Abstract
The aim of the present prospective observational study was to assess uptake and success of hepatitis C virus (HCV) treatment among a group of former and current injection drug users with chronic HCV infection at the Street Health Centre in Kingston, Ontario. The Street Health Centre offers hepatitis C education, assessment and treatment within a multidisciplinary, integrated and collaborative treatment model of care delivered by primary care professionals. The study enrolled a convenience sample of 34 patients. Seventy per cent of study patients had no postsecondary education, 85% were unemployed and one-third were unstably housed. A majority of study patients self-reported mental health problems. Of the 14 patients who initiated antiviral treatment in the study period, eight (57%) achieved sustained virological response. Regardless of virological outcome, patients who initiated treatment showed positive trends toward increased social and psychiatric stability, and decreases in high-risk behaviours. These results suggest that not only is successful treatment of chronic HCV infection in current and former injection drug users with concurrent psychiatric disorders possible, but the benefits of such treatment delivered in a community-based, multidisciplinary, primary care model may extend beyond narrowly defined virological outcomes.
Keywords: Canada, Community-based, Delivery of health care, Drug users, Hepatitis C virus, Kingston, Multidisciplinary, Treatment
Abstract
La présente étude prospective d’observation visait à évaluer l’utilisation et la réussite d’un traitement contre le virus de l’hépatite C (VHC) au sein d’un groupe d’utilisateurs actuels et passés de drogues injectables atteints d’une infection chronique par le VHC qui fréquentaient le Street Health Centre de Kingston, en Ontario. Le Street Health Centre offre de l’information sur l’hépatite C, une évaluation et un traitement contre cette maladie dans le cadre d’un modèle de traitement multidisciplinaire, intégré et coopératif prodigué par des professionnels de première ligne. L’étude a porté sur un échantillon de commodité de 34 patients. Soixante-dix pour cent des patients à l’étude n’avaient pas d’instruction postsecondaire, 85 % étaient sans emploi et le tiers n’avait pas de loge-ment stable. La majorité des patients à l’étude déclaraient eux-mêmes avoir des problèmes de santé mentale. Sur les 14 patients qui avaient amorcé une antivirothérapie pendant la période de l’étude, huit (57 %) ont obtenu une réponse virologique soutenue. Quelle que soit l’issue virologique, les patients qui avaient amorcé le traitement présentaient une tendance positive vers une meilleure stabilité sociale et psychia-trique et une diminution des comportements à haut risque. D’après ces résultats, non seulement le traitement de l’infection chronique par le VIH est-il concluant chez les utilisateurs actuels et passés de drogues injectables ayant peut-être un trouble de santé mentale concomitant, mais les bienfaits de ce traitement prodigué dans un modèle multidisci-plinaire de soins primaires communautaires pourraient aller au-delà des issues virologiques à la définition trop étroite.
Approximately 170 million people are chronically infected with hepatitis C virus (HCV) worldwide (1). Injection drug users (IDUs) represent 75% of incident cases of HCV in the developed world, 80% of whom will proceed to develop chronic disease (2). Approximately 250,000 individuals in Canada are living with HCV (3), including an estimated 111,000 people in Ontario; approximately one-third of those with the virus in Ontario are unaware they have been infected (4). In 2004, 80% of new HCV infections in Ontario were acquired by active IDUs and 98% of Ontarians living with both HIV and HCV are either past or present IDUs (5).
Research has shown that high-risk populations, including IDUs, can adhere to treatment programs as well as those who do not inject drugs, and can be safely and successfully treated (6–12). However, treatment uptake among IDUs remains very low and reaches <5% of those infected (13). Barriers to effective hepatitis C treatment may be influenced by patient, provider or system factors (14), and include past or ongoing drug use; concurrent mental illness; low socioeconomic status and homelessness; unstructured lifestyles; and lack of access to providers specialized in the treatment of hepatitis C. Other factors may include addiction (15), fear and mistrust of the health care system, stigmatization and marginalization (16–18).
Drug-using populations exhibit significantly higher rates of concurrent mental health disorders than the general population (19–22). In the United States Epidemiological Catchment Area study (23), approximately 45% of those with alcohol use disorders and 72% of those with drug use disorders had at least one concurrent psychiatric disorder. Psychiatric comorbidity rates are higher for those dependent on illicit drugs compared with alcohol dependence and highest for individuals with multiple dependencies (20,24). Psychiatric conditions commonly associated with substance use disorders include depression and mood disorders, post-traumatic stress disorder, attention deficit hyperactivity disorder, schizophrenia and personality disorders (20). Neuropsychiatric symptoms are commonly associated with HCV infection and treatment, including delirium, depression, anxiety, cognitive changes, mania and psychosis (25–27). In traditional treatment models, psychiatric illness and ongoing substance use disorders are often contraindications to treatment (28).
It has been argued that a client-centred, multidisciplinary approach linking drug treatment, mental health treatment and collaborative care to the HCV treatment of former and current IDUs is the most appropriate model of care for this population (16,18,29–32). Many programs successfully integrate hepatitis C care into a one-stop shop model in which the treatment of addiction, hepatitis C and other medical and psychiatric conditions is delivered by a team of nurses, counsellors, researchers and physicians (7,33–38). By providing care in a collaborative multidisciplinary framework, clinicians address the medical needs of HCV patients in the context of their complex bio-psychosocial needs (36,39).
The Street Health Centre
The Street Health Centre (SHC) located in Kingston Ontario (population 117,207) (40), began treating patients with chronic HCV infection in 1996, and initiated its current HCV Treatment Clinic in June 2006 using a unique, multidisciplinary, collaborative model (41). The Centre provides accessible and responsive health care to the communities of people who use injection and illicit drugs, as well as high-risk youth, individuals involved in the sex trade, and individuals who are homeless or have recently been released from incarceration.
SHC began as a needle exchange program in 1992 and now functions as a one-stop shop model of collaborative primary care, harm reduction and methadone maintenance therapy (MMT) delivered by nurses, nurse practitioners, physicians, a psychiatrist and counselling staff. Nurse practitioners are the main providers of ongoing primary care. The SHC also provides HCV treatment, palliative care, psychiatric treatment, addictions counselling, peer support and outreach services. The majority of the 1200 active patients have tested positive for antibodies to HCV.
As a part of Kingston Community Health Centres, the SHC is funded through several sources including core funding through the SouthEast Ontario Local Health Integration Network. Significant funds from the HIV/AIDs and Hepatitis C Programs Branch of the Ministry of Health and Long-Term Care provide for two dedicated hepatitis nurses, an outreach worker, a counselor and a network coordinator. The treating physician is paid on salary but the consulting psychiatrist bills the Ontario Hospital Insurance Plan on a fee-for-service basis.
The current study was initiated at a time when the sole option in Kingston for patients with chronic HCV infection desiring treatment was a traditional hospital-based outpatient clinic staffed by the members of the university-affiliated gastroenterology department. To access care, patients required a referral from a family physician who was asked to confirm that the patient was free of alcohol and illicit drug use for at least six months before being seen. Many HCV-positive clients at SHC did not have a family physician and were still actively using drugs and alcohol, thus effectively preventing them from being assessed for treatment. Patients who met the criteria for treatment were processed and referred to the hospital clinic by the SHC nurse practitioner. However, anecdotal reports from SHC patients suggest that they often failed to attend the initial assessment or follow-up appointments due to perceived stigmatization.
SHC established a treatment program specifically designed for such high-risk patients outside a hospital setting. The program was developed to provide an accessible, supportive, multidisciplinary model of care in which a team of clinicians would assess and address medical, psychiatric and social stability before initiating HCV treatment. Once treatment is underway, patients are carefully monitored and supported. Aftercare is also provided in the same clinic. The current study presents data collected during the first 30 months of the program.
The purpose of the present study was to assess uptake and success of hepatitis C treatment among a group of former and current IDUs with chronic HCV infection at the SHC within SHC’s multidisciplinary, integrated and collaborative treatment model of care centred on primary care professionals.
METHODS
The design was a prospective observational study of SHC clients with chronic HCV infection who were interested in pursuing hepatitis C treatment.
Patients
A convenience sample of 34 patients was enrolled in the study between June 2006 and December 2008. Patients who were self-referred to the hepatitis C treatment team or were referred through other SHC services (including MMT, needle exchange, counselling or primary care) were invited to participate in the study. To be eligible for the study, patients were SHC clients with chronic HCV infection, ≥18 years of age and had an interest in undergoing HCV treatment. HCV infection was confirmed by a positive HCV-RNA test, including genotype, during initial treatment assessment. Psychiatric comorbidities or ongoing illicit drug use did not exclude patients from the study or from proceeding to treatment. All patients were former or current IDUs. Participants provided informed consent to participate and the study was approved by the Queen’s University Health Sciences Research Ethics Board, Kingston, Ontario.
Data collection
A schedule of data collection is included in Table 1.
TABLE 1.
Schedule of data collection
| Data collection point | Data | Method |
|---|---|---|
| Baseline | Patient characteristics: Demographics (including sex, age, education, marital status, housing, employment, income, and legal status), injection drug use history, medical and psychiatric history, HCV transmission risk factors history, physical characteristics, medication use, and treatment motivation | Semistructured interview |
| Clinical characteristics: HCV genotype, alanine aminotransferase, aspartate aminotransferase, bilirubin, albumin, thyroid stimulating hormone, complete blood count including absolute neutrophil count, and international normalized ratio; liver biopsy grade and stage for patients who completed a liver biopsy | Laboratory test results | |
| Baseline and every 4 weeks throughout treatment | Beck Depression Inventory score (Version: BDI), a 21-question multiple choice self-report inventory used to measure the severity of depression. | Semistructured interview |
| Addiction Severity Index (Version: ASI-Lite), an interview-administered research assessment instrument to rate the extent to which treatment for addiction is needed. | Semistructured interview | |
| Weekly throughout prescribed treatment or until treatment was stopped | All baseline clinical characteristics | Laboratory test results |
| Urine toxicological screening for illicit drugs | High-performance liquid chromatography (Gamma Dynacare Medical Laboratories, London, Ontario) | |
| Prescribed psychotropic and nonpsychotropic medication | Abstracted from patient’s chart | |
| Ribavirin compliance | Semistructured interview | |
| Exposure to HCV transmission risk factors | Semistructured interview | |
| Treatment week 4 | Rapid virological response | Laboratory test results |
| Treatment week 12 | Early virological response | Laboratory test results |
| Treatment week 24 | End of treatment response for patients infected with HCV genotype 2 or 3 | Laboratory test results |
| Treatment week 48 | End of treatment response for patients infected with HCV genotype 1 | Laboratory test results |
| 24 weeks after completion of treatment | Sustained virologicial response | Laboratory test results |
HCV Hepatitis C virus
Assessing patients for treatment suitability and readiness
Suitability and readiness for treatment were assessed by SHC’s multi-disciplinary treatment team, which included a family physician who had completed the College of Family Physicians of Canada Hepatitis C Fellowship, a psychiatrist, a nurse practitioner, a counsellor and a registered nurse. Patients attended a series of appointments with members of the clinical treatment team in the pretreatment assessment stage over a period of weeks or months. Regular attendance at these appointments was used as one predictor of treatment adherence. Additional markers included housing stability and income status. If the patient was considered too socially unstable to begin treatment, the team provided assistance by completing Ontario Disability Support Program (ODSP) applications, advocating for priority housing status and establishing links to appropriate community agencies. Similarly, patients who were medically or psychiatrically unstable were offered assessment and therapy by the team’s psychiatrist and family physician, and their progress was monitored until they were deemed sufficiently stable to begin therapy.
Decisions regarding suitability for treatment were made at weekly clinical case conference meetings. Likelihood of success was evaluated against client motivation, medical safety, social stability and disease severity.
Treatment protocol
SHC’s standard HCV treatment protocol was used. Patients who proceeded to treatment received combination therapy with ribavirin (800 mg per day to 1200 mg per day, depending on weight) along with either pegylated interferon alpha 2a (Pegasys, Hoffman-La Roche Inc, USA) or pegylated interferon alpha 2b (Pegetro, Schering-Plough Corporation, USA). For patients infected with HCV genotype 1, prescribed treatment duration was 48 weeks; for those with genotype 2 or 3, duration was 24 weeks. The registered nurse administered the interferon injection weekly at the clinic, monitored side effects, and provided ongoing health education. Patients continued to see the SHC family physician and psychiatrist throughout the treatment period as needed, usually monthly. The counsellor provided practical and therapeutic support according to the patient’s needs. In addition, patients continued to access other SHC services as appropriate, including MMT, needle exchange, primary care and other counselling.
As per regular treatment protocol, the clinical treatment team met weekly for a case conference to discuss ongoing treatment concerns of each patient and to decide on interventions as necessary. These could include initiation of concurrent therapies (such as erythropoietin for symptomatic anemia), specialist referral, dose reduction or discontinuation of treatment.
Sustained virological response (SVR) was the primary outcome measure used to determine treatment success and was defined as an undetectable HCV viral load 24 weeks after completion of antiviral therapy for all genotypes. Nonresponse was defined in patients with HCV genotype 1 as the absence of early virological response (ie, a reduction of <2 log from baseline viral load at 12 weeks) or the persistence of a positive test for RNA at any level after 24 weeks of treatment. In patients with HCV genotype 2 or 3, nonresponse was defined as the persistence of viral RNA beyond 12 weeks. Reasons for discontinuation of therapy other than nonresponse were based on clinical chart notes from the staff physician or psychiatrist. End of treatment response (ETR) was defined as a negative test for HCV RNA at the end of the treatment course.
Statistics
Descriptive statistics are provided with the median and range. Nonparametric tests (χ2, Fisher’s exact and Mann-Whitney) were used where applicable; P<0.05 in a two-tailed comparison was considered to be statistically significant. All data were compiled and analyzed using SPSS version 16 (IBM Corporation, USA) for Windows (Microsoft Corporation, USA).
RESULTS
A total of 34 patients were enrolled in the study. Overall, patients in the study showed a positive willingness for HCV treatment.
Baseline characteristics of all study participants
Table 2 summarizes selected baseline characteristics of all 34 study patients.
TABLE 2.
Selected baseline characteristics (n=34)
| Male sex | 20 (59.0) |
| Age, years | |
| Median | 42 |
| Range | 21–67 |
| Education, ≤13 years | 15 (44.1) |
| Income | |
| Ontario Works | 15 (43.3) |
| Ontario Disability Support Program | 12 (36.7) |
| Unstable housing | 9 (33.3) |
| Unemployment | 23 (85.7) |
| On probation/parole | 8 (26.7) |
| History of incarceration | 27 (90.0) |
| Age at first injection drug use, years, median (range) | 19 (13–37) |
| Injection drug use in past six months | 12 (41.0) |
| Methadone maintenance therapy | 19 (57.6) |
| Hepatitis C genotype | |
| 1 | 25 (73.5) |
| 2 or 3 | 9 (26.5) |
| History of treatment for depression | 21 (75.0) |
| History of treatment for anxiety | 18 (62.1) |
| History of suicide attempt(s) | 15 (50.0) |
| Taking nonpsychotropic drugs | 12 (41.4) |
| Taking psychotropic drugs | 26 (76.5) |
Data presented as n (%) unless otherwise indicated
Demographics:
Only 30% of study patients had any postsecondary education, and slightly more than one-half had not completed high school. One-fifth (19%) of patients was married or in a common-law relationship, and the majority (52%) lived in an apartment. Eighty-five per cent of participants enrolled in the study were unemployed and received their income from provincially or municipally administered government income assistance programs.
One-third of patients was unstably housed, defined as living in a rooming house or shelter, ‘couch surfing’ or without a fixed address. The median number of months for all study participants in current housing was seven.
Injection drug use history:
All study patients were former or current IDUs. The median age of first injection drug use was 19 years (range 13 to 37 years). Slightly more than 40% of patients had used injection drugs within the past six months.
Exposure to HCV risk factors:
All patients reported exposure to at least one HCV transmission risk factor at some point in the past. The sharing of needles, water, or spoons/mixers was the most common among all the risk factors, with more than 60% of study patients having exposed themselves to these risks. In addition, almost one-half of patients had shared straws or bills for snorting, and 41% had shared crack pipes.
Medical and clinical characteristics:
Of the 34 study patients, 73% were infected with HCV genotype 1. The remaining 27% were infected with either HCV genotype 2 or 3. No other genotypes were found. The median body mass index of the group was 28.4 kg/m2. Tests for thyroid stimulating hormone, bilirubin, albumin, hemoglobin, white blood cells, absolute neutrophil count, platelets and international normalized ratio were all in normal range for study participants. Alanine aminotransferase levels were higher than normal clinical range, as would be expected for a group with chronic hepatitis C. The range for alanine aminotransferase was 27 U/L to 239 U/L, with a mean of 91.8 U/L.
Psychiatric characteristics:
Study patients self-reported complex psychiatric histories. A large majority – more than three-quarters – was on psychiatric medications at baseline. Fifty per cent had a history of attempted suicide, and 43% had been admitted to a psychiatric hospital or to a general hospital psychiatric ward. The most common psychiatric issues reported were depression (75%) and anxiety (62%). One-quarter of study participants also reported a history of psychosis. The median Beck Depression Inventory score was 17, which is at the upper end of the scale in the ‘mild-moderate’ depression category.
Characteristics of treatment group
Fourteen of the 34 patients initiated treatment in the study period. Table 3 summarizes selected characteristics of the treatment group. There was a significant difference in the length of time at current housing between the treatment group and study participants who did not initiate treatment (P=0.04). The median length of time in current housing for people who initiated treatment in the study period was 21 months, in contrast to four months for study participants who did not initiate treatment.
TABLE 3.
Characteristics of treatment group versus patients who did not initiate treatment in the study period
| Characteristic | Treatment group (n=14) | Did not initiate treatment (n=20) |
|---|---|---|
| Length in current housing*, months, median | 21 | 4 |
| Common-law or married† | 4 (28.6) | 2 (10.0) |
| Receiving Ontario Disability Support Program benefits† | 9 (64.3) | 4 (20.0) |
| Injection drug use in past six months† | 4 (28.6) | 8 (40.0) |
| Undergoing methadone maintenance therapy† | 8 (57.1) | 11 (55.0) |
| Taking nonpsychotropic medications† | 4 (28.6) | 8 (40.0) |
Data presented as n (%) unless otherwise indicated.
P<0.05;
Not statistically significant
Since completion of the study period, five of the 20 patients who did not originally initiate treatment at the time eventually went on to do so. This reflects the fact that the characteristics influencing selection for treatment are dynamic and subject to change over time, rather than indicative of an immutable distinction between two different populations: those who are deemed ‘suitable’ and those who are ‘beyond hope’. With the passage of time, the proportion of these patients with a greater length of time in current housing and receiving government disability pensions has increased such that many were able to commit to the treatment regimen and were ready and able to undergo treatment successfully. Those who have not yet reached this point continue to be engaged in therapeutic relationships with members of SHC staff who are working to address the very characteristics that delayed or prevented their treatment during the study period.
Treatment outcomes:
Treatment outcomes are depicted in Figure 1. Of the 14 patients enrolled in the study, 11 achieved an ETR, while three dropped out of the study due to adverse events or noncompliance. Of the 11 who reached ETR, eight achieved SVR as measured 24 weeks after completion of treatment. The other three experienced a relapse of the infection. Relapse was defined as the reappearance of detectable HCV RNA after an initial response.
Figure 1).
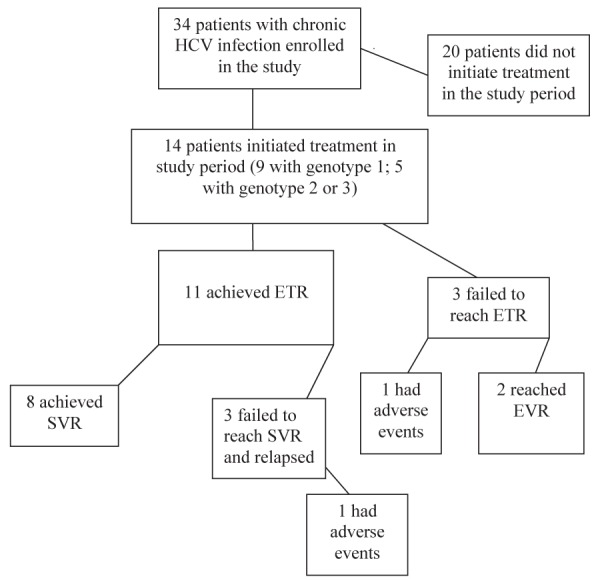
Hepatitis C treatment outcomes. HCV Hepatitis C virus; ETR End of treatment response; EVR Early virological response; SVR Sustained virological response
Table 4 summarizes selected characteristics of the 14 treatment patients. Twelve study participants were ‘responders’. Response was defined as either undetectable viral RNA by or before 12 weeks of treatment in the case of those infected with genotype 2 or 3; or undetectable RNA by or before 24 weeks of treatment in the case of infection with genotype 1. In all cases, SVR was defined as a negative RNA test 24 weeks after the end of treatment, regardless of genotype or treatment duration. Of the six patients who did not achieve an SVR, two failed to finish their treatment course and the physician discharged them because of a critical illness or psychosis. The other four patients responded to treatment but relapsed before achieving SVR.
TABLE 4.
Treatment for chronic hepatitis C virus (HCV) infection among 14 current and former injection drug users initiating therapy at the Street Health Centre in Kingston, Ontario
| Patient | Sex | Age, years | HCV genotype |
During treatment
|
Ribavirin compliance, % | Status | Outcome | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
Treatment, weeks
|
Self-reported use of illicit drugs* | Positive tox screen for illicit drugs | ||||||||
| Prescribed | Completed | |||||||||
| 1 | F | 39 | 1 | 48 | 48 | Yes | Yes | 98.2 | Completed | SVR |
| 2 | M | 54 | 1 | 48 | 48 | No | No | 100 | Completed | SVR |
| 3 | M | 49 | 3 | 24 | 20 | Yes | No | 97.1 | Stopped early due to relapse | N/A |
| 4 | M | 53 | 2/3 | 24 | 24 | No | Yes | 100 | Completed | SVR |
| 5 | F | 25 | 3 | 24 | 22 | No | Yes | 99 | Completed | SVR |
| 6 | M | 49 | 1 | 48 | 48 | Yes | Yes | 91 | Completed | ETR |
| 7 | F | 48 | 1 | 48 | 10 | No | No | 97.4 | Stopped early due to critical illness and psychosis | N/A |
| 8 | F | 29 | 3 | 24 | 24 | No | No | 97 | Completed | SVR |
| 9 | F | 31 | 1 | 48 | 48 | Yes | Yes | 81 | Completed | SVR |
| 10 | M | 48 | 1 | 48 | 48 | No | No | 100 | Completed | ETR |
| 11 | M | 56 | 1 | 48 | 19 | No | No | 100 | Stopped early due to illness | ETR |
| 12 | F | 48 | 1 | 48 | 48 | Yes | Yes | 99.8 | Completed | SVR |
| 13 | F | 41 | 1 | 48 | 39 | Yes | Yes | 100 | Stopped due to nonresponse | N/A |
| 14 | M | 43 | 3 | 24 | 24 | No | No | 100 | Completed | SVR |
Excludes cannabis. ETR End of treatment response; EVR Early virological response; F Female; M Male; N/A Not applicable; SVR Sustained virological response; tox Toxicology
Medical characteristics during treatment:
All treatment patients visited SHC weekly for interferon injections and other supports with two exceptions. Two study participants administered their own injections for a short period of time during the study: one patient for one week and the other over a three-week period.
A greater proportion of patients with HCV genotype 2 or 3 initiated treatment in the study period. All patients undergoing MMT who began HCV treatment continued to undergo MMT throughout the HCV treatment period.
Ten treatment study patients were on nonpsychotropic drugs at both baseline and during treatment. All but three study patients were prescribed erythropoietin (Eprex, Janssen-Ortho Inc, Canada) for symptomatic anemia during their treatment period and 13 patients experienced medical problems during the treatment period. Medical problems, as defined by the Addiction Severity Index, included ailments that would continue even if the patient were abstinent from drugs and alcohol.
Twelve of the 14 study patients demonstrated ribavirin compliance >96%. The other two patients showed 91% and 81% compliance to the treatment regimen.
Psychiatric characteristics during treatment:
Thirteen treatment study participants were on psychotropic medication at some point during their treatment period. Only one study participant did not have a self-reported psychiatric history, was not being treated with psycho-tropic drugs at baseline and did not take psychotropic drugs during treatment.
The frequencies of a history of psychosis (38.5% versus 9.1%) and depression (86.7% versus 61.5%) were higher in the group of study participants who did not initiate treatment. However, these differences were not statistically significant.
Thirteen of the treatment study patients received psychiatric assessment and follow-up treatment during their hepatitis C treatment period. Eight of these 13 also reported a history of psychiatric comorbidities at baseline.
All treatment study participants reported days when they experienced emotional/psychological problems during treatment and all reported having concerns about their psychological problems throughout the duration of their treatment. The frequency of these self-reported problems varied. None of the participants required a psychiatric hospitalization or attempted suicide during treatment. One patient ended treatment early (at 22 weeks, nonetheless progressing to achieve SVR) due to a psychiatric adverse event and one patient discontinued treatment due to a combination of critical illness and psychiatric symptoms.
Alcohol use during treatment:
Five study patients reported using alcohol during the treatment period. Three patients reported days when they were intoxicated during the treatment period. The frequency of alcohol use was low and only one patient used alcohol for 19% of their treatment days.
Drug use during treatment:
The study collected data on drug use during treatment through both monthly self-reports and weekly urine toxicology screens.
Six of the study participants reported illicit drug use (excluding cannabis) during treatment. Self-reported illicit drug use during treatment included: opiates/analgesics (four of 14), sedatives (three of 14), cocaine (three of 14), and amphetamines (one of 14). No study participants reported using heroin, barbiturates or hallucinogens during the treatment period. The frequency of illicit drug use varied, but was mostly infrequent.
Nine study patients reported using cannabis during treatment and tested positive for cannabinoids through urine toxicology screens. The frequency of use was wide ranging. Two participants reported using cannabis over 90% of the treatment period and others used it only episodically.
All 14 of the treatment patients had a history of injection drug use, but only three study participants reported using injection drugs during their treatment period. Two of these participants had reported using injection drugs six months before baseline data were collected; and one participant did not provide the injection drug use baseline six-month history. Two study participants who had reported using injection drugs in the six months before baseline did not use injection drugs during the treatment period.
Self-reported drug use versus positive urine toxicology tests for drug use are summarized in Table 4. Seven study participants tested positive for illicit drugs (excluding cannabis) at least once during treatment through urine toxicology screens. Five reported using illicit drugs during treatment and had positive toxicology screens during treatment. Two treatment patients reported not using illicit drugs during treatment but did test positive for illicit drugs in a urine toxicology screen. One study patient reported illicit drug use during treatment but did not test positive for illicit drugs in a urine toxicology screen.
Exposure to HCV transmission risk factors during treatment:
All study patients had reported exposure to at least one HCV transmission risk factor at some point in the past. However, study patients who initiated treatment reported a decrease in risk behaviours with fewer self-reported exposures to risk factors.
Ten study treatment patients had shared needles at some point before starting treatment, but once treatment commenced, only one reported sharing needles. No treatment patients reported sharing water, spoons/mixers or filters during treatment. In contrast, at baseline, the self-reported history of sharing this drug preparation equipment was eight of 14, nine of 14 and six of 14, respectively. At baseline, seven patients shared straws or bills for snorting, and four had shared crack/methamphetamine pipes. However, in treatment, only one study patient shared a straw/bill and one shared a crack/methamphetamine pipe.
DISCUSSION
The SHC began treating its client population for HCV at a time when HCV treatment for patients with active drug use and psychiatric diagnoses was rare. In the years since, literature attesting to the safety and efficacy of HCV treatment in similar patient populations has steadily accumulated (6–12). The data presented in the current study add to this evidence and emphasize that effective and successful HCV treatment of current and former IDUs, many with concurrent psychiatric disorders, is possible within a collaborative multidisciplinary model. The results show that 57% of patients in the present study achieved SVR and were cured of hepatitis C, yielding outcomes similar to treatment in non-drug-using populations (6–12).
Examining the specific indicators of social stability, psychiatric comorbidity and drug-related high-risk behaviours in our particular context, the results are instructive for those seeking to establish programs targeting similar populations elsewhere. Patients enrolled in the study had many of the hallmarks of high-risk behaviours and social instability seen in other studies conducted in urban centres (42–45). Importantly, several characteristics typical of such marginalized populations showed a trend toward improvement in the group of patients who initiated treatment. For example, the 14 patients who initiated treatment during the study period showed a significantly longer duration of time at their current place of residence than those who did not initiate treatment. Additionally, while not reaching statistical significance, a greater proportion of patients who initiated treatment in the study period were married or in a common-law relationship (27.3% versus 13.3%), were receiving higher levels of social assistance (ODSP 53.8%:Ontario Works [OW] 30.8% versus ODSP 23.5%:OW 52.9%) and were enrolled in MMT. Furthermore, fewer of the treatment patients used injection drugs in the six months before treatment.
While the greater degree of social stability may have biased the investigators toward selecting certain patients for therapy, the patients who initiated and adhered to the treatment protocol were, by no means, low risk. Only one patient did not have a history of psychiatric illness and was not prescribed psychotropic medication; one-half underwent urine drug screens indicating illicit substance use during treatment, including three who continued injection drug use; and five reported using alcohol, three to the point of intoxication.
Perhaps the most promising finding was the social improvement apparent in patients on treatment, and the suggestion that the HCV assessment and treatment process may have contributed to these positive outcomes. For example, the multidisciplinary treatment team made efforts to stabilize and improve patient incomes by completing documentation, investigations and recording functional status to help patients obtain ODSP benefits, which provide considerably higher benefits than those provided by OW. OW was the main source of income for most of the subjects at the start of the study.
Furthermore, the therapeutic relationships offered to patients with various members of the multidisciplinary team, including the staff psychiatrist, may have had a stabilizing effect. All study participants had a relationship with clinic staff before hepatitis C treatment was considered, as well as receiving assessment, treatment, follow-up and aftercare all at the same clinic.
A high proportion of patients enrolled in the study had histories of psychiatric illness. All study participants received psychiatric assessment, stabilization as needed and follow-up. The frequency of a history of psychosis and depression was lower in the group of study participants that initiated treatment during the study period. Nonetheless, all patients who initiated treatment reported emotional and psychological problems during treatment and the majority availed themselves of the services of the team psychiatrist. Patients continued to see the same psychiatrist throughout the treatment period, most at least monthly, which may have contributed to improved treatment adherence. Only one patient dropped out of treatment because of both critical illness and psychiatric complications. In spite of complex mental health issues and emerging symptoms, patients were able to complete treatment with a high ribavirin compliance rate. As other studies have demonstrated, drug using populations may adhere to complete treatment for hepatitis C at rates as high as those in other patient groups if their mental health is monitored closely and treatment is available (16,46).
Most encouraging are the behavioural changes suggested by the patients’ own reports. While all subjects had engaged in high-risk activities that had likely resulted in their HCV infection, patients who initiated treatment reported a decrease in high-risk behaviours during the treatment period. While the majority had shared both needles and other drug preparation equipment before starting treatment, only one reported sharing needles during treatment and none continued to share equipment such as water, spoons or filters. Also, while one-half of treatment patients had shared straws or bills for snorting drugs and four had shared pipes for smoking drugs, only one patient reported sharing this equipment during treatment. Despite the bias of health professionals against treating addicts, patients enrolled in our study demonstrated an ability to incorporate harm reduction strategies and completed treatment at rates similar to low-risk populations.
It is important to note that the 20 study participants who did not initiate treatment during the study period remain potential treatment patients. With the continuing support and care of the multidisciplinary treatment team, it is expected that most study participants will eventually achieve readiness to start treatment for their HCV infection; indeed, many of them have started antiviral treatment since the end of the period encompassed by the current study. Even if initiating treatment is not possible in every case, the positive trends toward greater social and psychiatric stability, as well as decreases in high-risk behaviours in patients will hopefully benefit all, regardless of whether SVR is ultimately achieved.
Future research should explore the most effective mix of services within a multidisciplinary approach to increase the likelihood of compliance and success of HCV treatment. Furthermore, outcomes, such as the prevention of HCV re-infection and other drug-related harms, should be evaluated through long-term follow-up of this population.
The primary limitation of the study methodology was the small sample size. The present study was designed to be an initial report of the effectiveness of treatment in a community health care setting. As a result, the study has weak external validity and the results may not be generalizable to other centres. The study also relied heavily on self-reported data and face-to-face interviews, leading to recall bias and the reporting of socially desirable responses. However, in terms of drug use, the comparison of urine toxicology screens to self-reported illicit drug use suggests reasonably high reliability of the self-reported data.
CONCLUSION.
The SHC’s approach shows that treatment of high-risk, marginalized, and traditionally underserved HCV patients is possible in a community-based clinic using a multidisciplinary collaborative model of care. Furthermore, the benefits of such a treatment model may extend beyond narrowly defined virological outcomes to improvements in other social determinants of health.
Acknowledgments
This study was funded, in part, by the Hepatitis C Secretariat, Ontario Ministry of Health and Long-Term Care. The Street Health Centre HCV treatment team also acknowledges Rosalyn Robertson for her formative contributions to the design of the study, and Regan Lavoie for her ongoing hard work and dedication.
REFERENCES
- 1.Schaefer M, Heinz A, Backmund M. Treatment of chronic hepatitis C in patients with drug dependence: Time to change the rules? Addiction. 2004;99:1167–75. doi: 10.1111/j.1360-0443.2004.00821.x. [DOI] [PubMed] [Google Scholar]
- 2.Grebely J, deVlaming S, Duncan F, Viljoen M, Conway B. Current approaches to HCV infection in current and former injection drug users. J Addict Dis. 2008;27:25–35. doi: 10.1300/J069v27n02_04. [DOI] [PubMed] [Google Scholar]
- 3.Public Health Agency of Canada < www.phac-aspc.gc.ca/hepc/index-eng.php> (Accessed March 23, 2009).
- 4.Ontario Ministry of Health and Long-Term Care Health Care Professionals. Hepatitis C. < www.health.gov.on.ca/english/providers/program/hepc/hepc_mn.html> (Accessed March 23, 2009).
- 5.Remis RS. The epidemiology of hepatitis C infection in Ontario, 2004: final report. Toronto: Hepatitis C Secretariat, Ontario Ministry of Health and Long-Term Care; Jan, 2007. [Google Scholar]
- 6.Backmund M, Meyer K, von Zielonka M, Eichenlaub D. Treatment of hepatitis C infection in injection drug users. Hepatology. 2001;34:183–8. doi: 10.1053/jhep.2001.25882. [DOI] [PubMed] [Google Scholar]
- 7.Edlin BR. Hepatitis C prevention and treatment for injection drug users. Hepatology. 2002;36(Suppl 1):S210–S219. doi: 10.1053/jhep.2002.36809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Thiel DH, Anantharaju A, Creech S. Response to treatment of hepatitis C individuals with a recent history of intravenous drug abuse. Am J Gastroenterol. 2003;98:2281–8. doi: 10.1111/j.1572-0241.2003.07702.x. [DOI] [PubMed] [Google Scholar]
- 9.Cournot M, Glibert A, Castel F, et al. Management of hepatitis C in active drug users: Experience of an addiction care hepatology unit. Gastroenterol Clin Biol. 2004;28:533–9. doi: 10.1016/s0399-8320(04)95008-7. [DOI] [PubMed] [Google Scholar]
- 10.Sylvestre DL, Clements BJ. Adherence to hepatitis C treatment in recovering heroin users maintained on methadone. Eur J Gastroenterol Hepatol. 2007;19:741–7. doi: 10.1097/MEG.0b013e3281bcb8d8. [DOI] [PubMed] [Google Scholar]
- 11.Bruggmann P, Falcato L, Dober S, et al. Swiss Hepatitis C Cohort Study Active intravenous drug use during chronic hepatitis C therapy does not reduce sustained virological response rates in adherent patients. J Viral Hepat. 2008;15:747–52. doi: 10.1111/j.1365-2893.2008.01010.x. [DOI] [PubMed] [Google Scholar]
- 12.Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199–207. doi: 10.1056/NEJMoa1009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer B, Kalousek K, Rehm J, et al. Hepatitis C, illicit drug use public health – does Canada really have a viable plan? Can J Public Health. 2006;97:485–8. doi: 10.1007/BF03405233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrill JA, Shrestha M, Grant R. Barriers to the treatment of Hepatitis C: Patient, provider, and system Factors. J Gen Inter Med. 2005;20:754–8. doi: 10.1111/j.1525-1497.2005.0161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper CL. Obstacles to successful HCV treatment in substance addicted patients. J Addict Dis. 2008;27:61–8. doi: 10.1300/J069v27n02_07. [DOI] [PubMed] [Google Scholar]
- 16.Edlin BR, Kresina TF, Raymond DB, et al. Overcoming barriers to prevention, care, and treatment of hepatitis C in illicit drug users. Clin Infect Dis. 2005;40:S276–S285. doi: 10.1086/427441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newman A, Mackenzie M, Shore R. Hepatitis C and injection drug use: Treatment is possible. Ont Med Rev. 2007;74:33–6. [Google Scholar]
- 18.Zevin B. Managing chronic hepatitis C in primary-care settings: More than antiviral therapy. Public Health Reports. 2007;122(Suppl 2):78–82. doi: 10.1177/00333549071220S215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozkan M, Corapcioglu A, Balcioglu I, et al. Psychiatric morbidity and its effect on the quality of life of patients with chronic hepatitis B and hepatitis C. Int J Psychiatry Med. 2006;36:283–97. doi: 10.2190/D37Y-X0JY-39MJ-PVXQ. [DOI] [PubMed] [Google Scholar]
- 20.Brady KT, Sinha R. Co-occurring mental and substance use disorders: The neurobiologicial effects of chronic stress. Am J Psychiatry. 2005;162:233–41. doi: 10.1176/appi.ajp.162.8.1483. [DOI] [PubMed] [Google Scholar]
- 21.Golub ET, Latka M, Hagan H, et al. STRIVE Project Screening for depressive symptoms among HCV-infected injection drug users: Examination of the utility of CES-D and the Beck Depression Inventory. J Urban Health. 2004;81:278–90. doi: 10.1093/jurban/jth114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brooner RK, King VL, Kidorf M, Schmidt CW, Bigelow GE. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54:71–80. doi: 10.1001/archpsyc.1997.01830130077015. [DOI] [PubMed] [Google Scholar]
- 23.Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse: Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–8. [PubMed] [Google Scholar]
- 24.Kandel DB, Huang FY, Davies M. Comorbidity between patterns of substance use dependence and psychiatric syndromes. Drug Alcohol Depend. 2001;64:233–41. doi: 10.1016/s0376-8716(01)00126-0. [DOI] [PubMed] [Google Scholar]
- 25.Sockalingam S, Shammi C, Stergiopoulos V. Managing the neuropsychatirc complications of Hepatitis C treatment. Br J Hosp Med. 2007;68:520–5. doi: 10.12968/hmed.2007.68.10.27321. [DOI] [PubMed] [Google Scholar]
- 26.Patten SB. Psychiatric side effects of interferon treatment. Curr Drug Saf. 2006;1:143–50. doi: 10.2174/157488606776930562. [DOI] [PubMed] [Google Scholar]
- 27.Dieperink E, Willenbring M, Ho SB. Neuropsychatric symptoms associated with Hepatitis C and interferon alpha: A review. Am J Psychiatry. 2000;157:867–75. doi: 10.1176/appi.ajp.157.6.867. [DOI] [PubMed] [Google Scholar]
- 28.Loftis JM, Matthews AM, Hauser P. Psychiatric and substance abuse disorders in individuals with Hepatitis C: Epidemiology and management. Drugs. 2006;66:155–74. doi: 10.2165/00003495-200666020-00003. [DOI] [PubMed] [Google Scholar]
- 29.Schaefer M, Schmidt F, Folwaczny C, et al. Adherence and mental side effects during hepatitis C treatment with interferon alfa and ribavirin in psychiatric risk groups. Hepatology. 2003;37:443–51. doi: 10.1053/jhep.2003.50031. [DOI] [PubMed] [Google Scholar]
- 30.Dore GJ. Enhancing hepatitis C treatment uptake and outcomes for injection drug users. Hepatology. 2007;45:3–5. doi: 10.1002/hep.21478. [DOI] [PubMed] [Google Scholar]
- 31.Fireman M, Indest DW, Blackwell A, Whitehead AJ, Hauser P. Addressing tri-morbidity (hepatitis C, psychiatric disorders, and substance use): The importance of routine mental health screening as a component of a comanagement model of care. Clin Infect Dis. 2005;40(Suppl 5):S286–S291. doi: 10.1086/427442. [DOI] [PubMed] [Google Scholar]
- 32.Guadagnino V, Trotta MP, Montesano F, et al. Nocchiero Study Group Effectiveness of a multi-disciplinary standardized management model in the treatment of chronic hepatitis C in drug addicts engaged in detoxification programmes. Addiction. 2007;102:423–31. doi: 10.1111/j.1360-0443.2006.01698.x. [DOI] [PubMed] [Google Scholar]
- 33.Moriarty H, Robinson G. Hepatitis services at an injecting drug user outreach clinic. N Z Med J. 2001;114:105–6. [PubMed] [Google Scholar]
- 34.Ewart A, Harrison L, Joyner B, Safe A. Providing treatment for hepatitis C in an Australian district centre. Postgrad Med J. 2004;80:180–2. doi: 10.1136/pgmj.2003.005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grebely J, Genoway K, Khara M, et al. Treatment uptake and outcomes among current and former injection drug users receiving directly observed therapy within a multidisciplinary group model for the treatment of hepatitis C virus infection. Int J Drug Policy. 2007;18:437–43. doi: 10.1016/j.drugpo.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Sylvestre DL, Zweben JE. Integrating HCV services for drug users: A model to improve engagement and outcomes. Int J Drug Policy. 2007;18:406–10. doi: 10.1016/j.drugpo.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Jack K, Willott S, Manners J, Varnam MA, Thomson BJ. Clinical trials: A primary care-based model for the delivery of anti-viral treatment to injecting drug users infected with hepatitis C. Aliment Pharmacol Ther. 2008;29:38–45. doi: 10.1111/j.1365-2036.2008.03872.x. [DOI] [PubMed] [Google Scholar]
- 38.Wilkinson M, Crawford V, Tippet A, et al. Community-based treatment for chronic hepatitis C in drug users: High rates of compliance with therapy despite ongoing drug use. Aliment Pharmacol Ther. 2008;29:29–37. doi: 10.1111/j.1365-2036.2008.03834.x. [DOI] [PubMed] [Google Scholar]
- 39.Nazareth S, Piercey C, Tibbet P, Cheng W. Innovative practice in the management of chronic hepatitis C: Introducing the nurse practitioner model. Aust J Adv Nurs. 2008;25:107–13. [Google Scholar]
- 40.Statistics Canada Census 2006 < www12.statcan.ca/census-recensement/2006/dp-pd/prof/92-591/details/Page.cfm?Lang=E&Geo1=CSD&Code1=3510010&Geo2=PR&Code2=35&Data=Count&SearchText=kingston&SearchType=Begins&SearchPR=01&B1=All&Custom=> (Accessed March 23, 2009).
- 41.Newman A, Beckstead S, Finch S, et al. Hepatitis C treatment of a marginalized population: A multi-disciplinary primary practitioner-led treatment model in practice. Can J Gastroenterology. 2009;23:85A. [Google Scholar]
- 42.Cooper CL, Mills EJ. Therapeutic challenges in hepatitis C-infected injection drug using patients. Harm Reduct J. 2007;3:31. doi: 10.1186/1477-7517-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grebely J, Conway B, Raffa JD, Lai C, Krajden M, Tyndall MW. Hepatitis C virus reinfection in injection drug users. Hepatology. 2006;44:1139–45. doi: 10.1002/hep.21376. [DOI] [PubMed] [Google Scholar]
- 44.Scheft H, Fontenette DC. Psychiatric barriers to readiness for treatment for hepatitis C virus infection among injection drug users: Clinical experience of an addiction psychiatrist in the HIV-HCV coinfection clinic of a public health hospital. Clin Infect Dis. 2005;40:S292–6. doi: 10.1086/427443. [DOI] [PubMed] [Google Scholar]
- 45.Hagan H, Latka MH, Campbell JC, et al. Eligibility for treatment of hepatitis C virus infection among young injection drug users in 3 US cities. Clin Infect Dis. 2006;42:669–72. doi: 10.1086/499951. [DOI] [PubMed] [Google Scholar]
- 46.Ebner N, Wanner C, Winklbaur B, et al. Retention rate and side effects in a prospective trial on hepatitis C treatment with pegylated interferon alpha-2a and ribavirin in opioid-dependent patients. Addict Bio. 2009;14:227–37. doi: 10.1111/j.1369-1600.2009.00148.x. [DOI] [PubMed] [Google Scholar]


