Abstract
Unilateral lesions to the medial forebrain bundle with 6-hydroxydopamine (6- OHDA) lead to force and timing deficits during a complex licking task. We hypothesized that training targeting tongue force generation during licking would improve timing and force measures and also lead to striatal dopamine sparing. Nine month-old male Fisher344/Brown Norway rats were used in this experiment. Sixteen rats were in the control condition and received tongue exercise (n=8) or no exercise (n=8). Fourteen rats were in the 6-OHDA lesion condition and underwent tongue exercise (n=7) and or no exercise (n=7). Following 4 weeks of training and post-training measures, all animals underwent bilateral stimulation of the hypoglossal nerves to measure muscle contractile properties and were then transcardially perfused and brain tissues collected for immunohistochemistry to examine striatal dopamine content. Results demonstrated that exercise animals performed better for maximal force, average force, and press rate than their no-exercise counterparts, and the 6-OHDA animals that underwent exercise performed as well as the control no exercise group. Interestingly, there were no group differences for tetanic muscle force, despite behavioral recovery of forces. Additionally, behavioral and neurochemical analyses indicate that there were no differences in striatal dopamine. Thus, targeted exercise can improve tongue force and timing deficits related to 6-OHDA lesions and this exercise likely has a central, versus peripheral (muscle strength) mechanism. However, this mechanism is not related to sparing of striatal dopamine content.
1. Introduction
Even in the early stages, Parkinson disease (PD) can lead to significant dysphagia that negatively impacts quality of life. [26,32,42] These deficits include difficulty with bolus formation and propulsion, as well as airway compromise. Clinically, impairments in tongue control contribute to swallowing deficits. As the disease progresses, these deficits cause debilitating health complications, including death from aspiration pneumonia. [4,14] The hallmark pathology in PD is characterized by loss of substantia nigra dopaminergic neurons with depletion of striatal dopamine. [15] However, the contribution of this pathology to the phenotypic expression of swallowing disorders is unclear, especially because swallow deficits do not reliably respond to surgical and pharmacological treatments that target nigrostriatal pathways. [9,13,16,25,28,33,36,39,48] Alternatively, exercise may be a promising intervention for these swallowing deficits. [17,40,46]
Importantly, animal studies using the unilateral 6-OHDA model have suggested that use of targeted training may reverse or slow disease progression as evidenced by striatal dopamine sparing for the limb. [1,11,50,52] However, targeted training for lingual deficits is largely understudied in both human and animal models. [46] Our recent work has shown that a unilateral lesion to the medial forebrain bundle with the catecholamine neurotoxin 6-hydroxydopamine (6- OHDA) leads to force and timing deficits during a complex licking task. [10] Specifically, maximal and average lingual forces were significantly diminished and average press time was significantly longer after neurotoxin administration, reflecting aspects of lingual bradykinesia and hypokinesia associated with PD. Because previous research in rat models of PD has shown that targeted training of the limb can rescue behavioral deficits and spare striatal dopamine content, [1,11,50,52] we explored the use of targeted training of the tongue on biological markers of striatal dopamine content (e.g., tyrosine hydroxylase) and behavioral outcomes during licking in a rat model of PD. We hypothesized that behavioral intervention targeting the tongue would improve timing and force outcomes during a complex licking task and lead to increased striatal dopamine content.
2. Materials and Methods
2.1 Animals
Nine month-old male Fisher344/Brown Norway rats (Charles River, Raleigh, NC) were used in this experiment (total n=30). Sixteen rats served in the Control condition and received tongue exercise (n=8) or no exercise (n=8). In the PD condition, one animal died during surgery and one animal was excluded from analysis because of an incomplete lesion (72% loss). As a result, there were a total of 14 animals in the PD condition that received tongue exercise (n=7) or no exercise (n=7). Thus, there were 4 experimental groups Control Exercise, Control No Exercise, PD Exercise, and PD No Exercise. Animals were housed in pairs in standard polycarbonate cages on a 12:12 light-dark reversed light cycle. Food was given ad libitum. Water was restricted to 3 hours per day during training, which has been shown to be safe while maintaining sufficient motivation. [53] Animal weight was monitored weekly; no rats exhibited signs of dehydration. All experiments were approved by the University of Wisconsin Institutional Animal Care and Use Committee (IACUC) and were conducted in accordance with the United States Public Health Service Guide for the Care and Use of Laboratory Animals
2.2 Experimental Overview
The experiment involved a 4-week introductory period including handling, light cycle reversal, and gradual water restriction/acclimation to the tongue force task. The introductory period was followed by 6-OHDA surgery for the animals in the PD condition. Animals were tested for behavioral tongue force and timing measures 72 hours after surgery and these data confirm deficits in the 6-OHDA animals as compared to pre-lesion (baseline) values which have been reported previously. [10] Rats in the Control and PD conditions were then randomized to undergo tongue exercise or no exercise, described below. After 4 weeks, rats in all conditions were tested for behavioral tongue force and timing measures during a complex licking task. Animals in the PD condition also underwent additional behavioral testing to confirm lesions, described below. After completion of all behavioral data collection, all rats underwent bilateral stimulation of the hypoglossal nerves to measure muscle contractile properties and were then transcardially perfused and brain tissues were collected for immunohistochemistry.
2.3.Parkinson disease model and behavioral testing
2.3.1 6-OHDA
In 14 animals (PD condition), severe degeneration of presynaptic striatal neurons was induced by unilateral infusion of 7 µg of 6-OHDA into the medial forebrain bundle. [21,22,27,30,31,43,52] 6-OHDA treated rats were anesthetized with 2–4% inhaled isoflurane, and were placed in a stereotaxic frame. Rats received unilateral infusions of 7 µg 6-OHDA hydrobromide (free base weight) dissolved in 3 µl artificial cerebrospinal fluid (composition: NaCl, KCl, CaCl2, MgCl2*6H20) containing 0.05% (w/v) ascorbic acid. Infusion coordinates were measured from bregma (−3.3 AP; ± 1.7 ML; −8.0 DV from dural surface), and infusions were delivered at a rate of .3 µl/min for 10 minutes. Infusions were into the nigrostriatal projections in the dominant hemisphere for forelimb use. Post-operation analgesia (0.1cc bupivacaine) was administered after suturing. Following surgery, animals were placed on a warm surface to prevent hypothermia, and upon waking were returned to their home cages.
2.3.2 Behavioral Tests
Rats treated with 6-OHDA (PD condition) underwent two behavioral tests to confirm severity of lesion. Forelimb-use asymmetry was tested before surgery to determine forelimb dominance for lesion target, post-surgery day 3 to confirm lesion (data reported elsewhere), [10] and after 4 weeks of training to confirm lesion severity (data reported here). Rats were placed in an upright acrylic cylinder (diameter 20 cm) to encourage rearing and exploratory movements with the forepaws. [49,55] The number of wall contacts made by either forelimb or by both forelimbs simultaneously was recorded. The percentage of contacts made by the non-impaired forelimb relative to the total number of contacts was calculated using the formula: (ipsilateral limb contacts + both (simultaneous or rapidly alternating) limb contacts)/ total number of contacts for the first 20 contacts. Scores were then multiplied by 100. Scores significantly above 50% indicate a greater reliance on the ipsilateral limb for voluntary movement and have been well-correlated with the degree of nigrostriatal dopamine depletion induced by 6-OHDA lesions. [49,55]
Apomorphine-induced rotational behavior was also examined at 7 days post-surgery and after 4 weeks of training. Rats were given 0.1 mg/kg apomorphine (s.c.), and the net number of contralateral turns made during a 2 min trial was recorded in revolutions per minute (25 min post injection) (modified from Herrera-Marschitz, Casas, & Ungerstedt, 1988). [27]
2.4 Tongue Force Testing and Training
After behavioral testing to determine PD lesion severity, rats from both the PD and Control conditions were randomized to undergo tongue force exercise or no exercise. Tongue force testing and tongue exercise employed a custom instrument that was designed based on previous research involving rodent models of licking behavior [8,12,27] that allowed us to modify and acquire tongue force and temporal measures during complex protrusive tongue movements. This set-up involved a traditional learning paradigm in which rats were trained to press a disk with their tongue by gradually restricting their access to water (see Connor et al, 2009 and Ciucci & Connor, 2009 for details). Throughout the experiment, animals were placed individually into a polycarbonate enclosure equipped with a 1×1 cm aperture and force operandum that delivered aliquots of water based on tongue press behaviors. Animals in the Exercise groups (Control Exercise and PD Exercise) were trained to press the tongue against the operandum to receive a water reward on a VR5 schedule with force requirements based on performance throughout the 4 weeks (see Connor, et al., 2009 for details). Animals were allowed 10 minutes to press the operandum and receive the water reward. The animals in the No Exercise groups (Control No Exercise and PD No Exercise) received water for 3 hours per day in an enclosure that resembled the force operandum enclosure but did not require a tongue press to extract water. Testing involved the same apparatus used in the training. However, forces were incremented quickly to obtain the maximal force during a 5 minute session. This was done for 3 days and the day with the highest forces was chosen for analysis (see Ciucci, 2011 for details).
2.5 Tongue force and temporal data analysis
Tongue presses were recorded at 200 Hertz (Hz) using custom-designed computer data acquisition software (Matrix Product Development, Cottage Grove, WI) and analyzed with Matlab software using custom designed algorithms. The following variables were measured: maximal tongue force in milliNewtons (mN), average tongue force (mN), and average press rate (presses/second). Maximum tongue force was the highest force achieved with a tongue press. Average tongue force was the average of the highest 10 tongue presses during a session. This was used as an alternative to averaging all data, as the water on the operandum registers a small force and forces are incremented up based on performance during testing. Further, average of the top 10 presses allows us to assess the ability to repeatedly press with a higher force across trials. Press rate was determined by the number of tongue presses that occurred per second and was determined over a two-minute period. For each rat, the two-minute period began when the rat initially approached the disk and initiated a press.
2.6 Bilateral hypoglossal stimulation
Tongue muscle contractile properties were recorded in vivo with supramaximal stimulation of the hypoglossal nerves, bilaterally. Rats were anesthetized via IP injection of sodium pentobarbital (70 mg/kg) and placed in a dorsal recumbent position on an operating table under an operating microscope (Zeiss, Opmi 6CH). This surgical approach has been documented previously in the research literature. [12] Hypoglossal nerves were exposed bilaterally using a ventral approach to allow placement of nerve cuff stimulation electrodes. A 2–5 mm section of the lateral branch of the hypoglossal nerve was cut and removed bilaterally. Following a 45-min stabilization period, a suture was placed into the tip of the tongue, for connection to a force transducer (Kent Scientific, Torrington, CT). Optimal direction and line tension on the suture was determined for each animal to yield maximum peak muscle forces. Whole hypoglossal nerves were then stimulated, but due to the cutting of lateral hypoglossal branch only the medial branch was effectively stimulated and protrusive muscle contractile properties were recorded. These isolated hypoglossal nerve stimulation pulses (1-Hz rectangular-wave pulses, pulse width 0.1 ms) were delivered at supramaximal levels. Supramaximal stimulation levels (1.5 times maximum stimulation level; generally between 300 and 500 µA; A-M Systems, Carlsborg, WA) controlled for small differences in stimulation electrode placement and contact. These stimulation parameters have been reported previously. [23] Three 10-sec trials, with a 1-min rest period between trials, were recorded per muscle.
Tetanic force, which is the maximal force of each stimulated fused wave, was measured and averaged across the three trials. The stimulation signal and force signal was acquired digitally on a dedicated laboratory computer equipped with an A/D converter (Data Translation, Marlboro, MA) using data acquisition software written and customized for our use (Acquire Ver. 1.3.0).
2.7 Immunohistochemistry
After completion of hypoglossal stimulation, rats were deeply anesthetized with 100mg/kg pentobarbital and intra-aortically perfused with 250 mL physiological saline one minute after an intracardial injection of 100 units of heparin. Immediately following, 500 mL of ice cold 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS) was perfused to fix brain tissue. Whole brains were removed and postfixed in ice cold fixative for 1–4 hours. Brains were then cryoprotected for 48–96 hours in a 20% sucrose/5% glycerol solution in 0.1M PBS at 4°C. Brains were mounted on a freezing microtome and one of every five 60 µm coronal slices throughout the basal ganglia were harvested and stored in PBS with 0.02% NaN3 at 4°C.
Floating slices were probed for tyrosine hydroxylase (TH) using a rabbit anti-tyrosine hydroxylase primary antibody (1:2000 dilution, Millipore, Billerica, MA, USA) and a biotinylated goat anti-rabbit secondary antibody (1:500 dilution, Millipore, Billerica, MA, USA). The signal was amplified using the VECTASTAIN Elite ABC avidin-biotin system (Vector Laboratories, Burlingame, CA, USA). Slices were incubated in primary antibody for 16 hours, in secondary for 3 hours, and avidin-biotin solution for 1 hour at room temperature. Labeling was visualized with 3,3'-diaminobenzidine (DAB) chromogen developed with a peroxidase reaction for 90 seconds. Slices were quenched, counterstained with haematoxylin, and mounted on gelatin-coated slides.
Brain slices were imaged on an Epson Perfection V500 Photo Scanner and uploaded to a computer (Dell Optiplex 960) and analyzed using ImageJ software (National Institutes of Health, Bethesda, MD). A custom-designed software program was developed in Image J to detect the optical density of thresholded values for neurons that were positive for TH immunoreactivity. The region of interest (striatum in each hemisphere) was identified manually, run through the analysis script, and values were expressed as a percent of thresholded values as compared with the non-injured hemisphere. Thus, the injured hemisphere is expressed as percent loss of TH in the striatum.
2.8 Statistical Analysis
A one-way ANOVA was performed after training to examine the effects of targeted tongue exercise on behavioral force and timing measures, as well as stimulated tongue forces. Post hoc analyses were performed with a Fisher’s LSD. In the case of unequal variances between groups, a log transformation was attempted. If unequal variances persisted, the non-parametric Kruskal Wallis Test One Way ANOVA by Ranks was used. Post-hoc testing was completed using the Fisher’s protected least significant difference (LSD) test or Wilcoxon rank sum test in the case of unequal variances.
Planned comparisons of behavioral measures of striatal dopamine loss (forelimb use asymmetry and apomorphine challenge) were performed with an independent samples t-test after surgery and after exercise between the PD No Exercise and PD Exercise groups. An independent samples t-test was used to compare striatal DA content for the PD No Exercise and PD Exercise groups. For all statistical tests an alpha level of less than 0.05 was set a priori for rejecting the null hypothesis.
3. Results
3.1 PD validation
3.1.1 Forelimb use asymmetry
As shown in Figure 1, the mean proportion of contralateral forelimb use (unimpaired limb) was 93% in the PD No Exercise group and 90% in the PD Exercise group after surgery (prior to training), indicating a severe unilateral Parkinsonian deficit in both groups and thus confirming lesion with behavior. [49,55] No differences were shown between groups prior to exercise (t (1, 16) = 0.29, p = 0.60), demonstrating that both groups were similar at baseline. After exercise, the mean proportion of contralateral forelimb use was 92% in the PD No Exercise group and 89% in the PD Exercise group, indicating that severe unilateral Parkinsonian deficits persisted in both groups equally. Again, no differences were shown between groups after exercise (t (1, 16) = 0.40, p = 0.54).
Figure 1.
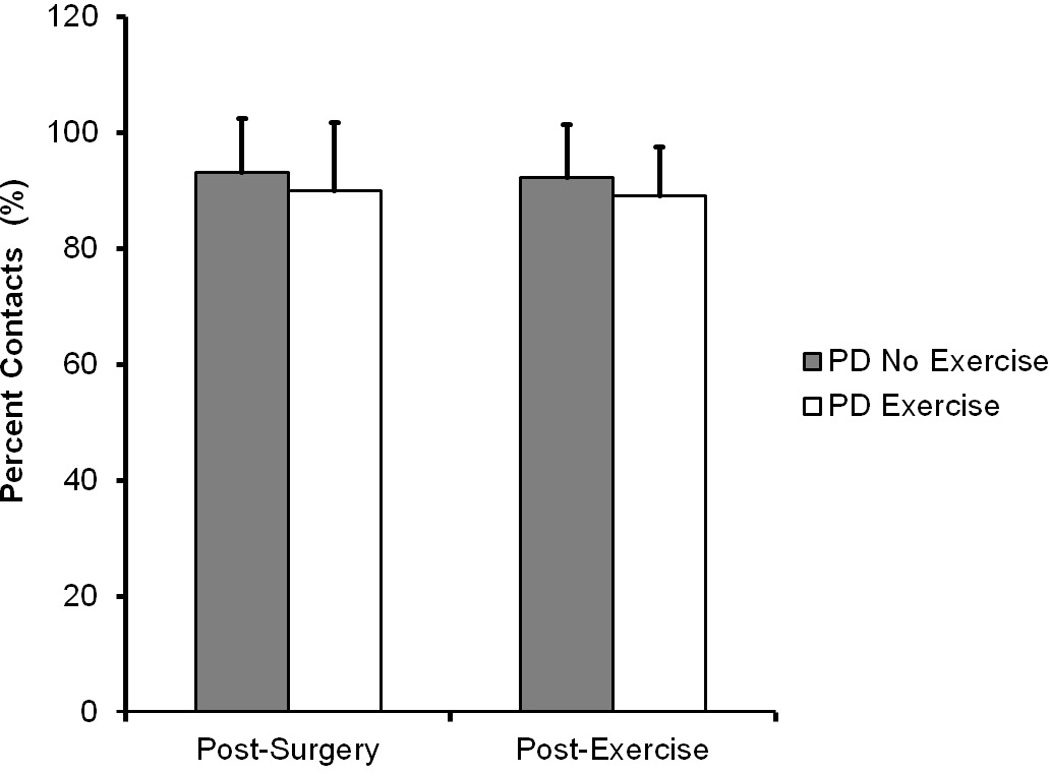
Forelimb Asymmetry Test (Cylinder test). Data are expressed as mean and standard deviatation of percentage of contacts made by the intact limb. A score of 50% indicates each forelimb is used to explore equally. 100% indicates use only by the intact limb and severe unilateral dopamine depletion. This figure shows severe forelimb deficits after surgery (left) and post-exercise (right) with no significant difference between groups. Comparisons were made with independent samples t-test.
3.1.2 Apomorphine
7 days after 6-OHDA infusions, average apomorphine rotations were 22 revolutions per minute for the PD No Exercise group and 23 revolutions per minute for the PD Exercise group, without any differences between the 2 groups (t (1, 16=.54). After exercise, average apomorphine rotations were 26 revolutions per minute for the PD No Exercise group and 33 revolutions per minute for the PD Exercise group, without any differences between the 2 groups(t (1, 16=.45), confirming that lesions were severe and unchanged during the study.
3.1.3 TH immunoreactivity
Percent loss of striatal dopamine as measured by TH immunoreactivity was 98% for the PD No Exercise group and 99% for the PD Exercise group (Figure 2). These results suggest severe lesions that were confirmed with behavioral testing after surgery persisted after exercise intervention, indicating no change in striatal dopamine synthesis as a result of exercise intervention.
Figure 2.
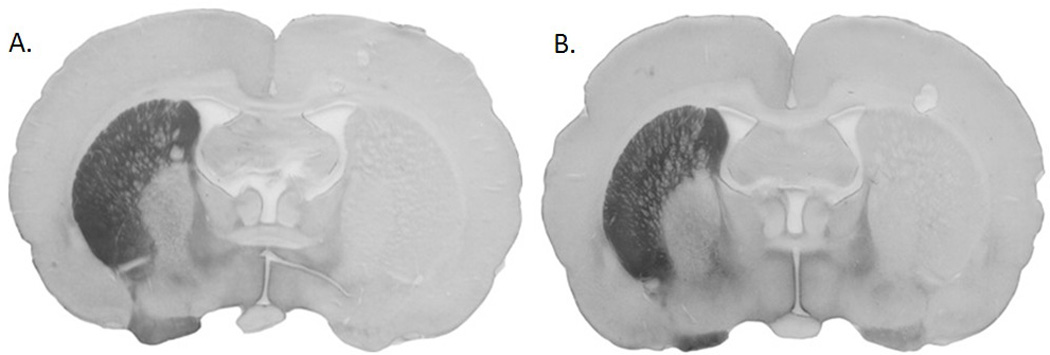
Coronal slices of rat brains labeled (brown) for immunoreactivity to tyrosine hydroxylase. Left side of slice (right striatum) is the intact hemisphere with tyrosine hydroxylase-positive labeling. Right side of each slice (left striatum) shows dopamine depletion. The rat represented on the left (A) was in the PD No Exercise group. Rat represented on the right (B) was in the PD Exercise group. This figure shows that striatal dopamine content was not associated with behavioral rescue of tongue forces.
3.2 Tongue Forces
3.2.1 Maximum Tongue Force
As shown in Figure 3, there were significant differences among groups for maximal tongue force after exercise (F (3, 26) = 16.51, p < 0.001). Maximal force for the PD Exercise group was significantly higher than the PD No Exercise group (p<0.001), demonstrating exercise-induced improvement in tongue force for the PD Exercise group. Importantly, maximal force in the PD Exercise group was not significantly different from the Control No Exercise group (p = 0.5), indicating recovery of function for the PD exercise group. Maximal forces in the PD No Exercise group were also lower than Control Exercise (p < 0.001) and Control No Exercise (p < 0.001) groups. Maximal force in the Control Exercise group was significantly higher than the Control No Exercise group (p =0.002), indicating animals that underwent exercise had higher maximal tongue forces than their no exercise counterparts.
Figure 3.
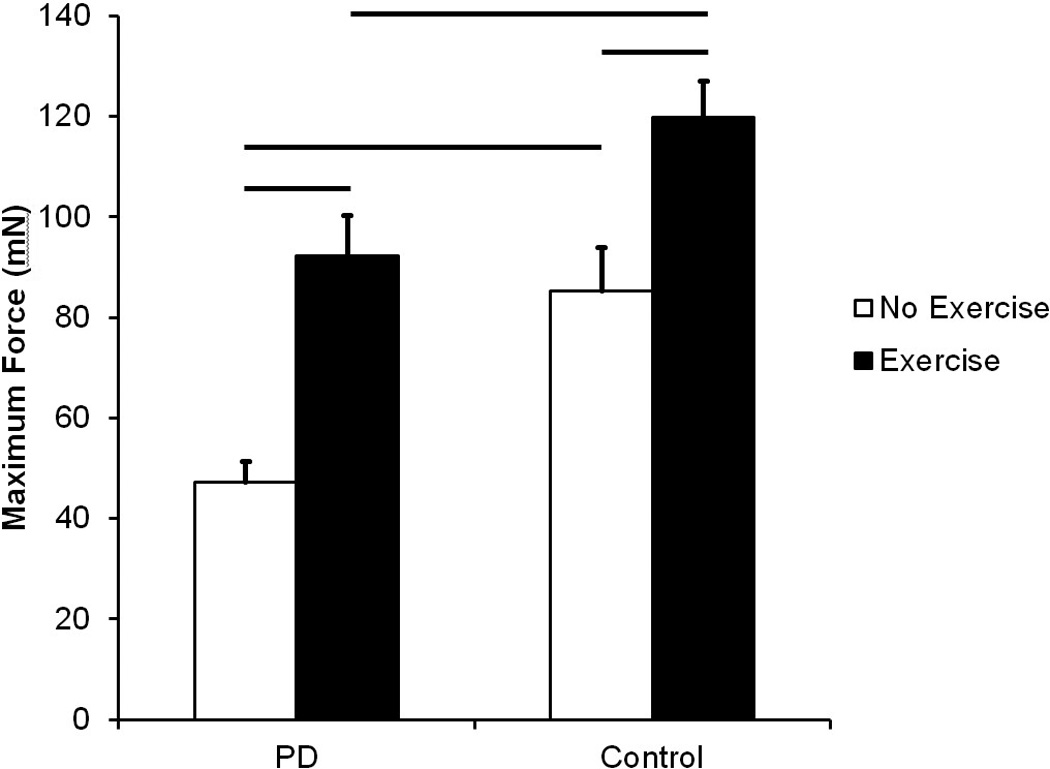
Maximum Tongue Force in milliNewtons (mN). Data are expressed as mean and SEM. Both the PD No Exercise group and the PD Exercise group had significantly lower maximum tongue forces than the Control No Exercise group and the Control Exercise group, respectively. Significant increases in maximum tongue force were found with exercise in both conditions. Comparisons were made using a Kruskal Wallis Test One Way analysis of variance (ANOVA) by Ranks and post-hoc testing was completed using Wilcoxon rank sum test. Bars represent p-value < 0.05.
3.2.2 Average Tongue Force
Figure 4 shows results for average tongue force. There were significant differences among groups for average tongue force after exercise (F (3, 26) = 6.68, p= 0.002). Average tongue force was significantly higher in the PD Exercise group than the PD No Exercise group (p = 0.01), demonstrating exercise-induced changes to tongue force. Akin to maximal tongue force measures, there was no significant difference in average force between PD Exercise group and the Control No Exercise group (p = 0.57), demonstrating recovery of deficits for the PD Exercise group. Average tongue force for the PD No Exercise group was significantly lower than the Control No Exercise group (p = 0.038) and the Control Exercise group (p < 0.001). Average tongue force was significantly higher in the Control Exercise group compared to the Control No Exercise group (p = 0.028), indicating rats that underwent tongue exercise had higher average forces than their no exercise counterparts.
Figure 4.
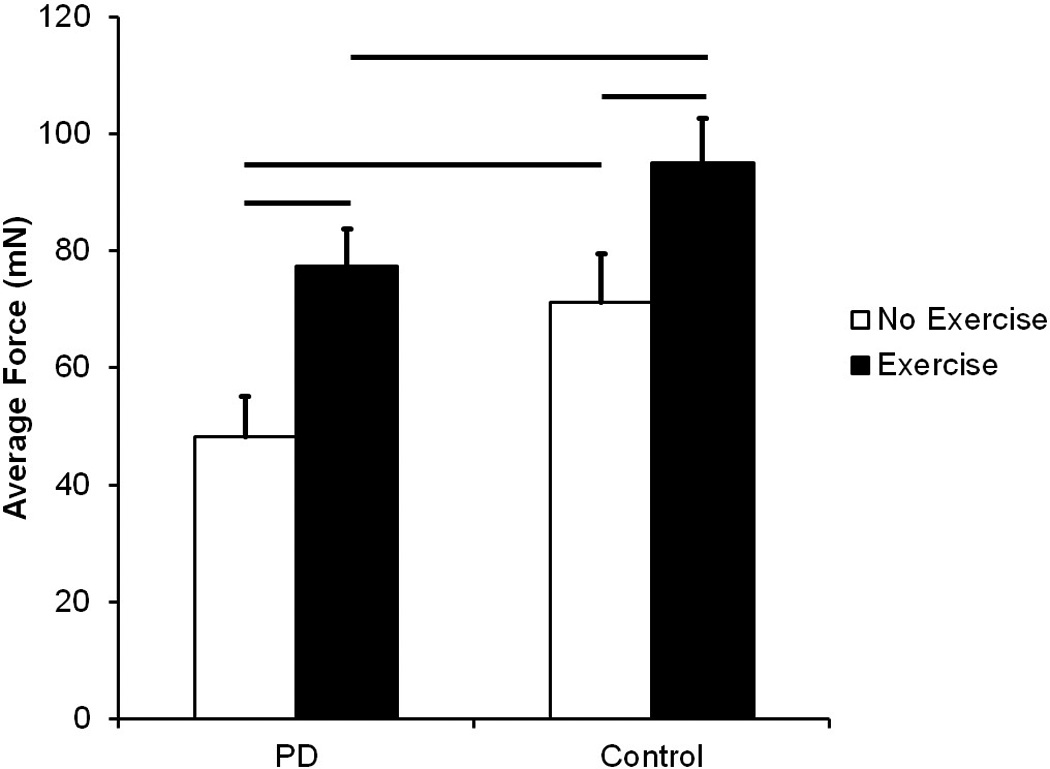
Average Tongue Force in milliNewtons (mN). Data are expressed as mean and SEM. Both the PD No Exercise group and the PD Exercise group had significantly lower average tongue forces than the Control No Exercise group and the Control Exercise group, respectively. Significant increases in average tongue force were found with exercise in both conditions. Comparisons were made using a one-way analysis of variance (ANOVA) and post hoc analyses were performed using Fisher’s LSD. Bars represent p-value < 0.05.
3.3 Timing
As shown in Figure 5, there were significant differences among groups in the rate of tongue press after exercise (F (3, 22) = 37.32, p <0.001). The rate of tongue press for the PD Exercise group was significantly higher than for the PD No Exercise group (p <0.001). Importantly, and similar to the force data, the rate of tongue press in the PD Exercise group was not significantly different from the rate of tongue press in the Control No Exercise group (p = 0.12), demonstrating recovery of function. The rate of tongue press for the PD No Exercise group was also significantly lower than the Control No Exercise (p < 0.001) and Control Exercise (p < 0.001) groups. The rate of tongue press in the Control Exercise group was significantly higher than the rate of tongue press in the Control No Exercise group (p < 0.001), demonstrating exercise was associated with significantly higher tongue press rates.
Figure 5.
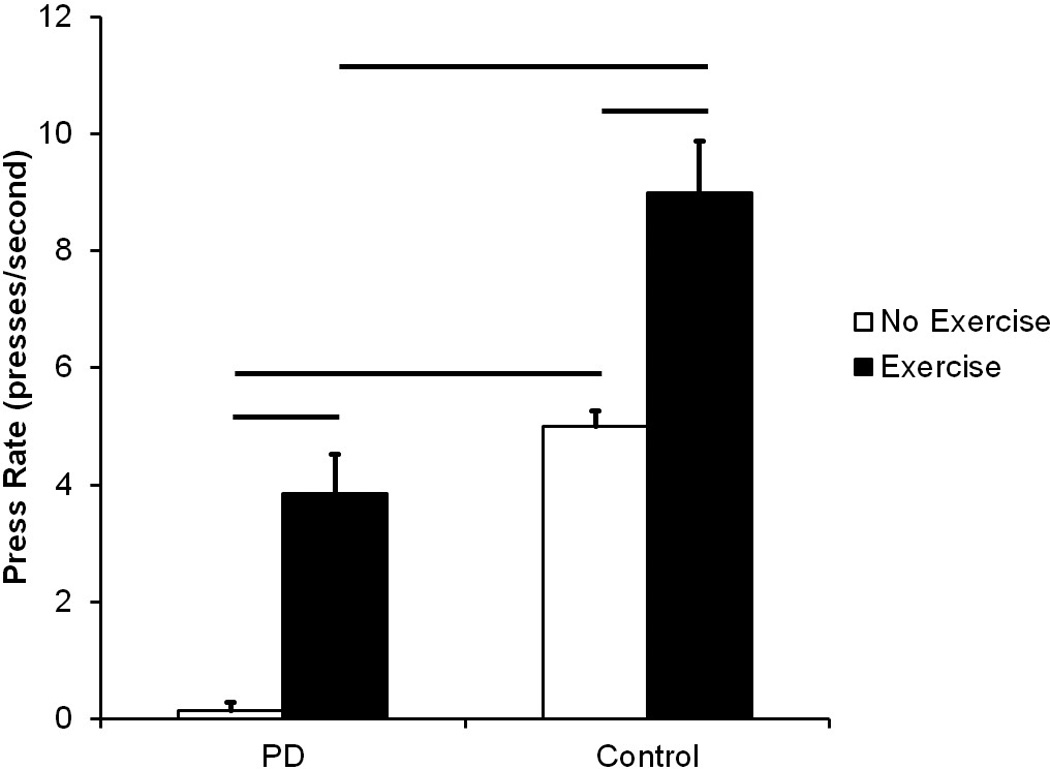
Rate of tongue press in presses/second. Data are expressed as mean and SEM. Both the PD No Exercise group and the PD Exercise group had significantly lower tongue press rates than the Control No Exercise group and the Control Exercise group, respectively. Significant increases in tongue press rates were found with exercise in both conditions. Comparisons were made using a Kruskal Wallis Test One Way analysis of variance (ANOVA) by Ranks and post-hoc testing was completed using Wilcoxon rank sum test. Bars represent p-value < 0.05.
3.4 Stimulation
As shown in Figure 6, no there were no significant differences between groups for tetanic force (F (3, 22) = 0.25, p = 0.86).
Figure 6.
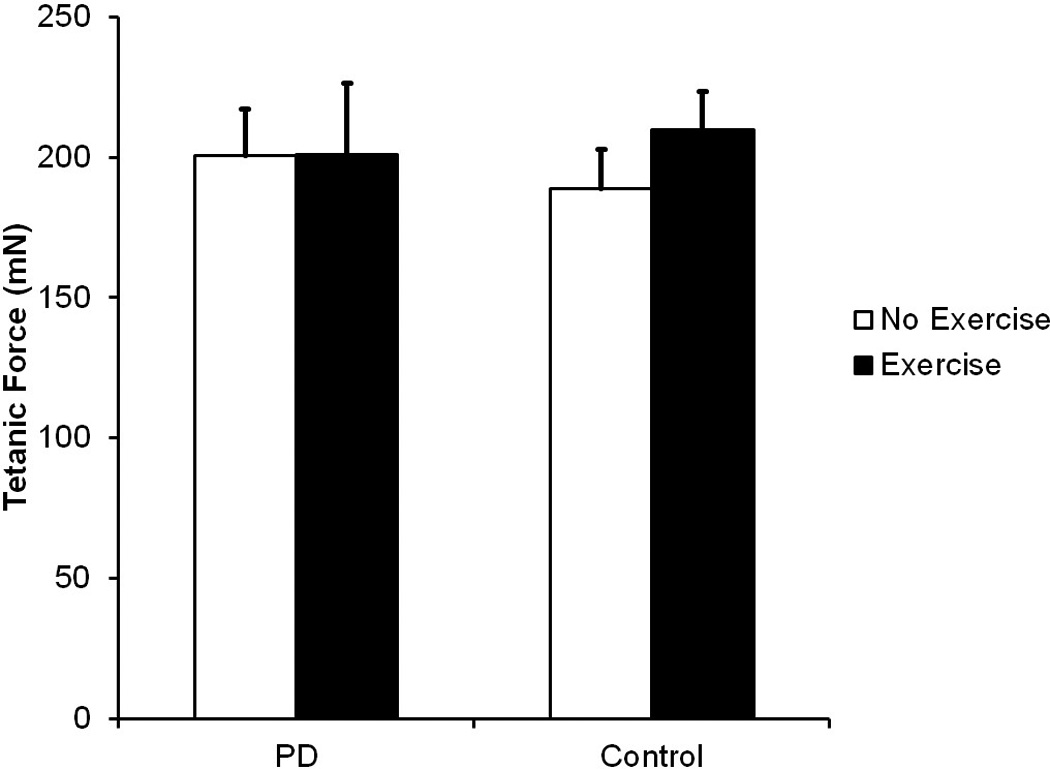
Tetanic Force in milliNewtons (mN). Data are expressed as mean and SEM. No significant differences were found. Comparisons were made using a one-way analysis of variance (ANOVA) and post hoc analyses were performed using Fisher’s LSD
4. Discussion
The aim of this study was to determine if tongue exercise could improve behavioral tongue force and timing characteristics and lead to increased striatal dopamine synthesis in the striatum in a 6-OHDA neurotoxin model of PD. Animals with unilateral 6-OHDA lesions to the medial forebrain bundle and control animals underwent a tongue exercise paradigm or no exercise. Behavioral tongue forces and press rate were measured after 4 weeks of exercise. Results demonstrated that the animals that underwent exercise had higher maximal force, average force, and press rate than their no exercise counterparts, for both the Control and PD conditions. In fact, the PD Exercise group performed as well as the Control No Exercise group on all parameters after tongue exercise. Specific to timing measures, the PD No Exercise group had very low press rates that were even lower than their baseline measures, reported previously. [10] We speculate that their deficits worsened over the 4 week period, whereas the PD Exercise group recovered function as a result of tongue exercise. Nonuse-related decline in function is documented in the rodent literature [51] and certainly the No Exercise animals had less opportunity with licking behavior than the Exercise animals. We explored whether the increased behavioral tongue forces were related to improved function at the level of the muscle. To test this, rats underwent bilateral stimulation of the hypoglossal nerve and we measured tetanic muscle force. Stimulated tetanic force assesses the muscle’s ability to maximally contract and is an indicator of overall muscle strength. This technique has been used to assess muscle capability after implementation of similar tongue exercise programs, and in young and aged rats; tetanic forces increase after 8 weeks of exercise, [3] indicating a peripheral mechanism of recovery. Interestingly, in the current study, there were no group differences for tetanic muscle force in the stimulated condition, despite behavioral recovery of forces. However, the current study only used 4 weeks of exercise to examine the acute effects of exercise on behavioral tongue forces. This could explain, in part, why we didn’t observe significant differences at the muscular level. The similar tetanic forces among groups despite behavior recovery indicate that changes within the sensorimotor system are likely occurring via modulation at the level of the neuromuscular junction, the cranial nerve nuclei, or perhaps centrally. This is an important finding in the parkinsonian rats, as PD typically does not affect muscle strength or muscle fiber type, and exercise does not necessarily need to be directed at changing these parameters. Rather, sensorimotor deficits associated with PD are related to an issue with central drive to the muscles. [24] Thus, training, even for a shorter period of time, can lead to rescue of behavior without changes to the muscle. This in part explains how exercise paradigms that use increased effort, calibration of internal sense of effort, or external targets can successfully lead to positive behavioral outcomes. [2,17,18,40,44–46,56]
However, contrary to our hypothesis, behavioral and neurochemical analyses indicate that there was no change to striatal dopamine synthesis. We did not observe significant differences in behavioral measures of striatal dopamine integrity (forelimb use asymmetry scores), normalization of up-regulated receptors in the lesioned hemisphere (apomorphine challenge), and importantly, we did not see any significant differences in striatal dopamine content measured by TH. One hypothesis is that our 1 in 5 sample was missing some potential recovery in orofacial regions, since the basal ganglia have a somatotopic arrangement. However, we sampled each slice throughout the entire striata in 4 of the PD Exercise animals and did not observe any increased levels of TH in orofacial regions. This is in stark contrast to what has been found in the limbs with targeted exercise. [1,11,50,52] Other studies of general exercise, such as treadmill running, show increased dopamine release. [19,35,38] However, our immunohistochemistry shows severe depletions (98, 99%), rendering this unlikely. It is also possible that other mechanisms of recovery may have occurred. Recent work suggests that with certain types of exercise, neural modulation may occur by down regulation of dopamine uptake and metabolism, [5,38,52] upregulation of post-synaptic receptors, [20,29] and decreased corticostriatal release of glutamate. [20,31,37,54] However, these experiments have not been done for cranial sensorimotor functions and were not done in this study. Regardless of the mechanism, this dissociation of modulation within limb versus cranial targeted training underscores the vital differences between limb and cranial sensorimotor control mechanisms.
It is also interesting to consider the bilateral innervation of the cranial sensorimotor system, and the intact hemisphere as possible source of compensation in this study. A bilateral lesion model would be an interesting method to examine this hypothesis. However, a unilateral model of dopamine depletion was chosen for several reasons. First, individuals with PD often present with asymmetric nigrostriatal dopamine depletion [6] and this unilateral model can represent earlier stages of PD. Second, unilateral 6-OHDA models in rats have consistently shown cranial sensorimotor deficits with regard to vocalization, [7] tongue use, [10,41] biting, [41] and oropharyngeal swallowing. [47] Bilateral models of dopamine depletion cause more severe deficits and are often associated with high levels of rodent mortality, rendering treatment studies challenging. A recent study compared unilateral to bilateral dopamine depletion in a licking task akin to the one used in the current study. [34] Both unilateral and bilateral lesions caused reductions in licking force, however, only the unilateral lesion group showed deficits in tongue motility. [34] These results suggested that unilateral deficits can represent an interhemispheric imbalance in terms of sensorimotor control. [34,41] Taken together with our results, a unilateral model is appropriate for studying aspects of PD, specifically nigrostriatal dopamine depletion. However, it would be interesting to examine the effects of targeted training with a bilateral model in future studies.
Our study shows that intensive targeted exercise of the tongue leads to improvement in behavioral tongue force and timing parameters, but not stimulated muscle forces or rescue of striatal dopamine content. Importantly, data from this study suggest that there are likely central, rather than peripheral (muscle) mechanisms of behavioral rescue of tongue force and timing characteristics that do not involve total striatal dopamine content. This study shows that exercise is a valuable treatment for lingual deficits and highlights that there are important differences between limb and cranial sensorimotor control that should be considered for investigation and treatment of PD.
Research Highlights.
Tongue exercise improves lingual force and timing deficits in a model of Parkinson Disease
Tongue exercise was not related to improved stimulated tetanic muscle forces
Tongue exercise was not related to striatal dopamine content rescue
Acknowledgements
We would like to thank Dr. Glen Leverson for statistical analysis. This work was funded by the National Institutes for Health, National Institute on Deafness and Other Communication Disorders P30DC010754 (Ciucci), R01 DC 005935 (Schaser, Russell), R01 DC 008149 (Ciucci, Schaser, Russell), T32 DC009401 (Schaser).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michelle R Ciucci, Assistant Professor, Department of Surgery-Division of Otolaryngology Head and Neck Surgery, Department of Communication Sciences and Disorders, 1975 Willow Drive, Madison, WI 53705, ciucci@surgery.wisc.edu, 608.262.6122, Fax: 608.265.0925.
Allison J Schaser, Department of Surgery-Division of Otolaryngology Head and Neck Surgery, Department of Communication Sciences Disorders, 1975 Willow Drive, Madison, WI 53705.
John A Russell, Department of Surgery-Division of Otolaryngology Head and Neck Surgery, Department of Communication Sciences and Disorders, 1975 Willow Drive, Madison, WI 53705.
References
- 1.Anstrom KK, Schallert T, Woodlee MT, Shattuck A, Roberts DC. Repetitive vibrissae-elicited forelimb placing before and immediately after unilateral 6-hydroxydopamine improves outcome in a model of Parkinson's disease. Behavioural Brain Research. 2007;179:183–191. doi: 10.1016/j.bbr.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner CA, Sapir S, Ramig TO. Voice quality changes following phonatory-respiratory effort treatment (LSVT) versus respiratory effort treatment for individuals with Parkinson disease. Journal of Voice. 2001;15:105–114. doi: 10.1016/s0892-1997(01)00010-8. [DOI] [PubMed] [Google Scholar]
- 3.Behan M, Moeser AE, Thomas CF, Russell JA, Wang H, Leverson GE, Connor NP. The effect of tongue exercise on serotonergic input to the hypoglossal nucleus in young and old rats. Journal of Speech, Language, and Hearing Research. 2012 doi: 10.1044/1092-4388(2011/11-0091). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beyer MK, Herlofson K, Arsland D, Larsen JP. Causes of death in a community-based study of Parkinson's disease. Acta Neurologica Scandinavica. 2001;103:7–11. doi: 10.1034/j.1600-0404.2001.00191.x. [DOI] [PubMed] [Google Scholar]
- 5.Bezard E, Dovero S, Belin D, Duconger S. Enriched environment confers resistance to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and cocaine: involvement of dopamine transporter and trophic factors. J Neurosci. 2003;23:10999–11007. doi: 10.1523/JNEUROSCI.23-35-10999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blesa J, Juri C, García-Cabezas MÁ, Adánez R, Sánchez-González MC1, Cavada C, Obeso JA. Inter-hemispheric asymmetry of nigrostriatal dopaminergic lesion: a possible compensatory mechanism in Parkinson's disease. Front Syst Neurosci. 2011;5:92. doi: 10.3389/fnsys.2011.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciucci MR, Ahrens AM, Ma ST, Kane JR, Windham EB, Woodlee MT, Schallert T. Reduction of dopamine synaptic activity: Degradation of 50-khz ultrasonic vocalization in rats. Behavioral Neuroscience. 2009;123:328–336. doi: 10.1037/a0014593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciucci MR, Connor NP. Dopaminergic influence on rat tongue function and limb movement initiation. Experimental Brain Research. 2009;194:587–596. doi: 10.1007/s00221-009-1736-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciucci MR, Ma ST, Kane JR, Ahrens AM, Schallert T. Limb use and complex ultrasonic vocalization in a rat model of Parkinson's disease: deficit-targeted training. Parkinsonism & Related Disorders. 2008;14(Suppl 2):S172–S175. doi: 10.1016/j.parkreldis.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciucci MR, Russell JA, Schaser AJ, Doll EJ, Vinney LM, Connor NP. Tongue force and timing deficits in a rat model of Parkinson disease. Behavioural Brain Research. 2011;222:315–320. doi: 10.1016/j.bbr.2011.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen AD, Tillerson JL, Smith AD, Schallert T, Zigmond MJ. Neuroprotective effects of prior limb use in 6-hydroxydopamine-treated rats: possible role of GDNF. J Neurochem. 2003;85:299–305. doi: 10.1046/j.1471-4159.2003.01657.x. [DOI] [PubMed] [Google Scholar]
- 12.Connor NP, Russell JA, Wang H, Jackson MA, Mann L, Kluender K. Effect of tongue exercise on protrusive force and muscle fiber area in aging rats. Journal of Speech, Language, and Hearing Research. 2009;52:732–744. doi: 10.1044/1092-4388(2008/08-0105). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Alatri L, Paludetti G, Contarino MF, Galla S, Marchese MR, Bentivoglio AR. Effects of bilateral subthalamic nucleus stimulation and medication on parkinsonian speech impairment. Journal of Voice. 2008;22:365–372. doi: 10.1016/j.jvoice.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 14.D'Amelio M, Ragonese P, Morgante L, Reggio A, Callari G, Salemi G, Savettieri G. Long-term survival of Parkinson's disease: a population-based study. Journal of Neurology. 2006;253:33–37. doi: 10.1007/s00415-005-0916-7. [DOI] [PubMed] [Google Scholar]
- 15.Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 16.Dromey C, Kumar R, Lang AE, Lozano AM. An investigation of the effects of subthalamic nucleus stimulation on acoustic measures of voice. Movement Disorders. 2000;15:1132–1138. doi: 10.1002/1531-8257(200011)15:6<1132::aid-mds1011>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 17.El Sharkawi A, Ramig L, Logemann JA, Pauloski BR, Rademaker AW, Smith CH, Pawlas A, Baum S, Werner C. Swallowing and voice effects of Lee Silverman Voice Treatment (LSVT): a pilot study. Journal of Neurology, Neurosurgery, and Psychiatry. 2002;72:31–36. doi: 10.1136/jnnp.72.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farley BG, Koshland GF. Training BIG to move faster: the application of the speed-amplitude relation as a rehabilitation strategy for people with Parkinson's disease. Experimental Brain Research. 2005;167:462–467. doi: 10.1007/s00221-005-0179-7. [DOI] [PubMed] [Google Scholar]
- 19.Fisher BE, Petzinger GM, Nixon K, Hogg E, Bremmer S, Meshul CK, Jakowec MW. Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse basal ganglia. Journal of Neuroscience Research. 2004;77:378–390. doi: 10.1002/jnr.20162. [DOI] [PubMed] [Google Scholar]
- 20.Fisher BE, Wu AD, Salem GJ, Song J, Lin CH, Yip J, Cen S, Gordon J, Jakowec M, Petzinger G. The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson's disease. Archives of Physical Medicine and Rehabilitation. 2008;89:1221–1229. doi: 10.1016/j.apmr.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleming SM, Delville Y, Schallert T. An intermittent, controlled-rate, slow progressive degeneration model of Parkinson's disease: antiparkinson effects of Sinemet and protective effects of methylphenidate. Behavioural Brain Research. 2005;156:201–213. doi: 10.1016/j.bbr.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 22.Fulceri F, Biagioni F, Lenzi P, Falleni A, Gesi M, Ruggieri S, Fornai F. Nigrostriatal damage with 6-OHDA: validation of routinely applied procedures. Ann N Y Acad Sci. 2006;1074:344–348. doi: 10.1196/annals.1369.032. [DOI] [PubMed] [Google Scholar]
- 23.Gilliam EE, Goldberg SJ. Contractile properties of the tongue muscles: effects of hypoglossal nerve and extracellular motoneuron stimulation in rat. Journal of Neurophysiology. 1995;74:547–555. doi: 10.1152/jn.1995.74.2.547. [DOI] [PubMed] [Google Scholar]
- 24.Hallett M, Khoshbin S. A physiological mechanism of bradykinesia. Brain. 1980;103:301–314. doi: 10.1093/brain/103.2.301. [DOI] [PubMed] [Google Scholar]
- 25.Hammer MJ, Barlow SM, Lyons KE, Pahwa R. Subthalamic nucleus deep brain stimulation changes velopharyngeal control in Parkinson's disease. Journal of Communication Disorders. 2011;44:37–48. doi: 10.1016/j.jcomdis.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartelius L, Svensson P. Speech and swallowing symptoms associated with Parkinson's disease and multiple sclerosis: a survey. Folia Phoniatrica et Logopaedica. 1994;46:9–17. doi: 10.1159/000266286. [DOI] [PubMed] [Google Scholar]
- 27.Herrera-Marschitz M, Casas M, Ungerstedt U. Caffeine produces contralateral rotation in rats with unilateral dopamine denervation: comparisons with apomorphine-induced responses. Psychopharmacology. 1988;94:38–45. doi: 10.1007/BF00735878. [DOI] [PubMed] [Google Scholar]
- 28.Klostermann F, Ehlen F, Vesper J, Nubel K, Gross M, Marzinzik F, Curio G, Sappok T. Effects of subthalamic deep brain stimulation on dysarthrophonia in Parkinson's disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2008;79:522–529. doi: 10.1136/jnnp.2007.123323. [DOI] [PubMed] [Google Scholar]
- 29.Li Q, Fisher B, Nacca A, Leahy RM, Walsh JP, Mukherjee J, Williams C, Jakowec MW, Petzinger GM. Exercise elevates dopamine D2 receptor in a mouse model of Parkinson's disease: in vivo imaging with [18F]fallypride. Movement Disorders. 2010;25:2777–2784. doi: 10.1002/mds.23407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marshall JF. Somatosensory inattention after dopamine-depleting intracerebral 6-OHDA injections: spontaneous recovery and pharmacological control. Brain Research. 1979;177:311–324. doi: 10.1016/0006-8993(79)90782-0. [DOI] [PubMed] [Google Scholar]
- 31.Meshul CK, Cogen JP, Cheng HW, Moore C, Krentz L, McNeill TH. Alterations in rat striatal glutamate synapses following a lesion of the cortico- and/or nigrostriatal pathway. Experimental Neurology. 2000;165:191–206. doi: 10.1006/exnr.2000.7467. [DOI] [PubMed] [Google Scholar]
- 32.Miller N, Noble E, Jones D, Burn D. Hard to swallow: dysphagia in Parkinson's disease. Age and Ageing. 2006;35:614–618. doi: 10.1093/ageing/afl105. [DOI] [PubMed] [Google Scholar]
- 33.Narayana S, Jacks A, Robin DA, Poizner H, Zhang W, Franklin C, Liotti M, Vogel D, Fox PT. A noninvasive imaging approach to understanding speech changes following deep brain stimulation in Parkinson's disease. American Journal of Speech-Language Pathology. 2009;18:146–161. doi: 10.1044/1058-0360(2008/08-0004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nuckolls AL, Worley C, Leto C, Zhang H, Morris JK, Stanford JA. Tongue force and tongue motility are differently affected by unilateral vs bilateral nigrostriatal dopamine depletion in rats. Behavioural Brain Research. 2012;234:343–348. doi: 10.1016/j.bbr.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Dell SJ, Gross NB, Fricks AN, Casiano BD, Nguyen TB, Marshall JF. Running wheel exercise enhances recovery from nigrostriatal dopamine injury without inducing neuroprotection. Neuroscience. 2007;144:1141–1151. doi: 10.1016/j.neuroscience.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 36.Pereira EA, Muthusamy KA, De Pennington N, Joint CA, Aziz TZ. Deep brain stimulation of the pedunculopontine nucleus in Parkinson's disease. Preliminary experience at Oxford. British Journal of Neurosurgery. 2008;22(Suppl 1):S41–S44. doi: 10.1080/02688690802448335. [DOI] [PubMed] [Google Scholar]
- 37.Petzinger GM, Fisher BE, Van Leeuwen JE, Vukovic M, Akopian G, Meshul CK, Holschneider DP, Nacca A, Walsh JP, Jakowec MW. Enhancing neuroplasticity in the basal ganglia: the role of exercise in Parkinson's disease. Movement Disorders. 2010;25(Suppl 1):S141–S145. doi: 10.1002/mds.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petzinger GM, Walsh JP, Akopian G, Hogg E, Jakowec MW. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci. 2007;27:5291–5300. doi: 10.1523/JNEUROSCI.1069-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinto S, Ozsancak C, Tripoliti E, Thobois S, Limousin-Dowsey P, Auzou P. Treatments for dysarthria in Parkinson's disease. Lancet Neurology. 2004;3:547–556. doi: 10.1016/S1474-4422(04)00854-3. [DOI] [PubMed] [Google Scholar]
- 40.Pitts T, Bolser D, Rosenbek J, Troche M, Okun MS, Sapienza C. Impact of expiratory muscle strength training on voluntary cough and swallow function in Parkinson disease. Chest. 2009;135:1301–1308. doi: 10.1378/chest.08-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plowman EK, Maling N, Rivera BJ, Larson K, Thomas NJ, Fowler SC, Manfredsson FP, Shrivastav R, Kleim JA. Differential sensitivity of cranial and limb motor function to nigrostriatal dopamine depletion. Behavioural Brain Research. 2013;237:157–163. doi: 10.1016/j.bbr.2012.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plowman-Prine EK, Sapienza CM, Okun MS, Pollock SL, Jacobson C, Wu SS, Rosenbek JC. The relationship between quality of life and swallowing in Parkinson's disease. Movement Disorders. 2009;24:1352–1358. doi: 10.1002/mds.22617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poulton NP, Muir GD. Treadmill training ameliorates dopamine loss but not behavioral deficits in hemi-parkinsonian rats. Experimental Neurology. 2005;193:181–197. doi: 10.1016/j.expneurol.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 44.Ramig LO, Countryman S, O'Brien C, Hoehn M, Thompson L. Intensive speech treatment for patients with Parkinson's disease: short-and long-term comparison of two techniques. Neurology. 1996;47:1496–1504. doi: 10.1212/wnl.47.6.1496. [DOI] [PubMed] [Google Scholar]
- 45.Ramig LO, Countryman S, Thompson LL, Horii Y. Comparison of two forms of intensive speech treatment for Parkinson disease. Journal of Speech and Hearing Research. 1995;38:1232–1251. doi: 10.1044/jshr.3806.1232. [DOI] [PubMed] [Google Scholar]
- 46.Russell JA, Ciucci MR, Connor NP, Schallert T. Targeted exercise therapy for voice and swallow in persons with Parkinson's disease. Brain Research. 2010;1341:3–11. doi: 10.1016/j.brainres.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russell JA, Ciucci MR, Hammer MJ, Connor NP. Videofluorographic assessment of rodent swallow in aging and Parkinson disease. Dysphagia. 2013;28:95–104. doi: 10.1007/s00455-012-9417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sapir S, Ramig L, Fox C. Speech and swallowing disorders in Parkinson disease. Current Opinion in Otolaryngology & Head and Neck Surgery. 2008;16:205–210. doi: 10.1097/MOO.0b013e3282febd3a. [DOI] [PubMed] [Google Scholar]
- 49.Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 50.Smith AD, Zigmond MJ. Can the brain be protected through exercise? Lessons from an animal model of parkinsonism. Experimental Neurology. 2003;184:31–39. doi: 10.1016/j.expneurol.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 51.Tillerson JL, Cohen AD, Caudle WM, Zigmond MJ, Schallert T, Miller GW. Forced nonuse in unilateral parkinsonian rats exacerbates injury. J Neurosci. 2002;22:6790–6799. doi: 10.1523/JNEUROSCI.22-15-06790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tillerson JL, Cohen AD, Philhower J, Miller GW, Zigmond MJ, Schallert T. Forced limb-use effects on the behavioral and neurochemical effects of 6-hydroxydopamine. J Neurosci. 2001;21:4427–4435. doi: 10.1523/JNEUROSCI.21-12-04427.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toth LA, Gardiner TW. Food and water restriction protocols: physiological and behavioral considerations. Contemporary Topics in Laboratory Animal Science. 2000;39:9–17. [PubMed] [Google Scholar]
- 54.VanLeeuwen JE, Petzinger GM, Walsh JP, Akopian GK, Vuckovic M, Jakowec MW. Altered AMPA receptor expression with treadmill exercise in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. Journal of Neuroscience Research. 2010;88:650–668. doi: 10.1002/jnr.22216. [DOI] [PubMed] [Google Scholar]
- 55.Woodlee MT, Schallert T. The interplay between behavior and neurodegeneration in rat models of Parkinson's disease and stroke. Restorative Neurology and Neuroscience. 2004;22:153–161. [PubMed] [Google Scholar]
- 56.Wu T, Wang L, Hallett M, Chen Y, Li K, Chan P. Effective connectivity of brain networks during self-initiated movement in Parkinson's disease. NeuroImage. 2011;55:204–215. doi: 10.1016/j.neuroimage.2010.11.074. [DOI] [PubMed] [Google Scholar]


