Abstract
Bisphenol A (BPA) is the backbone of polycarbonate plastic products and the epoxy resin lining of aluminum cans. Previous studies have shown that exposure to BPA decreases sex steroid hormone production in mouse antral follicles. The current study tests the hypothesis that BPA first decreases the expression levels of the steroidogenic enzyme cytochrome P450 side-chain cleavage (Cyp11a1) and steroidogenic acute regulatory protein (StAR) in mouse antral follicles, leading to a decrease in sex steroid hormone production in vitro. Further, the current study tests the hypothesis that these effects are acute and reversible after removal of BPA. Exposure to BPA (10μg/mL and 100μg/mL) significantly decreased expression of Cyp11a1 and StAR beginning at 18h and 72h, respectively, compared to controls. Exposure to BPA (10μg/mL and 100μg/mL) significantly decreased progesterone levels beginning at 24h and decreased androstenedione, testosterone, and estradiol levels at 72h and 96h compared to controls. Further, after removing BPA from the culture media at 20h, expression of Cyp11a1 and progesterone levels were restored to control levels by 48h and 72h, respectively. Additionally, expression of StAR and levels of androstenedione, testosterone, and estradiol never decreased compared to controls. These data suggest that BPA acutely decreases expression of Cyp11a1 as early as 18h and this reduction in Cyp11a1 may lead to a decrease in progesterone production by 24h, followed by a decrease in androstenedione, testosterone, and estradiol production and expression of StAR at 72h. Therefore, BPA exposure likely targets Cyp11a1 and steroidogenesis, but these effects are reversible with removal of BPA exposure.
Keywords: Bisphenol A, antral follicle, steroidogenesis, Cyp11a1, reversible
Introduction
Bisphenol A (BPA) is a mass-produced chemical used in the manufacturing of polycarbonate plastics and epoxy resins, as well other commonly used materials such as receipt paper and dental sealants. Humans are constantly exposed to BPA because it is present in food and drinks after leaching from polycarbonate containers or aluminum cans. Various studies have detected BPA in human blood, urine, fat, mammary tissue, and the placenta (Calafat et al., 2005; Ikezuki et al., 2002;Ouchi et al., 2002; Vandenberg et al., 2007). BPA can also reach the ovary and has been found in aspirated antral fluid of women undergoing in vitro fertilization treatment (Ikezuki et al., 2002). Once in the body, BPA can act as an endocrine disruptor, impairing processes such as sexual development (Kubo et al., 2003; Vandenberg et al., 2006), behavior (Fujimoto et al., 2006), and reproductive functioning. Few studies, however, have examined the effects of BPA on the ovary, and even fewer studies have examined the effects of BPA on adult antral follicles.
The follicle is the functional unit of the ovary. There are various stages of follicles that develop in cycles over the reproductive lifespan of a female: primordial, primary, pre-antral/secondary, and antral (Williams et al., 2012). The antral follicles are the main producers of sex steroid hormones, which are required for normal reproductive function as well as overall female health. Estradiol, for example, is required for estrous cyclicity (Glidewell-Kenney et al., 2007), proper bone health (Bagur et al., 1992), cardiovascular health (Hu FB et al., 1999), and brain functioning (Birge SJ, 1998). While the adrenal glands produce some sex steroid hormones, the majority of hormones in the female are produced by the follicles in the ovary.
Estradiol is the terminal sex steroid hormone produced in the estradiol biosynthesis pathway. In this pathway, cholesterol is brought into the mitochondria of thecal cells by steroidogenic acute regulatory protein (StAR), where it is metabolized into pregnenolone by cytochrome P450 side chain cleavage (Cyp11a1). Both StAR and Cyp11a1 are considered rate-limiting factors for steroidogenesis because their activities provide the necessary precursor for the rest of the hormones in the pathway, pregnenolone (LaVoie and King, 2009; Stocco and Clark, 1996b). In antral follicles, pregnenolone is further converted into hormones such as progesterone, androstenedione, and testosterone in the thecal cells, and then converted to estradiol in the granulosa cells by various enzymes in the pathway, such as 3β-hydroxysteroid dehydrogenase, 17β-hydroxysteroid dehydrogenase, and cytochrome P450 aromatase.
Follicular development to mature antral follicles and ovulation is mediated by positive and negative feedback of hormones throughout the menstrual or estrous cycle (Rajkovic et al., 2006). Further, uterine readiness for implantation and maintenance of pregnancy are dependent on proper hormonal signals (Cha et al., 2012; LaVoie and King, 2009). Thus, disruption of steroidogenesis and hormones will have deleterious effects on successful reproduction.
We previously have shown that exposure of antral follicles to BPA (10μg/mL and 100μg/mL) decreases levels of progesterone, dehydroepiandrosteone, androstenedione, estrone, testosterone, and estradiol produced by antral follicles after 120h in culture compared to controls, while a lower dose of BPA (1μg/mL) had no effect on hormone levels (Peretz et al. 2011). In addition, our previous studies have shown that BPA (10μg/mL and 100μg/mL) decreases expression of StAR and Cyp11a1 after 120h in culture, but it does not affect the other enzymes in the estradiol biosynthesis pathway compared to controls. We further confirmed this by showing that co-culturing BPA-treated follicles with pregnenolone protected the follicles from BPA-induced hormone inhibition.
Previous studies, however, did not determine the time-course of BPA-induced inhibition of steroidogenesis. Thus, we do not know how rapidly BPA inhibits steroidogenesis. Further, previous studies did not determine whether BPA first inhibits StAR or Cyp11a1 and how this inhibition relates to the observed reduction in hormone levels. Finally, previous studies only examined the effects of chronic exposure to BPA on steroidogenic gene expression and hormone levels. Thus, we do not know if acute exposure to BPA has similar effects to those observed with chronic exposure and whether the effects of BPA on the antral follicles are reversible. Therefore, the goals of the current study were to: 1) determine how quickly BPA exposure inhibits expression of steroidogenic enzymes and sex steroid hormone levels in mouse antral follicles, 2) determine whether BPA exposure first targets StAR or Cyp11a1, and 3) determine if the effects of BPA on the antral follicle are reversible. We specifically tested the hypothesis that BPA exposure rapidly inhibits StAR and Cyp11a1, leading to rapid decline in sex steroid hormone levels. We also tested the hypothesis that the effects of BPA on StAR and Cyp11a1 expression and hormone levels are reversible.
Methods
Chemicals
BPA powder (99%) was purchased from Sigma-Aldrich (St. Louis, MO). A stock solution of BPA was dissolved and diluted in the vehicle dimethyl sulfoxide (DMSO; Sigma-Aldrich) to achieve BPA treatment concentrations of 1.3, 13.3 and 133mg/mL for final working concentrations of 1.0, 10, and 100μg/mL BPA in the culture media. Using these treatment concentrations allowed each working concentration to contain the same final concentration of vehicle (0.075% DMSO).
The concentrations chosen were based on previous studies, which indicate that 1 and 10μg/mL BPA do not inhibit follicle growth or induce atresia, but that 10μg/mL BPA decreases hormone levels (Peretz et al., 2011). Thus, at 10μg/mL BPA, the observed effects on hormone production are due to direct effects on steroidogenesis and not due to inhibited follicle growth or increased atresia.
Animals
Adult cycling CD-1 mice were purchased from Charles River (Wilmington, MA) and allowed to acclimate to the facility for at least 5 days before use. The mice were housed at the University of Illinois at Urbana-Champaign, Veterinary Medicine Animal Facility. Food (Harlan Teklad: 8626) and water were provided for ad libitum consumption. Temperature was maintained at 22±1°C and animals were subjected to 12h light-dark cycles. The Institutional Animal Use and Care Committee at the University of Illinois at Urbana-Champaign approved all procedures involving animal care, euthanasia, and tissue collections.
Time-Course Cultures
Female CD-1 mice were euthanized on postnatal day (PND) 32–35 and their ovaries removed using aseptic technique. Antral follicles were mechanically isolated from the ovary based on relative size (250–400 μm), cleaned of interstitial tissue using fine watchmaker forceps, individually placed in wells of a 96-well culture plate, and covered with un-supplemented α-minimal essential medium (α-MEM) prior to treatment. Sufficient numbers of antral follicles for statistical power were isolated from unprimed mouse ovaries; follicles from 2–3 mice were isolated per experiment providing approximately 20–40 antral follicles from each mouse. Each experiment contained a minimum of 8–12 follicles per treatment group. Doses of vehicle control (DMSO) and BPA (1, 10, and 100μg/mL) were individually prepared in α-MEM supplemented media. Supplemented α-MEM was prepared with: 1% ITS (10 ng/ml insulin, 5.5 ng/ml transferrin, 5.5 ng/ml selenium), 100 U/ml penicillin, 100 mg/ml streptomycin, 5 IU/ml human recombinant follicle-stimulating hormone (FSH; Dr. A. F. Parlow, National Hormone and Peptide Program, Harbor- UCLA Medical Center, Torrance, CA), and 5% fetal calf serum (Atlanta Biologicals, Lawrenceville, GA). An equal volume of chemical was added for each dose to control for the amount of vehicle in each preparation (0.75 μL/mL of media: BPA treatments; 1.0 μL/mL of media: DMSO treatment). Antral follicles were cultured for 6h, 12h, 18h, 19h, 24h, 48h, 72h, or 96h in an incubator supplying 5% CO2 at 37°C. After cultures, follicles were snap-frozen in liquid nitrogen and subjected to quantitative PCR (qPCR) as described below. Media were collected and stored at −80°C until subjected to hormone assays as described below.
BPA Recovery Cultures
To evaluate if removal of BPA from the culture media would rescue follicles from the BPA-induced inhibition of hormones and decreased expression of StAR and Cyp11a1, female CD-1 mice were euthanized on PND32-35 and follicles isolated by aseptic technique based on relative size (250–400μm). Individual follicles were cleaned of interstitial tissue using fine watchmaker forceps and placed in wells of a 96-well culture plate, as described above. Doses of vehicle control (DMSO) and BPA (1μg/mL and 10μg/mL) were individually prepared in supplemented α-MEM as described above. We did not use 100μg/mL BPA in these experiments because our previous study indicates that it causes atresia, whereas 1 and 10μg/mL BPA do not cause atresia (Peretz et al., 2011). Therefore, by omitting 100μg/mL BPA, we can distinguish direct effects of BPA on steroidogenesis from indirect effects on steroidogenesis due to BPA-induced atresia. Antral follicles were cultured with BPA for 20h in an incubator supplying 5% CO2 at 37°C. We cultured follicles in BPA for 20h because our experiments indicated that BPA inhibits expression of Cyp11a1 by 18h in culture and we wanted to test if removal of BPA could recover both the decrease in gene expression and decrease in hormone levels over time. At 20h, media from the BPA treated groups were removed and replaced with supplemented α-MEM with DMSO at a concentration identical to the vehicle control group. After the media change, follicles were cultured for 4, 28, 52, and 76 more hours to mimic the 24h, 48h, 72h, and 96h total culture times used in previous studies (Peretz et al., 2011).
Analysis of hormone levels
Media were collected after 20h, 24h, 48h, 72h, and 96h of follicle culture and subjected to enzyme-linked immunosorbent assays (ELISA) for measurement of progesterone, androstenedione, testosterone, and estradiol levels. ELISA kits were obtained from DRG Instruments GmbH (Marburg, Germany). The assays were run using the manufacturer’s instructions. All samples were run in duplicate and all intra- and inter-assay coefficients of variability were less than 10%.
Analysis of gene expression by quantitative real-time PCR
Antral follicles were cultured as described above. To evaluate when expression of StAR and Cyp11a1 were altered in the BPA time-course or recovery cultures, follicles were collected and snap-frozen in liquid nitrogen for qPCR analysis. Total RNA was extracted from follicles using the RNeasy Micro Kit (Qiagen, Inc., Valencia, CA) according to the manufacturer’s protocol. Reverse transcriptase generation of complementary DNA (cDNA) was performed with 0.5μg of total RNA using an iScript RT Kit (Bio-Rad Laboratories, Inc., Hercules, CA). qPCR was conducted using the CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories) and accompanying software (CFX Manager Software) according to the manufacturer’s instructions. The amount of PCR product generated was quantified by measuring a dye (SYBR Green) that fluoresces when bound to double-stranded DNA. A standard curve was generated from five serial dilutions of a compilation of the samples, thus allowing analysis of the amount of cDNA in the exponential phase. qPCR analysis was performed using 2μL cDNA, EvaGreen, and forward and reverse primers for StAR (NM_011485.4) and Cyp11a1 ( NM_019779.3). An initial incubation of 95°C for 10 minutes (min) was followed by 94°C for 10 seconds (s), annealing at 60°C for 10s, and extension at 72°C for 10s, for 40 cycles, followed by a final extension at 72°C for 10min. A melting curve was generated at 55–90°C to monitor the generation of a single product. The software also generated a standard curve made from cDNA of samples within the experiment. β-actin (Actb; NM_007393) was used as a reference gene for each sample. Final values were calculated and expressed as the ratio normalized to Actb. All analyses were performed in duplicate for at least three separate experiments.
Statistical Analysis
Data were expressed as means ± standard error of the mean (SEM), and multiple comparisons between experimental groups were made using generalized linear model (GLM) univariate analysis and/or analysis of variance (ANOVA) followed by Tukey’s or Games-Howell post hoc comparisons, when appropriate. At least three separate experiments were conducted for each treatment prior to data analysis. Statistical significance was assigned at p≤ 0.05.
Results
Effect of BPA on sex steroid hormone production over time
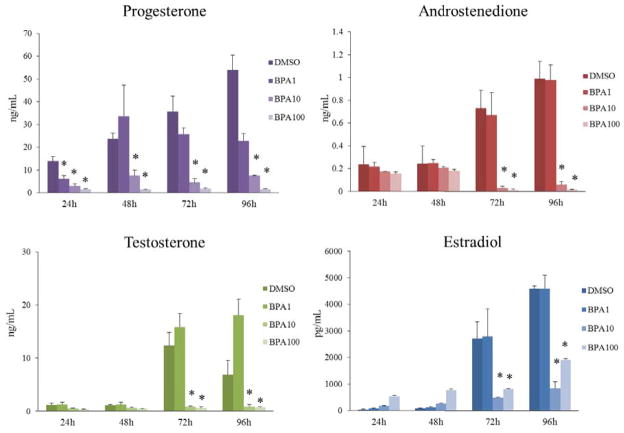
In previous studies, we have shown that BPA inhibits steroidogenesis at 120h in culture (Peretz et al., 2011), but we did not conduct time-course experiments and thus, did not know how rapidly BPA inhibits sex steroid hormone levels. Therefore, to test when BPA decreases levels of each hormone over time, we collected the media from the time-course study at 24h, 48, 72h, and 96h. At each time-point, levels of progesterone, androstenedione, testosterone, and estradiol were measured using ELISA. Exposure to BPA (10μg/mL and 100μg/mL) significantly decreased progesterone levels compared to control from 24–96h and significantly decreased androstenedione, testosterone, and estradiol levels compared to control from 72–96h (Figure 1). BPA (1μg/mL) significantly decreased progesterone levels at 24h, but did not significantly decrease progesterone levels compared to control at any other time-point. Further, BPA (1μg/mL) did not significantly decrease androstenedione, testosterone, or estradiol at any time-point compared to control.
Figure 1. Effect of BPA exposure on sex steroid hormone production over time.
After exposure of antral follicles to DMSO control or BPA (1–100μg/mL) for 24–96hrs in vitro, the media were collected at each time-point, pooled per treatment group, and subjected to enzyme-linked immunosorbent assays (ELISA) for progesterone, androstenedione, testosterone, and estradiol. The graphs represent means ± SEMs from at least three separate experiments. Asterisks (*) indicate p≤ 0.05 from DMSO control.
Effect of BPA on expression of StAR and Cyp11a1 over time
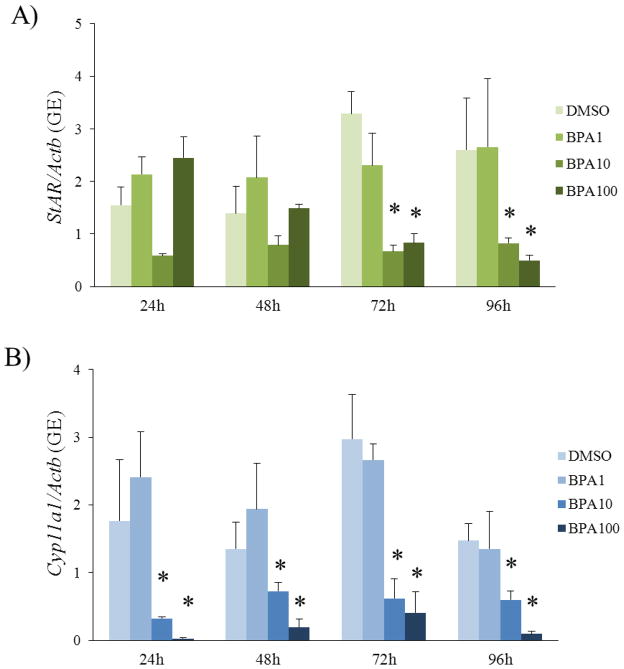
StAR and Cyp11a1 are the rate-limiting enzymes for the estradiol biosynthesis pathway in the follicles. StAR is required to transport cholesterol into the inner mitochondrial membrane where Cyp11a1 is present to convert cholesterol into pregnenolone, the sex steroid hormone precursor. If these are down-regulated, steroidogenesis will be impaired, preventing pregnenolone from being synthesized and metabolized into other hormones within the estradiol biosynthesis pathway. Previous studies have shown that BPA decreased StAR and Cyp11a1 expression at 120h in culture (Peretz et al., 2011). Therefore, we expanded on these findings by testing when these factors are initially decreased over time.
BPA (10 and 100μg/mL) significantly decreased expression of StAR compared to control beginning at 72h and continuing throughout 96h (Figure 2). Further, BPA (10 and 100μg/mL) significantly decreased expression of Cyp11a1 beginning at 24h and continuing throughout 96h (Figure 2). BPA (1μg/mL) did not significantly decrease expression of StAR and Cyp11a1 compared to control.
Figure 2. Effect of BPA exposure on expression of StAR and Cyp11a1 over time.
After exposure of antral follicles to DMSO control or BPA (1–100μg/mL) for 24–96hrs in vitro, the follicles were collected at each time-point and subjected to qPCR analysis for A) StAR and B) Cyp11a1 expression levels. All values were normalized to β-actin as a loading control. The graphs represent means ± SEMs from at least three separate experiments. Asterisks (*) indicate p≤ 0.05 from DMSO control.
Since BPA (10μg/mL) decreased expression of Cyp11a1 and progesterone at 24h, we wanted to determine if expression of Cyp11a1 was decreased any earlier, suggesting that BPA first decreases Cyp11a1 and then progesterone levels. To test if BPA decreased expression of Cyp11a1 earlier than 24h, we collected follicles from the time-course study at 6h, 12h, 18h, and 19h and subjected them to qPCR for Cyp11a1 gene expression analysis. Exposure to BPA (100μg/mL) decreased expression of Cyp11a1 as early as 6h compared to control (Figure 3). Exposure to BPA (10μg/mL) significantly decreased expression of Cyp11a1 as early as 18h compared to control (Figure 3).
Figure 3. Effect of short BPA exposure on expression of Cyp11a1.
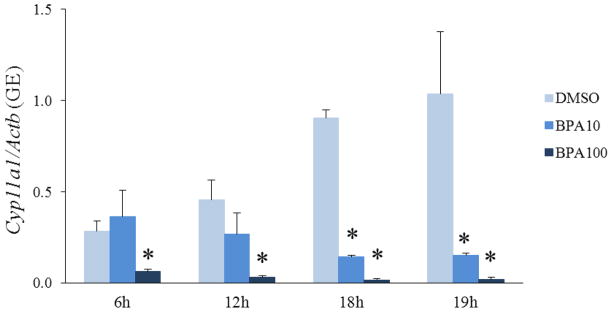
After exposure of antral follicles to DMSO control or BPA (10 or 100μg/mL) for 6–19hrs in vitro, the follicles were collected at each time-point and subjected to qPCR analysis for Cyp11a1 expression levels. All values were normalized to β-actin as a loading control. The graphs represent means ± SEMs from three separate experiments. Asterisks (*) indicate p≤ 0.05 from DMSO control.
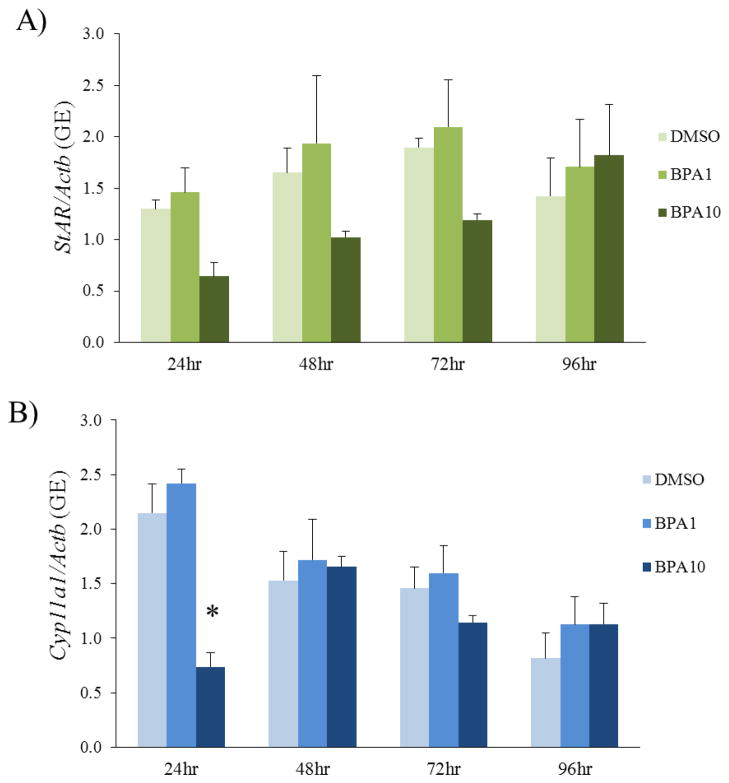
Effect of removing BPA from culture on expression of StAR and Cyp11a1
To determine if the effects of BPA are acute or chronic, we performed BPA recovery cultures. To test whether removing BPA from the cultures after 20h would restore expression of StAR and Cyp11a1 to control levels, media were collected at 24h, 48h, 72h, or 96h total times in culture (or 4h, 28h, 52h, or 76h after BPA removal, respectively) and subjected to qPCR. Acute BPA (10μg/mL) exposure for 20h did not significantly affect expression of StAR at any time-point in the culture (Figure 4). Acute BPA (10μg/mL) exposure for 20h significantly inhibited Cyp11a1 expression at 24h (Figure 4). However, removal of BPA (10μg/mL) from the culture resulted in normal expression of Cyp11a1 by 48h (Figure 4). Acute BPA (1μg/mL) exposure for 20h did not affect expression of StAR or Cyp11a1 at any time-point compared to control (Figure 4).
Figure 4. BPA recovery culture methods.
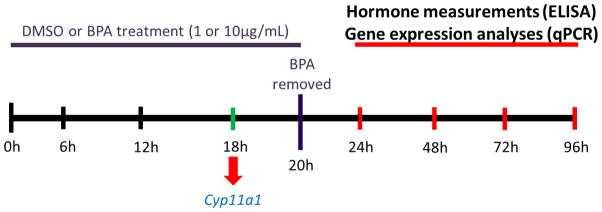
Antral follicles were exposed to DMSO control or BPA (1 or 10μg/mL) for 20h. After exposure for 20h, media from the BPA treated groups were removed and replaced with supplemented α-MEM with DMSO at a concentration identical to the vehicle control group. After the media change, follicles were then cultured for 4, 28, 52, or 76 more hours until total time in culture was 24h, 48h, 72h, or 96h. Follicles and media were collected at 24h, 48h, 72h, and 96h total culture time. At each time-point, hormone production and enzyme expression levels were measured.
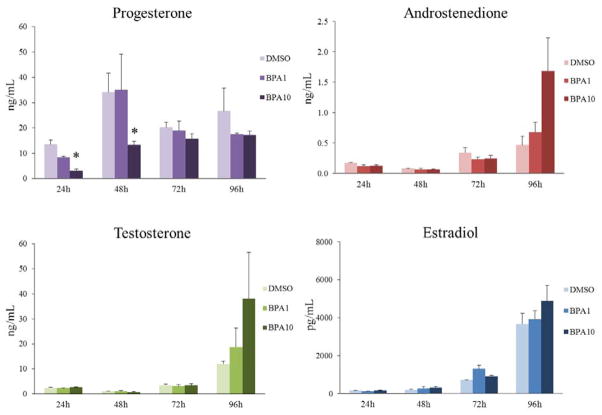
Effects of removing BPA from culture on sex steroid hormone production
To test whether removing BPA from the cultures after 20h would restore hormone levels to control levels, media were collected at 24h, 48h, 72h, or 96h total times in culture (or 4h, 28h, 52h, or 76h after BPA removal, respectively) and subjected to ELISA. Acute BPA (10μg/mL) exposure for 20h significantly decreased progesterone levels at 24h and 48h (Figure 5). However, removal of BPA (10μg/mL) from the culture resulted in normal levels of progesterone by 72h (Figure 5). Acute BPA (1μg/mL and 10μg/mL) exposure for 20h did not affect androstenedione, testosterone, or estradiol levels at any time-point compared to control (Figure 5).
Figure 5. Effect of BPA removal on expression of StAR and Cyp11a1 over time.
After exposure of antral follicles to DMSO control or BPA (1 or 10μg/mL) for 20h in vitro, the media were removed from follicles exposed to BPA (1 or 10μg/mL) and replaced with freshly prepared supplemented media with DMSO. Follicles were then cultured for 4, 28, 52, or 76 more hours until total time in culture was 24h, 48h, 72h, or 96h. At each time-point, follicles were collected and subjected to qPCR for analysis for A) StAR and B) Cyp11a1 mRNA expression levels. All values were normalized to beta-actin as a loading control. The graphs represent means ± SEMs from three separate experiments. Asterisk (*) indicates p≤ 0.05 from DMSO control.
Discussion
The results of our study indicate that BPA likely targets and decreases expression of Cyp11a1 prior to decreasing hormone production. BPA decreased Cyp11a1 beginning at 18h in culture and this continued throughout the culture period. Progesterone levels were decreased beginning at 24h and continuing throughout the culture period. Then, likely due to a lack of precursor hormone, androstenedione, testosterone, and estradiol levels decreased at 72h and 96h compared to controls. In addition, our data suggest that due to a lack of cholesterol conversion to pregnenolone without Cyp11a1, StAR is down-regulated at 72h to prevent a cholesterol build-up in the inner mitochondrial membrane. Further, these effects of BPA are reversible once BPA is removed from the culture media. BPA was removed after 20h of culture, long enough to ensure that expression of Cyp11a1 was decreased, and replaced by BPA-free media. Follicles then were cultured until 24h, 48h, 72h, or 96h total time in culture to evaluate if the follicles could recover Cyp11a1 expression, as well as produce normal levels of hormones. Expression of Cyp11a1 was recovered compared to control by 48h and expression of StAR never significantly decreased compared to control. Further, progesterone levels returned to control levels by 72h, and androstenedione, testosterone, and estradiol levels never decreased compared to controls. These data, in addition to the time-course results, suggest that BPA likely targets and decreases expression of Cyp11a1, which then mediates the decreases in hormone production.
The effects of BPA on steroidogenesis in antral follicles may be reversible because of how StAR and Cyp11a1 are regulated. Positive transcriptional regulation of StAR and Cyp11a1 in the ovary is controlled by cyclic-AMP dependent signaling pathways mediated through steroidogenic factor-1 and liver receptor homolog-1 (Amsterdam et al., 2003; Shih et al., 2011; Stocco and Clark, 1996a; Stocco and Clark, 1996b). Negative transcription regulation is controlled by factors such as prostaglandin F2α, lipopolysaccharide-induced endotoxemia, tumor necrosis factor α, activin A, extracellular signal-regulated kinase (ERK), and Dax-1 (LaVoie and King, 2009; Stocco and Clark, 1996b). Further, Dax-1 has been shown to inhibit SF-1 expression, which is required for StAR expression (Amsterdam et al., 2003; LaVoie and King, 2009), and to directly down-regulate Cyp11a1 expression (LaVoie and King, 2009). BPA has been shown to activate ERK and other cyclic-AMP dependent signaling factors in mouse hippocampal cells (Lee S et al., 2008) as well as to up-regulate expression of the gene encoding Dax-1, Nr0B1, in the brain and gonads of self-fertilizing fish (Rhee et al., 2011). As a result, BPA may decrease expression of Cyp11a1 and StAR through ERK or Dax-1 signaling pathways and second messenger signals, not by permanently impairing regulatory elements of each gene. Thus, removal of BPA may restore these pathways to their normal activities and, as our study indicates, the effects of BPA may be reversible. Further studies on ERK and Dax-1 on BPA-inhibited steroidogenesis in antral follicles are warranted.
Steroidogenesis is a tightly controlled, essential process for the development of antral follicles and female reproduction (Cain et al., 1995). All downstream hormones in the estradiol biosynthesis pathway, such as estrone and estradiol, are dependent on the presence or availability of upstream hormones such as testosterone, androstenedione, and progesterone. In addition, metabolism of these hormones through the pathway is dependent on steroidogenic enzymes such as Cyp11a1, 3β-hydroxysteroid dehydrogenase, aromatase, and the steroidogenic protein StAR. Without StAR, no cholesterol will be transported into the inner mitochondrial membrane (Stocco and Clark, 1996b). Further, without Cyp11a1 and thus, the conversion of cholesterol to pregnenolone, no further downstream hormones, such as progesterone, will be synthesized in the pathway (Simpson, 1979). Our study has shown that BPA decreases the expression of Cyp11a1 after 18h of culture. By preventing the conversion of cholesterol to pregnenolone, thecal cells cannot synthesize progesterone and, so, by 24h, it is likely that BPA exposure decreases progesterone levels. By 72h, the levels of androstenedione, testosterone, and estradiol, the downstream hormones dependent on the presence of progesterone, decrease as well, most likely for lack of available progesterone. We have previously shown that hormone levels can be rescued during exposure to BPA if precursor hormone, pregnenolone, was added to the cultures (Peretz et al., 2011), further supporting our hypothesis and these results.
Our results are consistent with data from human studies showing that BPA exposure may be associated with altered hormone levels in both males and females. BPA has been inversely associated with estradiol levels in women undergoing IVF (Ehrlich et al., 2012; Mok-Lin E et al., 2010), positively associated with androgen levels in women with polycystic ovarian syndrome (Kandaraki et al., 2011), and positively associated with decreases in the ratio of estradiol to testosterone in men within infertile partnerships utilizing assisted reproductive techniques (Meeker et al., 2010).
The goal of this study was to determine when and how the selected concentrations of BPA have the ability to inhibit steroidogenesis and steroidogenic enzyme expression because no other studies have investigated the effects of BPA on steroidogenesis of antral follicles. The results of the current study, in conjunction with our previous study (Peretz et al., 2011), suggest that the selected concentrations of BPA (10 and 100 μg/mL) may target the steroidogenic pathway. Thus, a range of concentrations, including those concentrations measured in fluids and various tissues, are needed for risk assessment of BPA and hormone production. While the BPA (10 and 100μg/mL) concentrations are higher than some other observed concentrations in other fluids and placental tissues, it is not known how much BPA is found in antral follicles of healthy women. Further, it is possible that concentrations could reach the selected BPA levels in this study because of bioaccumulation in fat and tissues. An estimated daily intake of BPA was reported to range from 0.6–71.4 μg/day, though this is only an estimate based on a single urine sample from human subjects and actual levels of BPA could be higher (Vandenberg et al., 2007; Ouchi and Watanabe, 2002). Additionally, it is highly likely that levels of BPA in fatty tissues may be higher than BPA levels measured in the serum due to bioaccumulation in the fatty tissues (Nunez et al., 2001; Fernandez et al., 2007). Antral follicles are highly vascularized structures housed within ovaries that are surrounded by fatty tissue. As such, antral follicles may be constantly exposed to BPA released from the fatty tissues surrounding the ovaries in addition to being exposed to circulating BPA (Tillet, 2009). Therefore, the concentrations of BPA used in our study may be plausible for human exposure, though more studies should be conducted to measure the levels of BPA reaching the ovaries and, thus, the antral follicles.
Our results are consistent with some in vivo and in vitro studies showing that BPA decreases hormone levels and expression of steroidogenic enzymes in mice and rats as well. BPA (100 and 200 mg/kg/day) exposure for four consecutive days has been shown to decrease testicular and plasma levels of testosterone as well as expression of StAR and Cyp11a1 in Wistar rats (Nakamura et al., 2010). In addition, BPA (10.125mg/day) exposure for 4 consecutive days decreases levels of progesterone in pregnant mice (Berger et al., 2008). Further, BPA (2.5μg/kg and 25μg/kg) exposure from gestational day 12 to post-natal day 21 decreases testosterone production in progenitor, immature, and adult Leydig cells of adult Long-Evans rats (Nanjappa et al., 2012). BPA (1–100μM) has also been shown to decrease estradiol levels in cultured mouse granulosa cells after 48h exposure and BPA (10 and 100μM) exposure has been shown to decrease progesterone levels in cultured granulosa cells from both mice (Mlynarcikova et al., 2005) and swine (Grasselli et al., 2010) at 48h and 72h exposure, respectively. Moreover, BPA (10 and 100μM) decreases testosterone levels in human and rat cultured Leydig cells after 3h exposure (Ye et al., 2011). In conjunction with these studies, our current findings provide a mechanism of action for BPA-inhibited steroidogenesis. Further, our results can serve as a bridge between whole animal in vivo effects from BPA exposure and single cell cultures with BPA treatments, indicating that in vitro studies are representative of in vivo results when similar concentrations of BPA are used over similar time periods.
Some effects of endocrine disrupting chemicals have been considered adaptive rather than adverse (Rhomberg and Goodman, 2012). Thus, it is possible that the recovery of enzyme expression and hormone production after removal of BPA was an adaptive response. However, in the current study, the effects of BPA on antral follicle steroidogenesis are adverse and may not be fully adaptive. In our previous study (Peretz et al., 2011), co-administration of pregnenolone with BPA protected some hormone levels in treated follicles, but did not ameliorate the effects of BPA on others, such as testosterone and estradiol, indicating that the follicles could not adjust fully to environment. Additionally, in that study, there were still dose-dependent effects on hormone production when BPA was co-administered with pregnenolone. Specifically, there were BPA dose-dependent decreases in DHEA-S, androstenedione, and estrone levels in follicles exposed to BPA (10 and 100μg/mL) co-administered with pregnenolone (Peretz et al., 2011). Therefore, the follicles could not adapt to BPA, even with the presence of the precursor hormone pregnenolone, indicating that the effects of BPA are not adaptive. However, the adverse effects are reversible. Once BPA was removed from the follicles in the current study, expression of Cyp11a1 and levels of all hormones tested returned to control levels, indicating that BPA does not have a permanent effect on steroidogenesis.
Though the effects of BPA may be reversible with the removal of BPA, exposure to BPA is thought to be constant and from a variety of sources (Tillet et al., 2009; Stahlhut et al., 2009), making eradication of BPA exposure extremely difficult, if not impossible. Further, in a recent study evaluating urinary levels of BPA during a dietary intervention to decrease BPA exposure, though the study design attempted to remove as much dietary exposure to BPA as possible, the authors commented that complete obliteration of BPA was impossible due to BPA contamination in food processing and packaging plants, as well as environmental BPA found in some foods such as eggs (Rudel et al., 2011). Collectively, these findings suggest that humans are constantly exposed to BPA and complete avoidance of BPA exposure is highly unlikely. However, in the published dietary intervention study, levels of measureable BPA were decreased by 66% in those participants that actively avoided BPA for two days, utilizing a diet of fresh food and foods packaged in glass containers. Within three days of returning to a diet that utilized food with BPA-containing packaging, measurable urinary BPA levels of the participants returned to their pre-dietary intervention levels (Rudel et al., 2011). Therefore, while it may be impossible to completely avoid BPA exposure, lessening dietary exposure to BPA could play a major role in lessening the adverse effects of BPA, especially if exposure to BPA can be lower than those BPA concentrations seen to have adverse effects.
Overall, this study shows that BPA likely targets Cyp11a1 to inhibit hormone production, though these effects can be reversed once BPA is removed. The presence of BPA in consumer products is a great concern due to the numerous studies investigating the potentially adverse of effects of BPA exposure. However, a recent study has indicated that BPA exposure in adults can be decreased with certain lifestyle choices. Urinary BPA concentrations were significantly lowered in participants when they ate fresh foods compared to when they ate canned and packaged foods or food from a restaurant (Rudel et al., 2011). Therefore, though further studies should be conducted, our data indicate that it may be possible to decrease exposure to BPA and recover from any adverse hormonal effects, potentially limiting adverse health effects associated with BPA.
Figure 6. Effect of BPA removal on sex steroid hormone production over time.
After exposure of antral follicles to DMSO control or BPA (1 or 10μg/mL) for 20h in vitro, the media were removed from follicles exposed to BPA (1 or 10μg/mL) and replaced with freshly prepared supplemented media with DMSO. Follicles were then cultured for 4, 28, 52, or 76 more hours until total time in culture was 24h, 48h, 72h, or 96h. At each time-point, the media were collected and subjected to ELISA for progesterone, testosterone, androstenedione, and estradiol. The graphs represent means ± SEMs from three separate experiments. Asterisk (*) indicates p≤ 0.05 from DMSO control.
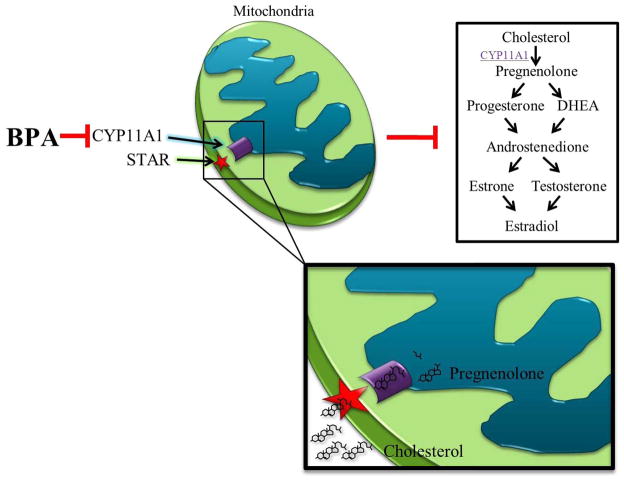
Figure 7. Summary of the effects of BPA on steroidogenesis.
BPA likely targets and down-regulates the expression of cytochrome P450 side-chain cleavage (Cyp11a1). CYP11A1 is tethered to the inner mitochondria membrane and works in conjunction with steroidogenic acute regulatory protein (STAR), tethered to the outer mitochondrial membrane. STAR transports cholesterol from the cytoplasm to the inner mitochondrial member where CYP11A1 removes the side-chain of cholesterol, synthesizing pregnenolone. Pregnenolone is the hormonal precursor required for synthesis of hormones with the estradiol biosynthesis pathway, including progesterone, dehydroepiandrosterone (DHEA), androstenedione, testosterone, estrone, and estradiol. Disruption of pregnenolone metabolism would negatively impact hormone production in antral follicles. Thus, BPA likely decreases the expression of Cyp11a1, leading to a decrease in hormones within the estradiol biosynthesis pathway due to a lack of pregnenolone.
Highlights.
Bisphenol A exposure significantly decreases expression of Cyp11a1 in ovarian antral follicles as early as 18 h
Bisphenol A exposure significantly decreases progesterone levels produced by antral follicles as early as 24 h
These effects of bisphenol A are reversible with removal of bisphenol A exposure
Acknowledgments
Funding
This work was supported by the National Institutes of Health [R01 ES019178, P20 ES 018163].
The authors would like to acknowledge the members of the Flaws’ lab and Dr. Steven Neese for their support and input throughout the project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jackye Peretz, Email: peretz@illinois.edu.
Jodi A. Flaws, Email: jflaws@illinois.edu.
References
- Amsterdam A, Tajima K, Frajese V, Seger R. Analysis of signal transduction stimulated by gonadotropins in granulosa cells. Mol Cell Endocrinol. 2003;202:77–80. doi: 10.1016/s0303-7207(03)00066-2. [DOI] [PubMed] [Google Scholar]
- Bagur AC, Mautzlen CA. Risk for developing osteoporosis in untreated premature menopause. Calcif Tiss Int. 1992;51:4–7. doi: 10.1007/BF00296207. [DOI] [PubMed] [Google Scholar]
- Berger RG, Shaw J, deCatanzaro D. Impact of acute bisphenol-A exposure upon intrauterine implantation of fertilized ova and urinary levels of progesterone and 17β-estradiol. Reprod Toxicol. 2008;26:94–99. doi: 10.1016/j.reprotox.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Birge SJ. Hormones and the aging brain. Geriatrics. 1998;53:S28–S30. [PubMed] [Google Scholar]
- Cain L, Chatterjee S, Collins TJ. In Vitro Folliculogenesis of Rat Preantral Follicles. Endocrinology. 1995;136:3369–3377. doi: 10.1210/endo.136.8.7628372. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary Concentrations of Bisphenol A and 4-Nonylphenol in a Human Reference Population. Environ Health Perspect. 2005;113:391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18:1754–1767. doi: 10.1038/nm.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S, Williams PL, Missmer SA, Flaws JA, Berry KF, Calafat AM, Ye X, Petrozza JC, Wright D, Hauser R. Urinary Bisphenol A Concentrations and Implantation Failure among Women Undergoing In Vitro Fertilization. Environ Health Perspect. 2012 doi: 10.1289/ehp.1104307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez MF, Arrebola JP, Taoufiki J, Navalón A, Ballesteros O, Pulgar R, Vilchez JL, Olea N. Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod Toxicol. 2007;24:259–264. doi: 10.1016/j.reprotox.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Kubo K, Aou S. Prenatal exposure to bisphenol A impairs sexual differentiation of exploratory behavior and increases depression-like behavior in rats. Brain Res. 2006;1068:49–55. doi: 10.1016/j.brainres.2005.11.028. [DOI] [PubMed] [Google Scholar]
- Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL. Nonclassical estrogen receptor α signaling mediates negative feedbback in the female mouse reproductive axis. Proc Natl Acad Sci USA. 2007;104:8173–8177. doi: 10.1073/pnas.0611514104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli F, Baratta L, Baioni L, Bussolati S, Ramoni R, Grolli S, Basini G. Bisphenol A disrupts granulosa cell function. Domest Anim Endocrinol. 2010;39:34–39. doi: 10.1016/j.domaniend.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Hu FB, Grodstein F, Hennekens CH, Colditz GA, Johnson M, Manson JE, Rosner B, Stampfer MJ. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med. 1999;159:1061–1066. doi: 10.1001/archinte.159.10.1061. [DOI] [PubMed] [Google Scholar]
- Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17:2839–2841. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- Kandaraki E, Chatzigeorgiou A, Livadas S, Palioura E, Economou F, Koutsilieris M, Palimeri S, Panidis D, Diamanti-Kandarakis E. Endocrine disruptors and polycystic ovary syndrome (PCOS): elevated serum levels of bisphenol A in women with PCOS. J Clin Endocrinol Metab. 2011;96:E480–E484. doi: 10.1210/jc.2010-1658. [DOI] [PubMed] [Google Scholar]
- Kubo K, Arai O, Omura M, Watanabe R, Ogata R, Aou S. Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats. Neurosci Res. 2003;45:345–356. doi: 10.1016/s0168-0102(02)00251-1. [DOI] [PubMed] [Google Scholar]
- LaVoie HA, King SR. (9 A.D.c). Transcriptional Regulation of Steroidogenic Genes: STARD1, CYP11A1, and HSD3B. Exp Biol Med. 234:880–907. doi: 10.3181/0903-MR-97. [DOI] [PubMed] [Google Scholar]
- Lee S, Suk K, Kim IK, Jang IS, Park JW, Johnson VJ, Kwon TK, Choi BJ, Kim SH. Signaling pathways of bisphenol A-induced apoptosis in hippocampal neuronal cells: role of calcium-induced reactive oxygen species, mitogen-activated protein kinases, and nuclear factor-kappaB. J Neurosci Res. 2008;86:2932–2942. doi: 10.1002/jnr.21739. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, Hauser R. Urinary bisphenol A concentrations in relation to serum thyroid and reproductive hormone levels in men from an infertility clinic. Environ Sci Technol. 2010;44:1458–1463. doi: 10.1021/es9028292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlynarcikova A, Kolena J, Fickova M, Scsukova S. Alterations in steroid hormone production by porcine ovarian granulosa cells caused by bisphenol A and bisphenol A dimethacrylate. Mol Cell Endocrinol. 2005;244:57–62. doi: 10.1016/j.mce.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Mok-Lin E, Ehrlich S, Williams PL, Petrozza J, Wright DL, Calafat AM, Ye X, Hauser R. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int J Androl. 2010;33:385–393. doi: 10.1111/j.1365-2605.2009.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura D, Yanagiba Y, Duan Z, Ito Y, Okamura A, Asaeda N, Tagawa Y, Li C, Taya K, Zhang S-Y, Naito H, Ramdhan DH, Kamijima M, Nakajima T. Bisphenol A may cause testosterone reduction by adversely affecting both testis and pituitary systems similar to estradiol. Toxicol Lett. 2010;194:16–25. doi: 10.1016/j.toxlet.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Nanjappa MK, Simon L, Akingbemi BT. The Industrial Chemical Bisphenol A (BPA) Interferes with Proliferative Activity and Development of Steroidogenic Capacity in Rat Leydig Cells. Biol Reprod. 2012;86:1–12. doi: 10.1095/biolreprod.111.095349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Toxicology Program. Carcinogenesis Bioassay of Bisphenol A (CAS No. 80–05–7) in F344 Rats and B6C3F1 Mice (Feed Study) Natl Toxicol Program. Tech Rep Ser. 1982;215:1–116. [PubMed] [Google Scholar]
- Nunez AA, Kannan K, Giesy JP, Fang J, Clemens LG. Effects of Bisphonel A on energy balance and accumulation in brown adipose tissue in rats. Chemosphere. 2001;42:917–922. doi: 10.1016/s0045-6535(00)00196-x. [DOI] [PubMed] [Google Scholar]
- Ouchi K, Watanabe H. Measurement of bisphenol A in human urine using liquid chromatography with multi-channel coulometric electrochemical detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;780:365–370. doi: 10.1016/s1570-0232(02)00547-0. [DOI] [PubMed] [Google Scholar]
- Peretz J, Gupta RK, Singh J, Hernandez-Ochoa I, Flaws JA. Bisphenol A impairs follicle growth, inhibits steroidogenesis, and down-regulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicol Sci. 2011;119:209–217. doi: 10.1093/toxsci/kfq319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkovic A, Pangas SA, Matzuk MM. Follicular Development: Mouse, Sheep, and Human Models. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. Elsevier; 2006. pp. 383–411. [Google Scholar]
- Rhee JS, Kim BM, Lee CJ, Yoon YD, Lee YM, Lee JS. Bisphenol A modulates expression of sex differentiation genes in the self-fertilizing fish, Kryptolebias marmoratus. Aquat Toxicol. 2011;104:218–219. doi: 10.1016/j.aquatox.2011.04.020. [DOI] [PubMed] [Google Scholar]
- Rudel RA, Gray JM, Engel CL, Rawsthorne TW, Dodson RE, Ackerman JM, Rizzo J, Nudelman JL, Brody JG. Food Packaging and Bisphenol A and Bis(2-Ethyhexyl) Phthalate Exposure: Findings from a Dietary Intervention. Environ Health Perspect. 2011;119:914–920. doi: 10.1289/ehp.1003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih M-CM, Chiu Y-N, Hu M-C, Guo I-C, Chung B-C. Regulation of steroid production: Analysis of Cyp11a1 promoter. Mol Cell Endocrinol. 2011;336:80–84. doi: 10.1016/j.mce.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Simpson ER. Cholesterol side-chain cleavage, cytochrome P450, and the control of steroidogenesis. Mol Cell Endocrinol. 1979;13:213–227. doi: 10.1016/0303-7207(79)90082-0. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Clark BJ. Regulation of the Acute Production of Steroids in Steroidogenic Cells. Endocr Rev. 1996a;17:221–244. doi: 10.1210/edrv-17-3-221. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Clark BJ. Role of the Steroidogenic Acute Regulatory Proten (StAR) in Steroidogenesis. Biochem Pharmacol. 1996b;51:197–205. doi: 10.1016/0006-2952(95)02093-4. [DOI] [PubMed] [Google Scholar]
- Tillet T, Bisphenol A. Chapter 2: New Data Shed Light on Exposure, Potential Bioaccumulation. Environews. 2009;117(5):A210. doi: 10.1289/ehp.117-a210b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Wadia PR, Sonnenschein C, Rubin BS, Soto AM. Exposure to environmentally relevant doses of the xenoestrogen bisphenol-A alters development of the fetal mouse mammary gland. Endocrinology. 2006;148:116–127. doi: 10.1210/en.2006-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Williams CJ, Erickson GF. Morphology and Physiology of the Ovary. Rebar RW, editor. Female Reproductive Endocrinology. 2012 http://www.endotext.org/female/female1/femaleframe1.htm.
- Ye L, Zhao B, Hu G, Chu Y, Ge R-S. Inhibition of human rat testicular steroidogenic enzyme activities by bisphenol A. Toxicol Lett. 2011;207:137–142. doi: 10.1016/j.toxlet.2011.09.001. [DOI] [PubMed] [Google Scholar]