Abstract
Withdrawal from opiates, such as heroin or oral narcotics, is characterized by a host of aversive physical and emotional symptoms. High rates of relapse and limited treatment success rates for opiate addiction have prompted a search for new approaches. For many opiate addicts, achieving abstinence may be further complicated by poly-drug use and co-morbid mental disorders. Research over the past decade has shed light on the influence of endocannabinoids on the opioid system. Evidence from both animal and clinical studies point towards an interaction between these two systems, and suggest that targeting the endocannabinoid system may provide novel interventions for managing opiate dependence and withdrawal. This review will summarize the literature surrounding the molecular effects of cannabinoids and opioids system on the locus coeruleus-norepinephrine system, a key circuit implicated in the negative sequelae of opiate addiction. A consideration of the trends and effects of marijuana use in those seeking treatment to abstain from opiates in the clinical setting will also be presented. In summary, the present review details how cannabinoid-opioid interactions may inform novel interventions in management of opiate dependence and withdrawal.
1) Opioid Addiction: A Persistent Societal Problem
a. Background
Overall, illicit drug abuse in the United States exceeded $180 billion in 2008 according to National Institutes of Health. Abuse of heroin and prescription opioids have long constituted a significant economic burden to society both through the direct and indirect consequences of illicit opioid use. These costs include not only direct medical expenses, but also the costs of criminal activities associated with drug acquisition, social welfare, secondary medical issues associated with high-risk needle sharing, and productivity losses. In 1996, the cumulative economic burden of heroin addiction in the United States was estimated to be $21.9 billion(Mark et al., 2001). In 2001, illicit use of prescription opioids cost the United States approximately $8.6 billion, and this number continues to rise (Birnbaum et al., 2006, Gilson and Kreis, 2009, Strassels, 2009).
Intravenous heroin use experienced a steady climb through the early 1980's in the United States, until rates began to decline concurrent with the implementation of programs designed to increase awareness of the risks associated with intravenous drug use and needle sharing. However, since the mid-1990's heroin use has experienced a resurgence, particularly among younger populations. In 2004, an estimated 3.7 million people in the United States had reported using heroin at some point in their lifetime according to data collected by the National Institute on Drug Abuse. The 2008 National Survey on Drug Use and Health determined that the number of heroin users over the age of 12 in the United States had increased dramatically from 153,000 in 2007 to 213,000 in 2008. Unlike prior surges in heroin use that were primarily characterized by injection drug use, recent climbs in heroin use rates are due to significant increases in inhaled or snorted heroin. Heroin purity increased dramatically during the 1990's and has remained stable(OAS, 2005). Meanwhile, the cost of heroin has decreased and is now less expensive relative to other opioid alternatives, potentially underlying the trends in increased inhalation drug use(OAS, 1998). The high abuse liability of heroin was demonstrated in a 2004 study of drug use, which found that 67% of those that used heroin also met the criteria for abuse or dependence, a statistic markedly higher than that for other drugs of abuse such as cocaine, marijuana, or sedatives(OAS). In 2008, 341,000 individuals received treatment for heroin dependence(OAS, 2009) and with recent increases in use, this number is likely to continue to climb.
b. Non-Medical Use of Prescription Opioids
Heroin use, while extremely problematic, is restricted to a very small percentage of the population. However, non-medical use of prescription opioids is now becoming more prevalent with rates of use rapidly increasing. The misuse or abuse of prescription drugs occurs when a person takes a prescription drug that was not prescribed or taken in one dose or for reasons other than those prescribed. Abuse of prescription drugs can produce serious health effects, including addiction. The classes of prescription drugs that are commonly abused include oral narcotics such as hydrocodone (Vicodin ®), oxycodone (OxyContin ®), propoxyphene (Darvon ®), hydromorphone (Dilaudid ®), meperidine (Demerol ®) and diphenoxylate (Lomotil ®) and their non-medical use has increased dramatically in recent years. For example, in 1990, the number of individuals initiating abuse of prescription opioids was 573,000. By the year 2000, the number had risen to over 2.5 million according to the National Institutes of Health. A 2009 nationwide study reported that 6.2 million individuals were recent non-medical users of prescription opioids(OAS, 2009). Among high school seniors, as many as 1 in 10 used prescription opioids for non-medical purposes in 2009. For the first time, the number of individuals initiating prescription opioid use nearly equaled that of marijuana; a previously unprecedented and alarming finding. Concurrently, emergency department visits due to complications from non-medical use of hydrocodone and oxycodone rose 170% and 450% respectively from 1994–2002. Furthermore, opioid-related deaths rose by more than 300% between 1999–2006 (OAS, 2009).
II. The Clinical Opioid Withdrawal Syndrome & Underlying Brain Circuitry
a. Clinical presentation and management
Abstinence following chronic exposure to opiates is accompanied by a pronounced syndrome of aversive physical and emotional symptoms. Characteristic signs of opiate withdrawal include yawning, rhinorrhea, perspiration, dilated pupils, anxiety and restlessness, nausea and vomiting, diarrhea, increased heart rate or blood pressure, as well as a host of flu-like symptoms such as chills, joint and muscle aches, and increased body temperature(Jasinski, 1981, Gossop, 1988, Farrell, 1994, Wesson and Ling, 2003). In opiate-dependent individuals, the experience of a pronounced and prolonged withdrawal syndrome often contributes to renewed illicit drug use and less-than-favorable treatment prognoses.
Pharmacotherapeutics designed to attenuate the severity of these opiate withdrawal signs can be used to promote improved outcome in the critical early phases of treatment. Currently, the pharmacotherapeutic options for opioid dependence and withdrawal are primarily designed around the principles of maintenance therapy and/or detoxification. Agonist replacement or maintenance therapy involves substitution of illicit opioid use with long-acting opioid-receptor agonists in a carefully controlled manner. Ideal agonist replacement drugs often bind to opioid receptors with greater affinity and are metabolized more slowly than commonly abused illicit opioids. Alternatively, opioid detoxification employs opioid receptor antagonists that provide potent and high-affinity blockade of mu-opioid receptor (MOR). These compounds work by inducing withdrawal, often through the displacement of illicit opioids from receptor binding sites in the nervous system.
To assist with opioid detoxification and the associated withdrawal syndrome, alpha-2 adrenergic receptor agonists are also used to address the noradrenergic hyperactivity that is a hallmark of opioid withdrawal. Clonidine and lofexidine are centrally-acting α-2 adrenergic receptor agonists used during rapid opioid detoxification to regulate noradrenergic hyperactivity associated with opioid withdrawal. These agents are often co-administered with opioid receptor antagonists during detoxification. Clonidine rapidly and effectively reduces withdrawal symptoms, but can cause postural hypotension through its actions on noradrenergic control of cardiovascular function(Gossop, 1988). Lofexidine, which works similarly to clonidine but involves lessened risk of hypotension, has shown greater efficacy than clonidine in recent studies(Strang et al., 1999, Gerra et al., 2001, Meader, 2010). The activity of these agents is heavily reliant on their activity within a key noradrenergic nucleus within the brain- the locus coeruleus (LC).
b. Noradrenergic circuitry
The LC is located in the anterior pons of the brainstem, near the fourth ventricle. This nucleus provides the majority of noradrenergic input to the central nervous system, and is highly interconnected with many different brain regions, allowing for the regulation of cognition, pain, emotional state, anxiety, arousal, and stress(Aston-Jones and Bloom, 1981, Aston-Jones et al., 1986, Nestler et al., 1999, Carrasco and Van de Kar, 2003, Dunn et al., 2004). The widespread downstream effects of LC neurotransmission occur as a result of the diverse network of projections sent to and from this nucleus to the entire neuraxis. The LC is a key anatomical locus where opioid and stress signaling intersect. Many of the neurons projecting from the nucleus paragigantocellularis (PGi) and nucleus prepositus hypoglossi (PrH) provide enkephalin to the LC(Aston-Jones et al., 1986, Aston-Jones et al., 1991), while corticotropin releasing factor (CRF) innervation arises predominantly from the PGi, paraventricular nucleus of the hypothalamus (PVN), and peri-coerulear region(Valentino et al., 1993, Valentino et al., 1998a). The LC is extremely sensitive to opioid and stress peptides delivered by afferent fibers due to the high density of opioid and CRF receptors (Pert et al., 1976, Valentino et al., 1983, Aston-Jones et al., 1986, Tempel and Zukin, 1987, Ding et al., 1996, Van Bockstaele et al., 1996a, Reyes et al., 2007). The kappa-opioid receptor (KOR) and delta-opioid receptor (DOR) have a prominent pre-synaptic distribution in the LC and are poised to auto-regulate neurotransmitter release from LC afferents(Van Bockstaele et al., 1997, Kreibich et al., 2008, Reyes et al., 2009a) (Kreibich et al., 2008). The mu-opioid receptor (MOR) has a dense post-synaptic distribution along the plasmalemma of somata and dendrites of LC neurons(Van Bockstaele et al., 1996a, Van Bockstaele et al., 1996b). The MOR-mediated sensitivity and response of this region to opioid exposure has been extensively studied and characterized.
c. Molecular Expression of Opioid Dependence and Withdrawal in LC Circuitry
MOR is a G-protein coupled receptor that typically signals through Gi/o alpha-subunit proteins to inhibit the activity of adenylyl cyclase (AC) I and VIII, thereby reducing cyclic adenosine monophosphate (cAMP) production, impacting Na+ current and neuronal excitability(Childers et al., 1992, Dhawan et al., 1996). Additionally, opioids also inhibit the LC by coupling opioid receptor activation to G-protein-gated inwardly rectifying K+ (GIRK) ion channels(Christie et al., 1987, North et al., 1987, Nestler, 1992, 2001). Direct effects of opioid exposure can include receptor desensitization and internalization via a clathrin-mediated mechanism(Van Bockstaele and Commons, 2001, Van Bockstaele et al., 2001, Alvarez et al., 2002, Dang and Williams, 2005, Johnson et al., 2005, Arttamangkul et al., 2006, Gintzler and Chakrabarti, 2006). In this manner, MOR activation by opioids has an acutely inhibitory effect in the LC, resulting in sedation, hypolocomotion, analgesia, and respiratory depression.
Following repeated exposure to opioids, a number of cellular adaptations occur within this region that significantly impact the development of opioid dependence and manifestation of opioid withdrawal. Suppression of AC-I and AC-VIII by opioid exposure leads to decreased cAMP levels and protein kinase-A (PKA) activity, and decreased phosphorylation of cAMP response element binding protein (CREB), a downstream effector of PKA. By binding to regulatory DNA sequences called cAMP response elements (CREs), CREB is able to alter the expression of key proteins in the cAMP pathway. Opioid exposure alters the phosphorylation level of CREB, thereby regulating the transcriptional activation of CREB target genes. These transcriptional changes may include increased expression of AC-VIII, PKA catalytic and regulatory subunits, CREB, and tyrosine hydroxylase (the rate-limiting enzyme in norepinephrine synthesis). As an end result of these adaptations, the cAMP pathway is upregulated and the electrical excitability in the LC increases (Widnell et al., 1994, Lane-Ladd et al., 1997, Nestler and Aghajanian, 1997, Boundy et al., 1998, Coven et al., 1998, Nestler, 2001).
Through its wide array of projections throughout the brain, hyperactivity in the LC during opiate withdrawal has the potential to impact signaling in a number of brain regions, primarily in the frontal cortex to which it provides the sole noradrenergic input [reviewed (Valentino and Aston-Jones, 2000, Berridge and Waterhouse, 2003, Van Bockstaele et al., 2010)]. Alterations in LC activity have been implicated in the somatic expression of withdrawal (Taylor et al., 1988, Rasmussen et al., 1990, Koob et al., 1992, Maldonado and Koob, 1993, Funada et al., 1994, Maldonado, 1997), and it has been shown that these effects are mediated by the excitatory input from the nucleus paragigantocellularis (PGi) (Rasmussen and Aghajanian, 1989, Maldonado, 1997, Van Bockstaele et al., 2000, Van Bockstaele et al., 2001, Johnson et al., 2002).
Upon the removal of opioids from the system, a surge in noradrenergic activity from the locus coeruleus contributes significantly to the somatic expression of opioid withdrawal (Crawley et al., 1979, Swann et al., 1982, Van Bockstaele et al., 2008). Within noradrenergic targets of the LC, opiate withdrawal-mediated LC disturbances can be appreciated in terms of increased tyrosine hydroxylase, c-Fos and FosB expression, along with enhanced activation (phosphorylation) of cyclic adenosine monophosphate (c-AMP) pathway members (Stornetta et al., 1993, Nestler et al., 1994, Maldonado et al., 1995, Nestler and Aghajanian, 1997, Nestler, 2001, Van Bockstaele et al., 2001, Maldonado, 2003). Both intrinsic and extrinsic factors to the LC contribute to the withdrawal phenomenon. The cAMP-related cellular adaptations that occur in response to chronic opioid exposure cause enhanced excitability in LC neurons. When coupled with the removal of inhibitory opioid input upon withdrawal or detoxification, this combination results in the dysregulation of the LC-noradrenergic circuit (Nestler et al., 1994, Nestler, 2001). In addition to upregulated cAMP pathway activity in the LC, excitatory amino acid influx to the LC from its afferents also contributes to the molecular expression of opioid withdrawal (Akaoka and Aston-Jones, 1991, Aghajanian et al., 1994, Maldonado, 1997, Van Bockstaele et al., 2001). Common therapeutic approaches employed in the management of opioid withdrawal are targeted to adrenergic receptors with the intent of counteracting cellular hyperactivity and restoring LC homeostasis.
Therapeutic agents targeting the brain's noradrenergic circuitry have demonstrated clinical efficacy in attenuating the noradrenergic hyperactivity that is observed during opiate withdrawal (Gold et al., 1979, Gossop, 1988, Gold, 1993, Gowing et al., 2004). However, in some individuals, the use of alpha-2 adrenergic receptor agonists is limited by the presence of side effects such as hypotension, sedation, and cognitive impairment, or by incomplete abolition of withdrawal symptoms (Gossop, 1988, Gerra et al., 2001, Lobmaier et al., 2010). It remains important to investigate other receptor systems that interact with the opioid system in noradrenergic brain regions and may serve as potential therapeutic targets.
III. Cannabinoid Modulation of Noradrenergic Circuitry
a. The endocannabinoid (EC) system
Through significant advances in our understanding of the cannabinoid system in recent years, we now understand that the endogenous cannabinoid system—or endocannabinoid system—is the primary regulator of cannabinoid functions in the brain. The endocannabinoid (EC) system has been implicated in a variety of physiological functions due to abundant expression of its receptors and endogenous ligands in the central nervous system(Herkenham et al., 1991, Mackie, 2005b, 2008). The EC consists of receptors, ligands and enzymes responsible for their biosynthesis and degradation. Endocannabinoids are lipophilic arachidonic acid derivatives that are released in an activity dependent fashion from the postsynaptic cell(Piomelli et al., 1998, Piomelli, 2003, Di Marzo et al., 2005, Piomelli, 2005, Basavarajappa, 2007). Two major endocannabinoids have been identified: anandamide(Devane et al., 1992, Di Marzo et al., 1994) and 2-arachidonoylglycerol (2-AG)(Mechoulam et al., 1995, Sugiura et al., 1995, Stella et al., 1997, Mechoulam et al., 1998, Martin et al., 1999). Endocannabinoids control emotional reactivity, motivated behaviors and energy homeostasis primarily by targeting brain CB1 type 1 receptors (CB1r)(Mechoulam et al., 1998, Giuffrida et al., 2001, Fride, 2002, Kreitzer and Regehr, 2002, Wilson and Nicoll, 2002, Piomelli, 2005, Witkin et al., 2005, Mackie, 2008, Steiner et al., 2008, Steiner and Wotjak, 2008). The CB1r is one of the most plentiful G-protein coupled receptors within the brain, and cannabinoid signaling through this receptor mediates a wide variety of peripheral and central processes (Howlett et al., 1990, Howlett et al., 2002, Freund et al., 2003, Szabo and Schlicker, 2005, Demuth and Molleman, 2006).
b. The EC, noradrenergic circuitry and stress
The EC and noradrenergic systems are significantly and dynamically impacted by stress (Hill and McEwen, Cassens et al., 1980, Flugge et al., 2004, Gorzalka et al., 2008, Shinba et al., 2010) and noradrenergic transmission is responsible for cannabinoid-induced activation of the HPA axis(McLaughlin et al., 2009). Under conditions of acute stress, NE is increased centrally and peripherally(Cassens et al., 1980, Abercrombie and Jacobs, 1987, Page and Valentino, 1994, Valentino et al., 1998b, Ferry et al., 1999, Nestler et al., 1999, Sands et al., 2000) while the EC system tonically constrains activation of neural circuits, including the hypothalamic-pituitary-adrenal (HPA) axis(Gorzalka et al., 2008, Steiner and Wotjak, 2008). However, disrupted noradrenergic and EC signaling is associated with an inability to adapt to chronic stress(Nestler et al., 1999, Wong et al., 2000, Flugge et al., 2004, Hill and Gorzalka, 2004, Gorzalka et al., 2008, Hill et al., 2008). We recently demonstrated that pre-treatment with a cannabinoid receptor agonist diminishes acute stress-induced noradrenergic transmission in the mPFC(Reyes et al., 2012). Cannabinoid pre-treatment also resulted in a decrease in climbing during a single exposure to swim, an arousal-related behavior that has been attributed to availability of NE(Detke et al., 1995). Furthermore, we tested the effect of acute stress on 2-AR-cannabinoid interactions in rats(Reyes et al., 2012). Chronic CB1r stimulation with WIN 55,212-2 in slices from unstressed animals desensitized the cortical 2-AR response, similar to the desensitization produced by acute WIN 55,212-2 pretreatment. However, when animals were exposed to chronic WIN 55,212-2 and then given a forced swim stress before brain slices were prepared for electrophysiology, the cortical 2-AR response did not desensitize and is similar in magnitude to subjects that are neither stressed nor exposed to CB1r stimulation. These findings indicate that stress sensitizes the cortical 2-AR response, making it resistant to desensitization by chronic activation of CB1rs. These data reveal the stress-dependent nature of cannabinoid interactions and implicate pre- and postsynaptic ARs. However, although information regarding interactions between the two systems is emerging, there remain gaps in our knowledge regarding how cannabinoids modulate noradrenergic circuitry under different physiological conditions.
Anatomical and electrophysiological studies within the coeruleo-cortical pathway demonstrate cannabinoid sensitivity within noradrenergic cells (Mendiguren and Pineda, 2004, Oropeza et al., 2005, Mendiguren and Pineda, 2006b, a, Muntoni et al., 2006, Mendiguren and Pineda, 2007, Oropeza et al., 2007, Page et al., 2007, Scavone et al., 2010). Convergent lines of evidence (Muntoni, 2001, Mendiguren and Pineda, 2006a, Muntoni et al., 2006) have demonstrated an interaction between the cannabinoid and noradrenergic systems in areas such as the mPFC(Oropeza et al., 2005, Oropeza et al., 2007, Page et al., 2007, Page et al., 2008), nucleus accumbens (Acb)(Carvalho and Van Bockstaele, Carvalho et al., 2010a, Carvalho et al., 2010b), locus coeruleus (LC)(Oropeza et al., 2005, Scavone et al., 2010) and the nucleus of the solitary tract (NTS)(Jelsing et al., 2009, Carvalho et al., 2010a, Carvalho et al., 2010b). Recent anatomical evidence demonstrates the presence of cannabinoid receptors in noradrenergic cells within the NAc and NTS (Carvalho et al., 2010a), frontal cortex (Oropeza et al., 2007, Page et al., 2008, Reyes et al., 2009b), and LC (Scavone et al., 2010).
While the interactions between the noradrenergic and opioid systems have been well characterized in addiction [reviewed in (Van Bockstaele et al., 2001, Berridge and Waterhouse, 2003, Weinshenker and Schroeder, 2007, Aston-Jones and Kalivas, 2008, Sara, 2009, Van Bockstaele et al., 2010)], the field of cannabinoid-opioid interactions in noradrenergic cells is relatively in its infancy. However, increasing numbers of groups have begun to uncover and probe the close interaction between the cannabinoid and opioid systems in the brain and periphery [reviewed in(Manzanares et al., 1999, Corchero et al., 2004a, Fattore et al., 2004, Fattore et al., 2005a, Vigano et al., 2005a, Welch, 2009, Parolaro et al., 2010)].
IV. Cannabinoid-Opioid Interactions
Opioid and cannabinoid receptors are major targets for many drugs of abuse and widely-used analgesics. These receptor systems are known to mediate common signaling pathways central to clinical issues of tolerance, dependence and addiction (Manzanares et al., 1999, Pasternak, 2005, Demuth and Molleman, 2006). Drugs that target both the CB1r and MOR systems possess shared pharmacological profiles. Agonists of both receptor types have been shown to cause antinociception, sedation, hypotension, motor depression, and drug reward/reinforcement (Manzanares et al., 1999, Massi et al., 2001, Maldonado and Valverde, 2003, Corchero et al., 2004a). Cannabinoids may be able to modulate opioid function at a number of different levels within the cell, ranging from direct receptor associations, to alterations in endogenous peptide release, or to post-receptor interactions via shared signal transduction pathways. Evidence of these interactions can be observed through the various studies demonstrating cross-tolerance, mutual potentiation, and receptor cross-talk (Manzanares et al., 1999, Cichewicz and McCarthy, 2003, Maldonado and Valverde, 2003, Corchero et al., 2004a, Vigano et al., 2005a, Roberts et al., 2006, Cox et al., 2007, Barta et al., 2009, Welch, 2009, Parolaro et al., 2010). Importantly, drugs that target the cannabinoid system often seem to affect the opioid system in tandem. Individual modulation of CB1r or MOR has been shown to alter indices of noradrenergic activity (Nestler, 1993, Nestler et al., 1999, Oropeza et al., 2005, Szabo and Schlicker, 2005). Since the LC and associated noradrenergic circuitry are critical sites of dysfunction during opioid addiction and withdrawal, it is particularly important to develop a comprehensive understanding of both the anatomical substrates and biochemical mechanisms through which cannabinoids may be used to specifically and effectively target the opioid system in this region.
a. Anatomical Considerations
Techniques used to discern the distribution of cannabinoid and opioid receptors and their endogenous ligands have provided valuable information pertaining to the interactions between these systems. Using immunohistochemical and immuno-electron microscopic techniques, anatomical data have revealed potential sites and systems where cannabinoids may be used to target the opioid system. Morphological substrates for cannabinoid-opioid interactions have been demonstrated through CB1r and MOR co-localization in multiple brain regions. These receptors co-localize within the dorsal horn of the spinal cord (Salio et al., 2001). In the nucleus accumbens (NAc), a region critical to the reward circuitry, CB1r and MOR are both localized within neurons and in synaptically-linked pairs of cells (Pickel et al., 2004). Additionally, an ultrastructural study of the caudate-putamen revealed CB1r and MOR labeling targeted to specific patches of post-synaptic neurons with approximately 50% of all CB1r-positive dendrites containing the MOR (Rodriguez et al., 2001). However, only recently has the field begun to generate evidence for cannabinoid-opioid interactions within noradrenergic loci within the brain.
The MOR is abundant within many of the noradrenergic brain regions responsible for mediating the adaptations that occur in response to opiate exposure and in the precipitation of opiate withdrawal (Tempel and Zukin, 1987, Maldonado et al., 1992, Mansour et al., 1995, Cheng et al., 1996, Ding et al., 1996, Moyse et al., 1997, Pickel and Colago, 1999 {Maldonado, 1992 #664). Recent anatomical and functional evidence demonstrates the presence of cannabinoid receptors in noradrenergic cells within the NAc and NTS (Carvalho et al., 2010a, Carvalho et al., 2010b), frontal cortex (Oropeza et al., 2007, Page et al., 2008, Reyes et al., 2009b), and LC (Scavone et al., 2010). Data from electrophysiological recordings in the locus coeruleus also support these anatomical findings, providing evidence of cannabinoid sensitivity in this opioid-sensitive nucleus (Mendiguren and Pineda, 2006b, a). Physiological data following local infusions of cannabinoids on to the opioidergic LC provide additional evidence of local cannabinoid effects (Muntoni et al., 2006). CB1r is expressed in the terminals of noradrenergic projections from the opioid-sensitive LC to the frontal cortex (Oropeza et al., 2007), suggesting that CB1r may be poised to modulate the norepinephrine release that occurs as a result of opioid dependence or withdrawal. Alternatively, indirect mechanisms involving GABA interneurons cannot be ruled out (Reyes et al, 2012). There is also evidence of CB1r and MOR co-localization in shared post-synaptic cellular compartments of the neurons that give rise to these projections (Scavone et al., 2010).
Co-localization of CB1r and MOR within noradrenergic brain regions is particularly noteworthy given the growing body of evidence that is strongly suggestive of heterodimerization and/or closely-associated CB1r-MOR signaling complexes in vitro and in vivo (Rios et al., 2001, Mackie, 2005a, Rios et al., 2006, Schoffelmeer et al., 2006, Hojo et al., 2008). Additionally, recent studies report increased abundance of CB1r-heterodimers and opioid receptor-heterodimers in response to chronic drug exposure or repeated receptor stimulation (Kearn et al., 2005, Gupta et al., 2010). Potential implications of GPCR heterodimerization include altered subcellular localization or ligand binding properties, in addition to changes in signal transduction (Rios et al., 2001, Terrillon and Bouvier, 2004).
b. Cannabinoid-opioid system cross-talk
Downstream evidence of cannabinoid modulation of the opioid system can be observed through the many accounts in the literature of cannabinoid-opioid synergy, cross-agonism, cross-antagonism, and cross-tolerance (Manzanares et al., 1999, Lichtman et al., 2001, Navarro et al., 2001, Valverde et al., 2001, De Vries et al., 2003, Cichewicz, 2004, Fattore et al., 2005b, Fattore et al., 2007b). Numerous studies have revealed synergistic effects of cannabinoid-opioid pairings such as CP55,940 & morphine (Welch and Stevens, 1992, Tham et al., 2005), delta-9-tetrahydrocannabinol (Δ9-THC, psychoactive component of marijuana) & morphine in an arthritis model (Cox et al., 2007), and Δ9-THC & morphine in humans (Roberts et al., 2006). Moreover, combined sub-analgesic doses of Δ9-THC and morphine possessed synergistic antinociceptive properties while circumventing the development of tolerance inherent to chronic morphine exposure (Cichewicz et al., 1999, Cichewicz and McCarthy, 2003, Cichewicz, 2004, Smith et al., 2007). Cannabinoids have also been shown to exert cross-antagonism within the opioid system (Vigano et al., 2005a). Similar studies have implicated the endogenous cannabinoid system in opioid dependence, as inhibition of cannabinoid receptor signaling with the cannabinoid receptor antagonist SR141716A during chronic opioid exposure reduced opioid withdrawal signs (Rubino et al., 2000, Mas-Nieto et al., 2001). Additionally, cross-sensitization to the behavioral effects of cannabinoids and opioids have also been demonstrated (Cadoni et al., 2001).
The mechanisms through which cannabinoids are able to modulate the opioid system may be through alterations in the level of endogenous opioids or their receptors, or via changes in G-protein mediated signaling through the opioid receptors or MOR-CB1r complexes (Figure 1). Numerous studies have reported changes in the levels of endogenous opioids following cannabinoid administration, suggesting a regulatory role for CB1r in endogenous opioid release (Corchero et al., 1997a, Manzanares et al., 1998, Corchero et al., 1999, Valverde et al., 2001). For instance, repeated Δ9-THC treatments increased the expression of endogenous opioid peptides in the rat spinal cord (Corchero et al., 1997a). Alternatively, the direct interaction of CB1r and MOR has also been postulated as the mechanism for cannabinoid modulation of the MOR system. Resonance energy transfer studies reveal close associations between CB1r and MOR, while anatomical studies display the co-localization of these receptors in shared cellular compartments (Rodriguez et al., 2001, Salio et al., 2001, Pickel et al., 2004). Devi's group has proposed that cannabinoid and opioid receptors exist as heterodimeric systems that physically interact to integrate independent signal pathways (Rios et al., 2006). These receptor systems may also interact at the level of their intracellular signaling molecules. In addition to baseline receptor similarities, several studies have revealed cannabinoid-induced changes in MOR-dependent G-protein activation through GTPγS binding assays (Corchero et al., 1999, Vigano et al., 2005b, Ellgren et al., 2006, Rios et al., 2006). Cannabinoid-opioid crosstalk can also be observed through receptor expression levels, as repeated administration of Δ9-THC regulates MOR density in a time and region-dependent manner (Corchero et al., 2004b). Although the functional relationship between opioid and cannabinoid systems is becoming better established, the specific mechanisms of their interaction in the LC and associated circuits remain to be elucidated.
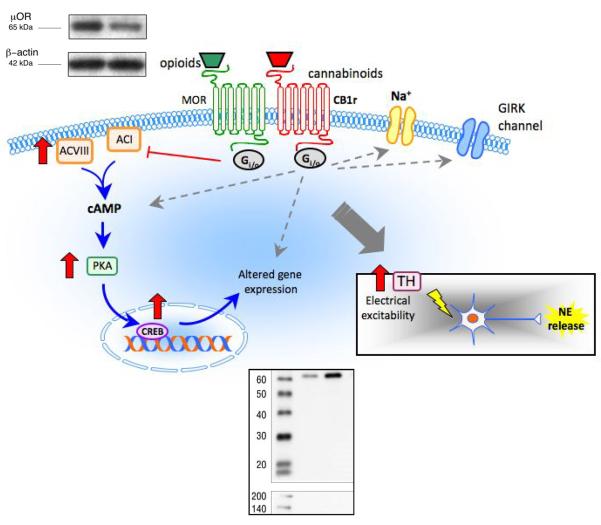
Figure 1.
Schematic illustration depicting putative interactions between the cannabinoid and opioid systems in LC neurons (see text for details). Using a combination of behavioral, biochemical and neurochemical approaches, chronic cannabinoid administration has been shown to increase multiple indices of noradrenergic activity, including increases in tyrosine hydroxylase (TH) expression in the LC (Western blot, bottom right). In contrast, chronic administration of a CB1r agonist decreases MOR expression in the LC (Western blot, top left). Opioid and cannabinoid receptors mediate common signaling pathways central to clinical issues of tolerance, dependence and addiction. The interaction of these receptor systems is supported by findings of cross-tolerance, mutual potentiation, and receptor cross-talk. Downstream effectors of CB1r modulation could lead to altered neuronal excitability and to alterations in opioid receptor signaling that are central to the etiology of anxiety, stress–related dysfunction and the management of pain.
Shifts in the subcellular distribution of MOR during opioid withdrawal may bring this receptor into close proximity with the cannabinoid receptor, promoting direct receptor associations. Unlike most cell-membrane bound GPCRs, the cannabinoid receptor has a predominantly intracellular distribution in the brain (Rozenfeld, 2010). The distribution of CB1r within somatodendritic structures in the LC agreed with this distribution pattern (Scavone et al., 2010). Interestingly, in the LC somatodendritic MOR was observed to shift from its typical plasmalemmal localization to an intracellular distribution in response to naltrexone precipitated opioid withdrawal (Scavone and Van Bockstaele, 2009). If CB1r and MOR have similar subcellular distribution patterns during opioid withdrawal situations, hetero-dimerization may be a distinct possibility. Using cannabinoids to target this heterodimer during opioid withdrawal may then influence not only cannabinoid signaling, but perhaps also restore normalcy to dysregulated opioid pathways.
In addition to the possibility of direct CB1r-MOR associations, cannabinoid-opioid interactions may also occur at the signal transduction level since both receptors signal through the Gi/o alpha subunit, targeting the cAMP pathway. During opioid withdrawal, studies have demonstrated increased abundance of Gi/o alpha in the coeruleo-cortical pathway during opioid withdrawal (Nestler et al., 1989, Nestler, 1992). If compensatory changes in CB1r signaling transduction occur during opioid withdrawal, this may also account in part for the efficacy of cannabinoids in reducing opioid withdrawal signs observed both in preclinical and clinical studies.
Cannabinoid modulation of endogenous opioid synthesis and release is yet another candidate mechanism that may contribute to WIN-mediated attenuation of opioid withdrawal signs (Figure 2). Cannabinoid agonists have been shown to facilitate endogenous opioid signaling. Acute exposure to cannabinoid agonists increased extracellular levels of endogenous opioids (Pugh et al., 1995, Pugh et al., 1996, Pugh et al., 1997, Houser et al., 2000), and chronic cannabinoid exposure increased the abundance of endogenous opioid precursors such as prodynorphin, proenkephalin and pro-opiomelanocortin (Corchero et al., 1997a, Corchero et al., 1997b, Manzanares et al., 1998). Cannabinoid stimulation of endogenous opioid release has been linked to the attenuation of opioid withdrawal in an animal model. Exposure to Δ9-THC enhanced enkephalin release in the NAc attenuated the signs of naloxone-precipitated withdrawal (Valverde et al., 2001). Similar approaches could be taken within the coeruleo-cortical pathway to determine whether the WIN interventions used to attenuate withdrawal in morphine-dependent rats also facilitate endogenous opioid release within the LC. Anatomical studies in the LC demonstrated that pre-synaptic CB1r-positive terminals contacted mu-opioid receptor-containing dendrites (Scavone et al., 2010). Presynaptic CB1r may be poised to regulate or facilitate neurotransmitter and neuropeptide release on to LC neuron; enkephalin release may help to stabilize the LC during opioid withdrawal.
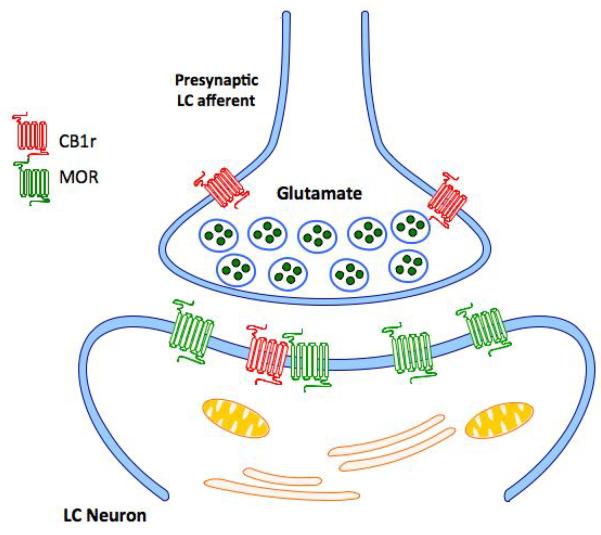
Figure 2.
Sites of action for CB1r modulation of noradrenergic circuitry include noradrenergic and GABAergic axon terminals in the frontal cortex, noradrenergic cell bodies in the LC as well as CB1r modulation of afferent input to the LC. Modulation of CB1r localized to presynaptic axon terminals in the LC, many of which are of the excitatory-type, may contribute to an attenuation of glutamatergic drive, a well-characterized hallmark of opiate withdrawal.
c. Implications of cannabinoid-induced alterations in opioid function in the LC
We previously identified important opposing CRF and opioid influences on LC activity and that changes in the sensitivity to one neuromodulator altered sensitivity to the other. Specifically, we showed that chronic opiate administration selectively increased the sensitivity of LC neurons to CRF (Xu et al., 2004). This was apparent as an increased magnitude of activation produced by microinfused CRF, as well as by stress. More importantly, sensitization of LC neurons to CRF in chronic morphine rats translated to a change in the behavioral repertoire in response to a challenge (Xu et al., 2004) Thus, exposure of morphine-dependent rats to swim stress resulted in excessive climbing at times when control rats would adopt a passive coping strategy of immobility. Climbing behavior in this model has been linked to activation of the brain norepinephrine system (Detke et al., 1995). This represents an example of how the level and mode of LC activity is the result of a balance of neuromodulators that are engaged and active at a particular time. The mechanism by which chronic opioids induce postsynaptic sensitization to CRF in LC neurons is currently unknown, although many changes in intracellular signaling systems within LC neurons have been documented to occur as a result of chronic opiate administration and these could contribute to altered postsynaptic sensitivity to CRF (Nestler et al., 1999).
Along these lines, it is critical to determine how repeated cannabinoid use affects MOR function in the LC. As cannabinoids significantly affect behavioral regulatory processes known to be LC-specific, such as the modulation of attention, arousal, anxiety and stress (Muntoni et al., 2006, Nestler, 1999), cannabinoid-induced alterations in MOR activity within the LC may likely sensitize this region to input from stress-related pathways, and may underlie the behavioral abnormalities and cognitive deficits seen with repeated cannabinoid use. Long-term cannabinoid use is associated with a syndrome of cognitive impairment that notably includes significant attentional dysfunction, and anxiety and stress disorders (Chaperon and Thiebot, 1999, Lundqvist, 2005, Demuth and Molleman, 2006).
V. Potential Therapeutic Targets of Cannabinoid-Opioid Interactions
a. Cannabinoid-induced decreases in opiate withdrawal expression
In the area of therapeutic utility, cannabis can be recommended as a palliative medical alternative for patients with multiple sclerosis and spinal cord problems where it has been shown to alleviate pain, muscle spasms and convulsions. In addition, it can be recommended to cancer and AIDS patients in order to prevent vomiting and nausea, which result as side effects of chemotherapy, radiation therapy and antiretroviral medications. It also stimulates appetite in people who suffer from this medical condition. Similarly, the therapeutic use of marijuana slows chronic pain and helps attenuate the tics in patients with Gilles Tourette's syndrome. It also may be useful both in the reduction of physical and stress-related symptoms, which occur following the cessation of drug abuse. However, cannabis plays no role in curing these conditions, but only acts to ease their symptoms. Even in the abovementioned cases, there exist contraindications, and cannabis use is not recommended for use in patients with psychotic disorders and psychological or heart problems, cardiac arrhythmias, heart failure or patients who have had angina or a heart attack. Currently Dronabinol and Nabilone as cannabinoids are available for clinical use, but there are few studies reporting on their real effectiveness.
Because the cannabinoid system interacts so closely with the opioid system, the cannabinoid receptors are attractive potential therapeutic targets. Despite this, assessment of cannabis for the reduction of opioid withdrawal has been limited to qualitative survey data rating the efficacy of illicit substances for withdrawal symptoms (Hermann et al., 2005). At present, strict regulation of cannabinoid compounds and political controversy prevent rigorous clinical assessment of cannabinoid testing in the clinical setting. However in the absence of controlled clinical studies, data from experimental animal models provide an excellent source of information regarding potential therapeutic cannabinoid regimens. A number of preclinical studies investigating cannabinoid modulation of opioid dependence and withdrawal may have therapeutic implications for the treatment of the opioid withdrawal syndrome in the clinical population. Clinically, withdrawal from heroin or prescription opioids is characterized by a host of quantifiable physical and emotional symptoms that may include muscle aches and pain, agitation and anxiety, nausea, gastrointestinal upset, tachycardia, rhinorrhea, and chills (Wesson and Ling, 2003). Similarly, in animal models of opioid dependence, withdrawal behaviors can be quantitatively assessed to determine whether cannabinoids can mediate the opioid withdrawal syndrome (Gellert and Holtzman, 1978, Fernandez Espejo et al., 1995). The use of various cannabinoid agents designed to augment or block cannabinoid signaling has been tested to determine their effects on the development of opioid dependence and the expression of opioid withdrawal.
By targeting the cannabinoid system and in turn modulating opioid signaling in noradrenergic cells, it may be possible to reduce the severity of opiate withdrawal signs (Figure 3). Numerous studies have demonstrated that endocannabinoid or delta-9-tetrahydrocannabinol (Δ9-THC) exposure during morphine withdrawal in both mice and rats reduces the severity of morphine withdrawal symptoms (Frederickson et al., 1976, Vela et al., 1995, Lichtman et al., 2001, Valverde et al., 2001, Yamaguchi et al., 2001, Del Arco et al., 2002, Cichewicz and Welch, 2003). Several studies have displayed the potential of endocannabinoid interventions for normalizing opioid system dysfunction. Attenuation of morphine-withdrawal has also been achieved via administration of the endogenous cannabinoids 2-arachidonoylglycerol and anandamide (Vela et al., 1995, Yamaguchi et al., 2001). Common withdrawal behaviors diminished due to Δ9-THC administration were jumping, weight loss, head shakes and paw tremor, although findings varied considerably depending upon dosing paradigm (Vela et al., 1995, Lichtman et al., 2001, Yamaguchi et al., 2001). Peripheral effects of naloxone-precipitated withdrawal were also attenuated by Δ9-THC in guinea pig ileum (Frederickson et al., 1976). Only one study to date has tested the efficacy of a synthetic cannabinoid agonist, and found that HU210 was similarly efficacious to Δ9-THC and the endocannabinoid 2-arichidonoylglycerol (2-AG) in reducing instances of paw tremor and jumping behavior. Interestingly, administration of Δ9-THC was shown to attenuate signs of morphine withdrawal through facilitation of endogenous enkephalin release (Valverde et al., 2001), an endogenous opioid that targets the opioid receptors in the LC (Johnson et al., 2002, Barr and Van Bockstaele, 2005). This finding is especially important given that endogenous peptide levels are decreased in response to chronic opioid exposure in LC pathways (Van Bockstaele et al., 2000).
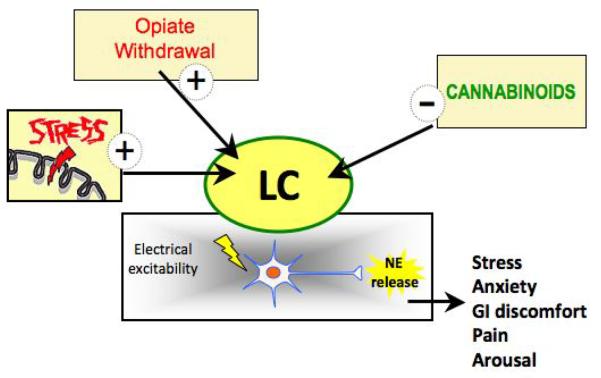
Figure 3.
Emerging knowledge of how the LC-norepinephrine system is regulated by stressors and determinants of sensitivity of LC neurons to prolonged opioid exposure is an important step in understanding and alleviating stress-induced relapse to drug abuse. Engagement of the endocannabinoid system in modulation of noradrenergic neurons could lead to altered neuronal excitability that improve behavioral dysfunction observed in anxiety, stress–related dysfunction and opiate dependence and withdrawal.
Cannabinoid antagonists may also have therapeutic utility if administered prior to the development of opioid withdrawal. Modulation of opiate withdrawal behavior, associated protein expression, and morphine-sensitivity has also been achieved by administration of cannabinoid receptor antagonist SR141716A in chronic opiate-exposed rodents (Rubino et al., 2000, Lichtman et al., 2001, Navarro et al., 2001, Singh et al., 2004, Vigano et al., 2004). Along similar lines, cannabinoids also appear to modulate heroin seeking behaviors, though there is debate in the literature as to the nature of these effects. Several groups have shown that SR141716A can negate the reinforcing and motivational properties of heroin in animals trained to self administer the drug (Navarro et al., 2001, De Vries et al., 2003, Fattore et al., 2005b). Others provide evidence for Δ9-THC-mediated enhancement of opioid self-administration (Solinas et al., 2004, Ellgren et al., 2007), which may also support a role for cannabinoid antagonists in the prevention of heroin-seeking. Collectively, these findings support the presence of functional crosstalk between cannabinoid and opioid systems and the ability to demonstrate this phenomenon using a variety of models and addiction paradigms.
b. Insights into Potential Cannabinoid-Opioid Therapeutic Targets from the Clinic
The debate surrounding medicinal cannabinoids has received added attention recently as regulatory concerns may prevent full consideration of studies that report multiple clinical applications for cannabinoids (Cohen, 2009a, b, 2010). Applications for medicinal cannabinoids that are already under investigation include the treatment of nausea, anorexia, neurodegeneration, inflammation, excito-toxicity and pain. The appetitive and anti-emetic properties of cannabinoids have led to the approval of their use in chemotherapy and AIDS patients. There is growing evidence for therapeutic cannabinoid effects on inflammatory and excito-toxic cellular processes that are linked to epilepsy, Parkinson's disease, amyotrophic lateral sclerosis, spasticity, and CNS injury (Robson, 2001, Ramirez et al., 2005, Steffens et al., 2005, Machado Rocha et al., 2008, Garcia-Arencibia et al., 2009, Onaivi, 2009, Bosier et al., 2010, Fernandez-Ruiz et al., 2010, Gowran et al., 2010). Increasing numbers of publications have reported on the critical role of the cannabinoid system in addiction and substance abuse (Fattore et al., 2007a, 2008, Bosier et al., 2010), as well as the potential for potent modulation of the opioid system by cannabinoids (Fattore et al., 2005a, Lopez-Moreno et al., 2010, Robledo, 2010, Tucci, 2010).
Despite increasing accumulations of preclinical data suggesting that cannabinoid benefits may outweigh the risks in certain serious medical conditions, political conflict over the legalization of medicinal marijuana continues to preclude a smooth transition of these preclinical studies into clinical trial testing. This may persist in the near future as state and federal government debates over cannabis regulations continue. However, there are many therapeutic alternatives to smoking or ingested cannabis. The development of compounds designed to modulate endocannabinoid levels or the use of synthetic cannabinoids with well-defined pharmacological properties may address many of the concerns associated with legalization of medical marijuana. In the meanwhile, indirect approaches can be taken to assess cannabinoid modulation of the opioid system among individuals in treatment for substance abuse. Co-abuse of cannabis and opioids is quite common in substance abusers, and investigation of this polydrug use may provide clues as to how cannabinoid-opioid interactions play out in the substance abuse treatment setting.
c. Concurrent Cannabis and Opioid Use
Cannabis use is particularly widespread among opioid-dependent individuals (Nurco et al., 1988, Nirenberg, 1996), and numerous studies have examined the concurrent use of cannabis and opioids. Within those seeking treatment for opioid dependence, cannabis is consistently reported to be among the most frequently co-abused substances, along with benzodiazepines and cocaine. Estimates of cannabis use in clients have ranged anywhere from 20% to 95% of the population (Saxon et al., 1990, Nirenberg, 1996, Budney et al., 1998, Church et al., 2001, Epstein and Preston, 2003, Nixon, 2003, Aharonovich et al., 2005). The prevalence of cannabis use varies by geographical location, likely due to the associated changes in drug availability, cost, and potency. Nonetheless, despite this variability, cannabis use remains a prominent characteristic of the drug use profile of many opioid users. Among those prescribed chronic opioid therapy for pain, cannabis use was significantly more common than in the general population in every age group (Reisfield et al., 2009). Based on these observations, co-abuse of cannabis and opioids has become the focus of many clinical research initiatives.
The prevalence of cannabis use has been established within a number of methadone maintenance programs by urinary drug screen (UDS). For example, Marammani observed within a cohort of 1090 heroin-dependent individuals presenting for methadone maintenance between 1994 and 2005, that 64.6% of men and 57.1% of females reported concurrent opioid and cannabis use when entering treatment (Maremmani et al., 2010). One study of 98 males on MMT found that 55.1% used cannabis during treatment (Saxon, 1993), while another study reported an even higher 79% rate of use (Nirenberg, 1996). Cannabis use was evaluated prior to and during treatment in a sample of 196 persons in an Israeli methadone maintenance program. At treatment intake, 25% reported current cannabis use, and 52% had at least one cannabis-positive UDS during the first year of treatment. Approximately one-third of the sample met the cannabis abuse criteria, and used cannabis for at least three consecutive months during treatment (Weizman et al., 2004). In a study designed to determine predictors of relapse in 74 heroin-dependent individuals, 47.3% of the methadone-maintained sample reported using cannabis during the 8-week study period (Wasserman et al., 1998). Finally, in a retrospective analysis of cannabis use from three clinical trial cohorts on methadone maintenance (n=408), 30.6% of the individuals in care were categorized as occasional users (≤1/6 UDS cannabis positive), while 23.3% demonstrated frequent use (>1/6 UDS positive) (Epstein and Preston, 2003).
Cannabis use has also been examined among individuals participating in other opioid-dependence treatment modalities. As part of a buprenorphine and behavioral therapy treatment trial, Budney, et al. reported that 66% of their sample of opioid dependent patients tested positive for cannabis use at least once during the 26–32 week study period; users of cannabis had an average of 45% of UDS result in cannabis-positive findings (Budney et al., 1998). Among 47 individuals on a 6-month outpatient naltrexone therapy program, cannabis use was also prevalent, observed in at least once in 68% of the sample with an average of 47% of all UDS's being cannabis-positive (Church et al., 2001). Raby (2009) observed only 38.1% of 63 opiate dependent individuals were cannabis abstinent during outpatient naltrexone treatment, while 28.6% were intermittent cannabis users, and 33.3% were classified as consistent cannabis users (Raby et al., 2009).
Longitudinal studies over the course of treatment have also revealed interesting trends. While some have observed levels of cannabis use to remain stable or increase early in treatment (Raby et al., 2009), other have failed to demonstrate this effect (Gottheil et al., 1993, Weinstein et al., 1993). In a study comparing the rates of cannabis use at months one, six, and twelve of maintenance treatment, there were differences between methadone and heroin maintenance. Among those on methadone maintenance, rates of cannabis-positive UDS fall from 63% at the start of treatment to 57.1% six months later, finally dropping to 48.5% at the one-year mark. Meanwhile those on heroin maintenance increase over the study period from 54.5% to 69.5% (Musshoff et al., 2009).
d. Cannabis Use During Treatment for Opioid Dependence
Investigations of the effects of cannabis in opioid-dependent individuals have often focused upon the impact of cannabis use on treatment for opioid dependence. Among the outcomes assessed were treatment retention, illicit drug use, medication compliance, relapse, risk behaviors and opioid withdrawal signs. However, to date the findings remain equivocal as the as to the impact of cannabis use on outcomes of medication assisted treatment. Additionally, numerous studies provide neutral evidence that cannabis use does not appear to significantly alter the course of treatment for opioid addiction.
Other illicit drug use rates do not appear to increase during treatment for opioid dependence among those using cannabis. There were no significant correlations between cannabis use and the likelihood of testing positive for opioid, benzodiazepine or cocaine use in a group of buprenorphine-treated persons (Budney et al., 1998), or in methadone-maintained individuals (Saxon, 1993, Epstein and Preston, 2003, Scavone et al., 2013). Findings from a meta-analysis on predictors of substance use in treatment-seeking opioid dependent individuals found evidence for concurrent cannabis and opioid use during treatment, but no prospective effects of cannabis on increased opioid use following completion of treatment (Brewer et al., 1998). Several studies have demonstrated that retention in treatment for opioid dependence was unaffected by the use of cannabis either prior to or during treatment (Saxon, 1993, Budney et al., 1998, Epstein and Preston, 2003, Weizman et al., 2004). However the long-term nature of MMT often renders retention a poor indicator of overall treatment outcome. Among MMT patients, chronic cannabis use also did not alter control of heroin craving and withdrawal signs (Nava et al., 2007). Additionally, no increases in psychological distress, infectious disease, and risk-taking behavior were observed within a cohort of methadone-maintained individuals testing positive for cannabis use (Weizman et al., 2004). Cognitive function and employment were not found to be significantly different between cannabis-users and cannabis-abstinent individuals undergoing MMT (Saxon, 1993). Moreover, regardless of cannabis use, disruptions in normal hypothalamic-pituitary-adrenal (HPA) axis function due to opioid abuse were restored to normal following methadone maintenance treatment (Nava et al., 2007).
We recently examined the question of cannabis use in a longitudinal fashion ina sample of individuals seeking outpatient medication assisted treatment. Retrospective chart analysis was used and examined past and present cannabis use and its association with opiate abstinence, dose stabilization, and indices of treatment compliance. Consistent with the literature, objective rates of cannabis use were high during methadone induction, dropping significantly following dose stabilization. History of cannabis use correlated with cannabis use during MMT, but did not appear to negatively impact the methadone induction process. Pilot data also suggested that objective ratings of opiate withdrawal decreased in MMT patients using cannabis during stabilization. The present findings may point to novel interventions to be employed during treatment for opiate dependence that specifically target cannabinoid-opioid system interactions (Scavone et al., 2013).
While much of the existing research finds a lack of evidence for harmful cannabis effects on treatment for opioid-dependence, several studies have revealed detrimental associations across diverse populations. Cannabis use was associated with more rapid lapse to heroin use within a cohort enrolled in MMT (Wasserman et al., 1998). While no impact of cannabis use on treatment outcome was noted within an outpatient buprenorphine treatment trial coupled with behavioral therapy, cannabis use was associated with drug dealing and needle sharing factors (Budney et al., 1998). Interestingly, following inpatient treatment for substance abuse, post-discharge cannabis use was associated with faster relapse to alcohol and cocaine use, but was unrelated to heroin use (Aharonovich et al., 2005). However, among individuals receiving chronic opioid therapy for pain, the presence of cannabis use was a positive predictor of future opioid misuse (Reisfield et al., 2009). Within the general population, cannabis use was also strongly associated with increased risk for other substance use and dependence (Degenhardt et al., 2001).
Interestingly, several studies have demonstrated that under certain circumstances, cannabis use can be associated with positive treatment prognosis among opioid-dependent cohorts. For example, Epstein and Preston (2003) found that cannabis abuse and dependence were predictive of decreased heroin and cocaine use during treatment. Intermittent use of cannabis (defined as ≥1 but ≤ 99% positive drug screens) was associated with a lower percentage of positive opioid UDS and improved medication compliance on naltrexone therapy (Church et al., 2001). Similarly, associations of intermittent or occasional cannabis use with improved retention in treatment for opioid dependence have also been reported (Ellner, 1977, Raby et al., 2009). Among opioid-dependent individuals undergoing naltrexone therapy, intermittent cannabis users (with 1–80% of UDS positive for cannabis) fared better than cannabis abstinent or consistent cannabis users in terms of treatment retention and medication compliance (Raby et al., 2009 010 #715, Lopez-Moreno et al., 2010, Robledo, 2010).
An important consideration is the response to cannabinoid therapies in the substance-dependent population, which may differ significantly from drug naïve or recreational substance users. Within adolescents, frequent cannabis use doubles the risk for depression and anxiety, and significantly decreases multiple indices of psychosocial functioning (Brook et al., 1999, Brook et al., 2002, Patton et al., 2002). Indisputably, chronic cannabis exposure in large doses has negative psychological and health consequences (Hollister, 1986, Kendler and Prescott, 1998, Budney and Moore, 2002, Patton et al., 2002, Lundqvist, 2005, Rubino and Parolaro, 2008). However, obvious differences would be expected in the responses to cannabinoid exposure in the opioid dependent population given the many physiological adaptations that occur with long-term drug exposure and typical psychiatric co-morbidity. The risks associated with cannabis use in the opioid-dependent population must be weighed against the prospective benefit of successful substance abuse treatment outcomes and the prevention of the much greater risk and harm association with opioid addiction.
VI. Conclusions
Opioid dependence and withdrawal are complex biological processes that appear to be subject to the influence of cannabinoids. The findings from basic and pre-clinical studies in rodent models highlight several potential mechanisms through which cannabinoids may modulate the phenomenon of opioid withdrawal, and call attention to the importance of cannabinoid-opioid interactions within noradrenergic brain circuits such as the coeruleo-cortical pathway. Preclinical studies that continue to explore the safest and most effective means of using cannabinoids to target disrupted noradrenergic circuits will be central to the progress within this field of research. Like many pharmacological therapeutics, the use of cannabinoids does not come without risk and will continue to require assessment of the impact of its use to treat conditions such as opioid withdrawal in humans. Determining whether cannabinoids have therapeutic efficacy in clinical populations similar to that reported in animal models will be extremely important. Ultimately, the knowledge gained from the preclinical and clinical research studies described within this review highlights important and exciting new avenues for future research that continue to investigate cannabinoid effects on noradrenergic circuit dysfunction during opioid dependence and withdrawal. Future studies may contribute to the development of novel cannabinoid-based therapeutics that provide clinicians an additional tool to support the recovery of opioid-dependent persons undergoing treatment.
Highlights 3–5 bullet points (maximum of 85 characters, including spaces, per highlight) to describe only core results of the paper
Review of the problem of opioid dependence and withdrawal
Review of cannabinoid effects on noradrenergic circuitry
Review of cannabinoid opioid cross-talk
Targeting cannabinoid opioid interactions in novel therapeutic approaches
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None.
References
- Abercrombie ED, Jacobs BL. Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. II. Adaptation to chronically presented stressful stimuli. J Neurosci. 1987;7:2844–2848. doi: 10.1523/JNEUROSCI.07-09-02844.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanian GK, Kogan JH, Moghaddam B. Opiate withdrawal increases glutamate and aspartate efflux in the locus coeruleus: an in vivo microdialysis study. Brain Res. 1994;636:126–130. doi: 10.1016/0006-8993(94)90186-4. [DOI] [PubMed] [Google Scholar]
- Aharonovich E, Liu X, Samet S, Nunes E, Waxman R, Hasin D. Postdischarge cannabis use and its relationship to cocaine, alcohol, and heroin use: a prospective study. Am J Psychiatry. 2005;162:1507–1514. doi: 10.1176/appi.ajp.162.8.1507. [DOI] [PubMed] [Google Scholar]
- Akaoka H, Aston-Jones G. Opiate withdrawal-induced hyperactivity of locus coeruleus is substantially mediated by neurons augmented excitatory amino acid input. J Neurosci. 1991;11:3830–3839. doi: 10.1523/JNEUROSCI.11-12-03830.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez VA, Arttamangkul S, Dang V, Salem A, Whistler JL, Von Zastrow M, Grandy DK, Williams JT. mu-Opioid receptors: Ligand-dependent activation of potassium conductance, desensitization, and internalization. J Neurosci. 2002;22:5769–5776. doi: 10.1523/JNEUROSCI.22-13-05769.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arttamangkul S, Torrecilla M, Kobayashi K, Okano H, Williams JT. Separation of mu-opioid receptor desensitization and internalization: endogenous receptors in primary neuronal cultures. J Neurosci. 2006;26:4118–4125. doi: 10.1523/JNEUROSCI.0303-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Nonrepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci. 1981;1:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Ennis M, Pieribone VA, Nickell WT, Shipley MT. The brain nucleus locus coeruleus: restricted afferent control of a broad efferent network. Science. 1986;234:734–737. doi: 10.1126/science.3775363. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Shipley MT, Chouvet G, Ennis M, van Bockstaele E, Pieribone V, Shiekhattar R, Akaoka H, Drolet G, Astier B, et al. Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology. Prog Brain Res. 1991;88:47–75. doi: 10.1016/s0079-6123(08)63799-1. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Kalivas PW. Brain norepinephrine rediscovered in addiction research. Biol Psychiatry. 2008;63:1005–1006. doi: 10.1016/j.biopsych.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr J, Van Bockstaele EJ. Vesicular glutamate transporter-1 colocalizes with endogenous opioid peptides in axon terminals of the rat locus coeruleus. Anat Rec A Discov Mol Cell Evol Biol. 2005;284:466–474. doi: 10.1002/ar.a.20184. [DOI] [PubMed] [Google Scholar]
- Barta WD, Kurth ME, Stein MD, Tennen H, Kiene SM. Craving and self-efficacy in the first five weeks of methadone maintenance therapy: a daily process study. J Stud Alcohol Drugs. 2009;70:735–740. doi: 10.15288/jsad.2009.70.735. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS. Neuropharmacology of the endocannabinoid signaling system-molecular mechanisms, biological actions and synaptic plasticity. Curr Neuropharmacol. 2007;5:81–97. doi: 10.2174/157015907780866910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Birnbaum HG, White AG, Reynolds JL, Greenberg PE, Zhang M, Vallow S, Schein JR, Katz NP. Estimated costs of prescription opioid analgesic abuse in the United States in 2001: a societal perspective. Clin J Pain. 2006;22:667–676. doi: 10.1097/01.ajp.0000210915.80417.cf. [DOI] [PubMed] [Google Scholar]
- Bosier B, Muccioli GG, Hermans E, Lambert DM. Functionally selective cannabinoid receptor signalling: therapeutic implications and opportunities. Biochem Pharmacol. 2010;80:1–12. doi: 10.1016/j.bcp.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Boundy VA, Gold SJ, Messer CJ, Chen J, Son JH, Joh TH, Nestler EJ. Regulation of tyrosine hydroxylase promoter activity by chronic morphine in TH9.0-LacZ transgenic mice. J Neurosci. 1998;18:9989–9995. doi: 10.1523/JNEUROSCI.18-23-09989.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer DD, Catalano RF, Haggerty K, Gainey RR, Fleming CB. A meta-analysis of predictors of continued drug use during and after treatment for opiate addiction. Addiction. 1998;93:73–92. [PubMed] [Google Scholar]
- Brook JS, Adams RE, Balka EB, Johnson E. Early adolescent marijuana use: risks for the transition to young adulthood. Psychol Med. 2002;32:79–91. doi: 10.1017/s0033291701004809. [DOI] [PubMed] [Google Scholar]
- Brook JS, Balka EB, Whiteman M. The risks for late adolescence of early adolescent marijuana use. Am J Public Health. 1999;89:1549–1554. doi: 10.2105/ajph.89.10.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Bickel WK, Amass L. Marijuana use and treatment outcome among opioid-dependent patients. Addiction. 1998;93:493–503. doi: 10.1046/j.1360-0443.1998.9344935.x. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA. Development and consequences of cannabis dependence. J Clin Pharmacol. 2002;42:28S–33S. doi: 10.1002/j.1552-4604.2002.tb06000.x. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Pisanu A, Solinas M, Acquas E, Di Chiara G. Behavioural sensitization after repeated exposure to Delta 9-tetrahydrocannabinol and cross-sensitization with morphine. Psychopharmacology (Berl) 2001;158:259–266. doi: 10.1007/s002130100875. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- Carvalho AF, Mackie K, Van Bockstaele EJ. Cannabinoid modulation of limbic forebrain noradrenergic circuitry. Eur J Neurosci. 2010a;31:286–301. doi: 10.1111/j.1460-9568.2009.07054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AF, Reyes AR, Sterling RC, Unterwald E, Van Bockstaele EJ. Contribution of limbic norepinephrine to cannabinoid-induced aversion. Psychopharmacology (Berl) 2010b;211:479–491. doi: 10.1007/s00213-010-1923-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AF, Van Bockstaele EJ. Direct intra-accumbal infusion of a beta-adrenergic receptor antagonist abolishes WIN 55,212-2-induced aversion. Neurosci Lett. 2011;500:82–85. doi: 10.1016/j.neulet.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassens G, Roffman M, Kuruc A, Orsulak PJ, Schildkraut JJ. Alterations in brain norepinephrine metabolism induced by environmental stimuli previously paired with inescapable shock. Science. 1980;209:1138–1140. doi: 10.1126/science.7403874. [DOI] [PubMed] [Google Scholar]
- Chaperon F, Thiebot MH. Behavioral effects of cannabinoid agents in animals. Crit Rev Neurobiol. 1999;13:243–281. doi: 10.1615/critrevneurobiol.v13.i3.20. [DOI] [PubMed] [Google Scholar]
- Cheng PY, Liu-Chen LY, Chen C, Pickel VM. Immunolabeling of Mu opioid receptors in the rat nucleus of the solitary tract: extrasynaptic plasmalemmal localization and association with Leu5-enkephalin. J Comp Neurol. 1996;371:522–536. doi: 10.1002/(SICI)1096-9861(19960805)371:4<522::AID-CNE3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Childers SR, Fleming L, Konkoy C, Marckel D, Pacheco M, Sexton T, Ward S. Opioid and cannabinoid receptor inhibition of adenylyl cyclase in brain. Ann N Y Acad Sci. 1992;654:33–51. doi: 10.1111/j.1749-6632.1992.tb25954.x. [DOI] [PubMed] [Google Scholar]
- Christie MJ, Williams JT, North RA. Mechanisms of tolerance to opiates in locus coeruleus neurons. NIDA Res Monogr. 1987;78:158–168. [PubMed] [Google Scholar]
- Church SH, Rothenberg JL, Sullivan MA, Bornstein G, Nunes EV. Concurrent substance use and outcome in combined behavioral and naltrexone therapy for opiate dependence. Am J Drug Alcohol Abuse. 2001;27:441–452. doi: 10.1081/ada-100104511. [DOI] [PubMed] [Google Scholar]
- Cichewicz DL, Martin ZL, Smith FL, Welch SP. Enhancement mu opioid antinociception by oral delta9-tetrahydrocannabinol: dose-response analysis and receptor identification. J Pharmacol Exp Ther. 1999;289:859–867. [PubMed] [Google Scholar]
- Cichewicz DL, McCarthy EA. Antinociceptive synergy between delta(9)-tetrahydrocannabinol and opioids after oral administration. J Pharmacol Exp Ther. 2003;304:1010–1015. doi: 10.1124/jpet.102.045575. [DOI] [PubMed] [Google Scholar]
- Cichewicz DL, Welch SP. Modulation of oral morphine antinociceptive tolerance and naloxone-precipitated withdrawal signs by oral Delta 9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2003;305:812–817. doi: 10.1124/jpet.102.046870. [DOI] [PubMed] [Google Scholar]
- Cichewicz DL. Synergistic interactions between cannabinoid and opioid analgesics. Life Sci. 2004;74:1317–1324. doi: 10.1016/j.lfs.2003.09.038. [DOI] [PubMed] [Google Scholar]
- Cohen PJ. Medical marijuana: the conflict between scientific evidence and political ideology. Part one of two. J Pain Palliat Care Pharmacother. 2009a;23:4–25. doi: 10.1080/15360280902727973. [DOI] [PubMed] [Google Scholar]
- Cohen PJ. Medical marijuana: the conflict between scientific evidence and political ideology. Part two of two. J Pain Palliat Care Pharmacother. 2009b;23:120–140. doi: 10.1080/15360280902900620. [DOI] [PubMed] [Google Scholar]
- Cohen PJ. Medical marijuana 2010: it's time to fix the regulatory vacuum. J Law Med Ethics. 2010;38:654–666. doi: 10.1111/j.1748-720X.2010.00519.x. [DOI] [PubMed] [Google Scholar]
- Corchero J, Avila MA, Fuentes JA, Manzanares J. delta-9-Tetrahydrocannabinol increases prodynorphin and proenkephalin gene expression in the spinal cord of the rat. Life Sci. 1997a;61:PL 39–43. doi: 10.1016/s0024-3205(97)00405-0. [DOI] [PubMed] [Google Scholar]
- Corchero J, Fuentes JA, Manzanares J. delta 9-Tetrahydrocannabinol increases proopiomelanocortin gene expression in the arcuate nucleus of the rat hypothalamus. Eur J Pharmacol. 1997b;323:193–195. doi: 10.1016/s0014-2999(97)00144-1. [DOI] [PubMed] [Google Scholar]
- Corchero J, Romero J, Berrendero F, Fernandez-Ruiz J, Ramos JA, Fuentes JA, Manzanares J. Time-dependent differences of repeated administration with Delta9-tetrahydrocannabinol in proenkephalin and cannabinoid receptor gene expression and G-protein activation by mu-opioid and CB1-cannabinoid receptors in the caudate-putamen. Brain Res Mol Brain Res. 1999;67:148–157. doi: 10.1016/s0169-328x(99)00053-4. [DOI] [PubMed] [Google Scholar]
- Corchero J, Manzanares J, Fuentes JA. Cannabinoid/opioid crosstalk in the central nervous system. Crit Rev Neurobiol. 2004a;16:159–172. doi: 10.1615/critrevneurobiol.v16.i12.170. [DOI] [PubMed] [Google Scholar]
- Corchero J, Oliva JM, Garcia-Lecumberri C, Martin S, Ambrosio E, Manzanares J. Repeated administration with Delta9-tetrahydrocannabinol regulates mu-opioid receptor density in the rat brain. J Psychopharmacol. 2004b;18:54–58. doi: 10.1177/0269881104040237. [DOI] [PubMed] [Google Scholar]
- Coven E, Ni Y, Widnell KL, Chen J, Walker WH, Habener JF, Nestler EJ. Cell type-specific regulation of CREB gene expression: mutational analysis of CREB promoter activity. J Neurochem. 1998;71:1865–1874. doi: 10.1046/j.1471-4159.1998.71051865.x. [DOI] [PubMed] [Google Scholar]
- Cox ML, Haller VL, Welch SP. Synergy between delta9-tetrahydrocannabinol and morphine in the arthritic rat. Eur J Pharmacol. 2007;567:125–130. doi: 10.1016/j.ejphar.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Laverty R, Roth RH. Clonidine reversal of increased norepinephrine metabolite levels during morphine withdrawal. Eur J Pharmacol. 1979;57:247–250. doi: 10.1016/0014-2999(79)90372-8. [DOI] [PubMed] [Google Scholar]
- Dang VC, Williams JT. Morphine-Induced mu-opioid receptor desensitization. Mol Pharmacol. 2005;68:1127–1132. doi: 10.1124/mol.105.013185. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Homberg JR, Binnekade R, Raaso H, Schoffelmeer AN. Cannabinoid modulation of the reinforcing and motivational properties of heroin and heroin-associated cues in rats. Psychopharmacology (Berl) 2003;168:164–169. doi: 10.1007/s00213-003-1422-1. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Hall W, Lynskey M. The relationship between cannabis use and other substance use in the general population. Drug Alcohol Depend. 2001;64:319–327. doi: 10.1016/s0376-8716(01)00130-2. [DOI] [PubMed] [Google Scholar]
- Del Arco I, Navarro M, Bilbao A, Ferrer B, Piomelli D, Rodriguez De Fonseca F. Attenuation of spontaneous opiate withdrawal in mice by the anandamide transport inhibitor AM404. Eur J Pharmacol. 2002;454:103–104. doi: 10.1016/s0014-2999(02)02483-4. [DOI] [PubMed] [Google Scholar]
- Demuth DG, Molleman A. Cannabinoid signalling. Life Sci. 2006;78:549–563. doi: 10.1016/j.lfs.2005.05.055. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Dhawan BN, Cesselin F, Raghubir R, Reisine T, Bradley PB, Portoghese PS, Hamon M. International Union of Pharmacology. XII. Classification of opioid receptors. Pharmacol Rev. 1996;48:567–592. [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L, Bisogno T. The biosynthesis, fate and pharmacological properties of endocannabinoids. Handb Exp Pharmacol. 2005:147–185. doi: 10.1007/3-540-26573-2_5. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Kaneko T, Nomura S, Mizuno N. Immunohistochemical localization of mu-opioid receptors in the central nervous system of the rat. J Comp Neurol. 1996;367:375–402. doi: 10.1002/(SICI)1096-9861(19960408)367:3<375::AID-CNE5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, Palamarchouk V. Brain circuits involved in corticotropin-releasing factor-norepinephrine interactions during stress. Ann N Y Acad Sci. 2004;1018:25–34. doi: 10.1196/annals.1296.003. [DOI] [PubMed] [Google Scholar]
- Ellgren M, Spano SM, Hurd YL. Adolescent Cannabis Exposure Alters Opiate Intake and Opioid Limbic Neuronal Populations in Adult Rats. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1301127. [DOI] [PubMed] [Google Scholar]
- Ellgren M, Spano SM, Hurd YL. Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology. 2007;32:607–615. doi: 10.1038/sj.npp.1301127. [DOI] [PubMed] [Google Scholar]
- Ellner M. Marijuana use by heroin abusers as a factor in program retention. J Consult Clin Psychol. 1977;45:709–710. doi: 10.1037//0022-006x.45.4.709. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL. Does cannabis use predict poor outcome for heroin-dependent patients on maintenance treatment? Past findings and more evidence against. Addiction. 2003;98:269–279. doi: 10.1046/j.1360-0443.2003.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell M. Opiate withdrawal. Addiction. 1994;89:1471–1475. doi: 10.1111/j.1360-0443.1994.tb03745.x. [DOI] [PubMed] [Google Scholar]
- Fattore L, Cossu G, Spano MS, Deiana S, Fadda P, Scherma M, Fratta W. Cannabinoids and reward: interactions with the opioid system. Crit Rev Neurobiol. 2004;16:147–158. doi: 10.1615/critrevneurobiol.v16.i12.160. [DOI] [PubMed] [Google Scholar]
- Fattore L, Deiana S, Spano SM, Cossu G, Fadda P, Scherma M, Fratta W. Endocannabinoid system and opioid addiction: behavioural aspects. Pharmacol Biochem Behav. 2005a;81:343–359. doi: 10.1016/j.pbb.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano S, Cossu G, Deiana S, Fadda P, Fratta W. Cannabinoid CB(1) antagonist SR 141716A attenuates reinstatement of heroin self-administration in heroin-abstinent rats. Neuropharmacology. 2005b;48:1097–1104. doi: 10.1016/j.neuropharm.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Deiana S, Melis V, Cossu G, Fadda P, Fratta W. An endocannabinoid mechanism in relapse to drug seeking: a review of animal studies and clinical perspectives. Brain Res Rev. 2007a;53:1–16. doi: 10.1016/j.brainresrev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Fattore L, Vigano D, Fadda P, Rubino T, Fratta W, Parolaro D. Bidirectional regulation of mu-opioid and CB1-cannabinoid receptor in rats self-administering heroin or WIN 55,212-2. Eur J Neurosci. 2007b;25:2191–2200. doi: 10.1111/j.1460-9568.2007.05470.x. [DOI] [PubMed] [Google Scholar]
- Fattore L, Fadda P, Spano MS, Pistis M, Fratta W. Neurobiological mechanisms of cannabinoid addiction. Mol Cell Endocrinol. 2008;286:S97–S107. doi: 10.1016/j.mce.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Fernandez Espejo E, Cador M, Stinus L. Ethopharmacological analysis of naloxone-precipitated morphine withdrawal syndrome in rats: a newly-developed “etho-score”. Psychopharmacology (Berl) 1995;122:122–130. doi: 10.1007/BF02246086. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Hernandez M, Ramos JA. Cannabinoid-dopamine interaction in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther. 2010;16:e72–91. doi: 10.1111/j.1755-5949.2010.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry B, Roozendaal B, McGaugh JL. Role of norepinephrine in mediating stress hormone regulation of long-term memory storage: a critical involvement of the amygdala. Biol Psychiatry. 1999;46:1140–1152. doi: 10.1016/s0006-3223(99)00157-2. [DOI] [PubMed] [Google Scholar]
- Flugge G, Van Kampen M, Mijnster MJ. Perturbations in brain monoamine systems during stress. Cell Tissue Res. 2004;315:1–14. doi: 10.1007/s00441-003-0807-0. [DOI] [PubMed] [Google Scholar]
- Frederickson RC, Hewes CR, Aiken JW. Correlation between the in vivo and an in vitro expression of opiate withdrawal precipitated by naloxone: their antagonism by l-(-)-delta9-tetrahydrocannabinol. J Pharmacol Exp Ther. 1976;199:375–384. [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Fride E. Endocannabinoids in the central nervous system--an overview. Prostaglandins Leukot Essent Fatty Acids. 2002;66:221–233. doi: 10.1054/plef.2001.0360. [DOI] [PubMed] [Google Scholar]
- Funada M, Suzuki T, Sugano Y, Tsubai M, Misawa M, Ueda H, Misu Y. Role of beta-adrenoceptors in the expression of morphine withdrawal signs. Life Sci. 1994;54:PL113–118. doi: 10.1016/0024-3205(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Garcia-Arencibia M, Garcia C, Fernandez-Ruiz J. Cannabinoids and Parkinson's disease. CNS Neurol Disord Drug Targets. 2009;8:432–439. doi: 10.2174/187152709789824642. [DOI] [PubMed] [Google Scholar]
- Gellert VF, Holtzman SG. Development and maintenance of morphine tolerance and dependence in the rat by scheduled access to morphine drinking solutions. J Pharmacol Exp Ther. 1978;205:536–546. [PubMed] [Google Scholar]
- Gerra G, Zaimovic A, Giusti F, Di Gennaro C, Zambelli U, Gardini S, Delsignore R. Lofexidine versus clonidine in rapid opiate detoxification. J Subst Abuse Treat. 2001;21:11–17. doi: 10.1016/s0740-5472(01)00178-7. [DOI] [PubMed] [Google Scholar]
- Gilson AM, Kreis PG. The burden of the nonmedical use of prescription opioid analgesics. Pain Med. 2009;10(Suppl 2):S89–100. doi: 10.1111/j.1526-4637.2009.00668.x. [DOI] [PubMed] [Google Scholar]
- Gintzler AR, Chakrabarti S. Post-opioid receptor adaptations to chronic morphine; altered functionality and associations of signaling molecules. Life Sci. 2006;79:717–722. doi: 10.1016/j.lfs.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Beltramo M, Piomelli D. Mechanisms of endocannabinoid inactivation: biochemistry and pharmacology. J Pharmacol Exp Ther. 2001;298:7–14. [PubMed] [Google Scholar]
- Gold MS. Opiate addiction and the locus coeruleus. The clinical utility of clonidine, naltrexone, methadone, and buprenorphine. Psychiatr Clin North Am. 1993;16:61–73. [PubMed] [Google Scholar]
- Gold MS, Redmond DE, Jr., Kleber HD. Noradrenergic hyperactivity in opiate withdrawal supported by clonidine reversal of opiate withdrawal. Am J Psychiatry. 1979;136:100–102. doi: 10.1176/ajp.136.1.100. [DOI] [PubMed] [Google Scholar]
- Gorzalka BB, Hill MN, Hillard CJ. Regulation of endocannabinoid signaling by stress: implications for stress-related affective disorders. Neurosci Biobehav Rev. 2008;32:1152–1160. doi: 10.1016/j.neubiorev.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Gossop M. Clonidine and the treatment of the opiate withdrawal syndrome. Drug Alcohol Depend. 1988;21:253–259. doi: 10.1016/0376-8716(88)90078-6. [DOI] [PubMed] [Google Scholar]
- Gottheil E, Sterling RC, Weinstein SP. Diminished illicit drug use as a consequence of long-term methadone maintenance. J Addict Dis. 1993;12:45–57. doi: 10.1300/J069v12n04_04. [DOI] [PubMed] [Google Scholar]
- Gowing L, Farrell M, Ali R, White J. Alpha2 adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2004:CD002024. doi: 10.1002/14651858.CD002024.pub2. [DOI] [PubMed] [Google Scholar]
- Gowran A, Noonan J, Campbell VA. The Multiplicity of Action of Cannabinoids: Implications for Treating Neurodegeneration. CNS Neurosci Ther. 2010 doi: 10.1111/j.1755-5949.2010.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Mulder J, Gomes I, Rozenfeld R, Bushlin I, Ong E, Lim M, Maillet E, Junek M, Cahill CM, Harkany T, Devi LA. Increased abundance of opioid receptor heteromers after chronic morphine administration. Sci Signal. 2010;3:ra54. doi: 10.1126/scisignal.2000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann D, Klages E, Welzel H, Mann K, Croissant B. Low efficacy of non-opioid drugs in opioid withdrawal symptoms. Addict Biol. 2005;10:165–169. doi: 10.1080/13556210500123514. [DOI] [PubMed] [Google Scholar]
- Hill MN, Carrier EJ, Ho WS, Shi L, Patel S, Gorzalka BB, Hillard CJ. Prolonged glucocorticoid treatment decreases cannabinoid CB1 receptor density in the hippocampus. Hippocampus. 2008;18:221–226. doi: 10.1002/hipo.20386. [DOI] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. Enhancement of anxiety-like responsiveness to the cannabinoid CB(1) receptor agonist HU-210 following chronic stress. Eur J Pharmacol. 2004;499:291–295. doi: 10.1016/j.ejphar.2004.06.069. [DOI] [PubMed] [Google Scholar]
- Hill MN, McEwen BS. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:791–797. doi: 10.1016/j.pnpbp.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo M, Sudo Y, Ando Y, Minami K, Takada M, Matsubara T, Kanaide M, Taniyama K, Sumikawa K, Uezono Y. mu-Opioid receptor forms a functional heterodimer with cannabinoid CB1 receptor: electrophysiological and FRET assay analysis. J Pharmacol Sci. 2008;108:308–319. doi: 10.1254/jphs.08244fp. [DOI] [PubMed] [Google Scholar]
- Hollister LE. Health aspects of cannabis. Pharmacol Rev. 1986;38:1–20. [PubMed] [Google Scholar]
- Houser SJ, Eads M, Embrey JP, Welch SP. Dynorphin B and spinal analgesia: induction of antinociception by the cannabinoids CP55,940, Delta(9)-THC and anandamide. Brain Res. 2000;857:337–342. doi: 10.1016/s0006-8993(00)01981-8. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Bidaut-Russell M, Devane WA, Melvin LS, Johnson MR, Herkenham M. The cannabinoid receptor: biochemical, anatomical and behavioral characterization. Trends Neurosci. 1990;13:420–423. doi: 10.1016/0166-2236(90)90124-s. [DOI] [PubMed] [Google Scholar]
- Jasinski DR. Opiate withdrawal syndrome: acute and protracted aspects. Ann N Y Acad Sci. 1981;362:183–186. doi: 10.1111/j.1749-6632.1981.tb12807.x. [DOI] [PubMed] [Google Scholar]
- Jelsing J, Galzin AM, Guillot E, Pruniaux MP, Larsen PJ, Vrang N. Localization and phenotypic characterization of brainstem neurons activated by rimonabant and WIN55,212-2. Brain Res Bull. 2009;78:202–210. doi: 10.1016/j.brainresbull.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Johnson AD, Peoples J, Stornetta RL, Van Bockstaele EJ. Opioid circuits originating from the nucleus paragigantocellularis and their potential role in opiate withdrawal. Brain Res. 2002;955:72–84. doi: 10.1016/s0006-8993(02)03367-x. [DOI] [PubMed] [Google Scholar]
- Johnson EE, Christie MJ, Connor M. The role of opioid receptor phosphorylation and trafficking in adaptations to persistent opioid treatment. Neurosignals. 2005;14:290–302. doi: 10.1159/000093044. [DOI] [PubMed] [Google Scholar]
- Kearn CS, Blake-Palmer K, Daniel E, Mackie K, Glass M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: a mechanism for receptor crosstalk? Mol Pharmacol. 2005;67:1697–1704. doi: 10.1124/mol.104.006882. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Cannabis use, abuse, and dependence in a population-based sample of female twins. Am J Psychiatry. 1998;155:1016–1022. doi: 10.1176/ajp.155.8.1016. [DOI] [PubMed] [Google Scholar]
- Koob GF, Maldonado R, Stinus L. Neural substrates of opiate withdrawal. Trends Neurosci. 1992;15:186–191. doi: 10.1016/0166-2236(92)90171-4. [DOI] [PubMed] [Google Scholar]
- Kreibich A, Reyes BA, Curtis AL, Ecke L, Chavkin C, Van Bockstaele EJ, Valentino RJ. Presynaptic inhibition of diverse afferents to the locus ceruleus by kappa-opiate receptors: a novel mechanism for regulating the central norepinephrine system. J Neurosci. 2008;28:6516–6525. doi: 10.1523/JNEUROSCI.0390-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde signaling by endocannabinoids. Curr Opin Neurobiol. 2002;12:324–330. doi: 10.1016/s0959-4388(02)00328-8. [DOI] [PubMed] [Google Scholar]
- Lane-Ladd SB, Pineda J, Boundy VA, Pfeuffer T, Krupinski J, Aghajanian GK, Nestler EJ. CREB (cAMP response element-binding protein) in the locus coeruleus: biochemical, physiological, and behavioral evidence for a role in opiate dependence. J Neurosci. 1997;17:7890–7901. doi: 10.1523/JNEUROSCI.17-20-07890.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Sheikh SM, Loh HH, Martin BR. Opioid and cannabinoid modulation of precipitated withdrawal in delta(9)-tetrahydrocannabinol and morphine-dependent mice. J Pharmacol Exp Ther. 2001;298:1007–1014. [PubMed] [Google Scholar]
- Lobmaier P, Gossop M, Waal H, Bramness J. The pharmacological treatment of opioid addiction--a clinical perspective. Eur J Clin Pharmacol. 2010;66:537–545. doi: 10.1007/s00228-010-0793-6. [DOI] [PubMed] [Google Scholar]
- Lopez-Moreno JA, Lopez-Jimenez A, Gorriti MA, de Fonseca FR. Functional interactions between endogenous cannabinoid and opioid systems: focus on alcohol, genetics and drug-addicted behaviors. Curr Drug Targets. 2010;11:406–428. doi: 10.2174/138945010790980312. [DOI] [PubMed] [Google Scholar]
- Lundqvist T. Cognitive consequences of cannabis use: comparison with abuse of stimulants and heroin with regard to attention, memory and executive functions. Pharmacol Biochem Behav. 2005;81:319–330. doi: 10.1016/j.pbb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Machado Rocha FC, Stefano SC, De Cassia Haiek R, Rosa Oliveira LM, Da Silveira DX. Therapeutic use of Cannabis sativa on chemotherapy-induced nausea and vomiting among cancer patients: systematic review and meta-analysis. Eur J Cancer Care (Engl) 2008;17:431–443. doi: 10.1111/j.1365-2354.2008.00917.x. [DOI] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptor homo- and heterodimerization. Life Sci. 2005a;77:1667–1673. doi: 10.1016/j.lfs.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol. 2005b:299–325. doi: 10.1007/3-540-26573-2_10. [DOI] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptors: where they are and what they do. J Neuroendocrinol. 2008;20(Suppl 1):10–14. doi: 10.1111/j.1365-2826.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Stinus L, Gold LH, Koob GF. Role of different brain structures in the expression of the physical morphine withdrawal syndrome. J Pharmacol Exp Ther. 1992;261:669–677. [PubMed] [Google Scholar]
- Maldonado R, Koob GF. Destruction of the locus coeruleus decreases physical signs of opiate withdrawal. Brain Res. 1993;605:128–138. doi: 10.1016/0006-8993(93)91364-x. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Garbay C, Roques BP. Protein kinases in the locus coeruleus and periaqueductal gray matter are involved in the expression of opiate withdrawal. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:565–575. doi: 10.1007/BF00169392. [DOI] [PubMed] [Google Scholar]
- Maldonado R. Participation of noradrenergic pathways in the expression of opiate withdrawal: biochemical and pharmacological evidence. Neurosci Biobehav Rev. 1997;21:91–104. doi: 10.1016/0149-7634(95)00061-5. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Valverde O. Participation of the opioid system in cannabinoid-induced antinociception and emotional-like responses. Eur Neuropsychopharmacol. 2003;13:401–410. doi: 10.1016/j.euroneuro.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Maldonado R. The neurobiology of addiction. J Neural Transm. 2003;(Suppl):1–14. doi: 10.1007/978-3-7091-0541-2_1. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Akil H, Watson SJ. Immunohistochemical localization of the cloned mu opioid receptor in the rat CNS. J Chem Neuroanat. 1995;8:283–305. doi: 10.1016/0891-0618(95)00055-c. [DOI] [PubMed] [Google Scholar]
- Manzanares J, Corchero J, Romero J, Fernandez-Ruiz JJ, Ramos JA, Fuentes JA. Chronic administration of cannabinoids regulates proenkephalin mRNA levels in selected regions of the rat brain. Brain Res Mol Brain Res. 1998;55:126–132. doi: 10.1016/s0169-328x(97)00371-9. [DOI] [PubMed] [Google Scholar]
- Manzanares J, Corchero J, Romero J, Fernandez-Ruiz JJ, Ramos JA, Fuentes JA. Pharmacological and biochemical interactions between opioids and cannabinoids. Trends Pharmacol Sci. 1999;20:287–294. doi: 10.1016/s0165-6147(99)01339-5. [DOI] [PubMed] [Google Scholar]
- Maremmani I, Stefania C, Pacini M, Maremmani AG, Carlini M, Golia F, Deltito J, Dell'Osso L. Differential substance abuse patterns distribute according to gender in heroin addicts. J Psychoactive Drugs. 2010;42:89–95. doi: 10.1080/02791072.2010.10399789. [DOI] [PubMed] [Google Scholar]
- Mark TL, Woody GE, Juday T, Kleber HD. The economic costs of heroin addiction in the United States. Drug Alcohol Depend. 2001;61:195–206. doi: 10.1016/s0376-8716(00)00162-9. [DOI] [PubMed] [Google Scholar]
- Martin BR, Mechoulam R, Razdan RK. Discovery and characterization of endogenous cannabinoids. Life Sci. 1999;65:573–595. doi: 10.1016/s0024-3205(99)00281-7. [DOI] [PubMed] [Google Scholar]
- Mas-Nieto M, Pommier B, Tzavara ET, Caneparo A, Da Nascimento S, Le Fur G, Roques BP, Noble F. Reduction of opioid dependence by the CB(1) antagonist SR141716A in mice: evaluation of the interest in pharmacotherapy of opioid addiction. Br J Pharmacol. 2001;132:1809–1816. doi: 10.1038/sj.bjp.0703990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massi P, Vaccani A, Romorini S, Parolaro D. Comparative characterization in the rat of the interaction between cannabinoids and opiates for their immunosuppressive and analgesic effects. J Neuroimmunol. 2001;117:116–124. doi: 10.1016/s0165-5728(01)00323-x. [DOI] [PubMed] [Google Scholar]
- McLaughlin RJ, Hill MN, Gorzalka BB. Monoaminergic neurotransmission contributes to cannabinoid-induced activation of the hypothalamic-pituitary-adrenal axis. Eur J Pharmacol. 2009;624:71–76. doi: 10.1016/j.ejphar.2009.09.055. [DOI] [PubMed] [Google Scholar]
- Meader N. A comparison of methadone, buprenorphine and alpha(2) adrenergic agonists for opioid detoxification: a mixed treatment comparison meta-analysis. Drug Alcohol Depend. 2010;108:110–114. doi: 10.1016/j.drugalcdep.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Fride E, Di Marzo V. Endocannabinoids. Eur J Pharmacol. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- Mendiguren A, Pineda J. Cannabinoids enhance N-methyl-D-aspartate-induced excitation of locus coeruleus neurons by CB1 receptors in rat brain slices. Neurosci Lett. 2004;363:1–5. doi: 10.1016/j.neulet.2004.02.073. [DOI] [PubMed] [Google Scholar]
- Mendiguren A, Pineda J. CB(1) cannabinoid receptors inhibit the glutamatergic component of KCl-evoked excitation of locus coeruleus neurons in rat brain slices. Neuropharmacology. 2006a doi: 10.1016/j.neuropharm.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Mendiguren A, Pineda J. Systemic effect of cannabinoids on the spontaneous firing rate of locus coeruleus neurons in rats. Eur J Pharmacol. 2006b;534:83–88. doi: 10.1016/j.ejphar.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Mendiguren A, Pineda J. CB(1) cannabinoid receptors inhibit the glutamatergic component of KCl-evoked excitation of locus coeruleus neurons in rat brain slices. Neuropharmacology. 2007;52:617–625. doi: 10.1016/j.neuropharm.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Moyse E, Marcel D, Leonard K, Beaudet A. Electron microscopic distribution of mu opioid receptors on noradrenergic neurons of the locus coeruleus. Eur J Neurosci. 1997;9:128–139. doi: 10.1111/j.1460-9568.1997.tb01361.x. [DOI] [PubMed] [Google Scholar]
- Muntoni AL, Pillolla G, Diana M, Pani L, Gessa GL. The cannabinoid Win 55,212-2 stimulates locus coeruleus noradrenergic neurons in rats. 2001:51. [Google Scholar]
- Muntoni AL, Pillolla G, Melis M, Perra S, Gessa GL, Pistis M. Cannabinoids modulate spontaneous neuronal activity and evoked inhibition of locus coeruleus noradrenergic neurons. Eur J Neurosci. 2006;23:2385–2394. doi: 10.1111/j.1460-9568.2006.04759.x. [DOI] [PubMed] [Google Scholar]
- Musshoff F, Trafkowski J, Lichtermann D, Madea B. Comparison of urine results concerning co-consumption of illicit heroin and other drugs in heroin and methadone maintenance programs. Int J Legal Med. 2009 doi: 10.1007/s00414-009-0361-8. [DOI] [PubMed] [Google Scholar]
- Nava F, Manzato E, Lucchini A. Chronic cannabis use does not affect the normalization of hypothalamic-pituitary-adrenal (HPA) axis induced by methadone in heroin addicts. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1089–1094. doi: 10.1016/j.pnpbp.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Navarro M, Carrera MR, Fratta W, Valverde O, Cossu G, Fattore L, Chowen JA, Gomez R, del Arco I, Villanua MA, Maldonado R, Koob GF, Rodriguez de Fonseca F. Functional interaction between opioid and cannabinoid receptors in drug self-administration. J Neurosci. 2001;21:5344–5350. doi: 10.1523/JNEUROSCI.21-14-05344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Erdos JJ, Terwilliger R, Duman RS, Tallman JF. Regulation of G proteins by chronic morphine in the rat locus coeruleus. Brain Res. 1989;476:230–239. doi: 10.1016/0006-8993(89)91243-2. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. J Neurosci. 1992;12:2439–2450. doi: 10.1523/JNEUROSCI.12-07-02439.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Cellular responses to chronic treatment with drugs of abuse. Crit Rev Neurobiol. 1993;7:23–39. [PubMed] [Google Scholar]
- Nestler EJ, Alreja M, Aghajanian GK. Molecular and cellular mechanisms of opiate action: studies in the rat locus coeruleus. Brain Res Bull. 1994;35:521–528. doi: 10.1016/0361-9230(94)90166-x. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Alreja M, Aghajanian GK. Molecular control of locus coeruleus neurotransmission. Biol Psychiatry. 1999;46:1131–1139. doi: 10.1016/s0006-3223(99)00158-4. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular neurobiology of addiction. Am J Addict. 2001;10:201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- Nirenberg TD, Liepman MR, Cellucci T, Swift RM, Sirota AD. Cannabis versus other illicit drug use among methadone maintenance patients. Psychology of Addictive Behaviors. 1996;10:222–227. [Google Scholar]
- Nixon LN. Cannabis use and treatment outcome in methadone maintenance. Addiction. 2003;98:1321–1322. doi: 10.1046/j.1360-0443.2003.00532.x. author reply 1322–1323. [DOI] [PubMed] [Google Scholar]
- North RA, Williams JT, Surprenant A, Christie MJ. Mu and delta receptors belong to a family of receptors that are coupled to potassium channels. Proc Natl Acad Sci USA. 1987;84:5487–5491. doi: 10.1073/pnas.84.15.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurco DN, Kinlock TW, Hanlon TE, Ball JC. Nonnarcotic drug use over an addiction career--a study of heroin addicts in Baltimore and New York City. Compr Psychiatry. 1988;29:450–459. doi: 10.1016/0010-440x(88)90060-0. [DOI] [PubMed] [Google Scholar]
- OAS . In: Analyses of Substance Abuse and Treatment Need Issues. Office of Applied Studies, S. A. a. M. H. S. A., editor. Substance Abuse and Mental Health Services Administration; 1998. [Google Scholar]
- OAS . Results from the 2004 National Survey on Drug Use and Health: National Findings. In: Office of Applied Studies, S. A. a. M. H. S. A., editor. National Survey on Drug Use and Health. Substance Abuse and Mental Health Services Administration, U.S. Department of Health and Human Services; 2005. [Google Scholar]
- OAS . Results from the 2008 National Survey on Drug Use and Health: National Findings. In: Office of Applied Studies, S. A. a. M. H. S. A., editor. National Survey on Drug Use and Health. Substance Abuse and Mental Health Services Administration, U.S. Department of Health and Human Services; 2009. [Google Scholar]
- Onaivi ES. Cannabinoid receptors in brain: pharmacogenetics, neuropharmacology, neurotoxicology, and potential therapeutic applications. Int Rev Neurobiol. 2009;88:335–369. doi: 10.1016/S0074-7742(09)88012-4. [DOI] [PubMed] [Google Scholar]
- Oropeza VC, Mackie K, Van Bockstaele EJ. Cannabinoid receptors are localized to noradrenergic axon terminals in the rat frontal cortex. Brain Res. 2007;1127:36–44. doi: 10.1016/j.brainres.2006.09.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oropeza VC, Page ME, Van Bockstaele EJ. Systemic administration of WIN 55,212-2 increases norepinephrine release in the rat frontal cortex. Brain Res. 2005;1046:45–54. doi: 10.1016/j.brainres.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Page ME, Valentino RJ. Locus coeruleus activation by physiological challenges. Brain Res Bull. 1994;35:557–560. doi: 10.1016/0361-9230(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Page ME, Oropeza VC, Sparks SE, Qian Y, Menko AS, Van Bockstaele EJ. Repeated cannabinoid administration increases indices of noradrenergic activity in rats. Pharmacol Biochem Behav. 2007;86:162–168. doi: 10.1016/j.pbb.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ME, Oropeza VC, Van Bockstaele EJ. Local administration of a cannabinoid agonist alters norepinephrine efflux in the rat frontal cortex. Neurosci Lett. 2008;431:1–5. doi: 10.1016/j.neulet.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolaro D, Rubino T, Vigano D, Massi P, Guidali C, Realini N. Cellular mechanisms underlying the interaction between cannabinoid and opioid system. Curr Drug Targets. 2010;11:393–405. doi: 10.2174/138945010790980367. [DOI] [PubMed] [Google Scholar]
- Pasternak GW. Molecular biology of opioid analgesia. J Pain Symptom Manage. 2005;29:S2–9. doi: 10.1016/j.jpainsymman.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Patton GC, Coffey C, Carlin JB, Degenhardt L, Lynskey M, Hall W. Cannabis use and mental health in young people: cohort study. Bmj. 2002;325:1195–1198. doi: 10.1136/bmj.325.7374.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pert CB, Kuhar MJ, Snyder SH. Opiate receptor: autoradiographic localization in rat brain. Proc Natl Acad Sci USA. 1976;73:3729–3733. doi: 10.1073/pnas.73.10.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Kash TL, Rodriguez JJ, MacKie K. Compartment-specific localization of cannabinoid 1 (CB1) and mu-opioid receptors in rat nucleus accumbens. Neuroscience. 2004;127:101–112. doi: 10.1016/j.neuroscience.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Colago EE. Presence of mu-opioid receptors in targets of efferent projections from the central nucleus of the amygdala to the nucleus of the solitary tract. Synapse. 1999;33:141–152. doi: 10.1002/(SICI)1098-2396(199908)33:2<141::AID-SYN4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Piomelli D, Beltramo M, Giuffrida A, Stella N. Endogenous cannabinoid signaling. Neurobiol Dis. 1998;5:462–473. doi: 10.1006/nbdi.1998.0221. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The endocannabinoid system: a drug discovery perspective. Curr Opin Investig Drugs. 2005;6:672–679. [PubMed] [Google Scholar]
- Pugh G, Jr., Abood ME, Welch SP. Antisense oligodeoxynucleotides to the kappa-1 receptor block the antinociceptive effects of delta 9-THC in the spinal cord. Brain Res. 1995;689:157–158. doi: 10.1016/0006-8993(95)00560-d. [DOI] [PubMed] [Google Scholar]
- Pugh G, Jr., Smith PB, Dombrowski DS, Welch SP. The role of endogenous opioids in enhancing the antinociception produced by the combination of delta 9-tetrahydrocannabinol and morphine in the spinal cord. J Pharmacol Exp Ther. 1996;279:608–616. [PubMed] [Google Scholar]
- Pugh G, Jr., Mason DJ, Jr., Combs V, Welch SP. Involvement of dynorphin B in the antinociceptive effects of the cannabinoid CP55,940 in the spinal cord. J Pharmacol Exp Ther. 1997;281:730–737. [PubMed] [Google Scholar]
- Raby WN, Carpenter KM, Rothenberg J, Brooks AC, Jiang H, Sullivan M, Bisaga A, Comer S, Nunes EV. Intermittent marijuana use is associated with improved retention in naltrexone treatment for opiate-dependence. Am J Addict. 2009;18:301–308. doi: 10.1080/10550490902927785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez BG, Blazquez C, Gomez del Pulgar T, Guzman M, de Ceballos ML. Prevention of Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005;25:1904–1913. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen K, Aghajanian GK. Withdrawal-induced activation of locus coeruleus neurons in opiate-dependent rats: attenuation by lesions of the nucleus paragigantocellularis. Brain Res. 1989;505:346–350. doi: 10.1016/0006-8993(89)91466-2. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Beitner-Johnson DB, Krystal JH, Aghajanian GK, Nestler EJ. Opiate withdrawal and the rat locus coeruleus: behavioral, electrophysiological, and biochemical correlates. J Neurosci. 1990;10:2308–2317. doi: 10.1523/JNEUROSCI.10-07-02308.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisfield GM, Wasan AD, Jamison RN. The prevalence and significance of cannabis use in patients prescribed chronic opioid therapy: a review of the extant literature. Pain Med. 2009;10:1434–1441. doi: 10.1111/j.1526-4637.2009.00726.x. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Glaser JD, Van Bockstaele EJ. Ultrastructural evidence for co-localization of corticotropin-releasing factor receptor and mu-opioid receptor in the rat nucleus locus coeruleus. Neurosci Lett. 2007;413:216–221. doi: 10.1016/j.neulet.2006.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BA, Chavkin C, van Bockstaele EJ. Subcellular targeting of kappa-opioid receptors in the rat nucleus locus coeruleus. J Comp Neurol. 2009a;512:419–431. doi: 10.1002/cne.21880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BA, Rosario JC, Piana PM, Van Bockstaele EJ. Cannabinoid modulation of cortical adrenergic receptors and transporters. J Neurosci Res. 2009b;87:3671–3678. doi: 10.1002/jnr.22158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes B, Szot P, Sikkema C, Cathel A, Kirby L, Bockstaele EV. Stress-induced sensitization of cortical adrenergic receptors following a history of cannabinoid exposure. Experimental Neurology. 2012 doi: 10.1016/j.expneurol.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios C, Gomes I, Devi LA. mu opioid and CB1 cannabinoid receptor interactions: reciprocal inhibition of receptor signaling and neuritogenesis. Br J Pharmacol. 2006;148:387–395. doi: 10.1038/sj.bjp.0706757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios CD, Jordan BA, Gomes I, Devi LA. G-protein-coupled receptor dimerization: modulation of receptor function. Pharmacol Ther. 2001;92:71–87. doi: 10.1016/s0163-7258(01)00160-7. [DOI] [PubMed] [Google Scholar]
- Roberts JD, Gennings C, Shih M. Synergistic affective analgesic interaction between delta-9-tetrahydrocannabinol and morphine. Eur J Pharmacol. 2006;530:54–58. doi: 10.1016/j.ejphar.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Robledo P. Cannabinoids, opioids and MDMA: neuropsychological interactions related to addiction. Curr Drug Targets. 2010;11:429–439. doi: 10.2174/138945010790980330. [DOI] [PubMed] [Google Scholar]
- Robson P. Therapeutic aspects of cannabis and cannabinoids. Br J Psychiatry. 2001;178:107–115. doi: 10.1192/bjp.178.2.107. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Mackie K, Pickel VM. Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat Caudate putamen nucleus. J Neurosci. 2001;21:823–833. doi: 10.1523/JNEUROSCI.21-03-00823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenfeld R. Type I cannabinoid receptor trafficking: all roads lead to lysosome. Traffic 2011. 2010 Jan;12(1):12–8. doi: 10.1111/j.1600-0854.2010.01130.x. doi: 10.1111/j.1600-0854.2010.01130.x. [DOI] [PubMed] [Google Scholar]
- Rubino T, Massi P, Vigano D, Fuzio D, Parolaro D. Long-term treatment with SR141716A, the CB1 receptor antagonist, influences morphine withdrawal syndrome. Life Sci. 2000;66:2213–2219. doi: 10.1016/s0024-3205(00)00547-6. [DOI] [PubMed] [Google Scholar]
- Rubino T, Parolaro D. Long lasting consequences of cannabis exposure in adolescence. Mol Cell Endocrinol. 2008;286:S108–113. doi: 10.1016/j.mce.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Salio C, Fischer J, Franzoni MF, Mackie K, Kaneko T, Conrath M. CB1-cannabinoid and mu-opioid receptor co-localization on postsynaptic target in the rat dorsal horn. Neuroreport. 2001;12:3689–3692. doi: 10.1097/00001756-200112040-00017. [DOI] [PubMed] [Google Scholar]
- Sands SA, Strong R, Corbitt J, Morilak DA. Effects of acute restraint stress on tyrosine hydroxylase mRNA expression in locus coeruleus of Wistar and Wistar-Kyoto rats. Brain Res Mol Brain Res. 2000;75:1–7. doi: 10.1016/s0169-328x(99)00255-7. [DOI] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Saxon AJ, Calsyn DA, Blaes PA, Haver VM, Greenberg DM. Marijuana use by methadone maintenance patients. NIDA Res Monogr. 1990;105:306–307. [PubMed] [Google Scholar]
- Saxon AJ, Calsyn DA, Greenberg D, Blaes P, Haver VM, Stanton V. Urine screening for marijuana among methadone-maintained patients. American Journal on Addictions. 1993;2:207–211. [Google Scholar]
- Scavone JL, Van Bockstaele EJ. Mu-opioid receptor redistribution in the locus coeruleus upon precipitation of withdrawal in opiate-dependent rats. Anat Rec (Hoboken) 2009;292:401–411. doi: 10.1002/ar.20860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scavone JL, Mackie K, Van Bockstaele EJ. Characterization of cannabinoid-1 receptors in the locus coeruleus: relationship with mu-opioid receptors. Brain Res. 2010;1312:18–31. doi: 10.1016/j.brainres.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scavone JL, Sterling R, Weinstein SP, Van Bockstaele E. Impact of Cannabis Use during Stabilization on Methadone Maintenance Treatment. Am J Addict. 2013 doi: 10.1111/j.1521-0391.2013.12044.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffelmeer AN, Hogenboom F, Wardeh G, De Vries TJ. Interactions between CB1 cannabinoid and mu opioid receptors mediating inhibition of neurotransmitter release in rat nucleus accumbens core. Neuropharmacology. 2006;51:773–781. doi: 10.1016/j.neuropharm.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Shinba T, Ozawa N, Yoshii M, Yamamoto K. Delayed increase of brain noradrenaline after acute footshock stress in rats. Neurochemical research. 2010;35:412–417. doi: 10.1007/s11064-009-0070-1. [DOI] [PubMed] [Google Scholar]
- Singh ME, Verty AN, Price I, McGregor IS, Mallet PE. Modulation of morphine-induced Fos-immunoreactivity by the cannabinoid receptor antagonist SR 141716. Neuropharmacology. 2004;47:1157–1169. doi: 10.1016/j.neuropharm.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Smith PA, Selley DE, Sim-Selley LJ, Welch SP. Low dose combination of morphine and delta9-tetrahydrocannabinol circumvents antinociceptive tolerance and apparent desensitization of receptors. Eur J Pharmacol. 2007;571:129–137. doi: 10.1016/j.ejphar.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Goldberg SR. Exposure to delta-9-tetrahydrocannabinol (THC) increases subsequent heroin taking but not heroin's reinforcing efficacy: a self-administration study in rats. Neuropsychopharmacology. 2004;29:1301–1311. doi: 10.1038/sj.npp.1300431. [DOI] [PubMed] [Google Scholar]
- Steffens S, Veillard NR, Arnaud C, Pelli G, Burger F, Staub C, Karsak M, Zimmer A, Frossard JL, Mach F. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature. 2005;434:782–786. doi: 10.1038/nature03389. [DOI] [PubMed] [Google Scholar]
- Steiner MA, Marsicano G, Wotjak CT, Lutz B. Conditional cannabinoid receptor type 1 mutants reveal neuron subpopulation-specific effects on behavioral and neuroendocrine stress responses. Psychoneuroendocrinology. 2008;33:1165–1170. doi: 10.1016/j.psyneuen.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Steiner MA, Wotjak CT. Role of the endocannabinoid system in regulation of the hypothalamic-pituitary-adrenocortical axis. Prog Brain Res. 2008;170:397–432. doi: 10.1016/S0079-6123(08)00433-0. [DOI] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Norton FE, Guyenet PG. Autonomic areas of rat brain exhibit increased Fos-like immunoreactivity during opiate withdrawal in rats. Brain Res. 1993;624:19–28. doi: 10.1016/0006-8993(93)90055-r. [DOI] [PubMed] [Google Scholar]
- Strang J, Bearn J, Gossop M. Lofexidine for opiate detoxification: review of recent randomised and open controlled trials. Am J Addict. 1999;8:337–348. doi: 10.1080/105504999305749. [DOI] [PubMed] [Google Scholar]
- Strassels SA. Economic burden of prescription opioid misuse and abuse. J Manag Care Pharm. 2009;15:556–562. doi: 10.18553/jmcp.2009.15.7.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Swann AC, Elsworth JD, Charney DS, Jablons DM, Roth RH, Redmond DE, Jr., Maas JW. Brain catecholamine metabolites and behavior in morphine withdrawal. Eur J Pharmacol. 1982;86:167–175. doi: 10.1016/0014-2999(82)90314-4. [DOI] [PubMed] [Google Scholar]
- Szabo B, Schlicker E. Effects of cannabinoids on neurotransmission. Handb Exp Pharmacol. 2005:327–365. doi: 10.1007/3-540-26573-2_11. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Elsworth JD, Garcia EJ, Grant SJ, Roth RH, Redmond DE., Jr. Clonidine infusions into the locus coeruleus attenuate behavioral and neurochemical changes associated with naloxone-precipitated withdrawal. Psychopharmacology (Berl) 1988;96:121–134. doi: 10.1007/BF02431544. [DOI] [PubMed] [Google Scholar]
- Tempel A, Zukin RS. Neuroanatomical patterns of the mu, delta, and kappa opioid receptors of rat brain as determined by quantitative in vitro autoradiography. Proc Natl Acad Sci U S A. 1987;84:4308–4312. doi: 10.1073/pnas.84.12.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrillon S, Bouvier M. Roles of G-protein-coupled receptor dimerization. EMBO Rep. 2004;5:30–34. doi: 10.1038/sj.embor.7400052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham SM, Angus JA, Tudor EM, Wright CE. Synergistic and additive interactions of the cannabinoid agonist CP55, 940 with mu opioid receptor and alpha2-adrenoceptor agonists in acute pain models in mice. Br J Pharmacol. 2005;144:875–884. doi: 10.1038/sj.bjp.0706045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucci S. Addiction and pain: cannabinoid and opioid interactions. Curr Drug Targets. 2010;11:392. doi: 10.2174/138945010790980286. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL, Aston-Jones G. Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus. Brain Res. 1983;270:363–367. doi: 10.1016/0006-8993(83)90615-7. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL, Page ME. The locus coeruleus as a site for integrating corticotropin-releasing factor and noradrenergic mediation of stress responses. Ann N Y Acad Sci. 1993;697:173–188. doi: 10.1111/j.1749-6632.1993.tb49931.x. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Curtis AL, Page ME, Pavcovich LA, Florin-Lechner SM. Activation of the locus ceruleus brain noradrenergic system during stress: circuitry, consequences, and regulation. Adv Pharmacol. 1998a;42:781–784. doi: 10.1016/s1054-3589(08)60863-7. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Curtis AL, Page ME, Pavcovich LA, Florin-Lechner SM. Activation of the locus ceruleus brain noradrenergic system during stress: circuitry, consequences, and regulation. Adv Pharmacol. 1998b;42:781–784. doi: 10.1016/s1054-3589(08)60863-7. [DOI] [PubMed] [Google Scholar]
- Valentino R, Aston-Jones G. Physiological and Anatomical Determinants of Locus Coeruleus Discharge: Behavioral and Clinical Implications. American College of Neuropsychopharmacology Psychopharmacology. 2000 [Google Scholar]
- Valverde O, Noble F, Beslot F, Dauge V, Fournie-Zaluski MC, Roques BP. Delta9-tetrahydrocannabinol releases and facilitates the effects of endogenous enkephalins: reduction in morphine withdrawal syndrome without change in rewarding effect. Eur J Neurosci. 2001;13:1816–1824. doi: 10.1046/j.0953-816x.2001.01558.x. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EE, Cheng P, Moriwaki A, Uhl GR, Pickel VM. Ultrastructural evidence for prominent distribution of the mu-opioid receptor at extrasynaptic sites on noradrenergic dendrites in the rat nucleus locus coeruleus. J Neurosci. 1996a;16:5037–5048. doi: 10.1523/JNEUROSCI.16-16-05037.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EE, Moriwaki A, Uhl GR. Mu-opioid receptor is located on the plasma membrane of dendrites that receive asymmetric synapses from axon terminals containing leucine-enkephalin in the rat nucleus locus coeruleus. J Comp Neurol. 1996b;376:65–74. doi: 10.1002/(SICI)1096-9861(19961202)376:1<65::AID-CNE4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Commons K, Pickel VM. Delta-opioid receptor is present in presynaptic axon terminals in the rat nucleus locus coeruleus: relationships with methionine5-enkephalin. J Comp Neurol. 1997;388:575–586. doi: 10.1002/(sici)1096-9861(19971201)388:4<575::aid-cne6>3.3.co;2-v. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Peoples J, Menko AS, McHugh K, Drolet G. Decreases in endogenous opioid peptides in the rat medullo-coerulear pathway after chronic morphine treatment. J Neurosci. 2000;20:8659–8666. doi: 10.1523/JNEUROSCI.20-23-08659.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Commons KG. Internalization of mu-opioid receptors produced by etorphine in the rat locus coeruleus. Neuroscience. 2001;108:467–477. doi: 10.1016/s0306-4522(01)00426-2. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Menko AS, Drolet G. Neuroadaptive responses in brainstem noradrenergic nuclei following chronic morphine exposure. Mol Neurobiol. 2001;23:155–171. doi: 10.1385/mn:23:2-3:155. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Qian Y, Sterling RC, Page ME. Low dose naltrexone administration in morphine dependent rats attenuates withdrawal-induced norepinephrine efflux in forebrain. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1048–1056. doi: 10.1016/j.pnpbp.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Reyes BA, Valentino RJ. The locus coeruleus: A key nucleus where stress and opioids intersect to mediate vulnerability to opiate abuse. Brain Res. 2010;1314:162–174. doi: 10.1016/j.brainres.2009.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vela G, Ruiz-Gayo M, Fuentes JA. Anandamide decreases naloxone-precipitated withdrawal signs in mice chronically treated with morphine. Neuropharmacology. 1995;34:665–668. doi: 10.1016/0028-3908(95)00032-2. [DOI] [PubMed] [Google Scholar]
- Vigano D, Rubino T, Parolaro D. Molecular and cellular basis of cannabinoid and opioid interactions. Pharmacol Biochem Behav. 2005a;81:360–368. doi: 10.1016/j.pbb.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Vigano D, Rubino T, Vaccani A, Bianchessi S, Marmorato P, Castiglioni C, Parolaro D. Molecular mechanisms involved in the asymmetric interaction between cannabinoid and opioid systems. Psychopharmacology (Berl) 2005b;182:527–536. doi: 10.1007/s00213-005-0114-4. [DOI] [PubMed] [Google Scholar]
- Vigano D, Valenti M, Cascio MG, Di Marzo V, Parolaro D, Rubino T. Changes in endocannabinoid levels in a rat model of behavioural sensitization to morphine. Eur J Neurosci. 2004;20:1849–1857. doi: 10.1111/j.1460-9568.2004.03645.x. [DOI] [PubMed] [Google Scholar]
- Wasserman DA, Weinstein MG, Havassy BE, Hall SM. Factors associated with lapses to heroin use during methadone maintenance. Drug Alcohol Depend. 1998;52:183–192. doi: 10.1016/s0376-8716(98)00092-1. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Schroeder JP. There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology. 2007;32:1433–1451. doi: 10.1038/sj.npp.1301263. [DOI] [PubMed] [Google Scholar]
- Weinstein SP, Gottheil E, Sterling RC, DeMaria PA., Jr. Long-term methadone maintenance treatment: some clinical examples. J Subst Abuse Treat. 1993;10:277–281. doi: 10.1016/0740-5472(93)90075-d. [DOI] [PubMed] [Google Scholar]
- Weizman T, Gelkopf M, Melamed Y, Adelson M, Bleich A. Cannabis abuse is not a risk factor for treatment outcome in methadone maintenance treatment: a 1-year prospective study in an Israeli clinic. Aust N Z J Psychiatry. 2004;38:42–46. doi: 10.1046/j.1440-1614.2003.01296.x. [DOI] [PubMed] [Google Scholar]
- Welch SP. Interaction of the cannabinoid and opioid systems in the modulation of nociception. Int Rev Psychiatry. 2009;21:143–151. doi: 10.1080/09540260902782794. [DOI] [PubMed] [Google Scholar]
- Welch SP, Stevens DL. Antinociceptive activity of intrathecally administered cannabinoids alone, and in combination with morphine, in mice. J Pharmacol Exp Ther. 1992;262:10–18. [PubMed] [Google Scholar]
- Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS) J Psychoactive Drugs. 2003;35:253–259. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- Widnell KL, Russell DS, Nestler EJ. Regulation of expression of cAMP response element-binding protein in the locus coeruleus in vivo and in a locus coeruleus-like cell line in vitro. Proc Natl Acad Sci U S A. 1994;91:10947–10951. doi: 10.1073/pnas.91.23.10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Tzavara ET, Nomikos GG. A role for cannabinoid CB1 receptors in mood and anxiety disorders. Behav Pharmacol. 2005;16:315–331. doi: 10.1097/00008877-200509000-00005. [DOI] [PubMed] [Google Scholar]
- Wong ML, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P, Karp B, McCutcheon IE, Geracioti TD, Jr., DeBellis MD, Rice KC, Goldstein DS, Veldhuis JD, Chrousos GP, Oldfield EH, McCann SM, Gold PW. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:325–330. doi: 10.1073/pnas.97.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu GP, Van Bockstaele E, Reyes B, Bethea T, Valentino RJ. Chronic morphine sensitizes the brain norepinephrine system to corticotropin-releasing factor and stress. J Neurosci. 2004;24:8193–8197. doi: 10.1523/JNEUROSCI.1657-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Hagiwara Y, Tanaka H, Sugiura T, Waku K, Shoyama Y, Watanabe S, Yamamoto T. Endogenous cannabinoid, 2-arachidonoylglycerol, attenuates naloxone-precipitated withdrawal signs in morphine-dependent mice. Brain Res. 2001;909:121–126. doi: 10.1016/s0006-8993(01)02655-5. [DOI] [PubMed] [Google Scholar]