Abstract
Objective
Lowered baroreflex sensitivity (BRS) predicts mortality and occurs with increasing age and diabetes. We examined whether aerobic exercise could restore arterial BRS in adults at high cardiovascular risk (diabetes, geriatric age group, hypercholesterolemia, and hypertension).
Design
Randomized, controlled, single-blind study.
Setting
VITALiTY (Vancouver Initiative to Add Life to Years) Research Laboratory.
Participants
Thirty-nine older adults (mean age, 71.5 ± 0.7 years) with diet-controlled or oral hypoglycemic-controlled type 2 diabetes, hypertension, and hypercholesterolemia.
Interventions
Subjects were recruited to each of 2 groups: an aerobic group (3 months of vigorous aerobic exercise as defined by 80% to 85% of maximal heart rate), and a nonaerobic (no aerobic exercise) group. Exercise sessions were supervised by a certified exercise trainer 3 times per week.
Main Outcome Measures
Baroreflex function was assessed using the spontaneous baroreflex method. Main outcome measures included BRS, BRSup, BRSdown, and V̇o2max.
Results
The aerobic group demonstrated an increase in BRS that was not demonstrated in the nonaerobic group (+60.9 ± 23.5 vs +2.2 ± 7.9%; P = 0.010).
Conclusions
Our findings indicate that a relatively short aerobic exercise intervention can reverse functional impairments of the arterial baroreflex function in older adults at high cardiovascular risk.
Keywords: arterial baroreflex, gerontology, type 2 diabetes, aerobic exercise
INTRODUCTION
Autonomic dysfunction occurs in almost half of all patients with diabetes and is associated with increased mortality.1 Cardiac autonomic function is primarily controlled by the arterial baroreflex, located in the aortic arch and carotid sinuses, which responds to a drop in blood pressure by eliciting inverse changes in heart rate (HR). Baroreflex sensitivity (BRS) has been shown to decrease with normal aging and with diabetes and is an independent predictor of mortality.2 In fact, lowered BRS was found to double mortality in patients who had other cardiovascular risk factors, such as diabetes or hypertension.2
One potential therapy for low BRS is aerobic training. Previous prospective work has demonstrated an increase in BRS with aerobic training in both young athletes3 and middle-aged4 healthy adults. Studies of middle-aged populations with type 2 diabetes have had mixed results, demonstrating either no change5 or an increase6 in resting BRS with aerobic training. To our knowledge, there has been no prospective examination of the ability to restore BRS in older adults with type 2 diabetes, especially in the presence of comorbid hypertension and hypercholesterolemia.
In the current study, we examined whether aerobic exercise can restore arterial BRS in adults with a high cardiovascular risk profile (long-standing diabetes, geriatric age group, hypercholesterolemia, and hypertension). We hypothesized that despite multiple cardiovascular risk factors, aerobic exercise would be an effective nonpharmacological therapy to increase BRS.
MATERIALS AND METHODS
Subjects
Forty-five older adults were recruited from the local community through advertisements in local publications (Table 1). All subjects had to be older than 65 years and were excluded if they had any history of angina, myocardial infarction, stroke, chronic pulmonary disease, current smoking, or exercise-limiting orthopedic impairment. All older subjects were required to have type 2 diabetes for at least 5 years, hypertension, and hyperlipidemia. Hypertension, diabetes, and hyperlipidemia were defined by current American Diabetes Association guidelines.7 Hypertension was defined as taking antihypertensive agents or having an elevated average blood pressure (based on the mean of 3 supine measurements taken on 3 different days). Each blood pressure reading was taken after 20 minutes of quiet supine rest and was recorded by automatic sphygmomanometer (BP Tru; VS Medtech, Ltd, Brooklyn, New York). Elevated blood pressure was defined as a systolic blood pressure (SBP) greater than 130 mm Hg or a diastolic blood pressure (DBP) greater than 80 mm Hg.7 Subjects were excluded if they took beta-blockers, calcium channel blockers, or any other agent with the potential to influence autonomic function. Entry requirements included a normal resting electerocardiogram, a normal Bruce protocol treadmill maximal exercise stress test as per current American Heart Association guidelines,8 a normal hematocrit, and a normal creatinine. Subjects had to be sedentary at the start of the study (as defined as no strength training and less than 30-minute brisk walking/moderate exercise per day and no vigorous exercise in the preceding 6 months). Five subjects were excluded on the basis of this screening, leaving a total of 40 subjects (25 men and 15 women; mean age, 71.5 ±0.7, ranging in age from 65 to 83 years) participating in the study. Twenty subjects were randomized to each of 2 groups: an aerobic group (AT), and a nonaerobic (NA) group. This study was approved by the Human Subjects Committee of the University of British Columbia, and all subjects gave written informed consent.
TABLE 1.
Subject Characteristics
| Measure | All Subjects | AT Subjects | NA Subjects | P |
|---|---|---|---|---|
| Age, y | 71.5 ± 0.7 | 71.8 ± 1.1 | 71.2 ± 0.9 | 0.420 |
| Body mass, kg | 81.0 ± 2.1 | 83.5 ± 2.6 | 79.3 ± 3.1 | 0.343 |
| Height, m | 1.68 ± 0.01 | 1.66 ± 0.03 | 1.69 ± 0.02 | 0.532 |
| Body mass index, kg/m2 | 28.6 ± 0.63 | 30.1 ± 1.0 | 27.7 ± 1.0 | 0.061 |
| Waist to hip ratio | 0.95 ± 0.01 | 0.97 ± 0.02 | 0.94 ± 0.02 | 0.291 |
| Blood pressure, mm Hg | ||||
| Systolic | 143 ± 3 | 149 ± 6 | 139 ± 4 | 0.123 |
| Diastolic | 85 ± 2 | 83 ± 2 | 86 ± 2 | 0.329 |
| Mean | 104 ± 2 | 105 ± 3 | 104 ± 3 | 0.723 |
| Heart rate, beats/min | 64.5 ± 1.7 | 66.1 ± 2.8 | 62.8 ± 1.9 | 0.340 |
| Fasting blood glucose, mEq1 | 7.5 ± 0.3 | 7.9 ± 0.6 | 7.1 ± 0.3 | 0.22 |
| Glycosylated hemoglobin, % | 6.5 ± 0.1 | 6.7 ± 0.2 | 6.4 ± 0.1 | 0.432 |
| Lipid profile, mmol/L | ||||
| Total cholesterol | 4.7 ± 0.2 | 5.0 ± 0.2 | 4.6 ± 0.3 | 0.290 |
| LDL cholesterol | 2.6 ± 0.2 | 2.6 ± 0.8 | 2.5 ± 0.2 | 0.592 |
| HDL cholesterol | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.5 ± 0.1 | 0.619 |
| BRS, ms/mm Hg | ||||
| All sequences | 9.12 ± 0.93 | 8.85 ± 1.67 | 9.31 ± 1.08 | 0.975 |
| Upward sequences | 8.65 ± 1.11 | 8.06 ± 2.03 | 9.08 ± 1.25 | 0.957 |
| Downward sequences | 9.46 ± 0.82 | 9.27 ± 1.37 | 9.60 ± 1.03 | 0.824 |
Baseline data for aerobically trained (AT), untrained (NA) and all subjects are shown as mean ± standard error. A P value of < 0.05 was considered significant.
HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Study Design
Each subject underwent 2 evaluation sessions before and after the 3-month intervention. Postintervention study sessions could be delayed up to 7 days to accommodate each subject’s schedule, as long as the training program was continued. All study sessions were performed with the subject supine and took place between 7 AM and noon for all subjects to avoid bias due to circadian rhythms. Each subject was supine for 45 minutes before the start of data collection to reach steady state. Subjects were fasting, had refrained from the consumption of alcohol or caffeine, and had not exercised for the 24 hours before each session. The technician responsible for performing all measures was blinded to subject group.
Training Program
The endurance training (AT group) intervention was designed to improve aerobic fitness according to current guidelines9 and consisted of moderate to vigorous intensity exercise on a treadmill and a cycle ergometer. Training sessions were 3 times per week for 12 weeks, and subjects had to attend 90% of all training sessions to remain enrolled in the study. The aerobic training sessions were 60 minutes in duration and consisted of 10-minute warm-up, 20-minute aeorobic treadmill training, 20-minute cycle ergometer training, and 10 minutes of cooldown/stretching. Target HRs for the AT group were based on maximal HR observed during maximal exercise treadmill testing (see below). The endurance training program used a target HR zone of 50% to 60% of maximal HR for the first 2 weeks, progressing to 80% to 85% of maximal HR for the remainder of the program. Heart rates were monitored at 60-second intervals during training using Polar Vantage Heart watches ( Polar Electro, Inc, Adelaide, Australia). Subjects in the NA group also attended sessions 3 times per week. The NA group sessions were specifically designed to have no aerobic component and consisted of nonstrenuous core (exercise ball) and nonstrenous strength training (very light dumbbells) exercises. We confirmed a lack of aerobic training in the NA group with a test of maximal oxygen consumption in each subject (V̇o2max see below). The trainer also contacted each subject weekly to ensure that they were not undertaking any additional exercise. For ethical reasons, each subject in the NA group was given the option of joining the aerobic exercise group after the 3-month intervention period was complete, to encourage them to increase their level of physical fitness.
Data Collection and Processing
Heart rate was monitored continuously using a 3-lead electrocardiogram. Blood pressure was monitored using a Finometer (Finapres Medical Systems BV, Amsterdam, the Netherlands). The Finometer measures beat-to-beat blood pressure noninvasively using infrared plethysmography through a finger cuff. Use of the Finometer and infrared plethysmography for monitoring blood pressure changes has been well established as a noninvasive measure of beat-to-beat blood pressure, has been extensively validated against intra-arterial blood pressure monitoring in older adults,10 and is validated for the assessment of arterial baroreflex function.11 The Finometer uses waveform filtering, level correction, and an additional return-to-flow calibration to reconstruct brachial artery pressures.12 The electrocardiogram and blood pressure signals were sampled at 1000 Hz (Powerlab; AD Instruments, Colorado Springs, Colorado) and digitized for later analysis. Using commercially available software, beat-to-beat measures of blood pressure (Beatscope; Finapres Medical Systems BV) and HR (Powerlab; AD Instruments) were calculated. All post-collection analyses of arterial baroreflex function were done in a blinded fashion. Before all derived measurements, each segment of raw blood pressure and electrocardiogram was manually examined to exclude artifacts.
The Sequence Method of Assessing Arterial Baroreflex Function
This method involves the analysis of “spontaneous” swings in blood pressure and HR that are mediated by the arterial baroreflexes. Data are examined for progressive increases in both SBP and R-R interval (RRI) or progressive decreases in SBP and RRI. Baroreflex sensitivity is defined as the mean slope of the regression lines for all these baroreflex-mediated sequences (+RRI/+SBP or −RRI/−SBP) and is measured in milliseconds per millimeter of mercury.13 Parameters used for our spontaneous baroreflex analysis were the inclusion of all baroreflex-mediated sequences of 3 or more beats that had a correlation coefficient greater than 0.80, a threshold of blood pressure change of 1 mm Hg, and a threshold for change in RRI of 4 milliseconds. For the purpose of our analysis, there is a +1 shift between the SBP data and the RRI data (the SBP pulse is plotted against the following RRI for the purpose of regression analysis). This set of conditions for spontaneous baroreflex is well established in the older adult population,10,13 maximally correlates with the bolus intravenous phenylephrine method,14 and has high intrasubject reproducibility.15 Spontaneous baroreflex measures allows for a separate examination of arterial BRS for sequences characterized by a decrease (BRSdown) or increase (BRSup) in blood pressure.
Heart rate, systolic (SBP), DBP, and mean (MAP) blood pressure were measured using an automated blood pressure cuff (BpTRU Medical Devices, Coquitlam, British Columbia). Each subject’s body mass was measured using a physician’s balance scale. Body mass index, waist circumference, hip circumference, and hip to waist ratio were measured and calculated as per established guidelines.16 V̇o2max was determined using a maximal Bruce treadmill protocol exercise test. The change in V̇o2max was examined in all groups, including the untrained and strength trained subjects.
Statistical Analysis
All data analysis was done in a blinded fashion. Results are expressed as the mean ± standard error. Our sample size calculations for our 4 primary outcome measures (BRS, BRSup, BRSdown, and V̇o2max) assumed a power of 90% and a 1.25% level of significance. We found that we required a sample size of at least 15 subjects to detect a 15% difference in our primary outcome measures. The effects of training on all measures were calculated by a 2-way analysis of variance for repeated measures (time × group).17 A value of P < 0.0125 was considered significant, due to a Bonferroni correction for multiple comparisons.17 Dropouts were handled on an intention-to-treat basis.
RESULTS
Subject Characteristics
There was 1 dropout from the study (AT group, female subject), which was handled on an intention-to-treat basis (Table 1). Therefore, 19 subjects from the AT group and 20 from the NA group completed the intervention. As shown in Table 1, at the time of entry into the study, there was no significant difference between AT and NA subjects with respect to demographic data, resting HR, resting blood pressure, fasting blood sugar, glycosylated hemoglobin, or lipid profile. There was also no difference in baseline BRS, BRSup, and BRSdown (Table 1).
Effects of Training on Measures of Arterial Baroreflex Function
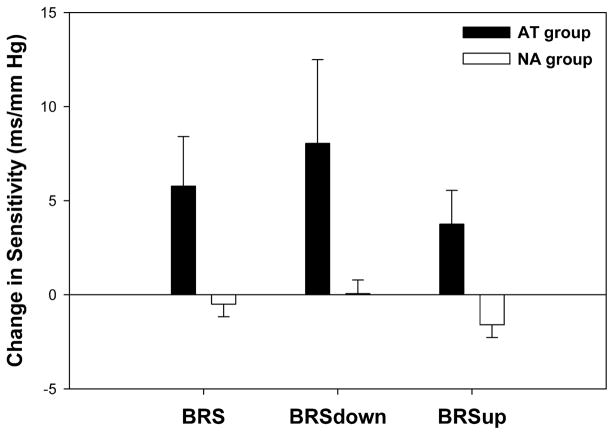
As shown in Figure 1, the AT group demonstrated an increase in BRS that was not demonstrated in the NA group (+60.9% ±23.5% vs +2.2% ±7.9%; P = 0.010). The increase in arterial BRS observed with training in the AT group was primarily due to an increase in BRSup (+50.1% ± 20.8% vs −10.1% ± 6.9%; P = 0.004). There was also a trend toward a training-induced increase in BRSdown (+67.0% ± 25.5% vs +7.6% ± 8.7%; P = 0.019) in the AT group that did not reach statistical significance.
FIGURE 1.
Training-induced changes in baroreflex sensitivity. The aerobically trained (AT) group demonstrated an increase in baroreflex sensitivity (BRS) that was not demonstrated in the nonaerobic (NA) group (+60.9% ± 23.5% vs +2.2% ± 7.9%; P = 0.010). The increase in BRS observed with training in the AT group was primarily due to an increase in the sensitivity of upward sequences (BRSup, +50.1% ± 20.8% vs −10.1% ± 6.9%; P = 0.004). There was also a trend toward a training-induced increase in downward sequences (BRSdown, +67.0% ± 25.5% vs +7.6% ± 8.7%; P = 0.019) in the AT group that did not reach statistical significance. A value of P < 0.0125 was considered significant, due to a Bonferroni correction for multiple comparisons.
Effects of Training on Measures of Fitness
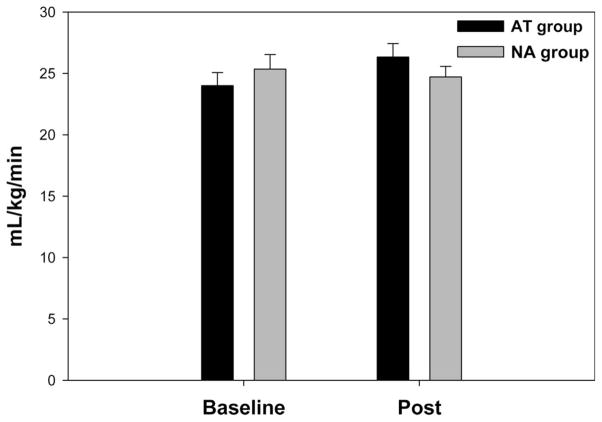
The 3-month training program resulted in no significant increase in V̇o2max in the AT group as compared with that in the NA group (Figure 2; P = 0.027). As shown in Table 2, there was no significant difference between the NA and AT groups with respect to changes in body mass (P = 0.998), body mass index (P = 0.518), waist to hip ratio (P = 0.689), or fasting blood glucose (P = 0.020). There was also no significant difference between the 2 groups with respect to changes in SBP (P = 0.104), MAP (P = 0.226), DBP (P = 0.086), or resting HR (P = 0.172).
FIGURE 2.
Maximum volume of oxygen uptake before and after intervention. The 3-month training program resulted in a trend toward improved aerobic fitness as demonstrated by a nonsignificant increase in maximal oxygen consumption (V̇o2max) in the AT group that was not observed in the NA group (P = 0.027). Black = AT group; White = NA group. A value of P < 0.0125 was considered significant, due to a Bonferroni correction for multiple comparisons.
TABLE 2.
Change in Fitness Measures After Intervention
| Measure | Delta for AT Subjects | Delta for NA Subjects | P |
|---|---|---|---|
| Body mass, kg | +0.14 ± 0.66 | +0.14 ± 0.36 | 0.998 |
| Body mass index, kg/m2 | −0.15 ± 0.26 | +0.05 ± 0.17 | 0.518 |
| Waist to hip ratio | −0.003 ± 0.005 | +0.002 ± 0.011 | 0.689 |
| Blood pressure, mm Hg | |||
| Systolic | −11 ± 4 | −2 ± 3 | 0.104 |
| Diastolic | −4 ± 2 | −1 ± 2 | 0.226 |
| Mean | −6 ± 2 | −1 ± 3 | 0.086 |
| Heart rate, beats/min | −4.1 ± 2.5 | −0.4 ± 1.1 | 0.172 |
| Fasting blood glucose, mEq | −1.1 ± 0.4 | −0.1 ± 0.2 | 0.020 |
Changes in measures of fitness for aerobically trained (AT) and untrained (NA) are shown as mean ± standard error. None of these measures demonstrated a significant training effect.
DISCUSSION
Three months of aerobic training restored arterial BRS in adults with multiple cardiovascular risk factors (geriatric age, type 2 diabetes, hypertension, and hypercholesterolemia). This improvement in BRS occurred without any significant reductions in body mass, body mass index, waist to hip ratio, or blood pressure, indicating that the effects of aerobic training on arterial baroreflex function vasculature may be independent of these other well-established benefits of exercise.
Previous animal and human studies on the effects of aerobic training on arterial baroreflex function have shown varying results depending on the age and cardiovascular risk status of the subjects trained. Most investigations are uncontrolled and involve young or middle-aged subjects. Previous examinations of vigorous aerobic training in both young healthy rats18 and young human athletes3 has demonstrated an increase in BRS, although the human study did not have a control group. Cross-sectional investigations comparing active to sedentary middle-aged subjects have shown either a benefit19 or a lack of benefit20 in subjects with higher levels of aerobic activity. An uncontrolled prospective study of middle-aged healthy women demonstrated an increase in arterial baroreflex function with aerobic training.4 Uncontrolled studies of aerobic training in young hypertensive subjects have demonstrated improved BRS in both rat21 and human studies.22 Aerobically training middle-aged populations with type 2 diabetes have resulted in either an increase6 or no change5 in resting BRS. To our knowledge, we are the first to demonstrate prospectively that subjects at very high cardiovascular risk due to type 2 diabetes, age, hypertension, and hypercholesterolemia can restore BRS with regular aerobic exercise.
Type 2 diabetes produces both functional and structural changes in the arterial baroreflex, with only functional changes likely to respond to exercise intervention.23 Certainly, there is some histological evidence for structural damage to peripheral vagal fibers,24 but restoration of cardiac autonomic function during sleep suggests that a large portion of impaired BRS is possibly reversible.23 Functional impairment of the arterial baroreflex in the setting of diabetes is likely due to a central resetting of the baroreflex.25 More recent work in diabetic rats has demonstrated that endurance training increases vagal tone to the sinus node by about 40%25 perhaps due to changes in muscarinic receptor number.26 Alternatively, changes in vascular compliance in the carotid sinus27 or the release of endothelial factors due to exercise-induced shear stress28 could be contributing to the restoration of baroreflex function with aerobic exercise. Aerobic training-induced alterations in neurotransmission at the level of the nucleus tractus solitarius in rats suggests that changes in central integration may also explain the observed increase in BRS,29 although this is beyond the scope of the present investigation.
Our study did not demonstrate a significant training-induced bradycardia, despite the fact that this is a commonly assessed measure of the autonomic nervous system’s response to training. One explanation is that while aerobic training clearly results in bradycardia in young subjects,30 evidence is mixed regarding training-induced bradycardia in older adults. Studies from both our laboratory31 and other investigators32 have questioned aerobic training–induced bradycardias in older adults. A meta-analysis examining this question found that aerobic training can induce bradycardia in sedentary older adults but mainly in training programs longer than 30 weeks.33 Because our intervention was only 12 weeks, this likely explains the lack of a significant training-induced bradycardia.
Clinical Implications
Although the relationship between low BRS and high cardiovascular event rates are well established,2 the mechanisms underlying this relationship are not well established. Certainly, a depressed BRS would theoretically imply both an increase in sympathetic activity and a decrease in vagal activity, shifting the autonomic balance toward sympathetic predominance.34 Animal studies after myocardial infarction have identified a role for increased sympathetic activity in triggering episodes of sudden death and a protective role for vagal activity in preventing ventricular arrhythmias.35 Low BRS might relate to nonfatal cardiovascular events by being a marker of neurohormonal activation, one of the factors involved in ventricular remodeling and the development of heart failure.36 High cardiac sympathetic outflow could also promote myocardial ischemia through increased platelet aggregation and elevated shear stress.37 Although training-induced elevations in BRS in postmyocardial patients has been associated with reductions in rates of sudden death,38 whether the improvements in arterial baroreflex function observed in our type 2 diabetes population will directly influence clinical outcomes (such as mortality or cardiovascular events) requires further study.
Patients with uncomplicated type 2 diabetes have also been shown to have the same cardiovascular mortality rates as postmyocardial infarction patients without diabetes likely due to cardiac autonomic dysfunction, determined mainly by the sensitivity of the arterial baroreflex.39 Lowered BRS predicts mortality in both subjects with and without diabetes40 and doubles mortality in patients with cardiovascular risk factors.2 In fact, the change in BRS induced by aerobic exercise in the current study is consistent with an approximately 10% difference in 3-year mortality in patients followed for 3 years.41 Aerobic exercise also resulted in about 50% of the improvement in BRS demonstrated by 2 months of drug treatment (angiotensin-converting enzyme inhibitors) in high-risk cardiac populations.42 Clearly, the results of the current study indicate that aerobic exercise should be first-line treatment to restore arterial baroreflex function in older adults with type 2 diabetes, even if the patient has additional cardiovascular risk factors such as hypertension and hypercholesterolemia.
Limitations
Further research is needed to determine the exact pathophysiological mechanism for the increase in arterial BRS with aerobic exercise in our population. Also, the contributory role of alterations in body mass index, blood pressure, and other cardiovascular risk factors with training need to be further explored because our study was only powered to examine 4 primary outcomes (BRS, BRSup, BRSdown, and V̇o2max). Although the primary findings of the study (improved arterial baroreflex function) were unaffected, the use of the standard Bruce protocol for measuring V̇o2max in an older adult population might have underestimated the magnitude of this variable both before and after the exercise intervention.
We only recruited subjects who were not taking medications that affect the autonomic nervous system (such as calcium channel blockers or beta-blockers). This obviously diminishes the ability to generalize our results to subjects with more comorbidities in addition to their diabetes. The fact that our subjects had a lower than expected average HR (65 beats per minute) also suggests that our subject were healthier than the average population. It is difficult to say whether this was due to small subject numbers or due to the well-documented phenomenon of volunteer bias.43 Baroreflex sensitivity is also not a recognized clinical end point; further work needs to be done to examine the clinical impact of aerobic exercise on cardiovascular health.
Summary
Three months of aerobic training results in a functional (as opposed to structural) improvement in the arterial baroreflex in subjects with multiple cardiovascular risk factors (type 2 diabetes, aging, hypertension, and hypercholesterolemia).
Acknowledgments
Study funded by the Canadian Institutes of Health Research.
Footnotes
None of the authors have relevant conflict of interests to disclose.
References
- 1.Ewing DJ, Campbell IW, Clarke BF. The natural history of diabetic autonomic neuropathy. Q J Med. 1980;49:95–108. [PubMed] [Google Scholar]
- 2.Gerritsen J, Dekker JM, TenVoorde BJ, et al. Impaired autonomic function is associated with increased mortality, especially in subjects with diabetes, hypertension, or a history of cardiovascular disease: the Hoorn Study. Diabetes Care. 2001;24:1793–1798. doi: 10.2337/diacare.24.10.1793. [DOI] [PubMed] [Google Scholar]
- 3.Raczak G, Danilowicz-Szymanowicz L, Kobuszewska-Chwirot M, et al. Long-term exercise training improves autonomic nervous system profile in professional runners [in Polish] Kardiol Pol. 2006;64:135–140. discussion 141–132. [PubMed] [Google Scholar]
- 4.Gulli G, Cevese A, Cappelletto P, et al. Moderate aerobic training improves autonomic cardiovascular control in older women. Clin Auton Res. 2003;13:196–202. doi: 10.1007/s10286-003-0090-x. [DOI] [PubMed] [Google Scholar]
- 5.Figueroa A, Baynard T, Fernhall B, et al. Endurance training improves post-exercise cardiac autonomic modulation in obese women with and without type 2 diabetes. Eur J Appl Physiol. 2007;100:437–444. doi: 10.1007/s00421-007-0446-3. [DOI] [PubMed] [Google Scholar]
- 6.Loimaala A, Huikuri HV, Koobi T, et al. Exercise training improves baroreflex sensitivity in type 2 diabetes. Diabetes. 2003;52:1837–1842. doi: 10.2337/diabetes.52.7.1837. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association. Clinical practice recommendations 2005. Diabetes Care. 2005;28(suppl 1):S1–S79. doi: 10.2337/diacare.28.suppl_1.s1. [DOI] [PubMed] [Google Scholar]
- 8.Gibbons RJ, Balady GJ, Beasley JW, et al. ACC/AHA guidelines for exercise testing: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing) Circulation. 1997;96:345–354. doi: 10.1161/01.cir.96.1.345. [DOI] [PubMed] [Google Scholar]
- 9.Christmas C, Andersen RA. Exercise and older patients: guidelines for the clinician. J Am Geriatr Soc. 2000;48:318–324. doi: 10.1111/j.1532-5415.2000.tb02654.x. [DOI] [PubMed] [Google Scholar]
- 10.Rongen GA, Bos WJ, Lenders JW, et al. Comparison of intrabrachial and finger blood pressure in healthy elderly volunteers. Am J Hypertens. 1995;8:237–248. doi: 10.1016/0895-7061(94)00000-2. [DOI] [PubMed] [Google Scholar]
- 11.Omboni S, Parati G, Frattola A, et al. Spectral and sequence analysis of finger blood pressure variability. Comparison with analysis of intra-arterial recordings. Hypertension. 1993;22:26–33. doi: 10.1161/01.hyp.22.1.26. [DOI] [PubMed] [Google Scholar]
- 12.Guelen I, Westerhof BE, Van Der Sar GL, et al. Finometer, finger pressure measurements with the possibility to reconstruct brachial pressure. Blood Press Monit. 2003;8:27–30. doi: 10.1097/00126097-200302000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Bertinieri G, di Rienzo M, Cavallazzi A, et al. A new approach to analysis of the arterial baroreflex. J Hypertens Suppl. 1985;3:S79–S81. [PubMed] [Google Scholar]
- 14.Davies LC, Francis D, Jurak P, et al. Reproducibility of methods for assessing baroreflex sensitivity in normal controls and in patients with chronic heart failure. Clin Sci (Lond) 1999;97:515–522. [PubMed] [Google Scholar]
- 15.Johnson P, Shore A, Potter J, et al. Baroreflex sensitivity measured by spectral and sequence analysis in cerebrovascular disease: methodological considerations. Clin Auton Res. 2006;16:270–275. doi: 10.1007/s10286-006-0351-6. [DOI] [PubMed] [Google Scholar]
- 16.Lau DC, Douketis JD, Morrison KM, et al. 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children [summary] CMAJ. 2007;176:S1–S13. doi: 10.1503/cmaj.061409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dawson-Saunders B, Trapp RG. Basic and Clinical Biostatistics. 2. London, United Kingdom: Prentice-Hall; 1994. [Google Scholar]
- 18.De Angelis K, Wichi RB, Jesus WR, et al. Exercise training changes autonomic cardiovascular balance in mice. J Appl Physiol. 2004;96:2174–2178. doi: 10.1152/japplphysiol.00870.2003. [DOI] [PubMed] [Google Scholar]
- 19.Ueno LM, Moritani T. Effects of long-term exercise training on cardiac autonomic nervous activities and baroreflex sensitivity. Eur J Appl Physiol. 2003;89:109–114. doi: 10.1007/s00421-002-0777-z. [DOI] [PubMed] [Google Scholar]
- 20.Christou DD, Jones PP, Seals DR. Baroreflex buffering in sedentary and endurance exercise-trained healthy men. Hypertension. 2003;41:1219–1222. doi: 10.1161/01.HYP.0000072011.17095.AE. [DOI] [PubMed] [Google Scholar]
- 21.Minami N, Yoshikawa T, Kataoka H, et al. Effects of exercise and beta-blocker on blood pressure and baroreflexes in spontaneously hypertensive rats. Am J Hypertens. 2003;16:966–972. doi: 10.1016/s0895-7061(03)01010-0. [DOI] [PubMed] [Google Scholar]
- 22.Laterza MC, de Matos LD, Trombetta IC, et al. Exercise training restores baroreflex sensitivity in never-treated hypertensive patients. Hypertension. 2007;49:1298–1306. doi: 10.1161/HYPERTENSIONAHA.106.085548. [DOI] [PubMed] [Google Scholar]
- 23.Bennett T, Farquhar IK, Hosking DJ, et al. Assessment of methods for estimating autonomic nervous control of the heart in patients with diabetes mellitus. Diabetes. 1978;27:1167–1174. doi: 10.2337/diab.27.12.1167. [DOI] [PubMed] [Google Scholar]
- 24.Duchen LW, Anjorin A, Watkins PJ, et al. Pathology of autonomic neuropathy in diabetes mellitus. Ann Intern Med. 1980;92:301–303. doi: 10.7326/0003-4819-92-2-301. [DOI] [PubMed] [Google Scholar]
- 25.Eckberg DL, Harkins SW, Fritsch JM, et al. Baroreflex control of plasma norepinephrine and heart period in healthy subjects and diabetic patients. J Clin Invest. 1986;78:366–374. doi: 10.1172/JCI112586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Favret F, Henderson KK, Clancy RL, et al. Exercise training alters the effect of chronic hypoxia on myocardial adrenergic and muscarinic receptor number. J Appl Physiol. 2001;91:1283–1288. doi: 10.1152/jappl.2001.91.3.1283. [DOI] [PubMed] [Google Scholar]
- 27.Studinger P, Lenard Z, Kovats Z, et al. Static and dynamic changes in carotid artery diameter in humans during and after strenuous exercise. J Physiol. 2003;550:575–583. doi: 10.1113/jphysiol.2003.040147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz SD. The role of endothelium-derived vasoactive substances in the pathophysiology of exercise intolerance in patients with congestive heart failure. Prog Cardiovasc Dis. 1995;38:23–50. doi: 10.1016/s0033-0620(05)80012-x. [DOI] [PubMed] [Google Scholar]
- 29.Mueller PJ, Hasser EM. Putative role of the NTS in alterations in neural control of the circulation following exercise training in rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R383–R392. doi: 10.1152/ajpregu.00455.2005. [DOI] [PubMed] [Google Scholar]
- 30.Shi X, Stevens GH, Foresman BH, et al. Autonomic nervous system control of the heart: endurance exercise training. Med Sci Sports Exerc. 1995;27:1406–1413. [PubMed] [Google Scholar]
- 31.Madden KM, Levy WC, Stratton JK. Exercise training and heart rate variability in older adult female subjects. Clin Invest Med. 2006;29:20–28. [PubMed] [Google Scholar]
- 32.Wilmore JH, Stanforth PR, Gagnon J, et al. Endurance exercise training has a minimal effect on resting heart rate: the HERITAGE Study. Med Sci Sports Exerc. 1996;28:829–835. doi: 10.1097/00005768-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Huang G, Shi X, Davis-Brezette JA, et al. Resting heart rate changes after endurance training in older adults: a meta-analysis. Med Sci Sports Exerc. 2005;37:1381–1386. doi: 10.1249/01.mss.0000174899.35392.0c. [DOI] [PubMed] [Google Scholar]
- 34.Berne RaLM. Cardiovascular Physiology. St Louis, MO: Mosby; 2001. [Google Scholar]
- 35.Vanoli E, De Ferrari GM, Stramba-Badiale M, et al. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res. 1991;68:1471–1481. doi: 10.1161/01.res.68.5.1471. [DOI] [PubMed] [Google Scholar]
- 36.Fraccarollo D, Bauersachs J, Kellner M, et al. Cardioprotection by long-term ET(A) receptor blockade and ACE inhibition in rats with congestive heart failure: mono-versus combination therapy. Cardiovasc Res. 2002;54:85–94. doi: 10.1016/s0008-6363(01)00553-3. [DOI] [PubMed] [Google Scholar]
- 37.Goto S, Ikeda Y, Murata M, et al. Epinephrine augments von Willebrand factor-dependent shear-induced platelet aggregation. Circulation. 1992;86:1859–1863. doi: 10.1161/01.cir.86.6.1859. [DOI] [PubMed] [Google Scholar]
- 38.La Rovere MT, Bersano C, Gnemmi M, et al. Exercise-induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation. 2002;106:945–949. doi: 10.1161/01.cir.0000027565.12764.e1. [DOI] [PubMed] [Google Scholar]
- 39.Haffner SM, Lehto S, Ronnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 40.Farrell TG, Bashir Y, Cripps T, et al. Risk stratification for arrhythmic events in postinfarction patients based on heart rate variability, ambulatory electrocardiographic variables and the signal-averaged electrocardiogram. J Am Coll Cardiol. 1991;18:687–697. doi: 10.1016/0735-1097(91)90791-7. [DOI] [PubMed] [Google Scholar]
- 41.Johansson M, Gao SA, Friberg P, et al. Baroreflex effectiveness index and baroreflex sensitivity predict all-cause mortality and sudden death in hypertensive patients with chronic renal failure. J Hypertens. 2007;25:163–168. doi: 10.1097/01.hjh.0000254377.18983.eb. [DOI] [PubMed] [Google Scholar]
- 42.Grassi G, Cattaneo BM, Seravalle G, et al. Effects of chronic ACE inhibition on sympathetic nerve traffic and baroreflex control of circulation in heart failure. Circulation. 1997;96:1173–1179. doi: 10.1161/01.cir.96.4.1173. [DOI] [PubMed] [Google Scholar]
- 43.Otto SJ, Schroder FH, de Koning HJ. Low all-cause mortality in the volunteer-based Rotterdam section of the European randomised study of screening for prostate cancer: self-selection bias? J Med Screen. 2004;11:89–92. doi: 10.1258/096914104774061074. [DOI] [PubMed] [Google Scholar]