Abstract
Drug use typically begins during adolescence, which is a period of ongoing neurobiological development that may confer heightened vulnerability to develop drug dependence. Previously, our lab has shown that amphetamine (AMPH)-induced deficits in a medial prefrontal cortex (mPFC)-sensitive working memory task are greater in rats exposed to the drug during adolescence compared to adulthood. Here, we examine potential age-dependent effects of AMPH exposure on behavioral flexibility tasks that are sensitive to disruptions in mPFC and orbitofrontal cortex (OFC) function. Male Sprague-Dawley rats were injected (i.p.) with saline or 3 mg/kg AMPH every other day between postnatal days (PNDs) 27–45 and PNDs 85–103. Starting around PND 125, rats were tested in an attentional set-shifting task and a subset of those was then tested in an operant strategy shifting task. Following completion of the operant task, rats were challenged with 3 mg/kg AMPH and monitored in open field chambers. Our results demonstrate that AMPH-exposed rats were faster to acquire simple and compound discriminations, but were impaired during the first stimulus-reward reversal when compared to controls. In the operant strategy shifting task, adolescent-exposed rats shifted more rapidly between strategies and completed reversals faster than adult-exposed and control rats, respectively. The final AMPH challenge revealed evidence for sensitization in drug pre-exposed rats, with adult-exposed animals exhibiting the most significant effects. Together, these results suggest that AMPH induces long-lasting changes in behavioral flexibility that are at least partially dependent on age of exposure and may be due to adaptations in OFC function.
Keywords: Adolescence, Amphetamine, Behavioral Flexibility, Prefrontal Cortex
1. Introduction
Adolescence is a transitional period between childhood and adulthood, which in humans is estimated to range from 12 to as late as 25 years of age [1,2]. There are normal, yet substantial, neurobiological and cognitive changes occurring during this period [2–4], which also coincides with the peak time period of initiation of drug use [5]. This is of significant concern because an early onset of drug use is associated with a number of negative outcomes, including continued use into late adulthood [6], a greater likelihood to transition to dependence [7] and the development of significant cognitive dysfunction [8]. Importantly, cognitive impairment resulting from drug exposure during adolescent development may play a significant role in the heightened vulnerability of adolescents to developing addiction. Indeed, cognitive deficits in tasks sensitive to the prefrontal cortex (PFC) are observed in drug abusers [9,10] and have been linked to poorer treatment outcomes [11].
Studies in humans and laboratory animals have demonstrated that the PFC is among the last brain regions to fully mature [12]. For example, longitudinal MRI studies have demonstrated that gray matter in the PFC follows an inverted U-shaped curve that peaks during early to mid-adolescence and declines to stable levels by the early twenties [13]. Furthermore, post-mortem findings have shown that synapse elimination continues to occur in the PFC during adolescence [14,15]. Similar effects have been observed in rodents during their adolescent period, which has been defined as broadly as postnatal days (PNDs) 21 to 60 [16], but more conservatively as PNDs 28–45 [2]. During this time, there is a loss of neurons in the medial PFC (mPFC) [17] and changes in the molecular composition of synapses in this region [18]. Widespread changes in neurochemical signaling and connectivity with other brain regions is also ongoing during this time. For example, there are significant changes in the density of monoamine transporters [19] and dopaminergic fibers in the PFC [20], as well as alterations in the number of receptor sites. In the case of dopamine D1 and D2 receptors in the PFC and nucleus accumbens (NAc), there is an initial period of overproduction followed by elimination as animals reach young adulthood [21,22]. Importantly, these receptors are known to differentially modulate NMDA receptors in the PFC and NAc between adolescence and adulthood [23–26].
In psychostimulant abusers, deficits have been observed in PFC-sensitive tasks assessing attention [27–29], behavioral inhibition [30], and decision-making [31]. In rodents exposed to amphetamine (AMPH) during adulthood, there is evidence for impairments in extradimensional shifts and reversal learning in an attentional set shifting task (ASST) [32], impaired visual attention in a five-choice serial reaction time task [33], and impaired impulse control in a differential reinforcement of low rates of responding task [34]. Recent studies, which have focused on the potential for age-dependent differences in the effects of repeated drug exposure on cognition, have suggested that adolescents may be more vulnerable to drug-induced deficits in performance [35]. For example, studies from our laboratory have shown that pre-exposure to AMPH leads to more significant deficits in working memory when that exposure occurs during adolescence compared to adulthood [36].
The goal of the current study was to further examine the potential for age of exposure-dependent effects of AMPH on cognition. To do this, we tested adult rats exposed to saline or AMPH during adolescence or young adulthood in an ASST that has previously been shown to be sensitive to manipulations that disrupt functioning in the PFC [37]. Specifically, performance on the extradimensional shift is known to be sensitive to lesions of the mPFC [38], whereas lesions to the orbitofrontal cortex (OFC) impair reversal learning [39]. Based on our previous findings of AMPH-induced disruptions in working memory [36] and the long lasting effects of adolescent AMPH exposure on response perseveration in adulthood [40], we hypothesized that rats treated with AMPH would be impaired on reversal learning and the extradimensional shift. A subset of rats that completed the ASST were tested in an operant strategy shifting task that has been argued [41] to assess behavioral flexibility and be sensitive to mPFC function in a manner similar to the ASST. Thus, we hypothesized that performance in the ASST would correlate to that on the operant-based task, with AMPH pre-exposure causing deficits in behavior. In order to determine if there were lasting effects of drug pre-exposure on AMPH-induced motor activity, rats that concluded operant testing were subsequently observed in an open-field arena following a challenge injection of AMPH.
2. METHODS
2.1 Subjects
Subjects were sixty-six male Sprague-Dawley rats born from vendor-obtained breeders (Harlan; Indianapolis, IN, USA) that were maintained in our facility. They were weaned on PND 22 and housed 2–3 per cage with same-sex littermates. Starting between PND 119–120, rats were food restricted so that they could be maintained at ≥ 85% of their free-feeding body weights; water was available ad libitum throughout the study. Rats were kept on a 12-hour light/dark cycle (lights on at 0800) with experiments performed between 0830 and 1830. Experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Illinois, Urbana-Champaign, and were consistent with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
2.2 Pre-treatment
Rats were assigned to one of three treatment groups – control, adolescent-exposed, or adult-exposed – such that all groups were represented in each litter. Injections (i.p.) were given every other day during both adolescence (PND 27–45) and young adulthood (PND 85–103) as follows: those assigned to the control group were given 0.9% saline (1 ml/kg) at both time points, those in the adolescent-exposed groups were given 3 mg/kg d-AMPH sulfate during adolescence and saline during adulthood, and those in the adult-exposed groups were given saline during adolescence and 3 mg/kg d-AMPH sulfate during adulthood. This dose of AMPH, which was calculated based on the weight of the salt, was chosen based on our previous studies demonstrating its ability to induce long-lasting changes in behavior following adolescent or adult exposure [40,42]. For this treatment, animals were transported to a testing room, given their assigned injection, and placed individually in a clear plastic tub (46 × 25 × 22 cm) lined with hardwood bedding. After 60 min, rats were returned to their home cages and the colony room.
2.3 Attentional set-shifting task
Starting on ~PND 125, which was at least 5 days after they began food restriction, rats started training in an ID/ED version of an ASST (adapted from [38] and [43]). Training and testing took place in a clear plastic arena (74 × 30 × 50 cm), with access to either side of the arena controlled by insertion or removal of a divider. Terra-cotta pots with a height of 10 cm and an internal diameter of 11 cm were used and outfitted with stimuli for discriminations as described below. Rats were first trained to dig in a pot filled with corncob bedding for food reinforcement (45-mg Bioserv pellets). Food pellets were buried increasingly deeper in the pot until rats were reliably digging to retrieve pellets. Subsequently, they were trained to discriminate between pots based on one of three stimulus dimensions (texture, odor, or digging medium). A pair of pots that differed along one stimulus dimension (e.g. digging medium) was presented to rats by removing the divider to expose one end of the testing arena. On each trial, one of the pots was baited with a food pellet and the other remained un-baited. Residue from a crushed food pellet was placed in the un-baited pot to encourage the use of the presented stimulus dimension as the basis for discrimination rather than olfactory cues of the food pellet. At the start of each discrimination, rats were given four discovery trials during which they could dig in both pots without consequence. Choice of a particular pot was defined as displacement of the digging medium with the snout or paws. Following discovery trials, rats were allowed to dig in one pot only, as the non-chosen pot was immediately removed from the testing arena. Rats were trained in each discrimination until they met a criterion of six consecutive correct choices.
On the following day, rats were trained on a series of seven discriminations, with their movement to the next discrimination dependent on them meeting the training criterion of six consecutive correct choices (Table 1). First, rats were given a simple discrimination (SD) where the pots differed on a single stimulus dimension (e.g. odor). Next, they were progressed to a compound discrimination (CD) where all three stimulus dimensions were present on the pots. In this discrimination, the correct stimulus during the SD was maintained, an irrelevant dimension was added (e.g. digging medium) and the third dimension added was kept constant across all pots (e.g. texture). Following the CD, the stimulus-reward association was reversed (CD reversal, or CDR) such that the previously non-rewarded stimulus was rewarded and the previously rewarded stimulus was no longer rewarded. Next, an intradimensional shift (IDS) was required. In this discrimination, the relevant stimulus dimension from the previous discriminations was maintained (e.g. odor), but all of the stimuli were changed for a total changeover design [44]. Following acquisition of the IDS, the stimulus-reward association was again reversed (intradimensional reversal, or IDR). Lastly, rats were progressed to an extradimensional shift (EDS), in which the previously irrelevant stimulus dimension (e.g. digging medium) predicted location of the food pellet and the previously relevant dimension (e.g. odor) became irrelevant. Entirely novel stimuli were used for the EDS. Following acquisition of the EDS, the stimulus-reward association was reversed one final time (extradimensional reversal; EDR).
Table 1.
Order of training for the seven discriminations sets used in the ID/ED set-shifting task. In the example illustrated here, texture (T) was the constant stimulus dimension across all discriminations and the stimulus depicted in bold was baited. For SD through IDR, odor (O) was the relevant dimension and digging medium (D) was irrelevant. For EDS and EDR, D was the relevant dimension and O was the irrelevant dimension. Numbers refer to specific stimuli (e.g., O1= Lavender).
| Discrimination | Odor | Digging Medium | Texture |
|---|---|---|---|
| Simple Discrimination (SD) | O1, O2 | None | None |
| Compound Discrimination (CD) | O1, O2 | D1, D2 | T1 |
| Compound Discrimination Reversal (CDR) | O1, O2 | D1, D2 | T1 |
| Intradimensional Shift (IDS) | O3, O4 | D3, D4 | T2 |
| Intradimensional Reversal (IDR) | O3, O4 | D3, D4 | T2 |
| Extradimensional Shift (EDS) | O5, O6 | D5, D6 | T3 |
| Extradimensional Reversal (EDR) | O5, O6 | D5, D6 | T3 |
2.4 Operant Strategy-Shifting
Following completion of ASST, a subset of rats (n = 29; between PND 128–132) began training in the operant strategy-shifting task as adapted from Floresco et al. [41]. Rats were trained and tested in standard operant chambers (Coulbourn Instruments; Whitehall, PA, USA) that were housed inside sound-attenuating cubicles. The chambers were identical to those described previously by our lab [40].
Training began with one session of magazine training, during which 60 pellets were delivered on a random time 30-sec schedule. Subsequently, rats were trained during four 30-min sessions (1 session/day) to lever press on both levers on a fixed ratio 1 (FR1) schedule of reinforcement. The order in which the levers were presented was counterbalanced across groups and alternated each day. Rats that did not acquire lever pressing by the end of the second session were manually shaped using successive approximations. Following lever press training, rat’s were trained during five sessions to respond within 10-sec of lever extension into the chamber. Each rat s side bias was then established in a single session as described by Floresco et al. [41].
Strategy shifting sessions, which were given once per day, consisted of 120 trials that were separated by a 20-sec ITI. During each trial, one of the two cue lights located above the levers was illuminated for 3 sec, followed by illumination of the houselight and extension of both levers into the chamber. Individual cue lights were presented pseudorandomly across trials, such that no cue light could be presented on more than two consecutive trials. Rats were first trained to use a visual strategy by reinforcing them (with delivery of a food pellet) for pressing the lever that had a cue light illuminated above it, regardless of the lever’s spatial location (i.e., left or right side of pellet delivery trough). Trials continued until rats achieved ten consecutive correct choices and had completed at least 30 trials. On the day following acquisition of this performance criterion, rats were required to shift to an egocentric response strategy. This required that rats press a lever relative to its position, regardless of cue light location (e.g. always press the left lever). The location of the reinforced lever for the response strategy was determined individually for each rat such that it was the lever opposite of the rat’s side bias that was observed during initial training (see above). Trials continued until rats achieved a criterion of eight consecutive correct choices. Following acquisition of the response strategy, the stimulus-reward association was reversed such that pressing on the previously non-rewarded lever now resulted in reinforcement (e.g. always press the right lever). As before, trials continued until rats achieved a criterion of eight consecutive correct choices. For all stages of the task, trials where rats failed to respond on a lever within 10-sec of trial onset were terminated, scored as an omission, and led to the ITI.
2.5 AMPH challenge
At the conclusion of operant testing, rats were returned to ad libitum feeding conditions. Between 10 and 16 days later (PND 152–157), they were tested for their behavioral response to challenge injections of saline and 3 mg/kg d-AMPH. Locomotor activity was assessed in one of four available open-field arenas (Coulbourn Instruments) that each consisted of a clear acrylic box (41 × 41 × 41 cm) fitted with a photobeam frame located 2.5 cm above the arena floor (16 beams/dimension; 2.5 cm between beams). These chambers were located inside a 76 × 80 × 63 cm sound attenuating cubicle that had a 76 mm speaker mounted on the inside of one wall and two ceiling-mounted white lights (4 W each) that provided dim illumination. White noise (70 dB) was played continuously through the speakers when rats were in the testing room. Each open-field apparatus was connected to a nearby computer running activity monitoring software (TruScan, v 2.01; Coulbourn Instruments) that recorded beam breaks with a 500 ms sampling rate and converted this to ambulatory distance (m).
On the day of open-field testing, rats were transported in their home-cages to the testing room and allowed to habituate for 30 min. Rats were then placed individually into an open-field chamber and allowed to behave undisturbed for 30 min. They were then briefly removed from the apparatus and injected (i.p.) with 0.9% saline and immediately returned to the chambers for an additional 60 min. Lastly, rats were removed from the chamber, injected with 3 mg/kg d-AMPH, and returned for an additional 60 min.
2.6 Data analysis
For each of the discriminations in the ASST, latency from the time the divider was lifted until rats dug in a pot and both the number of trials and errors to criterion were recorded. Because different components of this task assess separate cognitive domains [45], discriminations that occurred consecutively (SD and CD) were analyzed together in a two-way, mixed factor ANOVA with group as the between-subjects factor and discrimination as the repeated factor. The remaining discriminations were analyzed separately in one-way ANOVAs. Tukey’s post-hoc tests were used to investigate significant main effects and interactions.
For the operant strategy-shifting task, the number of trials and errors to criterion were recorded. Errors following the shift to the response strategy were classified as perseverative, regressive, or never-reinforced according to previously established criteria [41]. Perseverative errors were defined as those that would have been correct when the visual strategy was in force (e.g., responding on the left lever when the left cue light was illuminated, but the right lever response was the reinforced response). Trials in which this type of error could be made were divided into blocks of eight consecutive trials. A perseverative error was scored when six out of the eight trials in a trial block were errors because the rat was still using the previous (i.e., visual) strategy on 75% of the trials. However, once five or fewer errors were made in a trial block, subsequent errors were scored as regressive. All other errors in a session were scored as never-reinforced, as they would not have been correct in a visual strategy and they included responses on the non-reinforced lever during the response strategy. Rats that did not acquire the reinforced strategy within one session were removed from subsequent strategy shifting analyses (visual: n = 1 control, n = 1 adolescent-exposed, and n = 2 adult-exposed; reversal: n = 1 adult-exposed). Because the strategy shifts are mediated by distinct neural substrates [41] they were analyzed in separate one-way ANOVAs. Error types, however, were analyzed in a two-way repeated measures ANOVA because they occurred within the same session (shift from visual to response strategy). Tukey’s post-hoc tests were used to investigate significant main effects and interactions.
For the AMPH challenge, two dependent measures of behavior were taken from the activity monitoring software. The first was ambulation, which was calculated by tabulating consecutive photobeam breaks in the horizontal plane and converting this to distance (in meters). The second was stereotypy, which is a measure of repetitive behavior such as head and body swaying, head bobbing, and sniffing, and is calculated by tabulating repetitive beam breaks in a focused area that do not contribute to large changes in location in the open-field. Cumulative measures during the first 10- (novelty) and 30-min (habituation) in the open-field arena were analyzed in separate one-way ANOVAs. Cumulative measures following saline (60-min) or AMPH (60-min) injection were analyzed with two-way repeated measures ANOVAs to compare differences in responding following injection with saline or AMPH. Tukey’s post-hoc analyses were used to investigate main effects and interactions. For all statistical tests, p values ≤0.05 were considered significant.
3. RESULTS
3.1 ASST
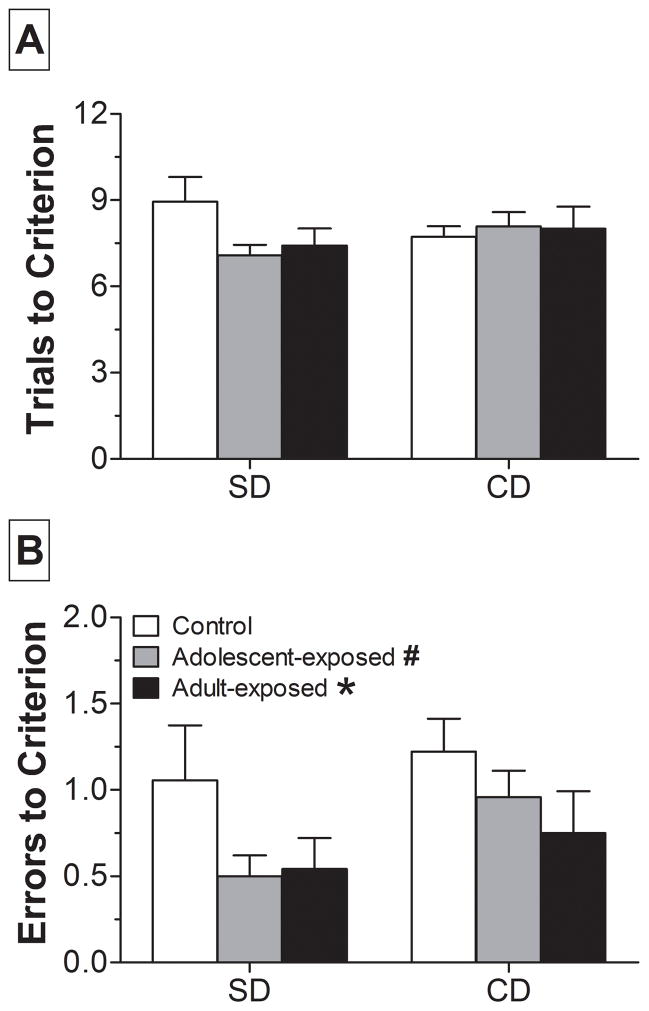
Rats were trained on a series of seven discriminations beginning with the SD and CD. As shown in Fig. 1A, rats treated with AMPH during adolescence or adulthood required fewer trials to reach criterion on the SD compared to controls, but this was not statistically significant. However, two-way repeated measures ANOVA of errors to criterion revealed a significant main effect of group (F2,126 = 3.05; p = 0.05). As shown in Fig. 1B, adult-exposed rats made significantly fewer errors than controls in both the SD and CD. Consistent with the tendency to require fewer trials, particularly on the SD, post-hoc analyses also revealed a trend for adolescent-exposed rats to make fewer errors than controls (p = 0.052). For both the SD and CD, there were no differences in latency to make a choice (data not shown).
Figure 1.
Performance on the simple and compound discrimination (SD and CD, respectively) in rats exposed to saline (control; n = 18) or AMPH during adolescence (n = 24) or adulthood (n = 24). Shown are trials (A) and errors (B) occurring before the performance criterion, which was defined as six consecutive correct pot choices. * p < 0.05, #p = 0.052 compared to control (collapsed across discrimination).
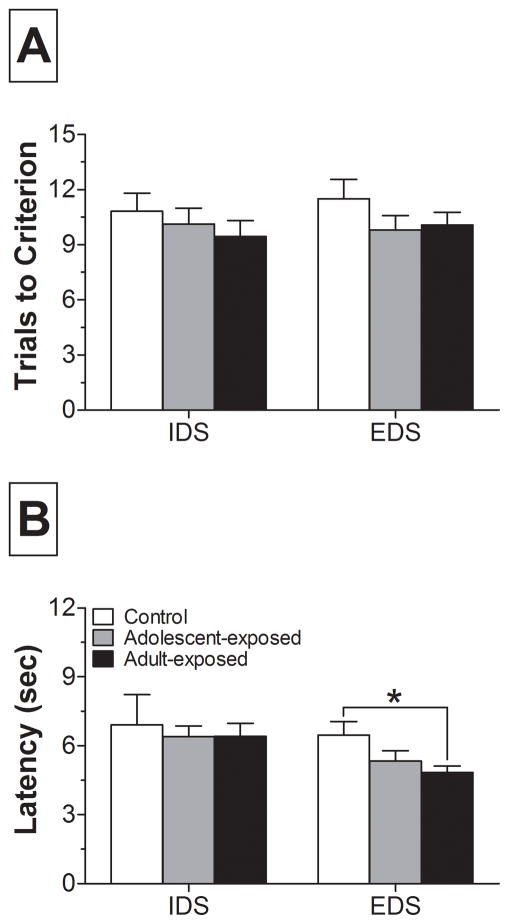
During the IDS and EDS, rats were presented with a set of novel stimuli and were required to maintain or shift from an attentional set, respectively. Pre-treatment with AMPH did not significantly affect trials or errors to criterion, although AMPH-exposed rats appeared to shift more readily between the IDS and EDS (Fig. 2; errors to criterion not shown). One-way ANOVA of latency to make a choice during the EDS revealed a significant main effect of group (F2,63 = 3.37; p < 0.05). Post-hoc analyses indicated that adult-exposed rats had significantly shorter latencies than controls (Fig. 2B).
Figure 2.
Comparison of performance on the intradimensional shift (IDS) and extradimensional shift (EDS), showing trials and average latency to criterion. (A) Trials to criterion and (B) average choice latency are presented for these two discriminations. * p < 0.05, versus control within EDS.
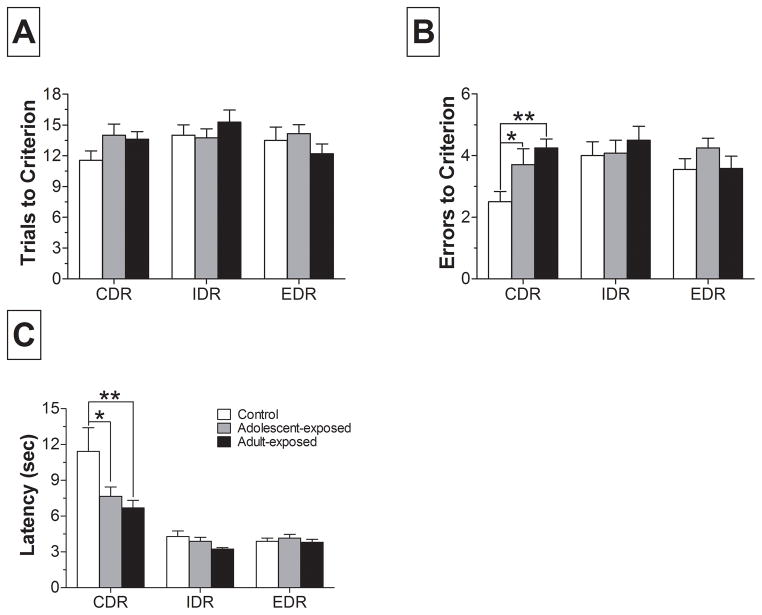
Following the CD, IDS, and EDS, rats were challenged with a reversal of the rule they had just acquired. As shown in Fig. 3, performance on the CDR was impaired in AMPH-treated groups of rats compared to controls. One-way ANOVA of errors to criterion revealed a main effect of group (F2,63 = 4.41; p < 0.05) and post-hoc tests confirmed that both the adolescent- and adult-exposed groups committed significantly more errors than controls (Fig. 3B). Consistent with this finding, AMPH pretreated rats appeared to require more trials to criterion, but this was not statistically significant. Furthermore, a one-way ANOVA of latency during the CDR revealed significant group differences (F2,63 = 4.49; p < 0.5). Post-hoc analyses indicated that during the CDR, both adolescent- and adult-exposed rats had significantly shorter latencies than controls (Fig. 3C). Analysis of trials, errors, and latency during the IDR and EDR revealed no significant differences.
Figure 3.
Performance on the three stimulus-reward reversals that followed the compound, intradimensional and extradimensional shifts. Data presented are (A) trials to criterion, (B) errors to criterion, and (C) average choice latency for the compound discrimination reversal (CDR), intradimensional reversal (IDR), and extradimensional reversal (EDR). * p < 0.05, ** p < 0.01, versus control within CDR.
3.2 Operant Strategy-Shifting
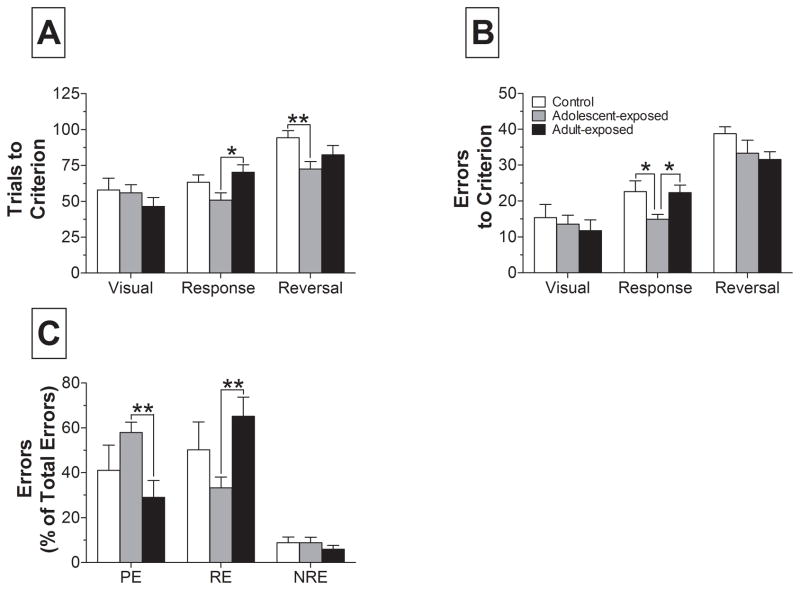
A subset of rats that completed the ASST (n = 29) were trained in an operant strategy-shifting task. In this task, rats were first reinforced for using a visual strategy, shifted to a response strategy, and then reinforced for shifting to the opposite response strategy. As shown in Fig. 4A, there were no statistically significant differences in the number of trials or errors to criterion during the visual strategy. However, analysis of trials and errors to criterion when rats were shifted to the response strategy (Fig. 4A–B) revealed significant differences between groups for both measures (trials: F2,22 = 3.82; p < 0.05; errors: F2,22 = 4.04; p < 0.05). Post-hoc analyses indicated that adolescent-exposed rats required significantly fewer trials to reach criterion than adult-exposed rats and committed significantly fewer errors than both adult-exposed and control rats. There were also significant group differences in performance during the reversal strategy (F2,21 = 4.15; p < 0.05) and post-hoc tests revealed that adolescent-exposed rats required significantly fewer trials to reach criterion than controls. Analysis of the number of errors to reach criterion during the reversal revealed no significant differences.
Figure 4.
Comparison of performance on the operant strategy-shifting task in rats exposed to saline (control; n = 8–9) or AMPH during adolescence (n = 9–10) or adulthood (n = 7–10). Rats began with a visual strategy, shifted to a response strategy, and finally reversed the response strategy. Rats that failed to acquire a strategy within one session were excluded from subsequent analyses. (A) Trials to criterion and (B) errors to criterion are shown for the three strategies: visual, response, and reversal. (C) Percent of total errors on the response strategy classified as perseverative errors (PE), regressive errors (RE), or never reinforced errors (NRE). * p < 0.05, ** p < 0.01 vs. adolescent-exposed within strategy.
Analysis of the relative contributions of different types of errors committed during the response strategy, which required rats to shift from the previously learned visual strategy, suggested group differences in the way rats performed this shift (Fig. 4C). A two-way repeated measures ANOVA revealed a significant effect of error type (F2,44= 42.9; p < 0.001) and a significant group x error type interaction (F4,44 = 4.22; p < 0.01). Post-hoc tests revealed that adolescent-exposed rats committed a greater and smaller proportion, respectively, of perseverative and regressive errors than adult-exposed rats. There were no group differences in the number of never-reinforced errors committed.
In order to investigate the association between behavior on the ASST and the operant strategy-shifting task in rats that performed both tasks, we performed a correlation analysis of trials to criterion on each of the reversals during ASST with trials to criterion during the operant strategy-reversal. We found no statistically significant correlations between the rat’s performance on any of the set-shifting reversals and the operant strategy reversal (range of r2 values: 0.01–0.03). We also correlated trials to criterion between the EDS and the operant shift from visual to response strategy, both of which have been shown to be sensitive to mPFC function [38,41]. There also was no statistically significant relationship between these factors (r2 = 0.002).
3.3 Amphetamine Challenge
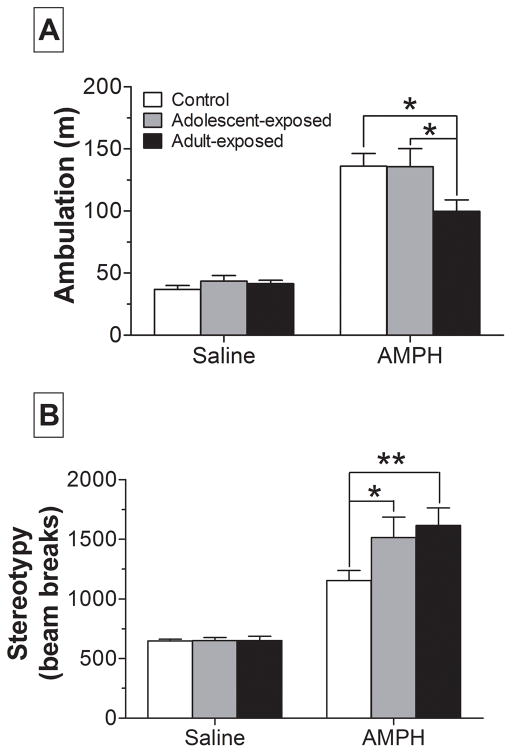
Approximately 2 weeks following completion of the operant strategy shifting task, rats (n = 29) were challenged with 3 mg/kg AMPH and their locomotor activity was assessed in an open-field arena. Analysis of cumulative activity during the first 10 min rats were in the open-field (i.e., novelty response) and the entire 30-min habituation period revealed no significant group differences in either ambulation or stereotypy (data not shown). AMPH, in contrast, had unique effects in AMPH-treated rats compared to controls (Fig. 5A). Two-way repeated measures ANOVA of ambulation revealed a main effect of time (F1,52 = 139.6; p < 0.001) and a group x time interaction (F2,52 = 3.23; p < 0.05). Post-hoc analyses demonstrated that adult-exposed rats traveled significantly less distance following AMPH injection than control and adolescent-exposed rats. This relative reduction in ambulation was likely due to a significant increase in a competing behavior, stereotypy. A two-way repeated measures ANOVA of stereotypy revealed a significant main effect of time (F1,52 = 85.8; p < 0.001) and a near group x time interaction (F2,52 = 2.65; p = 0.080). Based on the consistency of these data with recently published findings [36] we chose to further investigate the nature of this near significant interaction. As shown in Figure 5B, post-hoc analyses confirmed that both adolescent- and adult-exposed rats engaged in more stereotypy than controls following AMPH challenge.
Figure 5.
Response to a challenge injection of 3 mg/kg AMPH given ~2 weeks following completion of operant strategy-shifting (n = 9–10/group). Ambulatory distance (A) and stereotypy moves (B) are presented as cumulative activity during the 60-min period following AMPH injection. * p < 0.05, ** p < 0.01.
4. DISCUSSION
In this study, we examined age-dependent effects of AMPH exposure on behavioral flexibility during adulthood. We found that rats treated with AMPH exhibited faster acquisition (committed fewer errors) in simple and compound discriminations. In contrast to these apparent improvements in performance, rats pretreated with AMPH, regardless of age of exposure, exhibited deficits in reversal learning that were most significant in those exposed during adulthood. When a subset of these rats was trained on an operant strategy shifting task, we found that adolescent-exposed rats shifted more rapidly to an alternative strategy and a reversal of the previous strategy than adult-exposed and control rats, respectively. Furthermore, consistent with our previous findings [40], we found that adolescent-exposed rats committed a greater proportion of perseverative errors and fewer regressive errors compared to adult-exposed rats during strategy reversal learning. Lastly, when rats were given an AMPH challenge at the conclusion of operant testing, both adolescent- and adult-exposed rats exhibited sensitized behavioral responses to the drug. Together, these results demonstrate that AMPH exposure has a long-lasting impact on behavioral flexibility and locomotor plasticity that can be modulated by the age of exposure.
Our finding that rats exposed to AMPH did not exhibit impairments in the shift to the EDS is somewhat inconsistent with other studies in rats exposed to AMPH in adulthood [32,33], however, the treatment regimes in these studies covered a longer period of time and used escalating doses of AMPH (1–5 mg/kg). Furthermore, the extent to which the EDS used in the current experiment assessed mPFC functioning is unclear. In fact, our control group did not display a significant increase in trials to criterion between the IDS and EDS, indicating that our task may not have been sensitive to functioning in the mPFC. Failure to observe a significant increase in trials to criterion between IDS and EDS responding in controls has been observed in other studies as well [46,47], and appears to be a function of the number of IDSs presented [48–51]. Furthermore, rats with lesions to the OFC either fail to demonstrate [46] or require multiple IDSs in order to demonstrate the cost of shifting to an EDS [49]. Therefore, our attentional set-shifting results pertaining to AMPH’s influence on tasks sensitive to the mPFC are inconclusive. In spite of this, the burgeoning literature of age-dependent effects of drug exposure on cognition [35,52], including a recent study from our lab [36], suggests that adolescent drug exposure does differentially impair performance on tasks sensitive to mPFC function when the task is appropriately sensitive to function in this subregion of the PFC.
Our results demonstrating that rats exposed to AMPH are impaired during the first of three stimulus-reward associations are consistent with our recent findings of increased perseveration in adolescent-exposed rats [40] and reveal that this impairment is not dependent on the age of exposure, at least when rats are tested following different time periods of withdrawal. Moreover, these studies suggest that AMPH pretreatment results in deficits in behaviors that are sensitive to the OFC. Previously, reversal learning and perseverative responding have been associated with deficits in this subregion of the PFC [37,39,46,49,53,54] and both AMPH and cocaine exposure lead to deficits in these tasks [32,55,56]. Although the deficits observed in adolescent- and adult-exposed rats appear similar in magnitude, the time between the last AMPH injection and testing was ~8 weeks longer for adolescent-exposed rats than adult-exposed rats. Given that the effects of AMPH are known to dissipate with longer withdrawal periods [57,58], the lasting deficits in the adolescent-exposed group is striking and suggests that there may be differential effects of drug exposure during adolescence compared to adulthood.
Multiple lines of research suggest that the patterns of behavioral performance we observed in rats treated with AMPH could be mediated by drug-induced alterations in serotonergic functioning. Several studies have demonstrated that both systemic [59–64] and intra – OFC manipulations of serotonin [50,60,65] affect reversal learning and perseveration. Specifically, systemic and intra-OFC antagonism of 5-HT2C, but not 5-HT2A, receptors enhance reversal learning by reducing perseverative responses [59,65]. These data, together with AMPH’s known ability to stimulate the release of 5-HT and other monoamines, suggest that changes in serotonergic functioning are a likely mechanism for the changes in AMPH-exposed rats we observed here. Given the continued development of serotonergic innervation in the PFC during adolescence [19], we might expect age of exposure-dependent differences in AMPH’s effect on serotonin neurotransmission, but this hypothesis will require further testing.
The patterns of performance displayed throughout the ASST by AMPH-exposed rats converge on a common theme, indicating that their behavior is guided by heightened sensitivity to reward-associated cues. AMPH-exposed rats acquired SD and CDs faster than controls (committed fewer errors), but made significantly more errors when the stimulus-reward association was reversed. Faster acquisition of the SD and CD suggests that AMPH-exposed rats made associations between reward-paired cues significantly faster than control rats, which is consistent with studies of Pavlovian conditioning following psychostimulant exposure [66–68]. This interpretation is further supported by our observation that AMPH-exposed rats committed significantly more errors during initial reversal learning than controls, as this indicates that they had more trouble inhibiting responses to previously rewarded cues [59], although this deficit dissipated with training. Furthermore, adolescent- and adult-exposed rats had shorter latencies to make choices throughout many of the shifts compared to control rats, which reached significance during the CDR (adult-exposed) and EDS (AMPH-treated), suggesting that their behavior was controlled by features of reward cues rather than information about the stimulus dimension. If their behavior had been guided by features of the stimulus dimensions, then they should have demonstrated a ‘shift cost’ in the form of increased latency to make a choice [69]. These data are consistent with the notion that the behavior of AMPH-exposed rats was guided primarily by prepotent responses that were driven by reward-associated cues.
Performance during operant strategy-shifting revealed age of exposure-dependent differences in AMPH’s effects on cognitive functioning, but did not entirely parallel the findings from the ASST phase of the experiment. The most likely explanation for the disparate findings between our ASST and the operant strategy shifting task is that the operant task did not employ a total changeover design [44,70]. Without the use of a total changeover design, the rat’s performance may have been influenced by intermittent reinforcement of a previously reinforced strategy, thereby making direct comparison of the two tasks more difficult. In drug naïve rodents, intermittent reinforcement of a previously reinforced strategy slows the acquisition of a new strategy [71]. This may explain why our control group performed worse than AMPH-exposed groups on strategy shifts and reversals since, in both cases, previously correct strategies were intermittently reinforced. Moreover, our findings are consistent with a previous study [72] that also failed to observe deficits in strategy shifting following AMPH exposure.
The differences between adolescent- and adult-exposed rats during the strategy shifts may shed light on fundamental differences in their patterns of behavior. The intermittent reinforcement inherent in these shifts causes them to take on properties of a probabilistic learning paradigm, which suggests that sensitivity to positive and negative feedback could account for the patterns of behavior we observed [64]. Adopting alternative strategies in the face of a shift (lose-shift) would enhance performance on the strategy shift and reversal components of this task similar to the behavior observed in the adolescent-exposed rats. This suggests that, under conditions of probabilistic reinforcement, adolescent-exposed rats exhibit heightened sensitivity to negative feedback. Since adult-exposed rats performed similar to adolescent-exposed rats during set-shifting, but not strategy shifting, it is possible that they have heightened sensitivity to reward-associated cues like adolescent-exposed rats, but are less sensitive to negative feedback. Differential sensitivity to positive and negative feedback would suggest that there may be age of exposure-dependent differences in serotonergic functioning [64], which would be consistent with the knowledge that serotonergic innervation of the PFC continues to develop during adolescence [19]. However, the hypotheses that sensitivity to negative feedback and serotonin neurotransmission are differentially sensitive to drug-induced alterations depending upon the age of exposure will require further testing.
When we analyzed the types of errors committed during the shift from the visual to response strategy, adolescent-exposed rats made a greater proportion of perseverative responses compared to adult-exposed rats. This is consistent with the type of deficit we observed previously in rats exposed to AMPH during adolescence [40], both of which are indicative of alterations in OFC functioning [37,46,49]. Furthermore, this pattern of behavior is consistent with disruptions in serotonergic functioning, as several studies have linked serotonergic neurotransmission with changes in perseverative responding [59,61–63]. This evidence contributes to our hypothesis that there may be age of exposure-dependent alterations in OFC and serotonin functioning.
Following a challenge injection of 3 mg/kg AMPH, adolescent- and adult-exposed rats demonstrated sensitized behavioral responses compared to controls. Furthermore, although adolescent- and adult-exposed rats both displayed sensitization to the challenge injection, their responses were fundamentally different. Adult-exposed rats had significantly lower post-AMPH locomotion, but engaged in significantly more stereotyped behaviors than controls. Adolescent-exposed rats engaged in significantly more post-AMPH locomotion than adult-exposed rats and engaged in significantly more stereotyped behaviors than controls, similar to levels reached by adult-exposed rats. Studies that have examined age-dependent differences in psychostimulant sensitization differ considerably from the methodology of the current study and have yielded inconsistent findings [73–79], largely due to differences in pretreatment, dose, and withdrawal period. However, our findings are similar to a recently published study from our lab [36] with a few exceptions. In both studies, AMPH-exposed rats demonstrated sensitization to the challenge injection; however, in the Sherrill et al. [36] study adult-exposed rats reached similar levels of locomotion and higher levels of stereotypy than adolescent-exposed rats. These inconsistencies are likely due to minor differences in the studies. Prior to the AMPH challenge, rats in the Sherrill et al. [36] study were challenged with ketamine and low doses of AMPH during cognitive testing. This, and differences in subject acquisition (not all of the rats in the previous study were obtained from breeders maintained in our facilities), are likely responsible for the differences observed. In both studies we demonstrate long lasting sensitization following AMPH exposure (~3.5 and ~2 months in adolescent- and adult-exposed rats, respectively), and here we show that there are differences in the composition of behavioral plasticity depending upon the age of exposure. In conjunction with our strategy shifting data, these data suggest that there are age-dependent differences in the long-lasting effects of AMPH exposure.
In summary, the results of the current experiment suggest that drug-induced changes in cognitive flexibility and behavior are dependent on the age of exposure and the task being utilized. While we did not observe age-dependent effects of AMPH exposure on performance in the ASST, our data from the strategy shifting and locomotor testing suggest that some of AMPH’s effects are dependent upon the age of exposure. Indeed, we have demonstrated previously that adolescent-exposed rats have heightened working memory deficits [36] and a reduction in dopamine-mediated inhibition in mPFC pyramidal neurons [35] when compared to controls. Other studies that have examined the age-specific effects of drug exposure on cognition have typically compared treatment groups at different ages [52,56], but this approach is subject to an influence of age differences in task performance that exist prior to drug exposure [45,80,81]. To our knowledge, this is the first study to compare age of exposure-dependent differences in behavioral flexibility at the same endpoint (i.e. PND 125) and demonstrate the existence of long-lasting drug-induced alterations on behavioral flexibility. Future studies are needed to further elucidate the impact of these specific (and sometimes subtle) age-dependent changes in function and to determine the precise mechanisms through which AMPH acts to alter the normal trajectory of adolescent brain development.
Highlights.
Amphetamine impairs reversal learning early, but not late, in a set-shifting task
Adolescent amphetamine exposure facilitates strategy reversal learning
Amphetamine-induced sensitization is greater in rats exposed during adulthood
Results suggest that amphetamine induces lasting changes in cognitive flexibility
Acknowledgments
This study was supported by a grant from the National Institute on Drug Abuse (R01 DA029815). We thank Dr. Lori Newman for extensive guidance in our design and implementation of the attentional set shifting task. We also thank Courtney Hong, Jane Rivas, Alan Jarman, and Randy McCarthy for excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dahl RE. Adolescent Brain Development: A Period of Vulnerabilities and Opportunities. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- 2.Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 3.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nature Neuroscience. 2004;7:1040–7. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- 4.Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72:124–33. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Substance Abuse and Mental Health Services. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. NSDUH; 2011. Series H-4:11–4658. [Google Scholar]
- 6.Merline AC, O’Malley PM, Schulenberg JE, Bachman JG, Johnston LD. Substance use among adults 35 years of age: prevalence, adulthood predictors, and impact of adolescent substance use. American Journal of Public Health. 2004;94:96–102. doi: 10.2105/ajph.94.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the national longitudinal alcohol epidemiologic survey national institute on alcohol abuse and alcoholism. Journal of Substance Abuse. 1997;9:103–10. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- 8.Newton NC, O’Leary-Barrett M, Conrod PJ. Adolescent substance misuse: neurobiology and evidence-based interventions. Current Topics in Behavioral Neurosciences. 2013;13:685–708. doi: 10.1007/7854_2011_164. [DOI] [PubMed] [Google Scholar]
- 9.Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Current Opinion in Neurobiology. 2001;11:250–7. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- 10.Selby MJ, Azrin RL. Neuropsychological functioning in drug abusers. Drug and Alcohol Dependence. 1998;50:39–45. doi: 10.1016/s0376-8716(98)00002-7. [DOI] [PubMed] [Google Scholar]
- 11.McKellar JD, Harris AH, Moos RH. Predictors of outcome for patients with substance-use disorders five years after treatment dropout. Journal of Studies on Alcohol. 2006;67:685–93. doi: 10.15288/jsa.2006.67.685. [DOI] [PubMed] [Google Scholar]
- 12.Casey B, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54:241–57. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 13.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 14.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. The Journal of Comparative Neurology. 1997;387:167–78. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 15.Glantz LA, Gilmore JH, Hamer RM, Lieberman JA, Jarskog LF. Synaptophysin and postsynaptic density protein 95 in the human prefrontal cortex from mid-gestation into early adulthood. Neuroscience. 2007;149:582–91. doi: 10.1016/j.neuroscience.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neuroscience and Biobehavioral Reviews. 2003;27:163–78. doi: 10.1016/s0149-7634(03)00018-6. [DOI] [PubMed] [Google Scholar]
- 17.Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144:961–8. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Counotte DS, Li KW, Wortel J, Gouwenberg Y, Van Der Schors RC, Smit AB, et al. Changes in molecular composition of rat medial prefrontal cortex synapses during adolescent development. The European Journal of Neuroscience. 2010;32:1452–60. doi: 10.1111/j.1460-9568.2010.07404.x. [DOI] [PubMed] [Google Scholar]
- 19.Moll GH, Mehnert C, Wicker M, Bock N, Rothenberger A, Rüther E, et al. Age-associated changes in the densities of presynaptic monoamine transporters in different regions of the rat brain from early juvenile life to late adulthood. Brain Research Developmental Brain Research. 2000;119:251–7. doi: 10.1016/s0165-3806(99)00182-0. [DOI] [PubMed] [Google Scholar]
- 20.Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. The Journal of Comparative Neurology. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- 21.Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. The Journal of Neuroscience. 2008;28:2375–82. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. Neuroreport. 1997;8:1495–8. doi: 10.1097/00001756-199704140-00034. [DOI] [PubMed] [Google Scholar]
- 23.Huppé-Gourgues F, O’Donnell P. D1 -NMDA receptor interactions in the rat nucleus accumbens change during adolescence. Synapse. 2012;66:584–91. doi: 10.1002/syn.21544. [DOI] [PubMed] [Google Scholar]
- 24.Tseng K, O’Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cerebral Cortex. 2007;17:1235–40. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tseng KY, O’Donnell P. Post-pubertal emergence of prefrontal cortical up states induced by D1-NMDA co-activation. Cerebral Cortex. 2005;15:49–57. doi: 10.1093/cercor/bhh107. [DOI] [PubMed] [Google Scholar]
- 26.Benoit-Marand M, O’Donnell P. D2 dopamine modulation of corticoaccumbens synaptic responses changes during adolescence. The European Journal of Neuroscience. 2008;27:1364–72. doi: 10.1111/j.1460-9568.2008.06107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ornstein TJ, Iddon JL, Baldacchino AM, Sahakian BJ, London M, Everitt BJ, et al. Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology. 2000;23:113–26. doi: 10.1016/S0893-133X(00)00097-X. [DOI] [PubMed] [Google Scholar]
- 28.McKetin R, Mattick RP. Attention and memory in illicit amphetamine users: comparison with non-drug-using controls. Drug and Alcohol Dependence. 1998;50:181–4. doi: 10.1016/s0376-8716(98)00022-2. [DOI] [PubMed] [Google Scholar]
- 29.Hosak L, Preiss M, Bazant J, Tibenska A, Cermakova R, Cermakova E. Comparison of Wisconsin Card Sorting Test results between Czech subjects dependent on methamphetamine versus healthy volunteers. Psychiatria Danubina. 2012;24:188–93. [PubMed] [Google Scholar]
- 30.Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug and Alcohol Dependence. 2005;79:273–7. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, et al. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–39. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- 32.Fletcher PJ, Tenn CC, Rizos Z, Lovic V, Kapur S. Sensitization to amphetamine, but not PCP, impairs attentional set shifting: reversal by a D1 receptor agonist injected into the medial prefrontal cortex. Psychopharmacology. 2005;183:190–200. doi: 10.1007/s00213-005-0157-6. [DOI] [PubMed] [Google Scholar]
- 33.Fletcher PJ, Tenn CC, Sinyard J, Rizos Z, Kapur S. A sensitizing regimen of amphetamine impairs visual attention in the 5-choice serial reaction time test: reversal by a D1 receptor agonist injected into the medial prefrontal cortex. Neuropsychopharmacology. 2007;32:1122–32. doi: 10.1038/sj.npp.1301221. [DOI] [PubMed] [Google Scholar]
- 34.Peterson JD, Wolf ME, White FJ. Impaired DRL 30 performance during amphetamine withdrawal. Behavioural Brain Research. 2003;143:101–8. doi: 10.1016/s0166-4328(03)00035-4. [DOI] [PubMed] [Google Scholar]
- 35.Gulley JM, Juraska JM. The effects of abused drugs on adolescent development of corticolimbic circuitry and behavior. Neuroscience. doi: 10.1016/j.neuroscience.2013.05.026. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherrill LK, Stanis JJ, Gulley JM. Age-dependent effects of repeated amphetamine exposure on working memory in rats. Behavioural Brain Research. 2013;242C:84–94. doi: 10.1016/j.bbr.2012.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. The Journal of Neuroscience. 2008;28:11124–30. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. The Journal of Neuroscience. 2000;20:4320–4. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dias R, Roberts R. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 40.Hankosky ER, Gulley JM. Performance on an impulse control task is altered in adult rats exposed to amphetamine during adolescence. Developmental Psychobiology. 2012 doi: 10.1002/dev.21067. 10.1002/dev.21067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Floresco SB, Block AE, Tse MTL. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behavioural Brain Research. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Stanis JJ, Marquez AH, White MD, Gulley JM. Dissociation between long-lasting behavioral sensitization to amphetamine and impulsive choice in rats performing a delay-discounting task. Psychopharmacology. 2008;199:539–48. doi: 10.1007/s00213-008-1182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newman LA, Darling J, McGaughy J. Atomoxetine reverses attentional deficits produced by noradrenergic deafferentation of medial prefrontal cortex. Psychopharmacology. 2008;200:39–50. doi: 10.1007/s00213-008-1097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slamencka NJ. A methodological analysis of shift paradigms in human discrimination learning. Psychological Bulletin. 1968;69:423–38. doi: 10.1037/h0025762. [DOI] [PubMed] [Google Scholar]
- 45.Newman LA, McGaughy J. Adolescent rats show cognitive rigidity in a test of attentional set shifting. Developmental Psychobiology. 2011;53:391–401. doi: 10.1002/dev.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behavioural Brain Research. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 47.Parsegian A, Glen WB, Lavin A, See RE. Methamphetamine self-administration produces attentional set-shifting deficits and alters prefrontal cortical neurophysiology in rats. Biological Psychiatry. 2011;69:253–9. doi: 10.1016/j.biopsych.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bissonette GB, Lande MD, Martins GJ, Powell EM. Versatility of the mouse reversal/set-shifting test: Effects of topiramate and sex. Physiology & Behavior. 2012 doi: 10.1016/j.physbeh.2012.05.018. 10.1016/j.physbeh.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chase EA, Tait DS, Brown VJ. Lesions of the orbital prefrontal cortex impair the formation of attentional set in rats. The European Journal of Neuroscience. 2012;36:2368–75. doi: 10.1111/j.1460-9568.2012.08141.x. [DOI] [PubMed] [Google Scholar]
- 50.Clarke HF, Walker SC, Crofts HS, Dalley JW, Robbins TW, Roberts AC. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. The Journal of Neuroscience. 2005;25:532–8. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garner JP, Thogerson CM, Würbel H, Murray JD, Mench JA. Animal neuropsychology: validation of the Intra-Dimensional Extra-Dimensional set shifting task for mice. Behavioural Brain Research. 2006;173:53–61. doi: 10.1016/j.bbr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Counotte DS, Goriounova NA, Li KW, Loos M, Van der Schors RC, Schetters D, et al. Lasting synaptic changes underlie attention deficits caused by nicotine exposure during adolescence. Nature Neuroscience. 2011;14:417–9. doi: 10.1038/nn.2770. [DOI] [PubMed] [Google Scholar]
- 53.Freedman M, Black S, Ebert P, Binns M. Orbitofrontal function, object alternation and perseveration. Cerebral Cortex. 1998;8:18–27. doi: 10.1093/cercor/8.1.18. [DOI] [PubMed] [Google Scholar]
- 54.Clark L, Cools R, Robbins TW. The neuropsychology of ventral prefrontal cortex: decision-making and reversal learning. Brain and Cognition. 2004;55:41–53. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- 55.Jentsch JD, Olausson P, De La Garza R, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26:183–90. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- 56.Harvey RC, Dembro KA, Rajagopalan K, Mutebi MM, Kantak KM. Effects of self-administered cocaine in adolescent and adult male rats on orbitofrontal cortex-related neurocognitive functioning. Psychopharmacology. 2009;206:61–71. doi: 10.1007/s00213-009-1579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Black YD, Maclaren FR, Naydenov AV, Carlezon WA, Jr, Baxter MG, Konradi C. Altered attention and prefrontal cortex gene expression in rats after binge-like exposure to cocaine during adolescence. The Journal of Neuroscience. 2006;26:9656–65. doi: 10.1523/JNEUROSCI.2391-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Santucci AC, Capodilupo S, Bernstein J, Gomez-Ramirez M, Milefsky R, Mitchell H. Cocaine in adolescent rats produces residual memory impairments that are reversible with time. Neurotoxicology and Teratology. 2004;26:651–61. doi: 10.1016/j.ntt.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 59.Boulougouris V, Glennon JC, Robbins TW. Dissociable effects of selective 5-HT2A and 5-HT2C receptor antagonists on serial spatial reversal learning in rats. Neuropsychopharmacology. 2008;33:2007– 19. doi: 10.1038/sj.npp.1301584. [DOI] [PubMed] [Google Scholar]
- 60.Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cerebral Cortex. 2007;17:18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- 61.van der Plasse G, Feenstra MGP. Serial reversal learning and acute tryptophan depletion. Behavioural Brain Research. 2008;186:23–31. doi: 10.1016/j.bbr.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 62.Brown HD, Amodeo DA, Sweeney JA, Ragozzino ME. The selective serotonin reuptake inhibitor, escitalopram, enhances inhibition of prepotent responding and spatial reversal learning. Journal of Psychopharmacology. 2012 doi: 10.1177/0269881111430749. 10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brigman JL, Mathur P, Harvey-White J, Izquierdo A, Saksida LM, Bussey TJ, et al. Pharmacological or genetic inactivation of the serotonin transporter improves reversal learning in mice. Cerebral Cortex. 2010;20:1955–63. doi: 10.1093/cercor/bhp266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bari A, Theobald DE, Caprioli D, Mar AC, Aidoo-Micah A, Dalley JW, et al. Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacology. 2010;35:1290–301. doi: 10.1038/npp.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boulougouris V, Robbins TW. Enhancement of spatial reversal learning by 5-HT2C receptor antagonism is neuroanatomically specific. The Journal of Neuroscience. 2010;30:930–8. doi: 10.1523/JNEUROSCI.4312-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harmer CJ, Phillips GD. Enhanced appetitive conditioning following repeated pretreatment with d-amphetamine. Behavioural Pharmacology. 1998;9:299–308. [PubMed] [Google Scholar]
- 67.Taylor JR, Jentsch JD. Repeated Intermittent Administration of Psychomotor Stimulant Drugs Alters the Acquisition of Pavlovian Approach Behavior in Rats: Differential Effects of cocaine, d-amphetamine and 3,4-methylenedioxymethamphetamine (“Ecstasy”) Biological Psychiatry. 2001;50:137–143. doi: 10.1016/s0006-3223(01)01106-4. [DOI] [PubMed] [Google Scholar]
- 68.Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: increased cue-triggered “wanting” for sucrose reward. The Journal of Neuroscience. 2001;21:7831–40. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Monsell S. Task switching. Trends in Cognitive Sciences. 2003;7:134–40. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- 70.Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1991;29:993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- 71.Buss AH. Rigidity as a function of reversal and non-reversal shifts in the learning of successive discriminations. Journal of Experimental Psychology. 1953;45:153–156. doi: 10.1037/h0053784. [DOI] [PubMed] [Google Scholar]
- 72.Featherstone RE, Rizos Z, Kapur S, Fletcher PJ. A sensitizing regimen of amphetamine that disrupts attentional set-shifting does not disrupt working or long-term memory. Behavioural Brain Research. 2008;189:170–9. doi: 10.1016/j.bbr.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 73.Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behavioral Neuroscience. 1998;112:1152–66. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- 74.Mathews IZ, Kelly H, McCormick CM. Low doses of amphetamine lead to immediate and lasting locomotor sensitization in adolescent, not adult, male rats. Pharmacology, Biochemistry, and Behavior. 2011;97:640–6. doi: 10.1016/j.pbb.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 75.Kameda SR, Fukushiro DF, Trombin TF, Procópio-Souza R, Patti CL, Hollais AW, et al. Adolescent mice are more vulnerable than adults to single injection-induced behavioral sensitization to amphetamine. Pharmacology, Biochemistry, and Behavior. 2011;98:320–4. doi: 10.1016/j.pbb.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 76.Richetto J, Feldon J, Riva MA, Meyer U. Comparison of the long-term consequences of withdrawal from repeated amphetamine exposure in adolescence and adulthood on information processing and locomotor sensitization in mice. European Neuropsychopharmacology. 2013;23:160–70. doi: 10.1016/j.euroneuro.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 77.Zakharova E, Leoni G, Kichko I, Izenwasser S. Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behavioural Brain Research. 2009;198:45–50. doi: 10.1016/j.bbr.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Good R, Radcliffe R. Methamphetamine-induced locomotor changes are dependent on age, dose, and genotype. Pharmacology, Biochemistry and Behavior. 2011;98:101–11. doi: 10.1016/j.pbb.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Niculescu M, Ehrlich ME, Unterwald EM. Age-specific behavioral responses to psychostimulants in mice. Pharmacology, Biochemistry, and Behavior. 2005;82:280–8. doi: 10.1016/j.pbb.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 80.Andrzejewski ME, Schochet TL, Feit EC, Harris R, McKee BL, Kelley AE. A comparison of adult and adolescent rat behavior in operant learning, extinction, and behavioral inhibition paradigms. Behavioral Neuroscience. 2011;125:93–105. doi: 10.1037/a0022038. [DOI] [PubMed] [Google Scholar]
- 81.Hammerslag LR, Gulley JM. Age and Sex Differences in Reward Behavior in Adolescent and Adult Rats. Developmental Psychobiology. doi: 10.1002/dev.21127. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]