Summary
Inflammatory cell and cytokine cascade activation is present in humans with alcoholic liver disease as well as in animal models of alcohol-induced liver damage. Gut-derived lipopolysaccahride (LPS), a ligand of the Toll-like receptor 4 (TLR4), plays a central role in triggering and maintaining activation of Kupffer cells in alcoholic hepatitis. In this mini-review, we describe molecular mechanisms that lead to increased inflammatory cell activation by alcohol and LPS and discuss the mechanism for activation in alcohol-exposed macrophages. In alcohol-induced liver disease we discuss the role of MyD88-independent but IRF3-mediated TLR4 signaling in alcohol-related liver inflammation and liver damage.
Introduction
The history of alcohol consumption traces back to Mesopotamia but the ancient Egyptians were also drinkers as they invented the first straws for drinking beer that still contained wheat-husks. Some of Egyptian texts even refer to the social problems associated with drunkenness. Throughout human civilization to date alcohol use remained the most commonly used substance of abuse in all societies and remains to be one of the most common etiologies for liver disease. There are multiple factors contributing to alcoholic liver disease including genetic factors, environment, gender and co-existing diseases. The pioneering research of the late Charlie Lieber opened the stage for recent discoveries in alcoholic liver disease after showing that alcohol has direct biological effects on the liver as opposed to only causing nutritional impairment (Lieber, 1975). While the direct effects of alcohol and its metabolites have received significant attention in causing direct damage to hepatocytes in the liver, the pathomechanism of alcoholic liver disease is more complex and it involves interactions between the gut, the site of alcohol absorption, the portal circulation and the different cell types in the liver. Crosstalk between the various cell types in the liver including parenchymal cells such as hepatocytes, biliary epithelium, stellate cells, liver sinusoidal endothelial cells, and Kupffer cells adds another level of regulation disrupted during liver injury.
Liver-gut axis in alcoholic liver disease
The unique anatomical position, blood supply, and architecture of the liver expose hepatocytes and cells in the liver sinusoids not only to gut-derived nutrients required for metabolism but also to gut-derived microbial products. In health, a normal balance of gut barrier function, gut permeability and a balance of commensal and pathogenic microbes in the gut lumen is maintained. This prevents microbial translocation from the gut (Rao, 2009). In normal homeostasis, the liver participates by detoxification of gut-derived toxins and microbial products through uptake by hepatocytes and Kupffer cells in a manner that prevents cell damage or inflammation.
There is increasing evidence for a dysbalance of the gut-liver axis in various liver diseases including alcoholic liver disease, non-alcoholic fatty liver disease and steatohepatitis (Keshavarzian, 1999; Keshavarzian, 2009; Miele, 2009). It is believed that gut microbial translocation can be a result of gut barrier dysfunction, increased gut permeability, and/or microbial overgrowth. Hepatocyte dysfunction and Kupffer cell activation also contribute to the dysbalance of the gut-liver axis when increased microbial products enter the liver to activate immune cells, particularly Kupffer cells, the liver resident macrophages, and other immune cells to include production of oxygen radicals and pro-inflammatory mediators (Mandrekar, 2009; Nagy, 2003; Wu, 2009:). Liver-derived inflammatory cytokines, particularly TNFα by entering the systemic circulation, can further increase gut permeability by disrupting tight junctions of gut epithelial cells (Yajima, 2009). Alcohol consumption can directly impair the gut barrier function by disruption of epithelial cell tight junctions, bacterial overgrowth and potentially through other, yet to be explored mechanisms (Bode, 2003). Several studies indicate that bacterial translocation as well as Kupffer cell activation play a central role in the pathomechanism of alcoholic liver disease. Sterilization of the gut with antibiotics or elimination of Kupffer cells, respectively, attenuated alcoholic fatty liver in animal models (Adachi, 1994; Adachi, 1995).
LPS sensing and its modulation by alcohol use
Lipopolysaccharide, a component of Gram negative bacteria, is a potent activator of innate immune responses through its binding to the Toll-like receptor 4 (TLR4) complex. The lipid A component of LPS is sensed as a danger signal by the mammalian hosts via the TLR4 receptor complexes. While TLR4 cannot directly bind LPS, the co-receptors, CD14 or MD-2 bind LPS and upon LPS binding activate TLR4. TLR4 dimerization leads to downstream signaling of two pathways: the MyD88-dependent and MyD88-independent pathways. Association of the intracellular TIR domain of TLR4 with the adapter molecule MyD88 through TRAM, results in downstream activation of the IRAK1/4/TRAF6 complex and further activation of the IKK kinase complex that phosphorylates IκB to allow nuclear translocation of NF-κB (Verstak, 2009). NF-κB binding to the NF-κB responsive element in the promoter region of pro-inflammatory cytokine genes results in production of TNFα, and other pro-inflammatory cytokines and chemokines (Akira, 2006). The MyD88-independent signaling pathway is activated by TLR4 after recruitment of the TRIF adapter, TRAF6 and TBK/IKKε phosphorylation leading to phosphorylation of the interleukin regulatory factor 3 (IRF3) (Schafer, 1998). This leads to IRF3 nuclear translocation and induction of Type I IFNs.
There is increasing evidence that moderate/acute and heavy/chronic alcohol use have different effects on human health (Sazbo and Mandrekar, 2009). Moderate alcohol use is associated with benefits on overall mortality, cardiovascular diseases and diabetes, while chronic excessive alcohol intake can lead to damage of the liver, lung, pancreas and other organs. Many of these processes involve activation of inflammatory cells as a critical component of the disease pathomechanism. At the cellular and molecular level, acute alcohol administration inhibited while chronic alcohol use increased production of pro-inflammatory mediators particularly when LPS was used as an inflammatory insult (Messingham, 2002).
Previous studies demonstrated that consumption of a single dose of alcohol in vivo significantly attenuated LPS-induced production of TNFα and IL-1ß and NF-κB activation in human monocytes (Szabo, 1996). This was in contrast with the increased TNFα production and NF-κB activation found in monocytes of patients with alcoholic steatohepatitis or after prolonged in vitro alcohol treatment (McClain, 1989). The molecular mechanisms involved in the effects of acute and prolonged alcohol exposure were tested in normal human monocytes. It was shown that acute and prolonged alcohol use had opposite effects on TNFα production in monocytes (Mandrekar, 2009). Acute alcohol (25 mM) resulted in significant attenuation of LPS-induced TNFα production at the mRNA and protein levels. Assessment of the TLR4 signaling pathway revealed that acute alcohol treatment inhibited IRAK1 phosphorylation, IKK kinase activity and NF-κB phosphorylation and activation in response to a subsequent LPS stimulation (Mandrekar, 2009). Such attenuation of TLR4 signaling by acute alcohol was similar to LPS tolerance (Medvedev, 2002). Recent studies found that TLR/LPS tolerance is induced via upregulation of TLR inhibitor molecules. In monocytes/macrophages the negative regulator, IRAK-monocytes (IRAK-M) is upregulated after a single LPS dose resulting in tolerance to the second LPS dose (Escoll, 2003). In human monocytes after acute alcohol treatment, IRAK-M was upregulated both at the RNA and protein levels, and siRNA knock-down of IRAK-M prevented the alcohol-induced inhibition of TNFα production in response to LPS (Mandrekar, 2009). In contrast to acute alcohol, prolonged alcohol exposure of monocytes for 4 days or longer in vitro, augmented LPS-induced TNFα production compared to alcohol-naïve cells (Mandrekar, 2009). The involvement of the TLR4 signaling pathway was suggested by increased IRAK-1 phosphorylation, increased IKK kinase activity, increased NF-κB nuclear translocation and DNA transactivation (Mandrekar, 2009). Upregulation of TLR4 signaling occurred in the presence of diminished expression of IRAK-M in monocytes after prolonged alcohol treatment. Over-expression of IRAK-M prevented the increased LPS-induced TNFα production in chronic alcohol-treated cells suggesting that loss of IRAK-M is likely to contribute to the loss of TLR4 tolerance in monocytes after prolonged alcohol exposure (Mandrekar, 2009).
TLR4 signaling in alcoholic liver disease
The importance of LPS-induced cell signaling has been demonstrated in animal models where mutation in the LPS receptor or the LPS adapter, CD14, showed protection from early alcoholic liver steatosis (Uesugi, 2001; Yin, 2001). However, the involvement of the specific components of TLR4 signaling pathway have not been investigated. In a recent study we found that mice deficient in the expression of TLR4 were protected from development of ALD on a Lieber-DeCarli diet (Hritz, 2008). There were decreased steatosis and inflammation and significantly reduced liver triglyceride, TNFα and IL-6 serum levels in TLR4-deficient mice after chronic alcohol feeding, suggesting a key role for TLR4-mediated signals in alcoholic liver disease. Engagement of TLR4 with its ligand, LPS, leads to activation of NF-κB and pro-inflammatory cytokine induction via recruitment of the MyD88 adapter molecule to the TLR4 complex while recruitment of the TRIF adapter activates the TBK/IKKε kinase and IRF3 to induce Type I IFN production as well as NF-κB activation (Covert, 2005). To evaluate the hypothesis that MyD88-dependent and MyD88-independent TLR4 signaling pathways may have different involvement in alcoholic liver disease, first we tested MyD88-deficient mice because the role of NF-κB activation and TNFα induction has been identified as a major component in alcoholic liver disease (Szabo, 2009). Alcohol feeding with the Lieber-DeCarli diet (Lieber, 1975; Lieber, 1982) resulted in significant steatosis and liver damage indicated by ALT elevation in MyD88 deficient mice compared to pair-fed diet and the extent of alcohol-induced changes were comparable between alcohol-fed MyD88-deficient and wild-type mice. This observation suggests that TLR4-mediated signaling via MyD88-independent pathways is important in induction of alcoholic liver disease. The TLR4-induced MyD88-independent, TRIF-mediated pathway activation results in Type I IFN induction. Indeed, we found evidence for activation of the Type I IFN pathway in livers with alcoholic liver disease where increased mRNA expression of ISG56 was found (Szabo unpublished data).
Furthermore, cell-specific activation of the IFN-inducible gene was reported; IRF7 in Kupffer cells of alcohol-fed mice was increased compared to control diet-fed animals (Hritz, 2008). In a different study it was also reported that TRIF-deficient mice were protected against alcohol-induced liver disease and it is likely that IRF3, a transcription factor downstream to TLR4/TRIF, may bind to the TNFα promoter region resulting in induction of TNFα (Zhao, 2008). The role of TLR4 in alcoholic liver disease also extends to stellate cell activation (Seki, 2007). However, the role of IRF3 in stellate cell activation is yet to be evaluated.
Conclusion
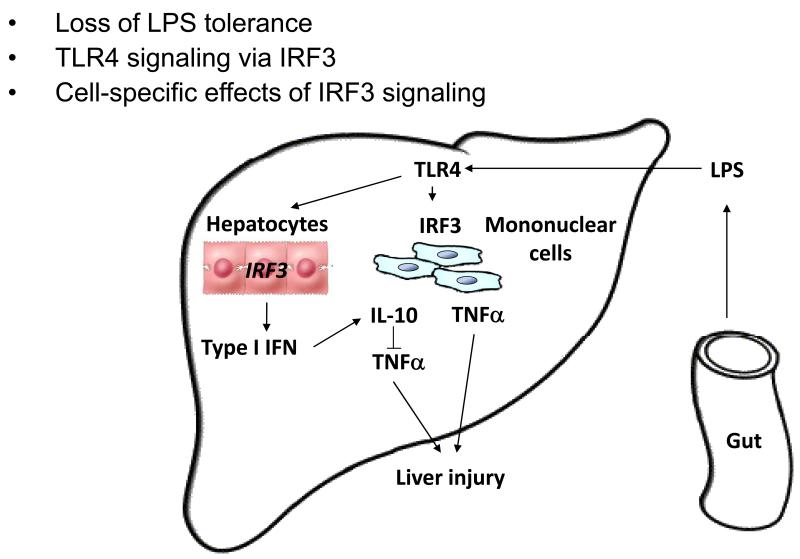
Both steatosis and inflammatory cascade activation are induced by TLR4-mediated intracellular signaling pathways in alcoholic liver disease. Studies with acute and prolonged alcohol administration suggest that in monocytes/macrophages acute alcohol results in attenuated while prolonged alcohol use sensitizes to LPS and TLR4 activation.. Recent data also suggests that the MyD88-independent TLR4 signaling cascade that induces Type I IFNs is important in the development of alcoholic liver injury. Further studies on cell-specific regulation and cross-cellular communication and determination of the cell-specific role of IRF3 in ALD await investigation.
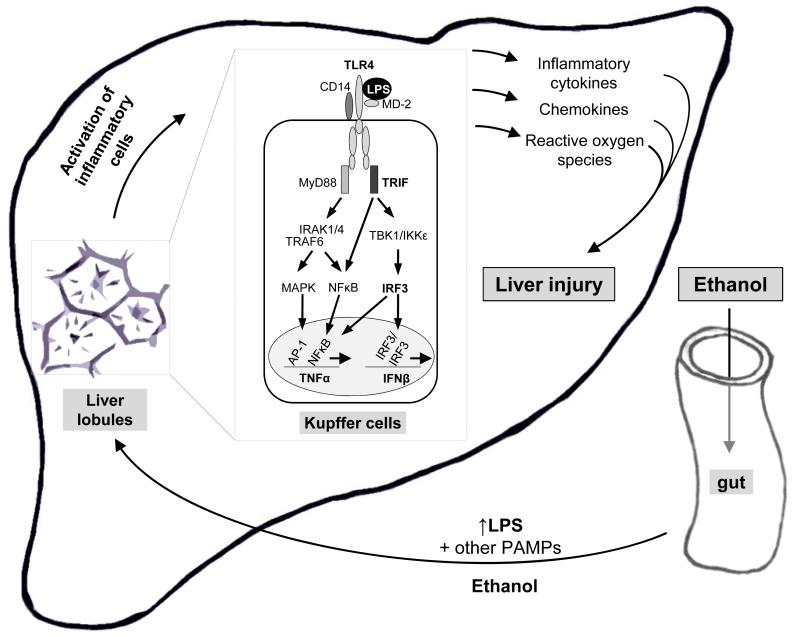
Fig. 1. New concepts in the pathophysiology of alcohol-induced liver injury.
Ethanol increases translocation of bacterial endotoxin (lipopolysaccharide, LPS) and other pathogen-associated molecular patterns (PAMPs) from the gut to the liver. In the liver, LPS activates inflammatory cells, in particular Kupffer cells. In Kupffer cells, TLR4 recognizes LPS in cooperation with its co-receptors, CD14 and MD-2. The signal is passed preferentially through the TRIF-dependent intracellular pathways, which activate various transcription factors, including IRF3 and NFκB, and induces pro-inflammatory cytokine and Type I interferon genes. Activated Kupffer cells are crucial in alcohol-induced liver injury by induction of inflammatory cytokines, chemokines and reactive oxygen species.
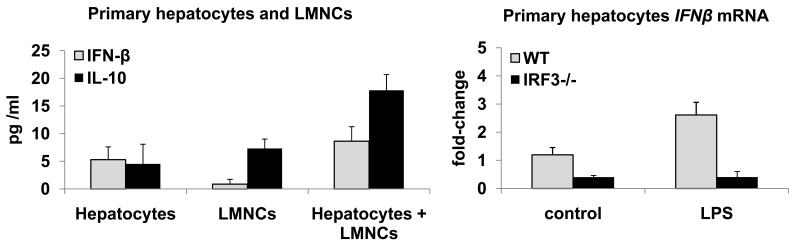
Fig. 2.
A. Cell-specific induction of IFN-ß and IL-10 in co-cultures of hepatocytes and liver mononuclear cells (LMNCs)
B. IRF3-dependent induction of IFN-β in hepatocytes
Fig. 3.
Pathogenesis of alcohol-induced liver injury: New concepts
Acknowledgements
This work was supported by NIAAA grants # AA017729 and AA011576
References
- Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. 1994;20:453–460. [PubMed] [Google Scholar]
- Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. 1995;108:218–224. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Bode C, Bode JC. Effect of alcohol consumption on the gut. 2003;17:575–592. doi: 10.1016/s1521-6918(03)00034-9. [DOI] [PubMed] [Google Scholar]
- Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolysaccharide-induced NF-kappaB activation. 2005;309:1854–1857. doi: 10.1126/science.1112304. [DOI] [PubMed] [Google Scholar]
- De Nardo D, Nguyen T, Hamilton JA, Scholz GM. Down-regulation of IRAK-4 is a component of LPS- and CpG DNA-induced tolerance in macrophages. 2009;21:246–252. doi: 10.1016/j.cellsig.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Dolganiuc A, Norkina O, Kodys K, Catalano D, Bakis G, Marshall C, Mandrekar P, Szabo G. Viral and host factors induce macrophage activation and loss of toll-like receptor tolerance in chronic HCV infection. 2007;133:1627–1636. doi: 10.1053/j.gastro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoll P, del Fresno C, Garcia L, Valles G, Lendinez MJ, Arnalich F, Lopez-Collazo E. Rapid up-regulation of IRAK-M expression following a second endotoxin challenge in human monocytes and in monocytes isolated from septic patients. 2003;311:465–472. doi: 10.1016/j.bbrc.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, Kurt-Jones E, Szabo G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. 2008;48:1224–1231. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Blatt LM, Taylor MW. Type 1 interferon as an antiinflammatory agent: inhibition of lipopolysaccharide-induced interleukin-1 beta and induction of interleukin-1 receptor antagonist. 1995;15:317–321. doi: 10.1089/jir.1995.15.317. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. 2009;50:538–547. doi: 10.1016/j.jhep.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. 1999;94:200–207. doi: 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Alcohol and malnutrition in the pathogenesis of liver disease. 1975;233:1077–1080. [PubMed] [Google Scholar]
- Lieber CS, DeCarli LM. The feeding of alcohol in liquid diets: two decades of applications and 1982 update. 1982;6:523–531. doi: 10.1111/j.1530-0277.1982.tb05017.x. [DOI] [PubMed] [Google Scholar]
- Lieber CS, DeCarli LM. Alcoholic liver injury: experimental models in rats and baboons. 1975;59:379–393. doi: 10.1007/978-1-4757-0632-1_27. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Bala S, Catalano D, Kodys K, Szabo G. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. 2009;183:1320–1327. doi: 10.4049/jimmunol.0803206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. 2009;50:1258–1266. doi: 10.1016/j.jhep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain CJ, Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. 1989;9:349–351. doi: 10.1002/hep.1840090302. [DOI] [PubMed] [Google Scholar]
- Medvedev AE, Lentschat A, Wahl LM, Golenbock DT, Vogel SN. Dysregulation of LPS-induced Toll-like receptor 4-MyD88 complex formation and IL-1 receptor-associated kinase 1 activation in endotoxin-tolerant cells. 2002;169:5209–5216. doi: 10.4049/jimmunol.169.9.5209. [DOI] [PubMed] [Google Scholar]
- Medvedev AE, Sabroe I, Hasday JD, Vogel SN. Tolerance to microbial TLR ligands: molecular mechanisms and relevance to disease. 2006;12:133–150. doi: 10.1179/096805106X102255. [DOI] [PubMed] [Google Scholar]
- Messingham KA, Faunce DE, Kovacs EJ. Alcohol, injury, and cellular immunity. 2002;28:137–149. doi: 10.1016/s0741-8329(02)00278-1. [DOI] [PubMed] [Google Scholar]
- Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Masciana R, Forgione A, Gabrieli ML, Perotti G, Vecchio FM, Rapaccini G, Gasbarrini G, Day CP, Grieco A. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- Nagy LE. Recent insights into the role of the innate immune system in the development of alcoholic liver disease. 2003;228:882–890. doi: 10.1177/153537020322800803. [DOI] [PubMed] [Google Scholar]
- Piao W, Song C, Chen H, Diaz MA, Wahl LM, Fitzgerald KA, Li L, Medvedev AE. Endotoxin tolerance dysregulates MyD88- and Toll/IL-1R domain-containing adapter inducing IFN-beta-dependent pathways and increases expression of negative regulators of TLR signaling. 2009;86:863–875. doi: 10.1189/jlb.0309189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. 2009;50:638–644. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J, McClellan JL, Kluger MJ, Zeisberger E. Attenuation of fever and release of cytokines after repeated injections of lipopolysaccharide in guinea-pigs. 1994;477(Pt 1):177–185. doi: 10.1113/jphysiol.1994.sp020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer SL, Lin R, Moore PA, Hiscott J, Pitha PM. Regulation of type I interferon gene expression by interferon regulatory factor-3. 1998;273:2714–2720. doi: 10.1074/jbc.273.5.2714. [DOI] [PubMed] [Google Scholar]
- Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. 2008;48:322–335. doi: 10.1002/hep.22306. [DOI] [PubMed] [Google Scholar]
- Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P. A recent perspective on alcohol, immunity, and host defense. 2009;33:220–232. doi: 10.1111/j.1530-0277.2008.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P, Girouard L, Catalano D. Regulation of human monocyte functions by acute ethanol treatment: decreased tumor necrosis factor-alpha, interleukin-1 beta and elevated interleukin-10, and transforming growth factor-beta production. 1996;20:900–907. doi: 10.1111/j.1530-0277.1996.tb05269.x. [DOI] [PubMed] [Google Scholar]
- Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. 2001;34:101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- van Rooijen N, Sanders A, van den Berg TK. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. 1996;193:93–99. doi: 10.1016/0022-1759(96)00056-7. [DOI] [PubMed] [Google Scholar]
- Verstak B, Nagpal K, Bottomley SP, Golenbock DT, Hertzog PJ, Mansell A. MyD88 adapter-like (Mal)/TIRAP interaction with TRAF6 is critical for TLR2- and TLR4-mediated NF-kappaB proinflammatory responses. 2009;284:24192–24203. doi: 10.1074/jbc.M109.023044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Cederbaum AI. Oxidative stress and alcoholic liver disease. 2009;29:141–154. doi: 10.1055/s-0029-1214370. [DOI] [PubMed] [Google Scholar]
- Yajima S, Morisaki H, Serita R, Suzuki T, Katori N, Asahara T, Nomoto K, Kobayashi F, Ishizaka A, Takeda J. Tumor necrosis factor-alpha mediates hyperglycemia-augmented gut barrier dysfunction in endotoxemia. 2009;37:1024–1030. doi: 10.1097/CCM.0b013e31819b53b6. [DOI] [PubMed] [Google Scholar]
- Yin M, Bradford BU, Wheeler MD, Uesugi T, Froh M, Goyert SM, Thurman RG. Reduced early alcohol-induced liver injury in CD14-deficient mice. 2001;166:4737–4742. doi: 10.4049/jimmunol.166.7.4737. [DOI] [PubMed] [Google Scholar]
- Zhao XJ, Dong Q, Bindas J, Piganelli JD, Magill A, Reiser J, Kolls JK. TRIF and IRF-3 binding to the TNF promoter results in macrophage TNF dysregulation and steatosis induced by chronic ethanol. 2008;181:3049–3056. doi: 10.4049/jimmunol.181.5.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]