Abstract
BACKGROUND:
Musculoskeletal symptoms belonging to the spectrum of ‘seronegative spondyloarthritis’ (SpA) are the most common extraintestinal manifestations in patients with inflammatory bowel disease (IBD) and may lead to important disease burden. Patients with suspected SpA should be referred to a rheumatologist for further evaluation.
OBJECTIVE:
To investigate the self-reported prevalence of musculoskeletal SpA features in a cohort of patients with IBD and to compare this with actual referrals to a rheumatologist.
METHODS:
Consecutive patients with IBD visiting the outpatient clinic were interviewed by a trained research nurse about possible SpA features using a standardized questionnaire regarding the presence or history of inflammatory back pain, peripheral arthritis, enthesitis, dactylitis, psoriasis, uveitis and response to nonsteroidal anti-inflammatory drugs. All patient files were verified for previous visits to a rheumatologist and any rheumatic diagnosis.
RESULTS:
At least one musculoskeletal SpA feature was reported by 129 of 350 (36.9%) patients. No significant differences between patients with Crohn disease and ulcerative colitis were found. Review of medical records showed that 66 (51.2%) patients had ever visited a rheumatologist. Axial SpA was diagnosed in 18 (27.3%) patients, peripheral SpA in 20 (30.3%) patients and another rheumatic disorder in 14 (21.2%) patients.
CONCLUSION:
Musculoskeletal SpA features are frequently present in patients with IBD. However, a substantial group of patients is not evaluated by a rheumatologist. Gastroenterologists play a key role in early referral of this often debilitating disease.
Keywords: Ankylosing spondylitis, Inflammatory bowel disease, Extraintestinal manifestations, Spondyloarthritis
Abstract
HISTORIQUE :
Les symptômes musculosquelettiques qui appartiennent au spectre de la spondyloarthrite séronégative (SpA) sont les principales manifestations extra-intestinales chez les patients atteints d’une maladie inflammatoire de l’intestin (MII) et peuvent s’associer à un important fardeau de maladie. Les patients chez qui on craint une SpA devrait être aiguillés vers un rhumatologue pour subir une évaluation plus approfondie.
OBJECTIF :
Examiner la prévalence autodéclarée de caractéristiques musculosquelettiques de SpA dans une cohorte de patients atteints d’une MII et comparer les résultats avec le nombre d’aiguillages vers un rhumatologue.
MÉTHODOLOGIE :
Une infirmière de recherche formée a passé en entrevue des patients consécutifs atteints d’une MII afin de connaître leurs caractéristiques éventuelles de SpA au moyen d’un questionnaire standardisé sur la présence ou les antécédents de douleurs dorsales inflammatoires, d’arthrite périphérique, d’enthésite, de dactylite, de psoriasis, d’uvéite et de réponse aux anti-inflammatoires non stéroïdiens. Les chercheurs ont vérifié le dossier de tous les patients afin d’établir s’ils avaient déjà vu un rhumatologue et obtenu un diagnostic de problème rhumatismal.
RÉSULTATS :
Au total, 129 des 350 patients (36,9 %) ont déclaré au moins une caractéristique musculosquelettique de SpA. Il n’y avait pas de différence significative entre les patients atteints de la maladie de Crohn et de la colite ulcéreuse. L’examen des dossiers médicaux a révélé que 66 patients (51,2 %) n’avaient jamais consulté un rhumatologue. On avait diagnostiqué une SpA axiale chez 18 patients (27,3 %), une SpA périphérique chez 20 patients (30,3 %) et un autre trouble rhumatismal chez 14 patients (21,2 %).
CONCLUSION :
Les patients ayant une MII présentent souvent des caractéristiques musculosquelettiques de SpA. Cependant, un groupe important de patients n’est pas évalué par une rhumatologue. Les gastroentérologues ont un rôle essentiel à jouer pour l’aiguillage rapide des patients en vue de traiter cette maladie souvent débilitante.
Musculoskeletal symptoms are the most common extraintestinal manifestations in patients with inflammatory bowel disease (IBD) (1). Arthritis and spondylitis associated with IBD belong to the spectrum of ‘seronegative spondyloarthritis’ (SpA) (2). SpA is a group of disorders that share several clinical features, show familial clustering and are linked to the human leukocyte antigen B27. The major subtypes of the SpA group are ankylosing spondylitis, psoriatic arthritis, reactive arthritis, arthritis/spondylitis associated with IBD and undifferentiated SpA. According to clinical presentation, patients with SpA can be divided into two groups: those with predominantly axial symptoms and those with predominantly peripheral symptoms (3). Axial involvement consists of inflammatory back pain reflecting inflammation of the sacroiliac joints and/or spine. Peripheral involvement consists of peripheral arthritis, dactylitis (‘sausage-like’ finger or toe) and enthesitis (frequently at the insertion of the Achilles tendon or the plantar fascia).
In daily practice, SpA symptoms are not always recognized in patients with IBD. To most patients, the relationship between joint and gut symptoms is unknown, and gastroenterologists do not always specifically ask about joint involvement. Subsequently, patients with symptoms of SpA may be underdiagnosed and effective treatment delayed, which may lead to a chronic debilitating disease course and decreased quality of life (4). To date, several studies have shown that dramatic improvements in disease activity and functioning can be achieved with antitumour necrosis factor-alpha (anti-TNF-α) treatment in patients with several forms of SpA, including the early stages of axial SpA (5–10). It has also been demonstrated that remission of symptoms with anti-TNF-α treatment can be achieved in a higher percentage of patients when treated early in the disease course and at a young age (11,12). Recognition and intervention of the disease at an early stage is, therefore, warranted.
Diagnosing SpA is not always easy and diagnostic criteria are currently lacking. Several criteria sets are available for classification of (subgroups of) SpA, but these have been developed mainly for study purposes. Ankylosing spondylitis, as the prototype of SpA, is classified by the modified New-York criteria (13). In this classification set, radiographic sacroiliitis is essential, together with the presence of at least one clinical criterion. However, it can take many years before sacroiliitis is visible on pelvic radiographs, resulting in a mean diagnostic delay of six to eight years (14). In the early 1990s, two other criteria sets were developed to classify patients with SpA: the European Spondyloarthropathy Group (ESSG) criteria (15) and the Amor criteria (16). The ESSG and Amor criteria perform well in groups of patients with a definite diagnosis of SpA (17–19), but also lack diagnostic value in patients with early, mild or ‘possible’ SpA (19,20).
Recently, an international group of experts in the field of SpA – the Assessment of SpondyloArthritis international Society (ASAS) – generated two new sets of criteria for the classification of SpA: one for patients with predominantly axial symptoms and one for patients with predominantly peripheral symptoms (Figure 1) (21,22). Both criteria sets have been developed to also capture early and mild cases of SpA and include several SpA features. These features can easily be asked for in daily practice, also by gastroenterologists, to recognize patients possibly suffering from SpA.
Figure 1).
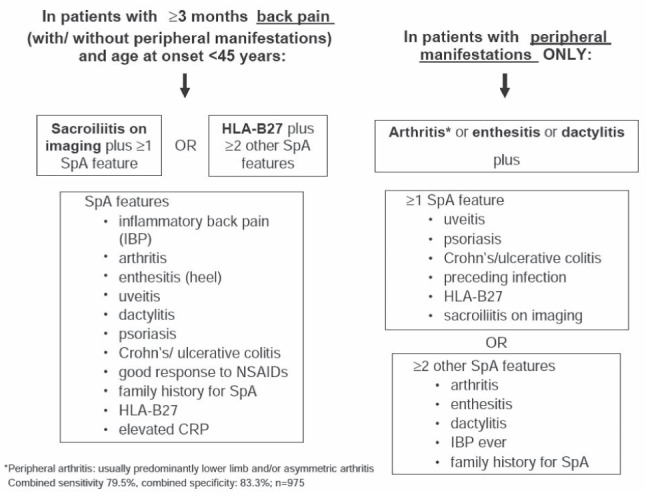
Assessment of SpondyloArthritis international Society (ASAS) criteria for axial and peripheral spondyloarthritis. CRP C-reactive protein; HLA-B27 Human leukocyte antigen B27; IBP Inflammatory back pain; NSAIDs Nonsteroidal anti-inflammatory drugs; SpA Spondyloarthritis. Reproduced with permission from reference 52
The aim of the present study was to first investigate the self-reported prevalence of musculoskeletal SpA features in a large cohort of patients with IBD, as included in the new ASAS criteria sets to obtain a better understanding of the size of this concomitant disease in daily practice. The second aim was to compare the self-reported prevalence with actual referrals to a rheumatologist and the final diagnosis in these referred patients.
METHODS
Patients included in the present study were part of an ongoing cohort of patients with IBD (IBD South Limburg cohort). The diagnosis of IBD, made by gastroenterologists, was based on clinical, endoscopic and histological evaluation. For the present study, all patients from the IBD South Limburg cohort who consecutively visited the outpatient clinic of the Maastricht University Medical Center, (Maastricht, The Netherlands) between October 2009 and June 2011 were interviewed by a trained research nurse about possible SpA features. A standardized questionnaire containing the following features from the ASAS criteria was used: presence or history of inflammatory back pain; duration of inflammatory back pain; (history of) peripheral arthritis; (history of) enthesitis (history of Achilles tendinitis, plantar fasciitis or inflammation of the anterior chest wall); (history of) dactylitis (history of a ‘sausage-like digit’); psoriasis; (history of) uveitis; response of arthritis or inflammatory back pain to nonsteroidal anti-inflammatory drugs (NSAIDs) and a family history of SpA. Inflammatory back pain was defined as low back pain existing for more than three months, which started before 45 years of age, is worst in the early morning and improves with exercise. From the database of the IBD South-Limburg cohort, information regarding age, sex, diagnosis (Crohn disease [CD], ulcerative colitis [UC] or IBD unclassified [IBDU]), duration of the IBD, current use of medication for IBD and IBD disease activity was extracted. IBD disease activity was calculated using the Harvey-Bradshaw index (HBI) for patients with CD (range 0 to infinite; score <5 is defined as CD in remission, a score >15 as severe disease) and the simple clinical colitis activity index (SCCAI) for patients with either UC or IBDU (range 0 to 20; score >4 is suggestive for active colitis) (23,24). Because joint symptoms are part of these disease activity scores (counting for one point if present), the total scores for both the HBI and the SCCAI were also recalculated excluding this item. All patient files were verified for previous visits to a rheumatologist and any rheumatic diagnosis (axial or peripheral SpA or any other rheumatic disease). The study was approved by the Ethics Committee of the Maastricht University Medical Center.
Statistics
Descriptive statistics were used to calculate the mean and SD for continuous data. Independent t tests and χ2 tests were used to compare differences between the groups for continuous and dichotomous data, respectively. Univariable followed by multivariable logistic regression analyses were performed to identify associations between any reported SpA feature and demographic and clinical variables. Similar analyses were performed to identify associations between a definite diagnosis of SpA and these variables. In multivariable analyses, models were stratified according to diagnosis of IBD (CD or UC), due to the different disease activity scores for CD and UC. To investigate the relationship between either self-reported peripheral or axial SpA features or the definite diagnosis of peripheral or axial SpA with duration of IBD, the cohort was subdivided in quartiles according to duration of IBD and subsequently the frequency of self-reported SpA features and diagnosis of SpA was calculated per quartile. Logistic regression analyses were performed to test the relationship between the disease duration of IBD (in quartiles) and the frequency of reported (peripheral or axial) SpA features or diagnosis of (peripheral or axial) SpA. In patients who reported at least one musculoskeletal SpA feature, associations between the individual SpA symptoms and referral to a rheumatologist were identified in univariable followed by multivariable logistic regression analysis while controlling for demographic and disease characteristics. All logistic regression analyses were performed using a stepwise backward likelihood ratio method. Possible interactions between the variables were tested in separate analyses. All analyses were performed using SPSS version 16.0 (IBM Corporation, USA). The level of statistical significance was set at 0.05.
RESULTS
Patient characteristics
In total, 365 consecutive patients with IBD who visited the outpatient clinic between October 2009 and June 2011 were asked to participate in the IBD South Limburg cohort, of whom 350 (95.9%) agreed. All 350 patients were interviewed about SpA features. Patient characteristics and self-reported SpA features are shown in Table 1. Of the 350 patients, 206 had CD, 136 had UC and eight had IBDU. Patients with CD were younger, more frequently using a biological and more frequently female compared with patients with UC.
TABLE 1.
Patients characteristics and prevalence of self-reported spondyloarthritis (SpA) features in patients with inflammatory bowel disease (IBD)
| Total IBD (n=350) | CD (n=206) | UC (n=136) | IBDU (n=8) | P (CD vs UC) | |
|---|---|---|---|---|---|
| Age, years, mean ± SD | 46.6±15.5 | 43.8±14.9 | 50.5±15.2 | 52.4±21.6 | <0.01 |
| Female sex | 197 (56.3) | 128 (62.1) | 65 (47.8) | 4 (50.0) | 0.01 |
| Duration of IBD, years, mean ± SD | 11.4±10.1 | 11.6±10.7 | 11.4±9.5 | 7.5±4.2 | 0.85 |
| IBD activity score*, mean ± SD | – | 3.3±3.5 | 2.7±2.7 | 0.9±0.9 | – |
| IBD activity excluding joint symptoms†, mean ± SD | – | 3.1±3.4 | 2.7±2.7 | 0.2±0.4 | – |
| Currently using medication for IBD | 291 (83.1) | 172 (83.5) | 111 (81.6) | 8 (100) | 0.65 |
| Thiopurines | 100 (28.6) | 75 (36.4) | 23 (16.9) | 2 (25) | <0.01 |
| Mesalazine | 133 (38) | 46 (22.3) | 82 (60.3) | 5 (62.5) | <0.01 |
| Methotrexate | 11 (3.1) | 10 (4.9) | 1 (0.7) | 0 (0) | 0.04 |
| Corticosteroids | 33 (9.4) | 23 (11.2) | 10 (7.4) | 0 (0) | 0.24 |
| Biologicals | 84 (24) | 68 (33) | 15 (11) | 1 (12.5) | <0.01 |
| Psoriasis | 27 (7.7) | 16 (7.8) | 11 (8.1) | 0 (0) | 0.91 |
| Uveitis | 5 (1.4) | 5 (2.4) | 0 (0) | 0 (0) | 0.06 |
| Family history of SpA | 159 (45.4) | 96 (46.6) | 58 (42.6) | 4 (50.0) | 0.49 |
| Family history of IBD | 105 (30.0) | 64 (31.1) | 37 (27.2) | 4 (50.0) | 0.42 |
| Any musculoskeletal SpA feature | 129 (36.9) | 82 (39.8) | 45 (33.1) | 2 (25.0) | 0.21 |
| Inflammatory back pain | 79 (22.6) | 49 (23.8) | 29 (21.3) | 1 (12.5) | 0.44 |
| Duration of inflammatory back pain, years | |||||
| <2 | 13 (3.7) | 9 (4.4) | 4 (2.9) | 0 (0) | 0.60 |
| 2–10 | 24 (6.9) | 13 (6.3) | 11 (8.1) | 0 (0) | 0.29 |
| >10 | 42 (12.0) | 27 (13.1) | 14 (10.3) | 1 (12.5) | 0.56 |
| Any peripheral SpA feature | 83 (23.7) | 54 (26.2) | 27 (19.9) | 1 (12.5) | 0.18 |
| Enthesitis | 47 (13.4) | 30 (14.6) | 16 (11.8) | 1 (12.5) | 0.46 |
| Dactylitis | 29 (8.3) | 19 (9.2) | 10 (7.4) | 0 (0) | 0.54 |
| Peripheral arthritis | 33 (9.4) | 25 (12.1) | 8 (5.1) | 0 (0) | 0.05 |
| Diagnosis of axial SpA by rheumatologist | 18 (5.1) | 13 (6.3) | 5 (3.7) | 0 (0) | 0.25 |
| Diagnosis of peripheral SpA by rheumatologist | 20 (5.7) | 15 (7.3) | 5 (3.7) | 0 (0) | 0.15 |
Data presented as n (%) unless otherwise indicated.
Harvey-Bradshaw index in patients with Crohn disease (CD) (data available for 184 patients);
Simple clinical colitis activity index in patients with ulcerative colitis (UC) and IBD unclassified (IBDU) (data available for 116 patients). vs Versus
Self-reported SpA features
At least one musculoskeletal SpA feature was reported by 129 of 350 (36.9%) patients. Seventy-nine (22.6%) patients reported axial symptoms and 83 (23.7%) patients reported at least one peripheral SpA feature. There were no statistically significant differences between patients with CD and UC with regard to self-reported SpA features. Figure 2A illustrates the relationship between self-reported axial or peripheral SpA features and duration of IBD. A trend toward more axial and peripheral SpA features with longer disease duration was found but was not statistically significant (P=0.28 and P=0.18, respectively).
Figure 2).
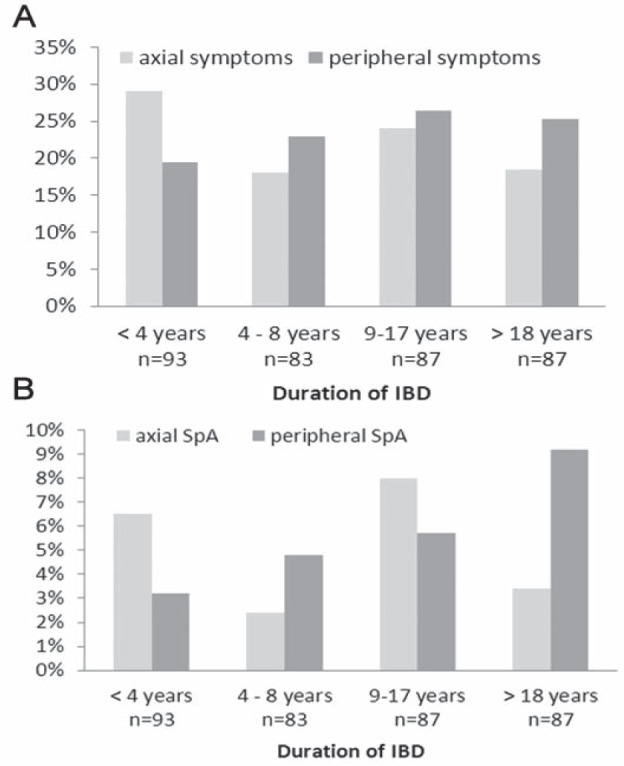
Presence of self-reported spondyloarthritis (SpA) features (A) and diagnosis of either axial or peripheral SpA per quartile of duration of inflammatory bowel disease (IBD) (B)
Table 2 shows the results from the regression analyses investigating the association between demographic and clinical variables on the presence of self-reported SpA features. The regression analysis was performed using data from 342 patients: the eight patients with IBDU were excluded. In the univariable analysis, male sex was associated with less frequently reported SpA features, whereas use of biologicals and a higher mean disease activity score (the latter only in patients with CD) were associated with more frequently reported SpA features. When joint symptoms were excluded from the disease activity score, the score remained significantly associated with self-reported SpA features. In multivariable analysis, which was stratified according to IBD diagnosis, male sex (OR 0.44 [95% CI 0.23 to 0.84]) and disease activity score (OR 1.14 [95% CI 1.03 to 1.24]) were both significantly associated with the presence of reported SpA features in patients with CD. In patients with UC, none of the variables were associated with self-reported SpA features. Interaction between the variables was not found.
TABLE 2.
Univariable and multivariable logistic regression analyses investigating the association between demographic and clinical variables and the presence of either self-reported spondyloarthritis (SpA) features or diagnosis of SpA
| Variables | Presence of musculoskeletal SpA features | Presence of axial or peripheral SpA diagnosis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Multivariable analysis | Multivariable analysis | |||||||||
|
|
|
|
|
|||||||
| Univariable analysis | CD | Univariable analysis | CD | Ulcerative colitis | ||||||
|
| ||||||||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Age | 1.00 (0.99–1.02) | 0.64 | – | – | 1.00 (0.98–1.02) | 0.98 | – | – | – | – |
| Male sex | 0.59 (0.37–0.92) | 0.02 | 0.44 (0.23–0.84) | 0.01 | 0.83 (0.42–1.65) | 0.59 | – | – | – | – |
| Duration of IBD | 1.00 (0.98–1.02) | 0.95 | – | – | 1.01 (0.98–1.05) | 0.37 | – | – | – | – |
| Diagnosis of CD | 1.34 (0.85–2.10) | 0.21 | – | – | 1.98 (0.93–4.23) | 0.08 | – | – | – | – |
| Currently using medication for IBD | 0.77 (0.43–1.36) | 0.36 | – | – | 0.91 (0.38–2.19) | 0.84 | – | – | – | – |
| Biologicals | 1.72 (1.04–2.85) | 0.03 | – | – | 1.52 (0.73–3.16) | 0.27 | – | – | – | – |
| HBI score* | 1.14 (1.04–1.24) | 0.04 | 1.14 (1.04–1.24) | 0.01 | 1.06 (0.95–1.19) | 0.27 | – | – | – | – |
| HBI score excluding joint symptoms* | 1.11 (1.01–1.21) | 0.02 | – | – | 1.04 (0.92–1.16) | 0.55 | – | – | – | – |
| SSCAI score† | 1.04 (0.90–1.20) | 0.61 | – | – | 1.08 (0.87–1.35) | 0.50 | – | – | – | – |
| SSCAI score excluding joint symptoms† | 1.01 (0.88–1.17) | 0.86 | – | – | 0.99 (0.76–1.30) | 0.96 | – | – | – | – |
| Family history of SpA | 1.19 (0.77–1.85) | 0.43 | – | – | 1.57 (0.80–3.09) | 0.19 | – | – | – | – |
| Uveitis | 2.58 (0.42–15.68) | 0.30 | – | – | 11.34 (1.83–70.30) | <0.01 | 9.06 (1.44–57.10) | 0.02 | – | – |
| Psoriasis | 1.93 (0.87–4.24) | 0.10 | – | – | 1.94 (0.69–5.47) | 0.21 | – | – | 6.32 (1.37–29.20) | 0.02 |
HBI Harvey-Bradshaw index (Crohn disease [CD]) (data available for 184 patients);
SSCAI Simple clinical colitis activity index (ulcerative colitis [UC] and inflammatory bowel disease [IBD] unclassified) (data available for 116 patients)
Referrals to rheumatologist and final diagnosis
Review of the medical records of all 350 patients showed that 66 (51.2%) of the 129 patients who reported at least one musculoskeletal SpA feature were ever seen by a rheumatologist in the hospital (Figure 3). Table 3 shows the regression analysis investigating which individual SpA symptoms were associated with a referral to a rheumatologist in patients who reported at least one musculoskeletal SpA feature, while controlling for demographic and disease characteristics. In the multivariable analysis, inflammatory back pain (OR 8.97 [95% CI 2.48 to 32.45]), peripheral arthritis (OR 44.56 [95% CI 8.57 to 231.56]) and enthesitis (OR 4.02 [95% CI 1.22 to 13.27]) were all independently associated with referral to a rheumatologist, whereas dactylitis was not. Interaction between the variables was not found.
Figure 3).
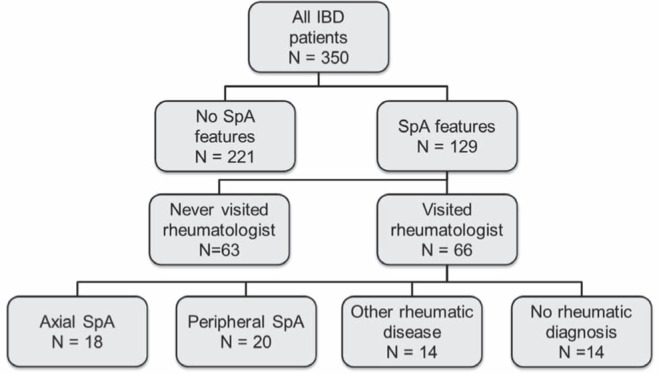
Flow-chart of all 350 patients with inflammatory bowel disease (IBD) included in the present study. SpA spondyloarthritis
TABLE 3.
Univariable and multivariable logistic regression analysis investigating the association among individual spondyloarthritis symptoms and referral to a rheumatologist
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
|
|
|
|||
| OR (95% CI) | P | OR (95% CI) | P | |
| Inflammatory back pain | 1.98 (0.96–4.08) | 0.07 | 8.97 (2.48–32.45) | <0.01 |
| Peripheral arthritis | 17.88 (5.07–63.09) | <0.01 | 44.56 (8.57–231.56) | <0.01 |
| Enthesitis | 1.22 (0.59–2.52) | 0.59 | 4.02 (1.22–13.27) | 0.02 |
| Dactylitis | 1.23 (0.54–2.84) | 0.63 | – | – |
| Uveitis | 0.00 (0.00–0.00) | 1.00 | – | – |
| Psoriasis | 1.83 (0.58–5.81) | 0.30 | – | – |
By default, the multivariable model was adjusted for age, disease duration, sex and diagnosis (ulcerative colitis or Crohn disease)
In the patients who were seen by a rheumatologist, axial SpA was diagnosed in 18 (27.3%) and peripheral SpA in 20 (30.3%). Fourteen of 66 (21.2%) patients suffered from another rheumatic disorder (rheumatoid arthritis [n=3], juvenile inflammatory arthritis [n=1] and fibromyalgia [n=10]) and, in 14 (21.2%) patients, no rheumatic disorder was diagnosed. Figure 2B illustrates the relationship between the duration of IBD and a final diagnosis of axial or peripheral SpA. Visually, a trend toward an increase in peripheral SpA diagnosis with increasing duration of IBD was found. However, this relationship was not statistically significant (P=0.09). For axial SpA diagnosis, no association with disease duration was found (P=0.73). Table 2 presents the logistic regression analysis investigating variables associated with a final diagnosis of SpA. In patients with CD, uveitis was independently associated with a diagnosis of SpA (OR 9.06 [95% 1.44 to 57.10]). In patients with UC, psoriasis was associated with a diagnosis of SpA (OR 6.32 [95% CI 1.37 to 29.20]).
DISCUSSION
The present study showed that more than one-third of 350 patients from an unselected IBD cohort reported musculoskeletal SpA features as included in the new ASAS criteria. Of these patients, only 51% were ever seen by a rheumatologist. Axial or peripheral SpA was diagnosed in 58% of the patients who were seen by a rheumatologist and, in 21% of the patients, another rheumatic disorder was diagnosed. Strikingly, almost 50% of the patients who reported musculoskeletal symptoms never visited a rheumatologist.
Referral to a rheumatologist is important because SpA may result in significant impairment in several aspects of quality of life and restrictions in social roles, including work participation (25). The disease course of axial SpA begins with inflammation of the sacroiliac joints. Disease progression is characterized by the development of (irreversible) structural damage of the sacroiliac joints and the spine, which is associated with worse physical function and limitation of spinal mobility (26). Importantly, patients with early axial SpA are not different from those with definite ankylosing spondylitis with respect to disease activity, pain, quality of life and response to treatment (27). Effective treatment is available for both axial and peripheral SpA and early diagnosis and treatment are important to modify disease progression and decrease the disease burden (28). Optimal management of SpA consists of a combination of nonpharmacological and pharmacological treatment modalities coordinated by a rheumatologist (29). The cornerstone of nonpharmacological treatment of patients with axial SpA is patient education and regular exercise. Physiotherapy interventions have proven to be effective for ankylosing spondylitis (30). Pharmacological treatment includes NSAIDs, disease-modifying antirheumatic drugs and anti-TNF therapy. NSAIDs are the first-line drug treatment for SpA and rapidly remove pain and stiffness. Traditional NSAIDs are relatively contraindicated in patients with IBD for fear of disease exacerbation. Cyclooxygenase-2 inhibitors (ie, ‘coxibs’) may be safe and beneficial in most patients with IBD (31). Conventional disease-modifying antirheumatic drugs, which have been shown to be effective for rheumatoid arthritis, have no proven effect for axial symptoms, but may be considered for peripheral symptoms. Anti-TNF therapy should be given to patients with persistently high disease activity despite conventional treatments (29). To start effective treatment at an early stage, however, patients with SpA must be diagnosed early by a rheumatologist. Several studies have shown that infliximab improves the severity of spinal pain, peripheral arthritis and enthesitis in CD (32–34). Moreover, it has been shown that treatment of axial SpA with anti-TNF-α treatment is more effective when started early in the disease course and at a younger age (11,12).
There may be several reasons why only 51% of the patients with self-reported SpA features were ever seen by a rheumatologist. First, gastroenterologists may not always specifically ask patients with IBD about possible SpA features or do not know exactly which symptoms belong to the spectrum of SpA. The present study showed that some SpA features were significantly associated with referral to a rheumatologist (eg, peripheral arthritis) whereas others were not (eg, dactylitis). Second, patients may have reported symptoms in the questionnaire that they have experienced in the past but are no longer present. If these patients were asymptomatic during their visit to the gastroenterologist, it is likely no referral was made. However, it is important to realize that SpA symptoms have a fluctuating course. For the diagnosis of SpA, it is not necessary to have the full range of symptoms present at the time of diagnosis, and the fluctuating character of some of the symptoms may still be an indication for referral. Third, it is possible that more patients were referred by gastroenterologists than were actually seen by a rheumatologist due to unwillingness of patients or a visit to a rheumatologist in another hospital. Fourth, a high percentage of patients were on immunosuppressive therapy, including biologicals, which may also influence SpA symptoms. Therefore, gastroenterologists may have believed that referral to a rheumatologist would not change management. However, we believe that every patient with possible SpA should be seen by a rheumatologist for final diagnosis and the coordination of multidisciplinary nonpharmacological and pharmacological treatment (29).
The prevalence of SpA features in patients with IBD varies widely in the literature. Any SpA manifestation was found in 17% to 62% of patients with IBD; inflammatory back pain was found in 5% to 30% of patients; peripheral arthritis in 5% to 30%; ‘definite’ SpA classification in 12% to 46% and ankylosing spondylitis in 2% to 10% of patients with IBD (35–45). Several factors may explain these large variations in prevalence among different studies. First, patient selection plays an important role. It is known that the cumulative probability of SpA increases with longer duration of IBD (46). Hence, studies including patients with longstanding IBD will find a higher prevalence of SpA compared with studies including patients with IBD of short duration. We also found a similar trend for the diagnosis of peripheral SpA in the present study, but not for axial SpA. Second, the prevalence may vary among different ethnic populations. In a large North American cohort of patients with IBD (47), it was shown that African-American patients were more likely than Caucasians to have a diagnosis of sacroiliitis. Third, the prevalence also depends on the definitions and criteria used. In most recent studies, classification of SpA and ankylosing spondylitis is based on the ESSG and modified New-York criteria, respectively. With the introduction of the new ASAS criteria for axial and peripheral SpA, these criteria sets are now more frequently being used, which may lead to differences in the prevalence of the disease among studies.
Most studies in the literature found a similar prevalence of SpA features in both CD and UC (36,38–41,45), although two studies showed a significantly higher prevalence of peripheral arthritis in CD (46,48). In our study, a trend toward more peripheral arthritis in CD was found, although this did not reach statistical significance (Table 1). Similar to previous studies, SpA features in our study were more frequently reported in female than in male patients with IBD (35,39).
Inflammatory back pain was the most frequently reported musculoskeletal SpA feature (22.6%) in our study. In comparable studies, the prevalence of inflammatory back pain in patients with IBD ranged widely from 5% to 30% (36,39,41,44).
At least one peripheral symptom was reported by 23.7% of the patients, and a definite diagnosis of peripheral SpA was made in 5.7%. Peripheral arthritis in SpA most frequently presents as an asymmetric oligoarthritis of the lower limbs that is nonerosive and nondeforming; however, small joint symmetrical polyarthritis or destructive lesions are also described (49). Historically, peripheral arthritis is frequently subdivided into type 1 and type 2. Type 1 is defined as acute and self-limiting attacks of oligoarthritis that often coincide with relapses of IBD and is reported to be strongly associated with extraintestinal manifestations of IBD (44). Type 2 is defined as a polyarthritis with symptoms persisting for months to years, running an independent course of IBD, and is also associated with uveitis but not with other extraintestinal manifestations (44). This subdivision is frequently used in gastroenterological studies but is not used by rheumatologists in daily practice; the clinical value is probably low due to significant overlap. With the development of the new ASAS classification criteria, it is recommended to no longer use the type 1 or 2 classification, but to classify SpA into the presenting symptoms (axial and/or peripheral) because this better reflects the need for treatment.
Some limitations of the present study need to be addressed. First, it must be emphasized that it was not the aim of the present study to validate the new ASAS criteria in patients with IBD. The present study was based on a self-reported questionnaire and, therefore, not all self-reported symptoms can automatically be interpreted as objective SpA features. The inflammatory character of chronic back pain, which was reported by 22.6% of patients, is especially challenging. Chronic back pain of more than three months’ duration is very common in the general population, and ankylosing spondylitis accounts for no more than 5% of all patients presenting with chronic back pain (50). An inflammatory character of back pain is present in 70% to 80% of patients with ankylosisng spondylitis, but also in 20% to 25% of patients with mechanical back pain (51). Therefore, not all patients reporting inflammatory back pain can be diagnosed with axial SpA. Similarly, peripheral reported symptoms of enthesitis are difficult to interpret without further evaluation. However, due to the high pretest probability of SpA in IBD, gastroenterologists should actively ask for the presence of musculoskeletal SpA features in IBD patients and, if present, refer to a rheumatologist for further evaluation. Second, recall bias may have occurred. In the present cross-sectional study, patients were interviewed about possible current and previous SpA features. Patients may have forgotten symptoms they may have experienced a long time ago. Also, the majority of the patients (83.1%) used immunosuppressive drugs, including biologicals (24.0%), which may have influenced SpA symptoms. It is, therefore, possible that the true prevalence of SpA may be higher than currently reported. Third, a substantial proportion of the group of patients who reported symptoms were never seen by a rheumatologist. It is possible that in some patients SpA can be diagnosed. This could have influenced our final results.
CONCLUSION.
SpA features are reported by more than one-third of patients with IBD. Review of medical records showed that one-half of the patients with self-reported SpA features were never seen by a rheumatologist; however, in those who were seen, a rheumatic disorder was diagnosed in almost 80%. Treatment for SpA is more effective when started early in the disease course; therefore, gastroenterologists play a key role in early recognition and referral of this often debilitating disease.
Footnotes
DISCLOSURES: The authors have no financial disclosures or conflicts of interest to declare.
REFERENCES
- 1.Greenstein AJ, Janowitz HD, Sachar DB. The extra-intestinal complications of Crohn’s disease and ulcerative colitis: A study of 700 patients. Medicine (Baltimore) 1976;55:401–12. doi: 10.1097/00005792-197609000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Moll JM, Haslock I, Macrae IF, Wright V. Associations between ankylosing spondylitis, psoriatic arthritis, Reiter’s disease, the intestinal arthropathies, and Behcet’s syndrome. Medicine (Baltimore) 1974:343–64. doi: 10.1097/00005792-197409000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Rudwaleit M. New approaches to diagnosis and classification of axial and peripheral spondyloarthritis. Curr Opin Rheumatol. 2010;22:375–80. doi: 10.1097/BOR.0b013e32833ac5cc. [DOI] [PubMed] [Google Scholar]
- 4.Ibn Yacoub Y, Amine B, Laatiris A, Bensabbah R, Hajjaj-Hassouni N. Relationship between diagnosis delay and disease features in Moroccan patients with ankylosing spondylitis. Rheumatol Int. 2012;32:357–60. doi: 10.1007/s00296-010-1635-7. [DOI] [PubMed] [Google Scholar]
- 5.Davis JC, van der Heijde DM, Braun J, et al. Sustained durability and tolerability of etanercept in ankylosing spondylitis for 96 weeks. Ann Rheum Dis. 2005;64:1557–62. doi: 10.1136/ard.2004.035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Heijde D, Dijkmans B, Geusens P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: Results of a randomized, placebo-controlled trial (ASSERT) Arthritis Rheum. 2005;52:582–91. doi: 10.1002/art.20852. [DOI] [PubMed] [Google Scholar]
- 7.van der Heijde D, Kivitz A, Schiff MH, et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: Results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2006;54:2136–46. doi: 10.1002/art.21913. [DOI] [PubMed] [Google Scholar]
- 8.Antoni CE, Kavanaugh A, Kirkham B, et al. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: Results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT) Arthritis Rheum. 2005;52:1227–36. doi: 10.1002/art.20967. [DOI] [PubMed] [Google Scholar]
- 9.Mease PJ, Gladman DD, Ritchlin CT, et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: Results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2005;52:3279–89. doi: 10.1002/art.21306. [DOI] [PubMed] [Google Scholar]
- 10.Mease PJ, Kivitz AJ, Burch FX, et al. Etanercept treatment of psoriatic arthritis: Safety, efficacy, and effect on disease progression. Arthritis Rheum. 2004;50:2264–72. doi: 10.1002/art.20335. [DOI] [PubMed] [Google Scholar]
- 11.Rudwaleit M, Listing J, Brandt J, Braun J, Sieper J. Prediction of a major clinical response (BASDAI 50) to tumour necrosis factor alpha blockers in ankylosing spondylitis. Ann Rheum Dis. 2004;63:665–70. doi: 10.1136/ard.2003.016386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudwaleit M, Schwarzlose S, Hilgert ES, Listing J, Braun J, Sieper J. MRI in predicting a major clinical response to anti-tumour necrosis factor treatment in ankylosing spondylitis. Ann Rheum Dis. 2008;67:1276–81. doi: 10.1136/ard.2007.073098. [DOI] [PubMed] [Google Scholar]
- 13.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–8. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 14.Mau W, Zeidler H, Mau R, et al. Clinical features and prognosis of patients with possible ankylosing spondylitis. Results of a 10-year followup. J Rheumatol. 1988;15:1109–14. [PubMed] [Google Scholar]
- 15.Dougados M, van der Linden S, Juhlin R, et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991;34:1218–27. doi: 10.1002/art.1780341003. [DOI] [PubMed] [Google Scholar]
- 16.Amor B, Dougados M, Mijiyawa M. Criteria of the classification of spondylarthropathies. Rev Rhum Mal Osteoartic. 1990;57:85–9. [PubMed] [Google Scholar]
- 17.Baddoura R, Awada H, Okais J, Habis T, Attoui S, Abi Saab M. Validation of the European Spondylarthropathy Study Group and B. Amor criteria for spondylarthropathies in Lebanon. Rev Rhum Engl Ed. 1997;64:459–64. [PubMed] [Google Scholar]
- 18.Erturk M, Alaca R, Tosun E, Duruoz MT. Evaluation of the Amor and ESSG classification criteria for spondylarthropathies in a Turkish population. Rev Rhum Engl Ed. 1997;64:293–300. [PubMed] [Google Scholar]
- 19.Collantes-Estevez E, Cisnal del Mazo A, Munoz-Gomariz E. Assessment of 2 systems of spondyloarthropathy diagnostic and classification criteria (Amor and ESSG) by a Spanish multicenter study. European Spondyloarthropathy Study Group. J Rheumatol. 1995;22:246–51. [PubMed] [Google Scholar]
- 20.Collantes E, Veroz R, Escudero A, et al. Can some cases of ‘possible’ spondyloarthropathy be classified as ‘definite’ or ‘undifferentiated’ spondyloarthropathy? Value of criteria for spondyloarthropathies. Spanish Spondyloarthropathy Study Group. Joint Bone Spine. 2000;67:516–20. doi: 10.1016/s1297-319x(00)00201-3. [DOI] [PubMed] [Google Scholar]
- 21.Rudwaleit M, van der Heijde D, Landewe R, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann Rheum Dis. 2009;68:777–83. doi: 10.1136/ard.2009.108233. [DOI] [PubMed] [Google Scholar]
- 22.Rudwaleit M, van der Heijde D, Landewe R, et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011;70:25–31. doi: 10.1136/ard.2010.133645. [DOI] [PubMed] [Google Scholar]
- 23.Harvey RF, Bradshaw JM. A simple index of Crohn’s disease activity. Lancet. 1980;1:514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 24.Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998;43:29–32. doi: 10.1136/gut.43.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boonen A, van der Linden SM. The burden of ankylosing spondylitis. J Rheumatol Suppl. 2006;78:4–11. [PubMed] [Google Scholar]
- 26.Landewe R, Dougados M, Mielants H, van der Tempel H, van der Heijde D. Physical function in ankylosing spondylitis is independently determined by both disease activity and radiographic damage of the spine. Ann Rheum Dis. 2009;68:863–7. doi: 10.1136/ard.2008.091793. [DOI] [PubMed] [Google Scholar]
- 27.Rudwaleit M, Haibel H, Baraliakos X, et al. The early disease stage in axial spondylarthritis: Results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum. 2009;60:717–27. doi: 10.1002/art.24483. [DOI] [PubMed] [Google Scholar]
- 28.Sieper J, Rudwaleit M, Khan MA, Braun J. Concepts and epidemiology of spondyloarthritis. Best Pract Res Clin Rheumatol. 2006;20:401–17. doi: 10.1016/j.berh.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Braun J, van den Berg R, Baraliakos X, et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis. 2011;70:896–904. doi: 10.1136/ard.2011.151027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dagfinrud H, Kvien TK, Hagen KB. Physiotherapy interventions for ankylosing spondylitis. Cochrane Database Syst Rev. 2008;23 doi: 10.1002/14651858.CD002822.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Miedany Y, Youssef S, Ahmed I, El Gaafary M. The gastrointestinal safety and effect on disease activity of etoricoxib, a selective cox-2 inhibitor in inflammatory bowel diseases. Am J Gastroenterol. 2006;101:311–7. doi: 10.1111/j.1572-0241.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 32.Generini S, Giacomelli R, Fedi R, et al. Infliximab in spondyloarthropathy associated with Crohn’s disease: An open study on the efficacy of inducing and maintaining remission of musculoskeletal and gut manifestations. Ann Rheum Dis. 2004;63:1664–9. doi: 10.1136/ard.2003.012450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herfarth H, Obermeier F, Andus T, et al. Improvement of arthritis and arthralgia after treatment with infliximab (Remicade) in a German prospective, open-label, multicenter trial in refractory Crohn’s disease. Am J Gastroenterol. 2002;97:2688–90. doi: 10.1111/j.1572-0241.2002.06064.x. [DOI] [PubMed] [Google Scholar]
- 34.Van den Bosch F, Kruithof E, De Vos M, De Keyser F, Mielants H. Crohn’s disease associated with spondyloarthropathy: Effect of TNF-alpha blockade with infliximab on articular symptoms. Lancet. 2000;356:1821–2. doi: 10.1016/s0140-6736(00)03239-6. [DOI] [PubMed] [Google Scholar]
- 35.Beslek A, Onen F, Birlik M, et al. Prevalence of spondyloarthritis in Turkish patients with inflammatory bowel disease. Rheumatol Int. 2009;29:955–7. doi: 10.1007/s00296-008-0811-5. [DOI] [PubMed] [Google Scholar]
- 36.de Vlam K, Mielants H, Cuvelier C, De Keyser F, Veys EM, De Vos M. Spondyloarthropathy is underestimated in inflammatory bowel disease: Prevalence and HLA association. J Rheumatol. 2000;27:2860–5. [PubMed] [Google Scholar]
- 37.Palm O, Moum B, Ongre A, Gran JT. Prevalence of ankylosing spondylitis and other spondyloarthropathies among patients with inflammatory bowel disease: A population study (the IBSEN study) J Rheumatol. 2002;29:511–5. [PubMed] [Google Scholar]
- 38.Rodriguez VE, Costas PJ, Vazquez M, et al. Prevalence of spondyloarthropathy in Puerto Rican patients with inflammatory bowel disease. Ethn Dis. 2008;18:S2-225–9. [PubMed] [Google Scholar]
- 39.Salvarani C, Vlachonikolis IG, van der Heijde DM, et al. Musculoskeletal manifestations in a population-based cohort of inflammatory bowel disease patients. Scand J Gastroenterol. 2001;36:1307–13. doi: 10.1080/003655201317097173. [DOI] [PubMed] [Google Scholar]
- 40.Turkcapar N, Toruner M, Soykan I, et al. The prevalence of extraintestinal manifestations and HLA association in patients with inflammatory bowel disease. Rheumatol Int. 2006;26:663–8. doi: 10.1007/s00296-005-0044-9. [DOI] [PubMed] [Google Scholar]
- 41.Lanna CC, Ferrari Mde L, Rocha SL, Nascimento E, de Carvalho MA, da Cunha AS. A cross-sectional study of 130 Brazilian patients with Crohn’s disease and ulcerative colitis: Analysis of articular and ophthalmologic manifestations. Clin Rheumatol. 2008;27:503–9. doi: 10.1007/s10067-007-0797-5. [DOI] [PubMed] [Google Scholar]
- 42.Protzer U, Duchmann R, Hohler T, et al. [Enteropathic spondylarthritis in chronic inflammatory bowel diseases: Prevalence, manifestation pattern and HLA association] Med Klin (Munich) 1996;91:330–5. [PubMed] [Google Scholar]
- 43.Scarpa R, del Puente A, D’Arienzo A, et al. The arthritis of ulcerative colitis: Clinical and genetic aspects. J Rheumatol. 1992;19:373–7. [PubMed] [Google Scholar]
- 44.Orchard TR, Wordsworth BP, Jewell DP. Peripheral arthropathies in inflammatory bowel disease: Their articular distribution and natural history. Gut. 1998;42:387–91. doi: 10.1136/gut.42.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palm O, Moum B, Jahnsen J, Gran JT. The prevalence and incidence of peripheral arthritis in patients with inflammatory bowel disease, a prospective population-based study (the IBSEN study) Rheumatology (Oxford) 2001;40:1256–61. doi: 10.1093/rheumatology/40.11.1256. [DOI] [PubMed] [Google Scholar]
- 46.Veloso FT, Carvalho J, Magro F. Immune-related systemic manifestations of inflammatory bowel disease. A prospective study of 792 patients. J Clin Gastroenterol. 1996;23:29–34. doi: 10.1097/00004836-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen GC, Torres EA, Regueiro M, et al. Inflammatory bowel disease characteristics among African Americans, Hispanics, and non-Hispanic Whites: Characterization of a large North American cohort. Am J Gastroenterol. 2006;101:1012–23. doi: 10.1111/j.1572-0241.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- 48.Mendoza JL, Lana R, Taxonera C, Alba C, Izquierdo S, Diaz-Rubio M. [Extraintestinal manifestations in inflammatory bowel disease: Differences between Crohn’s disease and ulcerative colitis] Med Clin (Barc) 2005;125:297–300. doi: 10.1157/13078423. [DOI] [PubMed] [Google Scholar]
- 49.Mielants H, Veys EM, Goethals K, Van Der Straeten C, Ackerman C. Destructive lesions of small joints in seronegative spondylarthropathies: Relation to gut inflammation. Clin Exp Rheumatol. 1990;8:23–7. [PubMed] [Google Scholar]
- 50.Underwood MR, Dawes P. Inflammatory back pain in primary care. Br J Rheumatol. 1995;34:1074–7. doi: 10.1093/rheumatology/34.11.1074. [DOI] [PubMed] [Google Scholar]
- 51.Rudwaleit M, van der Heijde D, Khan MA, Braun J, Sieper J. How to diagnose axial spondyloarthritis early. Ann Rheum Dis. 2004;63:535–43. doi: 10.1136/ard.2003.011247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rudwaleit M, van der Heijde D, Landewé R, et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011;70:25–31. doi: 10.1136/ard.2010.133645. [DOI] [PubMed] [Google Scholar]


