Abstract
Understanding the early cytokine response to lentiviral infections may be critical to the design of prevention and treatment strategies. By using the feline immunodeficiency virus (FIV) model, we have documented an interleukin 10 (IL10)-dominated response in lymphoid tissue CD4+ and CD8+ T lymphocytes within the first 4 weeks after mucosal FIV infection. This profile coincided with the period of high tissue viral replication. By 10 weeks postinfection, tissue viral levels decreased significantly, and gamma interferon (IFNγ) production in CD8+ T cells had increased to restore the IL10/IFNγ ratio to control levels. Concurrently, increased production of IL6 and viral RNA was detected in macrophages. These temporal associations of viral replication with cytokine balance in tissues suggest roles for IL10 in the permissive stage of infection and IFNγ in the subsequent down modulation of lentiviral infection.
Feline immunodeficiency virus (FIV) was identified as a lentivirus associated with immunodeficiency in cats soon after the initial detection of human immunodeficiency virus (HIV) in cohorts of homosexual men (46). While there are some differences between the two viruses in genomic complexity and apparent breadth of cellular targets, the two lentiviruses produce virtually identical clinical immunodeficiency disease. Acute FIV and HIV infections are both characterized by flu-like illness, lymphadenopathy, high levels of virus, and rapid loss of CD4+ T cells (1, 20, 58). This is followed by a long, clinically asymptomatic chronic infection marked by a further gradual decline in CD4 cells, which culminates in a terminal phase of clinical immunodeficiency, wasting, and opportunistic infections (47, 56, 64).
The cytokines elaborated early in a variety of infectious diseases are critical to the course of infection. A cytokine response dominated by gamma interferon (IFNγ), tumor necrosis factor alpha (TNFα), and interleukin 12 (IL12) (type 1 cytokine response) has been shown to be important in the clearance of intracellular pathogens, including viruses (5, 43, 55). These cytokines are instrumental in the generation of cytotoxic T lymphocytes (CTL), and multiple studies have shown a correlation between the emergence of CTL and the reduction of circulating HIV and simian immunodeficiency virus (SIV) (30, 40, 63). IL4, IL10, and IL13 have been shown to antagonize the production and effects of type 1 cytokines, and it is postulated that these cytokines play a role in viral escape from immune surveillance (55).
The study of the early events in HIV infection is difficult due to the exact timing of infection and the invasive nature of the assays that would be most informative. To model the early tissue responses to HIV infection, we used a rectal mucosal transmission model of FIV to examine cytokine responses in regional and systemic lymphoid tissue cell subpopulations. We found that progression from uncontrolled to down-regulated FIV infection correlated with characteristic changes in T-cell and macrophage cytokine profiles.
MATERIALS AND METHODS
Animals and sample collection.
Sixteen-week-old cats from a specific-pathogen-free breeding colony maintained at Colorado State University (Fort Collins, Colo.) were inoculated by atraumatic exposure of the rectal mucosa with either 1 ml of 200 TCID50 (50% tissue culture infective dose) (n = 4) per ml or 1 ml of 400 TCID50 per ml of cell-free infectious FIV-B supernatant (n = 10). Age-matched control cats received 1 ml of cell-free noninfectious cell culture medium (n = 15). Inoculations were repeated 24 h after the first exposure. Blood collection was performed every 5 days until FIV proviral DNA was detected by PCR, and then collections were performed every 14 days. Six FIV-infected and seven naive controls were euthanized at 4 weeks postinoculation (PI). The remaining eight FIV-infected and eight naive controls were sacrificed at 10 weeks PI. The colic lymph nodes and the spleens were collected immediately following euthanasia and placed in ice-cold Hanks’ balanced salt solution (GIBCO, Grand Island, N.Y.) containing 5 U of DNase (Sigma, St. Louis, Mo.) per ml.
Virus inocula.
The first cell-free cell culture virus inoculum (200 TCID50/ml) was generated by the coculture of naive feline peripheral blood mononuclear cells (PBMC) with PBMC from a cat acutely infected with FIV-B via the intravenous route. The second cell culture inoculum (400 TCID50/ml) was generated by the coculture of native PBMC with the PBMC from a pool of three cats acutely infected with FIV-B via the rectal mucosal route. The infectivity of the supernatants was determined via titration, and aliquots were frozen at −70° C.
Processing of PBMC and tissue.
PBMC were separated by density gradient centrifugation (Histopaque 1077; Sigma). Colic lymph node and spleen tissues were mechanically dispersed by passage through a fine wire mesh and resuspended in cold Hanks' balanced salt solution (GIBCO). The erythrocytes from the splenic samples were lysed by incubation in 5 ml of ACK lysis buffer (29) for 10 min. The isolated cells were washed three times in sterile Dulbecco's phosphate-buffered saline (PBS) (GIBCO) and placed in RPMI medium (GIBCO) supplemented with 10% fetal bovine serum (FBS), 1% penicillin-streptomycin, 2% glutamine, and 2 × 10−5 M 2-mercaptoethanol containing 20 ng of phorbol 12-myristate 13-acetate (Sigma) per ml and 1 μg of ionomycin (Sigma) per ml at a concentration of 4 × 106 cells/ml for 2 h at 37°C. After mitogen stimulation, the nonadherent cells were washed three times in PBS and subjected to magnetic sorting. The adherent cells were cultured for an additional 5 days in Dulbecco's modified Eagle's medium (Sigma) supplemented with 10% FBS and 1% penicillin-streptomycin. The purity of the adherent macrophages was assessed by cytochemical staining with the antibody Mac387 (Serotec Inc., Raleigh, N.C.). RNA was extracted from the macrophages by adding Trizol reagent (GIBCO) directly to the culture flasks and was stored at −70°C until further processing, according to the manufacturer's instructions.
Magnetic sorting of cells.
The isolated nonadherent cells were resuspended in 1 ml of PBS supplemented with 2% FBS (HyClone, Logan, Utah) and 4 μg of murine immunoglobulin G for 30 min at 4°C. The cells were washed two times with PBS and resuspended in 800 μl of PBS-2% FBS. Fluorescein isothiocyanate (FITC)-conjugated antibodies to either feline CD4 or CD8 (44) (Southern Biotechnology, Birmingham, Ala.) were added, and the cells were incubated at 4°C for an additional 30 min. After washing the cells three times, they were resuspended in 800 μl of PBS-2% FBS with 40 μl of anti-FITC magnetic beads (Miltenyi Biotec, Auburn, Calif.) and incubated for 15 min at 4°C. The cells were washed one time in PBS and resuspended in 500 μl of degassed PBS supplemented with 2 mM EDTA and 0.5% bovine serum albumin (separation buffer).
MACS LS separation columns (Miltenyi Biotec) were used according to the manufacturer's instructions. The cells were separated over two sequential columns to increase purity. The cells were quantified in the final eluants, and 1 × 105 cells were stained with the same FITC-labeled antibody used for sorting, a phycoerythrin-labeled antibody for either CD4 or CD8, and a tricolor-labeled antibody for B220 (BD Pharmingen, San Diego, Calif.). Flow cytometry with an EPICS XL-MCL system (Coulter, Hialeah, Fla.) was performed to assess the final purity of the sorted cells. The lymphocytes were pelleted and lysed in 1 ml of Trizol reagent (GIBCO) and stored at −70°C until further processing, according to the manufacturer's instructions. The RNA was lyophilized in 2-μg aliquots by using a Speed Vac concentrator (Savant, Farmingdale, N.Y.) immediately prior to performing the ribonuclease protection assay.
RNase protection assay.
Two probe sets were developed for feline cytokines. The set used with CD4+ and CD8+ T-cell RNA consisted of probes for IL10, IFNγ, TNFα, IL4, IL2, and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The set used with macrophage RNA consisted of probes for IL6, IL10, TNFα, IL12p40 and IL12p35, IFNβ, and GAPDH.
The primers for feline IFNγ (accession no. X86972), TNFα (X54000), IL2 (L19402), IL10 (U39569), IL6 (D13227), IFNβ (AB021707), and IL12 (U83185 and U83184) were designed based on sequences from the GenBank database. Partial sequences for feline IL12 and IL4 were kindly provided by Gregg Dean (North Carolina State University, Raleigh). A partial sequence for feline GAPDH was generated by amplifying GAPDH with species consensus primers (53) and sequencing the product (Macromolecular Resources, Fort Collins, Colo.). The selected primer pairs were as follows: for GAPDH, 5′-AATTCCACGGCACAGTCAAGG-3′ and 5′-CATTTGATGTTGGCGGGATC-3′; for IFNγ, 5′-TTCGCTTTCCAGCTTTGCAT-3′ and 5′-CTGGAGCTGGTATTTAACAA-3′; for TNFα, 5′-TGGCCTGCAACTAATCAACC-3′ and 5′-GTGTGGAAGGACATCCTTGG-3′; for IFNβ, 5′-TCTCGAAGTCTTTGCTTCAGCAC-3′ and 5′-GAGGTTCTGTTCAAGTTCACCAGG-3′; for IL2, 5′-ACTGACTCTTATACTCGTCAC-3′ and 5′-GTCAATTCTGTGGCCTTCTTG-3′; for IL4, 5′-GGTCTGCTTACTAGCATTTACCA-3′ and 5′-GGTGGAGCAGTTGTGATGTG-3′; for IL6, 5′-GCAGAAAACAACCTGAATCTTCCG-3′ and 5′-GAGAAAGGAATGCCCGTGAAC-3′; for IL10, 5′-GAGGACCCAGACATCAAAC-3′ and 5′-AGAGGTATGACCGGGTTCTCCAA-3′; for IL12p35, 5′-AGGAATGTTCCAGTGCCTCAAC-3′ and 5′-CACCTGGTACATCTTCAAGTCCTC-3′; and for IL12p40, 5′-ACCAGCAGCTTCTTCATCAGGG-3′ and 5′-GGACCTGTACGCCAAATGTTAA-3′.
The probe sets were labeled with [32P]UTP by using a Riboquant in vitro transcription kit (BD Pharmingen) according to the manufacturer's instructions. The probe mixture was used at a final concentration of 3 × 105 Cherenkov counts/μl in the Riboquant multiprobe RNase protection system. The samples were loaded on a denaturing 8% polyacrylamide sequencing gel along with a 1:150 dilution of the probe. The gel was dried on filter paper, loaded into a phosphor screen (Amersham Biosciences, Piscataway, N.J.), and incubated at room temperature for 72 to 96 h. Individual band intensity was determined by using ImageQuant software (Amersham Biosciences), and all results were expressed as a ratio of cytokine to GAPDH.
DNA PCR for provirus detection.
DNA was extracted from PBMC by using a QIAamp blood kit (QIAGEN, Chatsworth, Calif.). One microgram of DNA was amplified by nested PCR with FIV gag primers. The first-round primers were Gag129 (5′-CGTAACTACAGGACGAGAACCTGG-3′) and Gag802 (5′-CCAACTTTCCCAATGCTTCAAG-3′), and the second-round primers were Gag3 (5′-TTGACCCAAAAATGGTGTCCA-3′) and Gag4 (5′-TTCTGCTTGTTGTTCTTGAGT-3′), resulting in a 293-bp product. For both first- and second-round reactions, hot-start PCR was performed with Ampliwax PCR gems (Perkin Elmer, Norwalk, Conn.). Each round consisted of 35 cycles of 94°C for 15 s, 55°C (first round) or 60°C (second round) for 20 s, and 72°C for 30 s. The first- and second-round reaction mixtures contained 3 mM MgCl2, 200 μM each deoxynucleoside triphosphate, 1× gene amp, 10× PCR buffer II (Perkin Elmer), 2.5 U of AmpliTaq DNA polymerase (Perkin Elmer), and 0.1 μM each first-round primer or 0.05 μM each second-round primer. The product was visualized on a 1.2% agarose gel stained with ethidium bromide. Amplimer specificity has been previously shown via Southern blot analysis (42).
RT-PCR for viral RNA.
Viral RNA levels in plasma and tissue were quantified by using a quantitative competitive reverse transcriptase PCR (QC-RT-PCR) assay based on the method described by Diehl et al. (14, 15). A single-round, 40-cycle reaction was run as described above with the primers Gag3 and Gag4. Threefold serial dilutions of competitor RNA containing from 106 to 103.6 copies were added to viral RNA samples. Ethidium bromide-stained amplimers were quantified by using AlphaImager software (Alpha Innotech, San Leandro, Calif.), and the point of equivalence of competitor and virus was determined. Input amounts of RNA consisted of 25 μl of Alsever's plasma (Sigma) or 25 ng of total tissue RNA per PCR mixture.
FIV antigen enzyme-linked immunosorbent assay.
Productive in vitro infection was assessed by the capsid antigen (p26) capture enzyme-linked immunosorbent assay described by Dreitz et al. (18) performed on macrophage supernatants. Optical densities, measured by absorbance at 450 nm, were recorded with a Dynatech 5000MRTM microplate reader (Dynatech Corp., Chantilly, Va.). Positive reactions were defined as those with a minimum optical density of 0.1 and at least twice those of negative control supernatants run in parallel.
Statistical analysis.
Data were analyzed with StatView software (Adept Scientific, Bethesda, Md.). Samples were determined to be normally distributed by using the Kolmogorov-Smirnov test. Comparisons were made by using a two-tailed Student's t test, with significance defined as a P value of < 0.05. Correlations were calculated, and P values were determined by using Fisher's r-to-z transformation with significance defined as a P value of < 0.05.
RESULTS
Rectal mucosal infection.
Of the 30 cats inoculated with two doses of FIV-B-2542 via the rectal mucosal route, PBMC from 14 cats became DNA PCR positive (47% rate of infection), beginning 14 to 22 days PI. A virus stock generated from cats acutely infected via the rectal route did not demonstrate any augmented ability to cross the rectal mucosa and establish infection, i.e., 4 of 10 cats (40%) became infected after rectal exposure. To determine whether rectally inoculated, PCR-negative animals may have sequestered virus infection not revealed by the examination of peripheral blood, we necropsied five such animals and assayed regional and systemic lymphoid tissues for FIV DNA or RNA. Neither of these were detectable in any tissue sampled (data not shown).
Purity of sorted cell populations.
Magnetically sorted CD8+ lymphocytes were consistently >94 to 98% pure (mean, 95%). Sorted CD4+ lymphocytes ranged from 91 to 97% purity (mean, 93%). The size and forward scatter properties of the magnetically sorted cells were consistent with lymphocytes. Adherence-separated macrophages were 85 to 92% pure (mean, 88%).
Lymphocyte cytokine mRNA levels at 4 weeks PI.
At four weeks PI, IL10 was the only cytokine message that differed significantly for infected and control cats. This was true for IL10 in CD8+ T cells of the colonic lymph node and for both CD4+ and CD8+ T cells of the spleen (Fig. 1 and 2). IL4 was only detected in the CD4+ T cells of the colonic lymph node, and there were no significant differences in the levels for control and infected cats (data not shown). IFNγ, TNFα, and IL2 mRNA levels did not differ between FIV-positive and control cats (Table 1).
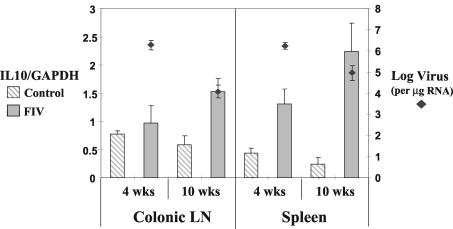
FIG. 1.
IL10 RNA levels were increased in CD4+ T cells of the spleen at 4 weeks PI (P < 0.05) and in both the spleen and colonic lymph nodes (LN) at 10 weeks PI (P < 0.05) in FIV-infected animals. Viral RNA levels decreased between 4 and 10 weeks PI in both the spleen (P < 0.05) and colonic lymph nodes (P < 0.01). Cytokine RNA was analyzed via RNase protection assay in purified cells from the colonic lymph nodes and spleens at 4 and 10 weeks after rectal inoculation with FIV-B or sham control. Cellular viral RNA levels were measured by QC-RT-PCR and expressed as log numbers of virus per microgram of total cellular RNA.
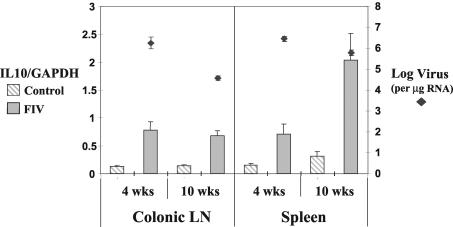
FIG. 2.
IL10 RNA levels were increased in CD8+ T cells of the spleen and colonic lymph nodes (LN) at 4 weeks PI (P < 0.01) and in the spleen (P < 0.01) and colonic lymph nodes (P < 0.001) at 10 weeks PI in FIV-infected animals. Viral RNA levels decreased between 4 and 10 weeks PI in both the spleen (P < 0.05) and colonic lymph nodes (P < 0.001). Cytokine and viral RNA levels were measured as per the legend to Fig. 1.
TABLE 1.
Cytokine RNA levels in splenic and colonic lymph node CD4+ and CD8+ T cells and splenic macrophages
| Cell type | Animal infection status | Cytokine ratio (SD) ata:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 wks PI
|
10 wks PI
|
||||||||||
| IL2 | IL10 | TNFα | IFNγ | IL6 | IL2 | IL10 | TNFα | IFNγ | IL6 | ||
| Colonic CD4 | Control | 3.12 (0.97) | 0.78 (0.059) | 1.70 (0.23) | 0.87 (0.25) | ND | 3.58 (1.4) | 0.58 (0.16) | 2.91 (0.73) | 1.32 (0.55) | ND |
| FIV infected | 2.33 (1.6) | 0.97 (0.3) | 1.50 (0.26) | 0.66 (0.37) | 2.64 (0.64) | 1.53 (0.24) | 2.85 (0.49) | 2.85 (1.11) | |||
| Colonic CD8 | Control | 0.89 (0.26) | 0.10 (0.016) | 0.86 (0.12) | 0.88 (0.24) | ND | 0.63 (0.05) | 0.14 (0.02) | 1.17 (0.16) | 0.77 (0.15) | ND |
| FIV infected | 0.64 (0.47) | 0.71 (0.16) | 1.17 (0.15) | 1.6 (0.66) | 0.89 (0.20) | 0.72 (0.09) | 1.9 (0.41) | 2.97 (0.52) | |||
| Spleen CD4 | Control | 3.0 (0.98) | 0.43 (0.09) | 1.60 (0.41) | 1.19 (0.40) | ND | 2.03 (1.13) | 0.23 (0.13) | 2.39 (0.77) | 1.24 (0.62) | ND |
| FIV infected | 1.70 (0.66) | 1.31 (0.27) | 1.33 (0.14) | 0.95 (0.24) | 2.40 (0.19) | 2.23 (0.51) | 1.94 (0.41) | 1.72 (0.83) | |||
| Spleen CD8 | Control | 0.52 (0.14) | 0.15 (0.03) | 1.25 (0.20) | 1.12 (0.30) | ND | 0.81 (0.24) | 0.31 (0.08) | 2.39 (0.87) | 1.04 (0.36) | ND |
| FIV infected | 0.21 (0.09) | 0.71 (0.19) | 1.00 (0.17) | 1.54 (0.61) | 0.63 (0.29) | 2.03 (0.48) | 4.25 (1.44) | 8.96 (3.41) | |||
| Spleen | Control | ND | 0.021 (0.009) | 0.049 (0.007) | ND | 0.010 (0.005) | ND | 0.013 (0.005) | 0.067 (0.014) | ND | 0.010 (0.007) |
| macrophage | FIV infected | 0.019 (0.005) | 0.072 (0.016) | 0.016 (0.003) | 0.018 (0.003) | 0.081 (0.016) | 0.052 (0.009) | ||||
Cytokine RNA was analyzed via RNase protection assay from purified CD4+ and CD8+ T cells in the colonic lymph nodes and spleen and from macrophages in the spleen at 4 and 10 weeks after rectal inoculation with FIV-B or sham control. All cytokine levels are expressed as a ratio of cytokine RNA to GAPDH RNA, and standard deviations are indicated in parentheses. Lymphocyte cytokine data were generated from six FIV-infected and seven control animals at 4 weeks PI and from eight FIV-infected and eight control animals at 10 weeks PI. Macrophage cytokine data were generated from four FIV-infected and six control animals at 4 weeks PI and from five FIV-infected and seven control animals at 10 weeks PI. The bolded figures indicate significant differences between control and infected animals (P < 0.05, two-tailed Student's t test).
Macrophage cytokine mRNA levels at 4 weeks PI.
The number of macrophages cultured from colonic lymph nodes was insufficient to allow the evaluation of cytokines in the vast majority of cats. The spleen yielded sufficient macrophages in 22 of the 29 cats. IL6, IL10, and TNFα were the only cytokines detectable in the adherence-purified macrophages. IL12 and IFNβ were consistently below the limits of detection (data not shown). There were no statistically significant differences in the cytokine RNA levels of FIV-positive macrophages when compared to the levels for controls at 4 weeks PI (Table 1).
Lymphocyte cytokine mRNA levels at 10 weeks PI.
IL10 mRNA levels in FIV-infected cats remained significantly elevated over those of controls in both CD4+ and CD8+ T cells in the colonic lymph node and spleen at 10 weeks PI (Fig. 1 and 2). By 10 weeks PI, a highly significant difference was detected in the levels of IFNγ mRNA in the CD8+ lymphocyte populations of FIV-positive and control cats (Fig. 3). This rise in IFNγ mRNA was not seen in the CD4+ T cells of infected cats (Fig. 4). There were no differences in TNFα or IL2 mRNA levels in the lymphocyte population in either the spleens or colonic lymph nodes (Table 1).
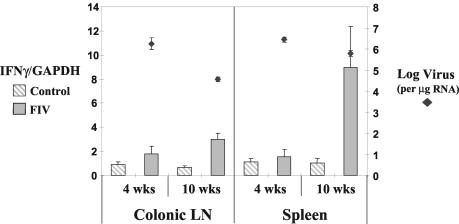
FIG. 3.
IFNγ RNA levels were increased in CD8+ T cells of the spleen (P < 0.05) and colonic lymph nodes (LN) (P < 0.001) at 10 weeks PI in FIV-infected animals. Viral RNA levels decreased between 4 and 10 weeks PI in both the spleen (P < 0.05) and colonic lymph nodes (P < 0.001). Cytokine and viral RNA levels were measured as per the legend to Fig. 1.
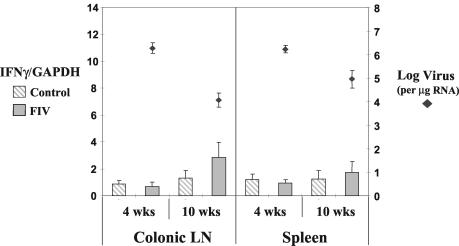
FIG. 4.
There were no significant differences in IFNγ RNA levels in CD4+ T cells of both spleen and colonic lymph nodes (LN) at 4 and 10 weeks PI (P > 0.05) in FIV-infected animals and controls. Viral RNA levels decreased between 4 and 10 weeks PI in both the spleen (P < 0.05) and colonic lymph nodes (P < 0.01). Cytokine and viral RNA levels were measured as per the legend to Fig. 1.
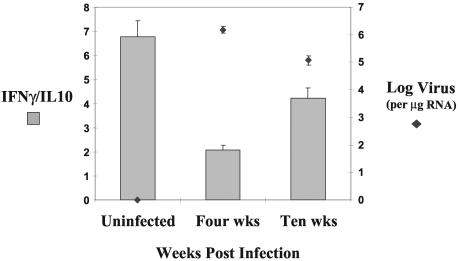
The early rise of IL10 mRNA with no corresponding increase in IFNγ mRNA resulted in a significantly decreased IFNγ/IL10 ratio within the CD8+ T cells at 4 weeks PI. The increased levels of IL10 persisted throughout the 10-week study, but because of an even larger rise in IFNγ, there was no statistical difference between the control cats and the infected cats at 10 weeks PI (Fig. 5). A significant reduction in viral RNA levels (log virus per microgram of RNA) within the CD8+ T cells was temporally associated with the rising IFNγ/IL10 ratio at 10 weeks PI (Fig. 5).
FIG. 5.
The ratio of IFNγ to IL10 decreased between 4 and 10 weeks after rectal inoculation with FIV-B in both colonic lymph node and splenic tissue CD8+ T cells in FIV-infected animals. There was a significant difference between the levels for the control and those of the infected animals at 4 weeks PI (P < 0.01), and also between the infected animals at 4 and 10 weeks PI (P < 0.001). Viral RNA levels decreased between 4 and 10 weeks PI in CD8+ T cells (P < 0.01). Cytokine and viral RNA levels were measured as per the legend to Fig. 1. Control animals consisted of the sham-inoculated cats at both 4 and 10 weeks PI.
Macrophage cytokine mRNA levels at 10 weeks PI.
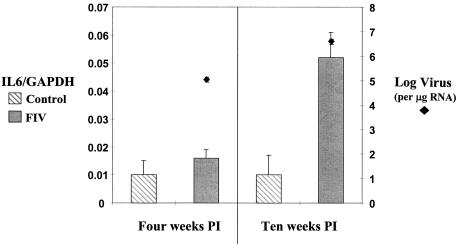
IL6 mRNA levels were significantly increased in the splenic macrophages of FIV-positive cats at week 10 PI (Fig. 6). Both TNFα and IL10 levels remained unchanged (Table 1).
FIG. 6.
IL6 RNA levels were increased in splenic macrophages 10 weeks after infection with FIV-B (P < 0.05). Viral RNA levels increased between 4 and 10 weeks PI in macrophages (P < 0.01). Cytokine and viral RNA levels were measured as per the legend to Fig. 1.
Viral RNA levels.
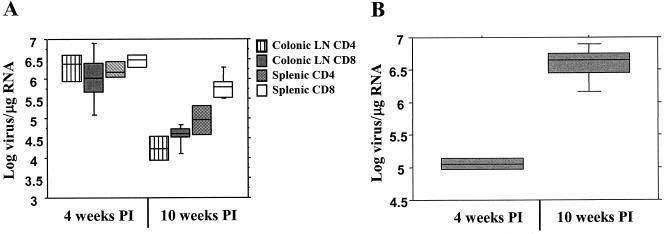
Viral RNA levels within the plasma of infected cats were high (7.7 × 105 to 4.7 × 106 viral copies/ml) at 4 weeks PI but were significantly decreased by 10 weeks PI (1 × 104 to 6.3 × 105 viral copies/ml) (data not shown). This reduction over time was mirrored in the tissue CD4+ and CD8+ T-cell viral levels in both colonic lymph node and spleen (Fig. 7A). At 10 weeks PI, there was more viral RNA in the spleens than in the colonic lymph nodes. Within the spleen, there was significantly more viral RNA in the CD8+ than in the CD4+ T-cell population (Fig. 7A).
FIG. 7.
Viral RNA levels decreased in tissue T cells between 4 and 10 weeks PI (A), while viral RNA levels increased in splenic macrophages during the same time period (B). There were significant differences between the 4- and 10-week viral levels in both T-cell subsets in the spleen and colonic lymph nodes (P < 0.05) and within the macrophages (P < 0.01). Cytokine and viral RNA levels were measured as per the legend to Fig. 1.
The pattern of viral RNA levels in splenic macrophages was distinct from that in lymphocytes and plasma. Macrophage viral RNA levels significantly increased between weeks 4 and 10 PI (Fig. 7B). Interestingly, no viral antigen could be detected in the supernatants from any of the infected macrophage cultures (data not shown).
Cytokines and viral levels.
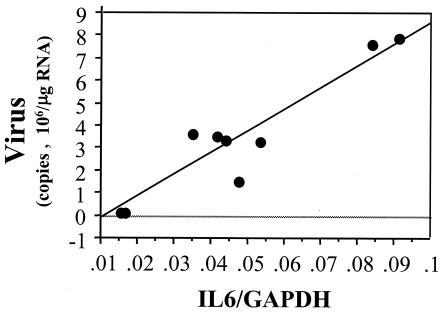
There were no significant correlations between cytokine mRNA levels and virus levels in infected CD4+ or CD8+ T cells. Macrophage IL6 mRNA levels were directly correlated with the amount of viral RNA within macrophages (r = 0.889, P < 0.05) (Fig. 8).
FIG. 8.
IL6 RNA levels were correlated with viral RNA levels within macrophages of the spleen and colonic lymph nodes at 4 and 10 weeks after rectal inoculation with FIV-B (r = 0.889, P < 0.05). Cytokine and viral RNA levels were measured as per the legend to Fig. 1.
DISCUSSION
The rate of rectal FIV-B-2542 transmission in the present study, and in concurrent work in this laboratory (39), is approximately 50%. This rate appeared to be independent of the TCID, and in vivo passage of the virus via rectal inoculation did not appear to increase the ability of the virus pool to cross the rectal mucosa. Because low-level or transient viremia has been seen in macaques rectally inoculated with SIV (60), we necropsied several exposed, PCR-negative cats and were unable to detect any evidence of a tissue virus reservoir. The intact rectal mucosa appears to provide a relatively effective barrier against retroviral transmission, and the transmission rates seen in sexually exposed people likely reflect repeated exposures and/or minor mechanical trauma to the mucosa.
The decrease in tissue viral levels within the lymphocyte subsets between weeks 4 and 10 PI mirrored that seen in the plasma, suggesting that T cells within the lymphoid tissue compartments directly contribute to the circulating pool of virus. While HIV can infect CD8+ T cells in some situations (27), CD8+ T cells are not considered one of the major cellular targets. FIV has been described as infecting CD8+ T cells, yet this study documents for the first time that levels of CD8+ T-cell-associated virus can actually exceed those of CD4+ T-cell virus at certain times PI. Although FIV is known to infect B cells (19), the contribution of B lymphocytes to tissue virus levels was not examined in this study.
In contrast to the lymphocytes, macrophage viral RNA levels increased between 4 and 10 weeks PI. An earlier study of FIV infection by Beebe et al. utilizing RNA in situ hybridization has documented the progressive increase in macrophage-associated virus during the early weeks and months postinfection (2). Monocyte/macrophages have been implicated as a major source of persistent HIV infection, particularly in those patients being treated with highly active antiretroviral treatment (48), and the ability of the virus to maintain low levels of replication in the long-lived population of monocytes is thought to contribute to the escape of immune clearance (54). Despite the high levels of viral RNA that we documented in the adherence-purified macrophages, we were unable to detect viral antigen in the supernatants. There is precedent for the adherence-stimulated up regulation of FIV viral RNA synthesis in macrophages without a concurrent release of virus (17), suggesting that these cells may be a persistent source of low-level virus infection. The ability of FIV to shift from the predominant infection of lymphocytes to macrophages may represent an in vivo evolution of tropism in order to avoid the apparent immune-mediated decrease in virus levels in lymphocytes.
The cytokine pattern at 4 weeks after FIV infection was dominated by increased lymphocyte IL10, which was initially described as a factor secreted by murine Th2 cells that inhibited the production of IFNγ (22). IL10 has since been shown to have a variety of inhibitory effects on activated macrophages, including the inhibition of nitric oxide production (23), the reduction in the surface expression of major histocompatibility complex class II molecules (13) and CD80/CD86 costimulatory molecules (16), and the inhibition of IL12 and TNFα production (12, 21, 50). Dendritic cells have been shown to be the most potent initiators of an immune response, and IL10 can decrease IL12 production and costimulatory molecule expression in these cells as well (8, 37).
Elevations of IL10 have been reported in HIV-infected patients in all stages of infection (36, 57). Many HIV-positive people have demonstrable defects in proliferative recall responses to HIV as well as non-HIV proteins such as tetanus toxoid and influenza virus (11). The in vitro neutralization of IL10 restores the defective recall responses in patients who have between 200 and 500 CD4 cells/μl (31), indicating that IL10 is playing a role in the generalized dampening of the immune response to HIV.
A subset of lymphocytes that produce high levels of IL10 have been termed Tr1 cells (25, 52), and recent work provided the first evidence of pathogen-specific Tr1 cells in a murine model of Bordetella pertussis infection (35). The pathogen-derived molecule was shown to specifically inhibit IL12 and augment IL10 production from dendritic cells, which then directed naive T cells into the Tr1 subtype. Type 1 cytokine responses were inhibited by the increased levels of IL10 in this system. Circulating Tr1 cells have been demonstrated to be present in HIV-infected people, and the numbers of Tr1 cells were significantly higher in those patients with active replication or progressive disease (45). Although dendritic cells were not examined in the present work, this model for the pathogen-specific skewing of dendritic cell cytokine production is an attractive explanation for the early IL10-dominated response in FIV infection.
The sum total of the effects described above of IL10 on monocyte/macrophages and dendritic cells would be to decrease their ability to direct a viral-specific, cell-mediated immune response. Any impaired ability to mount a cell-mediated immune response, shown to be critical for the control of HIV and SIV replication (30), would be advantageous to the virus. The fact that we have detected a selective increase in IL10 during peak FIV replication provides indirect evidence that FIV may exploit the immunosuppressive effects of IL10 in order to establish a persistent infection.
The cytokine response at 10 weeks after FIV infection evolves into a mixed type 1/type 2 response, with elevations in both IL10 and IFNγ mRNA levels. A similar pattern of cytokine response has been documented in the thymus of FIV-infected cats when examined beginning at 6 weeks PI (33). In our study, a significant elevation in IFNγ mRNA levels was only documented in the CD8-positive population, while both CD4+ and CD8+ T cells maintained elevated production of IL10 mRNA.
A recent study examining IFNγ production in PBMC from HIV-positive patients demonstrated that the increased IFNγ levels were found only in the CD8+ lymphocytes and, more specifically, in CD8+ CD28− cells (9). Cells with this phenotype have been shown to be effective cytolytic cells in HIV (26) and cytomegalovirus (34) infections. Increasing levels of CD8+ T-cell IFNγ mRNA as FIV levels are dropping raises the possibility that virus-specific CTL are emerging between 4 and 10 weeks. This would be consistent with observations in SIV and HIV infections in which the initial control of viremia is correlated with the emergence of viral peptide-specific CTL (6, 30, 40, 63).
While we cannot determine from our present data whether the delayed rise in CD8+ T-cell production of IFNγ mRNA is due to an emerging population of virus effector cells, the rise in IFNγ mRNA and the subsequent restoration of the IFNγ/IL10 ratio is temporally associated with a decreasing viral load. It is becoming more apparent that it is the relative proportions of cytokines rather than the absolute production of type 1 or type 2 cytokines that influence disease progression (3, 59). Recent work with HIV infection has confirmed this principle by showing that the relative balance of CD4+ T-cell production of IL10 versus IFNγ correlates with active replication and progression of disease and that highly active antiretroviral treatment can decrease viral loads and shift the balance towards IFNγ production (45). Our findings in the FIV model corroborate these findings in that the balance of IL10 versus IFNγ shifted back to a more normal ratio in CD8+ T cells as viral levels came under initial host control.
The elevated IL6 mRNA levels seen at 10 weeks PI in the macrophages are a phenomenon described in many viral infections. IL6 has been shown to be elevated in HIV (7), SIV (10, 28), and FIV (32, 41) infections. The in vitro infection of monocytes with HIV (38) and FIV (51) induces the production of IL6. In addition, HIV infection has been shown to have a priming effect, whereby increased amounts of IL6 are produced by the subsequent ligation of the CD40 receptor (4). The intravenous infection of macaques with SIV has been shown to lead to a rapid rise in PBMC and peripheral lymph node levels of IL6 (10, 28), whereas people naturally infected with HIV via the mucosal route tend to have a more delayed increase associated with the transition into the chronic phase of infection (24).
IL6 has the ability to increase the production of HIV in chronically infected ex vivo monocytes and in macrophages infected in vitro (49). The increase in macrophage HIV production occurs at the posttranscriptional level, increasing HIV proteins and RT activity without the accumulation of viral RNA (49). The feline macrophage cultures that we examined at 10 weeks PI had very high levels of intracellular viral RNA but undetectable levels of viral antigen in the supernatants. The correlation between macrophage IL6 mRNA levels and macrophage viral RNA levels in our cats supports the notion that the two may be linked, but either IL6 acts to stimulate viral replication via a different mechanism in feline macrophages or it is the infection of the macrophages that induces high levels of IL6 mRNA. IL10 has been reported to decrease HIV replication within monocyte/macrophages by interfering with IL6 and TNFα production (62). Despite this fact, when the amount of IL10 is titrated, IL10 can actually enhance HIV replication in a synergistic fashion with TNFα and IL6 (61). The elevated lymphocyte IL10 RNA levels seen throughout this study do not appear to have inhibited macrophage IL6 RNA production and in fact may have acted synergistically with IL6 to increase viral RNA levels.
In summary, we have documented an early IL10-dominated cytokine response in both CD4+ and CD8+ T cells during mucosal FIV infection. The IL10-dominated phase correlated with the period of peak viral replication. This cytokine response evolved to include concurrent increases in CD8+ T-cell levels of IFNγ mRNA, which was temporally related to decreasing lymphocyte and plasma virus levels. A delayed rise in macrophage IL6 mRNA was associated with a shift to increased macrophage viral RNA levels. Determining the potential role of the innate immune response in triggering these responses and the functional significance of these cytokine changes on the documented down regulation of viral replication are subjects for further study.
Acknowledgments
This work was supported by NIH K08AI01421 (P.R.A.) and NIH R01AI33773 (E.A.H.).
REFERENCES
- 1.Bach, J. M., M. Hurtrel, L. Chrakrabarti, J.-P. Ganiere, L. Montagnier, and B. Hurtrel. 1994. Early stages of feline immunodeficiency virus infection in lymph nodes and spleen. AIDS Res. Hum. Retrovir. 10:1731-1738. [DOI] [PubMed] [Google Scholar]
- 2.Beebe, A. M., N. Dua, T. G. Faith, P. F. Moore, N. C. Pedersen, and S. Dandekar. 1994. Primary stage of feline immunodeficiency virus infection: viral dissemination and cellular targets. J. Virol. 68:3080-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belkaid, Y., K. F. Hoffman, S. Mendez, S. Kamhavi, T. A. Wynn, and D. Sacks. 2001. The role of interleukin-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody. J. Exp. Med. 194:1497-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergamini, A., F. Bolacchi, B. Bongiovanni, V. Colizzi, G. Cappelli, I. Uccella, M. Cepparulo, M. Capozzi, G. Mancino, and G. Rocchi. 2000. Human immunodeficiency virus type 1 infection modulates the interleukin-1beta and IL-6 responses of human macrophages to CD40 ligand stimulation. J. Infect. Dis. 182:776-784. [DOI] [PubMed] [Google Scholar]
- 5.Berger, D. P., D. Homann, and M. B. A. Oldstone. 2000. Defining parameters for successful immunocytotherapy of persistent viral infection. Virology 266:257-263. [DOI] [PubMed] [Google Scholar]
- 6.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. A. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breen, E. C., A. R. Rezai, K. Nakajima, G. N. Beall, R. T. Mitsuyasu, T. Hirano, T. Kishimoto, and M. O. Martinez. 1990. Infection with HIV is associated with elevated IL-6 levels and production. J. Immunol. 144:480-484. [PubMed] [Google Scholar]
- 8.Buelens, C., F. Willems, G. Pierard, A. Delvaux, T. Velu, and M. Goldman. 1995. IL-10 inhibits the primary allogeneic T cell response to human peripheral blood dendritic cells. Adv. Exp. Med. Biol. 378:363-365. [DOI] [PubMed] [Google Scholar]
- 9.Caruso, A., S. Licenziati, A. D. Canaris, A. Cantalamessa, M. Corulli, B. Benzoni, L. Peroni, A. Balsari, and A. Turano. 1996. Characterization of T cell subsets involved in the production of IFN-γ in asymptomatic HIV-infected patients. AIDS Res. Hum. Retrovir. 12:135-141. [DOI] [PubMed] [Google Scholar]
- 10.Cheret, A., R. Le Grand, P. Caufour, N. Dereuddre-Bosquet, F. Matheux, O. Neildez, F. Theodoro, N. Maestrali, O. Benveniste, B. Vaslin, and D. Dormont. 1996. Cytokine mRNA expression in mononuclear cells from different tissues during acute SIV mac251 infection of macaques. AIDS Res. Hum. Retrovir. 12:1263-1272. [DOI] [PubMed] [Google Scholar]
- 11.Clerici, M., N. I. Stocks, R. A. Zajac, R. N. Boswell, D. R. Lucey, C. S. Via, and G. M. Shearer. 1989. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J. Clin. Investig. 84:1892-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Andrea, A., M. Aste-Amezaga, N. M. Valiente, X. Ma, M. Kubin, and G. Trinchieri. 1993. Interleukin-10 inhibits human lymphocyte IFN-γ production by suppressing interleukin-12 synthesis in accessory cells. J. Exp. Med. 178:1041-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Waal Malefyt, R., J. Haanen, H. Spits, M. Roncarlo, A. te Velde, C. Figdor, K. Johnson, R. Kastelein, H. Yssel, and J. E. de Vries. 1991. Interleukin 10 and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of moncytes via downregulation of class II major histocompatibility complex expression. J. Exp. Med. 174:915-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diehl, L. J., C. K. Mathiason-DuBard, L. L. O'Neil, and E. A. Hoover. 1995. Longitudinal assessment of feline immunodeficiency virus kinetics in plasma by use of a quantitative competitive reverse transcriptase PCR. J. Virol. 69:2328-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diehl, L. J., C. K. Mathiason-DuBard, L. L. O'Neil, and E. A. Hoover. 1996. Plasma viral RNA load predicts disease progression in an accelerated feline immunodeficiency virus model. J. Virol. 70:2503-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding, L., P. S. Linsley, L. Huang, R. N. Germain, and E. M. Shevach. 1993. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J. Immunol. 151:1224-1234. [PubMed] [Google Scholar]
- 17.Dow, S. W., C. K. Mathiason, and E. A. Hoover. 1999. In vivo monocyte tropism of pathogenic feline immunodeficiency viruses. J. Virol. 73:6852-6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dreitz, M. J., S. W. Dow, S. A. Fiscus, and E. A. Hoover. 1995. Development of monoclonal antibodies and capture immunoassays for feline immunodeficiency virus. Am. J. Vet. Res. 56:764-768. [PubMed] [Google Scholar]
- 19.English, R. V., C. M. Johnson, D. H. Gebhard, and M. B. Tompkins. 1993. In vivo lymphocyte tropism of feline immunodeficiency virus. J. Virol. 67:5175-5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.English, R. V., P. Nelson, C. M. Johnson, M. Nasisse, W. A. Tompkins, and M. B. Tompkins. 1994. Development of clinical disease in cats experimentally infected with feline immunodeficiency virus. J. Infect. Dis. 170:543-552. [DOI] [PubMed] [Google Scholar]
- 21.Fiorentino, D., A. Zlotnick, T. Mosmann, M. H. Howard, and A. O'Garra. 1991. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 147:3815-3822. [PubMed] [Google Scholar]
- 22.Fiorentino, D., A. Zlotnik, P. Vieira, T. Mosmann, M. Howard, K. Moore, and A. O'Garra. 1991. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 146:3444-3451. [PubMed] [Google Scholar]
- 23.Gazzinelli, R. T., I. Oswald, S. L. James, and A. Sher. 1992. IL-10 inhibits parasite killing and nitric oxide production by IFNγ-activated macrophages. J. Immunol. 148:1792-1796. [PubMed] [Google Scholar]
- 24.Graziosi, C., G. Pantaleo, K. R. Gantt, J.-P. Fortin, J. F. Demarest, O. J. Cohen, R. P. Sékaly, and A. S. Fauci. 1994. Lack of evidence for the dichotomy of TH1 and TH2 predominance in HIV-infected individuals. Science 265:248-252. [DOI] [PubMed] [Google Scholar]
- 25.Groux, H. 2001. An overview of regulatory T cells. Microbes Infect. 3:883-889. [DOI] [PubMed] [Google Scholar]
- 26.Ho, H. N., L. E. Hultin, R. T. Mitsuyasu, J. L. Matud, M. A. Hausner, D. Bockstoce, C. C. Chou, S. O'Rourke, J. M. G. Taylor, and J. V. Giorgi. 1993. Circulating HIV-specific CD8+ cytotoxic T cells express CD38 and HLA-DR antigens. J. Immunol. 150:3070-3079. [PubMed] [Google Scholar]
- 27.Imlach, S., S. McBreen, T. Shirafuji, C. Leen, J. E. Bell, and P. Simmonds. 2001. Activated peripheral CD8 lymphocytes express CD4 in vivo and are targets for infection with human immunodeficiency virus type 1. J. Virol. 75:11555-11564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khatissian, E., L. Chakrabati, and B. Hurtrel. 1996. Cytokine patterns and viral load in lymph nodes during the early stages of SIV infection. Res. Virol. 147:181-189. [DOI] [PubMed] [Google Scholar]
- 29.Kruisbeek, A. 1994. In vitro assays for mouse lymphocyte function, vol. 1. John Wiley and Sons, New York, N.Y. [DOI] [PubMed]
- 30.Kuroda, M. J., J. E. Schmitz, W. A. Charini, C. E. Nickerson, M. A. Lifton, C. I. Lord, M. A. Forman, and N. L. Letvin. 1999. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J. Immunol. 162:5127-5133. [PubMed] [Google Scholar]
- 31.Landay, A. L., M. Clerici, F. Hashemi, H. Kessler, J. A. Berzofsky, and G. M. Shearer. 1996. In vitro restoration of T cell immune function in human immunodeficiency virus-positive persons: effects of interleukin-12 and anti-IL10. J. Infect. Dis. 173:1085-1091. [DOI] [PubMed] [Google Scholar]
- 32.Lawrence, C. E., J. J. Callanan, B. J. Willett, and O. Jarrett. 1995. Cytokine production by cats infected with feline immunodeficiency virus: a longitudinal study. Immunology 85:568-574. [PMC free article] [PubMed] [Google Scholar]
- 33.Liang, Y., L. C. Hudson, J. K. Levy, J. W. Ritchey, W. A. Tompkins, and M. B. Tompkins. 2000. T cells overexpressing interferon-gamma and interleukin-10 are found in both the thymus and secondary lymphoid tissues of feline immunodeficiency virus-infected cats. J. Infect. Dis. 181:564-575. [DOI] [PubMed] [Google Scholar]
- 34.McFarland, H. I., S. R. Nahill, J. W. Maciaszek, and R. M. Welsh. 1992. CD11b (Mac-1): a marker for CD8+ cytotoxic T cell activation and memory in virus infection. J. Immunol. 149:1326-1330. [PubMed] [Google Scholar]
- 35.McGuirk, P., C. McCann, and K. H. G. Mills. 2002. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates IL10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J. Exp. Med. 195:221-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meroni, L., D. Trabattoni, C. Balotta, C. Riva, A. Gori, M. Moroni, M. Villa, M. Clerici, and M. Galli. 1995. Evidence for type 2 cytokine production and lymphocyte activation in the early phases of HIV-1 infection. AIDS 10:23-30. [DOI] [PubMed] [Google Scholar]
- 37.Mitra, R. S., T. A. Judge, F. O. Nestle, L. A. Turka, and B. Nickoloff. 1995. Psoriatic skin-derived dendritic cell function is inhibited by exogenous IL-10. Differential modulation of B7-1 and B7-2. J. Immunol. 154:2668-2677. [PubMed] [Google Scholar]
- 38.Nakajima, K., M. O. Martinez, T. Hirano, E. C. Breen, P. G. Nishanian, G. J. Salazar, J. L. Fahey, and T. Kishimoto. 1989. Induction of IL-6 (B cell stimulatory factor-2/IFN-beta 2) production in HIV. J. Immunol. 142:531-536. [PubMed] [Google Scholar]
- 39.Obert, L. A., and E. A. Hoover. 2000. Relationship of lymphoid lesions to disease course in mucosal feline immunodeficiency virus type C infection. Vet. Pathol. 37:386-401. [DOI] [PubMed] [Google Scholar]
- 40.Ogg, G., S. Kostense, M. R. Klein, S. Jurriaans, D. Hamann, A. McMichael, and F. Miedema. 1999. Longitudinal phenotypic analysis of human immunodeficiency virus type 1-specific cytotoxic T lymphocytes: correlation with disease progression. J. Virol. 73:9153-9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohashi, T., R. Goitsuka, T. Watari, H. Tsujimoto, and A. Hasegawa. 1992. Elevation of feline interleukin 6-like activity in feline immunodeficiency virus infection. Clin. Immunol. Immunopathol. 65:207-211. [DOI] [PubMed] [Google Scholar]
- 42.O'Neil, L. L., M. J. Burkhard, L. J. Diehl, and E. A. Hoover. 1995. Vertical transmission of feline immunodeficiency virus. AIDS Res. Hum. Retrovir. 11:171-182. [DOI] [PubMed] [Google Scholar]
- 43.Orange, J. S., S. F. Wolf, and C. A. Biron. 1994. Effects of IL-12 on the response and susceptibility to experimental viral infections. J. Immunol. 152:1253-1264. [PubMed] [Google Scholar]
- 44.O'Reilly, K. L., and E. A. Hoover. 1993. Characterization of a panel of monoclonal antibodies specific for subsets of feline leukocytes, p. 38. In W. A. Tompkins (ed.), The Second International Feline Retrovirus Symposium. North Carolina State University, Raleigh, N.C.
- 45.Ostrowski, M. A., J. X. Gu, C. Kovacs, J. Freedman, M. A. Luscher, and K. S. MacDonald. 2001. Quantitative and qualitative assessment of human immunodeficiency virus type 1-specific CD4+ T cell immunity to gag in HIV-1-infected individuals with differential disease progression: reciprocal IFNγ and interleukin-10 responses. J. Infect. Dis. 184:1268-1278. [DOI] [PubMed] [Google Scholar]
- 46.Pedersen, N. C., E. W. Ho, M. L. Brown, and J. K. Yamamoto. 1987. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science 235:790-793. [DOI] [PubMed] [Google Scholar]
- 47.Pedersen, N. C., J. K. Yamamoto, T. Ishida, and H. Hansen. 1989. Feline immunodeficiency virus infection. Vet. Immunol. Immunopathol. 21:111-129. [DOI] [PubMed] [Google Scholar]
- 48.Perelson, A. S., P. Essenger, Y. Cao, M. Vesanen, A. Hurley, K. Sakseal, M. Markowitz, and D. D. Ho. 1997. Decay characteristics of HIV-1 infected compartments during combination therapy. Nature 387:188-191. [DOI] [PubMed] [Google Scholar]
- 49.Poli, G., P. Bressler, A. Kinter, W. C. Timmer, A. Rabson, J. S. Justernet, S. Stanley, and A. S. Fauci. 1990. Interleukin 6 induces human immunodeficiency virus expression in infected monocytic cells alone and in synergy with tumor necrosis factor alpha by transcriptional and post-translational mechanisms. J. Exp. Med. 172:151-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ralph, P., I. Nakoinz, A. Sampson-Johannes, S. Fong, D. Lowe, H. Y. Min, and L. Lin. 1992. IL10, T lymphocyte inhibitor of human blood cell production of IL-1 and tumor necrosis factor. J. Immunol. 148:808-814. [PubMed] [Google Scholar]
- 51.Ritchey, J. W., J. K. Levy, S. K. Bliss, W. A. Tompkins, and M. B. Tompkins. 2001. Constitutive expression of types 1 and 2 cytokines by alveolar macrophages from feline immunodeficiency virus-infected cats. Vet. Immunol. Immunopathol. 79:83-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roncarlo, M., R. Bacchetta, C. Bordignon, S. Narula, and M. K. Levings. 2001. Type 1 T regulatory cells. Immunol. Rev. 182:68-79. [DOI] [PubMed] [Google Scholar]
- 53.Rottman, J., E. Freeman, S. Tonkonogy, and M. Tompkins. 1995. A reverse transcription-polymerase chain reaction technique to detect feline cytokine genes. Vet. Immunol. Immunopathol. 45:1-18. [DOI] [PubMed] [Google Scholar]
- 54.Schutten, M., C. A. van Baalen, C. Guillon, R. C. Huisman, P. H. Boers, K. Sintnicolaas, R. A. Gruters, and A. Osterhaus. 2001. Macrophage tropism of human immunodeficiency virus type 1 facilitates in vivo escape from cytotoxic T-lymphocyte pressure. J. Virol. 75:2706-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sher, A., R. T. Gazzinelli, I. P. Oswald, M. Clerici, M. Kullberg, E. J. Pearce, J. A. Berzofsky, T. A. Mosmann, and H. C. Morse III. 1992. Role of T-cell derived cytokines in the downregulation of immune responses in parasitic and retroviral infection. Immunol. Rev. 127:183-204. [DOI] [PubMed]
- 56.Sparger, E. E., P. A. Luciw, J. H. Elder, J. K. Yamamoto, L. J. Lowenstine, and N. C. Pedersen. 1989. Feline immunodeficiency virus is a lentivirus associated with an AIDS-like disease in cats. AIDS 3:S43-S49. [DOI] [PubMed] [Google Scholar]
- 57.Stylianou, E., P. Aukrust, D. Kvale, F. Muller, and S. S. Froland. 1999. IL-10 in HIV infection: increasing serum IL-10 levels with disease progression-down-regulatory effect of potent anti-retroviral therapy. Clin. Exp. Immunol. 116:115-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tompkins, M. B., P. D. Nelson, R. V. English, and C. Novotney. 1991. Early events in the immunopathogenesis of feline retrovirus infections. J. Am. Vet. Med. Assoc. 199:1311-1315. [PubMed] [Google Scholar]
- 59.Trinchieri, G. 2001. Regulatory role of T cells producing both interferon γ and interleukin 10 in persistent infection. J. Exp. Med. 194:F53-F57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trivedi, P., D. Horejsh, S. B. Hinds, P. W. Hinds II, M. S. Wu, M. S. Salvato, and C. D. Pauza. 1996. Intrarectal transmission of simian immunodeficiency virus in rhesus macaques: selective amplification and host responses to transient or persistent viremia. J. Virol. 70:6876-6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weissman, D., G. Poli, and A. S. Fauci. 1995. IL10 synergizes with multiple cytokines in enhancing HIV production in cells of monocytic lineage. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 9:442-449. [PubMed] [Google Scholar]
- 62.Weissman, D., G. Poli, and A. S. Fauci. 1994. Interleukin 10 blocks HIV replication in macrophages by inhibiting the autocrine loop of tumor necrosis factor α and interleukin 6 induction of virus. AIDS Res. Hum. Retrovir. 10:1199-1206. [DOI] [PubMed] [Google Scholar]
- 63.Wilson, J. D., G. S. Ogg, R. L. Allen, C. Davis, S. Shaunak, J. Downie, W. Dyer, C. Workman, S. Sullivan, A. J. McMichael, and S. L. Rowland-Jones. 2000. Direct visualization of HIV-1-specific cytotoxic T lymphocytes during primary infection. AIDS 14:225-233. [DOI] [PubMed] [Google Scholar]
- 64.Yamamoto, J. K., E. Sparger, E. W. Ho, P. R. Andersen, T. P. O'Connor, C. P. Mandell, L. Lowenstine, R. Munn, and N. C. Pedersen. 1988. Pathogenesis of experimentally induced feline immunodeficiency virus infection in cats. Am. J. Vet. Res. 49:1246-1258. [PubMed] [Google Scholar]