Abstract
Cancer cells commonly have characteristic changes in metabolism. Cellular proliferation, a common feature of all cancers, requires fatty acids for synthesis of membranes and signaling molecules. Here, we provide a view of cancer cell metabolism from a lipid perspective, and we summarize evidence that limiting fatty acid availability can control cancer cell proliferation.
Introduction
Although cancers are hugely diverse in type and etiology, cancer cells frequently share the attribute of metabolic abnormalities. For example, glucose metabolism is commonly altered to decouple glycolysis from pyruvate oxidation (the Warburg effect) so that carbohydrates are not used for maximal ATP generation via mitochondrial respiration, despite high oxygen availability. A better understanding of these metabolic changes has prompted new approaches toward cancer therapy (reviewed in Hsu and Sabatini, 2008; Schulze and Harris, 2012).
Alterations in fatty acid (FA) metabolism in cancer cells have received less attention but are increasingly being recognized. FAs consist of a terminal carboxyl group and a hydrocarbon chain, mostly occurring in even numbers of carbons, that can be either saturated or unsaturated. They are required for energy storage, membrane proliferation, and the generation of signaling molecules. Here, we provide a brief review of metabolism in cancer cells, focusing on pathways of FA synthesis and storage. Furthermore, we examine a model for attenuating cancer cell proliferation and metastasis by manipulating FA metabolism to diminish FA availability. Due to the great diversity of cancer cells, our perspective is meant to be provocative, not universal. Nevertheless, our intention is to provide a framework for the generation of new ideas on how to manipulate fatty acid metabolism in cancer cells.
Alterations in Energy Metabolism in Cancer Cells
Cancer is fundamentally a disorder of cell growth and proliferation, which requires cellular building blocks, such as nucleic acids, proteins, and lipids. Cancer cells often have perturbed metabolism that allows them to accumulate metabolic intermediates as sources of these building blocks.
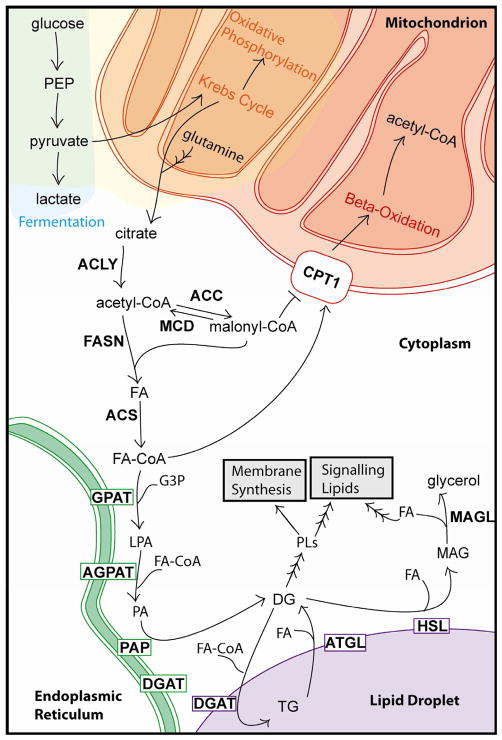
The most understood metabolic perturbation in cancer cells is the Warburg effect, an energetically wasteful alteration to glucose metabolism in which cancer cells use carbon from glucose to build other molecules instead of completely oxidizing them to carbon dioxide (Warburg, 1956). During normal cellular metabolism in the presence of oxygen, glucose undergoes glycolysis in the cytoplasm to produce pyruvate. After import into mitochondria, pyruvate is oxidized to acetyl-CoA, which then enters the Krebs cycle to produce reducing equivalents for oxidative phosphorylation (Figure 1). When oxygen is limiting, excess pyruvate is fermented to lactate in the cytoplasm. Differentiated cells typically use oxidative phosphorylation because of its efficiency, with one glucose molecule undergoing complete oxidation to yield ~36 ATP molecules versus 2 ATP that are obtained from anaerobic glycolysis. The Warburg effect is the use of fermentation even in the presence of oxygen and is characterized by an increase in glucose uptake and consumption, a decrease in oxidative phosphorylation, and the production of lactate1.
Figure 1. Overview of cellular fatty acid metabolism.
See text for description of depicted pathways. Enzymes are in bold. Enzymes with boxes around them are membrane localized.
Another commonly observed metabolic alteration in cancer is increased glutamine metabolism. In mammalian cells, glutamine is a major energy substrate through its metabolism to produce α-ketoglutarate, which feeds into the Krebs cycle. Glutamine-derived α-ketoglutarate contributes to the production of citrate by forward-flux through the Krebs cycle and malic enzyme-dependent production of pyruvate (DeBerardinis et al., 2007). Glutamine can also be converted to citrate by the reversal of the Krebs cycle reactions catalyzed by isocitrate dehydrogenase and aconitase (Wise et al., 2008; Mullen et al., 2012; Metallo et al., 2012). Citrate can then be used for the production of acetyl-groups for FA synthesis (see below).
Lipid metabolism is also altered in rapidly proliferating cells (for general reviews, see Swinnen et al., 2006; DeBerardinis and Thompson, 2012; Santos and Schulze, 2012). Here we focus on cancer and FA metabolism. In cancer cells, carbon must be diverted from energy production to FAs for biosynthesis of membranes and signaling molecules. The bulk of cell membrane lipids are phospholipids (PLs), such as phosphatidylcholine (PC) and phosphatidylethanolamine (PE), in addition to other lipids, such as sterols, sphingolipids, and lyso-PLs. All of these lipids are derived in part from acetyl CoA, and many contain FAs. The FA building blocks come from either exogenous sources or from de novo FA synthesis. While most normal human cells prefer exogenous sources, tumors synthesize FA de novo (Medes et al., 1953) and often exhibit a shift toward FA synthesis (Ookhtens et al., 1984). To enter the bioactive pool, FAs require “activation” by covalent modification by CoA via fatty acyl CoA synthetases. Once in the active pool, FAs can be esterified with glycerol or sterol backbones, generating triacylglycerols (TGs) or sterol esters (SEs), respectively, and then stored in lipid droplets (LDs) (See Figure 1). Within cells, FAs can have many fates, including being incorporated into membrane, storage, or signaling lipids, or oxidized to carbon dioxide as an energy source.
Although this review focuses on de novo FA synthesis pathways, some tumors scavenge lipids from their environment, rendering FA uptake pathways as a potential target. For example, fatty acid binding protein 4 (FABP4), a lipid chaperone, is implicated in providing FAs from surrounding adipocytes for ovarian tumors (Nieman et al., 2011). Also, prostate cancer cells show reduced viability in the presence of FASN (C75) or ACLY (SB-204990) inhibitors only when cultured in the absence of lipoproteins, an exogenous lipid source (Ros et al., 2012). CD36, a widely expressed transmembrane protein with diverse functions that include fatty acid uptake, has been implicated in breast cancer, and decreased levels of CD36 in stromal tissue are correlated with early steps in tumorigenesis (Defilippis et al., 2012). It is noteworthy that in vitro conditions for cell culture experiments are likely to be different than in vivo conditions, where exogenous uptake may be more important in some cancers.
Limiting Supplies of Fatty Acids to Limit Cancer Cell Proliferation
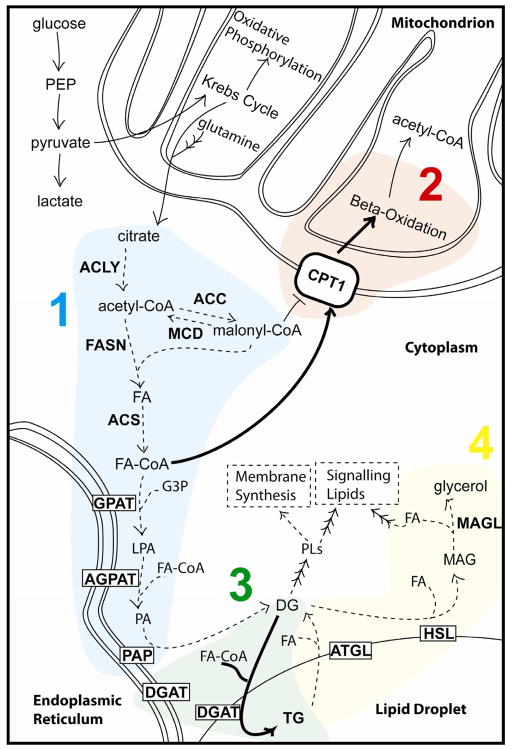
Since FAs are essential for cancer cell proliferation, limiting their availability could provide a therapeutic strategy. From the perspective of lipid metabolism, limiting FA availability could be achieved in several ways: 1) blocking FA synthesis, 2) increasing FA degradation via oxidation, 3) diverting FAs to storage, or 4) decreasing FA release from storage (Figure 2). Limiting FAs through these mechanisms could be accomplished in isolation or in a combinatorial manner. Using this as a framework, we review evidence relevant to this model.
Figure 2. Model showing how limiting FAs in the cell might limit cancer cell proliferation.
This may be done by 1) blocking the synthesis of fatty, 2) increasing the rate of FA degradation, 3) increasing FA storage in neutral TG, and/or 4) decreasing FA release from storage.
Blocking Fatty Acid Synthesis
The simplest way to reduce FA levels is to block their synthesis. Glucose metabolism feeds into FA metabolism at the point of citrate, an intermediate in the Krebs cycle (see Figure 1). Several steps are required to convert carbons from citrate to bioactive fatty acids. These steps involve ATP citrate lyase (ACLY, ACL, or ATPCL), acetyl-CoA carboxylase (ACC), fatty acid synthase (FASN or FAS), and acyl-CoA synthetase also known as fatty acid-CoA ligase (ACS, ACSL or FACL). In the model of decreasing FA availability, inhibiting these enzymes would limit cancer cell growth. Important for the clinical significance of these strategies, many inhibitors of these enzymes have minimal effects on non-cancer cells.
The subcellular localization of citrate determines its metabolic fate: mitochondrial citrate feeds into the Krebs cycle, and cytoplasmic citrate feeds into FA synthesis. Citrate is transported across the inner mitochondrial membrane for use in the cytoplasm in a regulated fashion by the transport protein CIC (citrate carrier). CIC levels are elevated in various cancer cell lines and tumors in a manner correlated with poor outcomes, and the inhibition of transport by benzene-tricarboxylate analog (BTA) shows anti-tumor effects in various tumor types and in vivo in xenograft mice (Catalina-Rodriguez et al., 2012).
ACLY
ACLY bridges glucose metabolism and FA metabolism by converting six-carbon citrate to oxaloacetate and two-carbon acetyl-CoA, the precursor for FA synthesis. Knockdown of ACLY reduces the ability of cells to metabolize glucose to lipid as shown by shRNA in murine lymphoid cells (Bauer et al., 2005) and siRNA in human adenocarcinoma cells (Hatzivassiliou et al., 2005). This alteration in metabolism impairs murine tumorigenesis and prevents xenograft tumor formation by human cancer cells when ACLY is knocked down by shRNA (Bauer et al., 2005; Hatzivassiliou et al., 2005) or siRNA (Migita et al., 2008) or chemically inhibited by SB-204990 (Hatzivassiliou et al., 2005). While ACLY is a promising therapeutic target, its product acetyl-CoA is an important metabolite for many molecules and a substrate for the acetylation of proteins and nucleic acids (Wellen et al., 2009). Thus, inhibiting its production may have consequences for other metabolic pathways as well.
ACC
ACC carboxylates acetyl-CoA to form malonyl-CoA, catalyzes the committed step, and is the most highly regulated enzyme in the fatty acid synthesis pathway (reviewed in Wakil and Abu-Elheiga, 2008). ACC is positively and allosterically regulated by citrate and glutamate and negatively and allosterically regulated by long- and short-chain fatty acyl CoAs such as palmitoyl-CoA. ACC is inactivated by phosphorylation by AMP-activated protein kinase (AMPK) and potentially regulated by many other kinases. There are two ACCs in the human genome, ACC1 (ACCα or ACACA) and ACC2 (ACCβ or ACACB). ACC1 is highly enriched in lipogenic tissues, and ACC2 occurs in oxidative tissues. Because they are primarily found in different specialized tissues, ACC1 and ACC2 have different metabolic roles. Malonyl-CoA made by ACC1 is thought to serve as a substrate for FA synthesis, whereas the malonyl-CoA made by ACC2 serves to inhibit CPT1 (see next section), thus preventing FA degradation.
Knockdown of ACC1 by siRNA induces apoptosis in prostate cancer (Brusselmans et al., 2005) and breast tumor (Chajès et al., 2006) cells but not in control non-malignant cells. Chemical inhibition of ACC1 and ACC2 by soraphen-A showed similar results in prostate cancer cells (Beckers et al., 2007). However, a contradictory result was observed with ACC inhibition by TOFA (5-(tetradecyloxy)-2-furoic acid) in breast cancer cells (Pizer et al., 2000). This may be attributable to epidermal growth factor receptor (EGFR) activation, as TOFA was observed by another group to block the growth of EGFR-activated human glioblastoma cell lines while not affecting non-EGFR activated cell lines (Guo et al., 2009a). The situation is further complicated by the observation that silencing of ACC1 or ACC2 accelerated tumor growth in lung cancer cells by promoting NADPH-dependent redox balance (Jeon et al., 2012).
While some aspects of the role of ACC in cancer cells still need to be elucidated, ACC activity might be controlled by promoting ACC phosphorylation. AMPK is activated by drugs, such as metformin, already widely used to treat diabetes. There is experimental evidence in vitro and in vivo in mice and humans, mainly in solid tumor models, that metformin treatment has anti-tumor activity, and clinical trials to further explore efficacy are underway (Pollak, 2012).
MCD
Malonyl-CoA decarboxylase (MCD) decarboxylates malonyl-CoA to acetyl-CoA, essentially reversing the reaction catalyzed by ACC. Thus, it is surprising that MCD inhibition yields similar data as ACC. siRNA against MCD and MPA treatment, a small-molecule inhibitor of MCD, are cytotoxic to breast cancer lines but not fibroblasts (Zhou et al., 2009).
FASN
FASN catalyzes successive condensation reactions to form a fatty acid from malonyl-CoA and acetyl-CoA substrates, producing mainly 16-carbon palmitate. It is perhaps the most studied FA metabolic enzyme with respect to cancer. Increased fatty acid synthesis due to increased levels of FASN has been observed in a multitude of cancers and is strongly correlated with a poor prognosis in many instances (reviewed in Menendez and Lupu, 2007). RNAi against FASN decreases levels of TG and phospholipids and inhibits cell growth and apoptosis in cells derived from a lymph node metastasis of prostate carcinoma (LNCaP) cells with no effects on growth rate or viability of non-malignant cultured skin fibroblasts (DeSchrijver et al., 2003). In many reports, chemical inhibitors of FASN preferentially killed cancer cells (reviewed in Lupu and Menendez, 2006). FASN is a particularly appealing therapeutic target because most cancer cells depend upon FASN-mediated de novo FA synthesis, whereas most non-cancer cells prefer exogenous FA. However, cell death induced after FASN inhibition might be due to the toxic accumulation of malonyl-CoA rather than a lack of FA (Pizer et al., 2000). Moreover, some inhibitors of FASN show severe side effects in animal models, including dramatic weight loss (Loftus et al., 2000), and FASN is required for adult neuronal stem cell function (Knobloch et al., 2012).
ACS
For FAs to enter bioactive pools, they must be activated by ACS enzymes, which generate FA-CoA. Bioactive FAs also contribute to protein palmitoylation, a post-translational modification that is important in certain cancers (Resh, 2012). Mammals have five ACS isoforms (ACSL1, 3, 4, 5, and 6) and also have fatty acid transport proteins with acyl CoA synthetase activity. ACSL4 is upregulated in some colon adenocarcinomas (Cao et al., 2000), and ACSL5 levels are increased in glioblastomas (Yamashita et al., 2000). Overexpression of ACSL4 promotes tumor cell survival by preventing apoptosis, likely through depletion of unesterified arachidonic acid (AA), which yields a pro-apoptotic signal (Cao et al., 2000). Chemical inhibition of ACS by Triacsin C (inhibitor of ACSL1, 3, and 4 but not 5 or 6 (Van Horn et al., 2005; Kim et al., 2001) preferentially induces apoptotic cell death in lung, colon, and brain cancer cells (Mashima et al., 2005). Several thiazolidinediones (TZDs) directly bind and inhibit rat ACSL4 (but not ACSL1 or ACSL5) in vitro (Kim et al., 2001). TZDs activate peroxisome proliferator-activated receptors (PPARs), particularly PPARγ, and are already in wide use for the treatment of diabetes. TZD use is correlated with decrease incidence of certain cancers in diabetics in what is likely to be a PPARγ-independent manner (Weng et al., 2006). When considering treatment through inactivation of ACS, it is important to note that different drugs have different isoform specificities so they may have differential effects, as the various isoforms have different tissue specificities, responses to nutritional state (Mashek et al., 2006), and preferred substrates (notably, ACSL4 prefers AA).
SCD
SCD catalyzes the introduction of double bonds into short-chain FAs in the C9 position (mainly converting stearoyl-CoA to oleoyl-CoA) (Paton and Ntambi, 2009). This alters the physical properties of FAs and has profound effects on lipid function. There are two isoforms of SCD in human cells (SCD1 and SCD5). SCD expression and activity is upregulated in some cancers, and its importance for cancer biology is increasingly recognized (Igal, 2010). Inhibition of SCD function causes cell death in cancer cells, probably by inducing the accumulation of unsaturated fatty acids (Ariyamo et al., 2010). Pharmacological inhibition of SCD limits tumor growth in pre-clinical cancer models (Fritz et al., 2010) without affecting overall body weight (Roongta et al., 2011). Since cancer cells rely considerably on de novo FA synthesis, SCD inhibition will likely show some degree of selectivity.
FAs are also substrates for sphingolipid synthesis. While sphingolipid metabolism is not a focus of this review, it is noteworthy that specific sphingolipids, such as ceramides and sphingosine-1-phosphate, are bioactive signaling molecules that generally suppress or promote tumors, respectively (Ogretmen and Hannun, 2004). Moreover, accumulation of ceramides is implicated in the therapeutic effects of various chemotherapeutic treatments of cancer.
Blocking Expression of Fatty Acid Synthesis Genes
In addition to directly targeting enzymes of fatty acid synthesis, their activities could be reduced by reducing transcription levels. The master transcriptional regulators of FA synthesis are sterol regulatory element-binding protein 1 (SREBP-1) transcription factors (Horton et al., 2002). SREBP-1 has two isoforms: SREBP-1a is the predominant isoform in most cultured cell lines and SREBP-1c is predominant in liver and most tissues. At normal levels, SREBP-1c activates the FA biosynthetic pathway with responsive genes including ACLY, ACC, FAS, SCD-1, and GPAT. Therefore, inhibiting SREBP-1 in cancer cells could decrease fatty acid synthesis gene expression and possibly prevent cancer cell proliferation. Indeed, shRNA knockdown of SREBP-1 decreases abundance of ACC and FAS and promotes tumor cell death of glioblastoma cells that overexpress SREBP-1 because of constitutively active EGFR, and 25-hydroxycholesterol (25-HC), an inhibitor of activation of SREBP-1 and -2, causes cell death in high EGFR (and therefore high SREBP-1) expressing cancer cell lines (Guo et al., 2009b). Additionally, higher levels of SREBP-1 are seen in prostate cancer tissue and both SREBP-1 and -2 play a role in the prostate cancer progression to androgen independence (Ettinger et al., 2004). Interestingly, recent work suggests that a mechanism for SREBP-1 repression preventing cancer cell proliferation is through loss of SCD-1 and FA desaturation, thereby causing lipotoxicity due to abnormally high levels of saturated FAs (Williams et al., 2013; Griffiths et al., 2013). Inhibition of SREBP by 25-HC, fatostatin, and FGH10019 all cause a decrease in expression of SREBP-1 and -2 target genes and significantly reduce cellular growth in a variety of cancer cell lines (Williams et al., 2013) and SREBP1 knockdown by shRNA reduces tumor growth in vivo in nude mice (Griffiths et al., 2013).
Further upstream, SREBP transcription factors and FA synthesis can be regulated by many signaling pathways, including growth factor signaling, which is reviewed in depth elsewhere (Shao and Espenshade, 2012; Kumar-Sinha et al., 2003; Peterson et al., 2011; Laplante and Sabatini, 2009; Lewis et al., 2011). Another transcription factor, liver X-activated receptor (LXR), activates fatty acid synthesis by inducing SREBP-1c (Liang et al., 2002). Therefore, cancer cell proliferation might be attenuated by preventing LXR activation. However, activation of LXR, particularly through T0901317, inhibits cancer cell proliferation in breast, colon, and prostate cancers (Viennois et al., 2012). These findings likely reflect functions of LXR other than regulating FA synthesis.
Increasing Fatty Acid Degradation
FA levels might be decreased in cancer cells by increasing the rate at which they are degraded. Activated FAs are broken by mitochondrial β-oxidation. FA-CoAs are transported from the cytosol across the outer mitochondrial membrane after they are converted to FA carnitines by carnitine palmitoyl transferase 1 (CPT1). Within the mitochondria, FAs are then repeatedly cleaved to produce acetyl-CoAs that feed into the Krebs cycle and produce reducing equivalents for oxidative phosphorylation. Increasing FA oxidation to limit FA abundance could in theory be beneficial, but data from experiments testing this idea are mixed.
CPT1
CPT1 is the first and rate-limiting step of fatty acid transport into mitochondria for oxidation to carbon dioxide. It is inhibited by malonyl-CoA. β-Oxidation of FAs is increased when ACC2 is inhibited because of the depletion of malonyl-CoA, the direct product of ACC. Therefore, the attenuation of cancer cell proliferation by inhibiting ACC (discussed previously) may also be due in part to an increase in degradation of FAs.
It is yet unclear whether increased FA oxidation in cancer cells will block proliferation. Cancer types likely differ in their clinical response to increasing FA oxidation, depending upon their energy requirements and ACC isoform expression patterns. In some types, increased FA oxidation may diminish FA availability and be beneficial. On the other hand, etomoxir, an inhibitor of CPT1, and ranolazine, an indirect inhibitor of FA oxidation, may kill cancer cells (Samudio et al., 2010; Pike et al., 2011). A further caveat of increasing the FA oxidation rate is that it could increase cellular ATP levels, thus providing energy for further cellular proliferation. Indeed, CPT1C, the brain isoform of CPT1, is important for the survival of cancer cells under energy stress (Zaugg et al, 2011).
It has long been known that PPARα is a major transcriptional regulator of FA oxidation with activation inducing oxidation. In keeping with the uncertainty regarding the role of FA oxidation in cancer cell proliferation, extended PPARα activation causes hepatocellular carcinoma in mice and rats by an unclear mechanism that involves perturbation of the cell cycle and production of reactive oxygen species (reviewed by Michalik et al., 2004). However, humans taking PPARα agonists do not develop similar cancers, and in fact, PPARα activation inhibits tumor growth in several models (reviewed in Yokoyama and Mizunuma, 2010).
Diverting Fatty Acids to Storage
Once made, FAs can be used for membrane lipid synthesis, degraded, or stored. Conceivably, increased storage of FAs in neutral lipids, such as TGs or sterol esters, could lead to a reduction in FAs available for use as membrane building blocks or signaling lipids and inhibit cellular proliferation. Most cells store FAs in TGs in the cytosolic lipid droplet (LD), an organelle whose major function is lipid storage (see Farese and Walther, 2009). The role of LDs in cancer cells is unclear. While increased numbers of LDs have been reported in many cancer cells (reviewed in Bozza and Viola, 2010), and this accumulation has been proposed to be pathogenic, the accumulation of LDs per se, might not be the culprit. The readily available pool of FAs that they represent might be pathogenic. LD accumulation might also reflect a cellular response to stress (Hapala et al., 2011). Future studies should also carefully delineate whether LD accumulation occurs within cancer cells or in surrounding cells.
The major TG synthesis pathway is known as the Kennedy or glycerol-phosphate pathway. It condenses FAs with glycerol 3-phosphate using the enzymes glycerol-3-phosphate acyltransferase (GPAT), acylglycerolphosphate acyltransferase (AGPAT), phosphatidic acid phosphohydrolase (Lipin or PAP), and diacylglycerol acyltransferase (DGAT). The products of all but the most distal enzyme (DGAT) feed into PL synthesis. Therefore, GPAT, AGPAT and Lipin might be inhibited to limit PL production, while efforts to increase FA storage would be focused on activating DGAT. Additionally, the potential benefits of increasing FA storage may only be realized while concomitantly inhibiting the release of FA from storage.
AGPAT
AGPAT esterifies lysophosphatidic acid (LPA) and a FA-CoA to form phosphatidic acid (PA). There may be as many as 11 human AGPATs. Elevated AGPAT2 expression is associated with poor prognosis of ovarian cancers, and AGPAT2 inhibitors have antitumor activity in xenograft mice (reviewed in Takeuchi and Reue, 2009). Additionally, AGPAT9 and AGPAT11 are upregulated in a variety of cancers (reviewed in Agarwal, 2012). As with any enzyme with multiple isoforms, differences in expression patterns of the isoforms may have a profound influence on the effectiveness of inhibition/activation of a particular isoform in a particular cancer.
PAP
Lipin removes a phosphate group from PA to form diacylglycerol (DG). It is one of the least-studied enzymes in the lipid storage pathway with respect to cancer, and little is known about how blocking or overexpressing this step of lipid synthesis affects cancer progression. However, lipin is involved in the regulation of the activity of sterol regulatory element binding proteins (SREBP), a family of transcription factors that regulate the expression of many enzymes involved in fatty acid and cholesterol biosynthesis (Ishimoto et al., 2009). Lipin is phosphorylated and inhibited by the mammalian target of rapamycin complex 1, resulting in activation of SREBP transcriptional activity (Peterson, et al., 2011). Modulating lipin activity may therefore have significant effects on cellular lipid homeostasis.
DGAT
DGAT enzymes esterify DG and a FA-CoA to form TG. Mammals have two DGATs (DGAT1 and DGAT2). DGAT catalyzes the only dedicated step in TG formation and thus provides a key target for decreasing available lipids by increasing lipid storage. Transformed human fibroblasts overexpressing DGAT1 had increased TG and decreased phospholipids, as well as reduced proliferation and invasiveness (Bagnato and Igal, 2003). Unpublished data from the Farese laboratory suggests that DGAT1-deficient mice have increased levels of LPA and PGE2 in mammary fat and develop some breast cancers more rapidly (Sylvaine Cases, unpublished). DGAT1 inhibition might also favor the accumulation of its substrate diacylglycerol in cells, which might have signaling effects. These findings would suggest caution, from a cancer standpoint, for the use of DGAT1 inhibitors, which are being explored clinically for use in metabolic diseases.
PLs are the other major products of glycerolipid synthesis and are important for membrane expansion in rapidly proliferating cells. The major mammalian membrane phospholipid is PC. Many cancers have increased PC levels and increased activity of any of several enzymes in the PC synthesis pathway, while inhibition or knockdown of many of the enzymes decrease cancer phenotypes (Glunde et al., 2011). An inhibitor of choline kinase alpha (CKα), the first step of choline activation for PC synthesis, is currently in Phase I trials for use against advanced solid tumors (http://clinicaltrials.gov/show/NCT01215864).
Blocking Fatty Acid Release From Storage
Once stored, FAs can be released for use by specific lipases. By preventing lipolysis, the active FA pool available for cancer cell proliferation might be decreased. FAs derived from lipolysis can also serve as precursors for important signaling lipids (see Wymann and Schneiter, 2008). Most knowledge on lipolysis is derived from work on adipocytes where each TG molecule in the LD can be fully hydrolyzed to release three FAs by the sequential action of adipose triglyceride lipase (ATGL), hormone sensitive lipase (HSL) and monoacylglycerol lipase (MAGL). Although each of these lipases also has important functions in other tissues, it is yet unclear whether other lipases might operate in other cell types. Currently, most data addressing lipases and cancer are for MAGL.
MAGL
MAGL hydrolyzes the final FA from MG leaving the glycerol backbone. MAGL expression and activity are increased in several aggressive cancer cell lines and primary tumors (Nomura et al., 2010). Knockdown and chemical inhibition of MAGL by JZL184 lowered free FA levels and reduced pathogenicity of melanoma and ovarian cancer cells in vitro and in vivo, while overexpression showed the opposite phenotype. Interestingly, a high-fat diet in mice reversed the reduced tumor growth of MAGL-inhibited tumors in mice. This observation raises the question of whether targeting lipid metabolism for cancer therapy may only be effective in combination with specific dietary regimes. Additionally, MAGL has a role in the regulation of signaling lipids: more invasive tumors have increased LPA and PGE2 levels, and those are decreased in the presence of MAGL inhibitors.
ATGL and HSL
Although their roles in cancer cell proliferation are unclear, ATGL and HSL play an important role in cancer cachexia, a wasting syndrome that is an adverse prognostic factor in cancer. Cancer patients with cachexia show increased HSL and ATGL activity when compared to non-cancer patients, and genetic ablation of ATGL (and HSL to a lesser extent) protects mice from cancer-associated loss of adipose tissue and skeletal muscle (Das et al., 2011). Therefore, pharmacological inhibition of ATGL and/or HSL may help to prevent cancer-associated cachexia.
Conclusion and Perspective
Cancer cells rely on FAs as cellular building blocks for membrane formation, energy storage, and the production of signaling molecules. Our review highlights this requirement and provides a framework for the investigation of limiting the supply of FAs. If the model that FAs are required for cancer cell proliferation is correct, cancer cells might be targeted at multiple points within the pathway of FA metabolism to subvert rapid proliferation, and many chemical inhibitors for specific steps already exist (Table 1). Much like glucose metabolism, targeting FA metabolism might be more selective for highly proliferative cells. Alternatively, delivery of FA metabolism inhibitors might be done in a cell-specific and targeted manner.
Table 1.
Examples of chemical inhibitors of lipid enzymes that could reduce fatty acid availability. Shown are selected inhibitors for enzymes mentioned in text.
| Enzyme | Inhibitor | Comments | Selected References |
|---|---|---|---|
| ACC | Soraphen-A | (Beckers et al., 2007) | |
| TOFA (5-(tetradecyloxy)- 2-furoic acid) | (Pizer et al., 2000), (Guo et al., 2009a) | ||
| A-769662 | (Göransson et al., 2007) | ||
| Metformin | Indirect, activates AMPK | (Pollak, 2012) | |
| AICAR | Indirect, activates AMPK | (Jose et al., 2011)(Swinnen et al., 2005) | |
| ACLY | SB-204990 | (Hatzivassiliou et al., 2005), (Ros et al., 2012) | |
| LY294002 | Indirect, PI3K inhibitor | (Migita et al., 2008) | |
| ACS | Triacscin C | (Mashima et al., 2005) | |
| Thiazolidinediones (TZDs) | ACSL4 specific, also activates PPARγ, FDA approved | (Kim et al, 2001) | |
| AGPAT | CT-32501 | AGPAT2 specific | (Takeuchi and Reue, 2009) |
| CKα | TCD-717 | Currently in phase I trials | http://clinicaltrials.gov/show/NCT01215864 |
| MN58B | (Glunde et al., 2011) | ||
| CIC | Benzene-tricarboxylate analog (BTA) | (Catalina-Rodriguez et al., 2012) | |
| CPT1 | Etomoxir | (Samudio et al., 2010) (Pike et al., 2011) | |
| Ranolazine | FDA approved | (Samudio et al., 2010) | |
| FASN | Cerulenin and its derivative C75 | (Lupu and Menendez, 2006) (Ros et al., 2012) | |
| Orlistat | FDA approved | (Lupu and Menendez, 2006) | |
| Flavonoids | Naturally occurring | (Lupu and Menendez, 2006) | |
| Epigallocatechin-3-gallate (EGCG) | Found in green tea | (Lupu and Menendez, 2006) | |
| MAGL | JZL184 | (Nomura et al., 2010) | |
| SCD | BZ36 | (Fritz et al., 2010) | |
| A939572 | (Roongta et al., 2011) | ||
| SREBP | Fatostatin | Inhibits processing of SREBP-1 and -2 | (Williams et al, 2013) |
| FGH10019 | Inhibits processing of SREBP-1 and -2 | (Williams et al, 2013) (Kamisuki et al, 2011) |
Cancers are diverse in type and underlying genetic alterations. Lipid metabolism is complex, with many different feedback mechanisms and points of regulation. Additionally, most of the lipid metabolic enzymes have multiple isoforms, and these may be coupled to different lipid metabolic processes and can have different cellular localization or tissue distribution. Therefore, successful therapies may be dependent upon understanding the specific metabolic abnormalities for a particular type of cancer.
Acknowledgments
We thank Gary Howard for editorial assistance.
Abbreviations
- AA
arachidonic acid
- ACC
acetyl-CoA carboxylase. Carboxylates Acetyl-CoA to form malonyl-CoA
- ACS/ACSL
acyl-CoA synthetase. Also known as Fatty Acid Co-A Ligase. Activates a fatty acid to a fatty acyl-CoA
- ACL/ATPCL/ACLY
ATP citrate lyase. Converts citrate to acetyl-CoA
- AGPAT
acylglycerophosphate acyltransferase. Condenses LPA and a FA-CoA to form PA
- ATGL
adipose triglyceride lipase. Hydrolyzes TG to DG and a FA
- CIC
citrate carrier protein
- CPT1
carnitine palmitoyl transferase 1. Transports FA-CoAs across the mitochondrial membrane for degradation
- DG/DAG
diacylglycerol. Contains a glycerol backbone and two fatty acids
- DGAT
diacylglycerol acyltransferase. Adds a FA to DG to form TG
- FA
fatty acid
- FACL
fatty acid Co-A ligase. See ACS
- FAS/FASN
fatty acid synthase. Condenses malonyl-CoA and acetyl-CoA to form a fatty acid
- GPAT
glycerol-3-phosphate acyltransferase. Condenses glycerol-3-phosphate and a FA-CoA to make LPA
- HSL
hormone sensitive lipase. Hydrolyzes DG to MG and a FA
- LD
lipid droplet. An organelle whose major functions include lipid storage
- LPA
lysophosphatidic acid
- LXR
liver X-activated receptor
- MG/MAG
monoacylglycerol. Contains a glycerol backbone and one fatty acid
- MAGL
monoacylglycerol lipase. Hydrolyzes MG to glycerol and a FA
- MCD
malonyl-CoA decarboxylase. Decarboxylates malonyl-CoA to acetyl-CoA
- PA
phosphatidic acid
- PAP
phosphatidic acid phosphohydrolase. Removes a phosphate from PA to form DG. Also known as lipin
- PGE2
prostaglandin E2
- PL
phospholipid
- PPAR
peroxisome proliferator-activated receptor. Three family members include α, β /δ, and γ
- PPP
pentose phosphate pathway. Also known as phosphogluconate pathway or the hexose monophosphate shunt. Generates NADPH for fatty acid synthesis
- SCD
stearoyl-CoA desaturase. Introduces double bonds into short-chain FAs
- SE
sterol ester. A sterol backbone and a FA
- SREBP-1
sterol response element binding protein-1. The major FA regulatory transcription factor. Has two isoforms, SREBP-1a and SREBP1-c
- TG/TAG
triacylglycerol. The major lipid stored in most lipid droplets. A glycerol backbone and three fatty acids
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal AK. Lysophospholipid acyltransferases: 1-acylglycerol-3-phosphate O-acyltransferases. From discovery to disease. Curr Opin Lipidol. 2012;23:290–302. doi: 10.1097/MOL.0b013e328354fcf4. [DOI] [PubMed] [Google Scholar]
- Ariyama H, Kono N, Matsuda S, Inoue T, Arai H. Decrease in membrane phospholipid unsaturation induces unfolded protein response. J Biol Chem. 2010;285:22027–22035. doi: 10.1074/jbc.M110.126870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnato C, Igal RA. Overexpression of diacylglycerol acyltransferase-1 reduces phospholipid synthesis, proliferation, and invasiveness in simian virus 40-transformed human lung fibroblasts. J Biol Chem. 2003;278:52203–52211. doi: 10.1074/jbc.M305760200. [DOI] [PubMed] [Google Scholar]
- Bauer DE, Hatzivassiliou G, Zhao F, Andreadis C, Thompson CB. ATP citrate lyase is an important component of cell growth and transformation. Oncogene. 2005;24:6314–6322. doi: 10.1038/sj.onc.1208773. [DOI] [PubMed] [Google Scholar]
- Beckers A, Organe S, Timmermans L, Scheys K, Peeters A, Brusselmans K, Verhoeven G, Swinnen JV. Chemical inhibition of acetyl-CoA carboxylase induces growth arrest and cytotoxicity selectively in cancer cells. Cancer Res. 2007;67:8180–8187. doi: 10.1158/0008-5472.CAN-07-0389. [DOI] [PubMed] [Google Scholar]
- Bozza PT, Viola JP. Lipid droplets in inflammation and cancer. Prostag Leukotr Ess. 2010;82:243–250. doi: 10.1016/j.plefa.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Brusselmans K, De Schrijver E, Verhoeven G, Swinnen JV. RNA interference-mediated silencing of the acetyl-CoA-carboxylase-alpha gene induces growth inhibition and apoptosis of prostate cancer cells. Cancer Res. 2005;65:6719–6725. doi: 10.1158/0008-5472.CAN-05-0571. [DOI] [PubMed] [Google Scholar]
- Cao Y, Pearman AT, Zimmerman GA, McIntyre TM, Prescott SM. Intracellular unesterified arachidonic acid signals apoptosis. Proc Natl Acad Sci. 2000;97:11280–11285. doi: 10.1073/pnas.200367597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalina-Rodriguez O, Kolukula VK, Tomita Y, Preet A, Palmieri F, Wellstein A, Byers S, Giaccia AJ, Glasgow E, Albanese C, Avantaggiati ML. The mitochondrial citrate transporter, CIC, is essential for mitochondrial homeostasis. Oncotarget. 2012;3:1220–1235. doi: 10.18632/oncotarget.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chajès V, Cambot M, Moreau K, Lenoir GM, Joulin V. Acetyl-CoA carboxylase alpha is essential to breast cancer cell survival. Cancer Res. 2006;66:5287–5294. doi: 10.1158/0008-5472.CAN-05-1489. [DOI] [PubMed] [Google Scholar]
- Das SK, Eder S, Schauer S, Diwoky C, Temmel H, Guerti B, Gorkiewicz G, Tamilarasan KP, Kumari P, Trauner M, Zimmerman R, Vesely P, Haemmerle G, Zechner R, Hoefler G. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science. 2011;333:233–8. doi: 10.1126/science.1198973. [DOI] [PubMed] [Google Scholar]
- DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Thompson CB. Cellular Metabolism and Disease: What Do Metabolic Outliers Teach Us? Cell. 2012;148:1132–1144. doi: 10.1016/j.cell.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defilippis R, Chang H, Dumont N, Rabban J, Chen Y, Fontenay G, Berman H, Gauthier M, Zhao J, Hu D, Marx J, Tjoe J, Ziv E, Febbraio M, Kerlikowske K, Parvin B, Tlsty T. CD36 Repression Activates a Multicellular Stromal Program Shared by High Mammographic Density and Tumor Tissues. Cancer Discov. 2012;2:826–839. doi: 10.1158/2159-8290.CD-12-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schrijver E, Brusselmans K, Heyns W, Verhoeven G, Swinnen JV. RNA interference-mediated silencing of the fatty acid synthase gene attenuates growth and induces morphological changes and apoptosis of LNCaP prostate cancer cells. Cancer Res. 2003;63:3799–3804. [PubMed] [Google Scholar]
- Ettinger SL, Sobel R, Whitmore TG, Akbari M, Bradley DR, Gleave ME, Nelson CC. Dysregulation of sterol response element-binding proteins and downstream effectors in prostate cancer during progression to androgen independence. Cancer Res. 2004;64:2212–2221. doi: 10.1158/0008-5472.can-2148-2. [DOI] [PubMed] [Google Scholar]
- Farese RV, Walther TC. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell. 2009;139:855–860. doi: 10.1016/j.cell.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz V, Benfodda Z, Rodier G, Henriquet C, Iborra F, Avances C, Allory Y, de la Taille A, Culine S, Blancou H, Cristol JP, Michel F, Sardet C, Fajas L. Abrogation of de novo lipogenesis by stearoyl-CoA desaturase 1 inhibition interferes with oncogenic signaling and blocks prostate cancer progression in mice. Mol Cancer Ther. 2010;9:1740–1754. doi: 10.1158/1535-7163.MCT-09-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glunde K, Bhujwalla ZM, Ronen SM. Choline metabolism in malignant transformation. Nat Rev Cancer. 2011;11:835–848. doi: 10.1038/nrc3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göransson O, McBride A, Hawley SA, Foss FA, Shpiro N, Foretz M, Viollet B, Hardie DG, Sakamoto K. Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J Biol Chem. 2007;282:32549–32560. doi: 10.1074/jbc.M706536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths B, Lewis CA, Bensaad K, Ros S, Zhang Q, Ferber EC, Konisti S, Peck B, Miess H, East P, Wakelam M, Harris AL, Schulze A. Sterol regulatory element binding protein-dependent regulation of lipid synthesis supports cell survival and tumor growth. Cancer & Metabolism. 2013;1 doi: 10.1186/2049-3002-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Hildebrandt IJ, Prins RM, Soto H, Mazzotta MM, Dang J, Czernin J, Shyy JY, Watson AD, Phelps M, Radu CG, Cloughesy TF, Mischel PS. The AMPK agonist AICAR inhibits the growth of EGFRvIII-expressing glioblastomas by inhibiting lipogenesis. Proc Natl Acad Sci. 2009a;106:12932–12937. doi: 10.1073/pnas.0906606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Prins RM, Dang J, Kuga D, Iwanami A, Soto H, Lin KY, Huang TT, Akhavan D, Hock MB, Zhu S, Kofman AA, Bensinger SJ, Yong WH, Vinters HV, Horvath S, Watson AD, Kuhn JG, Robins HI, Mehta MP, Wen PY, DeAngelis LM, Prados MD, Mellinghoff IK, Cloughesy TF, Mischel PS. EGFR signaling through an Akt-SREBP-1-dependent, rapamycin-resistant pathway sensitizes glioblastomas to antilipogenic therapy. Sci Signal. 2009b;2:ra82. doi: 10.1126/scisignal.2000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapala I, Marza E, Ferreira T. Is fat so bad? Modulation of endoplasmic reticulum stress by lipid droplet formation. Biol Cell. 2011;103:271–285. doi: 10.1042/BC20100144. [DOI] [PubMed] [Google Scholar]
- Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA, Thompson CB. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8:311–321. doi: 10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Horton J, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Igal RA. Stearoyl-CoA desaturase-1: a novel key player in the mechanisms of cell proliferation, programmed cell death and transformation to cancer. Carcinogenesis. 2010;31:1509–1515. doi: 10.1093/carcin/bgq131. [DOI] [PubMed] [Google Scholar]
- Ishimoto K, Nakamura H, Tachibana K, Yamasaki D, Ota A, Hirano K, Tanaka T, Hamakubo T, Sakai J, Kodama T, Doi T. Sterol-mediated Regulation of Human Lipin 1 Gene Expression in Hepatoblastoma Cells. J Biol Chem. 2009;284:22195–22205. doi: 10.1074/jbc.M109.028753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose C, Hebert-Chatelain E, Bellance N, Larendra A, Su M, Nouette-Gaulain K, Rossignol R. AICAR inhibits cancer cell growth and triggers cell-type distinct effects on OXPHOS biogenesis, oxidative stress and Akt activation. BBA - Bioenergetics. 2011;1807:707–718. doi: 10.1016/j.bbabio.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Kamisuki S, Shirakawa T, Kugimiya A, Abu-Elheiga L, Choo HY, Yamada K, Shimogawa H, Wakil SJ, Uesugi M. Synthesis and evaluation of diarylthiazole derivatives that inhibit activation of sterol regulatory element-binding proteins. J Med Chem. 2011;54:4923–4927. doi: 10.1021/jm200304y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lewin TM, Coleman RA. Expression and characterization of recombinant rat Acyl-CoA synthetases 1, 4, and 5. Selective inhibition by triacsin C and thiazolidinediones. J Biol Chem. 2001;276:24667–24673. doi: 10.1074/jbc.M010793200. [DOI] [PubMed] [Google Scholar]
- Knobloch M, Braun SM, Zurkirchen L, von Schoultz C, Zamboni N, Arauzo-Bravo MJ, Kovacs WJ, Karalay O, Suter U, Machado RA, Roccio M, Lutolf MP, Semenkovich CF, Jessberger S. Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature. 2012;493:226–230. doi: 10.1038/nature11689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar-Sinha C, Ignatoski KW, Lippman ME, Ethier SP, Chinnaiyan AM. Transcriptome analysis of HER2 reveals a molecular connection to fatty acid synthesis. Cancer Res. 2003;63:132–139. [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. An Emerging Role of mTOR in Lipid Biosynthesis. Curr Biol. 2009;19:R1046–R1052. doi: 10.1016/j.cub.2009.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C, Griffiths B, Santos C, Pende M, Schulze A. Regulation of the SREBP transcription factors by mTORC1. Biochem Soc Trans. 2011;39:495–499. doi: 10.1042/BST0390495. [DOI] [PubMed] [Google Scholar]
- Liang G, Yang J, Horton J, Hammer RE, Goldstein JL, Brown MS. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J Biol Chem. 2002;277:9520–9528. doi: 10.1074/jbc.M111421200. [DOI] [PubMed] [Google Scholar]
- Loftus TM, Jaworsky DE, Frehywot GL, Townsend CA, Ronnett GV, Lane MD, Kuhajda FP. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science. 2000;288:2379–2381. doi: 10.1126/science.288.5475.2379. [DOI] [PubMed] [Google Scholar]
- Lupu R, Menendez JA. Pharmacological inhibitors of fatty acid synthase (FASN)-catalyzed endogenous fatty acid biogenesis: a new family of anti-cancer agents? Curr Pharm Biotechnol. 2006;7:483–493. doi: 10.2174/138920106779116928. [DOI] [PubMed] [Google Scholar]
- Mashek DG, Li LO, Coleman RA. Rat long-chain acyl-CoA synthetase mRNA, protein, and activity vary in tissue distribution and in response to diet. J Lipid Res. 2006;47:2004–2010. doi: 10.1194/jlr.M600150-JLR200. [DOI] [PubMed] [Google Scholar]
- Mashima T, Oh-hara T, Sato S, Mochizuki M, Sugimoto Y, Yamazaki K, Hamada J, Tada M, Moriuchi T, Ishikawa Y, Kato Y, Tomoda H, Yamori T, Tsuruo T. p53-defective tumors with a functional apoptosome-mediated pathway: a new therapeutic target. J Natl Cancer Inst. 2005;97:765–777. doi: 10.1093/jnci/dji133. [DOI] [PubMed] [Google Scholar]
- Medes G, Thomas A, Weinhouse S. Metabolism of neoplastic tissue. IV. A study of lipid synthesis in neoplastic tissue slices in vitro. Cancer Res. 1953;13:27–29. [PubMed] [Google Scholar]
- Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L, Kelleher JK, Vander Heiden M, Iliopoulos O, Stephanopoulos G. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2012;481:380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator-activated receptors and cancers: complex stories. Nat Rev Cancer. 2004;4:61–70. doi: 10.1038/nrc1254. [DOI] [PubMed] [Google Scholar]
- Migita T, Narita T, Nomura K, Miyagi E, Inazuka F, matsuura M, Ushijima M, Mashima T, Seimiya H, Satoh Y, Okumura S, Nakagawa K, Ishikawa Y. ATP citrate lyase: Activation and therapeutic implications in non-small cell lung cancer. Cancer Res. 2008;68:8547–8554. doi: 10.1158/0008-5472.CAN-08-1235. [DOI] [PubMed] [Google Scholar]
- Mullen AR, Wheaton WW, Jin ES, Chen PH, Sullivan LB, Cheng T, Yang Y, Linehan WM, Chandel NS, DeBerardinis RJ. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2012;481:385–388. doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB, Hotamisligil GS, Yamada SD, Peter ME, Gwin K, Lengyel E. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura DK, Long JZ, Niessen S, Hoover HS, Ng S, Cravatt BF. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140:49–61. doi: 10.1016/j.cell.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogretmen B, Hannun Y. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- Ookhtens M, Kannan R, Lyon I, Baker N. Liver and adipose tissue contributions to newly formed fatty acids in an ascites tumor. Am J Physiol. 1984;247:R146–R153. doi: 10.1152/ajpregu.1984.247.1.R146. [DOI] [PubMed] [Google Scholar]
- Paton CM, Ntambi JM. Biochemical and physiological function of stearoyl-CoA desaturase. Am J Physiol Endocrinol Metab. 2009;297:E28–E37. doi: 10.1152/ajpendo.90897.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, Guertin DA, Madden KL, Carpenter AE, Finck BN, Sabatini DM. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike LS, Smift AL, Croteau NJ, Ferrick DA, Wu M. Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim Biophys Acta. 2011;1807:726–734. doi: 10.1016/j.bbabio.2010.10.022. [DOI] [PubMed] [Google Scholar]
- Pizer ES, Thupari J, Han WF, Pinn ML, Chrest FJ, Frehywot GL, Townsend CA, Kuhajda FP. Malonyl-coenzyme-A is a potential mediator of cytotoxicity induced by fatty-acid synthase inhibition in human breast cancer cells and xenografts. Cancer Res. 2000;60:213–218. [PubMed] [Google Scholar]
- Pollak MN. Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov. 2012;2:778–790. doi: 10.1158/2159-8290.CD-12-0263. [DOI] [PubMed] [Google Scholar]
- Resh MD. Targeting protein lipidation in disease. Trends Mol Med. 2012;18:206–214. doi: 10.1016/j.molmed.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roongta UV, Pabalan JG, Wang X, Ryseck RP, Fargnoli J, Henley BJ, Yang WP, Zhu J, Madireddi MT, Lawrence RM, Wong TW, Rupnow BA. Cancer cell dependence on unsaturated fatty acids implicates stearoyl-CoA desaturase as a target for cancer therapy. Mol Cancer Res. 2011;9:1551–1561. doi: 10.1158/1541-7786.MCR-11-0126. [DOI] [PubMed] [Google Scholar]
- Ros S, Santos CR, Moco S, Baenke F, Kelly G, Howell M, Zamboni N, Schulze A. Functional metabolic screen identifies 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 as an important regulator of prostate cancer cell survival. Cancer Discov. 2012;2:328–343. doi: 10.1158/2159-8290.CD-11-0234. [DOI] [PubMed] [Google Scholar]
- Samudio I, Harmancey R, Fiegl M, Kantarjian H, Konopleva M, Korchin B, Kaluarachchi K, Bornmann W, Duvvuri S, Taegtmeyer H, Andreeff M. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J Clin Invest. 2010;120:142–156. doi: 10.1172/JCI38942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J. 2012;279:2610–2623. doi: 10.1111/j.1742-4658.2012.08644.x. [DOI] [PubMed] [Google Scholar]
- Schulze A, Harris AL. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature. 2012;491:364–373. doi: 10.1038/nature11706. [DOI] [PubMed] [Google Scholar]
- Shao W, Espenshade PJ. Expanding roles for SREBP in metabolism. Cell Metabolism. 2012;16:414–419. doi: 10.1016/j.cmet.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen JV, Bekers A, Brusselmans K, Organe S, Segers J, Timmermans L, Vanderhoydonc F, Deboel L, Derua R, Waelkens E, De Schrijver E, Van de Sande T, Noel A, Foufelle F, Verhoeven G. Mimicry of a cellular low energy status blocks tumor cell anabolism and suppresses the malignant phenotype. Cancer Res. 2005;65:2441–2448. doi: 10.1158/0008-5472.CAN-04-3025. [DOI] [PubMed] [Google Scholar]
- Swinnen JV, Brusselmans K, Verhoeven G. Increased lipogenesis in cancer cells: new players, novel targets. Curr Opin Clin Nutr Metab Care. 2006;9:358–365. doi: 10.1097/01.mco.0000232894.28674.30. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Reue K. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am J Physiol Endocrinol Metab. 2009;296:E1195–E1209. doi: 10.1152/ajpendo.90958.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Horn CG, Caviglia JM, Li LO, Wang S, Granger DA, Coleman RA. Characterization of recombinant long-chain rat acyl-CoA synthetase isoforms 3 and 6: identification of a novel variant of isoform 6. Biochemistry. 2005;44:1635–1642. doi: 10.1021/bi047721l. [DOI] [PubMed] [Google Scholar]
- Viennois E, Mouzat K, Dufour J, Morel L, Lobacaro J, Baron S. Selective liver X receptor modulators (SLiMs): What use in human health? Mol Cell Endocr. 2012;351:129–141. doi: 10.1016/j.mce.2011.08.036. [DOI] [PubMed] [Google Scholar]
- Wakil SJ, Abu-Elheiga LA. Fatty acid metabolism: target for metabolic syndrome. J Lipid Res. 2008;50:S138–S143. doi: 10.1194/jlr.R800079-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng J, Chen CY, Pinzone JJ, Ringel MD, Chen CS. Beyond peroxisome proliferator-activated receptor gamma signaling: the multi-facets of the antitumor effect of thiazolidinediones. Endocr Relat Cancer. 2006;13:401–413. doi: 10.1677/erc.1.01182. [DOI] [PubMed] [Google Scholar]
- Williams K, Argus J, Zhu Y, Wilks M, Marbois B, York A, Kidani Y, Pourzia A, Akhavan D, Lisiero D, Komisopouou E, Henkin A, Soto H, Chamberlain B, Vergnes l, Jung M, Torres J, Liau L, Christofk H, Prins R, Mischel P, Reue K, Graeber T, Bensinger S. An essential requirement for the SCAP/SREBP signaling axis to protect cancer cells from lipotoxicity. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-13-0382-T. Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, Thompson CB. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Kumabe T, Cho YY, Watanabe M, Kawagishi J, Yoshimoto T, Fujino T, Kang MJ, Yamamoto TT. Fatty acid induced glioma cell growth is mediated by the acyl-CoA synthetase 5 gene located on chromosome 10q25.1–q25.2, a region frequently deleted in malignant gliomas. Oncogene. 2000;19:5919–5925. doi: 10.1038/sj.onc.1203981. [DOI] [PubMed] [Google Scholar]
- Yokoyama Y, Mizunuma H. Peroxisome proliferator-activated receptor and epithelial ovarian cancer. Eur J Gynaecol Oncol. 2010;31:612–615. [PubMed] [Google Scholar]
- Zaugg K, Yao Y, Reilly PT, Kannan K, Kiarash R, Mason J, Huang P, Sawyer SK, Fuerth B, Faubert B, Kalliomaki T, Elia A, Luo X, Nadeem V, Bungard D, Yalavarthi S, Growney JD, Wakeham A, Moolani Y, Silvester J, Ten AY, Bakker W, Tsuchihara K, Berger S, Hill RP, Jones RG, Tsao M, Robinson MO, Thompson CB, Pan G, Mak TW. Carnitine palmitoyltransferase 1C promotes cell survival and tumor growth under conditions of metabolic stress. Genes Dev. 2011;25:1041–1051. doi: 10.1101/gad.1987211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Tu Y, Simpson PJ, Kuhajda FP. Malonyl-CoA decarboxylase inhibition is selectively cytotoxic to human breast cancer cells. Oncogene. 2009;28:2979–2987. doi: 10.1038/onc.2009.160. [DOI] [PubMed] [Google Scholar]