Abstract
Purpose
Gliomas are known to induce local and systemic immunosuppression, inhibiting T cell-mediated cytotoxic responses to tumor growth. Tumor-associated macrophages are a significant component of the immune infiltrate in gliomas and may express immunosuppressive surface ligands, such as B7-H1.
Experimental Design
Tumor and peripheral blood samples from patients with glioblastoma (GBM) were analyzed by flow cytometry to evaluate the expression of B7-H1 in circulating and tumor-infiltrating macrophages. Human monocytes from healthy patients were stimulated with conditioned media from glioma cells to evaluate B7-H1 expression. Production of IL-10 by stimulated monocytes was measured by ELISA, and stimulation with IL-10 alone was evaluated for the ability to induce B7-H1 expression. The effect of inhibiting IL-10 and its receptor on glioma-induced B7-H1 expression in monocytes was evaluated.
Results
Circulating monocytes in patients with GBM had significantly increased expression of B7-H1 compared to healthy control patients. Tumor-associated macrophages from matched GBM tissue had even greater B7-H1 expression. Treatment of normal monocytes with glioma conditioned media could significantly increase B7-H1 expression. Stimulation of monocytes with conditioned media resulted in substantial production of IL-10 and upregulation of the IL-10 receptor. Stimulation of monocytes with IL-10 alone could significantly increase B7-H1 expression, sufficient to induce T cell apoptosis when co-cultured with stimulated monocytes. Inhibition of IL-10 and the IL-10 receptor could knock down the effect of glioma media on B7-H1 by greater than 50%.
Conclusions
Gliomas can upregulate B7-H1 expression in circulating monocytes and tumor-infiltrative macrophages through modulation of autocrine/paracrine IL-10 signaling, resulting in an immunosuppressive phenotype.
Keywords: Glioma, macrophage, B7-H1, PD-L1, interleukin-10, immunosuppression
INTRODUCTION
Malignant gliomas are highly aggressive tumors with a uniformly poor prognosis.(1, 2) Depsite advances in medical and surgical management over the past decade, the median survival for the most aggressive form of glioma, glioblastoma (GBM), remains approximately 12-15 months.(3, 4) One significant challenge to the development of new therapies for gliomas is their ability to induce local and systemic immunosuppression, limiting the innate defense to tumor growth and the efficacy of adaptive immunotherapy.(5, 6) In the local tumor microenvironment, gliomas have been shown to suppress immune responses through upregulation of anti-inflammatory proteins (7-10), downregulation of antigen presentation (11, 12), and expansion of immunosuppressive effectors cells such as regulatory T cells.(13, 14) Gliomas can also express immunosuppressive ligands at the cell surface, including the co-stimulatory molecule B7-homologue 1 (B7-H1), also known as the programmed death ligand 1 (PD-L1).(15) Loss of the tumor suppressor phosphatase and tensin homolog (PTEN) results in upregulated PI(3)kinase activity in glioma cells and increased surface expression of B7-H1.(16) This ligand can bind to and stimulate the programmed death-1 (PD-1) receptor on activated T cells resulting in T cell quiescence and apoptosis.(17, 18) Expression of B7-H1 by tumor cells is a common mechanism of immunoresistance in cancer, and has been demonstrated in numerous malignancies including renal, lung, breast, and prostate cancer.(19-22)
Despite adaptations to evade immune surveillance, gliomas do stimulate a significant immune response and are known to be infiltrated by immune effector cells.(8) In addition to the expected lymphocytic response, high-grade gliomas have substantial macrophage and microglial infiltration.(23, 24) Tumor-associated macrophages account for > 10% of infiltrative cells in peri-tumoral GBM tissue, in comparison to <1% of cells in tumor-free brain.(23) A common finding in multiple malignancies, tumor-associated macrophages can be paradoxically both immune-activating and immunosuppressive.(25-28) Macrophages respond to signals from tumor cells by polarizing into pro-inflammatory (M1) or anti-inflammatory (M2) phenotypes, defined by their cascade of cytokine production and secondary effects on other immune cells.(29, 30) Recent studies of the phenotype of glioma-associated macrophages suggest that they are largely immunosuppressive.(26, 29, 31) In gliomas, the degree of macrophage infiltration has been positively correlated with tumor grade (32) and within high-grade lesions, higher levels of macrophage infiltration are associated with decreased survival.(33)
While the standard definition of an anti-inflammatory (M2) tumor associated-macrophage is based on expression of immunosuppressive cytokines such as IL-10 and TGF-β, there is evidence that these macrophages can also express B7-H1.(31, 34) Studies from patients with hepatocellular carcinoma (HCC) demonstrate elevated levels of B7-H1 expression by tumor-infiltrating macrophages in human tumor samples, which correlate with poor outcomes.(35) While peripheral blood monocytes are known to express low levels of B7-H1, exposure of monocytes to conditioned media from HCC cells is sufficient to significantly upregulate B7-H1 expression in culture.(35) To date, substantial expression of B7-H1 in circulating monocytes and tumor-infiltrating macrophages in glioma patients has not been documented. Based on in vitro studies and results from patients with other tumor types, we hypothesized that glioma-infiltrating macrophages can be stimulated by a glioma-derived soluble factor, inducing B7-H1 expression and rendering these macrophages capable of suppressing T cell activity. Through this mechanism, gliomas may be capable of inducing immunosuppression locally and in circulation, independent of PTEN status and B7-H1 expression on the glioma cell itself. Here we present evidence from GBM patients supporting this hypothesis.
METHODS
Cell Lines and Specimens
Glioma cell lines (U251, U87) were obtained through the UCSF Brain Tumor Research Center, and normal human astrocytes (NHA) were obtained from Sciencell Laboratories. Fresh tumor tissue was obtained at surgery from patients undergoing initial operation for newly diagnosed GBM. Peripheral blood samples were also obtained at surgery from GBM patients or from healthy donors. All patient specimens were obtained with written, informed consent under approval of the UCSF Committee on Human Research.
Cell Sorting
Peripheral blood leukocytes (PBL) were isolated from whole blood using Ficoll-Paque Plus (GE Healthcare) centrifugation. Tumor-infiltrating leukocytes (TIL) were isolated from resected tissue using a three-step density gradient, as previously described.(36) Monocytes were then extracted from the PBL of healthy donors by CD14 positive selection using magnetic nanoparticles (EasySep, Stem Cell Technologies) according to the manufacturer’s instructions. Once separated, monocytes were suspended in RMPI-1640 25 mM Hepes, 2.0 g/L NaHCO3 supplemented with 1% penicillin-streptomycin, 1 mM sodium pyruvate, 10 mM nonessential amino acids, and 2.5% fetal bovine serum (UCSF Cell Culture Facility).
Co-Culture
Glioma cells were plated at 5 × 104 cells/well in 24-well plates in RPMI 1640 media with 10% FBS and allowed to adhere 24 hours prior to co-culture. Isolated CD14+ monocytes were plated with glioma cells at 2×105 cells/well in contact or above a 0.2μm pore Transwell permeable insert (Costar) and incubated for 24 hours at 37°C. Monocytes were then harvested from co-culture suspension, spun at 300g × 5min, and resuspended in RPMI prior to staining for flow cytometry.
Conditioned Media Culture
Supernatant from NHA and glioma cells cultured in T75 flasks was harvested at 90% confluence. Conditioned media was spun at 300g × 5min to pellet any cellular material and the supernatant was transferred to 10 kDa Amicon ultrafiltration tubes (Milipore) and spun at 35000 rpm × 30 min to concentrate the media 20-fold. Isolated CD14+ monocytes were plated at 1×105 cells/well in 96-well plates and treated with the concentrated conditioned media added at 1:10 with normal media. In addition, monocytes were stimulated with varying doses of human recombinant cytokines including MCP-1 (BD Biosciences), MCP-3 (BD Biosciences), M-CSF (eBioscience), IL-6 (eBioscience), or IL-10 (eBioscience). Cells were incubated with conditioned media and/or cytokines for 24 hours at 37°C prior to staining for flow cytometry.
Flow Cytometry
Cells were harvested and stained extracellularly with CD45 FITC (clone HI30, eBioscience), CD11b PeCy (clone ICRF44, eBioscience), HLA-DR APC (clone LN3, eBioscience), and B7-H1 PE (clone MIH1, eBioscience) or isotype control (eBioscience) in phosphate buffered saline with 2% bovine serum albumin on ice for 30 min. After washing, cells were fixed with 2% paraformaldehyde (Sigma) and read using a BD FACScaliber flow cytometer with CellQuest Software (Beckton Dickinson). Data was analyzed using FlowJo software (Treestar). For measurement of IL-10 receptor expression, IL-10R (CDw210) PE (clone 3F9, BD Bioscience) was used as an extracellular stain.
T-cell Apoptosis
T cells were enriched from donor PBL using a negative magnetic nanoparticle selection kit (StemCell Technologies). These cells were then activated by culture in 96-well plates with plate bound human anti-CD3 (clone OKT3, 2 ug/ml) and soluble anti-CD28 (clone CD28.2, 4 ug/ml) for 48 hours (eBioscience). Activation was confirmed by blast formation. Concurrently, monocytes were extracted from the same donor PBL by CD14 positive selection and stimulated as described. At 48 hours, activated monocytes and T cells were combined (1:1) in 96-well plates at a concentration of 1×105 cells/well in the presence of 10 U/ml recombinant human IL-2 (BD Bioscience). The co-culture was allowed to incubate for 12 hours. Cells were harvested, washed, and resuspended in phosphate buffered saline with 2% bovine serum albumin. Cells were surface stained on ice for 30 minutes with CD3 PE (clone SK7, BD Bioscience) and CD11b FITC (clone ICRF44, eBioscience). After washing with Annexin buffer, cells were stained with Annexin V APC (eBioscience) for 10 minutes at room temperature and fixed in 2% paraformaldehyde prior to analysis by flow cytometry. The percentage of Annexin positive cells was determined as a fraction of the total number of CD3+ T cells.
Enzyme-linked Immunosorbent Assay
IL-10 concentration in glioma-conditioned media and in the media of treated monocytes was determined using a human IL-10 ELISA kit (BD Bioscience) according to manufacturer’s instructions. Human cytomegalovirus (HCMV)-derived IL-10 was also measured in the glioma-conditioned media by ELISA. hcmvIL-10 specific IgG (100ng/well; affinity purified, polyclonal; R&D systems, cat# AF117) was plated as a capture antibody and biotinylated hcmvIL-10 specific polyclonal IgG (10ng/well; R&D systems, cat# BAF117) was used as a detection antibody. Standard curves were constructed using recombinant hcmvIL-10 (R&D systems, cat# 117-VL-025), as previously published.(37)
Reverse Transcribed-Quantitative PCR
B7-H1 and IL-10 mRNA was extracted from treated monocytes using the RNeasy Mini Kit (Qiagen) and cDNA was generated using the Superscript III Kit (Invitrogen). Transcript levels were detected using the SYBR Green (Applied Biosystems) and the CFX96 Real-Time System (Bio-Rad Laboratories). Values of transcript levels were standardized using 18S ribosomal RNA. Sequences of primers used were: B7-H1 (5′-GCTGTT-GAAGGA-CCAGCT-CT/TGCTTG-TCCAGA-TGACTT-CG-3′), IL-10 (5′-GTGGAG-CAGGTG-AAGAAT-GC/ATAGAG-TCGCCA-CCCTGA-TG-3′), and 18S rRNA (5′-GTAACC-CGTTGA-ACCCCA-TT/CCATCC-AATCGG-TAGTAG-CG 3′).
Immunohistochemistry
Paraffin-embedded specimens were deparaffinized and rehydrated, followed by antigen retrieval for 30 minutes at 95° C using 10 mM citrate buffer. Endogenous peroxidases were quenched with hydrogen peroxide. Samples were blocked in tyramide buffer (Invitrogen) with 1% Triton-X for 1 hour at room temperature. Incubation with a single primary antibody was performed overnight at 4° C. Slides were washed in PBS with 0.1% Tween-20 then incubated with a biotinylated secondary antibody (Vector Labs) at 1:500 for 30 minutes. Visualization was performed using tyramide signal amplification (TSA) kit #21 (Invitrogen) per manufacturer’s protocol. Samples were washed then incubated with ABC-Elite (Vector Labs) for 30 minutes, then final labeling performed using TSA kits #22 (Alexa Fluor 488), #25 (Alexa Fluor 594), or #26 (Alex Fluor 647). Samples were then blocked for 1 hour in tyramide buffer with avidin (Vector labs) and incubated overnight with a second primary antibody diluted in tyramide blocking buffer with biotin (Vector labs) at 4° C. The procedure above was repeated for the second and third primary antibodies. Dilution of primary antibodies was as follows: B7-H1 (Abcam ab55810, 1:500), CD163 (Abcam ab74604, prediluted), IL-10 (Thermo Scientific MA1-82664, 1:100). After completion of triple staining, nuclear visualization was performed with DAPI (Invitrogen) then mounted using VectaMount AQ (Vecor labs). Confocal images were generated on a Zeiss LSM 510 META laser-scanning microscope.
IL-10 Inhibition
Monocytes extracted from PBL by CD14 positive selection were plated at 1 × 105 cells/well in 96-well plates and pretreated with a soluble IL-10 neutralizing antibody (clone JES3-9D7, eBioscience) at 5 μg/ml or an IL-10 recepter anatagonist (clone 37607.11, Thermo Pierce Antibody) at 5 μg/ml for 2 hours. Cells were then stimulated with glioma-conditioned media and incubated for 24 hours at 37°C prior to staining for flow cytometry. The soluble IL-10 neutralizing antibody was re-dosed (5 μg/ml) at 12 hours to maintain an inhibitory effect.
Statistics
Data for each experiment was collected from at least three independent specimens with testing of each patient specimen repeated in triplicate. The mean value from the multiple repetitions for each specimen was used for statistical analysis. Differences between treated specimens and controls were compared using a paired t-test. Differences in the degree of change from control between different treatment conditions was evaluated by comparing the magnitude of change (Δ) using an independent t-test for 2 test conditions, or ANOVA from multiple groups. Correlations between protein expression and magnitude of functional change were evaluated using a Pearson’s correlation coefficient. Statistical significance was accepted for p<0.05. Comparisons were performed using SPSS version 19 (IBM). Data is reported and demonstrated graphically as mean ± SEM.
RESULTS
Identification of peritumoral macrophage infiltrates
Total leukocytes were isolated from peripheral blood and tumor of patients undergoing resection of GBM without prior therapy (n=16) and analyzed by flow cytometry. The infiltrating macrophage fraction in the tumor was determined by gating on CD45bright/CD11b+ cells, distinguished from CD45dim/CD11b+ resident microglia (Figure 1A). The cutoff intensity used to define CD45bright cells was selected from the lower boundary of CD45 positivity in the matched peripheral blood monocytes. A clear distinction between CD45bright and CD45dim groups was not always visible in the scatter plots, and therefore the macrophage fraction may contain some activated microglia as well.
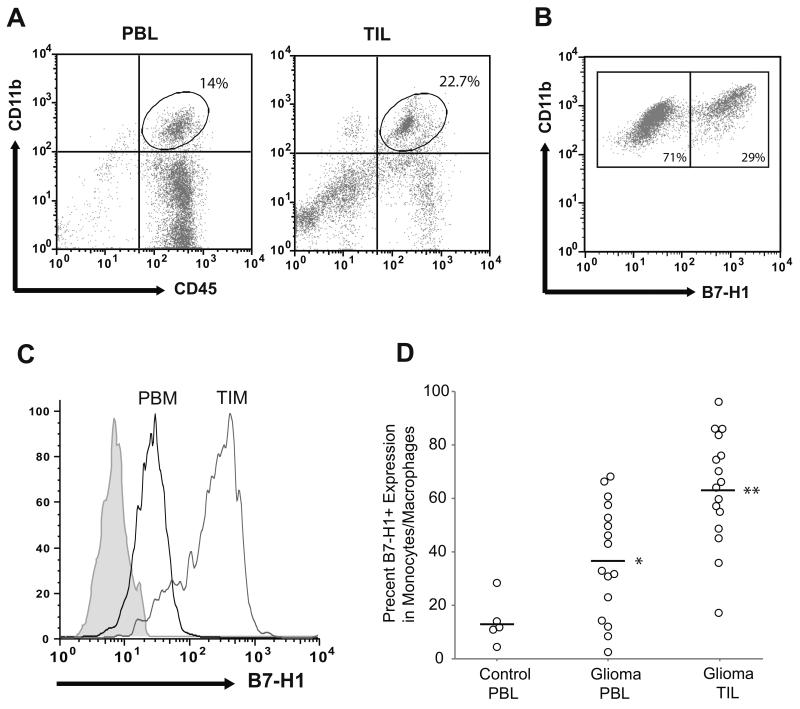
Figure 1. Peripheral blood monocytes and tumor-infiltrating macrophages in GBM patients express elevated levels of B7-H1.
(A) Representative gating of peripheral blood leukocytes (PBL) and tumor-infiltrating leukocytes (TIL) from GBM patients analyzed by flow cytometry is shown. Monocytes/macrophages were identified by co-positive CD45 and CD11b staining. (B) Representative analysis of the percent of B7-H1 positive cells among the CD45+/CD11b+ monocytes gated in panel (A) is shown. (C) Representative analysis of B7-H1 expression intensity in peripheral blood monocytes (PBM) and tumor-infiltrating macrophages (TIM) of a GBM patient is shown. B7-H1 intensity was measured after gating on CD45+/CD11b+ cells. Curves depict the isotype control (gray shaded region), peripheral monocytes (black line), and tumor-infiltrating macrophages (gray line). (D) The fraction of B7-H1 expressing cells as a percentage of the total number of monocytes/macrophages in peripheral blood and tumor from GBM patients (n=16), as well as from the peripheral blood of non-glioma control patients (n=5) is shown. Absolute percentage for each patient and group means are depicted. B7-H1 expression in peripheral monocytes from GBM patients is significantly greater than healthy control patients (* p < 0.05). B7-H1 expression in tumor-associated macrophages is significantly greater than in peripheral monocytes among GBM patients (** p < 0.001).
Tumor-infiltrating macrophages have increased expression of B7-H1
Expression of B7-H1 on circulating monocytes from the peripheral blood of non-glioma control patients was found to be low (<15% B7-H1 positive cells). By comparison, peripheral blood and tumor-infiltrating monocytes/macrophages from GBM patients had significantly increased expression of B7-H1 (Figure 1B and C). Although there was significant variability in absolute expression between patients, in 15/16 (94%) patients B7-H1 expression was higher in the tumor-infiltrative macrophages compared to peripheral circulating monocytes, with nearly double the number of B7-H1 positive cells in tumor (63 ± 5.2% vs. 36.6 ± 5.3%, p<0.001).
B7-H1 expression confers T cell immunoresistance
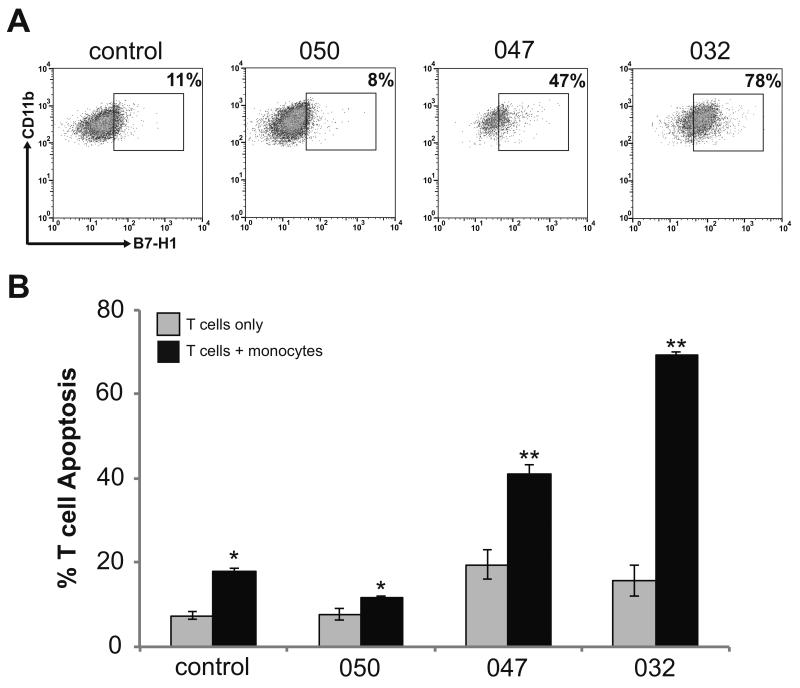
Peripheral blood monocytes from a non-glioma control patient and three GBM patients were isolated from PBL by CD14 positive selection. These selected patient monocytes had variable B7-H1 expression ranging from 8-78% positive cells (Figure 2A). Peripheral T cells were also isolated from the same patients and co-cultured with their autologous monocytes after T cell activation. The percentage of T cells undergoing apoptosis was evaluated after 12 hours of culture among solitary T cells and T cells co-cultured with monocytes (Figure 2B). For all patients, the degree of T cell apoptosis was greater for cells co-cultured with monocytes than in solitary culture (p<0.01). The magnitude of increase in apoptosis (Δ % apoptosis) correlated linearly with degree of B7-H1 expression (R=0.96, p<0.001).
Figure 2. Peripheral blood monocytes from GBM patients induce autologous T cell apoptosis relative to B7-H1 expression.
(A) Gating demonstrating the percentage of B7-H1 positive monocytes isolated from peripheral blood of three GBM patients and a single non-glioma control patient. Monocytes were isolated from total peripheral blood leukocytes by CD14 positive selection. Cells were stained for flow cytometry and monocytes were identified by gating on CD45+/CD11b+ cells. This figure demonstrates the subgate of CD11b+/B7-H1+ cells. The cutoff for B7-H1 positivity was determined based on isotype control staining. (B) The percentage of T cells undergoing apoptosis when monocytes from patients in panel (A) were co-cultured with autologous activated T cells is shown. The percentage of T cell undergoing apoptosis was determined by co-staining cells for CD3 and Annexin V and analyzed by flow cytometry. Columns represent mean apoptotic T cell counts ± SEM from 3 independent trials. T cells co-cultured with monocytes demonstrated significantly increased apoptosis as compared to the same cells in culture alone (* p<0.05, ** p<0.01).
Gliomas can induce B7-H1 expression in peripheral monocytes
Having shown that monocytes/macrophages in GBM patients have increased expression of B7-H1 that can functionally induce T cell apoptosis, we investigated the role of the glioma cell in stimulating expression of B7-H1 in macrophages. Peripheral monocytes from non-glioma control patients with low B7-H1 levels were co-cultured with U251 glioma cells in contact or through a 0.2 μm filter. After 24 hours, the number of B7-H1 expressing cells had increased by greater than 2-fold in monocytes co-cultured through a filter (48.0 ± 5.2% vs. 13.0 ± 3.9%, p<0.005), and greater than 4-fold in monocytes co-cultured in direct contact (83.9 ± 6.2% vs. 13.0 ± 3.9%, p<0.001; Supplementary Figure 1). The results of the filtered co-culture suggest that gliomas can induce B7-H1 expression in monocytes through a secreted soluble factor. However, the increase in B7-H1 expression from the contact co-culture was significantly greater than the filtered co-culture (p<0.01).
Glioma-derived soluble factors can induce B7-H1 expression in peripheral monocytes
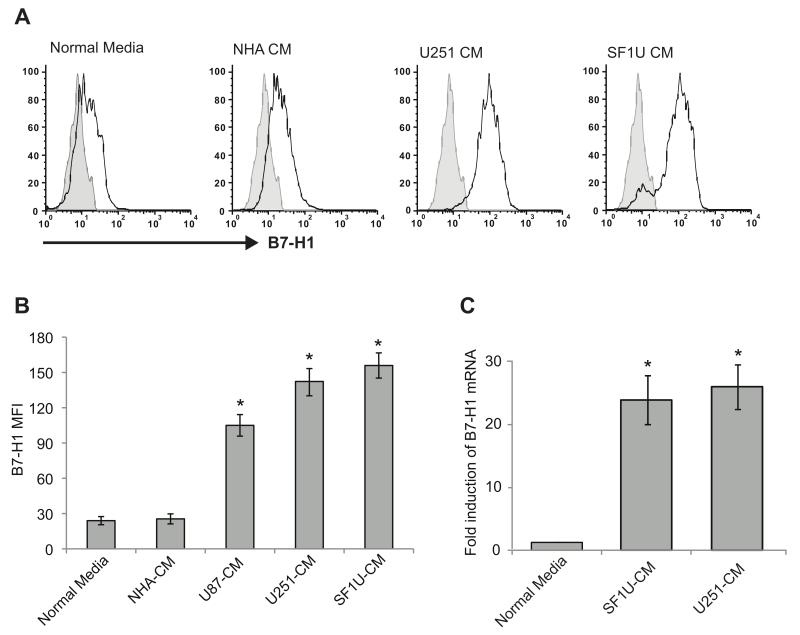
To further investigate the role of a soluble glioma-derived factor that could upregulate macrophage B7-H1, peripheral monocytes were stimulated with conditioned media from glioma cells after removal of all cellular material. Conditioned media (CM) was obtained from normal human astrocytes (NHA), established glioma cell lines (U87, U251), and a primary GBM cell culture (SF1U). The conditioned media was concentrated 20-fold and added to peripheral monocytes from non-glioma control patients at a concentration of 1:10 with normal media. B7-H1 surface expression was assessed by flow cytometry after 24 hours of culture. There was no significant change in B7-H1 expression with the addition of NHA CM; however, the addition of CM from all three glioma cell lines resulted in significant increases in B7-H1 protein expression (Figures 3A and 3B). Additionally, using quantitative RT-PCR, B7-H1 (CD274) mRNA was shown to increase nearly 15-fold with the addition of glioma CM as compared to unstimulated monocytes, suggesting activation at the transcriptional level (Figure 3C).
Figure 3. Glioma-dervied soluble factor(s) can induce B7-H1 expression in peripheral monocytes from healthy donors.
Monocytes were stimulated for 24 hours with normal media (control) or conditioned media from NHA or glioma cells lines. (A) Representative histograms of B7-H1 expression from stimulated monocytes are depicted (black), as well as isotype controls (gray shaded region). (B) Quantification of mean fluorescence intensity for B7-H1 staining measured by flow cytometry is shown for monocytes stimulated with various conditioned media. B7-H1 intensity was significantly increased for monocytes treated with media from all three glioma cell lines (* p<0.01) but not for media from NHA when compared to unstimulated cells. Columns represent mean fluorescence intensity ± SEM from 5 independent samples. Each sample was tested in triplicate and averaged as a single data point. (C) Quantification of B7-H1 mRNA levels from control monocytes and glioma conditioned monocytes using quantitative RT-PCR. mRNA levels were normalized to 18S rRNA and are reported relative to control monocyte expression. Monocytes treated with glioma-conditioned media have significantly increased B7-H1 mRNA expression (* p <0.05). Columns represent mean relative expression ± SEM from 3 independent samples.
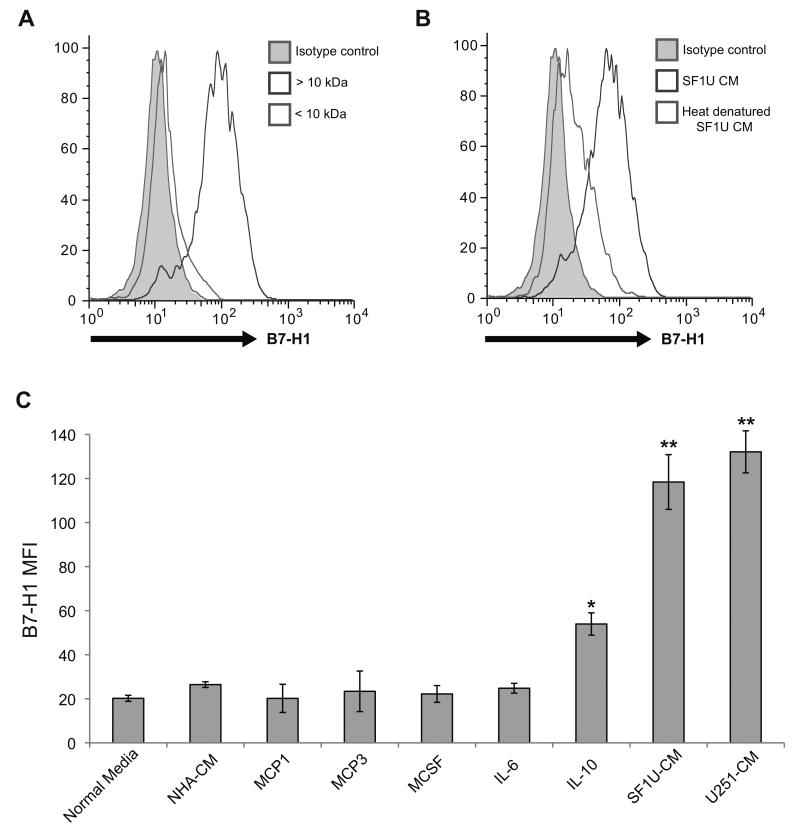
We then evaluated the physical properties of the soluble glioma-derived factor to identify the active component of the glioma CM responsible for induction of B7-H1 expression in macrophages. First, the glioma CM was spun through a 10 kDa filter and both filtered components were tested for the ability to upregulate B7-H1 expression in monocytes. Only the fraction with a molecular weight > 10 kDa demonstrated efficacy (Figure 4A). The active fraction was then subjected to heat denaturation at 100° C for 10 minutes, which resulted in loss of activity (Figure 4B). The glioma CM was also added to monocytes at various concentrations demonstrating increases in B7-H1 in a dose-response relationship (data not shown). These characteristics suggested that the inducing factor was likely a protein with a molecular weight > 10 kDa.
Figure 4. The glioma-derived B7-H1 inducing factor has properties consistent with a soluble protein.
(A) B7-H1 expression in normal monocytes treated for 24 hours with conditioned media from SF1U cells filtered to separate solutes with a molecular weight >10 kDa (dark gray) from solutes <10 kDa (light gray). The isotype control (gray shaded region) is also depicted. As shown, B7-H1 expression is significantly increased by a component in the > 10 kDa fraction. (B) B7-H1 expression in normal monocytes treated for 24 hours with unmodified conditioned media from SF1U cells (dark gray) or heat denatured media (light gray), demonstrating that heat denaturation eliminates the stimulatory effect of the conditioned media. (C) Mean fluorescence intensity of B7-H1 staining in normal monocytes treated with glioma-conditioned media or the following cytokines: MCP1 (200 ng/ml), MCP3 (200 ng/ml), M-CSF (50 ng/ml), IL-6 (10 ng/ml), IL-10 (10 ng/ml). Among the cytokines tested, only IL-10 stimulates a significant increase in B7-H1 expression (* p < 0.01). Compared to the response to IL-10, the increase seen with glioma-conditioned media stimulation was significantly greater (** p < 0.05). Columns represent mean fluorescence intensity ± SEM from 4 independent samples. Each sample was tested in triplicate and averaged as a single data point.
We subsequently used a candidate approach to screen a series of cytokines produced by gliomas that have been shown to stimulate monocytes/macrophages in previous studies. Monocyte chemotactic protein 1 and 3 (MCP1 and MCP3) as well as monocyte colony stimulating factor (M-CSF) are produced by a variety of tumors, including gliomas, and activate the PI3(k)/Akt pathway, which regulates B7-H1 expression in glioma cells.(38, 39) Additionally, interleukin-6 (IL-6) and interleukin-10 (IL-10) are produced by gliomas and activate the STAT1/3 pathways, which have been shown to drive anti-inflammatory phenotypes in monocytes/macrophages.(40, 41) Therefore, these cytokines were screened for their ability to induce B7-H1 expression in monocytes at a range of concentrations compared to glioma CM. Even at supra-physiologic levels of individual cytokines, B7-H1 levels were not significantly increased in monocytes treated with MCP1, MCP3, MCSF, or IL-6 (Figure 4C). However, monocytes treated with IL-10 did significantly upregulate B7-H1 expression (p<0.01), albeit less than the induction observed with glioma CM treatment (p<0.05).
Glioma-derived factors enhance IL-10 autocrine signaling in monocytes
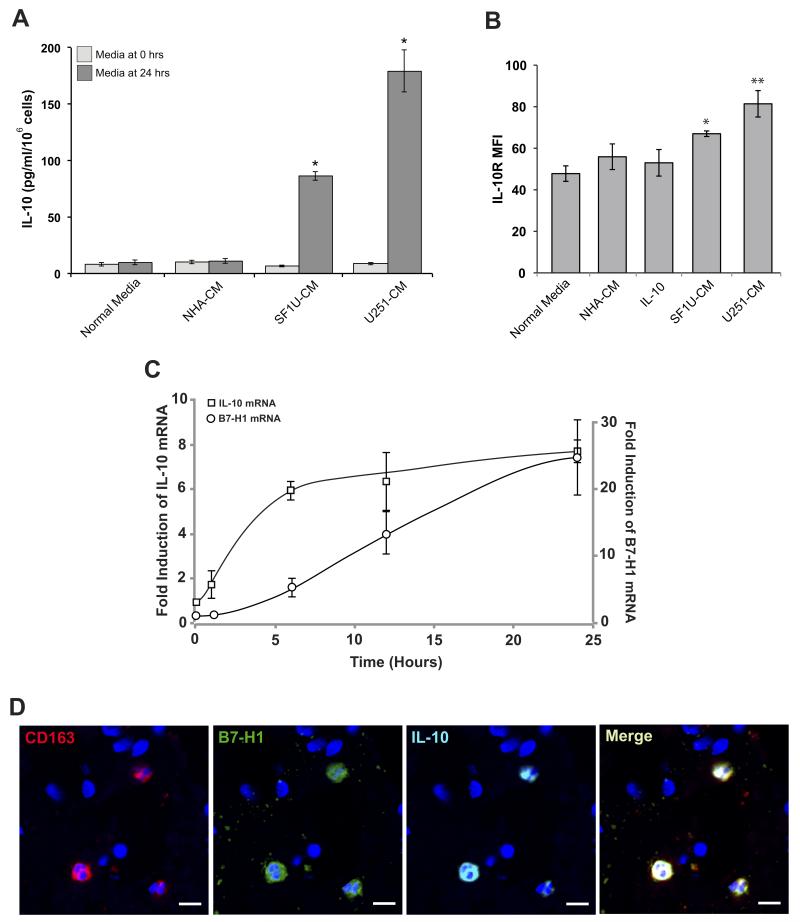
Having shown that IL-10 can stimulate B7-H1 expression in monocytes, the levels of IL-10 produced by glioma cells were investigated. Using an ELISA, conditioned media from NHA, U251, U87, and SF1U cell cultures were analyzed and found to have no appreciable human IL-10. The CM was then added to unstimulated monocytes in culture for 24 hours and IL-10 levels were again measured from the culture media. Following stimulation with U251 CM and SF1U CM, monocyte production of IL-10 was significantly increased (Figure 5A). Previous studies have shown that glioma stem cells from patients with prior exposure to cytomegalovirus (CMV) produce viral IL-10, which can phenotypically alter peripheral monocytes similar to human IL-10.(34) Therefore, the conditioned media from glioma cells was re-tested before and after stimulation of monocytes using an ELISA to specifically detect hcmvIL-10. No hcmvIL-10 was detected in the conditioned media before or after stimulation of monocytes.
Figure 5. Glioma-derived soluble factor(s) enhance IL-10 signaling.
(A) Human IL-10 expression measured by ELISA from culture media of normal monocytes stimulated with glioma-conditioned media before and after 24 hours of culture. None of the conditioned media initially contained appreciable levels of IL-10. After 24 hours, IL-10 expression was significantly increased in cells stimulated with glioma-conditioned media compared to normal media (* p<0.01). Columns represent mean total IL-10 ± SEM from 3 independent samples. (B) IL-10 receptor expression was measured by flow cytometry in monocytes stimulated with glioma-conditioned media. Compared to unstimulated cells, treated monocytes had a modest but significant increase in IL-10R mean fluorescence intensity (* p < 0.05, ** p < 0.01). Columns represent mean fluorescence intensity ± SEM from 3 independent samples. Each sample was tested in triplicate and averaged as a single data point. (C) Time course of IL-10 and B7-H1 mRNA expression in normal monocytes stimulated with SF1U conditioned media. Transcript levels were measured at 0, 1, 6, 12, and 24 hours after stimulation using quantitative RT-PCR and normalized to 18S rRNA. Normalized expression is reported as a relative increase over initial expression. Each point represents mean relative expression ± SEM from 3 independent samples. (D) Representative section of GBM tissue with fluorescent immunestaining for CD163 (red), B7-H1 (green), IL-10 (cyan), and DAPI (blue) demonstrating co-localization of all three probes to the same subset of tumor-associated macrophages (scale bar = 10μm).
In addition to IL-10, expression of the IL-10 receptor on monocytes was analyzed before and after stimulation with glioma CM. Although modest, there was a statistically significant increase in IL-10R expression after glioma CM stimulation (Figure 5B). Together, these results suggest that a glioma-derived soluble factor enhances monocyte IL-10 production and IL-10R expression, possibly leading to a synergistic increase in IL-10 autocrine/paracrine signaling.
IL-10 expression precedes B7-H1 expression in stimulated monocytes
To determine the relative timing of IL-10 and B7-H1 expression following stimulation, normal monocytes were stimulated with glioma CM and both IL-10 and B7-H1 mRNA levels were evaluated by quantitative RT-PCR at 1, 6, 12, and 24 hours following stimulation (Figure 5C). IL-10 transcript levels rose first, reaching near-peak expression by 6 hours, whereas B7-H1 expression lagged behind, rising steadily over 24 hours.
IL-10 and B7-H1 are co-expressed in immunosuppressive tumor-associate macrophages
To determine the source of IL-10 and B7-H1 in vivo, histopathological specimens from patients with GBM were immunostained for CD163, B7-H1, and IL-10 (Figure 5D). Immunosuppressive M2 macrophages, stained by CD163, were all found to be co-positive for B7-H1 and IL-10.
IL-10 signaling is a necessary component of glioma-induced upregulation of B7-H1
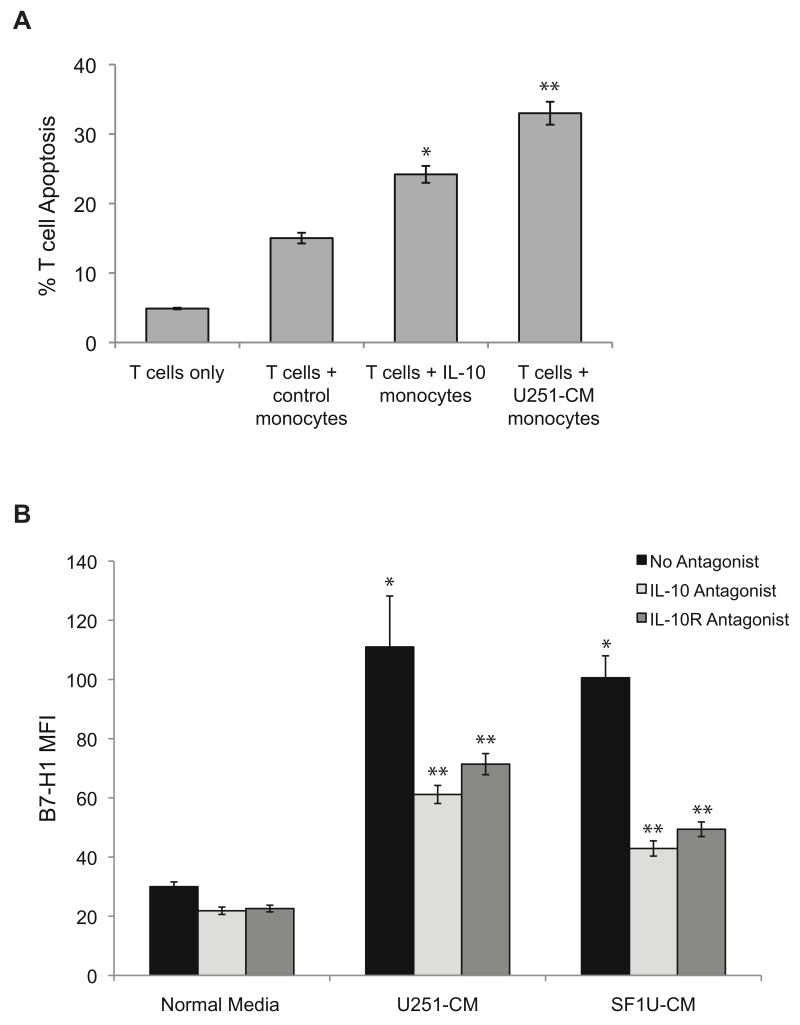
To assess the functional consequences of upregulated B7-H1 expression in stimulated monocytes/macrophages, monocytes were co-cultured with activated T cells and T cell apoptosis was measured by Annexin staining. Significant increases in T cell apoptosis were seen when co-cultured with monocytes stimulated with IL-10 or glioma CM (Figure 6A).
Figure 6. IL-10 is necessary and sufficient to stimulate an immunosuppressive phenotype in monocytes.
(A) The percentage of T cells undergoing apoptosis was measured by Annexin V staining after co-culture with stimulated or unstimulated (control) monocytes. Monocytes stimulated with IL-10 or glioma-conditioned media induce significantly more T cell apoptosis compared to control monocytes (* p < 0.05, ** p < 0.005). Columns represent mean apoptotic T cell counts ± SEM from 3 independent samples. Each sample was tested in triplicate and averaged as a single data point. (B) Soluble IL-10 and binding to the IL-10 receptor were inhibited to evaluate the effect on glioma-induced B7-H1 expression in monocytes. Monocytes were treated with normal media or glioma-conditioned media in the presence of no inhibitor (black bars), a soluble IL-10 neutralizing antibody at 5 μg/ml (light gray bars), or an IL-10 receptor antagonist at 5 μg/ml (dark gray bars) for 24 hours and B7-H1 expression was measured by flow cytometry. As previously shown, treatment with glioma-conditioned media in the presence of no inhibitors significantly increases the expression of B7-H1 (* p<0.01). However, the addition of either an IL-10 or IL-10R antagonist reduces the expression of B7-H1 by greater than 50% (** p<0.05). Columns represent mean fluorescence intensity ± SEM from 3 independent samples. Each sample was tested in triplicate and averaged as a single data point.
To determine if IL-10 was a necessary component of the response to glioma CM, monocytes were stimulated with glioma CM in the presence of a soluble IL-10 neutralizing antibody or an IL-10 receptor inhibitor. With inhibition of IL-10 and the IL-10 receptor, monocyte expression of B7-H1 in response to stimulation was significantly reduced (Figure 6B).
DISCUSSION
T cell-mediated cancer immunotherapy is dependent on initiation of a specific CD8+ T cell response that can effectively target and destroy the tumor cells.(42) Immunoresistance in the local tumor microenvironment is a significant impediment to successful immunotherapy, allowing the tumor to evade a cytotoxic response. Gliomas have been shown to resist immune responses through several mechanisms including downregulation of immune-stimulating antigens and expression of anti-inflammatory proteins.(12, 13, 40, 41) We have previously linked expression of the immunosuppressive protein B7-H1 in glioma cells with activation of the PI3K/mTOR pathway, leading to quiescence and apoptosis of infiltrating T cells.(16) This mechanism of local immunoresistance is well-recognized in multiple cancers including breast, lung, prostate, and renal cell.(19, 20, 22) However, in addition to local immune resistance driven by the tumor, glioma patients are known to have suppressed immune cell function, exhibiting a lack of response to normal immune activators.(5) Therefore, gliomas must modulate the function of immune effectors in a global fashion. There have been several reports in the literature of mechanisms by which gliomas affect immunity, including expansion of circulating regulatory T cells and phenotypic modulation of circulating monocytes to myeloid-derived suppressor cells.(14, 31, 43) Additionally, studies have shown that monocytes exposed to glioma cells in vitro can be induced to express B7-H1.(31, 34) However, despite demonstrating an association of anti-inflammatory macrophages with gliomas, the mechanism by which gliomas modulate macrophage function and overall immunity remains largely unknown. Expression of B7-H1 by circulating monocytes or infiltrating macrophages has not previously been demonstrated in glioma patients. We hypothesized that gliomas could induce expression of B7-H1 in macrophages/monocytes locally and systemically through production of a soluble cytokine.
The results presented in this study support our initial hypothesis. Circulating monocytes and tumor-infiltrating macrophages from GBM patients have significantly increased expression of B7-H1 compared to monocytes from healthy control patients. An increase in B7-H1 can be induced in normal monocytes through stimulation with a soluble factor(s) produced by malignant glioma cells, and this factor demonstrates the physical properties of a cytokine. Since B7-H1 expression has been shown to be dependent on PI3K/Akt and STAT3 activity, we investigated activators of these signaling pathways as candidates for the glioma-derived stimulatory cytokine. Despite evidence that they are produced by glioma cells, MCP-1, MCP-3, M-CSF, TGF-β, and IL-6 all failed to modulate B7-H1 expression in normal monocytes. Only stimulation with IL-10 resulted in a significant increase in B7-H1 expression in monocytes with the functional consequence of increasing T cell apoptosis when stimulated monocytes were co-cultured with activated T cells. These results suggested that IL-10 has a role regulating B7-H1 expression in glioma-associated macrophages.
We initially assumed that the glioma cells were the source of IL-10, however no IL-10 was detectable in the conditioned media from several glioma cell lines and low-passage primary cultures. IL-10 was produced by monocytes after stimulation with glioma conditioned media. Previously published studies of IL-10 expression in human glioma tissue have demonstrated that the primary source of IL-10 in tumors is tumor-infiltrative macrophages/microglia.(44) Therefore, it is not surprising to find that gliomas can stimulate normal monocytes to produce IL-10, which has been described as part of the anti-inflammatory phenotype of M2 macrophages in gliomas. Using immunofluorescence, we have confirmed that CD163 positive M2 macrophages in patient tumors do, in fact, express IL-10 and B7-H1.
The finding that IL-10 can stimulate B7-H1 expression in monocytes is also not entirely surprising, as this has been demonstrated in macrophages associated with hepatocellular carcinoma.(35) The novel finding in this study is that a glioma-derived factor can increase B7-H1 expression in macrophages indirectly by enhancing autocrine/paracrine IL-10 signaling, thereby promoting an immunosuppressive effect in the local microenvironment and systemic circulation. Furthermore, we have demonstrated that by antagonizing IL-10 signaling, we can block upregulation of B7-H1 expression.
Supporting our findings, recent publications in the immunology literature have demonstrated that stimulation of dendritic cells with agonists of the innate immune system (toll-like receptor ligands) such as lippopolysacharides results in production of IL-10.(45) These IL-10 expressing dendritic cells can then induce a phenotypic shift in naïve dendritic cells to B7-H1 expressing tolerogenic cells in the absence of a toll-like receptor agonist. This feedback system to suppress the response to pathogenic antigens likely exists to prevent over-stimulation of the immune system and protect against auto-immunity. We now demonstrate that tumors can capitalize on this system to evade immune monitoring and promote tumor growth.
One limitation in our findings is that the degree of B7-H1 expression induced by stimulation with IL-10 alone, while enough to affect T cell apoptosis, is less than the expression achieved with glioma-conditioned media stimulation. Although this may be explained by the concurrent upregulation in the IL-10 receptor associated with glioma-conditioned media stimulation, we cannot rule out the possibility of a second glioma-derived stimulatory factor. Since we have not yet identified the glioma-dervied factor that induces IL-10 production by monocytes, we cannot investigate the direct role that binding of this ligand has on B7-H1 expression. Having unsuccessfully used a candidate approach to identify this factor, we are now undertaking a protein purification approach to determine the active factor in glioma-conditioned media. Based on the known signaling pathways for IL-10, we hypothesize that B7-H1 expression is, at least in part, dependent on STAT3 activation. However, without having identified the IL-10 stimulating factor, we cannot predict what other pathways may be activated upstream and may modulate STAT3 activity. Additionally, the degree of B7-H1 upregulation in monocytes co-cultured with gliomas in contact is greater than the effect of culture with a soluble factor alone, suggesting that there may be further modulation by surface-bound co-factor on glioma cells. This has been shown in previous studies as well.(31) However, inhibition of IL-10 signaling unquestionably resulted in a greater than 50% reduction in B7-H1 expression, suggesting that targeting IL-10 may be a useful strategy to combat glioma-induced local and systemic immunosuppression. Further studies are necessary to clearly define all factors in this complex signaling pathway, and there is still much work to be done prior to developing a clinical strategy to decrease B7-H1 expression and increase the efficacy of adaptive immunity.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
Patients with malignant gliomas are known to be systemically immunosuppressed and their tumors demonstrate multiple mechanisms of local immunoresistance. Innate immune monitoring and adaptive immunotherapy are rendered ineffective by these immune suppressing factors, promoting tumor growth. Expression of B7-H1 by glioma cells has been shown to be a significant mechanism of local immunoresistance, resulting in quiescence and apoptosis of tumor-infiltrating cytotoxic T cells. In this study we demonstrate that circulating monocytes and tumor-infiltrating macrophages in glioma patients are induced to express B7-H1 as well, establishing a local and systemic immunosuppressive environment. Monocytes/macrophages are stimulated to express B7-H1 in response to a soluble factor produced by glioma cells that can amplify IL-10 autocrine/paracrine signaling in monocytes, resulting in upregulated B7-H1 expression. Inhibition of this pathway may represent a novel target to overcome glioma-mediated immune suppression and may be a necessary component of immunotherapy in the future.
Acknowledgments
Support: This work was supported by the National Institutes of Health grants 1F32 CA153839-01, 1K99 NS078055-01 (O.B.), 1K99 CA151412-02 (C.A.C.), and the Reza and Georgianna Khatib Endowed Chair in Skull Base Surgery (A.T.P.). The funding organizations had no role in study design, data collection and analysis, or preparation of the manuscript.
Footnotes
Disclosures: The authors declare they have no financial interests in the outcomes of this study.
REFERENCES
- 1.Surawicz TS, Davis F, Freels S, Laws ER, Jr., Menck HR. Brain tumor survival: results from the National Cancer Data Base. J Neurooncol. 1998;40:151–60. doi: 10.1023/a:1006091608586. [DOI] [PubMed] [Google Scholar]
- 2.Deorah S, Lynch CF, Sibenaller ZA, Ryken TC. Trends in brain cancer incidence and survival in the United States: Surveillance, Epidemiology, and End Results Program, 1973 to 2001. Neurosurg Focus. 2006;20:E1. doi: 10.3171/foc.2006.20.4.E1. [DOI] [PubMed] [Google Scholar]
- 3.Laws ER, Parney IF, Huang W, Anderson F, Morris AM, Asher A, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003;99:467–73. doi: 10.3171/jns.2003.99.3.0467. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Gousias K, Markou M, Arzoglou V, Voulgaris S, Vartholomatos G, Kostoula A, et al. Frequent abnormalities of the immune system in gliomas and correlation with the WHO grading system of malignancy. J Neuroimmunol. 2010;226:136–42. doi: 10.1016/j.jneuroim.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 6.Wei J, Barr J, Kong LY, Wang Y, Wu A, Sharma AK, et al. Glioma-associated cancer-initiating cells induce immunosuppression. Clin Cancer Res. 2010;16:461–73. doi: 10.1158/1078-0432.CCR-09-1983. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Chahlavi A, Rayman P, Richmond AL, Biswas K, Zhang R, Vogelbaum M, et al. Glioblastomas induce T-lymphocyte death by two distinct pathways involving gangliosides and CD70. Cancer Res. 2005;65:5428–38. doi: 10.1158/0008-5472.CAN-04-4395. [DOI] [PubMed] [Google Scholar]
- 8.Giometto B, Bozza F, Faresin F, Alessio L, Mingrino S, Tavolato B. Immune infiltrates and cytokines in gliomas. Acta Neurochir (Wien) 1996;138:50–6. doi: 10.1007/BF01411724. [DOI] [PubMed] [Google Scholar]
- 9.Ueda R, Fujita M, Zhu X, Sasaki K, Kastenhuber ER, Kohanbash G, et al. Systemic inhibition of transforming growth factor-beta in glioma-bearing mice improves the therapeutic efficacy of glioma-associated antigen peptide vaccines. Clin Cancer Res. 2009;15:6551–9. doi: 10.1158/1078-0432.CCR-09-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou JP, Morford LA, Chougnet C, Dix AR, Brooks AG, Torres N, et al. Human glioma-induced immunosuppression involves soluble factor(s) that alters monocyte cytokine profile and surface markers. J Immunol. 1999;162:4882–92. [PubMed] [Google Scholar]
- 11.Mehling M, Simon P, Mittelbronn M, Meyermann R, Ferrone S, Weller M, et al. WHO grade associated downregulation of MHC class I antigen-processing machinery components in human astrocytomas: does it reflect a potential immune escape mechanism? Acta Neuropathol. 2007;114:111–9. doi: 10.1007/s00401-007-0231-8. [DOI] [PubMed] [Google Scholar]
- 12.Zagzag D, Salnikow K, Chiriboga L, Yee H, Lan L, Ali MA, et al. Downregulation of major histocompatibility complex antigens in invading glioma cells: stealth invasion of the brain. Lab Invest. 2005;85:328–41. doi: 10.1038/labinvest.3700233. [DOI] [PubMed] [Google Scholar]
- 13.Crane CA, Ahn BJ, Han SJ, Parsa AT. Soluble factors secreted by glioblastoma cell lines facilitate recruitment, survival, and expansion of regulatory T cells: implications for immunotherapy. Neuro Oncol. 2012;14:584–95. doi: 10.1093/neuonc/nos014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294–302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 15.Wintterle S, Schreiner B, Mitsdoerffer M, Schneider D, Chen L, Meyermann R, et al. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63:7462–7. [PubMed] [Google Scholar]
- 16.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–8. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 17.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 18.Tsushima F, Yao S, Shin T, Flies A, Flies S, Xu H, et al. Interaction between B7-H1 and PD-1 determines initiation and reversal of T-cell anergy. Blood. 2007;110:180–5. doi: 10.1182/blood-2006-11-060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crane CA, Panner A, Murray JC, Wilson SP, Xu H, Chen L, et al. PI(3) kinase is associated with a mechanism of immunoresistance in breast and prostate cancer. Oncogene. 2009;28:306–12. doi: 10.1038/onc.2008.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–8. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10:5094–100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 22.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, et al. Costimulatory molecule B7-H1 in primary and metastatic clear cell renal cell carcinoma. Cancer. 2005;104:2084–91. doi: 10.1002/cncr.21470. [DOI] [PubMed] [Google Scholar]
- 23.Badie B, Schartner JM. Flow cytometric characterization of tumor-associated macrophages in experimental gliomas. Neurosurgery. 2000;46:957–61. doi: 10.1097/00006123-200004000-00035. discussion 61-2. [DOI] [PubMed] [Google Scholar]
- 24.Parney IF, Waldron JS, Parsa AT. Flow cytometry and in vitro analysis of human glioma-associated macrophages. Laboratory investigation. J Neurosurg. 2009;110:572–82. doi: 10.3171/2008.7.JNS08475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 26.Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. 2006;8:261–79. doi: 10.1215/15228517-2006-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25:315–22. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 28.Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, et al. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18:349–55. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;216:15–24. doi: 10.1002/path.2370. [DOI] [PubMed] [Google Scholar]
- 30.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717–27. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Rodrigues JC, Gonzalez GC, Zhang L, Ibrahim G, Kelly JJ, Gustafson MP, et al. Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro Oncol. 2010;12:351–65. doi: 10.1093/neuonc/nop023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okada H, Kohanbash G, Zhu X, Kastenhuber ER, Hoji A, Ueda R, et al. Immunotherapeutic approaches for glioma. Crit Rev Immunol. 2009;29:1–42. doi: 10.1615/critrevimmunol.v29.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagai T, Tanaka M, Tsuneyoshi Y, Xu B, Michie SA, Hasui K, et al. Targeting tumor-associated macrophages in an experimental glioma model with a recombinant immunotoxin to folate receptor beta. Cancer Immunol Immunother. 2009;58:1577–86. doi: 10.1007/s00262-009-0667-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dziurzynski K, Wei J, Qiao W, Hatiboglu MA, Kong LY, Wu A, et al. Glioma-associated cytomegalovirus mediates subversion of the monocyte lineage to a tumor propagating phenotype. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-11-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206:1327–37. doi: 10.1084/jem.20082173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ford AL, Goodsall AL, Hickey WF, Sedgwick JD. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J Immunol. 1995;154:4309–21. [PubMed] [Google Scholar]
- 37.Chang WL, Baumgarth N, Yu D, Barry PA. Human cytomegalovirus-encoded interleukin-10 homolog inhibits maturation of dendritic cells and alters their functionality. J Virol. 2004;78:8720–31. doi: 10.1128/JVI.78.16.8720-8731.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leung SY, Wong MP, Chung LP, Chan AS, Yuen ST. Monocyte chemoattractant protein-1 expression and macrophage infiltration in gliomas. Acta Neuropathol. 1997;93:518–27. doi: 10.1007/s004010050647. [DOI] [PubMed] [Google Scholar]
- 39.Okada M, Saio M, Kito Y, Ohe N, Yano H, Yoshimura S, et al. Tumor-associated macrophage/microglia infiltration in human gliomas is correlated with MCP-3, but not MCP-1. Int J Oncol. 2009;34:1621–7. doi: 10.3892/ijo_00000292. [DOI] [PubMed] [Google Scholar]
- 40.Lilja A, Nordborg C, Brun A, Salford LG, Aman P. Expression of the IL-6 family cytokines in human brain tumors. Int J Oncol. 2001;19:495–9. doi: 10.3892/ijo.19.3.495. [DOI] [PubMed] [Google Scholar]
- 41.Qiu B, Zhang D, Wang C, Tao J, Tie X, Qiao Y, et al. IL-10 and TGF-beta2 are overexpressed in tumor spheres cultured from human gliomas. Mol Biol Rep. 2011;38:3585–91. doi: 10.1007/s11033-010-0469-4. [DOI] [PubMed] [Google Scholar]
- 42.Lohr J, Ratliff T, Huppertz A, Ge Y, Dictus C, Ahmadi R, et al. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived transforming growth factor-betas. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-10-2557. [DOI] [PubMed] [Google Scholar]
- 43.Jacobs JF, Idema AJ, Bol KF, Grotenhuis JA, de Vries IJ, Wesseling P, et al. Prognostic significance and mechanism of Treg infiltration in human brain tumors. J Neuroimmunol. 2010;225:195–9. doi: 10.1016/j.jneuroim.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 44.Wagner S, Czub S, Greif M, Vince GH, Suss N, Kerkau S, et al. Microglial/macrophage expression of interleukin 10 in human glioblastomas. Int J Cancer. 1999;82:12–6. doi: 10.1002/(sici)1097-0215(19990702)82:1<12::aid-ijc3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 45.Wolfle SJ, Strebovsky J, Bartz H, Sahr A, Arnold C, Kaiser C, et al. PD-L1 expression on tolerogenic APCs is controlled by STAT-3. Eur J Immunol. 2011;41:413–24. doi: 10.1002/eji.201040979. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.