Abstract
Rationale
Rapid-response impulsivity, characterized by inability to withhold response to a stimulus until it is adequately appraised, is associated with risky behavior and may be increased in a state-dependent manner by norepinephrine.
Objective
We assessed effects of yohimbine, which increases norepinephrine release by blocking alpha-2 noradrenergic receptors, on plasma catecholamine metabolites, blood pressure, subjective symptoms, and laboratory-measured rapid-response impulsivity.
Methods
Subjects were twenty-three healthy controls recruited from the community, with normal physical examination and ECG, and negative history for hypertension, cardiovascular illness, and Axis I or II disorder. Blood pressure, pulse, and behavioral measures were obtained before and periodically after 0.4 mg/kg oral yohimbine or placebo in a randomized, counterbalanced design. Metabolites of norepinephrine (3-methoxy-4-hydroxyphenylglycol, MHPG; vanillylmandelic acid, VMA) and dopamine (homovanillic acid, HVA) were measured by high pressure liquid chromatography with electrochemical detection. Rapid-response impulsivity was measured by commission errors and reaction times on the Immediate Memory Task (IMT), a continuous performance test designed to measure impulsivity and attention.
Results
Yohimbine increased plasma MHPG and VMA but not HVA. Yohimbine increased systolic and diastolic blood pressure and pulse rate. On the IMT, yohimbine increased impulsive errors and impulsive response bias and accelerated reaction times. Yohimbine-associated increase in plasma MHPG correlated with increased impulsive response rates. Time courses varied; effects on blood pressure generally preceded those on metabolites and test performance.
Conclusions
These effects are consistent with increased rapid-response impulsivity after pharmacological noradrenergic stimulation in healthy controls. Labile noradrenergic responses, or increased sensitivity to norepinephrine, may increase risk for impulsive behavior.
Keywords: Norepinephrine, blood pressure, continuous performance test, impulsive behavior, yohimbine, receptors, noradrenergic, alpha
Introduction
Impulsivity, a pattern of inability to conform action to its context or consequences, is prominent in affective, substance-use, and personality disorders (Moeller et al. 2001). Susceptibility to state-dependent changes in impulsive behavior may have consequences including substance use, and suicidal or aggressive behavior. Yet, while impulsivity as a trait characteristic has been extensively investigated, less is known about potential causes of state-dependent changes in expression of impulsivity. Mechanisms of immediate, or state-dependent, risk for impulsive behavior can identify markers of risk and targets for treatment aimed at reducing likelihood or severity of episodic increase in impulsive behaviors. In this paper we explored effects of norepinephrine on changes in impulsive responses on laboratory tasks.
Impulsivity is complex and includes trait-like impulsivity measured by questionnaires and more state-dependent laboratory measures of inability to fully appraise a stimulus before responding (rapid-response impulsivity), or inability to withhold response for a delayed larger reward over a smaller immediate one (reward-delay impulsivity) (Dougherty et al. 2003a; Swann et al. 2002). Compared to questionnaire measures of trait impulsivity, responses on laboratory measures have advantages of being pharmacologically modifiable, relatively unaffected by recall or other biases, and having animal analogs for translational studies.
Rapid-response impulsivity can be measured by commission errors on continuous performance tests (CPT) while controlling for effects on attention, which could also affect commission errors (Dougherty et al. 2003a; Swann et al. 2002). Increased commission (impulsive) errors are associated with many aspects of impulsive and risky behavior, and are increased in disruptive behavior disorders (Dougherty et al. 2003a), alcohol-use disorders (Bjork et al. 2004), and patients with bipolar disorder (Malloy-Diniz et al. 2011) and their siblings (Doyle et al. 2009). Further, commission errors are increased in subjects with history of severe suicidal behavior, both with (Swann et al. 2005b) and without (Dougherty et al. 2004) histories of bipolar disorder. Increased commission errors appear to be familial (Dougherty et al. 2003b) and associated with stress (Schepis et al. 2011). Therefore, CPT commission errors are a robust measure of risk for impulsive behavior, with potential value in exploring its mechanisms.
Impulsivity results from imbalance between mechanisms that facilitate and inhibit the initiation of action (Fineberg et al. 2010). Table 1 summarizes effects of noradrenergic manipulations on animal models of impulsivity and related phenomena. The most consistent effects of NE appear to be on rapid-response impulsivity, so we will focus on those effects.
Table 1.
Norepinephrine and Animal Models of Impulsivity-related Behavior
| Mechanism | Examples | Behavioral effect | Pharmacology | LC effect |
|---|---|---|---|---|
| NE Reuptake blockade | Atomoxetine (Bari and Aston-Jones 2013;Economidou et al. 2012;Paterson et al. 2012), reboxetine (Liu et al. 2009), nortriptyline (Roychowdhury et al. 2012) | Improve response- inhibition, working memory, decreased 5CSRT impulsivity but no effect on impulsive choice | Reversed by alpha-1, alpha-2, or beta-2 blockade | Decrease FR, reduce MHPG, decrease phasic/tonic NE release |
| Alpha-2 receptor stimulation | Guanfacine (Avery et al. 2000), clonidine (Birnbaum et al. 2000) | Improve attention and working memory; reverse stress-induced PFC dysfunction | Reduced by alpha-1 blockade (medial PFC) or beta-2 blockade (DL PFC) | Decrease FR, reduce MHPG, decrease tonic/phasic NE release |
| Alpha-2 receptor blockade | Yohimbine (Arnsten and Dudley 2005;Arnsten and Li 2005;Ma et al. 2005;Mantsch et al. 2010;Sun et al. 2010) | Increase stress- induced drug reinstatement and 5CSRT impulsivity; block beneficial effect of MPH | Reversed by beta-2 blockade or reduced CREB expression | Increase FR and MHPG; increase tonic/phasic release |
| Alpha-1 receptor stimulation | Phenylephrine (Arnsten et al. 1999;Birnbaum et al. 1999) | Impaired delayed alternation, working memory and attention | Reversed by alpha-1 blockade or lithium | |
| Acute stress | Experimental or pharmacological (Arnsten 2000;Mantsch et al. 2010;Otte et al. 2005) | Increase drug reinstatement, PFC impairment | Reversed by alpha-2 agonist (all effects), beta-2 antagonist (drug reinstatement), alpha-1 antagonist, resembles yohimbine | Increase MHPG |
Abbreviations: NE Norepinephrine, LC locus coeruleus, MHPG 3,methoxy,4-hydroxyphenylglycol, FR firing rate, PFC prefrontal cortex, DL Dorsolateral, 5CSRT 5-choice serial reaction time test, MPH methylphenidate
Depending on arousal and the degree of increase, norepinephrine (NE) can increase or decrease impulsive behavior (Berridge and Waterhouse 2003). For example, NE released in response to stress can impair inhibitory functions of the prefrontal cortex (Arnsten and Li 2005;Fitzgerald 2011). Accordingly, blockade of alpha-2 NE receptors, which increases NE release, has been shown to increase analogs of rapid-response impulsivity in rats (Arnsten and Li 2005;Sun et al. 2010), and in a preliminary study in humans (Swann et al. 2005a).
Presynaptic alpha-2 NE receptors are part of a negative feedback mechanism whereby synaptic NE inhibits NE release (Aghajanian 1978), potentially stabilizing CNS and peripheral autonomic NE function. Blockade of alpha-2 NE receptors increases NE release (Aghajanian 1978), concentration of NE or its metabolite 3-methoxy-4-hydroxyphenylglycol (MHPG) in plasma (Charney et al. 1982;Gurguis and Uhde 1990) and CSF (Peskind et al. 1989), and blood pressure (Charney et al. 1982). Behavioral effects in susceptible individuals can include transient anxiety, panic (Gurguis and Uhde 1990), or manic symptoms (Price et al. 1984).
Treatments that increase synaptic NE have varying, even contrasting, effects on overall NE activity and behavior. For example, blockade of NE reuptake increases synaptic NE, but firing rate and NE release are reduced by stimulation of presynaptic alpha-2 autoreceptors; this reduction in NE release is prevented by alpha-2 antagonists (Owen and Whitton 2003).
These results suggest that alpha-2 receptor blockade is a promising strategy for initial investigations of possible noradrenergic effects on impulsivity in humans. As noted in Table 1, NE appears to increase impulsivity-related behavior via several receptor subtypes. Blockade of alpha-2 receptors increases NE release in general, potentially stimulating both alpha-1 and beta-receptors. Further, turnover of NE, as measured by its stable metabolite MHPG, can provide an index of the effectiveness of alpha-2 blockade.
Blockade of alpha-2 heteroceptors can also increase release of dopamine, serotonin, or acetylcholine, but generally at higher doses than those increasing NE release (Kalsner and Abdali 2001). In addition, compounds that inhibit alpha-2 receptors, including yohimbine, can interact with other receptors, perhaps especially serotonergic. Behavioral studies of yohimbine should therefore measure some index of NE turnover, and must interpret the results in terms of known effects of other transmitter systems. With these caveats, alpha-2 noradrenergic blockade is an effective strategy to investigate noradrenergic effects on state-like transient changes in impulsive behavior in humans, with potential for translation between human and animal effects (Sun et al. 2010).
In a pilot study with eight subjects, we showed that yohimbine, an alpha-2 receptor antagonist which increases NE, could increase laboratory-measured impulsivity (Swann et al. 2005a). However, straightforward interpretation of data from that study was limited by 1) a single time point after yohimbine administration, making it difficult to compare maximal effects on measures that could have different time courses, 2) no measures of transmitter effects of yohimbine, which can increase release of dopamine (de Villiers et al. 1995), another potential mechanism for increased impulsivity (Fineberg et al. 2010), and 3) inadequate sample size for investigating correlations between effects of yohimbine on autonomic measures and behavior. Therefore, we examined neurochemical, autonomic, and behavioral responses to yohimbine, in a larger group of healthy subjects, with multiple observations after administration of placebo or yohimbine, and measurement of plasma NE and DA metabolites. Our hypotheses were that yohimbine would increase rapid-response impulsivity, correlating with change in MHPG but not with change in HVA, and that yohimbine would increase subjective activation.
Methods
Subjects
Twenty-three healthy subjects (age 33.8 ± 10.6; education 13.9 ± 1.8 years; 10 men, age 34.4 ± 10.5 years, 13 women, age 32.7 ± 11.1 years), were recruited from the community by advertisement. None had participated in our pilot study. Subjects gave written informed consent after full presentation of the study and opportunity to ask questions. The study was approved by the Committee for the Protection of Human Subjects, Institutional Review Board for the University of Texas Houston Health Science Center (project HSC-MS-05-0036). Subjects received community standard reimbursement for time and parking/bus transportation and were given a light lunch. Diagnoses used the Structured Clinical Interview for DSMIV (SCID-1 and -2) (First et al. 1996); subjects could not meet present or past DSMIV criteria for any Axis I or Axis II disorder. Four subjects were smokers but none met criteria for nicotine dependence or abuse. Subjects had medical screening, including history, physical exam, ECG, and cardiology consultation. Hypertension (by history or examination) or treatment with noradrenergic, anti-noradrenergic, cardiovascular, or psychotropic drugs was exclusionary. Yohimbine/placebo administration was not scheduled until subjects had completed all baseline psychiatric and medical evaluations. Negative urine drug screens and breath alcohol were required on study days.
Yohimbine administration
Yohimbine (0.4 mg/kg) or matching placebo was given orally, double-blind, in counterbalanced order. Average weight was 78.5±14.7 kg (range 54.1–126.9); dose was 30.2±5.0 mg (range 21.6–43.2 mg). Dose did not correlate significantly with baseline or change in catecholamine metabolites, vital signs, or IMT performance. The dose was based on our preliminary study where 20 mg yohimbine had only small effects and 40 mg had unpleasant side effects, especially nausea and activation (Swann et al. 2005a). Study medication was randomized and dispensed by the pharmacy of the Memorial Hermann Hospital. Study personnel were blinded to study medication during data acquisition and analysis. Procedures were performed at the Memorial Hermann Clinical Research Unit, part of the Center for Clinical and Translational Sciences (CCTS) for the University of Texas Health Science Center at Houston.
Metabolite assays
Blood was obtained (EDTA anticoagulant), placed on ice, and centrifuged; plasma was separated and promptly frozen and stored at −80 °C until assay. Samples were shipped overnight on dry ice for assay by Martin Javors, Ph.D., at the University of Texas Health Science Center at San Antonio. Concentrations of homovanillic acid (HVA, a dopamine metabolite), 3-methoxy-4-hydroxyphenylglycol (MHPG, a norepinephrine metabolite), and vanillylmandelic acid (VMA, a metabolite of peripherally released norepinephrine) were measured using high pressure liquid chromatography (HPLC) with electrochemical detection (Scheinin et al. 1983). Plasma VMA values were only available for 5 men and 8 women because of a technical problem. Baseline MHPG, HVA, autonomic, impulsivity, and other psychiatric measures did not differ between subjects with versus without plasma VMA data.
Behavioral measures
Trait impulsivity was assessed during initial evaluation, using the Barratt Impulsiveness Scale (BIS-11), a well validated 30-item self-report (Stanford et al. 2009). The BIS-11, which is not designed to measure short-term changes, measures impulsivity as three constructs: attentional (lack of sustained attention or cognitive perseverance); motor (tendency to act on the spur of the moment); and nonplanning (lack of future sense).
Rapid-response impulsivity
Immediate Memory Task (IMT) is a computerized continuous performance test designed to assess impulsivity and attention (Dougherty et al. 2003a). Subjects are shown 5 digit numbers, for 0.5 sec, 0.5 sec apart, and instructed to respond as quickly as possible when a number matches the previous one. Responses are correct detections (accurate responses to a matching number), commission errors (4 of 5 digits match), and random errors (no digits match). Commission errors are considered impulsive responses (Dougherty et al. 2003a;Swann et al. 2002). Reaction times to responses, and signal detection parameters including discriminability (A′, ability to detect signal from noise), and response bias (β, with negative numbers representing impulsive bias) (Green and Swets 1966), were also determined.
Symptom scales
Subjects were given the Internal State Scale (ISS),a self-rated visual analog scale with 4 factors: Activation, Depression, Perceived Conflict, and Well-being (Bauer et al. 1991). Changes in symptoms were measured using the Profile of Mood States (van Kammen and Murphy 1975), with 6 factors: Tension (T), Depression (D), Anger-hostility (A), Vigor-activity (V), Fatigue (F), and Confusion (C). Baseline psychiatric symptoms were measured using mania, depression, anxiety, and psychosis factors from the Schedule for Affective Disorders and Schizophrenia, Change version (SADS-C) (Spitzer and Endicott 1978); Change in SADS-C scores was not investigated because this instrument is not designed to be given repeatedly over a short period.
General procedure
Subjects were fasting on study days because food alters plasma HVA (Swann et al. 1980). Between 0800 and 0900 (due to diurnal variations in plasma HVA and MHPG (Swann et al. 1980)), an intravenous catheter was inserted. Thirty minutes after blood pressure was stable, baseline procedures (IMT, event-related potentials to be reported elsewhere, vital signs, and symptom scales) were conducted, and 5 minutes later, subjects were given placebo or yohimbine. Procedures from baseline were repeated 30, 60, 120, and 180 minutes after yohimbine/placebo. Each testing session lasted 20 minutes. Blood pressure, temperature, and heart rate were monitored continuously and were recorded at baseline, 30, 60, 120, and 180 minutes. Subjects were observed on the unit until blood pressure was less than 10 mm Hg above baseline. IRB-approved procedures were in place for hypertensive urgencies, but were never required.
Statistical analyses
For each variable, differences between placebo and yohimbine administration were calculated at baseline and each of the four post-administration sessions. Repeated measures ANOVA was performed with Session (5 levels) as within subjects variable. Covariates, based on correlations with baseline demographic variables, are given in the Tables. A significant Session effect was taken as evidence for a significant effect of yohimbine administration. If a significant Session main effect was found, significance of differences between individual session times and baseline were assessed using the Newman-Keuls test. The session of the maximal net change with yohimbine compared to placebo was determined. Univariate maximal effect sizes were calculated as differences divided by pooled standard deviations for the peak effect session versus baseline.
To compare timing of yohimbine effects across variables, post-administration sessions were designated as 1, 2, 3, and 4 (30, 60, 120, and 180 minutes). Times of maxima for each variable were compared using nonparametric statistics. Friedman’s analysis of variance with peak net-change times as within-subjects measures was conducted first. If this was significant, maximum times for individual variables were compared using Wilcoxon tests.
For IMT or subjective response variables with significant Session effects, relationships to yohimbine-associated catecholamine metabolite change were assessed using forward stepwise multiple regression analysis. Maximal change in the behavioral variable was the dependent variable; independent variables were age, years of education, and maximal changes in HVA and MHPG.
Before analyses, normality of distributions for baseline and change measures was tested using the Shapiro-Wilks test. Change in ISS Activation, and POMS Depression, Anger, and Fatigue scales were not normally distributed. As these variables could not be normalized by natural log, inverse, or square root transformation, the nonparametric Friedman’s repeated measures analysis of variance was used.
Results
Subject characteristics
Baseline characteristics
BIS-11 total score was 56.9 ± 8.8 (SD), GAF was 88.8 ± 4.5, and SADS-C factor scores for depression, mania, anxiety, and psychosis did not differ significantly from zero. Baseline vital signs, metabolites, and symptom scores did not differ between the two testing days. Men and women did not differ in any of the variables studied. Age correlated with baseline plasma HVA (n=23, r=0.516, p=0.012), plasma VMA (n=13, r=0.582, p=0.037), and reaction times to correct detections (r=0.506, p=0.016) and commission errors (r=0.454, p=0.034), as previously reported (Swann et al. 1980;Swann et al. 2002). Years of education correlated positively with baseline plasma MHPG (n=22, r=0.619, p=0.002) and negatively with commission errors (r=−0.426, p=0.048). BIS total and subscale scores did not correlate significantly with baseline catecholamine metabolite or IMT performance measures.
Subject characteristics and effects of yohimbine
Yohimbine-induced peak change in plasma MHPG correlated with years of education (r=0.620, p=0.002). Age correlated significantly with peak change in IMT correct detections (r=−0.479, p=0.028), commission errors (r=−0.428, p=0.027), and response bias (r=0.458, p=0.032). BIS total and subscale scores did not correlate with yohimbine-associated changes in catecholamine metabolites, blood pressure, or IMT performance. Age and/or education were included as covariates when appropriate, as shown in the relevant Tables.
Effects of yohimbine on catecholamine metabolites and autonomic function
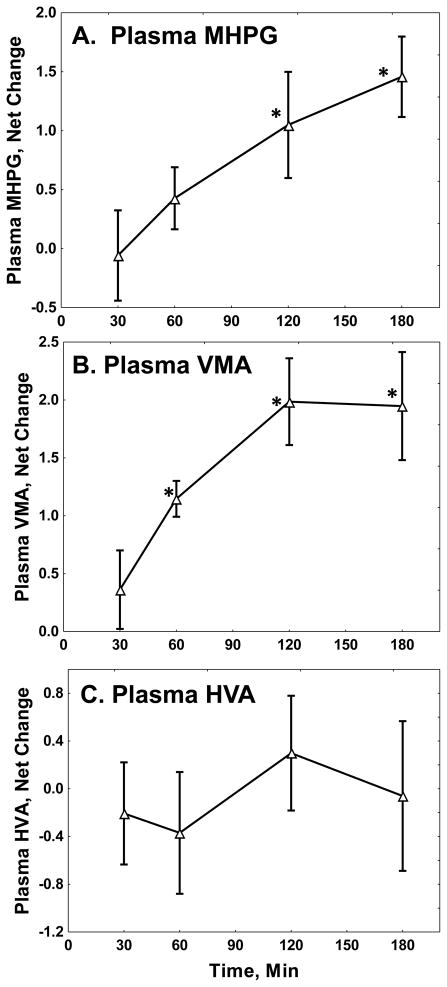
As summarized in Table 2, yohimbine significantly increased plasma MHPG and VMA, without significantly affecting plasma HVA. Figure 1 shows time courses of the effects. Plasma MHPG had median and modal peak increases at three hours after yohimbine administration. Time course for VMA had modal and median peak increases at two hours.
Table 2.
Effects of yohimbine on catecholamine metabolite levels and autonomic function
| Measure (n=23 or as shown) | Baseline | Maximum Change (Δ) | Univariate Effect Size for Δ | Repeated measures | |
|---|---|---|---|---|---|
| F (DF) | P | ||||
| MHPG (n=21), ng/mL | 4.47 ± 1.38 | 1.60 ± 2.12 | 0.75 | 2.64 (4,68) | 0.041 |
| VMA (n=13), ng/mL | 6.90 ± 2.20 | 1.99 ± 1.18 | 1.66 | 11.7 (4,44) | 0.00001 |
| HVA (n=22), ng/mL | 8.89 ± 2.57 | 0.31 ± 2.15 | 0.14 | 0.66 (4,84) | 0.63 |
| Systolic BP, mm Hg | 117.1 ± 11.1 | 11.65 ± 13.6 | 0.86 | 9.72 (4,88) | 0.00001 |
| Diastolic BP, mm Hg | 73.2 ± 8.0 | 5.7 ± 9.2 | 0.62 | 2.47 (4,88) | 0.05 |
| Pulse, b/min | 68.3 ± 9.9 | 7.09 ± 10.8 | 0.65 | 3.58 (4,88) | 0.009 |
| Temperature, degrees F | 97.4 ± 0.7 | 0.46 ± 1.24 | 0.37 | 1.82 (4,88) | 0.13 |
Means are given with standard deviations. Analysis for MHPG used education as covariate. Effect size (for Δ) is the effect size for the maximum difference between net change from baseline with administration of yohimbine versus placebo. Significant effects (p < 0.05) are shown in bold face.
Fig 1. Yohimbine Effects on Plasma Catecholamine Metabolites.
The Figure shows net changes in plasma (A) MHPG, (B) VMA, and (C) HVA, in ng/ml, with standard errors. Statistics are summarized in Table 1. *: Different from baseline, Newman-Keuls test, p < 0.05.
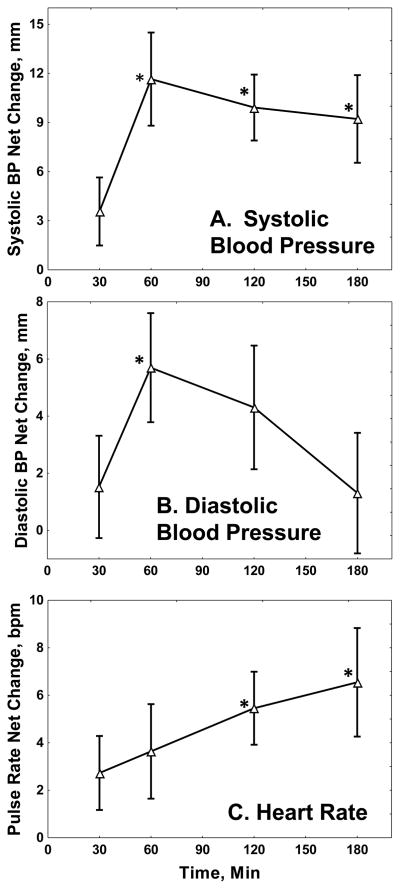
Table 2 also shows that yohimbine significantly increased systolic blood pressure, diastolic blood pressure, and pulse rate. Figure 2 shows the time course of these effects. Systolic and diastolic blood pressure peaked at about one hour after yohimbine, while pulse rate increased steadily over the three hours.
Fig 2. Yohimbine Effects on Blood Pressure and Pulse.
The Figure shows net changes in (A) systolic and (B) diastolic blood pressure in mm Hg, and (C) pulse rate in beats/min, with standard errors. Statistics are summarized in Table 1. *: Different from baseline, Newman-Keuls test, p < 0.05.
Effects of yohimbine on rapid-response impulsivity
Table 3 shows that, after covarying for age and education, yohimbine increased commission errors (impulsive responses) and impulsive response bias, and reduced reaction times for both correct detections and commission errors. Yohimbine had no effect on correct detections, random errors, or discriminability. Thus, yohimbine increased impulsivity measures but did not affect other performance indices.
Table 3.
Effects of yohimbine on IMT performance
| Measure (n=22) | Baseline | Maximum Change (Δ) | Univariate Effect Size for Δ | Repeated measures | |
|---|---|---|---|---|---|
| F (df) | P | ||||
| Correct detections (%) | 82.1 ± 13.6 | −2.17 ± 8.27 | 0.262 | 1.47 (4,68) | 0.23 |
| Commission errors (%) | 22.1 ± 14.4 | 2.64 ± 6.04 | 0.447 | 4.41 (4,76) | 0.003 |
| Random errors (%) | 0.49 ± 0.99 | 0.61 ± 4.18 | 0.146 | 0.48 (4,80) | 0.75 |
| Commission Errors/Corr. Detections | 0.276 ± 0.166 | 0.044 ± 0.103 | 0.427 | 2.64 (4,80) | 0.044 |
| Reaction time, CD (msec) | 473 ± 83 | −42 ± 39 | −1.077 | 3.05 (4,80) | 0.022 |
| Reaction time, CE (msec) | 477 ± 89 | −33 ± 55 | −0.600 | 3.53 (4,76) | 0.011 |
| Discriminability | 0.873 ± 0.065 | −0.023 ± 0.065 | −0.354 | 0.78 (4,76) | 0.54 |
| Response Bias | −0.083 ± 0.555 | −0.196 ± 0.422 | −0.465 | 3.02 (4,76) | 0.023 |
Means are given with standard deviations. Analyses for correct detections, commission errors, random errors, disciminability, and response bias used age and education as covariates; analyses of reaction times used age as covariate. Effect size (for Δ) is the effect size for the maximum difference between net change from baseline with administration of yohimbine versus placebo. Significant effects (p < 0.05) are shown in bold face.
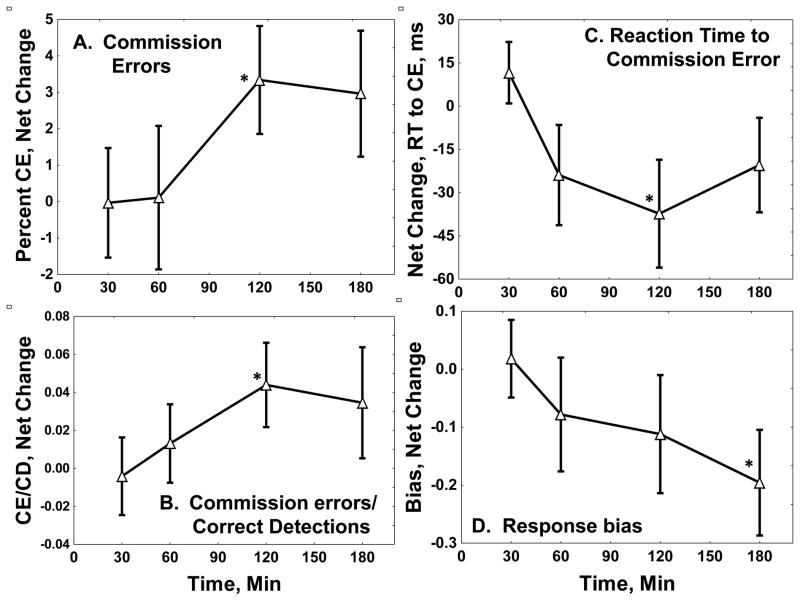
Time courses of yohimbine effects are shown in Figure 3. Modal time of maximal increase in commission errors after yohimbine was 180 minutes; median was 120 minutes. Reaction time to a commission error had median and modal maximal times of 120 minutes; reaction times to correct detections (not shown) had similar timing.
Fig 3. Yohimbine Effects on Immediate Memory Task Performance.
The Figure shows net changes in (A) percent commission errors, (B) commission errors/correct detections (CE/CD) ratio, (C) reaction time to a commission error (CE, in ms),and (D) response bias, with standard errors. Negative bias scores represent impulsive responding. Statistics are summarized in Table 2. *: Different from baseline, Newman-Keuls test, p < 0.05.
Relationships between yohimbine-induced MHPG or HVA and IMT performance changes were investigated as described in Methods. Change in MHPG was related significantly to change in commission errors (beta = 0.727 ± 0.243; for the model, F(2,18) = 4.67, P=0.023), while change in HVA did not contribute to the model (F < 1).
Subjective effects of yohimbine
Yohimbine had no significant effects on POMS or ISS.
Timing of yohimbine effects
The analyses used the dummy variables for times of maximal yohimbine effects for plasma MHPG, systolic blood pressure, and IMT commission errors, as described in Methods. Friedman’s analysis of variance was significant across these variables (X2(2 df)=7, p=0.03). Yohimbine effects peaked significantly later for plasma MHPG than for systolic blood pressure (Wilcoxon z=2.67, p=0.008). There were no other significant differences among these variables (for plasma MHPG vs commission errors, z=1.82, p=0.069; for systolic blood pressure vs commission errors, z=1, p=0.3).
Discussion
Relative to our hypotheses, yohimbine administration increased rapid-response impulsivity and accelerated reaction times (Table 3), consistent with results of our pilot study (Swann et al. 2005a). Increase in plasma MHPG, but not plasma HVA, predicted increase in impulsive IMT responses. Yohimbine had no effect on discriminability or correct detections. This is consistent with animal results where NE depletion by 6-hydroxydopamine reduced speed and impulsivity, but not discriminability (Cole and Robbins 1989). As anticipated (Charney et al. 1982;Gurguis and Uhde 1990), yohimbine increased plasma NE metabolites, blood pressure, and pulse. Yohimbine had no significant subjective effects. We will discuss these results in terms of relationships between NE and mechanisms of impulsivity, effects of yohimbine on catecholaminergic activity, relationships between effects of yohimbine on autonomic function and behavior, and specificity of yohimbine effects.
Rapid-response impulsivity and NE
Impulsivity, related to control of the initiation of action, is complex. Three interacting aspects potentially related to NE are behavioral mechanisms, neural mechanisms, and time course. Each will be discussed below.
Behavioral mechanisms
Rapid-response impulsivity, measured by procedures like stop-signal tasks or continuous performance tests, represents inability to conform the response to a stimulus to its context or to assess the stimulus adequately before responding; reward-based impulsivity, sometimes called impulsive choice, is related to inability to delay response for a larger reward (Evenden 2000;Swann et al. 2002). These mechanisms of impulsivity differ in their apparent responses to neurotransmitters, with NE potentially related to analogs of rapid-response, but not reward-based, impulsivity (Evenden 2000;Sun et al. 2010). Because of time constraints, we did not measure choice impulsivity, and thus cannot compare yohimbine effects across these two types of impulsivity.
Neural mechanisms
Impulsivity can be related to dysregulation of limbic arousal, imbalance between facilitatory and inhibitory behavioral systems, or impairment of attention (Fineberg et al. 2010). These mechanisms vary in neurotransmitter sensitivity (Evenden 2000). NE, for example, can have opposite effects on impulsivity or attention depending on context, by enhancing attention or orientation (Berridge and Waterhouse 2003;Riba et al. 2005), or by disrupting inhibitory prefrontal cortex function (Arnsten and Li 2005;Fitzgerald 2011).
Time course
Potential for impulsivity may be a trait-like characteristic, but its expression at a given time can depend on internal or external conditions (Barratt 1985), potentially including increased NE due to stress or increased arousal (Soltis et al. 1997). NE-mediated state-dependent increase in impulsive behavior could be reflected in phenomena such as transient hypomania following yohimbine administration in subjects with bipolar disorder (Price et al. 1984).
NE and yohimbine effects
Our results suggest that excessive NE can increase potential for impulsive behavior in a state-dependent manner, consistent with the model proposed by Arnsten (Arnsten and Li 2005). Rapid-response impulsivity is increased in mania (Swann et al. 2003), and manic symptoms correlate with NE metabolite levels (Swann et al. 1987). The results in Table 3 and Figure 3 show that pharmacologically stimulated NE release increased rapid-response impulsivity, confirming an earlier pilot study (Swann et al. 2005a) and more recent studies in rats (Sun et al. 2010;Torregrossa et al. 2012) (see Table 1). This increase correlated with change in plasma MHPG but not plasma HVA. Laboratory behavioral models of impulsivity may therefore provide a useful strategy for translation between animal and human models of impulsive behavior (Winstanley 2011).
Yohimbine-induced maximal changes in plasma MHPG and impulsive errors were significantly related, but neither correlated with baseline plasma MHPG. This is consistent with models of NE regulation positing two types of NE response, phasic and tonic (Aston-Jones and Cohen 2005;Berridge and Waterhouse 2003). The tonic response may be related to baseline NE, while the phasic effect may represent specific task-focused NE release. The phasic effect could enhance task performance (Aston-Jones and Cohen 2005); excessive tonic release, associated with acute stress or pharmacological alpha-2 receptor blockade, could result in impulsivity and poor task performance (Arnsten and Li 2005) (Table 1).
BIS-11 scores did not correlate with catecholamine metabolites or IMT performance, either at baseline or after yohimbine. Previous studies showed that IMT commission errors, but not BIS-11 scores, correlated with severity of ASPD (Swann et al. 2009) and severity of personality disorder symptoms in a mixed population (Swann et al. 2002). Further, history of criminal conviction in bipolar disorder (Swann et al. 2011), or of severe suicidal behavior with (Swann et al. 2005b) or without (Dougherty et al. 2004) bipolar disorder, was associated with increased IMT commission errors and faster reaction times, but not with higher BIS-11 scores. These results are consistent with previous work showing lack of correspondence between laboratory and psychometric markers of impulsivity (Lane et al. 2003;Reynolds et al. 2008).
Yohimbine and catecholamine function
Blockade of alpha-2 NE receptors increases NE release (Aghajanian 1978) and peripheral (Charney et al. 1982;Gurguis and Uhde 1990) and central NE metabolite levels (Peskind et al. 1989), providing a potential probe for effects of increased endogenous NE.
NE released in the brain leaves the CNS as MHPG, which is also produced in the sympathetic nervous system (Maas and Landis 1968). Analogously, DA from either the CNS or periphery is metabolized to HVA. Plasma MHPG, HVA, and VMA are reliable measures in healthy subjects (Baker et al. 1988). Studies using human brain arteriovenous differences (Maas et al. 1979) and peripheral monoamine oxidase inhibition (Swann et al. 1980) show that roughly half of plasma MHPG and about 20–25% of plasma HVA are from the brain. Plasma VMA is, essentially, exclusively from the peripheral sympathetic nervous system and may be the most sensitive metabolite in response to stressors (Fukuda et al. 1996).
Plasma MHPG is an integrated measure of NE turnover over at least 60 minutes (Szemeredi et al. 1991). In contrast, effects of yohimbine on blood pressure or performance occur at the time of measurement. Because the effect being measured is of NE rather than MHPG, this complicates studies measuring effects with different time courses. The results suggest that human laboratory studies of oral yohimbine need at least three hours.
Like yohimbine, selective NE reuptake inhibitors such as atomoxetine can increase synaptic NE (Owen and Whitton 2003). However, their effects on integrated NE function relative to impulsive behavior are quite different from those of yohimbine. For example, Arnsten has reported opposing effects of alpha-1 and alpha-2 NE receptors in prefrontal cortex, where stimulation of alpha-1 receptors disrupts inhibitory prefrontal cortex function, while stimulation of alpha-2 receptors enhances it (Arnsten and Pliszka 2011). NE reuptake blockers increase alpha-2 receptor stimulation, and their effects on impulsive behavior resemble those of alpha-2 agonists (Arnsten and Pliszka 2011;Fernando et al. 2012). Yohimbine, an alpha-2 antagonist, has opposite effects. Further, it blocks the feedback regulation by alpha-2 receptors of NE release, which limits the increase in overall NE function associated with NE reuptake blockade, resulting in reversal of the reduction in NE release by atomoxetine (Owen and Whitton 2003). Therefore, compared to atomoxetine, yohimbine increases net NE release, and blocks any direct behavioral effect of alpha-2 receptor stimulation. Although yohimbine and atomoxetine are both considered to be NE-enhancing drugs, they have opposite effects on NE-induced cyclic AMP production and CREB phosphorylation that run parallel to their opposite effects on impulsivity (Sun et al. 2012).
Relationships between autonomic and behavioral effects of yohimbine
Effects of yohimbine could have interacted with stress of testing. Yohimbine was reported to increase autonomic responses to mental arithmetic in controls, but had no interaction with a continuous performance task (Albus et al. 1992). Cognitive task administration was reported not to alter salivary MHPG (Li et al. 2004).
Yohimbine had no significant effects on POMS or ISS symptom measures. In our pilot study, yohimbine increased ISS Activation (Swann et al. 2005a). The studies differed in doses of yohimbine, order of administration (counterbalanced in this study, progressive in the earlier study), timing of measures (a single post-treatment measure at 90 minutes in the earlier study, instead of serial measures), and in the greater number and frequency of procedures during the current study. Repeated structured non-stressful task performance reduces subjective effects of pharmacologically altered NE (Albus et al. 1992;Li et al. 2004), consistent with the lack of subjective effects in the present study. Behavioral responses to yohimbine in healthy subjects have varied, with relatively modest effects in controls, especially in low-anxiety subjects (Mizuki et al. 1996), and more prominent effects in anxiety disorders (Goddard et al. 1995;Gurguis and Uhde 1990) or controls with high baseline anxiety (Mizuki et al. 1996). Therefore, the combination of multiple structured procedures and low baseline anxiety may have contributed to the lack of significant subjective effects.
Timing of yohimbine effects varied. Effects on blood pressure preceded those on IMT performance and plasma MHPG. IMT effects might be expected to take longer than those on blood pressure, as they involved more complex attentional and stimulus discrimination processes in the CNS. Effects on metabolites, requiring reuptake of released NE, oxidative metabolism, and equilibration of metabolites into plasma, were the slowest to appear.
Non-noradrenergic effects of yohimbine
Heterosynaptic alpha-2 receptors can also inhibit release of dopamine, serotonin, and acetylcholine (de Villiers et al. 1995;Kalsner and Abdali 2001). Stimulation of postsynaptic alpha-2 receptors also has endocrine effects, including release of growth hormone (Baumann et al. 2004) and inhibition of CRH release (Vythilingam et al. 2000). Many alpha-2 noradrenergic receptor ligands also act through imidazoline receptors, which alter blood pressure but not attentional state (Tibirica et al. 1991). Yohimbine is relatively selective for alpha-2 relative to imidazoline receptors (Szabo and Urban 1995), but effects such as increased blood pressure, which did not correlate with MHPG change, could have resulted from non-noradrenergic effects of yohimbine.
Yohimbine is also a ligand for other behaviorally relevant receptors. Yohimbine was reported to bind to monoaminergic receptors with greatest affinity for alpha-2 NE receptors, followed by 5HT-1A (agonist), 5HT-1B and 1-D, and D3 receptors, with lowest affinity for D2 receptors (Millan et al. 2000). While affinity was highest for alpha-2 receptors, the 5HT1A effect appears to account for disruption of prepulse inhibition of acoustic startle by yohimbine (Powell et al. 2005). Serotonergic and noradrenergic effects of yohimbine may interact in anxiety (Goddard et al. 1995) and alcohol reinstatement (Dzung et al. 2009). However, 5HT1A receptors have effects on accuracy (conservative bias) and speed of responding (slowed) that are opposite to those of yohimbine reported here (Carli and Samanin 2000).
Limitations
1) While effect sizes for NE metabolites and autonomic effects were substantial, the number of subjects was too small to investigate complex interactions across time; 2) these results cannot distinguish direct behavioral effects of alpha-2 receptor blockade from indirect effects mediated by increased NE release; 3) roles of other possible results of alpha-2 receptor binding, such as growth hormone and heteroceptor effects (except for plasma HVA), and other potential non-alpha-2 effects, were not determined; 4) while effects of yohimbine on impulsive responding correlated with those on NE metabolites, contributions of serotonergic and other systems cannot be ruled out.
Conclusions
Yohimbine, at a dose that increased plasma MHPG, VMA, and blood pressure, without effect on plasma HVA, elicited an impulsive pattern of responding on a test of rapid-response impulsivity. Noradrenergic reactivity may be related to state-dependent risk for impulsive behavior. Future studies are needed linking possible noradrenergic effects to specific noradrenergic effector systems.
Acknowledgments
This study was supported in part by the Pat R Rutherford, Jr. Chair in Psychiatry (ACS) and by NIH grants R01-MH69944 (ACS), K02-DA00403 (FGM), and UL1-RR024148 (CTSA; General Clinical Research Center UT Houston).
We thank Martin Javors, Ph.D. (University of Texas Health Science Center at San Antonio) for performaning catecholamine metabolite assays; Stephen Hecht, M.D., Department of Emergency Medicine, for assisting with medical backup; and Stacy Meier, Leslie Paith, Irshad Prasla, Tammy Souter, R.N., Anthony Zamudio, R.N., and the nursing staff of the Clinical Research Unit, for their skilled assistance.
Footnotes
Conflicts of Interest: Dr. Swann has served on Data Safety Monitoring Boards for Pfizer Laboratories and Teva Pharmaceuticals; as a speaker for Abbott Laboratories, Cortexcongress, Merck, and Sanofi-Aventis; as a consultant for Merck; and has received grant support from Elan Pharmaceuticals and the NIH. Dr. Moeller has acted as a consultant for Boeringer Ingelheim and has received funding from the NIH. Drs. Lane and Steinberg have received funding from the NIH. Dr. Lijffijt and Mr. Cox report no potential conflicts.
References
- Aghajanian GK. Feedback regulation of central monoaminergic neurons: evidence from single cell recording studies. Essays Neurochem Neuropharmacol. 1978;3:1–32. [PubMed] [Google Scholar]
- Albus M, Zahn TP, Breier A. Anxiogenic properties of yohimbine. II. Influence of experimental set and setting. Eur Arch Psychiatry Clin Neurosci. 1992;241:345–351. doi: 10.1007/BF02191959. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Stress impairs prefrontal cortical function in rats and monkeys: role of dopamine D1 and norepinephrine alpha-1 receptor mechanisms. Prog Brain Res. 2000;126:183–192. doi: 10.1016/S0079-6123(00)26014-7. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Dudley AG. Methylphenidate improves prefrontal cortical cognitive function through alpha2 adrenoceptor and dopamine D1 receptor actions: Relevance to therapeutic effects in Attention Deficit Hyperactivity Disorder. Behav Brain Funct. 2005;1:2. doi: 10.1186/1744-9081-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Mathew R, Ubriani R, Taylor JR, Li BM. Alpha-1 noradrenergic receptor stimulation impairs prefrontal cortical cognitive function. Biol Psychiatry. 1999;45:26–31. doi: 10.1016/s0006-3223(98)00296-0. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Pliszka SR. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol Biochem Behav. 2011;99:211–216. doi: 10.1016/j.pbb.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J Comp Neurol. 2005;493:99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- Avery RA, Franowicz JS, Studholme C, van Dyck CH, Arnsten AF. The alpha-2A-adrenoceptor agonist, guanfacine, increases regional cerebral blood flow in dorsolateral prefrontal cortex of monkeys performing a spatial working memory task. Neuropsychopharmacology. 2000;23:240–249. doi: 10.1016/S0893-133X(00)00111-1. [DOI] [PubMed] [Google Scholar]
- Baker NJ, Adler LE, Waldo M, Gerhardt G, Drebing C, Cox B, Berry S, Phillips W, Freedman R. Reproducibility of the measurement of plasma noradrenergic and dopaminergic metabolites in normal subjects. Psychiatry Res. 1988;23:119–130. doi: 10.1016/0165-1781(88)90001-7. [DOI] [PubMed] [Google Scholar]
- Bari A, Aston-Jones G. Atomoxetine modulates spontaneous and sensory-evoked discharge of locus coeruleus noradrenergic neurons. Neuropharmacology. 2013;64:53–64. doi: 10.1016/j.neuropharm.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt ES. Impulsiveness subtraits: arousal and information processing. In: Spence JT, Izard CE, editors. Motivation, emotion, and personality. Elsevier Science publishers; Amsterdam: 1985. pp. 137–146. [Google Scholar]
- Bauer MS, Crits Christoph P, Ball WA, Dewees E, McAllister T, Alahi P, Cacciola J, Whybrow PC. Independent assessment of manic and depressive symptoms by self-rating. Scale characteristics and implications for the study of mania. Arch Gen Psychiatry. 1991;48:807–812. doi: 10.1001/archpsyc.1991.01810330031005. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Milchanowski AB, Rothman RB. Evidence for alterations in alpha2-adrenergic receptor sensitivity in rats exposed to repeated cocaine administration. Neuroscience. 2004;125:683–690. doi: 10.1016/j.neuroscience.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Birnbaum S, Gobeske KT, Auerbach J, Taylor JR, Arnsten AF. A role for norepinephrine in stress-induced cognitive deficits: alpha-1-adrenoceptor mediation in the prefrontal cortex. Biol Psychiatry. 1999;46:1266–1274. doi: 10.1016/s0006-3223(99)00138-9. [DOI] [PubMed] [Google Scholar]
- Birnbaum SG, Podell DM, Arnsten AF. Noradrenergic alpha-2 receptor agonists reverse working memory deficits induced by the anxiogenic drug, FG7142, in rats. Pharmacol Biochem Behav. 2000;67:397–403. doi: 10.1016/s0091-3057(00)00306-3. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C. Impulsivity in abstinent alcohol-dependent patients: relation to control subjects and type 1-/type 2-like traits. Alcohol. 2004;34:133–150. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Carli M, Samanin R. The 5-HT(1A) receptor agonist 8-OH-DPAT reduces rats’ accuracy of attentional performance and enhances impulsive responding in a five-choice serial reaction time task: role of presynaptic 5-HT(1A) receptors. Psychopharmacology (Berl) 2000;149:259–268. doi: 10.1007/s002139900368. [DOI] [PubMed] [Google Scholar]
- Charney DS, Heninger GR, Sternberg DE. Assessment of alpha 2 adrenergic autoreceptor function in humans: effects of oral yohimbine. Life Sci. 1982;30:2033–2041. doi: 10.1016/0024-3205(82)90444-1. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi on performance of a 5-choice serial reaction time task in rats: implications for theories of selective attention and arousal. Behav Brain Res. 1989;33:165–179. doi: 10.1016/s0166-4328(89)80048-8. [DOI] [PubMed] [Google Scholar]
- de Villiers AS, Russell VA, Sagvolden T, Searson A, Jaffer A, Taljaard JJ. Alpha 2-adrenoceptor mediated inhibition of [3H]dopamine release from nucleus accumbens slices and monoamine levels in a rat model for attention-deficit hyperactivity disorder. Neurochem Res. 1995;20:427–433. doi: 10.1007/BF00973098. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Bjork JM, Harper RA, Marsh DM, Moeller FG, Mathias CW, Swann AC. Behavioral impulsivity paradigms: A comparison in hospitalized adolescents with disruptive behavior disorders. J Child Psychol Psychiatry. 2003a;44:1145–1157. doi: 10.1111/1469-7610.00197. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Bjork JM, Moeller FG, Harper RA, Marsh DM, Mathias CW, Swann AC. Familial transmission of Continuous Performance Test behavior: attentional and impulsive response characteristics. J Gen Psychol. 2003b;130:5–21. doi: 10.1080/00221300309601271. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Marsh DM, Papageorgiou TD, Swann AC, Moeller FG. Laboratory-measured behavioral impulsivity relates to suicide attempt history. Suicide and Life-Threatening Behavior. 2004;34:374–385. doi: 10.1521/suli.34.4.374.53738. [DOI] [PubMed] [Google Scholar]
- Doyle AE, Wozniak J, Wilens TE, Henin A, Seidman LJ, Petty C, Fried R, Gross LM, Faraone SV, Biederman J. Neurocognitive impairment in unaffected siblings of youth with bipolar disorder. Psychol Med. 2009;39:1253–1263. doi: 10.1017/S0033291708004832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzung LA, Funk D, Harding S, Juzytsch W, Fletcher PJ. The role of noradrenaline and 5-hydroxytryptamine in yohimbine-induced increases in alcohol-seeking in rats. Psychopharmacology (Berl) 2009;204:477–488. doi: 10.1007/s00213-009-1481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Theobald DE, Robbins TW, Everitt BJ, Dalley JW. Norepinephrine and Dopamine Modulate Impulsivity on the Five-Choice Serial Reaction Time Task Through Opponent Actions in the Shell and Core Sub-Regions of the Nucleus Accumbens. Neuropsychopharmacology. 2012:npp201253. doi: 10.1038/npp.2012.53. [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden J. Varieties of impulsivity. Psychopharmacol. 2000;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Fernando AB, Economidou D, Theobald DE, Zou MF, Newman AH, Spoelder M, Caprioli D, Moreno M, Hipolito L, Aspinall AT, Robbins TW, Dalley JW. Modulation of high impulsivity and attentional performance in rats by selective direct and indirect dopaminergic and noradrenergic receptor agonists. Psychopharmacology (Berl) 2012;219:341–352. doi: 10.1007/s00213-011-2408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, Sahakian BJ, Robbins TW, Bullmore ET, Hollander E. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology. 2010;35:591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders Patient Edition. Biometrics Research Institute, New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- Fitzgerald PJ. A neurochemical yin and yang: does serotonin activate and norepinephrine deactivate the prefrontal cortex? Psychopharmacology (Berl) 2011;213:171–182. doi: 10.1007/s00213-010-1856-1. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Hata A, Niwa S, Hiramatsu K, Honda H, Nakagome K, Iwanami A. Plasma vanillylmandelic acid level as an index of psychological stress response in normal subjects. Psychiatry Res. 1996;63:7–16. doi: 10.1016/0165-1781(96)02527-9. [DOI] [PubMed] [Google Scholar]
- Goddard AW, Charney DS, Germine M, Woods SW, Heninger GR, Krystal JH, Goodman WK, Price LH. Effects of tryptophan depletion on responses to yohimbine in healthy human subjects. Biol Psychiatry. 1995;38:74–85. doi: 10.1016/0006-3223(94)00223-P. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. Wiley; New York: 1966. [Google Scholar]
- Gurguis GN, Uhde TW. Plasma 3-methoxy-4-hydroxyphenylethylene glycol (MHPG) and growth hormone responses to yohimbine in panic disorder patients and normal controls. Psychoneuroendocrinology. 1990;15:217–224. doi: 10.1016/0306-4530(90)90032-5. [DOI] [PubMed] [Google Scholar]
- Kalsner S, Abdali SA. Rate-independent inhibition by norepinephrine of 5-HT release from the somadendritic region of serotonergic neurons. Brain Res Bull. 2001;55:761–765. doi: 10.1016/s0361-9230(01)00567-6. [DOI] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Rhoades HM, Pietras CJ, Tcheremissine OV. Relationships among laboratory and psychometric measures of impulsivity: Implications in substance abuse and dependence. Addictive Disorders and Their Treatment. 2003;2:33–40. [Google Scholar]
- Li GY, Ueki H, Kawashima T, Sugataka K, Muraoka T, Yamada S. Involvement of the noradrenergic system in performance on a continuous task requiring effortful attention. Neuropsychobiology. 2004;50:336–340. doi: 10.1159/000080962. [DOI] [PubMed] [Google Scholar]
- Liu YP, Lin YL, Chuang CH, Kao YC, Chang ST, Tung CS. Alpha adrenergic modulation on effects of norepinephrine transporter inhibitor reboxetine in five-choice serial reaction time task. J Biomed Sci. 2009;16:72. doi: 10.1186/1423-0127-16-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CL, Arnsten AF, Li BM. Locomotor hyperactivity induced by blockade of prefrontal cortical alpha2-adrenoceptors in monkeys. Biol Psychiatry. 2005;57:192–195. doi: 10.1016/j.biopsych.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Maas JW, Hattox SE, Greene NM, Landis DH. 3-Methoxy-4-hydroxyphenethyleneglycol production by human brain in vivo. Science. 1979;205:1025–1027. doi: 10.1126/science.472724. [DOI] [PubMed] [Google Scholar]
- Maas JW, Landis DH. In vivo studies of the metabolism of norepinephrine in the central nervous system. J Pharmacol Exp Ther. 1968;163:147–162. [PubMed] [Google Scholar]
- Malloy-Diniz LF, Neves FS, de Moraes PH, De Marco LA, Romano-Silva MA, Krebs MO, Correa H. The 5-HTTLPR polymorphism, impulsivity and suicide behavior in euthymic bipolar patients. J Affect Disord. 2011;133:221–226. doi: 10.1016/j.jad.2011.03.051. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Weyer A, Vranjkovic O, Beyer CE, Baker DA, Caretta H. Involvement of noradrenergic neurotransmission in the stress- but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for beta-2 adrenergic receptors. Neuropsychopharmacology. 2010;35:2165–2178. doi: 10.1038/npp.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Newman-Tancredi A, Audinot V, Cussac D, Lejeune F, Nicolas JP, Coge F, Galizzi JP, Boutin JA, Rivet JM, Dekeyne A, Gobert A. Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states. Synapse. 2000;35:79–95. doi: 10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Mizuki Y, Suetsugi M, Ushijima I, Yamada M. Differential effects of noradrenergic drugs on anxiety and arousal in healthy volunteers with high and low anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:1353–1367. doi: 10.1016/s0278-5846(96)00131-5. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Otte C, Neylan TC, Pole N, Metzler T, Best S, Henn-Haase C, Yehuda R, Marmar CR. Association between childhood trauma and catecholamine response to psychological stress in police academy recruits. Biol Psychiatry. 2005;57:27–32. doi: 10.1016/j.biopsych.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Owen JC, Whitton PS. Reboxetine modulates norepinephrine efflux in the frontal cortex of the freely moving rat: the involvement of alpha 2 and 5-HT1A receptors. Neurosci Lett. 2003;348:171–174. doi: 10.1016/s0304-3940(03)00792-4. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Wetzler C, Hackett A, Hanania T. Impulsive action and impulsive choice are mediated by distinct neuropharmacological substrates in rat. Int J Neuropsychopharmacol. 2012;15:1473–1487. doi: 10.1017/S1461145711001635. [DOI] [PubMed] [Google Scholar]
- Peskind ER, Veith RC, Dorsa DM, Gumbrecht G, Raskind MA. Yohimbine increases cerebrospinal fluid and plasma norepinephrine but not arginine vasopressin in humans. Neuroendocrinology. 1989;50:286–291. doi: 10.1159/000125235. [DOI] [PubMed] [Google Scholar]
- Powell SB, Palomo J, Carasso BS, Bakshi VP, Geyer MA. Yohimbine disrupts prepulse inhibition in rats via action at 5-HT1A receptors, not alpha2-adrenoceptors. Psychopharmacology (Berl) 2005;180:491–500. doi: 10.1007/s00213-005-2193-7. [DOI] [PubMed] [Google Scholar]
- Price LH, Charney DS, Heninger GR. Three cases of manic symptoms following yohimbine administration. Am J Psychiatry. 1984;141:1267–1268. doi: 10.1176/ajp.141.10.1267. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Penfold RB, Patak M. Dimensions of impulsive behavior in adolescents: laboratory behavioral assessments. Exp Clin Psychopharmacol. 2008;16:124–131. doi: 10.1037/1064-1297.16.2.124. [DOI] [PubMed] [Google Scholar]
- Riba J, Rodriguez-Fornells A, Morte A, Munte TF, Barbanoj MJ. Noradrenergic stimulation enhances human action monitoring. J Neurosci. 2005;25:4370–4374. doi: 10.1523/JNEUROSCI.4437-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychowdhury S, Pena-Contreras Z, Tam J, Yadlapalli A, Dinh L, Nichols JA, Basu D, Atzori M. alpha(2)- and beta-adrenoceptors involvement in nortriptyline modulation of auditory sustained attention and impulsivity. Psychopharmacology (Berl) 2012;222:237–245. doi: 10.1007/s00213-012-2635-y. [DOI] [PubMed] [Google Scholar]
- Scheinin M, Chang W, Jimerson DC, Linnoila M. Measurement of 3-methoxy-4-hydroxyphenylglycol in human plasma with high performance liquid chromatography using electrochemical detection. Analytical Biochemistry. 1983;132:165–170. doi: 10.1016/0003-2697(83)90442-6. [DOI] [PubMed] [Google Scholar]
- Schepis TS, McFetridge A, Chaplin TM, Sinha R, Krishnan-Sarin S. A pilot examination of stress-related changes in impulsivity and risk taking as related to smoking status and cessation outcome in adolescents. Nicotine Tob Res. 2011;13:611–615. doi: 10.1093/ntr/ntr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis RP, Cook JC, Gregg AE, Sanders BJ. Interaction of GABA and excitatory amino acids in the basolateral amygdala: role in cardiovascular regulation. J Neurosci. 1997;17:9367–9374. doi: 10.1523/JNEUROSCI.17-23-09367.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Endicott J. Biometrics Research. New York State Psychiatric Institute; New York: 1978. Schedule for Affective Disorders and Schizophrenia: Change Version. [Google Scholar]
- Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH. Fifty years of the Barratt Impulsiveness Scale: An update and review. Personality and Individual Differences. 2009;47:385–395. [Google Scholar]
- Sun H, Cocker PJ, Zeeb FD, Winstanley CA. Chronic atomoxetine treatment during adolescence decreases impulsive choice, but not impulsive action, in adult rats and alters markers of synaptic plasticity in the orbitofrontal cortex. Psychopharmacology (Berl) 2012;219:285–301. doi: 10.1007/s00213-011-2419-9. [DOI] [PubMed] [Google Scholar]
- Sun H, Green TA, Theobald DE, Birnbaum SG, Graham DL, Zeeb FD, Nestler EJ, Winstanley CA. Yohimbine increases impulsivity through activation of cAMP response element binding in the orbitofrontal cortex. Biol Psychiatry. 2010;67:649–656. doi: 10.1016/j.biopsych.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann AC, Birnbaum D, Jagar AA, Dougherty DM, Moeller FG. Acute yohimbine increases laboratory-measured impulsivity in normal subjects. Biol Psychiatry. 2005a;57:1209–1211. doi: 10.1016/j.biopsych.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Swann AC, Bjork JM, Moeller FG, Dougherty DM. Two models of impulsivity: Relationship to personality traits and psychopathology. Biol Psychiatry. 2002;51:988–994. doi: 10.1016/s0006-3223(01)01357-9. [DOI] [PubMed] [Google Scholar]
- Swann AC, Dougherty DM, Pazzaglia PJ, Pham M, Steinberg JL, Moeller FG. Increased impulsivity associated with severity of suicide attempt history in patients with bipolar disorder. Am J Psychiatry. 2005b;162:1680–1687. doi: 10.1176/appi.ajp.162.9.1680. [DOI] [PubMed] [Google Scholar]
- Swann AC, Koslow SH, Katz MM, Maas JW, Javaid J, Secunda SK, Robins E. Lithium carbonate treatment of mania. Cerebrospinal fluid and urinary monoamine metabolites and treatment outcome. Arch Gen Psychiatry. 1987;44:345–354. doi: 10.1001/archpsyc.1987.01800160057008. [DOI] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Kjome KL, Steinberg JL, Moeller FG. Criminal conviction, impulsivity, and course of illness in bipolar disorder. Bipolar Disord. 2011;13:173–181. doi: 10.1111/j.1399-5618.2011.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG. Trait impulsivity and response inhibition in antisocial personality disorder. J Psychiatr Res. 2009;43:1057–1063. doi: 10.1016/j.jpsychires.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann AC, Maas JW, Hattox SE, Landis DH. Catecholamine metabolites in human plasma as indices of brain function: Effects of debrisoquin. Life Sci. 1980;27:1857–1862. doi: 10.1016/0024-3205(80)90430-0. [DOI] [PubMed] [Google Scholar]
- Swann AC, Pazzaglia P, Nicholls A, Dougherty DM, Moeller FG. Impulsivity and phase of illness in bipolar disorder. J Affect Disord. 2003;73:105–111. doi: 10.1016/s0165-0327(02)00328-2. [DOI] [PubMed] [Google Scholar]
- Szabo B, Urban R. Mechanism of sympathoinhibition by imidazolines. Ann N Y Acad Sci. 1995;763:552–565. doi: 10.1111/j.1749-6632.1995.tb32449.x. [DOI] [PubMed] [Google Scholar]
- Szemeredi K, Komoly S, Kopin IJ, Bagdy G, Keiser HR, Goldstein DS. Simultaneous measurement of plasma and brain extracellular fluid concentrations of catechols after yohimbine administration in rats. Brain Res. 1991;542:8–14. doi: 10.1016/0006-8993(91)90990-d. [DOI] [PubMed] [Google Scholar]
- Tibirica E, Feldman J, Mermet C, Gonon F, Bousquet P. An imidazoline-specific mechanism for the hypotensive effect of clonidine: a study with yohimbine and idazoxan. J Pharmacol Exp Ther. 1991;256:606–613. [PubMed] [Google Scholar]
- Torregrossa MM, Xie M, Taylor JR. Chronic corticosterone exposure during adolescence reduces impulsive action but increases impulsive choice and sensitivity to yohimbine in male Sprague-Dawley rats. Neuropsychopharmacology. 2012;37:1656–1670. doi: 10.1038/npp.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kammen DP, Murphy DL. Attenuation of the euphoriant and activating effects of d- and l-amphetamine by lithium carbonate treatment. Psychopharmacologia. 1975;44:215–224. doi: 10.1007/BF00428897. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Anderson GM, Owens MJ, Halaszynski TM, Bremner JD, Carpenter LL, Heninger GR, Nemeroff CB, Charney DS. Cerebrospinal fluid corticotropin-releasing hormone in healthy humans: effects of yohimbine and naloxone. J Clin Endocrinol Metab. 2000;85:4138–4145. doi: 10.1210/jcem.85.11.6968. [DOI] [PubMed] [Google Scholar]
- Winstanley CA. The utility of rat models of impulsivity in developing pharmacotherapies for impulse control disorders. Br J Pharmacol. 2011;164:1301–1321. doi: 10.1111/j.1476-5381.2011.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]