Abstract
BACKGROUND:
Differences between American (United States [US]) and European guidelines for colonoscopy surveillance may create confusion for the practicing clinician. Under- or overutilization of surveillance colonoscopy can impact patient care.
METHODS:
The Canadian Association of Gastroenterology (CAG) convened a working group (CAG-WG) to review available guidelines and provide unified guidance to Canadian clinicians regarding appropriate follow-up for colorectal cancer (CRC) surveillance after index colonoscopy. A literature search was conducted for relevant data that postdated the published guidelines.
RESULTS:
The CAG-WG chose the 2012 US Multi-Society Task Force (MSTF) on Colorectal Cancer to serve as the basis for the Canadian position, primarily because the US approach was the simplest and comprehensively addressed the issue of serrated polyps. Aspects of other guidelines were incorporated where relevant. The CAG-WG recommendations differed from the US MSTF guidelines in three main areas: patients with negative index colonoscopy should be followed-up at 10 years using any of the appropriate screening tests, including colonos-copy, for average-risk individuals; among patients with >10 adenomas, a one-year interval for subsequent colonoscopy is recommended; and for long-term follow-up, patients with low-risk adenomas on both the index and first follow-up procedures can undergo second follow-up colonos-copy at an interval of five to 10 years.
DISCUSSION:
The CAG-WG adapted the US MSTF guidelines for colonoscopy surveillance to the Canadian health care environment with a few modifications. It is anticipated that the present article will provide unified guidance that will enhance physician acceptance and encourage appropriate utilization of recommended surveillance intervals.
Keywords: Canada, Colonoscopy, Colorectal cancer, Guideline, Surveillance
Abstract
HISTORIQUE :
Les différences entre les lignes directrices américaines et européennes sur la surveillance de la coloscopie peuvent susciter la confusion chez les cliniciens praticiens. La sous-utilisation ou la surutili-sation de la coloscopie de surveillance peut avoir une incidence sur les soins aux patients.
MÉTHODOLOGIE :
L’Association canadienne de gastroentérologie (ACG) a formé un groupe de travail (GT-ACG) pour examiner les lignes directrices disponibles et fournir des conseils unifiés aux cliniciens canadiens au sujet du suivi pertinent de la surveillance du cancer colorec-tal après une coloscopie de référence. Les chercheurs ont effectué une analyse bibliographique pour trouver des données pertinentes publiées après la diffusion des lignes directrices.
RÉSULTATS :
Le GT-ACG a sélectionné le Multi-Society Task Force (MSTF) on Colorectal Cancer de 2012 aux États-Unis comme base des principes canadiens, principalement parce que l’approche américaine était la plus simple et traitait de fond en comble de la question des polypes dentelés. Il y a intégré des aspects des autres lignes directrices lorsqu’ils étaient pertinents. Les recommandations du GT-ACG dif-féraient de celles des lignes directrices du MSTF des États-Unis dans trois grands secteurs : les patients dont la coloscopie de référence était négative devraient subir un suivi au bout de dix ans au moyen de l’un des tests de dépistage pertinents, y compris la coloscopie, lorsque leur risque correspond à la moyenne. Il est recommandé de faire subir une nouvelle coloscopie aux patients ayant plus de dix adénomes. Pour ce qui est du suivi à long terme, les patients ayant des adénomes à faible risque au moment de la coloscopie de référence et de la première intervention de suivi peuvent subir une deuxième coloscopie de suivi au bout de cinq à dix ans.
EXPOSÉ :
Le GT-ACG a adapté les lignes directrices du MSTF des États-Unis sur la surveillance de la coloscopie au milieu de la santé cana-dien en y apportant quelques modifications. On prévoit que le présent article fournisse des conseils unifiés qui amélioreront l’acceptation des médecins et favoriseront l’utilisation pertinente des intervalles de surveillance recommandés.
Colonoscopy is an integral part of gastroenterology practice. While a normal examination does not preclude the development of subsequent premalignant or malignant lesions, detection of premalig-nant polyps or cancer should result in the patient entering a surveillance program. The timing of follow-up colonoscopy is an important issue because follow-up intervals that are too long may reduce the rate of cancer prevention, and those that are too short may expose patients to colonoscopy risks without potential benefit and are an ineffective use of available limited resources, which may negatively impact wait times for colonoscopy (1).
Results of the Survey of Access to GastroEnterology program, conducted on an ongoing basis by the Canadian Association of Gastroenterology (CAG) (2–4), continues to demonstrate that wait times for colonoscopy in Canada continue to exceed recommended consensus targets. This underscores the need for Canadian-specific guidance for appropriate surveillance based on findings at the index colonoscopy. In addition to the issue of appropriate use of endoscopic resources, it was believed to be important to incorporate guidelines for follow-up after removal of different types of serrated polyps – a topic not comprehensively addressed previously.
Current guidelines for colonoscopy surveillance (5–7) differ in some aspects, which may create confusion for the practicing clinician. To provide clarity, the CAG organized a working group of Canadian experts to review the published guidelines from the United States Multi-Society Task Force (US MSTF) on Colorectal Cancer (5), the British Society of Gastroenterology (BSG) (6) and the European Commission (7). The goal was to provide unified guidance to clinicians regarding appropriate follow-up for colorectal cancer (CRC) surveillance after index colonoscopy in the Canadian setting. Consideration was given to embarking on a formal guideline development process; however, it was believed that this would be resource intensive and not add significantly to the existing literature.
METHODS
In September 2012, CAG convened a working group of gastroenterolo-gists with expertise in the delivery of health care related to CRC surveillance to review the US MSTF (published in 2012 [5]), and the BSG (6) and European Union (published in 2010 [7]) guidelines, to provide guidance for Canadian clinicians. In addition, a literature search was conducted from May 2008 to June 2012 to ensure that there were no relevant data that postdated the published guidelines. Key words used in the search included: “colonoscopy”, “colorectal neoplasms/epidemiology”, “neoplasm recurrence, local”, “polyps”, “adenomatous polyps/surgery”, “polyps/surgery”, “occult blood”, “colorectal”, “colonic polyps, neoplasm, tumour or cancer” and “colorectal neoplasm, tumour or cancer”. The search yielded 234 citations, of which 43 were deemed relevant and reviewed further; however, no new data that impacted recommendations for surveillance intervals were found.
Using the 2012 US MSTF guidelines (5) as a base, the working group reviewed and discussed each specific recommendation, compared them with the European and BSG recommendations, and determined whether the recommendation was compatible with previous guidance given to Canadian practitioners on average-risk screening and follow-up (8). If a change was found to be warranted, the recommendation was modified based on the other published guidelines (6,7) and current data relevant to Canadian patients. The present article does not reflect an attempt to create entirely new guidelines but rather seeks to provide unified guidance from current published guidelines that will be relevant to the Canadian CRC surveillance setting.
RECOMMENDATIONS
The 2012 US MSTF guidelines (5) were chosen over those from Europe (6,7) to serve as the basis for the Canadian position primarily because the US approach was less complex and addressed the issue of serrated polyps more comprehensively. It is hoped that simpler guidelines will enhance physician acceptance and utilization of recommended surveillance intervals.
Both the BSG (6) and European recommendations (7) classified patients into one of three groups. The BSG did not include histology in their risk stratification, but were otherwise very similar to the European recommendations. The European guidelines stratified patients into three groups based on adenoma number, size and histology (low risk: one to two adenomas <10 mm in size; intermediate risk: three to four adenomas <10 mm or one ≥10 mm and <20 mm or villous histology or high-grade dysplasia (HGD); and high risk: ≥5 adenomas or one ≥20 mm in size) (Table 1) (7). The US MSTF guidelines identified two adenoma risk groups: low-risk adenomas, one to two tubular adenomas <10 mm; and high-risk adenomas, adenoma with villous histology, HGD, ≥10 mm, or ≥3 adenomas and, in addition, addressed the issue of serrated lesions in detail (Table 1) (5). The US MSTF recommended follow-up intervals for first postindex colonoscopy, which are detailed in Table 2 according to findings at the index procedure. The European recommendations are shown in Appendix 1 (7).
TABLE 1.
United States Multi-Society Task Force (US MSTF) and European guidelines risk stratification criteria
| Risk | US MSTF guidelines risk groups (5) | European guidelines risk groups (7) |
|---|---|---|
| Low | 1–2 tubular adenomas <10 mm | 1–2 adenomas <10 mm |
| Intermediate | 3–4 adenomas <10 mm or 1 adenoma ≥10 mm and <20 mm or villous histology or high-grade dysplasia | |
| High | Adenoma with villous histology or high-grade dysplasia or ≥10 mm or ≥3 adenomas | ≥5 adenomas or ≥1 adenoma ≥20 mm |
TABLE 2.
United States Multi-Society Task Force recommendations for surveillance intervals in individuals with baseline average risk
While missed lesions at colonoscopy will always remain a concern, guidance cannot be based on a perpetual fear of missing a lesion. Similar to the US MSTF guidelines, the present Canadian guidance assumes a well-trained clinician performing a high-quality index colonoscopy according to previous published CAG quality indicators (9), including a thorough, complete examination of a properly prepared bowel in addition to an accurate pathological interpretation of any removed tissue.
If the index colonoscopy is normal and the patient is not at increased CRC risk for personal or familial reasons, then follow-up can occur as previously defined for individuals at average risk (8). Surveillance should use testing methods in accordance with recommendations outlined by the CAG (8), which include fecal immuno-chemical test, flexible sigmoidoscopy or colonoscopy, as appropriate.
The following is the CAG guidance for CRC surveillance after an index colonoscopy detecting ≥1 polyp(s). It results from the modifications to the US MSTF recommendations (5) as suggested by the working group.
Effect of positive family history on surveillance intervals
Patients with a history of CRC in a first-degree relative (FDR) are at a higher risk of adenoma (10,11). Although the age of the affected FDR is important in estimating risk, unfortunately, there is no agreement as to what age warrants more frequent screening. The BSG guidelines suggest a cut-off age of 50 years for the FDR (6); the US MSTF guidelines use 60 years (5). The working group agreed with the US MSTF guidelines, that for patients with a family history of CRC in an FDR <60 years of age, low-risk findings with recommendations for 10-year surveillance intervals should be shortened to five years and colonos-copy should be the method used (Table 2). All other surveillance intervals were unchanged.
The CAG has previously published recommendations for individuals at increased risk (12). Among patients with both a personal history of an adenoma and a positive family history, the dominant recommendation is the one that produces the shortest surveillance interval.
No adenomas or polyps, OR no adenomas; distal small (<10 mm) hyperplastic polyps
The CAG working group agreed with the US MSTF recommendation of a 10-year interval for follow-up surveillance in patients with negative findings or distal (sigmoid or rectum) hyperplastic polyps <10 mm in size. However, the CAG recommendation differs in that colonoscopy represents only one of the options that can be used for follow-up. At the 10-year point, it is recommended that these patients re-enter the screening pool and resume screening using an appropriate test for average-risk individuals according to local resource availability.
One to two tubular adenomas <10 mm in size
While the CAG working group believed that setting the interval as a range (five to 10 years) can be less helpful than a precise value, they agreed with this recommendation from the US MSTF. The group recognized that clinicians may want to individualize the surveillance interval based on adenoma size, family history and patient preference. There are data suggesting that 10 years may be appropriate for most individuals (13,14).
Three to 10 adenomas
The CAG working group agreed with the US MSTF recommendation of follow-up at three years for patients whose index colonoscopy reveals three to 10 adenomas. In addition, it was agreed that splitting this category into ‘intermediate’ and ‘high’, as per the European guidelines (Appendix 1) (7), was not warranted based on the available evidence.
>10 adenomas
The CAG working group believed that the US MSTF guidelines recommended interval of <3 years (5) was too vague for this patient group and, therefore, agreed with the European recommendation of one year for subsequent screening (7). The group believed that in the clinical practice setting, even after a high-quality examination, it is likely that the chances of having missed an adenoma would be higher in patients with this degree of multiple adenomas. In addition, this is a relatively infrequent finding among patients undergoing screening colonoscopy and, therefore, the more frequent interval of one year would add minimal burden to colonoscopy resources compared with a three-year interval.
≥ 1 tubular adenoma(s) ≥10 mm; OR ≥1 adenoma(s) with villous features of any size; OR ≥1 adenoma(s) with HGD
The CAG working group agreed with the US MSTF recommended interval of three years (5) for a patient with any of these findings (Table 2). If polyps are very large or removed piecemeal, this interval for follow-up may need to be shortened. In addition, this assumes the entire polyp has been removed.
Serrated polyps
Serrated lesions exhibit a distinct endoscopic appearance, and may be more difficult to detect than conventional adenomatous polyps (5,15). There are several classifications, one of which, the Canadian Partnership Against Cancer (CPAC) Pan-Canadian consensus guideline criteria, is shown in (Table 3) (5,15,16). Subtypes of serrated lesions, the sessile serrated adenoma/polyps (SSA/P) and the traditional serrated adenomas, may be associated with up to one-third of CRCs (15). The CPAC classification includes a category of serrated polyp – unclassified. The US MSTF does not make specific recommendations for this category; therefore, the CAG working group recommend treating these as SSA/P pending further information.
TABLE 3.
Classification of colorectal serrated lesions according to Canadian Paternership Against Cancer (CPAC) Pan-Canadian consensus guidelines
| CPAC classification (22) | Polyp type (22) | Qualification regarding dysplasia (22) | Prevalence (5,15) | Distribution (5,15) |
|---|---|---|---|---|
| Hyperplastic polyps | Very common | Mostly distal | ||
| Serrated adenomas/polyps | SSA/P | ± dysplasia (low-/high-grade) | Common | Mostly proximal |
| TSA | ± high-grade dysplasia | Rare | Mostly distal | |
| Serrated polyp, unclassified* |
The WHO definition of serrated polyposis syndrome (SPS) represents any one of the following: at least five serrated polyps proximal to sigmoid, with two or more ≥10 mm in size; any serrated polyps proximal to sigmoid with family history of SPS; and >20 serrated polyps of any size throughout the colon (5,15,16). The CAG working group agreed with the US MSTF recommended surveillance interval of one year for SPS (5).
Surveillance intervals for serrated polyps not associated with SPS are based on lesion size, the presence of dysplasia and serrated histology (Table 1). The CAG working group agreed with the US MSTF recommended follow-up intervals of five years for SSA/P <10 mm in size with no dysplasia, and three years for larger (≥10 mm) or dysplastic SSA/P and traditional serrated adenomas (5). In addition, the CAG working group has used the nomenclature from the US MSTF. One area of difficulty is that many proximal polyps, formerly classified as hyperplastic may, in fact, be serrated adenomas (17). A close working relationship with pathology is required.
Timing of subsequent surveillance
Subsequent follow-up intervals based on the findings at the index and follow-up colonoscopies are shown in Table 4 for the US MSTF and Appendix 1 for the European Union recommendations. The CAG recommendations are largely unchanged from those provided by the US MSTF (5), with the exception that for patients with low-risk adenomas on both the index and the follow-up procedure, an interval range of five to 10 years for a second follow-up is advised, whereas the US MSTF specifically recommended a five-year interval (5). The CAG working group believed that for patients with persistent low-risk findings, there was no evidence that the interval should be shortened.
TABLE 4.
United States Multi-Society Task Force recommendations for polyp surveillance after first surveillance colonoscopy
| Findings at index colonoscopy | Findings at first surveillance | Recommended interval for second surveillance, years |
|---|---|---|
| Low-risk adenoma | High-risk adenoma | 3 |
| Low-risk adenoma | 5* | |
| No adenoma | 10 | |
| High-risk adenoma | High-risk adenoma | 3 |
| Low-risk adenoma | 5 | |
| No adenoma | 5† |
Reprinted from reference 5 with permission from Elsevier.
The Canadian Association of Gastroenterology working group suggested that an interval of five to 10 years would be sufficient;
If the findings on the second surveillance are negative, there is insufficient evidence to make a recommendation
Other issues
Stopping surveillance:
The CAG Task Force agreed with the US MSTF that the decision to continue colonoscopy surveillance in older patients (75 to 85 years of age) should weigh the benefits of CRC detection or prevention given the reduced years of life expectancy against the increased risk of complications from the procedure (5). The risk of adverse gastrointestinal events (bleeding and perforation) after colonoscopy increases with age and comorbid conditions (18). Therefore, individual risk stratification should consider previous colonoscopy findings, comorbidities and patient life expectancy.
Poor bowel preparation:
The guidance provided in the present article is premised on colonoscopy being conducted according to published quality indicators (9), including adequate bowel preparation. However, poor-quality bowel preparation does occur and can lead to missed lesions. The CAG working group agreed with the US MSTF guidelines based on the adequacy of bowel preparation (5). If the preparation is very poor, then colonoscopy should be repeated as soon as possible (within one year); however, if it is fair to adequate (able to detect lesions <5 mm in size), then surveillance can proceed as recommended based on findings.
It is suggested that centres monitor the rate of inadequate colonos-copy preparation to ensure it is consistent with national averages, and that this is not being used as a rationale for shorter follow-up intervals for surveillance colonoscopy.
Positive fecal occult blood test (FOBT) result:
It is not recommended to perform repeat FOBT testing before the recommended surveillance interval. The CAG working group recommended that the surveillance interval, based on findings at index colonoscopy, should be adhered to regardless of a positive FOBT result, which led to the index colonoscopy. Fecal occult blood can be the result of other factors, such as hemorrhoidal bleeding, and patients with a negative colonoscopy remain at very low risk of CRC; the benefits of resuming FOBT testing at <10 years in those with an initial positive FOBT and subsequent normal good quality colonoscopy are likely very small.
Symptomatic patients:
Patients who develop symptoms before scheduled surveillance should be reassessed and the need for colonoscopy should be determined on an individual basis.
Risk factors for CRC (lifestyle, race, ethnicity or sex):
The CAG working group agreed with the US MSTF finding that there is currently insufficient evidence to require any changes to the colonoscopy surveillance intervals based on these factors (5).
DISCUSSION
Compliance with colonoscopy surveillance guidelines in Canada is suboptimal (1), and the fact that there are numerous and varying international guidelines can create confusion and may negatively impact compliance. There is a need for clear guidance for the Canadian clinician, as well as strategies to raise awareness and increase adherence.
Current data reveal substantial inappropriate use of colonoscopy services in Canada (1), the US (19) and Europe (20,21). In a survey of Canadian gastroenterologists (1), up to 60% of respondents chose surveillance intervals that were too short for some clinical scenarios, while up to 75% chose intervals that were too long for other scenarios. In addition, although respondents stated that they were following guideline recommendations, the surveillance interval was often incorrect, suggesting inadequate awareness of the guidelines. Surveys conducted in the US (19) and Europe (20,21) have demonstrated similar results.
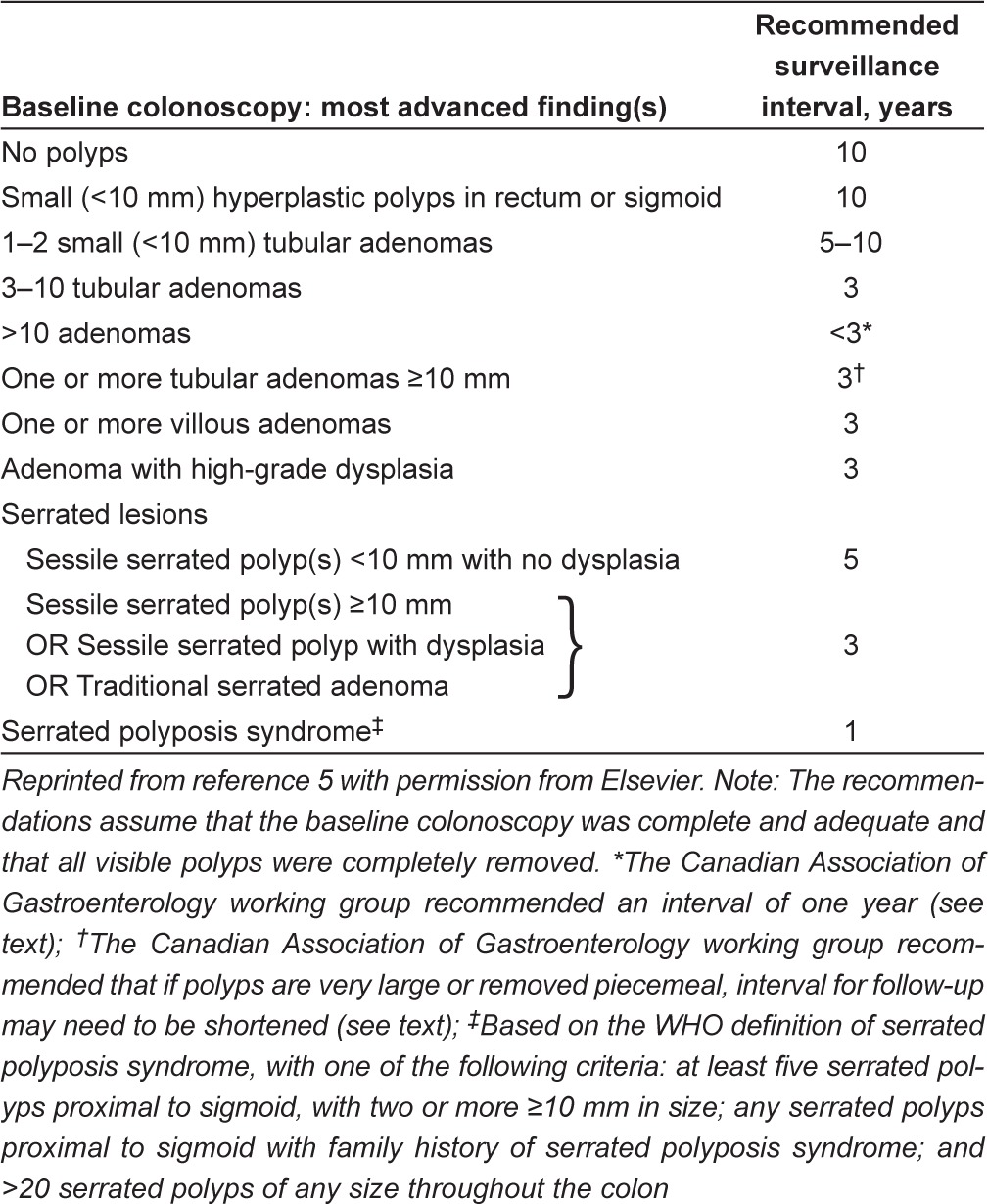
The present article is designed to provide unified guidance on colon-oscopy surveillance intervals for the prevention and detection of CRC from current published guidelines that will be relevant to the Canadian CRC surveillance setting (summarized in Tables 5 and 6). This guidance assumes a well-trained clinician performing a high-quality index colon-oscopy according to CAG quality indicators. The CAG working group recommends that all provincial screening programs and centres conduct quality monitoring programs and prospectively collect data on adherence to recommended surveillance intervals.
TABLE 5.
Summary of Canadian Association of Gastroenterology working group modifications to United States Multi-Society Task Force recommendations for surveillance intervals in individuals with baseline average risk*
| Baseline colonoscopy: most advanced finding(s) | Recommended surveillance interval, years |
|---|---|
| No polyps | 10* |
| Small (<10 mm) hyperplastic polyps in rectum or sigmoid | 10* |
| 1–2 small (<10 mm) tubular adenomas | 5–10* |
| 3–10 tubular adenomas | 3 |
| >10 adenomas | 1† |
| One or more tubular adenomas ≥10 mm | 3‡ |
| One or more villous adenomas | 3 |
| Adenoma with HGD | 3 |
| Serrated lesions | |
| Sessile serrated polyp(s) <10 mm with no dysplasia | 5 |
| Sessile serrated polyp(s) ≥10 mm | 3 |
| OR sessile serrated polyp with dysplasia | |
| OR traditional serrated adenoma | |
| Serrated polyposis syndrome§ | 1 |
Modified and reprinted from reference 5 with permission from Elsevier. Note: The recommendations assume that the baseline colonoscopy was complete and adequate, and that all visible polyps were completely removed.
In patients with a positive family history in a first-degree relative <60 years of age or in ≥2 first-degree relatives of any age, 10-year surveillance interval should be shortened to five years and colonoscopy should be the method used;
Change from United States Multi-Society Task Force recommendations;
If polyps are very large or removed piecemeal, interval for follow-up may need to be shortened;
Based on the WHO definition of serrated polyposis syndrome, with one of the following criteria: at least five serrated polyps proximal to sigmoid, with two or more ≥10 mm in size; any serrated polyps proximal to sigmoid with family history of serrated polyposis syndrome; and >20 serrated polyps of any size throughout the colon.
TABLE 6.
Summary of Canadian Association of Gastroenterology working group modifications to United States Multi-Society Task Force recommendations for polyp surveillance after first surveillance colonoscopy
| Findings at index colonoscopy | Findings at first surveillance | Recommended interval for second surveillance, years |
|---|---|---|
| Low-risk adenoma | High-risk adenoma | 3 |
| Low-risk adenoma | 5–10* | |
| No adenoma | 10 | |
| High-risk adenoma | High-risk adenoma | 3 |
| Low-risk adenoma | 5 | |
| No adenoma | 5† |
Modified and reprinted from reference 5 with permission from Elsevier.
Change from United States Multi-Society Task Force recommendation;
If the findings on the second surveillance are negative, there is insufficient evidence to make a recommendation
Acknowledgments
The authors thank Pauline Lavigne and Steven Portelance for editorial assistance.
Appendix 1).
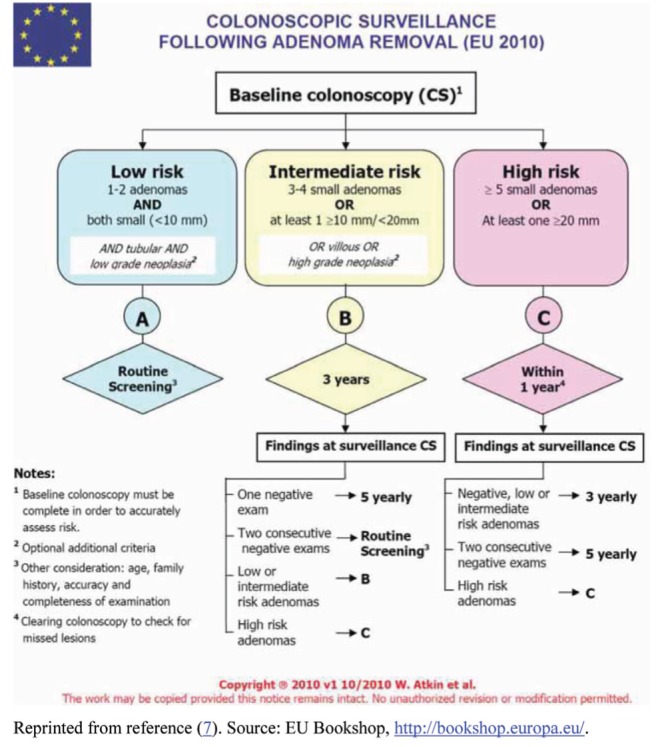
European Union (EU) recommended surveillance following adenoma removal. Reprinted from reference 7. Source: EU Bookshop, http://bookshop.europa.eu/
REFERENCES
- 1.van Kooten H, de Jonge V, Schreuders E, et al. Awareness of postpolypectomy surveillance guidelines: A nationwide survey of colonoscopists in Canada. Can J Gastroenterol. 2012;26:79–84. doi: 10.1155/2012/919615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leddin D, Armstrong D, Barkun AN, et al. Access to specialist gastroenterology care in Canada: Comparison of wait times and consensus targets. Can J Gastroenterol. 2008;22:161–7. doi: 10.1155/2008/479684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leddin D, Bridges RJ, Morgan DG, et al. Survey of access to gastroenterology in Canada: The SAGE wait times program. Can J Gastroenterol. 2010;24:20–5. doi: 10.1155/2010/246492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leddin D, Armstrong D, Borgaonkar M, et al. The 2012 SAGE wait times program: Survey of access to gastroenterology in Canada. Can J Gastroenterol. 2013;27:83–9. doi: 10.1155/2013/143018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–57. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002) Gut. 2010;59:666–89. doi: 10.1136/gut.2009.179804. [DOI] [PubMed] [Google Scholar]
- 7.Segnan N, Patnick J, von Karsa L. European guidelines for quality assurance in colorectal cancer screening and diagnosis. Office for official publications of the European Communities. < http://bookshop.europa.eu/en/european-guidelines-for-quality-assurance-in-colorectal-cancer-screening-and-diagnosis-pbND3210390/> (Accessed January 25, 2013). [DOI] [PubMed]
- 8.Leddin DJ, Enns R, Hilsden R, et al. Canadian Association of Gastroenterology position statement on screening individuals at average risk for developing colorectal cancer: 2010. Can J Gastroenterol. 2010;24:705–14. doi: 10.1155/2010/683171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong D, Barkun A, Bridges R, et al. Canadian Association of Gastroenterology consensus guidelines on safety and quality indicators in endoscopy. Can J Gastroenterol. 2012;26:17–31. doi: 10.1155/2012/173739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilschut JA, Habbema JD, Ramsey SD, et al. Increased risk of adenomas in individuals with a family history of colorectal cancer: Results of a meta-analysis. Cancer Causes Control. 2010;21:2287–93. doi: 10.1007/s10552-010-9654-y. [DOI] [PubMed] [Google Scholar]
- 11.Imperiale TF, Ransohoff DF. Risk for colorectal cancer in persons with a family history of adenomatous polyps: A systematic review. Ann Intern Med. 2012;156:703–9. doi: 10.7326/0003-4819-156-10-201205150-00006. [DOI] [PubMed] [Google Scholar]
- 12.Leddin D, Hunt R, Champion M, et al. Canadian Association of Gastroenterology and the Canadian Digestive Health Foundation: Guidelines on colon cancer screening. Can J Gastroenterol. 2004;18:93–9. doi: 10.1155/2004/983459. [DOI] [PubMed] [Google Scholar]
- 13.Brenner H, Haug U, Arndt V, et al. Low risk of colorectal cancer and advanced adenomas more than 10 years after negative colonoscopy. Gastroenterology. 2010;138:870–6. doi: 10.1053/j.gastro.2009.10.054. [DOI] [PubMed] [Google Scholar]
- 14.Brenner H, Chang-Claude J, Seiler CM, Hoffmeister M. Long-term risk of colorectal cancer after negative colonoscopy. J Clin Oncol. 2011;29:3761–7. doi: 10.1200/JCO.2011.35.9307. [DOI] [PubMed] [Google Scholar]
- 15.Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: Review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315–29. doi: 10.1038/ajg.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snover D, Ahnen D, Burt R, Odze R. Serrated polyps of the colon and rectum and serrated polyposis. In: Bosman F, Carneiro F, Hruban R, et al., editors. WHO Classification of tumours of the digestive system. Lyon: IARC; 2010. [Google Scholar]
- 17.Singh H, Bay D, Ip S, et al. Pathological reassessment of hyperplastic colon polyps in a city-wide pathology practice: Implications for polyp surveillance recommendations. Gastrointest Endosc. 2012;76:1003–8. doi: 10.1016/j.gie.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 18.Warren JL, Klabunde CN, Mariotto AB, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009;150:849–57. W152. doi: 10.7326/0003-4819-150-12-200906160-00008. [DOI] [PubMed] [Google Scholar]
- 19.Saini SD, Nayak RS, Kuhn L, Schoenfeld P. Why don’t gastroenterologists follow colon polyp surveillance guidelines?: Results of a national survey. J Clin Gastroenterol. 2009;43:554–8. doi: 10.1097/MCG.0b013e31818242ad. [DOI] [PubMed] [Google Scholar]
- 20.John BJ, Irukulla S, Pilgrim G, Swift I, Abulafi AM. Surveillance colonoscopies for colorectal polyps – too often, too many! An audit at a large district general hospital. Colorectal Dis. 2008;10:898–900. doi: 10.1111/j.1463-1318.2008.01516.x. [DOI] [PubMed] [Google Scholar]
- 21.O’Connor A, Keane RA, Egan B, et al. Adherence to colorectal polyp surveillance guidelines: Is there a ‘scope’ to increase the opportunities for screening? Eur J Cancer Prev. 2011;20:40–5. doi: 10.1097/CEJ.0b013e32833ecc5f. [DOI] [PubMed] [Google Scholar]
- 22.Driman D, Marcus V, Hilsden R, Owen D. Pathological reporting of colorectal polyps: Pan-Canadian consensus guidelines. Can J Pathology. 2012;4:81–90. [Google Scholar]


