Abstract
As personalized medicine becomes more applicable to oncologic practice, image-guided biopsies will be integral for enabling predictive and pharmacodynamic molecular pathology. Interventional radiology has a key role in defining patient-specific management. Advances in diagnostic techniques, genomics, and proteomics enable a window into subcellular mechanisms driving hyperproliferation, metastatic capabilities, and tumor angiogenesis. A new era of personalized medicine has evolved whereby clinical decisions are adjusted according to a patient’s molecular profile. Several mutations and key markers already have been introduced into standard oncologic practice. A broader understanding of personalized oncology will help interventionalists play a greater role in therapy selection and discovery.
The incidence of cancer and deaths from cancer is projected to increase globally (1). Although there have been major advances in both understanding of cancer biology and technologic achievements in diagnosis and treatment (1,2), this knowledge has been translated only slowly and incrementally into successful therapies or outcomes. Traditional chemotherapeutic drugs were aimed nonspecifically at cell division processes; however, newer targeted drugs have been engineered selectively for specific cellular pathways and processes (proteins, genes, organs, or stromal cells) important for tumor growth (1). Many of these so-called targeted therapies employ unique characteristics of the cancer cells to inhibit them more efficiently, and these therapies may improve survival (1,3). In the late 1990s, a new era of personalized oncology began with the approval of the anti–human epidermal growth factor receptor-2 (HER-2) –targeted monoclonal antibody (mAb) agent trastuzumab in the treatment of breast cancer (4). A companion diagnostic test for HER-2 was subsequently approved. Over the last 2 decades, numerous new tests and anticancer agents based on biomarker profiles have been investigated (3,4). Imatinib treatment for gastrointestinal stromal tumor or chronic myelogenous leukemia was another early successful drug to be specifically engineered and designed for a very specific target. Targeted drugs have since become standard therapies in a range of malignancies, including liver cancer, breast cancer, lung cancer, lymphoma, and melanoma. Despite certain successes, however, the full potential for targeted therapies on overall cancer mortality has yet to be realized (1,3,5). This article defines the basic concepts, reviews the current status of major targeted therapies affecting personalized oncology, and defines the vital role played by the interventional radiologist.
CONCEPTS
The Cancer Genome Atlas project was launched in 2006 by the National Institutes of Health to explore genetic variance specific to individual cancers (6). Key in this process has been the identification of unanticipated driver mutations in some cancers. Mutations are 100-fold to 500-fold more frequent in cancer cells compared with normal cells (7). The genomes of cancer cells within a tumor are extremely variable both temporally and spatially, across histologies as well as within specific tumors. This variability has led to distinction between driver or causal and passenger mutations (2,5,7). Driver mutations actively drive the neoplastic process conferring increased growth rate or the ability to invade surrounding tissues and metastasize. Passenger mutations do not initially contribute to the disease process but may become important in the context of resistance or other mutations (2,5,7). Identification of causal mutations would help stratify patients’ risk, prognosis, and the likelihood of response but is complex because increased background mutations in cancer cells decreasing the “signal-to-noise ratio” (3,5,8). Identifying subcellular mechanisms and developing effective therapies is challenging (8). The ideal targeted therapy would focus on a unique characteristic of subcellular mechanisms specific to the neoplastic process, enabling a selective destruction of tumor cells without nonspecific toxicity (1,3).
An array of new terminology has emerged, such as “pharmacogenomics” (9), the influence of genetic variance on drug response, and “theragnostics or theranosis” (10), combining diagnostic and therapeutic interventions to predict responses and determine patient selection (2,7). The goal of personalized medicine is to apply data mining to the large amounts of data collected about individuals to enable prediction of potential disease, prevention by improved surveillance and assessment of high-risk groups, and personalized care according to a patient’s profile with active participation from patients in the decision-making process (7). Personalized medicine is based on the “4 P’s”: predictive, preventive, personalized, and participatory (2,7). Although all treatments in medicine are in theory “personalized,” cancer has become the focus for a more selective and rationally engineered personalization process. The ability to apply personalized therapy to date has been made possible through key partnerships such as with interventional radiology (IR). Investigators engaging IR colleagues early during protocol development can optimize the timing, placement, and use of specialized tissue acquisition through this multidisciplinary collaboration.
Interventional radiologists need to be appraised of these concepts to contribute in a significant manner. In this article, general concepts pertaining to biomarkers, subcellular pathways, and targeted therapies are initially outlined. Then specific biomarkers and targeted therapies approved by the U.S. Food and Drug Administration (FDA) are discussed for solid tumors most frequently encountered in IR practice. Finally, the role of IR is reviewed.
BIOMARKERS
The two major categories of biomarkers informing the process of a patient’s care are prognostic and predictive biomarkers (11). There is a plethora of prognostic biomarkers—biomarkers that provide information about potential outcome, such as survival or metastatic potential. More important to success of personalized therapeutic direction is development and validation of predictive biomarkers—biomarkers that inform potential to respond to an intervention. Critical to the success of targeted therapy application, validated predictive biomarkers are currently few in number and generally require tissue for discovery and validation, sometimes in the form of paired tissue sampling. Validated predictive biomarkers may also have prognostic potential (12,13).
Some predictive biomarkers are the targets of drugs involved in molecular pathways, DNA repair, or polymorphisms in genes involved in drug metabolism (2,12). For example, patients with colorectal carcinoma carrying UGT1A1*28 polymorphism showed higher risk of hematologic toxicity compared with patients who were not carriers, although this was only for the first treatment cycle. More importantly, these patients also showed a higher response rate to chemotherapy (14). Such biomarkers may be used to predict response to therapy and determine optimal or patient-specific drug cocktails (13). Prognostic biomarkers predict the natural course of disease, such as molecules involved in angiogenesis, dedifferentiation, and invasiveness (12,13). Genetic variability of vascular endothelial growth factor (VEGF) receptors has been linked with differing therapeutic responses and toxicity. For example, patients with breast cancer and VEGF-2578AA genotype showed increased overall median survival with bevacizumab and paclitaxel versus paclitaxel alone compared with patients with other VEGF genotypes (15). Prognostic biomarkers are biomarkers that describe outcome differences independently of therapeutic intervention. For example, before the introduction of HER-2–targeted therapy, amplification of HER-2 was a negative prognostic sign in breast cancer, associated with worse overall survival and response to all therapy compared with tumors without HER-2 amplification.
MAJOR PATHWAYS
Protein products of mutated genes interact with one another and define a biochemical or developmental pathway that confers growth or antiapoptotic advantages to cells (8). Several pathways exist, and an exhaustive list is beyond the scope of this article, but epidermal growth factor receptor (EGFR) activation plays a major role in cell proliferation and growth in several solid tumors. EGFR is a family of tyrosine kinase receptors that includes HER-2 (1,16). After ligand binding, EGFR downstream signal activates major cellular pathways, including the RAS/RAF/MAPK pathway responsible for cell proliferation and metastasis and the PI3K/AKT/mTor pathway responsible for cell survival (16). Other cellular and receptor kinases also have been targeted successfully, including platelet-derived growth factor receptor, vascular endothelial growth factor receptor-2 (VEGFR2), mammalian target of rapamycin (mTOR), and tyrosine-protein kinase kit (c-KIT) (16).
VEGF is a family of six related glycoproteins, VEGF-A through VEGF-F, and placenta growth factor 1 and 2. VEGF family members are expressed by tumor cells in response to hypoxia, nutritional stress, and acidosis (17). They stimulate members of the VEGF receptor family, VEGFR1, VEGFR2, and VEGFR3, found on hematopoietic, lymphoendothelial, and endothelial cells and some cancer cells, and result in angiogenesis and lymphangiogenesis (17). VEGF may also recruit endothelial progenitor cells.
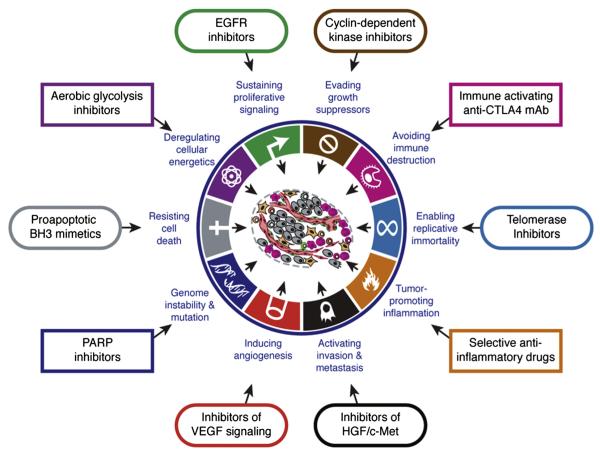
An evolving appreciation of the underlying molecular tumorigenic mechanisms of cancer outlines the complexities and oversimplifications associated with targeted therapies. For example, EGFR expression by immunohistochemistry did not predict response to anti-EGFR therapy in colon cancer (18). Drugs may target myriad cellular, molecular, and genetic or epigenetic pathways. The activation status of these pathways or subpathways may provide the explanation for either treatment sensitivity or treatment resistance. The hallmarks of cancer outline pathways and targets for drug interventions (Fig 1) (19). Factoring imaging biomarkers and using imageguided biopsy may help address the tumor heterogeneity, which is inherent to many cancers (20). Prospective correlation of specific imaging characteristics and specific tissue findings can build a library of information potentially leading in the long-term to the replacement of direct tumor sampling by noninvasive imaging.
Figure 1.
Targeting the hallmarks of cancer. Drugs that interfere with, or target, the hallmarks of cancer growth, progression, or resistance have been developed. (From Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646–674. Copyright © 2011 Elsevier. Reprinted with permission from Elsevier.)
TARGETED THERAPIES
Targeted anticancer therapies is a generic term with a variety of potential meanings, mostly related to drugs targeting specific biologic pathways that cause regression of malignant processes (1,3,9). Targeted therapies can directly alter molecular pathways of tumor cells or indirectly interfere with the tumor stroma or microenvironment by various mechanisms, such as inhibiting angiogenesis or causing vascular disruption (1). Different types of targeted therapies include (i) small molecule kinase inhibitors (SMKIs) targeting receptor or cytosolic kinase, (ii) mAbs targeting surface molecules or neutralizing circulating growth factors or cytokines, and (iii) antibody-drug conjugate.
Small Molecule Kinase Inhibitors
Examples of SMKIs are imatinib, gefitinib, erlotinib, crizotinib, and vemurafenib (1). SMKIs attach to the adenosine triphosphate (ATP)–binding site or adjacent small pocket within the kinase domain preventing ATP binding necessary for autophosphorylation and inhibit the action of protein tyrosine kinases. The latter are molecules that catalyze the transfer of phosphate from ATP to tyrosine residues (1). Protein tyrosine kinases have a different molecular constitution and mechanism of activation; however, their ATP binding site is similar. They are mutated and overexpressed in several cancers (1,5,12). For example, in chronic myelogenous leukemia, characterized by the Philadelphia chromosome, a fusion protein of bcr and Abelson (abl) kinase yields a mutant constitutively activated kinase, the driver mutation in the disease process. By binding to the kinase site of the fusion protein and inhibiting Abl tyrosine kinase activity, imatinib prohibits proliferation of leukemic progenitor cells and induces apoptosis of cells expressing this mutation (1,5). Several large-scale clinical studies have examined the effectiveness of imatinib for chronic myelogenous leukemia, with reports of a complete hematologic response in 95% of patients and a complete cytogenetic response in > 40% of patients, leading to FDA approval of this canonical targeted agent (9,21). Imatinib can also target c-KIT mutation present in gastrointestinal stromal tumors (1,3,9).
EGFR/HER1 activates the Ras signal transduction cascade and influences cell motility, invasiveness, survival, and angiogenesis. EGFR overexpression is present in many solid tumors (1,16). Gefitinib binds to the ATP-binding site of EGFR tyrosine kinase blocking EGFR from activating the Ras cascade (1,16). Table 1 lists SMKIs and their targets.
Table 1.
Small Molecule Kinase Inhibitors
| Name | Targets | Oncology Uses | Predictive Biomarkers |
|---|---|---|---|
| Dasatinib | BCR-ABL, c-KIT, PDGFR | CML, ALL | |
| Imatinib | BCR-ABL, c-KIT, PDGFR | CML, ALL, GIST | c-Kit mutation, Philadelphia chromo- some (bcr-abl) |
| Nilotinib | BCR-ABL | CML | |
| Erlotinib | EGFR | NSCLC, pancreatic cancer | EGFR mutation |
| Gefitinib | EGFR, RET | NSCLC, medullary thyroid cancer | EGFR mutation, RET oncogene |
| Lapatinib | EGFR/HER-2 | Breast cancer | HER-2 |
| Sorafenib | c-RAF, VEGFR2, EGFR, PDGFR |
HCC, RCC | |
| Sunitinib | VEGFR2, PDGFR, c-KIT | GIST, HCC, pancreatic neuroendocrine tumors |
|
| Crizotinib | ALK, cMET | NSCLC | EML4-ALK translocation |
| Vemurafenib | BRAF | Melanoma | B-Raf mutation |
| Regorafenib | VEGF | CRC | |
| Aflibercept | VEGF | CRC |
ALK, analplastic lymphoma factor, ALL, acute lymphoblastic leukemia, BCR-ABL, breakpoint cluster region/the Abelson tyrosine, BRAF, v-raf murine sarcoma viral oncogene, c-KIT, tyrosine-protein kinase kit or mast/stem cell growth factor receptor, cMET, mesenchymal epithelial transition factor, CML, chronic myelogenous leukemia, c-RAF, RAF oncogene, CRC, colorectal carcinoma, EGFR, epidermal growth factor receptor, EML4, echinoderm microtubule-associated protein-like 4, GIST, gastrointestinal stromal tumors, HCC, hepatocellular carcinoma, HER2, antihuman epidermal growth factor receptor 2, NSCLC, non–small cell lung carcinoma, PDGFR, platelet-derived growth factor receptor, RCC, renal cell carcinoma, RET, ret proto-oncogene, VEGFR2, vascular endothelial growth factor receptor-2.
mAbs Targeting Antigens and Growth Factors
Examples of targeted and clinically successful mAbs include bevacizumab, cetuximab, alemtuzumab, and rituximab. mAbs target specific antigens on key circulating growth factors or cytokines or both and cell surface receptors neutralizing their function and often yielding a proapoptotic or antimitotic effect (1). These antigens may be located within the tumor or in peritumoral tissue (ie, vessels or circulating antigens). For example, CD20 is a transmembrane protein vital to B-cell proliferation and differentiation and is overexpressed in 85% of patients with B-cell non-Hodgkin lymphoma. Rituximab is a mAb directed at CD20 (22). Table 2 lists mAbs and their targets as well as clinical indications.
Table 2.
Monoclonal Antibodies Targeted Therapies
| Name | Targets | Oncology Uses | Predictive Markers |
|---|---|---|---|
| Bevacizumab | VEGF | NSCLC, CRC, renal cancer, breast cancer, cervical cancer, ovarian cancer |
|
| Cetuximab | EGFR | CRC, head and neck cancer, NSCLC | KRAS mutation (CRC), mutation EGFR (NSCLC) (negative prediction) |
| Panitumumab | EGFR | CRC | KRAS mutation (negative prediction) |
| Rituximab | CD20 | Non-Hodgkin lymphoma | |
| Ipilimumab | CTLA-4 | Melanoma | |
| Trastuzumab | HER-2 | Breast cancer, gastric cancer | HER-2 amplification |
| Pertuzumab | HER-2 | Breast cancer | HER-2 amplification |
CD-20, clusters of differentiation-20 (antigen of B-cells), CRC, colorectal carcinoma, CTLA-4, cytotoxic T-lymphocyte associated antigen-4, EGFR, epidermal growth factor receptor, HER-2, antihuman epidermal growth factor receptor 2, KRAS, Kirsten rat sarcoma viral oncogene, NSCLC, non–small cell lung carcinoma, VEGFR2, vascular endothelial growth factor receptor-2.
Angiogenesis Inhibitors
Several biomarkers, such as fibroblast growth factor and VEGF, promote angiogenesis, a key process for tumor growth and metastatic potential. For tumors to grow > 2 mm3, they must acquire an angiogenic phenotype (1). Several agents, including mAbs and SMKIs, have been developed to block angiogenesis, tumor growth, and metastatic potential. Bevacizumab is the most heralded example of a mAb whose putative mode of action is inhibiting angiogenesis by preventing VEGF ligand from binding to and activating its receptors on vascular endothelium (1,5).
Vascular Disrupting Agents
Vascular disrupting agents (ie, combretastatin) target unique receptors on the surface of tumoral endothelial cells and take advantage of unique characteristics of tumor vasculature to reduce blood flow to the tumor, causing central necrosis (1). Often a peripheral rim of viable cancer cells persists, outlining the potential molecular and morphologic heterogeneity within solid tumors. Only a few vascular disrupting agents have reached clinical trials so far because of lack of specificity, lack of clinical efficacy, toxicity, and high cost (23,24).
Antibody-Drug Conjugates
Antibody-drug conjugates consist of cytotoxic drugs connected by chemical linkers to mAbs specific to a tumor antigen. The FDA approved trastuzumab emtansine (T-DM1) for HER-2+ breast tumors in February 2013. This drug is a combination of trastuzumab, a mAb directed against HER-2+, and DM1, a potent antimicrotubule agent, using a stable linker. Once trastuzumab emtansine binds to HER-2+, it is internalized by endocytosis, trastuzumab cleaves, and the emtansine is released leading to apoptosis of cancer cells (25).
SPECIFIC SOLID TUMORS
Certain tumors may be associated with a set of predictive and prognostic biomarkers (2,3). Only a small fraction of biomarkers and pathways have been identified and may be pharmacologically targeted. Of > 800 targeted therapies that have been developed, > 140 are undergoing clinical evaluation (3). The clinical roles of several biomarkers in tumors frequently encountered in IR practice are briefly reviewed.
Colon Cancer
FDA-approved targeted therapies for colon cancer include bevacizumab, aflibercept, cetuximab, panitumumab, and regorafenib. Bevacizumab was shown in several trials to increase progression-free and overall survival when added to first-line chemotherapy for metastatic colon cancer, albeit the gains were modest—1–4 months depending on the study (26–28).
Cetuximab and panitumumab are mAbs targeting the extracellular domain of EGFR, a receptor tyrosine kinase that when triggered causes activation of two pathways (3,28–30). The first is RAS/RAF/MAPK pathway, and the second is PI3KT/AKT/mTOR axis, vital in cell proliferation, adhesion, angiogenesis, and migration (28). Blocking EGFR is a rational approach to targeting prescreened and sensitive cancers. Cetuximab and panitumumab have been studied extensively with unimpressive results when applied to a wide unscreened population (28). Cetuximab and panitumumab may benefit only 1 in 10 patients, with a marginal improvement of progression-free and overall survival of a few months (3,12,28,30). The low response rate prompted a search for predictive biomarkers (28–30). KRAS mutation was identified as a major negative predictive biomarker because it can activate the mitogen-activated protein kinase pathway independent of EGFR status. Three major trials examined chemotherapy with or without these mAbs (cetuximab or panitumumab) in patients with KRAS mutant versus wild-type colon cancer. All three trials found that KRAS mutant tumors had significantly lower response rates, reduced progression-free and overall survival, with the addition of anti-EGFR mAbs (31–33).
BRAF, PIK3CA, and NRAS have been identified as associated prognostic markers, but their predictive value is still under investigation (3,28,30). KRAS is present in approximately 40% of colorectal carcinomas, PIK3CA mutation is present in 14.5%, BRAF is present in 4.7%, and NRAS is present in 2.6%. At the present time, available colorectal biomarkers enable identification of nonresponders but predict responders poorly (3,28,30).
Aflibercept, a novel anti-VEGF agent, was studied and deemed acceptable as a component of second-line chemotherapy for metastatic colorectal cancer (34) with statistically significant improved overall survival of 13.5 months versus 12.06 months. Finally, regorafenib, a SMKI of VEGF, was approved more recently as third-line therapy.
Hepatocellular Carcinoma
The only FDA-approved targeted therapy for hepatocellular carcinoma (HCC) is sorafenib, which blocks the c-RAF kinase, a critical component of the RAF/MEK/ERK signaling pathway and VEGFR2 involved in angiogenesis signaling (35). Sorafenib increases apoptosis, decreases angiogenesis and cell proliferation, and interferes with several tumoral pathways (36,37). The Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol trial was a prospective randomized, double-blind, placebo-controlled trial in which chemo-therapy-naïve patients with HCC who were ineligible for transarterial chemoembolization or whose tumor had progressed with transarterial chemoembolization, were randomly assigned to sorafenib or placebo. The trial demonstrated significant improvement in overall and progression-free survival of 3 months with addition of sorafenib (38). An additional prospective randomized, double-blind, placebo-controlled trial confirmed these results (39). EGFR is overexpressed in 40%–70% of HCC and protects the tumor cells from oxidative apoptosis and induces angiogenesis (37). Insulin-like growth factor, which is mutated in a third of HCC, also confers antiapoptotic properties and VEGF overexpression capabilities to the tumor cells.
Sunitinib, a multikinase inhibitor targeted against angiogenesis, seemed promising, but its use in HCC was discontinued because of side effects in phase II and III trials (40). Linifanib and brivanib, VEGF receptor (VEGFR1, VEGFR2, VEGFR3) inhibitors, have demonstrated encouraging results in phase II clinical trials and showed increased efficacy when combined with sorafenib (37). Another well-known signaling pathway involved in the development and maintenance of HCC is PI3K/AKT/mTOR, which is active in 30%–50% of HCC (36,37). Several drugs targeting this pathway are in clinical trials. Temsirolimus and everolimus are rapamycin analogues approved for kidney and breast cancers. They have shown promising results in combination with sorafenib in phase I and II clinical trials (36,37). Several SMKIs are currently in development targeting the PI3K/ AKT/mTOR pathway including PI-103 and OST 906 (37).
Non–Small Cell Lung Carcinoma
FDA-approved agents for non–small cell lung carcinoma (NSCLC) include gefitinib, erlotinib, crizotinib, and bevacizumab. Lung cancer mutation analysis is leading to personalized therapies for advanced NSCLCs in the clinic (41). A molecular panel associated with NSCLC has been identified, including KRAS (22% of tumors), EGFR (17%), EML4-ALK (7%), EGFR/HER-2 (2%), and AKT1, among others (41–44).
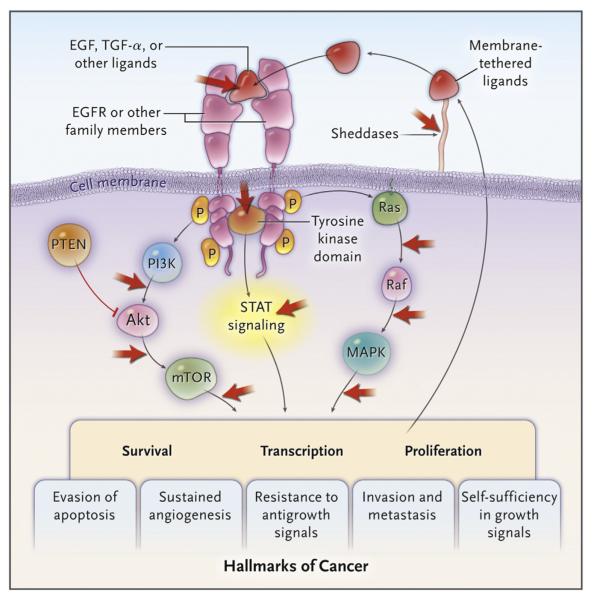
A prospective phase III randomized trial using gefinitib as single-agent first-line therapy versus platinum-based chemotherapy in patients with EGFR+ tumors showed prolonged median progression-free survival (10.8 mo vs 5.4 mo) and increased response rate (73.7% vs 30.7%) with SMKI versus chemotherapy (14). Erlotinib has shown similar results with improved tolerability (Fig 2) (41).
Figure 2.
Signaling pathways of EGFR and interaction sites of various targeted therapies. (Reprinted with permission from Gazdar AF. Personalized medicine and inhibition of EGFR signaling in lung cancer. N Engl J Med 2009; 361:1018–1020. Copyright © 2011 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.) (Available in color online at www.jvir.org.)
EML4-ALK mutations and HER-2 overexpression are more common in young patients, women, non-smokers or light smokers, and patients with EGFR– and KRAS– tumors (13,43). EML4-ALK tumors are resistant to EGFR SMKI but very sensitive to crizotinib, a selective inhibitor of this kinase (3,41,42). Overall, 40%–50% of NSCLC express one mutation and may benefit from a targeted therapy (Fig 3) (3).
Figure 3.
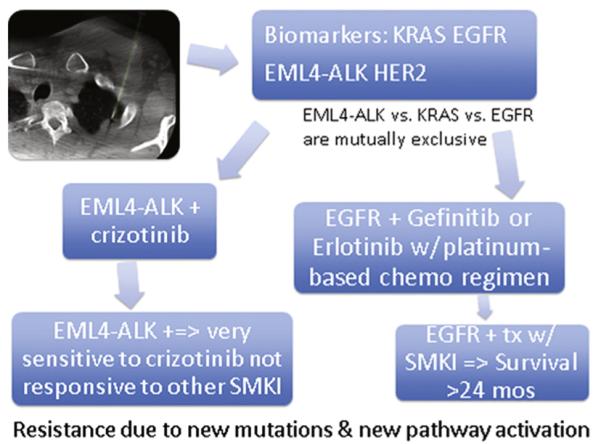
Molecular profiling lung cancer biopsy with potential outcomes and therapeutic regimens. (Available in color online at www.jvir.org.)
Breast Cancer
FDA-approved drugs for breast cancer include trastuzumab, lapatinib, pertuzumab, and trastuzumab emtansine. Bevacizumab was conditionally approved, but approval was rescinded by the FDA in December 2011; it is still often used in breast cancer treatment.
EGFR and HER-2 are overexpressed in many breast cancers with aggressive behavior (3,12). The first targeted therapy was trastuzumab, a mAb against HER-2 approved by the FDA for metastatic breast cancer in 1998 (4,12). Despite encouraging initial response, resistance to trastuzumab with subsequent increase in metastatic potential and invasiveness was observed (3). Lapatinib, a SMKI, promotes apoptosis by inhibiting activation of EGFR and HER-2 and the downstream pathways PI3K/AKT/mTOR and RAS/RAF/MAPK (45). Each targeted therapy alone has shown modest improvements, but the combination of trastuzumab and lapatinib has demonstrated a synergetic effect with a complete pathologic response of 51% in patients with HER-2+ breast tumor randomly assigned to paclitaxel with lapatinib and trastuzumab before surgery versus 29.5% with paclitaxel and lapatinib or 24.7% with paclitaxel and trastuzumab (46). Pertuzumab, another inhibitor of EGFR/HER-2, was approved more recently for use in combination with trastuzumab and docetaxel in women who have had progression of disease on trastuzumab-based therapy. Several other new agents are currently being evaluated; however, combination of agents often seems to produce the best outcomes (45). As stated earlier, trastuzumab emtansine was approved more recently with very promising results (25).
ROLE OF IR
The FDA now mandates that targeted therapies have a companion diagnostic test to select patients (11). This is generally a molecular pathology test (eg, immunohistochemistry, polymerase chain reaction, fluorescence in situ hybridization, sequencing, messenger RNA arrays, methylation) that is tissue-based and often requires image-guided biopsy (47). Interventional radiologists are seminal members of the multidisciplinary oncology team for patients considered for targeted therapy. Their expertise in targeting a specific part of a tumor is critical, whether a nonnecrotic or positron emission tomography–avid area, using imaging tools such as fusion with cone-beam computed tomography or electromagnetic tracking (48). A biopsy may be more likely to reflect relevant cellular and molecular signatures if tissue comes from an area of increased positron emission tomography activity than an area of little perfusion or from necrotic cells that provide no information at all (48).
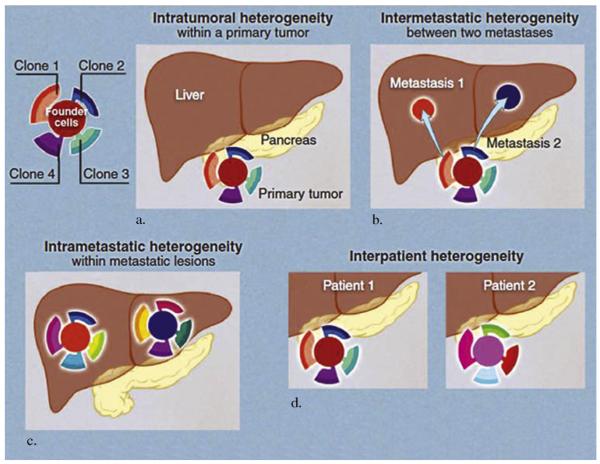
More recent discoveries have revealed that although tumors may have similar morphology, they may differ in oncogenic driver mutations and in their response to the same therapies (9). Some targeted therapies have met with unsuccessful clinical trial results, possibly partly because of unselected population or histology rather than selective administration to patients based on mutation status (ie, EGFR or KRAS status for lung and colon cancer, respectively). Biomarkers may facilitate, expedite, and validate targeted therapies and drug development. Biopsy tissue can be used before and after drug administration to “credential” a drug as producing its intended effect via an intended mechanism (49). Sequential biopsies before and after candidate drug administration may be faster than waiting for clinical or imaging responses (50). For example, a phase II clinical trial of vandetanib, targeting EGFR, VEGFR2, and ret, in ovarian cancer incorporated IR-directed biopsies before treatment and 1 month after daily drug administration (51). Biopsy specimens of the same site were obtained on both occasions, and the tissue was examined for proteomic evidence of pharmacodynamic activity. The study demonstrated a lack of clinical benefit correlated by imaging and IR. Tumor perfusion remained unchanged on dynamic contrastenhanced magnetic resonance imaging and computed tomography. VEGFR2 activation was also stable from analysis of IR-directed biopsies. This example demonstrates the power of multidisciplinary intervention where IR and imaging specialists are critical to the ability to understand the success, or in this case lack of success, of the targeted therapy. Clinical trials should consider sequential biopsies as a tool for validating biomarkers and translational endpoints and speeding development of personalized targeted therapies. The success of targeted therapies may have been limited by lack of validation of predictive biomarkers, acquired resistance, and molecular site heterogeneity (differences between primary and metastatic tumors) (Fig 4a–d) (3,8). Additional confounding factors explaining limited success of targeted therapies include intratumoral heterogeneity (8). Although a tumor may appear homogeneous on imaging, expression of mutations may be heterogeneous (20). Administration of targeted therapies induces secondary changes in mutations and molecular profiling (1). This characteristic can be used to study drug efficacy but also demonstrates the evolving nature of this process. Local therapies such as chemoembolization and radiofrequency have been documented to elicit an immune response that may be used to modulate response to other systemic therapies and increase susceptibility to targeted therapies (52). IR in personalized medicine is currently limited to tissue procurement but has the potential to expand further.
Figure 4.
(a–d) Different types of tumor heterogeneity are illustrated using pancreatic tumor and its metastasis as an example. (a) Mutations during cell growth result in heterogeneity within tumor. (b) As metastases are derived from different clones of primary tumors, intermetastatic heterogeneity is observed. (c) As metastases grow, they derive various mutations resulting in intrametastatic heterogeneity. (d) Finally, differences between patients result in interpatient heterogeneity. (From Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr, Kinzler KW. Cancer genome landscapes. Science 2013; 339:1546–1558. Copyright © 2013 The American Society for the Advancement of Science. Reprinted with permission from The American Society for the Advancement of Science.) (Available in color online at www.jvir.org.)
CONCLUSIONS
The era of personalized medicine presents a great challenge and opportunity for imaging and biopsy. Radiology and IR have a long history of innovation in minimally invasive image-guided procedures. The evolution of new roles for imaging is driven by an appreciation of the overlap between subspecialties and the opportunities to meet emerging clinical needs. In oncology, the biology and spatial heterogeneity of tumors presents an opportunity to use multimodality fusion or image guidance to shed light on biomarker status for specific tumors and to be the link between imaging and predictive molecular pathology (20). By correlating the imaging to the tissue biomarker, radiologists may play a key role as the specific fingerprints are identified for each patient’s disease process. A better understanding of the specific targeted therapies and tissue biomarkers would better inform the discovery of novel drugs and early imaging biomarkers and the role of predictive interventional biopsy in the care of patients with cancer. Sequential biopsy before and after experimental drugs in clinical trials can validate mechanism and efficacy early (50).
Imaging and image-guided biopsies may define earlier patient-specific tumor phenotypes or may predict prognosis or define sensitivities to targeted agents. This early recognition could avoid toxicities when no benefit is expected and can aid appropriate selection of therapy according to molecular profile (12,47). Patient-specific therapies are slowly replacing standard histology or organ-based algorithms (3). A rational approach for use and development of biomarkers and targeted therapies will be facilitated by informed interventional radiologists performing multimodality image-guided biopsies as part of a multidisciplinary team. Interventional radiologists will be central to this process by linking tissue and imaging biomarkers together to deliver patient-specific care better based on predictive molecular pathology.
Acknowledgments
This work is supported in part by the National Institutes of Health Center Interventional Oncology and the Intramural Research Program of the Institutes of Health (grant: 1Z01CL046011-01).
ABBREVIATIONS
- ATP
adenosine triphosphate
- ALK
analplastic lymphoma factor
- BCR-ABL
breakpoint cluster region/the Abelson tyrosine
- BRAF
v-raf murine sarcoma viral oncogene
- CD-20
clusters of differentiation-20 (antigen of B-cells)
- c-KIT
tyrosine-protein kinase kit or mast/stem cell growth factor receptor
- CML
chronic myelogenous leukemia
- CRC
colorectal carcinoma
- EGFR
epidermal growth factor receptor
- EML4
echinoderm microtubule-associated protein-like 4
- FDA
Food and Drug Administration
- HCC
hepatocellular carcinoma
- HER-2
human epidermal growth factor receptor-2
- KRAS
Kirsten rat sarcoma viral oncogene
- mAb
monoclonal antibody
- mTOR
mammalian target of rapamycin
- NSCLC
non–small cell lung carcinoma
- PDGFR
platelet-derived growth factor receptor
- SMKI
small molecule kinase inhibitor
- VEGF
vascular endothelial growth factor
- VEGFR2
vascular endothelial growth factor receptor-2
Footnotes
None of the authors have identified a conflict of interest.
REFERENCES
- 1.Li J, Chen F, Cona MM, et al. A review on various targeted anticancer therapies. Target Oncol. 2012;7:69–85. doi: 10.1007/s11523-012-0212-2. [DOI] [PubMed] [Google Scholar]
- 2.Kalia M. Personalized oncology: recent advances and future challenges. Metabolism. 2013;62(suppl 1):S11–S14. doi: 10.1016/j.metabol.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Gasparini G, Longo R. The paradigm of personalized therapy in oncology. Expert Opin Ther Targets. 2012;16(suppl 1):S7–S16. doi: 10.1517/14728222.2011.637921. [DOI] [PubMed] [Google Scholar]
- 4.Cronin M, Ross JS. Comprehensive next-generation cancer genome sequencing in the era of targeted therapy and personalized oncology. Biomark Med. 2011;5:293–305. doi: 10.2217/bmm.11.37. [DOI] [PubMed] [Google Scholar]
- 5.Saijo N. Present status and problems on molecular targeted therapy of cancer. Cancer Res Treat. 2012;44:1–10. doi: 10.4143/crt.2012.44.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science (New York, NY) 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian Q, Price ND, Hood L. Systems cancer medicine: towards realization of predictive, preventive, personalized and participatory (P4) medicine. J Intern Med. 2012;271:111–121. doi: 10.1111/j.1365-2796.2011.02498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science (New York, NY) 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nair SR. Personalized medicine: striding from genes to medicines. Perspect Clin Res. 2010;1:146–150. doi: 10.4103/2229-3485.71775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeNardo GL, DeNardo SJ. Concepts, consequences, and implications of theranosis. Semin Nucl Med. 2012;42:147–150. doi: 10.1053/j.semnuclmed.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Lee JM, Han JJ, Altwerger G, Kohn EC. Proteomics and biomarkers in clinical trials for drug development. J Proteomics. 2011;74:2632–2641. doi: 10.1016/j.jprot.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Awada A, Vandone AM, Aftimos P. Personalized management of patients with solid cancers: moving from patient characteristics to tumor biology. Curr Opin Oncol. 2012;24:297–304. doi: 10.1097/CCO.0b013e3283521349. [DOI] [PubMed] [Google Scholar]
- 13.Sudhindra A, Ochoa R, Santos ES. Biomarkers, prediction, and prognosis in non-small-cell lung cancer: a platform for personalized treatment. Clin Lung Cancer. 2011;12:360–368. doi: 10.1016/j.cllc.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Toffoli G, Cecchin E, Corona G, et al. The role of UGT1A1*28 polymorphism in the pharmacodynamics and pharmacokinetics of irinotecan in patients with metastatic colorectal cancer. J Clin Oncol. 2006;24:3061–3068. doi: 10.1200/JCO.2005.05.5400. [DOI] [PubMed] [Google Scholar]
- 15.Schneider BP, Wang M, Radovich M, et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol. 2008;26:4672–4678. doi: 10.1200/JCO.2008.16.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ilie M, Hofman P. Pitfalls in lung cancer molecular pathology: how to limit them in routine practice? Curr Med Chem. 2012;19:2638–2651. doi: 10.2174/092986712800493002. [DOI] [PubMed] [Google Scholar]
- 17.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 18.Chung KY, Shia J, Kemeny NE, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23:1803–1810. doi: 10.1200/JCO.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 19.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kantarjian H, Sawyers C, Hochhaus A, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346:645–652. doi: 10.1056/NEJMoa011573. [DOI] [PubMed] [Google Scholar]
- 22.Cheson BD, Leonard JP. Monoclonal antibody therapy for B-cell non-Hodgkin’s lymphoma. N Engl J Med. 2008;359:613–626. doi: 10.1056/NEJMra0708875. [DOI] [PubMed] [Google Scholar]
- 23.McKeage MJ. Clinical trials of vascular disrupting agents in advanced non–small-cell lung cancer. Clin Lung Cancer. 2011;12:143–147. doi: 10.1016/j.cllc.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Bhakta S, Flick SM, Cooney MM, et al. Myocardial stunning following combined modality combretastatin-based chemotherapy: two case reports and review of the literature. Clin Cardiol. 2009;32:E80–E84. doi: 10.1002/clc.20685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burris HA, 3rd, Tibbitts J, Holden SN, Sliwkowski MX, Lewis Phillips GD. Trastuzumab emtansine (T-DM1): a novel agent for targeting HER2+ breast cancer. Clin Breast Cancer. 2011;11:275–282. doi: 10.1016/j.clbc.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 26.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 27.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 28.De Mattos-Arruda L, Dienstmann R, Tabernero J. Development of molecular biomarkers in individualized treatment of colorectal cancer. Clin Colorect Cancer. 2011;10:279–289. doi: 10.1016/j.clcc.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 29.Holubec L, Liska V, Matejka VM, et al. The role of cetuximab in the treatment of metastatic colorectal cancer. Anticancer Res. 2012;32:4007–4011. [PubMed] [Google Scholar]
- 30.Ballestrero A, Garuti A, Cirmena G, et al. Patient-tailored treatments with anti-EGFR monoclonal antibodies in advanced colorectal cancer: KRAS and beyond. Curr Cancer Drug Targets. 2012;12:316–328. doi: 10.2174/156800912800190956. [DOI] [PubMed] [Google Scholar]
- 31.Adams RA, Meade AM, Seymour MT, et al. Intermittent versus continuous oxaliplatin and fluoropyrimidine combination chemotherapy for first-line treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet Oncol. 2011;12:642–653. doi: 10.1016/S1470-2045(11)70102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bokemeyer C, Bondarenko I, Hartmann JT, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22:1535–1546. doi: 10.1093/annonc/mdq632. [DOI] [PubMed] [Google Scholar]
- 33.Van Cutsem E, Kohne CH, Lang I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 34.Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499–3506. doi: 10.1200/JCO.2012.42.8201. [DOI] [PubMed] [Google Scholar]
- 35.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48(suppl):s20–s37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 36.Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology (Baltimore, Md) 2008;48:1312–1327. doi: 10.1002/hep.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sengupta B, Siddiqi SA. Hepatocellular carcinoma: important biomarkers and their significance in molecular diagnostics and therapy. Curr Med Chem. 2012;19:3722–3729. doi: 10.2174/092986712801661059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 39.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 40.Villanueva A, Llovet JM. Targeted therapies for hepatocellular carcinoma. Gastroenterology. 2011;140:1410–1426. doi: 10.1053/j.gastro.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Subramanian J, Waqar SN, Morgensztern D, Govindan R. Recent advances in lung cancer: summary of presentations from the 47th annual meeting of the American Society of Clinical Oncology (ASCO) 2011. J Thorac Oncol. 2012;7:260–265. doi: 10.1097/JTO.0b013e31823a40a6. [DOI] [PubMed] [Google Scholar]
- 42.Black A, Morris D. Personalized medicine in metastatic non-small-cell lung cancer: promising targets and current clinical trials. Curr Oncol. 2012;19:S73–S85. doi: 10.3747/co.19.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toyooka S, Mitsudomi T, Soh J, et al. Molecular oncology of lung cancer. Gen Thorac Cardiovasc Surg. 2011;59:527–537. doi: 10.1007/s11748-010-0743-3. [DOI] [PubMed] [Google Scholar]
- 44.Rosell R, Vergnenegre A, Liu B, et al. Biomarkers in lung oncology. Pulm Pharmacol Ther. 2010;23:508–514. doi: 10.1016/j.pupt.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Konecny GE. Emerging strategies for the dual inhibition of HER2-positive breast cancer. Curr Opin Obstet Gynecol. 2013;25:55–65. doi: 10.1097/GCO.0b013e32835c5e90. [DOI] [PubMed] [Google Scholar]
- 46.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 47.Dietel M, Johrens K, Laffert M, et al. Predictive molecular pathology and its role in targeted cancer therapy: a review focussing on clinical relevance. Cancer Gene Ther. 2013 Mar 15; doi: 10.1038/cgt.2013.13. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 48.Bilbault P, Castelain V, Schenck-Dhif M, Schneider F, Charpiot A. Life-threatening cervical necrotizing fasciitis after a common dental extraction. Am J Emerg Med. 2008;26:971.e5–971.e7. doi: 10.1016/j.ajem.2008.01.053. [DOI] [PubMed] [Google Scholar]
- 49.Yardley DA, Raefsky E, Castillo R, et al. Phase II study of neoadjuvant weekly nab-paclitaxel and carboplatin, with bevacizumab and trastuzumab, as treatment for women with locally advanced HER2+ breast cancer. Clin Breast Cancer. 2011;11:297–305. doi: 10.1016/j.clbc.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 50.Lee JM, Hays JL, Noonan AM, et al. Feasibility and safety of sequential research-related tumor core biopsies in clinical trials. Cancer. 2013;119:1357–1364. doi: 10.1002/cncr.27916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Annunziata CM, Walker AJ, Minasian L, et al. Vandetanib, designed to inhibit VEGFR2 and EGFR signaling, had no clinical activity as mono-therapy for recurrent ovarian cancer and no detectable modulation of VEGFR2. Clin Cancer Res. 2010;16:664–672. doi: 10.1158/1078-0432.CCR-09-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fatourou EM, Koskinas JS. Adaptive immunity in hepatocellular carcinoma: prognostic and therapeutic implications. Expert Rev Anticancer Ther. 2009;9:1499–1510. doi: 10.1586/era.09.103. [DOI] [PubMed] [Google Scholar]