Abstract
For optimal stimulation of T cells, protein-based vaccines must deliver protein antigens to antigen-presenting cells while simultaneously providing immunostimulatory signals. Listeriolysin O (LLO)-containing liposomes have been utilized to efficiently deliver protein antigens to the cytosolic pathway for antigen processing and major histocompatibility complex class I-dependent presentation while co-delivering immunostimulatory CpG-oligodeoxyribonuceotides (ODNs). In this report, we describe the synthesis of lipid-CpG-ODN conjugates utilizing maleimide-phosphatidylethanolamine (PE) lipids and 5′ sulfhdryl-containing CpG-ODNs as a method for facile incorporation of CpG-ODNs in liposomal vaccine carriers, an alternative to co-encapsulation inside liposomes, and as a means to enhance delivery of CpG-ODNs to their major receptor, Toll-like receptor 9 (TLR9), in the endosome. The characterization and biological evaluation of the vaccine delivery system made of liposomes, which contain the lipid-CpG-ODN conjugates inserted in the liposomal membrane, is described. We demonstrate in vitro in bone marrow-derived macrophages that the lipid-CpG-ODN conjugates incorporated onto the liposome bilayers interact with their receptor TLR9 as readily as liposome-encapsulated ODNs and exert their immunostimulatory capabilities. The liposomal vaccine delivery systems were evaluated in mice using ovalbumin (OVA) as a model antigen, and the results indicate equally robust OVA-specific cytotoxic T lymphocyte responses and similar Th1 immune skewing capabilities between liposomes containing lipid-conjugated or encapsulated CpG-ODNs. Overall, this work indicates that conjugating PE lipids and CpG-ODNs results in an efficient method that allows facile incorporation of CpG-ODNs into a liposome-based delivery platform while retaining the immune-stimulating capabilities of CpG-ODNs.
Introduction
The immune system generates humoral and cellular immune responses to viruses and bacteria by recognizing a variety of the pathogen’s components. Traditional vaccination strategies have successfully exploited these responses by utilizing whole live attenuated or inactivated pathogens for the treatment and prevention of a large number of diseases; however, both safety and production concerns have led to the development of protein antigen or subunit vaccines.1 Compared to whole pathogens, protein-based vaccines are inherently limited in: (i) their ability to stimulate a robust immune response and (ii) their ability to deliver antigen(s) to the cytosolic pathway of antigen presentation. Hence, adjuvants have been utilized in protein vaccines as an effective means for enhancing the immunogenicity of the antigens and modulating the types of immune responses. Classical adjuvants such as aluminum hydroxide (alum) and oil/water emulsions enhance the humoral response to protein antigens but not the cellular response, thus limiting their utility.1, 2 A cellular immune response, particularly the T helper (Th)1-type that is associated with interferon (IFN)-γ production and cytotoxic T lymphocytes (CTLs), is desirable in a number of cases and is often needed to clear tumors or intracellular pathogens such as viruses.3 Traditional vaccines rely on the molecular composition of the whole pathogen to stimulate the innate immune system through an array of pathogen-associated molecular patterns (PAMPs) such as unmethylated cytosine-phosphate-guanine (CpG)-containing DNA, lipopolysaccharides, lipoproteins, flagellin and double-stranded RNA.4 Recognition of one or more PAMPs induces antigen-presenting cell (APC) maturation resulting in cytokine secretion, co-stimulatory molecule expression, and enhanced antigen presentation, ultimately stimulating both the humoral and cellular arms of the immune system. The ability of some PAMPs to stimulate cellular immune responses has led to an increased interest in their utilization in vaccines as adjuvants that target innate immune receptors.5 Our current approach is to generate a vaccine delivery system emulating traditional whole pathogen-based vaccines to stimulate both humoral and cellular immune responses, while reducing the relative toxicity by including in the protein-based vaccine only essential, well-defined components for eliciting an antigen-specific immune response.
Synthetic CpG-oligodeoxyribonuceotides (ODNs) that mimic bacterial CpG-containing unmethylated DNA have been shown to activate monocytes/macrophages, dendritic cells, natural killer cells and B cells, induce the production of proinflammatory cytokines (interleukin (IL)-6, IL-12, IFNs, TNF-α) and upregulate the expression of major histocompatibility complex (MHC) class I, MHC class II and co-stimulatory molecules.6 CpG-ODNs may be co-administered with antigens to enhance the immune response [reviewed by Krieg6]. When APCs receive antigen without concomitant co-stimulation, however, T cell anergy, rather than the development of a memory T cell response, may occur.7 To address this issue various methods of co-delivering CpG-ODNs and antigens are being explored, including, but not limited to, antigen-CpG-ODN conjugates,8, 9 liposomal encapsulation,10–12 and microparticle loading.13, 14
Liposomes, which are internalized via endocytosis and disrupted inside the endosomal compartment,15 have tremendous potential for delivering co-encapsulated CpG-ODNs to facilitate their interaction with Toll-like receptor 9 (TLR9) in the endosome.16 We have previously explored and demonstrated the ability of listeriolysin O (LLO)-containing pH-sensitive liposomes to efficiently deliver antigen into the cytosol of APCs to enhance MHC class I processing and presentation as a means to augment CTL activity.17–19 LLO, the pore-forming cytolysin of the facultative intracellular bacterium Listeria monocytogenes (Lm), exerts optimal activity at pH 5.5 to temporarily disrupt endosome membranes and promote Lm’s escape from the endolysosome uptake pathway into the cytosol.20 LLO can thus be exploited to mimic the cytosol-invading aspect of Lm’s infectivity to efficiently deliver macromolecules to the cytosol. We have also shown that co-encapsulating ovalbumin (OVA) and CpG-ODNs within LLO-containing pH-sensitive liposomes significantly enhances the CTL response, while also skewing the immunity towards the Th1-type, as compared with OVA and CpG-ODN administered in solution (C.D. Andrews and M.S. Huh; unpublished results).
Although the previous results obtained from LLO-liposomes with co-encapsulated antigen and CpG-ODN were promising, the encapsulation efficiency of CpG-ODN was relatively low, resulting in a significant loss of material during the removal of un-encapsulated ODNs. Furthermore, because of its encapsulation within the liposomes, the access of CpG-ODN to TLR9 in the endosome may be limited both spatially and temporally, in effect reducing the overall targeting efficiency. Therefore, in order to increase the recovery of CpG-ODN and enhance the probability of CpG-ODN binding to TLR9, CpG-ODN was conjugated to lipid via an irreversible thioether bond and incorporated into the liposomal membrane. The hypothesis tested in this report is that lipid-conjugated CpG-ODNs on the surface of the liposomes will promote the interaction between CpG-ODN and TLR9, before the endosomal contents are released into the cytosol upon LLO-mediated perforation of the endosomal membrane. Here we show that we can conjugate CpG-ODN to phosphatidylethanolamine (PE), yielding an efficient process for incorporating CpG-ODNs in liposome membranes. The lipid-CpG-ODN conjugates incorporated in the liposomal membrane of the vaccine delivery system exhibited robust immunostimulatory activity as tested in bone marrow-derived macrophages (BMM) and are capable of readily interacting with TLR9. In mice, we use the model antigen OVA and show that liposomes containing OVA and LLO with either encapsulated or conjugated CpG-ODN enhance CTL activity compared with liposomes containing only OVA and LLO. Both conjugated and encapsulated CpG-ODNs are capable of stimulating a Th1-type response as indicated by enhanced IFN-γ secretion and anti-OVA IgG2a antibody production.
Experimental Procedures
Mice, cell culture, media and reagents
C57BL/6 (female, 8–9 weeks old; Harlan, Indianapolis, IN) and C57BL/10ScNJ (Tlr4Lps-del, H-2Kb, female, 6–8 weeks old; Jackson Laboratory, Bar Harbor, ME) were used in this study and were handled according to Institutional Guidelines. All tissue culture media and reagents were purchased from Invitrogen (Carlsbad, CA), and all cells were maintained and experimental incubations were conducted in a humidified incubator at 37°C and 5% CO2, unless otherwise noted. Bone marrow was harvested from the femurs and tibia of C57BL/10ScNJ mice and differentiated into bone marrow-derived macrophages (BMM) in BMM media (DMEM with 20% heat-inactivated FBS (HI-FBS), 30% L-cell conditioned media, 2 mM glutamine, 100 μg/mL streptomycin, 100 U/mL penicillin and 55 μM 2-mercaptoethanol) as described by Stier et al.21 BMM were harvested on day six and frozen in liquid nitrogen until the experiment. Unmodified and 5′-disulfide ODNs used in this study were composed of 22 bases with a phosphorothioate-modified backbone (Dynavax Technologies; Berkeley, CA). The positive control, CpG-ODN ISS 1018 (5′TGACTGTGAACGTTCGAGATGA), contains unmethylated CpG sequences, and the negative control, non-CpG-ODN ISS 1040 (5′TGACTGTGAACCTTAGAGATGA), lacks unmethylated CpG sequences.
Lipid-ODN conjugate synthesis
The disulfide precursor to 5′ sulfhydryl ISS 1018 CpG-ODN and 5′ sulfhydryl ISS 1040 non-CpG-ODN was a gift from Dynavax Technologies (Berkeley, CA). A 700 mM tris-(2-carboxyethyl) phosphine (TCEP; Pierce, Rockford, IL) solution was made in HBSE (140 mM NaCl buffered with 10 mM HEPES containing 1 mM EDTA) pH 7, and used at a five molar excess to reduce 5′-disulfide-ODN at 40°C for 2 h. Residual TCEP was removed using either a PD-10 or a NAP-5 desalting column (GE Healthcare, Piscataway, NJ) and eluted in HBSE pH 6.5. Reduced 5′SH-ODN was used immediately or stored at −80°C until use. Chloroform was removed from 1,2-dioleoyol-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidophenyl)butyramide] (MPB-PE; Avanti Polar Lipids, Alabaster, AL) using rotary evaporation at < 10 mm Hg pressure at RT followed by dissolving in ether, and a water-in-oil emulsion was formed by adding reduced 5′SH-ODN in HBSE at a 2:1 organic:aqueous ratio. The optimal MPB-PE:5′SH-ODN ratios were identified as 1:10 and 1:20 for 5′SH-CpG-ODN and 5′SH-non-CpG-ODN, respectively. The emulsion was formed by brief sonication, and the MPB-PE and 5′SH-ODN reacted for 4–5 h while continuously vortexing at RT. Ether was removed using rotary evaporation, after which water was removed by lyophilization. Two chloroform washes (25 mL) were performed to remove unreacted MPB-PE from the PE-ODN, and residual chloroform was removed by evaporation. Thin-layer chromatography was performed to verify the removal of unconjugated lipids. Both the reduced 5′SH-ODN and the MPB-PE and 5′SH-ODN reaction were resolved in a Tris-Borate-EDTA (TBE) polyacrylamide gel (BioRad, 15%) and stained with ethidium bromide. Conjugation efficiency was assessed by using known concentrations of ODNs resolved in the same gel. Unconjugated 5′SH-ODN was removed from PE-ODN conjugates by Sepharose CL-4B size-exclusion chromatography (1 × 25 cm; GE Healthcare). A TBE polyacrylamide gel (15%) stained with ethidium bromide was used to identify fractions containing conjugated PE-ODN, which were then combined, lyophilized, and the dried solid was dissolved in methanol:N-methyl-2-pyrrolidone (MeOH:NMP; 3.3:1). The amount of PE-ODN recovered was determined by measuring phosphate as described by Bartlett.22
Preparation of LLO
The hly gene encoding for LLO was inserted into the bacterial expression plasmid pET29b with a His6 tag. Recombinant LLO was purified from E. coli as described by Mandal et al.,18 with the following exceptions. LLO expression was induced in bacterial culture for 4–6 h with 1 mM isopropyl β-D-thiogalactopyranoside, the cell pellet was collected and resuspended in wash buffer (50 mM sodium phosphate, 300 mM sodium chloride, 20 mM imidazole, pH 8) containing 2 mM PMSF and 1 mM 2-mercaptoethanol and then lysed using a French press. Lysate supernatant was incubated with to Ni-NTA agarose (Qiagen) for 2 h and the Ni-NTA extensively washed with 200 bed volumes of wash buffer. His6-tagged LLO was eluted in wash buffer containing 400 mM imidazole and extensively dialyzed against HBSE pH 8.4 at 4°C. Protein purity and activity were assessed by SDS-PAGE and hemolysis assay, respectively, as previously described.18
Liposome preparation
Lipid films containing a 2:1 (mol:mol) mixture of egg phosphatidylethanolamine: cholesteryl hemisuccinate (ePE:CHEMS; Avanti Polar Lipids, Alabaster, AL and Sigma-Aldrich, respectively) were made by removing chloroform and methanol using rotary evaporation at < 10 mm Hg pressure at RT. PE-ODNs dissolved in MeOH:NMP were incorporated in the lipid film at 0.2 or 0.3 mole percent for in vivo or in vitro studies, respectively, and solvent was removed by rotary evaporation at 40°C and < 10 mm Hg pressure. The films were rehydrated with HBSE pH 8.4 containing a combination of ovalbumin (OVA; 10 mg (in vitro studies) or 2 mg (in vivo studies), Sigma-Aldrich, Grade VI), LLO (0.1 mg), and ODN (2 mg), and the resulting liposomes were subjected to five freeze-thaw cycles followed by 7 × 1 min bursts of bath sonication. Unencapsulated materials were separated from the liposomes in Sepharose CL-4B size-exclusion columns (1 × 25 cm). OVA and LLO encapsulation was determined by staining proteins resolved in SDS-PAGE with Krypton staining (Pierce) and measuring band intensities using a Molecular Dynamics Typhoon 9200 with ImageQuant (GE Healthcare). Calculations were based on known concentrations of proteins resolved in the same gel. ODN encapsulation was determined by generating a standard curve from free ODN or PE-ODN depending on the type of ODN to be quantified. All samples and standards contained normalized lipid amounts and C12E8 (1%, Sigma-Aldrich). SYBR Green I (Invitrogen) was added to the plate at a final dilution of 1:15,000 and the fluorescence quantified in a Synergy HT plate reader (BioTek Instruments, Winooski, VT) using an excitation of 485 nm and emission of 528 nm.
IL-12p40 secretion by ELISA
BMM (2 × 105/well) from C57BL/10ScNJ mice were plated in 96-well tissue culture plates in complete DMEM one day prior to liposome treatment. BMM (in triplicate) were pulsed with liposomes containing PE-ODN for 3 h in DMEM containing penicillin and streptomycin. BMM were washed three times and incubated for 20 h in complete DMEM (DMEM + 10% HI- FBS, 100 μg/mL streptomycin and 100 U/mL penicillin). Supernatants were collected and analyzed for IL-12 secretion by ELISA using IL-12p40 antibodies (R&D Systems, Minneapolis, MN) and IL-12p40 standard (Peprotech, Rocky Hill, NJ). Briefly, ELISA plates were coated with anti-mouse IL-12/IL-23p40 monoclonal antibody diluted in PBS, washed and then blocked with 5% sucrose and 1% BSA in PBS prior to incubating with supernatant. Plates were then washed and incubated with biotinylated anti-mouse IL-12/IL-23p40 antibody, followed by streptavidin-horse radish peroxidase (HRP, eBioscience, San Diego, CA) and TMB SureBlue Substrate (KPL, Gaithersburg, MD). The reaction was stopped using 2 N sulfuric acid, and the absorbance at 450 nm was measured using a Molecular Devices Emax plate reader.
Immunization protocol
C57BL/6 mice were primed with 8 μg of OVA on day 0 via the subcutaneous route at the base of the tail. Mice were euthanized on day 10, sera were collected via cardiac puncture, and spleens were isolated for assays.
IN VIVO cytotoxic T lymphocyte assay
RBC-lysed, single-cell spleen suspensions from naïve mice were pulsed at 1 × 107 cells/mL with either SIINFEKL or influenza nucleoprotein (NP)147–155 peptide (1 μg/mL; Anaspec, Fremont, CA) for 1 h at 37°C. The cells were washed and diluted to 1 × 108 cells/mL in PBS with 0.1% FBS. The SIINFEKL peptide-pulsed and NP147–155 peptide-pulsed populations were labeled with 4 or 0.4 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen), respectively for 10 min at RT. CFSE labeling was stopped by adding an equal volume of FBS for 5 min on ice. Cells were washed thrice with PBS + 5% FBS, counted, and 1 × 107 cells of each peptide-pulsed population were mixed together and injected via tail vein into immunized or naïve mice. Mice were euthanized 8.5 h post-adoptive transfer; spleens were harvested and single cell suspensions were obtained by grinding spleens through a 70 μm mesh screen containing mouse media. Splenocytes were washed, RBCs were removed using Ficoll-Paque Premium (GE Healthcare), and purified splenocytes were analyzed by FACSCalibur. Ten thousand viable CFSE-labeled cells were analyzed for each mouse. The percentage of specific lysis for each mouse was calculated by: 100 × [1−(ratio of cells recovered from naïve mice/ratio of cells recovered from immunized mice)].
Antigen-specific cytokine secretion
Splenocytes (5 × 105/well) were added to 96-well tissue culture plates in duplicate in 200 μL mouse media (RPMI 1640, 10% HI-FBS, 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 55 μM 2-mercaptoethanol (Sigma-Aldrich) supplemented with media, NP147–155 or MHC class I OVA peptide SIINFEKL, (5 μM; Anaspec), or concanavalin A (10 μg/mL, Sigma-Aldrich). After 76 h, splenocyte supernatants from a given mouse and treatment were combined and analyzed for IFN-γ secretion by ELISA as previously described.23 Briefly, samples were assayed in duplicate using ELISA plates coated with purified rat anti-mouse IFN-γ capture antibody (BD Biosciences, San Jose, CA). Cytokines were detected in dilutions of the cell culture supernatants with biotinylated rat anti-mouse IFN-γ (BD Biosciences) followed by streptavidin-HRP, and absorbance values were obtained as described above.
Anti-OVA Ig ELISA
Post-immune sera were analyzed by ELISA as described18 with the following modifications. Briefly, ELISA plates were coated with OVA (10 μg/mL), blocked, and incubated with serial dilutions of sera. Anti-OVA isotype-specific secondary antibodies (goat anti-mouse IgG2a-biotin or goat anti-mouse IgG1-biotin; Southern Biotechnology Associates, Birmingham, AL) were detected with streptavidin-HRP and absorbance values were determined as described above. The titer was defined as the reciprocal of the serum dilution that produced an absorbance value of 0.3 using a 4-parameter analysis (Softmax Pro5.2, Molecular Devices, Sunnyvale, CA). If no titer was observed, the lowest dilution tested was reported.
Statistical analyses
Grubb’s test was used to analyze for outliers in animal studies. Data from liposome-treated animals were compared by ANOVA and Tukey’s post-test for data with homogeneous variance. In the case of non-homogeneous variance, the data were compared using the Kruskal-Wallis test followed by the Dunn’s multiple comparison test. A p-value < 0.05 was considered significant; * p<0.05, ** p<0.01, *** p<0.001.
Results and Discussion
Lipid-ODN conjugate synthesis and liposome incorporation
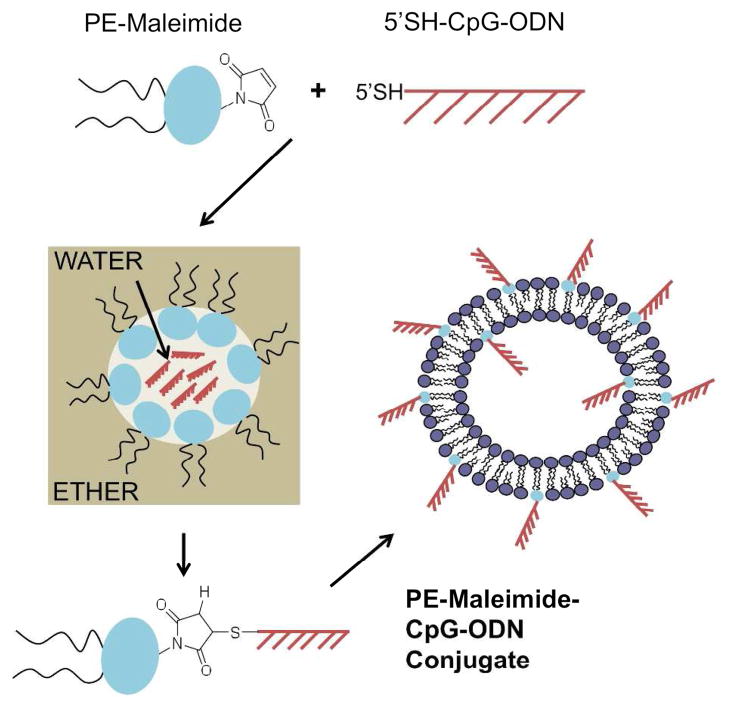
We recently investigated the co-encapsulation of CpG-ODN within LLO-containing pH-sensitive liposomes as a strategy for co-delivery of a protein antigen and the adjuvant to the same APC (C.D. Andrews and M.S. Huh; unpublished results). CpG-ODN was chosen as an adjuvant in this system because its receptor, TLR9, is located in the endocytic pathway, the primary route of uptake of liposomes by cells.15, 24, 25 However, the efficiency of co-encapsulation of CpG-ODNs in LLO-liposomes is relatively low (<10%). Furthermore, after the pH-sensitive LLO-liposomes are taken up by APCs and the liposomal contents are released in the acidic endosome, the perforation of the endosomal membrane by LLO leads to release of the contents, including CpG-ODN, into the cytosol where there is as yet no known CpG-ODN receptor. Therefore, we hypothesized that we could promote CpG-ODN interaction with TLR9, before endosomal rupture by LLO, using the strategy of incorporating lipid-CpG-ODN conjugates in the liposomal membrane (Figure 1).
Figure 1.
Schematic of PE-maleimide and 5′SH-CpG-ODN conjugation procedure and liposome incorporation. Freshly reduced 5′SH-CpG-ODN in aqueous buffer was added to ether containing PE-maleimide. A water-in-ether emulsion was formed by briefly sonicating and the conjugation reaction proceeded ~4h while vortexing at room temperature. The solution containing purified PE-maleimide-CpG-ODN conjugates was added to the lipid mixture, which was then dried to form lipid films. The rehydrated liposomes contained PE-maleimide-CpG-ODN conjugates in the membrane.
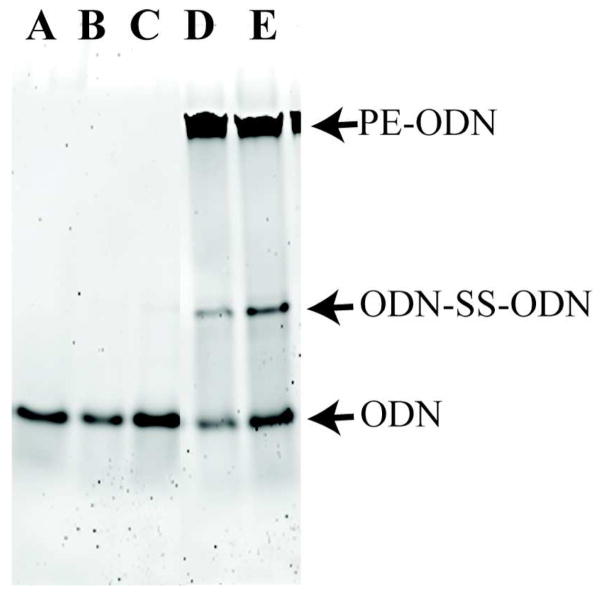
CpG-ODNs with 5′ sulfhydryls were conjugated to maleimide-PE (sulfhydryl-reactive maleimide group conjugated to the lipid headgroup of PE) through the formation of a thioether bond; this typically resulted in 90–95% of the CpG-ODN being conjugated, as determined by ethidium bromide staining of ODN and its lipid-conjugate after separation in TBE gels (Figure 2). In order to ensure that the enhanced immunopharmacological effects were not due to contaminating free CpG-ODNs, the PE-CpG-ODN conjugates were purified using size-exclusion chromatography to remove unconjugated monomeric and/or homodimeric (disulfide-bonded) ODNs. Fractions from size-exclusion chromatography were resolved using TBE-PAGE to identify the fractions that contain PE-CpG-ODN conjugate. The overall CpG-ODN recovery after pooling all fractions with conjugates was approximately 50% (data not shown). After lyophilization and dissolving in methanol:NMP, PE-CpG-ODNs were found to be stable under argon at −20°C for at least 26 days (data not shown).
Figure 2.
Representative PAGE gel of PE-CpG-ODN conjugation. Conjugation was assessed by resolving PE-CpG-ODNs from disulfide bonded (ODN-SS-ODN) and unconjugated 5′SH-CpG-ODN in a 15% TBE polyacrylamide gel stained with ethidium bromide. The amounts of disulfide-bonded and unconjugated ODN were calculated from a standard curve of known ODN amounts. (A) 176 ng CpG-ODN standard, (B,C) reduced 5′SH-CpG-ODN added to MPB-PE (0.03, 0.06 μL, respectively), (D,E) PE-CpG-ODN conjugation reaction (5.25, 10.5 μL, respectively). These results indicate that >95% 5′SH-CpG-ODN is conjugated.
The synthesized PE-CpG-ODNs were added at 0.2 to 0.3 mole percent (see method section for details) to the lipid mixture and dried by rotary evaporation to make lipid films used for liposome preparation. After liposome rehydration, we then tested whether PE-CpG-ODNs were incorporated and remained associated with the liposomes; liposomes were resolved in a Ficoll step density gradient (0, 10, 20% Ficoll) and ODNs were identified in the gradient fractions by ethidium bromide staining of TBE gels. In this assay, liposomes floated to the interface of the 0 and 10% layers, whereas non-liposomal PE-CpG-ODNs remained in the 20% Ficoll fractions (data not shown); all of the liposomal PE-CpG-ODNs detected were in the 0 and 10% interface, indicating that all lipid-CpG-ODN conjugates were incorporated in the liposome membrane. In agreement with previous work reporting the asymmetric distribution of gangliosides in liposome bilayers,26 using a nuclease protection assay27 against nuclease P1 we found the membrane leaflet distribution of PE-CpG-ODN to be ~80% extra-liposomal and ~20% luminal (data not shown).
Overall, these data indicate that PE-CpG-ODNs are efficiently synthesized with approximately 50% recovery after purification, and no loss of the conjugate was incurred during incorporation into the liposome membrane using the traditional method of liposome preparation. The majority of the loss of CpG-ODNs resulted from the size-exclusion chromatography step, which may not in fact be required as the efficiency of the conjugation process was so high (typically 90–95%). Overall, the process of lipid conjugation and incorporation into liposome bilayers is much more efficient than the more commonly employed passive encapsulation technique, which typically results in less than 10% CpG-ODN encapsulated.
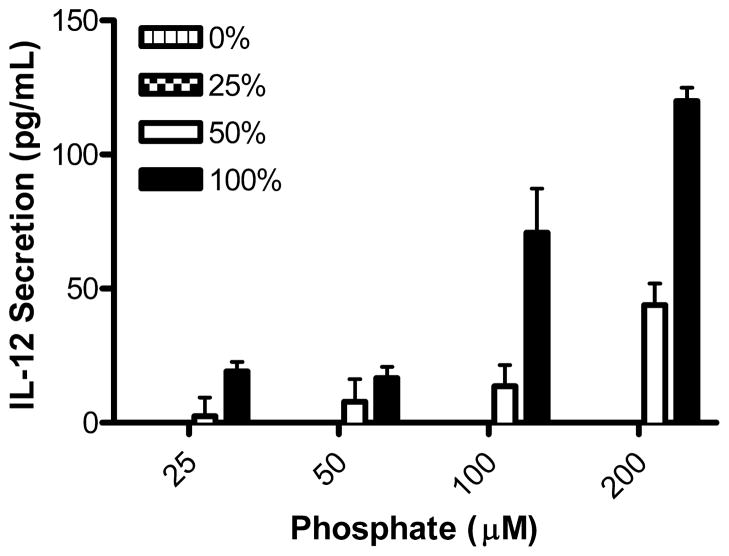
IL-12 secretion is PE-CpG-ODN dose-dependent
Next, we asked whether CpG-ODN incorporated onto the liposomes in the form of PE-CpG-ODN conjugates retain the capacity of CpG-ODN to stimulate and modulate an immune response. The liposomes were evaluated for their ability to induce the secretion of the Th1-type proinflammatory cytokine IL-12 in BMM in culture. It has been shown that the surface charge of liposomes affects their uptake by macrophages.28 Therefore, to eliminate uptake differences the surface charge of the liposomes was held constant by maintaining the total percentage of conjugated ODNs in the liposome membrane (total PE-ODN kept at 0.3 mole percent of total phospholipids) while varying the percentage of PE-ODNs that contain the CpG sequence at 0, 25, 50 and 100%; the remaining PE-ODNs consisted of PE-non-CpG-ODN conjugates. Increasing the percent of PE-CpG-ODN in the liposomal membranes increased the amount of IL-12 secreted in a linear fashion (Figure 3). No IL-12 was detected when treated with the 0% PE-CpG-ODN sample (100% PE-non-CpG-ODN). As the fraction PE-CpG-ODN of the total PE-ODNs (PE-CpG-ODN plus PE-non-CpG-ODNs) was increased in these experiments, greater than 25% PE-CpG-ODN was required to stimulate detectable IL-12 secretion; this was shown by the lack of detectable IL-12 when cells were treated with 25% PE-CpG-ODN and by the presence of detectable IL-12 secretion at 50% PE-CpG-ODN. These data suggest that a certain threshold or minimum number of PE-CpG-ODN conjugates per liposome are required to achieve optimal BMM activation. It is noteworthy that a similar dose responsiveness phenomenon has been observed when dendritic cells were treated with acid-degradable microparticles loaded with CpG-ODNs.29
Figure 3.
IL-12 secretion is PE-CpG-ODN dose-dependent. The percentage of PE-CpG-ODN incorporated in liposomes was varied while maintaining total PE-ODN (PE-CpG-ODN plus PE-non-CpG-ODN) fixed at 0.3 mole percent of phospholipids. BMM were treated in serum-free media with serial dilutions of liposomes for 3 h, washed and incubated in complete media for 20 h. The supernatant was collected and analyzed for secreted IL-12p40 by ELISA.
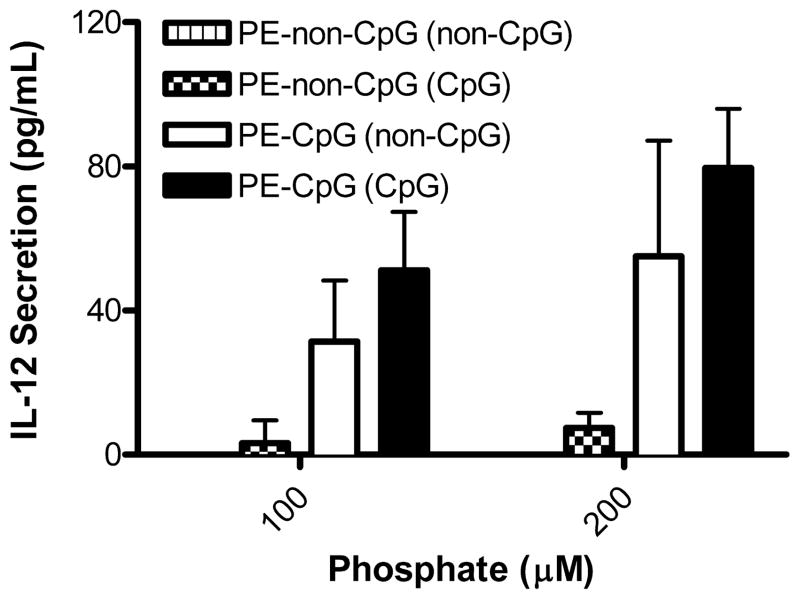
Lipid-conjugated ODNs likely interact with TLR9 before encapsulated ODNs
We hypothesized and investigated that conjugating CpG-ODN to PE lipids would promote the interaction between CpG-ODN and its presumptive receptor TLR9 in the endosomal compartment, before the pH-sensitive liposomes are destabilized by the low pH of the endosome and release their LLO. Ultimately, the effects of LLO breaching the endosomal membrane may have negative implications for the binding of CpG-ODN and signaling by TLR9. First, it is established that protease inhibitors or inhibitors of endosomal acidification prevent the activation of TLR9,30, 31 and when LLO forms pores in the endosomal membrane a transient rise in pH occurs.32 Secondly, LLO-mediated membrane perforation results in the release of endosomal contents,17 in this case CpG-ODNs, into the cytosol where there is no known receptor for single-stranded ODNs (reviewed by Ishii and Akira33). To test this hypothesis, we made PE-CpG-ODN-containing liposomes that contained either encapsulated CpG-ODN or encapsulated non-CpG-ODN; we also made PE-non-CpG-ODN-containing liposomes that encapsulated either CpG-ODN or non-CpG-ODN. Previous reports strongly suggest that phosphorothioate ODNs bind the ectodomain of TLR9 regardless of sequence composition (i.e. CpG-containing or not); however, activation of TLR9 is CpG-dependent,34, 35 indicating that non-CpG-ODNs do not stimulate IL-12 secretion despite their binding to TLR9. Based on these data, we predicted that there would be reduced IL-12 secretion with PE-non-CpG-ODNs if these ODNs were to bind TLR9 before release of encapsulated CpG-ODN, thus reducing both the interaction and the effect of encapsulated CpG-ODNs via TLR9. The results from Figure 4 support our hypothesis that the lipid-conjugated ODNs on the surface of the liposomes interact with TLR9 before encapsulated ODNs do, as we observed significantly attenuated IL-12 secretion with non-CpG-ODNs on the outside of liposomes encapsulating CpG-ODNs. Importantly, antigen presentation assays confirmed that the incorporation of conjugated ODNs did not affect the intracellular delivery of LLO-containing PE:CHEMS liposomes as shown by cytosolic delivery of the model antigen OVA (data not shown); therefore, we expected co-encapsulated ODNs to also have been efficiently released from the liposomes to interact with TLR9.
Figure 4.
Lipid-conjugated ODNs interact with TLR9 before encapsulated ODNs. Liposomes encapsulating either CpG-ODN or non-CpG-ODN [encapsulation denoted by the ODN type in parentheses in the figure caption] were made containing 0.3 mole percent PE-CpG-ODN or PE-non-CpG-ODN. BMM were treated in serum-free media with serial dilutions of liposomes for 3 h, washed and incubated in complete media for 20 h. The supernatant was collected and analyzed for secreted IL-12p40 by ELISA.
PE-CpG-ODNs stimulate CTL immune responses similar to encapsulated CpG-ODNs
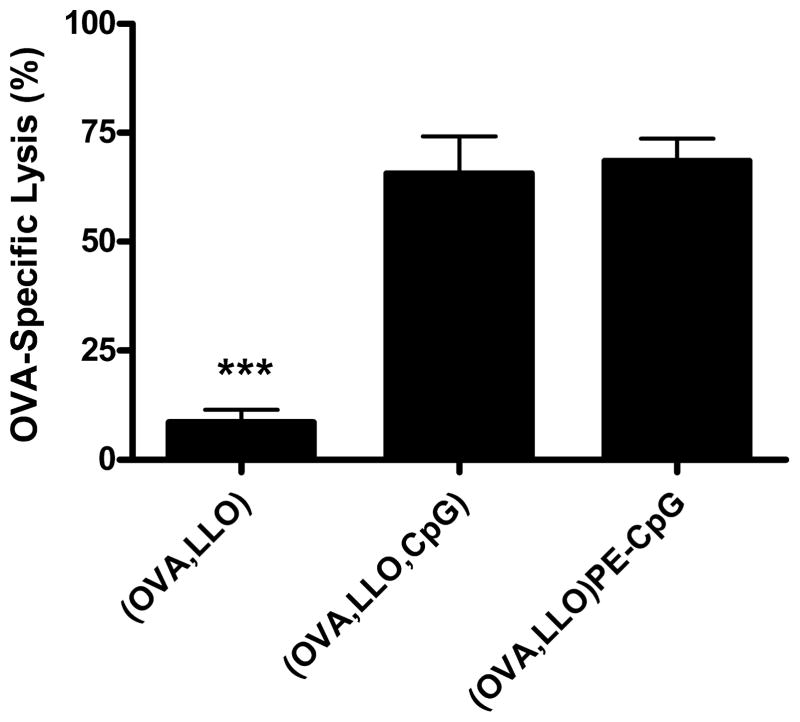
The in vitro studies indicated that lipid-conjugated CpG-ODNs on the liposome membrane exhibit immunostimulatory capabilities; to corroborate our results in vivo, we immunized mice to compare the two methods for including CpG-ODNs in the liposomes; incorporation of the CpG-ODN lipid conjugate onto liposome membranes versus incorporation via encapsulation. OVA and LLO were co-encapsulated in pH-sensitive liposomes with either lipid-conjugated or co-encapsulated CpG-ODNs as methods for simultaneously delivering antigen and co-stimulatory molecules to the same APC. These liposomes were tested for their capacity to stimulate OVA-specific CTL activity relative to that of OVA-containing LLO-liposomes without CpG-ODN. Mice were vaccinated subcutaneously with liposomes normalized for the amount of OVA (8 μg), and ten days later equal populations of CFSEHIGH-and CFSELOW-labeled splenocytes were injected intravenously. The CFSEHIGH target cells were pulsed with the CD8+ T cell epitope SIINFEKL (OVA257–264 peptide), while CFSELOW control cells were pulsed with a non-specific influenza NP peptide. The CTL responses were comparable when mice were immunized with liposomes co-encapsulating CpG-ODN or with lipid-conjugated CpG-ODNs; specific lysis was 66% ± 22 or 69% ± 13, respectively (Figure 5). The OVA-specific lysis was significantly enhanced (p<0.001) when mice were immunized with either of the liposome formulations containing CpG-ODN compared with liposomes containing only OVA and LLO (specific lysis of 9% ± 6). These results indicate that lipid-conjugated CpG-ODNs are capable of interacting with TLR9, providing T cells with the additional co-stimulation required for optimal activation in vivo.
Figure 5.
Encapsulated and lipid-conjugated CpG-ODNs induce an OVA-specific CTL response. Mice were immunized subcutaneously on day 0 with liposomes containing OVA and LLO (8 μg OVA) or OVA, LLO and CpG-ODN (8 μg OVA and 3.8 μg CpG-ODN) or OVA and LLO with PE-CpG-ODN conjugates (8 μg OVA and 4.6 μg CpG-ODN). OVA-specific CTL activity was monitored in vivo on day 10 by lysis of intravenously injected CFSEHIGH-labeled SIINFEKL-pulsed splenocytes in comparison to CFSELOW-labeled influenza NP peptide-pulsed splenocytes. The CFSE profiles were analyzed by FACS 8.5 h after adoptive transfer. The mean ± SEM is shown (n = 5–7 mice per group).
PE-CpG-ODNs stimulate a Th1-type immune response similar to encapsulated CpG-ODNs
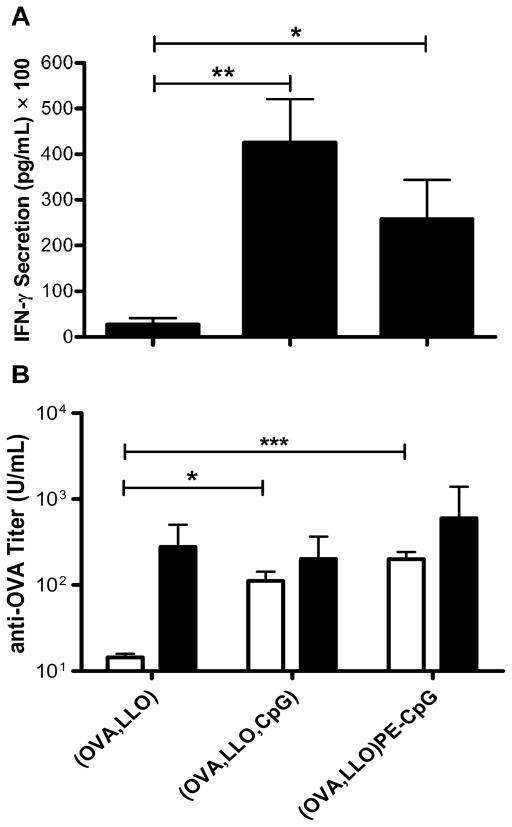
In addition to antigen-specific CTL induction, we evaluated the ability of CD8+ T cells from the splenocytes of immunized mice to secrete IFN-γ ex vivo upon antigen-specific CD8 peptide stimulation. Splenocytes were incubated with the OVA CD8 peptide SIINFEKL for 76 h in culture, and the supernatants were harvested and analyzed for the amount of secreted IFN-γ by ELISA. Splenocytes from mice immunized with liposomes co-encapsulating OVA and LLO secreted 2,702 (± 1,401) pg/mL IFN-γ (Figure 6A). IFN-γ secretion was significantly enhanced when CpG-ODN was co-encapsulated or lipid-conjugated: 42,599 (± 9,460) and 25,848 (± 8,534) pg/mL, respectively. As negative controls, media alone or the irrelevant NP peptide generated no detectable IFN-γ in any of the groups, while the positive control mitogen concanavalin A stimulate splenocytes from all groups to secrete IFN-γ (data not shown)
Figure 6.
PE-CpG-ODNs stimulate a Th1-type immune response similar to that of encapsulated CpG-ODNs. (A) Mice were immunized with liposome formulations on day 0, and spleens were harvested on day 10. Isolated splenocytes were stimulated with SIINFEKL for 76 h and the resulting IFN-γ secretion was monitored by ELISA. The mean ± SEM is shown. (B) Sera were obtained from blood of immunized mice harvested by cardiac puncture on day 10. Anti-OVA IgG2a (open bars) and IgG1 (closed bars) were monitored using ELISA. The geometric mean titer ± SEM is shown (n = 7–8 mice per group).
The induction of anti-OVA Th1- and Th2-type antibodies in the immunized mice was monitored, in addition to investigating the cytokine milieus induced by the APCs. CpG-ODNs stimulate a Th1-type immune response mediated by the cytokine IFN-γ, which drives the production of Th1-type antibodies such as IgG2a. Mice immunized with (i) liposomal OVA and LLO only, (ii) liposomes encapsulating OVA and LLO with CpG-ODN, or (iii) liposomes encapsulating OVA and LLO with lipid-conjugated CpG-ODN all produced similar levels of anti-OVA IgG1 (Figure 6B). However, adding CpG-ODN, either encapsulated or conjugated, significantly enhanced the IgG2a antibody response: 80 (± 31) U/mL or 158 (± 43) U/mL, respectively, compared with mice immunized with liposomal OVA and LLO, 15 (± 2) U/mL. Note that these antibody responses are relatively weak which is due to the fact that prime only, and not prime-boost, was employed; similar studies performed with prime-boost regimens resulted in stronger anti-OVA antibody responses with similar trends, confirming the results illustrated here by the prime-only study. These results are consistent with the IFN-γ secretion data, indicating that conjugating CpG-ODN to lipid and incorporating it onto the liposomal membrane does not alter the ability of the CpG-ODN to stimulate a Th1-type response as shown by the ex vivo IFN-γ secretion upon CD8 peptide stimulation and the Th1-type antibody induction.
Although reduced immunostimulatory activity of CpG-ODNs has been reported for some CpG-containing sequences after conjugation at the 5′end,29, 36 the activity of the CpG-ODN ISS 1018 used in this study appears to be unaffected by the choice of terminus (5′ or 3′) used for coupling when conjugated to proteins (G. Ott personal communication). Consistent with these findings, we observed similar CTL and Th1 responses when CpG-ODN were conjugated through the 5′ end or encapsulated. It is possible that the predicted reduction in immunostimulatory activity observed with 5′-conjugated CpG-ODNs was offset here by: (i) the liposomal surface position of PE-CpG-ODN more readily allowing interactions with TLR9 vis-à-vis encapsulated CpG-ODN, or (ii) PE-CpG-ODNs on the liposome surface may enhance uptake by APCs, similar to that which has been observed with antigen-CpG-ODN-conjugates.37 Regardless, of any potential mechanistic implications, this study demonstrates that liposomes containing lipid-conjugated CpG-ODNs are capable of stimulating an immune response that is skewed toward the Th1-type, and future studies may include conjugation at the 3′ end, which typically results in a relatively smaller reduction in immunostimulatory activity.29, 36 The study reported here was performed solely to compare the effects of incorporating CpG-ODNs in the liposomal formulation through either lipid-conjugation or encapsulation; however, future studies are required to determine the optimal PE-CpG-ODN density in the liposome membrane for LLO-liposomes as well as the orientation of the conjugation.
Conclusion
We have evaluated two methods for including CpG-ODNs in an LLO-liposome vaccine carrier. Conjugating CpG-ODNs to lipid allows the design of a liposome-based delivery platform that results in greater recovery of CpG-ODNs than does passive encapsulation. Moreover, lipid-conjugated CpG-ODNs in the liposome membrane exhibit immunostimulatory activity both in vitro and in vivo. The results in this report strongly suggest that LLO-containing liposomes with lipid-conjugated CpG-ODN are a versatile and efficient delivery vehicle that may be used with a variety of antigens for vaccination. The strong CTL and Th1-type immune responses indicate the potential for this vaccine delivery system for a variety of vaccines against viruses or cancer.
Acknowledgments
The authors would like to thank Zachary F. Walls, Ph.D. for helpful discussions. This work was supported by NIH R01 AI047173 to KDL. CDA was supported by NIH GM008353 and the American Foundation for Pharmaceutical Education.
References
- 1.Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol. 2007;5:505–17. doi: 10.1038/nrmicro1681. [DOI] [PubMed] [Google Scholar]
- 2.Ott G, Barchfeld GL, Van Nest G. Enhancement of humoral response against human influenza vaccine with the simple submicron oil/water emulsion adjuvant MF59. Vaccine. 1995;13:1557–62. doi: 10.1016/0264-410x(95)00089-j. [DOI] [PubMed] [Google Scholar]
- 3.O’Hagan DT, Rappuoli R. Novel approaches to vaccine delivery. Pharm Res. 2004;21:1519–30. doi: 10.1023/B:PHAM.0000041443.17935.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–45. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 5.Reed SG, Bertholet S, Coler RN, Friede M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009;30:23–32. doi: 10.1016/j.it.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–60. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–34. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 8.Cho HJ, Takabayashi K, Cheng PM, Nguyen MD, Corr M, Tuck S, Raz E. Immunostimulatory DNA-based vaccines induce cytotoxic lymphocyte activity by a T-helper cell-independent mechanism. Nat Biotechnol. 2000;18:509–14. doi: 10.1038/75365. [DOI] [PubMed] [Google Scholar]
- 9.Tighe H, Takabayashi K, Schwartz D, Marsden R, Beck L, Corbeil J, Richman DD, Eiden JJ, Jr, Spiegelberg HL, Raz E. Conjugation of protein to immunostimulatory DNA results in a rapid, long-lasting and potent induction of cell-mediated and humoral immunity. Eur J Immunol. 2000;30:1939–47. doi: 10.1002/1521-4141(200007)30:7<1939::AID-IMMU1939>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Li WM, Dragowska WH, Bally MB, Schutze-Redelmeier MP. Effective induction of CD8+ T-cell response using CpG oligodeoxynucleotides and HER-2/neu-derived peptide co-encapsulated in liposomes. Vaccine. 2003;21:3319–29. doi: 10.1016/s0264-410x(03)00172-5. [DOI] [PubMed] [Google Scholar]
- 11.de Jong S, Chikh G, Sekirov L, Raney S, Semple S, Klimuk S, Yuan N, Hope M, Cullis P, Tam Y. Encapsulation in liposomal nanoparticles enhances the immunostimulatory, adjuvant and anti-tumor activity of subcutaneously administered CpG ODN. Cancer Immunol Immunother. 2007;56:1251–64. doi: 10.1007/s00262-006-0276-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gursel I, Gursel M, Ishii KJ, Klinman DM. Sterically stabilized cationic liposomes improve the uptake and immunostimulatory activity of CpG oligonucleotides. J Immunol. 2001;167:3324–8. doi: 10.4049/jimmunol.167.6.3324. [DOI] [PubMed] [Google Scholar]
- 13.Standley SM, Mende I, Goh SL, Kwon YJ, Beaudette TT, Engleman EG, Frechet JM. Incorporation of CpG oligonucleotide ligand into protein-loaded particle vaccines promotes antigen-specific CD8 T-cell immunity. Bioconjug Chem. 2007;18:77–83. doi: 10.1021/bc060165i. [DOI] [PubMed] [Google Scholar]
- 14.Zwiorek K, Bourquin C, Battiany J, Winter G, Endres S, Hartmann G, Coester C. Delivery by cationic gelatin nanoparticles strongly increases the immunostimulatory effects of CpG oligonucleotides. Pharm Res. 2008;25:551–62. doi: 10.1007/s11095-007-9410-5. [DOI] [PubMed] [Google Scholar]
- 15.Straubinger RM, Papahadjopoulos D, Hong KL. Endocytosis and intracellular fate of liposomes using pyranine as a probe. Biochemistry. 1990;29:4929–39. doi: 10.1021/bi00472a025. [DOI] [PubMed] [Google Scholar]
- 16.Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–8. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 17.Lee KD, Oh YK, Portnoy DA, Swanson JA. Delivery of macromolecules into cytosol using liposomes containing hemolysin from Listeria monocytogenes. J Biol Chem. 1996;271:7249–52. [PubMed] [Google Scholar]
- 18.Mandal M, Lee KD. Listeriolysin O-liposome-mediated cytosolic delivery of macromolecule antigen in vivo: enhancement of antigen-specific cytotoxic T lymphocyte frequency, activity, and tumor protection. Biochim Biophys Acta. 2002;1563:7–17. doi: 10.1016/s0005-2736(02)00368-1. [DOI] [PubMed] [Google Scholar]
- 19.Mandal M, Kawamura KS, Wherry EJ, Ahmed R, Lee KD. Cytosolic delivery of viral nucleoprotein by listeriolysin O-liposome induces enhanced specific cytotoxic T lymphocyte response and protective immunity. Mol Pharm. 2004;1:2–8. doi: 10.1021/mp034021m. [DOI] [PubMed] [Google Scholar]
- 20.Portnoy DA, Jones S. The cell biology of Listeria monocytogenes infection (escape from a vacuole) Ann N Y Acad Sci. 1994;730:15–25. doi: 10.1111/j.1749-6632.1994.tb44235.x. [DOI] [PubMed] [Google Scholar]
- 21.Stier EM, Mandal M, Lee KD. Differential cytosolic delivery and presentation of antigen by listeriolysin O-liposomes to macrophages and dendritic cells. Mol Pharm. 2005;2:74–82. doi: 10.1021/mp049896v. [DOI] [PubMed] [Google Scholar]
- 22.Bartlett GR. Organization of red cell glycolytic enzymes; cell coat phosphorus transfer. Ann N Y Acad Sci. 1958;75:110–4. doi: 10.1111/j.1749-6632.1958.tb36855.x. [DOI] [PubMed] [Google Scholar]
- 23.Sun X, Provoda C, Lee KD. Enhanced in vivo gene expression mediated by listeriolysin o incorporated anionic LPDII: Its utility in cytotoxic T lymphocyte-inducing DNA vaccine. J Control Release. 2010;148:219–225. doi: 10.1016/j.jconrel.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barton GM, Kagan JC, Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat Immunol. 2006;7:49–56. doi: 10.1038/ni1280. [DOI] [PubMed] [Google Scholar]
- 25.Kumagai Y, Takeuchi O, Akira S. TLR9 as a key receptor for the recognition of DNA. Adv Drug Deliv Rev. 2008;60:795–804. doi: 10.1016/j.addr.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Thomas PD, Poznansky MJ. Curvature and composition-dependent lipid asymmetry in phosphatidylcholine vesicles containing phosphatidylethanolamine and gangliosides. Biochim Biophys Acta. 1989;978:85–90. doi: 10.1016/0005-2736(89)90502-6. [DOI] [PubMed] [Google Scholar]
- 27.Semple SC, Klimuk SK, Harasym TO, Dos Santos N, Ansell SM, Wong KF, Maurer N, Stark H, Cullis PR, Hope MJ, Scherrer P. Efficient encapsulation of antisense oligonucleotides in lipid vesicles using ionizable aminolipids: formation of novel small multilamellar vesicle structures. Biochim Biophys Acta. 2001;1510:152–66. doi: 10.1016/s0005-2736(00)00343-6. [DOI] [PubMed] [Google Scholar]
- 28.Lee KD, Hong K, Papahadjopoulos D. Recognition of liposomes by cells: in vitro binding and endocytosis mediated by specific lipid headgroups and surface charge density. Biochim Biophys Acta. 1992;1103:185–97. doi: 10.1016/0005-2736(92)90086-2. [DOI] [PubMed] [Google Scholar]
- 29.Beaudette TT, Bachelder EM, Cohen JA, Obermeyer AC, Broaders KE, Frechet JM, Kang ES, Mende I, Tseng WW, Davidson MG, Engleman EG. In vivo studies on the effect of co-encapsulation of CpG DNA and antigen in acid-degradable microparticle vaccines. Mol Pharm. 2009;6:1160–9. doi: 10.1021/mp900038e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ewald SE, Lee BL, Lau L, Wickliffe KE, Shi GP, Chapman HA, Barton GM. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature. 2008;456:658–62. doi: 10.1038/nature07405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park B, Brinkmann MM, Spooner E, Lee CC, Kim YM, Ploegh HL. Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat Immunol. 2008;9:1407–14. doi: 10.1038/ni.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beauregard KE, Lee KD, Collier RJ, Swanson JA. pH-dependent perforation of macrophage phagosomes by listeriolysin O from Listeria monocytogenes. J Exp Med. 1997;186:1159–63. doi: 10.1084/jem.186.7.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishii KJ, Akira S. Innate immune recognition of, and regulation by, DNA. Trends Immunol. 2006;27:525–32. doi: 10.1016/j.it.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Latz E, Verma A, Visintin A, Gong M, Sirois CM, Klein DC, Monks BG, McKnight CJ, Lamphier MS, Duprex WP, Espevik T, Golenbock DT. Ligand-induced conformational changes allosterically activate Toll-like receptor 9. Nat Immunol. 2007;8:772–9. doi: 10.1038/ni1479. [DOI] [PubMed] [Google Scholar]
- 35.Haas T, Metzger J, Schmitz F, Heit A, Muller T, Latz E, Wagner H. The DNA sugar backbone 2′ deoxyribose determines toll-like receptor 9 activation. Immunity. 2008;28:315–23. doi: 10.1016/j.immuni.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 36.Kandimalla ER, Bhagat L, Yu D, Cong Y, Tang J, Agrawal S. Conjugation of ligands at the 5′-end of CpG DNA affects immunostimulatory activity. Bioconjug Chem. 2002;13:966–74. doi: 10.1021/bc0200374. [DOI] [PubMed] [Google Scholar]
- 37.Shirota H, Sano K, Hirasawa N, Terui T, Ohuchi K, Hattori T, Shirato K, Tamura G. Novel roles of CpG oligodeoxynucleotides as a leader for the sampling and presentation of CpG-tagged antigen by dendritic cells. J Immunol. 2001;167:66–74. doi: 10.4049/jimmunol.167.1.66. [DOI] [PubMed] [Google Scholar]