ||
| Insula, Inferior Frontal G. | 13, 47 | −36 | 26 | 1 | 5.4 | 453 | |
|
| |||||||
| Temporal Cortex | |||||||
| Superior Temporal G. | 41 | 42 | −37 | 13 | 7.2 | 224 | |
| 41 | −33 | −34 | 22 | 4.3 | 156 | ||
| 22 | −42 | −40 | 13 | 4.9 | 567 | ||
| Inferior Temporal G. | 20 | 42 | −1 | −23 | 4.0 | 124 | |
| Parahippocampal G. | 36 | −21 | −49 | −5 | 5.1 | 1179 | |
|
| |||||||
| Parietal Cortex | |||||||
| Postcentral G. | 1,2,3 | 58 | −28 | 40 | 4.6 | 99 | |
| Precuneus | 7 | 27 | −52 | 52 | 5.0 | 178 | |
| 7 | −9 | −61 | 49 | 5.5 | 2556 | ||
| Inferior Parietal Lobule | 39, 40 | 51 | −61 | 28 | 5.0 | 3370 | |
| 40 | 24 | −37 | 55 | 4.1 | 137 | ||
| 40 | −54 | −43 | 19 | 4.3 | 146 | ||
| 40 | −57 | −25 | 19 | 4.8 | 174 | ||
|
| |||||||
| Occipital Cortex | |||||||
| Cuneus | 18 | 18 | −82 | 16 | 5.1 | 2406 | |
| 18 | −9 | −85 | 19 | 4.0 | 171 | ||
| Lingual G. | 19 | 15 | −58 | −5 | 4.3 | 424 | |
| 18 | −9 | −67 | −5 | 3.7 | 112 | ||
| 18 | −15 | −76 | −8 | 5.4 | 105 | ||
|
| |||||||
| Thalamus | 9 | −16 | 1 | 3.7 | 62 | ||
|
| |||||||
| Caudate Nucleus | 6 | 2 | 10 | 4.0 | 75 | ||
|
| |||||||
| Claustrum | −27 | −13 | 16 | 4.2 | 52 | ||
|
| |||||||
| Neutral >Smoking-related Images | |||||||
|
| |||||||
| Frontal | |||||||
| Subcallosal G. | 25 | 6 | 11 | −14 | 3.7 | 135 | |
| Temporal | |||||||
| Parahippocampal G. | 36 | 30 | −31 | −14 | 3.4 | 59 | |
| 35 | −24 | −22 | −20 | 3.7 | 43 | ||
| Parietal | |||||||
| Fusiform G. | 37 | −42 | −37 | −20 | 4.9 | 376 | |
| Occipital | |||||||
| Inferior Occipital G. | 17 | −18 | −91 | −8 | 4.0 | 40 | |
When fMRI activity to smoking versus neutral images was compared across scans, several brain areas were found to exhibit increased activation during extended smoking abstinence in comparison to the pre-quit state (z ≥ 3.1, p < 0.005; Figure 1; Table 3). Increased activation was found cortically in the frontal (BA 6, 9, 44, 46), anterior cingulate (BA 24, 32), posterior cingulate (BA 31), temporal (BA 22, 41, 42), and parietal (BA 1, 2, 3, 7, 40) cortex, and subcortically in the caudate nucleus. Reduced fMRI responses to smoking versus neutral images were found bilaterally in hippocampus.
Table 3. Between-scan fMRI Reactivity Differences: (Smoking-Related > Neutral Images).
Brain areas, Brodmann areas and Talairach coordinates (Talairach & Tournoux, 1988) refer to the peak activation voxel within each cluster of continuous voxels. The t values refer to the maximum t statistic in each cluster. Voxel numbers refer to the total number of voxels per cluster. Gyrus (G).
| Scan 2 > Scan 1 | Brodmann Area | Talairach Coordinates | t | Voxels | ||
|---|---|---|---|---|---|---|
|
|
||||||
| Brain Area | x | y | z | |||
|
| ||||||
| Frontal Cortex | ||||||
| Superior Frontal Gyrus | 9, 46 | 21 | 47 | 28 | 5.0 | 171 |
| Precentral Gyrus | 6 | −21 | −10 | 55 | 3.9 | 37 |
| 44 | −51 | 2 | 7 | 4.7 | 285 | |
| Middle Frontal Gyrus | 6 | −21 | −16 | 59 | 3.8 | 31 |
| 9 | −21 | 23 | 28 | 4.1 | 36 | |
| 9 | −33 | 20 | 25 | 4.1 | 185 | |
| Inferior Frontal Gyrus | 6, 44 | −42 | 11 | 19 | 4.3 | 212 |
|
| ||||||
| Cingulate | ||||||
| Anterior Cingulate | 32 | 21 | 11 | 28 | 5.1 | 56 |
| 24 | 21 | −25 | 40 | 3.5 | 31 | |
| 32 | −6 | −22 | 16 | 4.4 | 31 | |
| Posterior Cingulate | 31 | 18 | −31 | 46 | 4.6 | 65 |
|
| ||||||
| Temporal Cortex | ||||||
| Superior Temporal Gyrus | 22, 42 | 60 | −28 | 10 | 4.9 | 174 |
| 22 | −54 | −10 | 1 | 4.9 | 249 | |
|
| ||||||
| Parietal Cortex | ||||||
| Postcentral Gyrus | 1, 2, 3 | −12 | −34 | 62 | 5.1 | 160 |
| Inferior Parietal Lobe | 7, 40 | 42 | −31 | 43 | 4.0 | 147 |
| 7, 40 | 42 | −43 | 37 | 3.9 | 120 | |
| 7, 40 | 33 | −58 | 40 | 5.1 | 272 | |
| Supramarginal Gyrus | 40 | −36 | −43 | 31 | 4.8 | 222 |
|
| ||||||
| Caudate Nucleus, White Matter | Head | −15 | 32 | 4 | 4.5 | 292 |
| Tail | −21 | −31 | 22 | 6.1 | 74 | |
|
| ||||||
| Scan 1 > Scan 2 | ||||||
|
| ||||||
| Hippocampus | −15 | −22 | −14 | 6.8 | 719 | |
| −27 | −37 | 1 | 4.4 | 97 | ||
Discussion
We used fMRI to detect brain activity in response to smoking-related versus neutral images in tobacco-dependent women prior to a quit attempt and then again during extended smoking abstinence aided by NRT. Both scans revealed increased activity in response to smoking-related versus neutral images in regions shown previously to be activated by smoking-related and other drug-related cues (Due et al., 2002; Franklin et al., 2007; Garavan et al., 2000; George et al., 2001; Grusser et al., 2004; McClernon, Kozink, Lutz, & Rose, 2009; McClernon, Kozink, & Rose, 2008).
We also found between-scan differences in fMRI reactivity patterns. At scan 1, fMRI reactivity to smoking versus neutral images was increased in regions involved in emotional processing, including the frontal and anterior cingulate cortex (Fichtenholtz et al., 2004; Keightley et al., 2003), visuospatial processing areas within parietal and occipital cortices (McClernon, Kozink, & Rose, 2008; Thiel, Zilles, & Fink, 2005) as well as in temporal areas which have been correlated with increased reactivity to smoking cues (McClernon, Kozink, & Rose, 2008). In comparison to the pre-quit state, during extended smoking abstinence subjects exhibited significantly greater fMRI activation to smoking versus neutral images in prefrontal, anterior and posterior cingulate, temporal, and parietal cortex, as well as in caudate nucleus. Those regions are known to be involved in action planning, habit learning, and craving (Fuchs, Branham, & See, 2006; Garavan et al., 2000; Kosten et al., 2006; See, Elliott, & Feltenstein, 2007; Smolka et al., 2006; Weible, Weiss, & Disterhoft, 2007), and may reflect smoking cue-induced activation of circuits that maintain or increase relapse vulnerability.
While we show that brain reactivity to smoking-related images differs between smoking states, we did not include a placebo condition and thus are unable to differentiate the effects of extended abstinence from those of NRT on fMRI reactivity. However, we believe that our finding of increased fMRI reactivity in caudate nucleus and several other brain areas to smoking versus neutral images during the second versus the first scan likely is a consequence of extended smoking abstinence rather than extended NRT use. In this regard, animal and human studies have demonstrated that brain reactivity to drug cues, including smoking cues, increases following drug abstinence (Fuchs, Branham, & See, 2006; McClernon, Kozink, Lutz, & Rose, 2009; See, Elliott, & Feltenstein, 2007). Additionally, if NRT increased smoking cue-induced brain reactivity over time, its therapeutic usage likely would decrease smoking abstinence, which is not observed clinically (Etter & Stapleton, 2006; Hughes, Shiffman, Callas, & Zhang, 2003). Collectively, this suggests that the increased fMRI reactivity we observed at scan 2 more likely is due to extended smoking abstinence than NRT usage.
Although mechanisms underlying the observed effects are not clear at this time, the increased caudate nucleus reactivity to smoking versus neutral images during extended smoking abstinence versus the pre-quit state is interesting in light of prior observations implicating the dorsal striatum in drug cue-induced reactivity. In this regard, dorsal striatal neurons activate both in expectation of and in preparation for responding to a reward (Apicella, Scarnati, Ljungberg, & Schultz, 1992). Dorsal striatal dopamine levels increase during drug-related cue responding (Ito, Dalley, Robbins, & Everitt, 2002), during cue-induced drug craving (Volkow et al., 2006; Wong et al., 2006), and such increases may predict increased relapse vulnerability (Volkow et al., 2006). Additionally, the dorsal striatum may be especially important for maintaining cue-induced reactivity once drug seeking has become habitual (Porrino, Lyons, Smith, Daunais, & Nader, 2004). Further, this region is involved in maintaining drug-seeking following a period of abstinence (Fuchs, Branham, & See, 2006; See, Elliott, & Feltenstein, 2007). Dorsal striatal reactivity to smoking-related cues is enhanced following 24 hours of smoking abstinence (McClernon, Kozink, Lutz, & Rose, 2009). While our study differs from McClernon et al. (McClernon, Kozink, Lutz, & Rose, 2009) in that our subjects were abstinent for longer time periods, collectively, the data demonstrate that both acute and extended smoking abstinence are associated with enhanced dorsal striatal reactivity to smoking-related cues. Since all subjects were receiving NRT at scan 2, albeit at different dose levels that were self-titrated to minimize relapse, these data suggest that dorsal striatal reactivity to smoking-related cues is not ameliorated by the presence of nicotine in the form of NRT.
We also found increased scan 2 versus scan 1 fMRI smoking cue reactivity in other brain regions implicated in drug cue-induced reactivity, motor behavior, and craving, such as anterior and posterior cingulate cortices, prefrontal cortex, temporal, and parietal cortex. The anterior cingulate has been shown to be involved in cue-induced motor responses (Devinsky, Morrell, & Vogt, 1995) and posterior cingulate neurons become active during attention to reward-associated stimuli (Smith, Freeman, Nicholson, & Gabriel, 2002). Prefrontal cortex (BA 6, 9, 44, 46), parietal cortex (BA 7, 40) and primary somatosensory cortex (BA 1, 2, 3) are involved in imagined and executed movement (Gerardin et al., 2000). If smoking-related cues observed in the real world (outside the scanner) are capable of activating these circuits during extended smoking abstinence, this could promote smoking re-initiation and relapse.
During extended smoking abstinence, smoking-related images also activated the insula. This region has been shown to be involved in maintaining smoking behavior (Naqvi, Rudrauf, Damasio, & Bechara, 2007) and it is activated during various forms of craving and/or exposure to drug-related or other incentive-related cues (Craig, 2009). While we found no between-scan differences in insula activation, the data suggest that extended smoking abstinence is unable to abolish cue-associated interoceptive sensations mediated by the insula, which may trigger or accompany craving responses. Together, our data imply that a number of brain areas continue to be reactive to smoking-related cues and some regions exhibit increased reactivity in comparison to the pre-quit state, during extended smoking abstinence. These brain areas may facilitate craving and habitual responding to smoking cues and predispose people to relapse while trying to quit smoking (Hughes, Shiffman, Callas, & Zhang, 2003), and may be useful targets for relapse prevention medications.
Our findings suggest that developing treatments that modulate dorsal striatal activity during smoking abstinence may help to suppress cue reactivity and decrease relapse potential. Since dorsal striatal D2-like receptor binding is increased in response to drug-related cues (Volkow et al., 2006; Wong et al., 2006), it is conceivable that dopamine D2-like receptor antagonists may attenuate smoking cue-induced activity. We are conducting studies with D2-like receptor antagonists to determine whether they reduce smoking cue fMRI reactivity in the caudate nucleus and in other brain regions as well as alter relapse potential.
Our study has limitations that merit discussion. First, only women were enrolled. Since sex differences in smoking cue reactivity have been identified (McClernon, Kozink, & Rose, 2008), our findings may not extend to men. We are conducting parallel studies in men that will attempt to address whether sex differences in smoking cue reactivity during extended smoking abstinence exist. Another important limitation is that we did not obtain subjective ratings of craving proximate to scan sessions. Thus, we cannot relate craving to our fMRI data. However, associations between smoking cue fMRI data (including images that are part of the image set we used) and craving responses have been reported (McClernon, Hiott, Huettel, & Rose, 2005), suggesting that craving data would have minimally informed this study. As noted above, NRT treatment was individualized to maximize abstinence. This is typical and even desirable in NRT-using populations since individualized NRT appears to increase therapeutic efficacy (McClure and Swan 2006), but may cofound research studies such as this. Future studies including placebo controls will attempt to better characterize the effects of NRT dose, duration, and formulation on fMRI cue reactivity. Notwithstanding these limitations, our results indicate that female smokers maintain fMRI smoking cue reactivity in a number of brain areas during extended smoking abstinence and exhibit greater fMRI reactivity during extended smoking abstinence versus the pre-quit state in dorsal caudate nucleus and other brain areas involved in learning, action planning, and motor behavior, which may contribute to relapse vulnerability.
Acknowledgments
This research was supported in part by National Institute on Drug Abuse grants U01DA019378, R01DA022276, R01DA014674, R01DA09448, K02DA017324, K24DA015116, T32DA015036, by funding from the Counter-Drug Technology Assessment Center (CTAC), an office within the Office of National Drug Control Policy (ONDCP) via Army Contracting Agency contract BK39-03-C-0075, and by research support from GlaxoSmithKline. We thank Dr. Anne Andorn (GSK) for her comments and support.
References
- Apicella P, Scarnati E, Ljungberg T, Schultz W. Neuronal activity in monkey striatum related to the expectation of predictable environmental events. Journal of Neurophysiology. 1992;68(3):945–960. doi: 10.1152/jn.1992.68.3.945. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clinical Pharmacology and Therapeutics. 2008;83(4):531–541. doi: 10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, et al. Neural substrates of resisting craving during cigarette cue exposure. Biological Psychiatry. 2007;62(6):642–651. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, et al. Cue dependency of nicotine self-administration and smoking. Pharmacology, Biochemistry, and Behavior. 2001;70(4):515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- CDC. Press Release: Smoking Deaths Cost Nation $92 Billion in Lost Productivity Annually. 2005 Retrieved December, 2008, from http://www.cdc.gov/od/oc/media/pressrel/r050630.htm.
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nature reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. The American Journal of Psychiatry. 2002;159(6):954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Etter J, Stapleton JA. Nicotine replacement therapy for long-term smoking cessation: a meta-analysis. Tob Control. 2006;15(4):280–285. doi: 10.1136/tc.2005.015487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addictive Behaviors. 1978;3(3-4):235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Fichtenholtz HM, Dean HL, Dillon DG, Yamasaki H, McCarthy G, LaBar KS. Emotion-attention network interactions during a visual oddball task. Brain Research Cognitive Brain Research. 2004;20(1):67–80. doi: 10.1016/j.cogbrainres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, et al. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32(11):2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. The Journal of Neuroscience. 2006;26(13):3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. The American Journal of Psychiatry. 2000;157(11):1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, et al. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Archives of General Psychiatry. 2001;58(4):345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- Gerardin E, Sirigu A, Lehericy S, Poline JB, Gaymard B, Marsault C, et al. Partially overlapping neural networks for real and imagined hand movements. Cerebral Cortex. 2000;10(11):1093–1104. doi: 10.1093/cercor/10.11.1093. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Rabinovich NE. International smoking image series (with neutral counterparts) version 1.2: Integrative Neuroscience Laboratory. Dept. of Psychology, Southern Illinois Univ; 1999. [Google Scholar]
- Glover ED, Laflin MT, Schuh KJ, Schuh LM, Nides M, Christen AG, et al. A randomized, controlled trial to assess the efficacy and safety of a transdermal delivery system of nicotine/mecamylamine in cigarette smokers. Addiction. 2007;102(5):795–802. doi: 10.1111/j.1360-0443.2007.01763.x. [DOI] [PubMed] [Google Scholar]
- Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, et al. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berlin) 2004;175(3):296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. The British Journal of Social and Clinical Psychology. 1967;6(4):278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Gulliver SB, Fenwick JW, Valliere WA, Cruser K, Pepper S, et al. Smoking cessation among self-quitters. Health Psychology. 1992;11(5):331–334. doi: 10.1037//0278-6133.11.5.331. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Shiffman S, Callas P, Zhang J. A meta-analysis of the efficacy of over-the-counter nicotine replacement. Tobacco Control. 2003;12(1):21–27. doi: 10.1136/tc.12.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. The Journal of Neuroscience. 2002;22(14):6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keightley ML, Winocur G, Graham SJ, Mayberg HS, Hevenor SJ, Grady CL. An fMRI study investigating cognitive modulation of brain regions associated with emotional processing of visual stimuli. Neuropsychologia. 2003;41(5):585–596. doi: 10.1016/s0028-3932(02)00199-9. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, et al. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31(3):644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Ledberg A, Akerman S, Roland PE. Estimation of the probabilities of 3D clusters in functional brain images. Neuroimage. 1998;8(2):113–128. doi: 10.1006/nimg.1998.0336. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Huettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology. 2005;30(10):1940–1947. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Lutz AM, Rose JE. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology (Berlin) 2009;204(1):25–35. doi: 10.1007/s00213-008-1436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33(9):2148–2157. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- McClure JB, Swan GE. Tailoring nicotine replacement therapy: rationale and potential approaches. CNS Drugs. 2006;20(4):281–291. doi: 10.2165/00023210-200620040-00002. [DOI] [PubMed] [Google Scholar]
- Morissette SB, Palfai TP, Gulliver SB, Spiegel DA, Barlow DH. Effects of transdermal nicotine during imaginal exposure to anxiety and smoking cues in college smokers. Psychology of Addictive Behaviors. 2005;19(2):192–198. doi: 10.1037/0893-164X.19.2.192. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315(5811):531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poline JB, Mazoyer BM. Analysis of individual positron emission tomography activationmaps by detection of high signal-to-noise-ratio pixel clusters. Journal of Cerebral Blood Flow and Metabolism. 1993;13(3):425–437. doi: 10.1038/jcbfm.1993.57. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. The Journal of Neuroscience. 2004;24(14):3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Ross RS, Slotnick SD. The hippocampus is preferentially associated with memory for spatial context. Journal of Cognitive Neuroscience. 2008;20(3):432–446. doi: 10.1162/jocn.2008.20035. [DOI] [PubMed] [Google Scholar]
- See RE, Elliott JC, Feltenstein MW. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology (Berlin) 2007;194(3):321–331. doi: 10.1007/s00213-007-0850-8. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology. 1996;64(2):366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews. 2004;(3):CD000146. doi: 10.1002/14651858.CD000146.pub2. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart J., Jr Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cognitive Brain Research. 2003;17(1):75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Smith DM, Freeman JH, Jr, Nicholson D, Gabriel M. Limbic thalamic lesions, appetitively motivated discrimination learning, and training-induced neuronal activity in rabbits. The Journal of Neuroscience. 2002;22(18):8212–8221. doi: 10.1523/JNEUROSCI.22-18-08212.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolka MN, Buhler M, Klein S, Zimmermann U, Mann K, Heinz A, et al. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology (Berlin) 2006;184(3-4):577–588. doi: 10.1007/s00213-005-0080-x. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Thiel CM, Zilles K, Fink GR. Nicotine modulates reorienting of visuospatial attention and neural activity in human parietal cortex. Neuropsychopharmacology. 2005;30(4):810–820. doi: 10.1038/sj.npp.1300633. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Cox LS, Elash CA. Effects of transdermal nicotine patches on abstinence-induced and cue-elicited craving in cigarette smokers. Journal of Consulting and Clinical Psychology. 2000;68(2):233–240. doi: 10.1037//0022-006x.68.2.233. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. The Journal of Neuroscience. 2006;26(24):6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weible AP, Weiss C, Disterhoft JF. Connections of the caudal anterior cingulate cortex in rabbit: neural circuitry participating in the acquisition of trace eyeblink conditioning. Neuroscience. 2007;145(1):288–302. doi: 10.1016/j.neuroscience.2006.11.046. [DOI] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, et al. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31(12):2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]