Abstract
Recent studies demonstrated that the excitotoxic amino acid homocysteine induces apoptotic death of retinal ganglion cells in vivo. In the present study, an in vitro rat retinal ganglion cell (RGC-5) culture system was used to analyze the toxicity of acute exposure to high levels of homocysteine, the mechanism of homocysteine-induced toxicity and the usefulness of σR1 ligands as neuroprotectants. When cultured RGC-5 cells were subjected to treatment with 1 mM D, L- homocysteine, a significant increase in cell death was detected by TUNEL analysis and analysis of activated caspase. When cells were treated with homocysteine- or glutamate in the presence of MK-801, an antagonist of the NMDA receptor, the cell death was inhibited significantly. In contrast, NBQX, an antagonist of the AMPA/Kainate receptor, and nifedipine, a calcium channel blocker, did not prevent the homocysteine- or glutamate-induced cell death. Semi-quantitative RT-PCR and immunocytochemical analysis demonstrated that RGC-5 cells exposed to homocysteine or glutamate express type 1 sigma receptor at levels similar to control cells. Treatment of RGC-5 cells with 3 µM or 10 µM concentrations of the σR1-specific ligand (+)-pentazocine inhibited significantly the apoptotic cell death induced by homocysteine or glutamate. The results suggest that homocysteine is toxic to ganglion cells in vitro, that the toxicity is mediated via NMDA receptor activation, and that the σR1-specific ligand (+)-pentazocine can block the RGC-5 cell death induced by homocysteine and glutamate.
Keywords: homocysteine, glutamate, retinal ganglion cells, apoptosis, NMDA receptor, sigma receptor, pentazocine
Introduction
Homocysteine, a non-protein sulfur amino acid, is a metabolite of the essential amino acid methionine. Methionine plays a key role in generation of methyl groups required for synthesis of DNA and this process results in the formation of homocysteine. Homocysteine can either be remethylated to methionine by enzymes that require folate or cobalamin or catabolized by cystathionine β-synthase to form cysteine [43]. There has been considerable interest in homocysteine in recent years because moderate increases in plasma levels of homocysteine increase the risk of cardiovascular disease [16]. Hyperhomocysteinemia is associated with increased incidence and progression of arterial occlusive disease [8], atherosclerosis [50], and central retinal vein occlusion [52]. Assessment of the effects of homocysteine in cultured endothelial cells suggests that homocysteine causes reductive stress and inhibits antioxidant enzymes in these cells [39].
In addition to its association with cardiovascular diseases, hyperhomocysteinemia is implicated also in neurological diseases [30] such as stroke [40], Alzheimer’s disease [47], and Parkinson’s disease [32]. In vitro studies show that homocysteine induces neuronal apoptosis in cultured hippocampal neurons [22], cerebellar granule cells [19] and cortical neurons [18]. Lipton et al [26] demonstrated that homocysteine causes direct neurotoxicity of cortical neurons by activating the N-methyl-D-aspartate (NMDA) subtype of glutamate receptor. Under these conditions, neuronal damage derives from excessive calcium influx and reactive oxygen generation [26].
Recent studies from our laboratory demonstrated the in vivo toxicity of homocysteine on retinal ganglion cells, the second order neurons of the visual system. Intravitreal injection of homocysteine in mice led to ∼25% decrease in the number of cells in the retinal ganglion cell layer within 5–6 days of injection [36]. Examination of homocysteine-injected eyes revealed many TUNEL-positive ganglion cells and ganglion cells positive for activated caspase-3. Ultrastructural analysis revealed several of the morphological features of apoptosis in these cells. This was the first report of homocysteine-induced retinal ganglion cell death in vivo. Additional experiments in which homocysteine and glutamate were injected simultaneously revealed even greater induction of apoptotic ganglion cell death [36].
In the present work, we extend these findings to a retinal ganglion cell culture system to test whether an acute exposure to high levels of homocysteine induces ganglion cell death by activating the NMDA receptor. In addition, we examined whether sigma receptor ligands could prevent the homocysteine- and glutamate-induced death of cultured ganglion cells.
Sigma receptors are nonopiate and nonphencyclidine binding sites that mediate the psychotomimetic actions of certain opioid derivatives [34]. Sigma receptors consist of several subtypes that are distinguishable by biochemical and pharmacological means [41]. Among these, the type 1 sigma receptor (σR1) is the best characterized. σR1 is believed to mediate the immunosuppressant, antipsychotic and neuroprotective effects elicited by σ ligands such as (+)-pentazocine, ditolyguanidine and haloperidol [11,48]. The neuroprotective effects of σR1 ligands are thought to include modulation of NMDA receptors as well as muscarinic receptors [5,35]. Sigma receptor ligands demonstrate robust neuroprotective properties including inhibition of ischemia-induced glutamate release [27,28], attenuation of postsynaptic glutamate-evoked calcium influx [10,20], depressed neuronal responsivity to NMDA receptor stimulation [3,7,54], and reduced NO production [14].
σR expression has been demonstrated in eye. Schoenwald et al. [42] detected σR1 in lacrimocytes isolated from rabbit lacrimal gland using binding assays. More recently, Bucolo et al. [6] used binding assays to demonstrate the presence of σR1 in iris-ciliary body isolated from rabbit. Their findings were particularly important as they showed a decrease in intraocular pressure when the σR1 ligands pentazocine and (+)-N-allynormetazocine were applied topically. In retina, binding assays have demonstrated the presence of σR in bovine [44] and rat retina [56]. These investigators indicated that the retina has the highest density of σR in central and peripheral tissue suggesting an important function for these receptors. There is evidence that amacrine cells express σR based on observations that pentazocine and SA4503, two σR1 ligands, conferred neuroprotective effects against glutamate-induced damage of cultured amacrine cells [45]. Recent work in our lab demonstrated for the first time that σR1 is expressed abundantly in ganglion cells in vivo and in vitro and established the molecular identity of σR1 in retina [37]. Additionally, we demonstrated that in retinas of diabetic mice, in which ganglion cell death occurs (Moore unpublished observations), σR1 continues to be expressed in ganglion cells [38] and hence may prove to be a useful target in therapeutic intervention of ganglion cell death.
Results of the present studies demonstrate that homocysteine-induced death of cultured ganglion cells occurs by activation of the NMDA subtype of glutamate receptor and that the σR1 ligand (+)-pentazocine can prevent homocysteine-induced death of cultured retinal ganglion cells.
Materials and Methods
Reagents
Reagents used in these studies were from the following sources: DMEM:F12, TRIzol reagent, and penicillin-streptomycin (Gibco-Life Technologies, Rockville, MD); Nunc Lab-Tek II CC2 4 well or 8-well chamber slides and 75cm2 culture flasks (Fisher, Pittsburgh, PA); ApopTAG in situ Apoptosis Detection Kit-Fluorescein (Intergen, Purchase, NY); CaspACE FITC-VAD-FMK in situ marker (Promega, Madison, WI); FITC-conjugated AffiniPure goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories Inc., West Grove, PA); Vectashield Mounting Medium for fluorescence (Vector Laboratories, Burlingame, CA); fetal bovine serum, succinyl concanavalin A (sConA), L-glutamate, D,L-homocysteine, (+)-pentazocine, haloperidol, MK-801 and all other chemicals (Sigma Chemical Corporation, St. Louis, MO).
Cell Culture
The development of the rat ganglion cell line (RGC-5) has been described [21]. RGC-5 cells were maintained at 37°C in a humidified chamber of 5% CO2. They were cultured in 75 cm2 flasks with Dulbecco’s modified Eagle’s medium: nutrient mixture F12 (DMEM: F12), supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 µg/ml streptomycin. The culture medium was replaced with fresh medium every other day. Upon confluency, cultures were passaged by dissociation in 0.05% (w/v) trypsin in 0.01M phosphate-buffered saline (PBS).
Homocysteine excitotoxicity in cultured RGCs
RGC-5 cells were cultured in Nunc Lab-Tek II CC2 4 or 8-well chamber slides. The differentiation of RGC-5 cells was promoted following the method of Krishnamoorthy et al. [21]. Briefly, RGC-5 cells, which had been grown as described above, were cultured in the absence of serum for 24 h, after which they were cultured in DMEM (4 mM glutamine, 1% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin) supplemented with 50 µg/ml succinyl concanavalin A (sConA) for 7 days. Differentiated RGC-5 cells were treated with D, L-homocysteine or L-glutamate (1 mM solutions prepared in the DMEM). Incubation of cells with L-glutamate (1 mM) served as a positive control for induction of RGC-5 apoptosis. To determine whether homocysteine-induced RGC-5 cell death was mediated by NMDA receptor activation, homocysteine-treated cells were incubated with the NMDA-specific antagonist dizocilpine (MK-801) (3, 10 or 25 µM), dosages found to be neuroprotective in rat neuronal cells [26]. Additional homocysteine-treated cells were incubated with 25 µM NBQX, an antagonist of AMPA/kainate receptors or 25 µM nifedipine, a known calcium channel blocker, to determine whether RGC-5 cell apoptosis induced by acute exposure to homocysteine or glutamate is mediated by stimulation of NMDA receptors alone, or through several other glutamate receptors as well. To test the neuroprotective effects of σR1 ligands, homocysteine-treated cells were incubated with the σR1-specific agonist (+)-pentazocine, or the non-selective σR1 ligand haloperidol. Differentiated RGC-5 cells were cultured in serum-free DMEM: F12 for 24 h. Cells were then pre-treated for 1 h with (+)-pentazocine or haloperidol (3 or 10 µM each), followed by 24 h exposure to culture medium containing homocysteine or glutamate (1 mM) in the presence and absence of 3 or 10 µM (+)-pentazocine or haloperidol. These dosages were selected based upon reports that they were neuroprotective in other systems [10, 29].
TUNEL Assay for in situ Detection of DNA fragmentation
The presence of DNA fragmentation in differentiated RGC-5 cells was evaluated in homocysteine-treated and control cultures via TUNEL (terminal dUTP nick end labeling) analysis [13] using the ApopTAG in situ Apoptosis Detection Kit-Fluorescein following our previously described method [35]. Prior to TUNEL analysis, treated cells were fixed in 1% paraformaldehyde in 0.01M PBS for 5 min at room temperature and washed in two changes of 0.01M PBS for 5 min each. For negative stain control, deionized water was substituted for the TdT enzyme in the reaction mixture. Upon completion of the TUNEL assay, coverslips were mounted using Vectashield Mounting Medium for fluorescence. Cells subjected to TUNEL analysis were viewed by epifluorescence using standard fluorescein excitation and emission filters. Each field was examined systematically for the presence of green positively-labeled cells indicating apoptosis. Images were photographed (10 fields per chamber) at using the 40X objective. Data were expressed as the number of positive cells per 100 cells. Images were captured using Spot software.
In Situ Detection of Activated Caspases
To further evaluate the treated cells for apoptosis, we used the CaspACE FITC-VAD-FMK in situ marker to detect caspase activity. CaspACE FITC-VAD-FMK in situ marker was added to the medium of homocysteine- and glutamate-treated cells at a final concentration of 10 µM. Cells were protected from light and re-incubated for 20 min at 37°C in a humidified chamber of 5% CO2. Cells were then fixed in 1% paraformaldehyde for 5 min, washed with PBS, and coverslips were mounted using Vectashield Mounting Medium for fluorescence containing 1 µg ml−1 propidium iodide. Cells were viewed by epifluorescence using standard fluorescein excitation and emission filters. The number of green, positively-labeled cells indicating localization of activated caspases was counted in each field and as with the TUNEL analysis, the data were expressed as the number of caspase positive cells per 100 cells. Spot software was used for image capture and analysis.
Neutral Red Assay
The effect of acute exposure to glutamate or homocysteine on RGC-5 cell viability was tested using the neutral red assay [4]. RGC-5 cells were seeded on laminin-coated 24-well plates and induced to differentiate. Differentiated RGC-5 cells were cultured in serum-free media for 24 h, then treated with glutamate or homocysteine (1 mM) for 24 h, both in the presence and absence of MK-801, a selective antagonist of the NMDA receptor, nifedipine (a calcium channel blocker), or NBQX (an antagonist of AMPA/kainate glutamate receptors). Neutral red dye was added to a final concentration of 0.033% to cell culture media after the cells were treated with the indicated compounds. Cells were incubated for 2 h at 37°C, then washed gently with HEPES buffer (125 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 2 mM MgCl2, 0.5 mM NaH2PO2, 5 mM NaHCO3, 10 mM d-glucose, 10 mM HEPES, pH 7.2). The cells were allowed to air dry for 20 min, then were treated with ice-cold solubilization buffer (ethanol: acetic acid; 5:1; 300 µl/well) for 20 minutes. 100 µl aliquots were then transferred to the wells of flat-bottomed 96-well plates and optical densities of samples were read at 570 nm.
Image capture/Data Analysis
Images were obtained using a Zeiss Axioplan 2 fluorescent microscope (Carl Zeiss, West Germany) equipped with a Spot Camera and Spot Software version 2.2 (Diagnostic Instruments, Sterling Heights, MI). ANOVA was used to determine if there was a difference in the number of TUNEL-positive cells between compound-treated and control cells at each of the differing concentrations (ANOVA: factors = compound treatment, concentration). P < 0.05 was considered significant. Tukey-Kramer’s multiple comparison test was the post hoc statistical test.
Semiquantitative RT-PCR analysis of σR1 mRNA in RGC-5 cells exposed to homocysteine or glutamate
Confluent RGC-5 cells were cultured in medium containing 1 mM D, L-homocysteine or L-glutamate for 24 h. Total RNA was prepared using the TRIzol reagent. RT-PCR was carried out using primer pairs specific for rat σR1 and rat glyceraldehye-3-phospate dehydrogenase (GAPDH). The primers for σR1 were 5’-GTTTCTGACTATTGTGGCGGTGCTG-3’ (sense) and 5’-CAAATGCCAGGGTAGACGGAATAAC-3’ (antisense) corresponding to nucleotide positions 80–104 and 567–591 respectively of the cloned rat σR1 cDNA [45]. RT-PCR was performed over cycles 12 through 32, with denaturing phase of 30 sec at 94°C, annealing phase of 30 sec at 60.5°C and an extension of 2 min at 72°C. 10 µl of the PCR products were gel electrophoresed and subjected to Southern hybridization with a 32P-cDNA probe specific for rat σR1. Rat GAPDH was utilized as an internal control. The upstream primer 5’-TGGAGTCTA CTGGCGTCTTC-3’(sense) and the downstream primer 5’-TCATGAGCCCTTCCACGATG-3’ (antisense) correspond to nucleotide positions 1130–1140 and 1350–1369 respectively, in rat GAPDH cDNA (GenBank accession number: AF106860). The hybridization signals were quantified using STORM Phosphorimaging System (Molecular Dynamics, Sunnyvale, CA) and processed using ImageQuaNT (Version 4.2a) software application. The relationship between the intensity of the signal and the PCR cycle number was analyzed to determine the linear range for the PCR product formation. The intensities of the signals within the linear range were used for data analysis.
Immunocytochemistry
The σR1 was detected in differentiated RGC-5 cells exposed to 1 mM homocysteine or 1 mM glutamate using immunocytochemical methods. Differentiated RGC-5 cells were serum-deprived for 24 h, followed by 24 h exposure to culture medium containing 1 mM homocysteine or glutamate. Treated and control RGC-5 cells were then fixed in ice-cold methanol for 5 min, washed with 0.01M PBS (pH 7.4) and blocked with 10 % normal goat serum for 60 min. Cells were incubated with the σR1 specific polyclonal antibody [38] at a dilution of 1:250 overnight at 4°C. Negative control sections were treated identically with buffer only or normal goat serum. Cells were rinsed and incubated overnight at 4°C with a fluorescein isothiocyanate (FITC)-conjugated AffiniPure goat anti-rabbit IgG at a dilution of 1:100. Slides were examined using a Zeiss Axioplan 2 fluorescent microscope equipped with a Spot Camera and Spot Software version 2.2.
Results
Homocysteine-induced death of cultured RGCs
To assess the consequences of acute exposure to high levels of homocysteine on cultured rat ganglion cells, differentiated RGC-5 cells were exposed to 1 mM homocysteine for 24 h. Exposure to glutamate (1 mM) served as a positive control for induction of RGC-5 death. Cells were fixed and processed for determination of apoptosis using the TUNEL assay. Figure 1A depicts fluorescent microscopic detection of TUNEL-positive RGC-5 cells in control cells and RGC-5 cells following 24 h exposure to 1 mM glutamate or homocysteine. There were very few TUNEL positive control cells; however treatment with 1 mM homocysteine increased the number of TUNEL-positive cells considerably. Analysis of activated caspase using CaspACE showed similar results (Figure 1B) and suggested that homocysteine-treated cells were dying by apopotosis. The number of TUNEL-positive RGC-5 cells per 100 cells was determined in each chamber (Figure 1C) and there was a significant increase in the number of TUNEL-positive RGC-5 cells in cultures exposed to 1 mM homocysteine as well as glutamate compared to controls. Similar results were obtained via in situ detection of activated caspases (data not shown). These data suggest that homocysteine is toxic to ganglion cells in vitro as it is in vivo [36].
Figure 1. Detection of apoptosis in RGC-5 cells following acute exposure to homocysteine or glutamate.
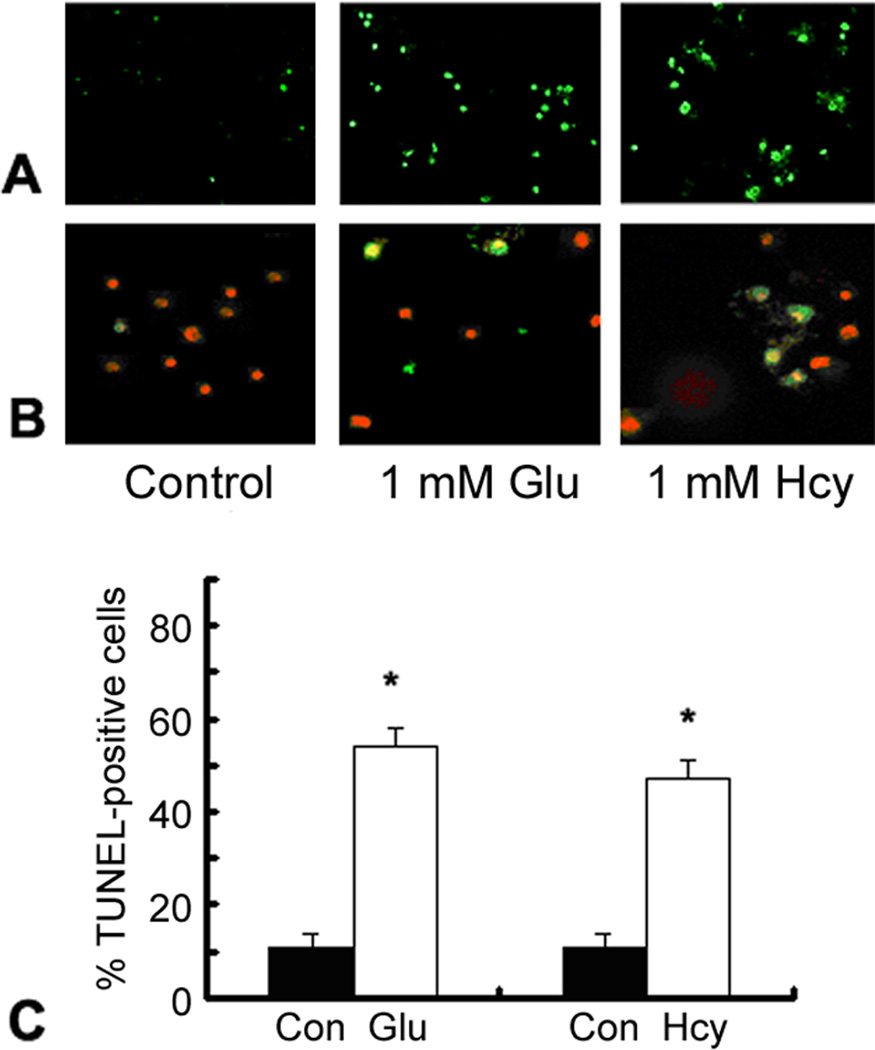
(A) Fluorescent microscopic detection of TUNEL-positive and (B) activated caspase-positive RGC-5 cells following 24 h exposure to 1 mM glutamate (Glu) or 1 mM homocysteine (Hcy). (Magnification 400x). In panel B, the red staining reflects propidium iodide, which labels all cells, the yellow cells reflect cells that are positive also for activated caspase. (Magnification 630x). (C) Quantitation of RGC-5 cells exposed to hyc or glu (1 mM) for 24 h and subjected to TUNEL analysis. The number of TUNEL-positive cells per 100 cells counted was determined in representative images of each chamber. *Significantly different from control, untreated cultures (ANOVA: F=50.56, p<0.001). Data are mean ± S.E. from three separate experiments.
Homocysteine-induced RGC toxicity is mediated by NMDA receptor
To determine whether homocysteine-induced death of ganglion cells was mediated via activation of NMDA receptors, RGC-5 cells were exposed to culture medium containing 1 mM homocysteine in the presence and absence of MK-801, a selective antagonist of the NMDA receptor. MK-801 is neuroprotective against glutamate-mediated toxicity in retina via blockade of NMDA receptor activity [23,1]. Differentiated RGC-5 cells were serum-deprived for 24 h, followed by 1 h pretreatment with MK-801 (3 or 10 µM). RGC-5 cells were incubated for 24 h in culture medium containing 1 mM homocysteine or 1 mM glutamate in the presence or absence of (+)-MK-801 (3 or 10 µM). The number of TUNEL-positive cells per 100 cells was determined for each chamber (Figure 2). Apoptosis of RGC-5 cells exposed to 1 mM homocysteine was attenuated significantly by treatment of the cells with MK-801. Treatment of RGC-5 cells exposed to 1 mM homocysteine with 3 µM MK-801 resulted in a significant reduction in the percentage of TUNEL-positive cells, while 10 µM MK-801 further attenuated the cell death. Glutamate-induced cell death was prevented by MK-801 in a similar manner. To confirm these observations, additional RGC-5 cells were exposed to 1 mM homocysteine in the presence or absence of 3, 10 or 25 µM MK-801 and cell viability was assessed using the Neutral Red assay (Figure 3). Cell survival under various treatment conditions was calculated as percent of cell survival in control cultures. RGC-5 cells subjected to acute exposure to 1 mM homocysteine exhibited ∼50% cell death. Culture of cells with 1 mM homocysteine in the presence of 3 or 10 µM MK-801 attenuated the cell death somewhat, although cell survival was still significantly less than control cells. Treatment of RGC-5 cells exposed to 1 mM homocysteine with 25 µM MK-801 completely abrogated RGC-5 cell death induced by homocysteine. Additional RGC-5 cells were treated with 1 mM homocysteine in the presence of 25 µM NBQX (an AMPA/kainate receptor antagonist) or nifedipine (a calcium channel blocker) and cell viability analyzed via Neutral Red assay (Figure 3). NBQX or nifedipine failed to provide protection against homocysteine-induced cell death. These data suggest that in vitro ganglion cell death induced by homocysteine is mediated by stimulation of NMDA receptors.
Figure 2. Comparison of TUNEL-positive RGC-5 cells exposed to homocysteine or glutamate in the presence or absence of (+)-MK-801, an NMDA receptor antagonist.
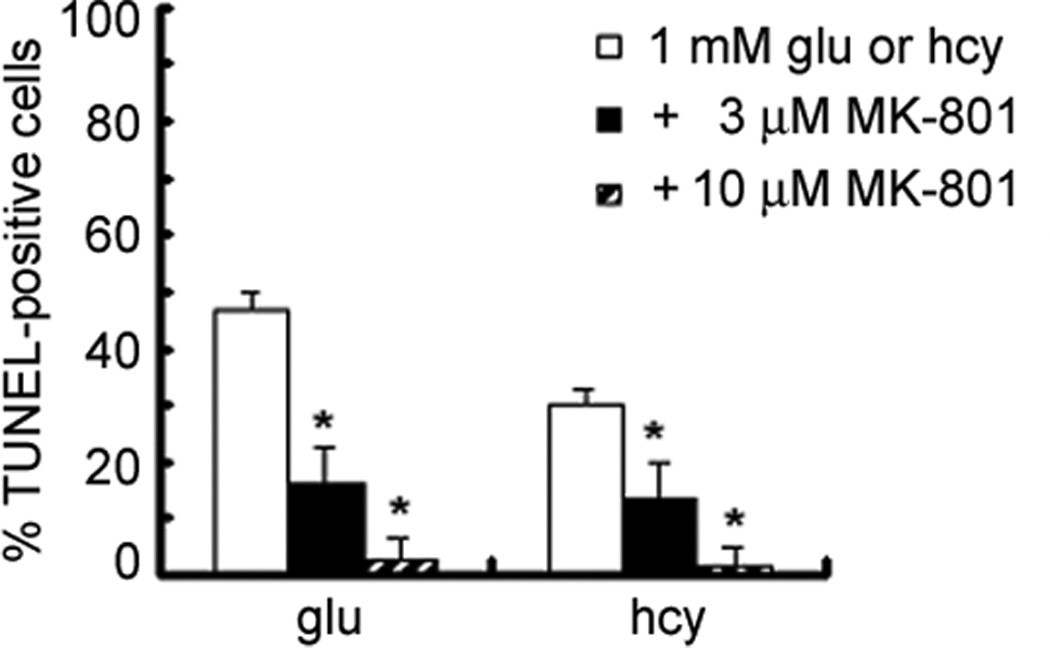
Differentiated RGC-5 cells were cultured on chamber slides in the absence of serum for 24 h, followed by 24 h exposure to either 1 mM homocysteine (Hcy) or 1.0 mM (Glu) in the presence or absence of (+)-MK-801 (3 or 10 µM). The number of TUNEL-positive cells was determined per 100 cells counted. * Significantly different from glutamate- or homocysteine-treated cells (ANOVA: F=29.17, p<0.001). Data are mean ± S.E. from three separate experiments.
Figure 3. Assessment of RGC-5 cell survival via neutral red assay following treatment with MK-801, NBQX or nifedipine.
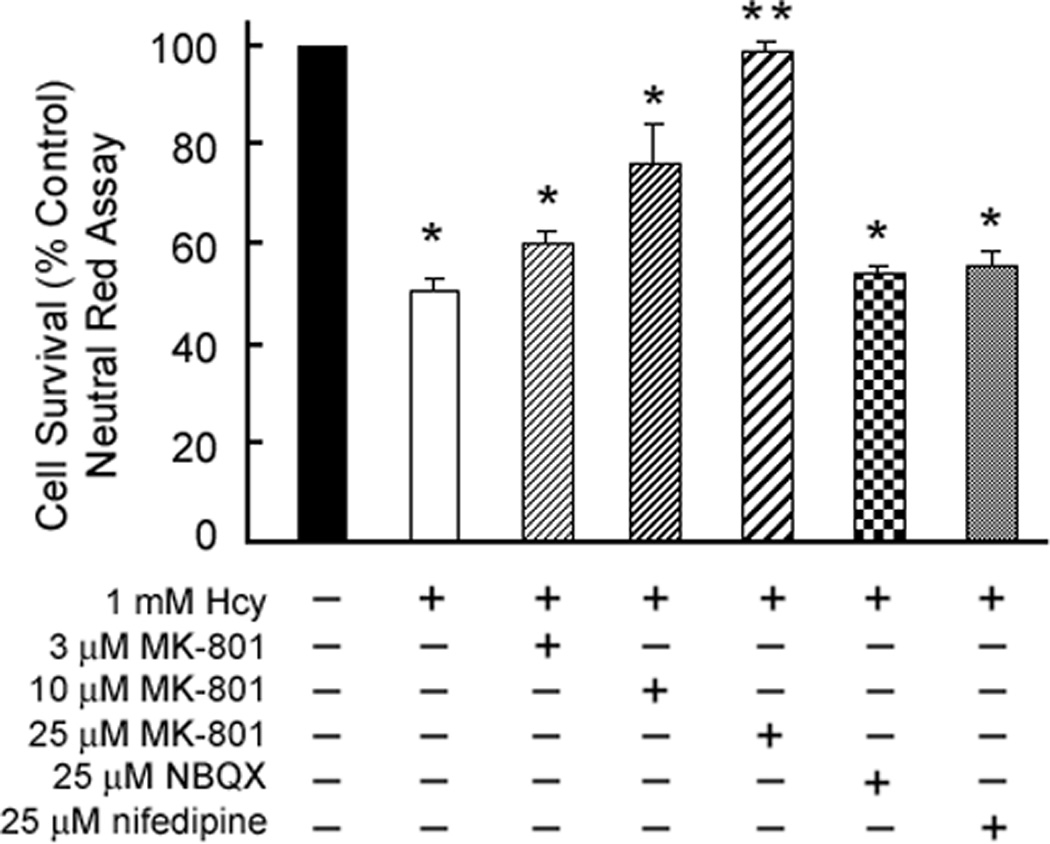
Differentiated RGC-5 cells were subjected to 24 h exposure to 1 mM homocysteine in the presence or absence of MK-801 (3, 10, 25 µM), NBQX, (25 µM) or nifedipine (25 µM). Cell survival was analyzed using the Neutral Red assay. *Significantly different from control, ** significantly different from cells exposed to 1 mM homocysteine (ANOVA: F= 14.23, p<0.001). Data are mean ± S.E. from three separate experiments (n = 6).
σR1 expression in cells exposed to homocysteine or glutamate
σR1 is expressed abundantly in normal RGCs in vivo and in vitro [37]. To determine whether exposure to homocysteine or glutamate alters this expression, RGC-5 cells were exposed to culture medium containing 1 mM homocysteine or glutamate for 24 h. The expression of mRNA encoding σR1 was determined by semiquantitative RT-PCR (Figure 4A). GAPDH mRNA was analyzed as an internal control. The hybridization signals were quantified using the STORM Phosphorimaging system. The relationship between the intensity of the signal and the PCR cycle number was analyzed to determine the linear range for the PCR product formation. The intensities of the signal within the linear range were used for data analysis (Figure 4B). The expression of mRNA encoding σR1 in RGC-5 cells exposed to 1 mM homocysteine or glutamate did not differ significantly from that of control, untreated RGC-5 cells (F=2.06, p=0.11). Therefore, exposure of RGC-5 cells to high levels of homocysteine or glutamate does not alter σR1 mRNA expression.
Figure 4. Semiquantitative RT-PCR of σR1 mRNA in RGC-5 cells after 24 h exposure to 1mM homocysteine or glutamate.
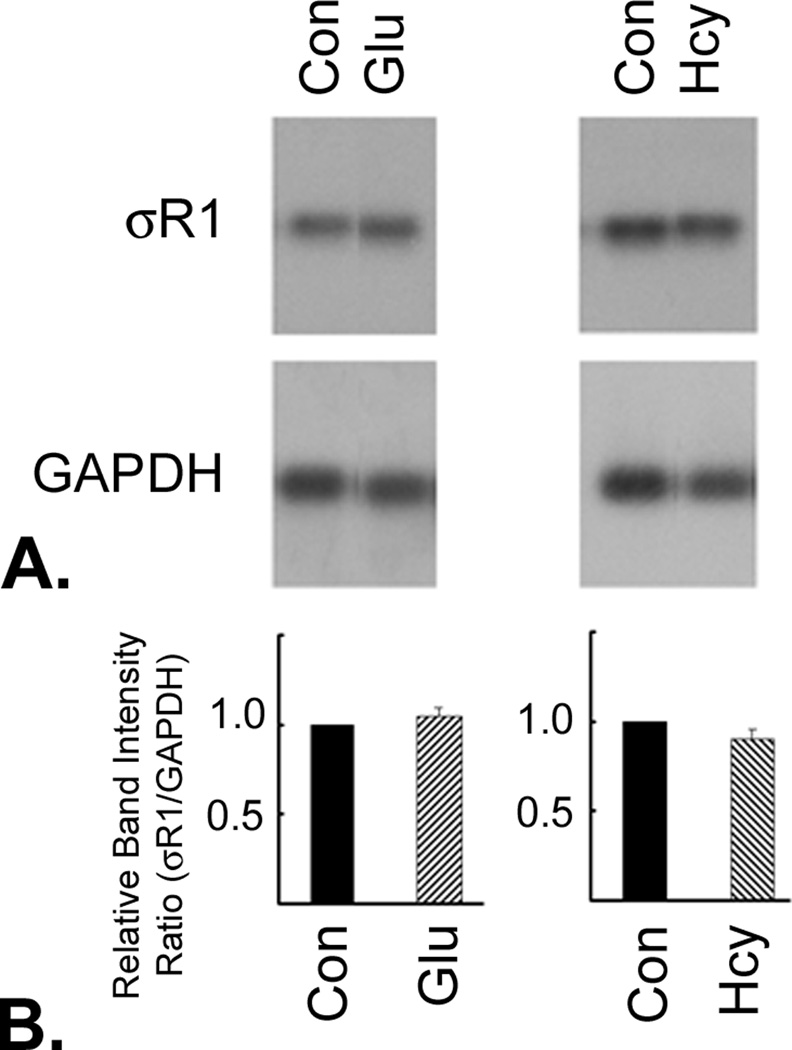
RGC-5 cells exposed to 1mM glutamate (Glu) or 1 mM homocysteine (Hcy) for 24 h. (A) The expression of mRNA encoding σR1 was determined by semiquantitative RT-PCR. GAPDH was used as an internal control. (B) The hybridization signals were quantified and the data represent the intensity of the σR1-specific band after adjusting for the variations in the GAPDH-specific band. The relationship between the intensity of the signal and the PCR cycle number was analyzed to determine the linear range for the PCR product formation. The intensities of the signals within the linear range were used for data analysis.
Immunocytochemical analysis of σR1 protein in cells treated with homocysteine or glutamate
After determining that σR1 mRNA expression was not altered in the presence of homocysteine, the presence and distribution of σR1 protein were analyzed immunocytochemically. Differentiated RGC-5 cells were cultured in serum-free media for 24 h, followed by 24 h exposure to 1 mM homocysteine or glutamate. Cells were fixed and incubated with polyclonal antibody against σR1. Figure 5 depicts fluorescent microscopic immunodetection of σR1 protein in control RGC-5 cells (5A) and in RGC-5 cells exposed to 1 mM glutamate (5B) or homocysteine (5C) for 24 h. In control cells, the antibody labeled the cell membrane. In cells treated with glutamate (5B), there were fewer cells owing to apoptotic death of the cells, however the cells that remain demonstrate the same σR1 distribution pattern as untreated control cells (5A). Cells treated with either glutamate or homocysteine continued to be immunopositive for σR1. Cells incubated with pre-immune rabbit serum only showed no positive immunoreactvity (data not shown). Thus, the immunocytochemical analysis for σR1 supported the results obtained via semi-quantitative RT-PCR and demonstrate that expression and localization of σR1 is not altered by 24 h exposure to high concentrations of homocysteine or glutamate.
Figure 5. Immunocytochemical analysis of σR1 expression in RGC-5 cells after 24 h exposure to homocysteine or glutamate.
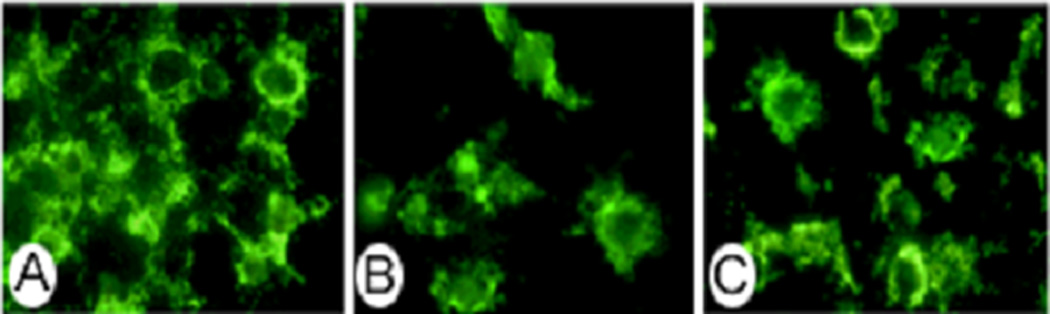
Differentiated RGC-5 cells were cultured in serum-free media for 24 h, followed by 24 h exposure to (A) no compound (B) 1 mM homocysteine or (C) 1 mM glutamate and subjected to immunofluorescence detection of σR1 using a polyclonal antibody to σR1 and an FITC-labeled secondary antibody
σR1 ligands as protective agents against homocysteine- and glutamate-induced RGC apoptosis
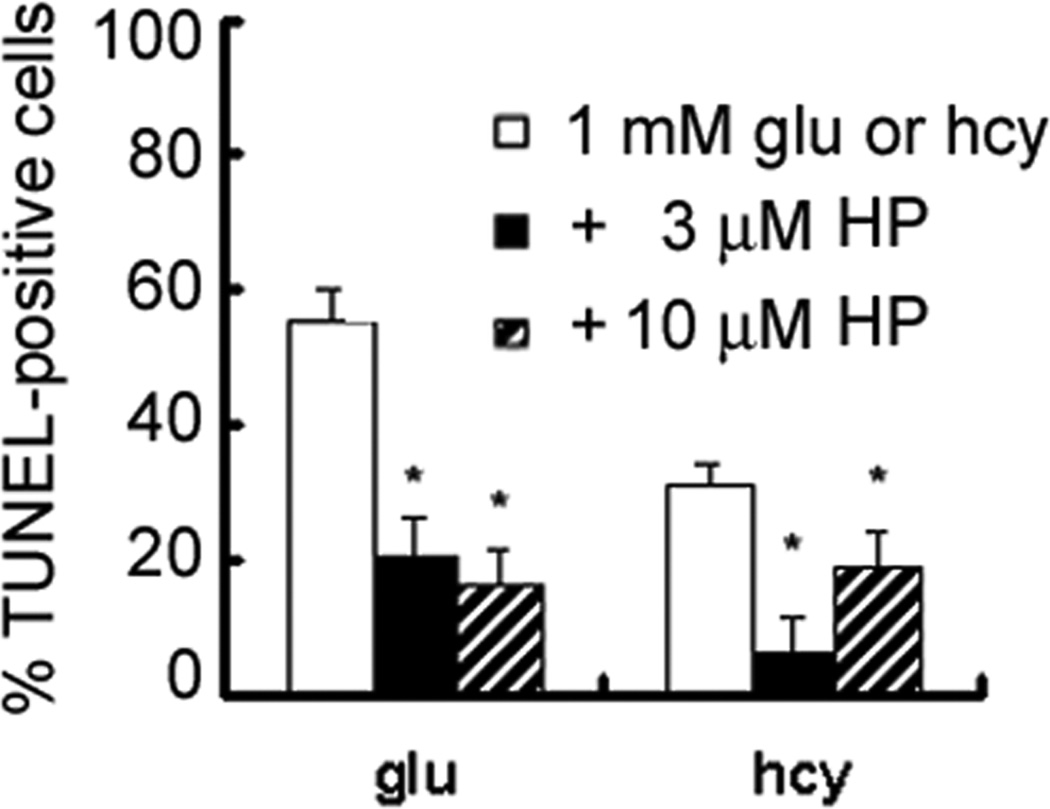
Results of the semi-quantitative RT-PCR and immunocytochemical analyses suggest that σR1 expression and protein distribution are not altered by exposure to homocysteine or glutamate at least up to 24 h. Thus, σR1 may be an available therapeutic target to prevent the toxic effects of these excitatory amino acids. To test this, we determined whether the non-selective σR1 ligand haloperidol and the σR1-specific ligand (+)-pentazocine would prevent RGC apoptosis induced by homocysteine. Differentiated RGC-5 cells were pretreated for 1 h with haloperidol (3 or 10 µM) followed by 24 h co-incubation with 1 mM homocysteine or glutamate. Cells were fixed and subjected to TUNEL analysis. The number of TUNEL-positive cells was determined per 100 cells counted. As shown in Figure 6, the number of TUNEL-positive cells following pre-treatment with either 3 µM or 10 µM haloperidol decreased significantly compared to glutamate- and homocysteine-exposed cells in the absence of haloperidol. Interestingly, cells treated with homocysteine showed a better response to the lower dosage of haloperidol (3 µM) than to the 10 µM concentration.
Figure 6. Neuroprotective effect of haloperidol (HP) against homocysteine or glutamate toxicity.
Differentiated RGC-5 cells were cultured on chamber sides in the absence of serum for 24 h, followed by 24 h exposure to 1 mM homocysteine or glutamate in the presence or absence of haloperidol (3 or 10 µM). The number of TUNEL-positive RGC-5 cells was determined in representative images of each chamber (n = 6). * Significantly different from glutamate- or homocysteine-treated cells (p<0.05).
Additional RGC-5 cells were pretreated for 1 h with (+)-pentazocine (3 or 10 µM) followed by co-incubation with 1 mM homocysteine or glutamate. Cells were fixed and subjected to TUNEL analysis. The number of TUNEL-positive cells was expressed per 100 cells examined (Figure 7). Treatment of RGC-5 cells with (+)-pentazocine attenuated significantly RGC-5 apoptosis induced by exposure to homocysteine or glutamate. Treatment of cultures with 3 µM (+)-pentazocine resulted in a 2–3-fold reduction in the percentage of apoptotic cells in cultures exposed to 1 mM homocysteine or glutamate for 24 h. This reduction was even greater in cultures treated with 10 µM (+)-pentazocine. These results demonstrate that the σR1-specific ligand (+)-pentazocine is efficacious in preventing RGC-5 cell death induced by in vitro exposure to homocysteine and glutamate. The neuroprotective effects conferred by treatment of RGC-5 cells with (+)-pentazocine are dose-dependent.
Figure 7. Neuroprotective effects of the σR1 ligand (+)-pentazocine against homocysteine or glutamate toxicity.
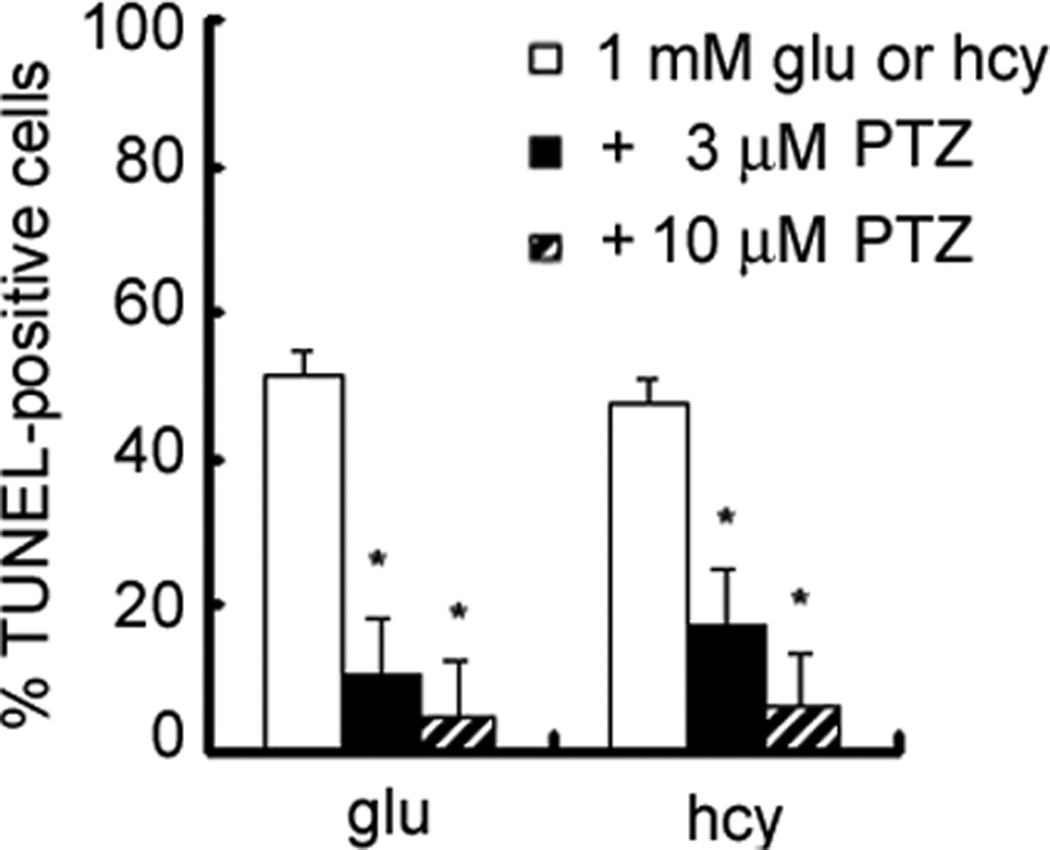
Differentiated RGC-5 cells were cultured on chamber sides in serum-free media for 24 h, followed by exposure to 1 mM homocysteine or glutamate in the presence or absence of (+)-pentazocine (3 or 10 µM). The number of TUNEL-positive cells was determined in representative images of each chamber (n = 6). * Significantly different from glutamate- or homocysteine-treated cells (p<0.05).
Discussion
There were three important findings of the present study. The first was that in vitro, as in vivo [36], homocysteine induced death of retinal ganglion cells. The second was that the homocysteine-induced excitotoxicity in ganglion cells was mediated by NMDA receptor activation. The third was that σR1 ligands could prevent the homocysteine-induced ganglion cell death.
Regarding the first finding, while we had demonstrated previously that homocysteine induced apoptosis of ganglion cells when injected into the vitreal chamber of mice [36], we wanted to test an established retinal ganglion cell line for the effects of acute exposure to high levels of homocysteine. It has been reported that the RGC-5 cell line expresses many proteins characteristic of ganglion cells, such as THY-1, Brn 3C and NMDA-receptor [21] . As described in the original publication, when the cells are not differentiated in culture, they do not demonstrate the characteristic susceptibility to glutamate that intact ganglion cells demonstrate. When differentiated, the cells still require higher levels of glutamate (500 µM – 1 mM) to induce approximately 50% death of the cells. Nonetheless, for initial studies, particularly for neuroprotection, they offer a screening system that is much more rapid than studies using an intact animal system. It was for that reason that we tested a high dosage of homocysteine (1 mM) and readily acknowledge that this dosage is much higher than physiologic levels of homocysteine and higher than the dosage which induced death of ganglion cells in vivo [36]. Nevertheless, from these acute studies we have confirmed the susceptibility of the cultured cell line to homocysteine toxicity. It now remains to establish primary ganglion cells in culture to test the sensitivity of the cells to homocysteine, particularly to levels likely to be seen under disease states.
The second finding of the study was that homocysteine-induced cell death was likely occurring via a mechanism similar to that of glutamate. Glutamate’s neurotoxicity via NMDA receptor stimulation is well documented [49]. The NMDA overstimulation is thought to trigger an increase of intracellular calcium that leads to the apoptotic cell death cascade. While homocysteine has been shown to act via the NMDA receptor in other tissues and cell types [18,26], its mechanism of action on ganglion cells has not been examined. To test whether the NMDA receptor was involved in the homocysteine-induced ganglion cell death, pharmacologic methods were used. RGC-5 cells were pretreated with MK-801, an NMDA-specific antagonist. Our data showed attenuation of cell death at lower MK-801 concentrations (3 or 10 µM) and complete protection from cell death when homocysteine-incubated cells were treated with 25 µM MK-801. These data suggest that the mechanism of homocysteine-induced toxicity in cultured RGC-5 cells is via NMDA receptor stimulation. It is possible however, that several pathways contribute to homocysteine-induced toxicity, and that blocking any one of them is sufficient to lower toxicity. Therefore, we examined the effect of blocking the AMPA/kainate receptor using NBQX and observed no neuroprotective effect. In addition, since it is generally thought that NMDA receptor-mediated toxicity occurs through calcium influx through the receptor channel, additional cells were treated with homocysteine in the presence of nifedipine, a blocker of voltage-gated calcium channels. Interestingly, our data demonstrated that treatment of RGC-5 cultures for 24 h with homocysteine in the presence of nifedipine, which blocks receptor-mediated calcium influx, did not reverse the cell death. This suggests that the acute treatment with high dosages of homocysteine may not be leading to increased calcium, however it can not be ruled out that homocysteine may have induced oxidative stress by increasing for example nitric oxide production.
The third finding of this study was that homocysteine-induced RGC-5 cell death could be prevented by σR1 ligands. Prior to testing any σR1 ligands, it was necessary to determine whether the gene encoding σR1 continued to be expressed following exposure to homocysteine. Exposure to homocysteine, as well as glutamate, neither down-regulated nor upregulated σR1 expression indicating that these excitotoxins did not alter σR1 gene expression. Additional immunocytochemical studies suggested that the σR1 protein is detected in homocysteine- and glutamate- treated cells. These findings set the stage for additional experiments to use σR1 ligands as potential protective agents against homocysteine-induced cell death in ganglion cells.
Pretreatment of RGC-5 cells with either (+)-pentazocine or haloperidol, followed by co-incubation with these compounds plus homocysteine or glutamate attenuated cell death significantly. In the case of glutamate treated cells, the effects of the two dosages of haloperidol were similar, whereas in cells treated with homocysteine the 3 µM dosage actually decreased the number of TUNEL positive cells more than the 10µM dosage. Haloperidol is known to induce apoptosis in neuronal cells [33], thus, it is possible that in the presence of homocysteine the higher dosage of haloperidol is less neuroprotective. Haloperidol can interact with dopamine type 2 receptors [9] as well as σR1 [51,53] and has been shown to have toxic side effects [31,17]. We were interested in testing the influence of haloperiodol because it is known to interact with σR1. The much more specific σR1 ligand, (+)-pentazocine, was neuroprotective at 3 and 10 µM concentrations. σR1 ligands have broad neuroprotective effects. They not only reduce NMDA receptor activation [5,34], but also can inhibit ischemia-induced glutamate release [27,28], attenuate postsynaptic glutamate-evoked Ca2+ influx [10,20], and reduce NO production [14]. These beneficial effects of σR1 ligands are noteworthy for diseases in which ganglion cells die, such as in glaucoma [24, 25] and, as has been reported recently, in diabetic retinopathy [2,12,15,55].
In summary, this study represents the first use of σR1 ligands in reversing the toxic effects of homocysteine. In their recent review of homocysteine’s role in neurodegenerative disorders, Mattson and Shea [30] list a number of compounds including antioxidants, NMDA receptor blockers, PARP inhibitors and Vitamin E, that have been used as neuroprotective agents to reverse homocysteine-induced degenerative conditions. It may be that certain neurodegenerative conditions, associated with increased levels of homocysteine, will benefit also from the robust neuroprotective effects of σR1 ligands. Our future studies will establish primary cultures of retinal ganglion cells, test their sensitivity to homocysteine, and explore the role of σR1 as neuroprotectants.
Acknowledgements
This work was supported by National Institutes of Health Grants EY12830 and EY014560.
Literature Cited
- 1.Adachi K, Fujita Y, Morizane C, Akaike A, Ueda M, Satoh M, Masai H, Kashii S, Honda Y. Inhibition of NMDA receptors and nitric oxide synthase reduces ischemic injury of the retina. Eur. J. Pharmacol. 1998;350:53–57. doi: 10.1016/s0014-2999(98)00317-3. [DOI] [PubMed] [Google Scholar]
- 2.Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. The Penn State Retina Research Group, Neural apoptosis in the retina during experimental and human diabetes: early onset and effect of insulin. J. Clin. Invest. 1998;102:783–791. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhardwaj A, Sawada M, London ED, Koehler RC, Traystman RJ, Kirsch JR. Potent σ1-receptor ligand 4-phenyl-1-(4-phenylbutyl)-piperidine modulates basal and N-methyl-D-aspartate–evoked nitric oxide production in vivo. Stroke. 1998;29:2404–2411. [PubMed] [Google Scholar]
- 4.Borenfreund E, Puerner JA. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol Lett. 1985;24:119–124. doi: 10.1016/0378-4274(85)90046-3. [DOI] [PubMed] [Google Scholar]
- 5.Bowen WD, Kirschner BN, Newmen AH, Rice KC. Sigma receptors negatively modulate agonist-stimulated phosphoinositide metabolism in rat brain. Eur. J. Pharmacol. 1985;149:399–400. doi: 10.1016/0014-2999(88)90678-4. [DOI] [PubMed] [Google Scholar]
- 6.Bucolo C, Campana G, Di Toro R, Cacciaguera S, Spampinato S. Sigma 1 recognition sites in rabbit iris-ciliary body: topical sigma 1-site ligands lower intraocular pressure. J. Pharmacol. Exp. Ther. 1999;289:1362–1369. [PubMed] [Google Scholar]
- 7.Carter C, Benavides J, Legendre P, Vincent JD, Noel F, Thuret F, Lloyd KG, Arbilla S, Zivkovic B, MacKenzie ET. Ifenprodil and SL 82.0715 as cerebral anti-ischemic agents, II: evidence for N-methyl-D-aspartate σ-receptor antagonist properties. J. Pharmacol. Exp. Ther. 1988;247:1222–1232. [PubMed] [Google Scholar]
- 8.Cook JW, Taylor LM, Orloff SL, Landry GJ, Moneta GL, Porter JM. Homocysteine and arterial disease. Experimental mechanisms. Vascul Pharmacol. 2002;38:293–300. doi: 10.1016/s1537-1891(02)00254-9. [DOI] [PubMed] [Google Scholar]
- 9.Creese L, Burt D, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- 10.DeCoster MA, Klette KL, Knight KS, Tortella SC. σ Receptor-mediated neuroprotection against glutamate toxicity in primary rat neuronal cultures. Brain Res. 1995;671:45–53. doi: 10.1016/0006-8993(94)01294-r. [DOI] [PubMed] [Google Scholar]
- 11.Ferris CD, Hirsch DJ, Brooks BP, Snyder SH. σ Receptors: from molecule to man. J. Neurochem. 1991;57:729–737. doi: 10.1111/j.1471-4159.1991.tb08213.x. [DOI] [PubMed] [Google Scholar]
- 12.Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW. Diabetic retinopathy. More than meets the eye. Surv Ophthalmol. 2002;47:S253–S262. doi: 10.1016/s0039-6257(02)00387-9. [DOI] [PubMed] [Google Scholar]
- 13.Gavrieli Y, Shermanm Y Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goyagi T, Goto S, Bhardwaj A, Dawson VL, Hurn PD, Kirsch JR. Neuroprotective effect of sigma(1)-receptor ligand 4-phenyl-1-(4-phenylbutyl) piperidine (PPBP) is linked to reduced neuronal nitric oxide production. Stroke. 2001;32:1613–1620. doi: 10.1161/01.str.32.7.1613. [DOI] [PubMed] [Google Scholar]
- 15.Hammes HP, Federoff HJ, Brownlee M. Nerve growth factor prevents both neuroretinal programmed cell death and capillary pathology in experimental diabetes. Mol Med. 1995;1:527–534. [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobsen DW. Homocysteine and vitamins in cardiovascular disease. Clin Chem. 1998;44:1833–1843. [PubMed] [Google Scholar]
- 17.Kelley JJ, Gao XM, Tamminga CA, Roberts RC. The effect of chronic haloperidol treatment on dendritic spines in the rat striatum. Exp. Neurol. 1997;146:471–478. doi: 10.1006/exnr.1997.6552. [DOI] [PubMed] [Google Scholar]
- 18.Kim WK. S-nitrosation ameliorates homocysteine-induced neurotoxicity and calcium responses in primary culture of rat cortical neurons. Neurosci Lett. 1999;265:99–102. doi: 10.1016/s0304-3940(99)00212-8. [DOI] [PubMed] [Google Scholar]
- 19.Kim WK, Pae YS. Involvement of N-methyl-D-aspartate receptor and free radical in homocysteine-mediated toxicity on rat cerebellar granule cells in culture. Neurosci. Lett. 1996;216:117–120. doi: 10.1016/0304-3940(96)13011-1. [DOI] [PubMed] [Google Scholar]
- 20.Klette KL, DeCoster MA, Moreton JE, Tortella FC. Role of calcium in sigma-mediated neuroprotection in rat primary cortical cultures. Brain Res. 1995;704:31–41. doi: 10.1016/0006-8993(95)01103-x. [DOI] [PubMed] [Google Scholar]
- 21.Krishnamoorthy RR, Agarwal P, Prasanna G, Vopat K, Lambert W, Sheedlo HJ, Pang IH, Shade D, Wordinger RJ, Yorio T, Clark AF, Agarwal N. Characterization of a transformed rat retinal ganglion cell line. Brain Res. Mol. Brain Res. 2001;86:1–12. doi: 10.1016/s0169-328x(00)00224-2. [DOI] [PubMed] [Google Scholar]
- 22.Kruman II, Culmsee C, Chan SL, Kruman Y, Guo Z, Penix L, Mattson MP. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J Neurosci. 2000;20:6920–6926. doi: 10.1523/JNEUROSCI.20-18-06920.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwong JM, Lam TT. N-methyl-D-aspartate (NMDA) induced apoptosis in adult rabbit retinas. Exp. Eye Res. 2000;1:437–444. doi: 10.1006/exer.2000.0894. [DOI] [PubMed] [Google Scholar]
- 24.Levin LA. Relevance of the site of injury of glaucoma to neuroprotective strategies. Surv Ophthalmol. 2001;45:S243–S249. doi: 10.1016/s0039-6257(01)00197-7. [DOI] [PubMed] [Google Scholar]
- 25.Lipton SA. Retinal ganglion cells, glaucoma and neuroprotection. Prog. Brain Res. 2001;131:712–718. [PubMed] [Google Scholar]
- 26.Lipton SA, Kim WK, Choi YB, Kuman S, D’Emilia DM, Rayudu PV, Arnelle DR, Stamler JS. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc. Natl. Acad. Sci. USA. 1997;94:5923–5928. doi: 10.1073/pnas.94.11.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lobner D, Lipton P. σ-Ligands and non-competitive NMDA antagonists inhibit glutamate release during cerebral ischemia. Neurosci. Lett. 1990;117:169–174. doi: 10.1016/0304-3940(90)90139-z. [DOI] [PubMed] [Google Scholar]
- 28.Lockhart BP, Soulard P, Benicourt C, Privat A, Junien J-L. Distinct neuroprotective profiles for σ-ligands against N-methyl-D-aspartate (NMDA) and hypoxia-mediated neurotoxicity in neuronal culture toxicity studies. Brain Res. 1995;675:110–120. doi: 10.1016/0006-8993(95)00049-v. [DOI] [PubMed] [Google Scholar]
- 29.Lysko PG, Gagnon RC, Yue TL, Gu JL, Feuerstein G. Neuroprotective effects of SKF 10,047 in cultured rat cerebellar neurons and in gerbil global brain ischemia. Stroke. 1992;3:414–419. doi: 10.1161/01.str.23.3.414. [DOI] [PubMed] [Google Scholar]
- 30.Mattson MP, Shea TB. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003;26:137–146. doi: 10.1016/S0166-2236(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 31.Meshul CK, Tan SE. Haloperidol-induced morphological alterations are associated with changes in calcium/calmodulin kinase II activity and the glutamate immunoreactivity. Synapse. 1994;18:205–217. doi: 10.1002/syn.890180306. [DOI] [PubMed] [Google Scholar]
- 32.Miller JW. Homocysteine, folate deficiency, and Parkinson's disease. Nutr. Rev. 2002;60:410–413. doi: 10.1301/002966402320964089. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell IJ, Cooper AC, Griffiths MR, Cooper AJ. Acute administration of haloperidol induces apoptosis of neurones in the striatum and substantia nigra in the rat. Neuroscience. 2002;109:89–99. doi: 10.1016/s0306-4522(01)00455-9. [DOI] [PubMed] [Google Scholar]
- 34.Moebius FF, Striessnig J, Glossmann JH. The mysteries of sigma receptors: new family members reveal a role in cholesterol synthesis. Trends Pharm. Sci. 1997;18:67–70. doi: 10.1016/s0165-6147(96)01037-1. [DOI] [PubMed] [Google Scholar]
- 35.Monnet FP, Debonnel G, Fournier A, De Montigny C. Neuropeptide Y potentiates the N-methyl-D-aspartate response in the CA3 dorsal hippocampus. II. Involvement of a subtype of sigma receptors. J. Pharmacol. Exp. Ther. 1992;263:1219–1225. [PubMed] [Google Scholar]
- 36.Moore P, El-Sherbeny A, Roon P, Schoenlein PV, Ganapathy V, Smith VSB. Apoptotic retinal ganglion cell death is induced in vivo by the excitatory amino acid homocysteine. Exp. Eye Res. 2001;73:45–57. doi: 10.1006/exer.2001.1009. [DOI] [PubMed] [Google Scholar]
- 37.Ola MS, Moore PM, El-Sherbeny A, Roon P, Agarwal N, Sarthy VP, Casellas P, Ganapathy V, Smith SB. Expression pattern of sigma receptor 1 mRNA and protein in mammalian retina. Mol. Brain Res. 2001;95:86–95. doi: 10.1016/s0169-328x(01)00249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ola MS, Moore PM, Maddox DM, El-Sherbeny A, Huang W, Roon P, Agarwal N, Ganapathy V, Smith SB. Analysis of Sigma Receptor (σR1) expression in retinal ganglion cells cultured under hyperglycemic conditions and in diabetic mice. Mol. Brain Res. 2002;107:97–107. doi: 10.1016/s0169-328x(02)00444-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Outinen PA, Sood SK, Liaw PC, Sarge KD, Maeda N, Hirsh J, Ribau J, Podor TJ, Weitz JI, Austin RC. Characterization of the stress-inducing effects of homocysteine. Biochem J. 1998;332:213–221. doi: 10.1042/bj3320213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parnetti L, Caso V, Amici S, Lanari A, Gallai V, Bottiglieri T. Hyperhomocyst(e)inemia: a risk factor for cerebrovascular disease. Clin Exp Hypertens. 2002;24:501–509. doi: 10.1081/ceh-120015326. [DOI] [PubMed] [Google Scholar]
- 41.Quirion R, Bowen WD, Itzhak YT, Junien JL, Musacchio JM, Rothman RB, Su T-P, Tam SW, Taylor DP. A proposal for the classification of sigma binding sites. Trends Pharmacol. Sci. 1992;13:85–86. doi: 10.1016/0165-6147(92)90030-a. [DOI] [PubMed] [Google Scholar]
- 42.Schoenwald RD, Barfknecht CR, Xia E, Newton RE. The presence of σ receptor in the lacrimal gland. J. Ocular Pharmacol. 1993;9:125–139. doi: 10.1089/jop.1993.9.125. [DOI] [PubMed] [Google Scholar]
- 43.Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- 44.Senda T, Matsuno K, Mita S. The presence of σ receptor subtypes in bovine retinal membranes. Exp. Eye Res. 1997;64:857–860. doi: 10.1006/exer.1996.0272. [DOI] [PubMed] [Google Scholar]
- 45.Senda T, Mita K, Kaneda M, Kikuchi M, Akaike A. Effect of SA4503, a novel σ1 receptor agonist, against glutamate neurotoxicity in cultured rat retinal neurons. Eur. J. Pharmacol. 1998;342:105–111. doi: 10.1016/s0014-2999(97)01450-7. [DOI] [PubMed] [Google Scholar]
- 46.Seth P, Fei YJ, Li HW, Huang W, Leibach FH, Ganapathy V. Cloning and functional characterization of a sigma receptor from rat brain. J. Neurochem. 1998;70:922–931. doi: 10.1046/j.1471-4159.1998.70030922.x. [DOI] [PubMed] [Google Scholar]
- 47.Shea TB, Lyons-Weiler J, Rogers E. Homocysteine, folate deprivation and Alzheimer neuropathology. J Alzheimers Dis. 2002;4:261–267. doi: 10.3233/jad-2002-4401. [DOI] [PubMed] [Google Scholar]
- 48.Su Sigma TP. Putative links between nervous receptors endocrine and immune systems. Eur. J. Biochem. 1991;200:633–642. doi: 10.1111/j.1432-1033.1991.tb16226.x. [DOI] [PubMed] [Google Scholar]
- 49.Sucher NJ, Lipton SS, Dreyer EB. Molecular basis of glutamate toxicity in retinal ganglion cells. Vis. Res. 1997;37:3483–3493. doi: 10.1016/S0042-6989(97)00047-3. [DOI] [PubMed] [Google Scholar]
- 50.Temple MF, Luzier AB, Kazierad DJ. Homocysteine as a risk factor for atherosclerosis. Ann Pharmacother. 2000;34:57–65. doi: 10.1345/aph.18457. [DOI] [PubMed] [Google Scholar]
- 51.Vilner BJ, Costa BR, Bowen WD. Cytotoxic effects of sigma ligands: Sigma receptor-mediated alterations in cellular morphology and viability. J. Neurosci. 1995;15:117–134. doi: 10.1523/JNEUROSCI.15-01-00117.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vine AK. Hyperhomocysteinemia: a risk factor for central retinal vein occlusion. Am J Ophthalmol. 2000;129:640–644. doi: 10.1016/s0002-9394(99)00476-6. [DOI] [PubMed] [Google Scholar]
- 53.Walker JM, Bowen WD, Walker FO, Matsumoto RR, De Costa B, Rice CK. Sigma receptors: biology and function. Pharmacol. Rev. 1992;42:355–402. [PubMed] [Google Scholar]
- 54.Yamamoto H, Yamamoto T, Sagi N, Klenerova V, Goji K, Kawai N, Baba A, Takamori E, Moroji T. Sigma ligands indirectly modulate the NMDA receptor-ion channel complex on intact neuronal cells via sigma 1 site. J. Neurosci. 1995;15:731–736. doi: 10.1523/JNEUROSCI.15-01-00731.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang L, Inoue M, Dong K, Yamamoto M. Retrograde axonal transport impairment of large- and medium-sized retinal ganglion cells in diabetic rat. Curr. Eye Res. 2000;20:131–136. [PubMed] [Google Scholar]
- 56.Zuo P, Ogita K, Yoneda Y. Presence of the binding of a variety of ligands related to ionotropic excitatory amino acid receptors in rat retina. Brain Res. 1992;576:168–172. doi: 10.1016/0006-8993(92)90626-k. [DOI] [PubMed] [Google Scholar]