Abstract
Numerous secretory cells use the regulated secretory pathway to release signaling molecules. Regulated secretion is an essential component of the intercellular communication network of a multicellular organism and serves diverse functions in neurobiology, endocrinology, and many other aspects of animal physiology. Probes that can monitor a specific exocytotic event with high temporal and spatial resolution would be invaluable tools for studying the molecular and cellular mechanisms underlying stimulus-secretion coupling, and for characterizing secretion defects that are found in different human diseases. This review summarizes different strategies and recent progress in developing fluorescent sensors for imaging regulated cell secretion.
Introduction
Secretion is a fundamental cellular process that is essential to almost all cell types of a living organism. Agenome-wide assessment of the secretory process in human cells estimated that ~15% of the proteins encoded by the human genome contribute to the process of secretion [1]. Cell secretion can be broadly classified into two pathways. The constitutive secretory pathway is used by essentially all eukaryotic cells and involves transporting secretory vesicles to the cell surface and releasing them independently of a biochemical stimulus [2]. In contrast, the regulated secretory pathway mainly operates in specialized secretory cells such as neurons, endocrine cells, exocrine cells and hematopoietic cells [3]. These cells produce secretory granules and store them in the cytoplasm including the subplasmalemmal area, and only secrete the content of these secretory granules into the extracellular milieu or the blood circulation upon receiving a bio-chemical or electrical stimulation. The released biomolecules, among others, may include neurotransmitters, peptide or protein hormones, growth factors, cytokines, and digestive enzymes. This regulated secretory pathway is an essential component of the intercellular communication network of a multicellular organism and serves diverse functions in neurobiology, endocrinology, and many other aspects of animal physiology. Probes that can monitor a specified exocytotic event with high temporal and spatial resolution would be invaluable tools for studying the molecular and cellular mechanisms under-lying stimulus-secretion coupling, and for characterizing secretion defects that are found in different human diseases.
Traditional electrical methods including patch-clamp capacitance measurement and carbon fiber amperometry have been used to monitor regulated secretion at the cellular level [4,5]. These methods provide very high temporal resolution, yet they are limited in their spatial resolution and can only be applied to a single cell at a time. Fluorescence microscopy, on the other hand, offers the major advantages including superb sensitivity of detection, high spatial and temporal resolution, and the capability of tracking multiple cells simultaneously in cell populations. Moreover, recent developments in nonlinear laser scanning microscopy have further facilitated visualizing cells deep within tissues that are inaccessible to electrophysiological approaches. Together, these benefits bring forth a drastic improvement in the throughput of assaying cell secretion by using optical techniques.
This review is focused on fluorescent probes and related imaging methods for monitoring exocytosis. We will summarize recent developments of three conceptually different strategies for imaging regulated secretion in living cells. These strategies include labeling secretory vesicles with fluorescent cargos, crafting fluorescent sensors responsive to a secreted product, and engineering reporter cells that express specific cell surface receptors and intracellular indicators for sensing secreted molecules.
Imaging fluorescently labeled secretory vesicles
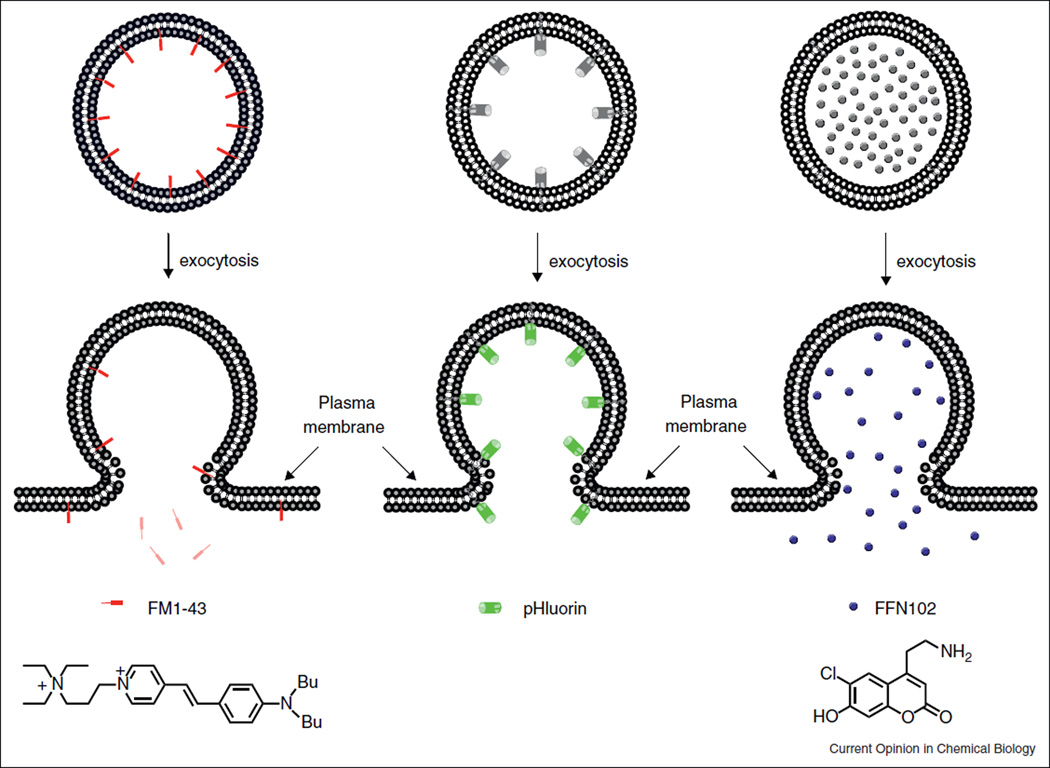
FM1–43 is an amphiphilic, membrane impermeable fluorescent styryl pyridinium dye. It has been widely used to image activity-dependent synaptic vesicle cycling [6]. FM1–43 can be introduced into the secretory vesicles through endocytosis. Once incorporated into the inner leaflet of the vesicle membrane, the dye exhibits much higher fluorescence intensity than in aqueous solutions. This facilitates imaging the dynamics of single labeled vesicles. Subsequent triggering of exocytosis promotes the fusion of the labeled vesicular membrane with the plasma membrane, during which FM1–43 rapidly diffuses into the surrounding plasma membrane and then, over a period of a few seconds, dissipate into the extracellular solution, losing fluorescence (Figure 1) [6•]. In addition to synaptic vesicles, FM1–43 has been applied to label other secretory vesicles in different cell types. In some cells, however, the dye may enter cells through other mechanism such as Ca2+ channels [7].
Figure 1.
Fluorescent probes for labeling and imaging secretory vesicles. FM1-43 is fluorescent in the membrane but nonfluorescent in the solution. pHluorin and FFN102 are weakly fluorescent in the lumen of secretory vesicles (pH < 6), but emit much stronger fluorescence during exocytosis when they are exposed to the extracellular medium (pH 7.3).
The secretory granule of mammalian cells is normally acidic. Since extracelluar fluids are neutral (pH ~7.3), secretory granules experience alkalinization during exocytosis. This phenomenon was first exploited by Miesenbock and co-workers to devise a fluorescent assay of exocytosis by using pH-sensitive mutants of EGFP, named pHluorins (pka ~7) [8••]. When fused with a vesicle membrane protein such as synaptobrevin (synapto-pHluorin), pHluorins were sorted to synaptic vesicles where they resided in the vesicle lumen and remained weakly fluorescent in this acidic compartment (pH ~5.6). Fusion of the vesicle with the plasma membrane led to a sudden rise of vesicular pH and a concomitant increase in the fluorescence signal (Figure 1). In this way, synapto-pHluorins provide a prompt readout of secretion and synaptic transmission. pHluorins have since been applied to tag a number of vesicle proteins to monitor exocytic events in different cells. These include synaptagmin [9], syncollin-pHluorin for pancreatic acinar cells [10], phogrin or neuropeptide Y for insulin release islet beta cells [11]. When combined with the spinning disk confocal microscopy, pHluorin fusion proteins allowed the visualization of exocytic events in real-time in three dimensions [10,11].
Recently, a pH sensitive red fluorescent protein, pHTomato, has been developed as an alternative to pHluorins for monitoring exocytosis [12•]. pHTomato has a pka of 7.8 so it is practically nonfluorescent below pH 6.3. Fusion of pHTomato with synaptophysin leads to sypHTomato which operates similarly as synapto-pHluorin. Its red-shifted excitation and emission is advantageous for tissue imaging where light scattering drops rapidly at longer wavelengths. Moreover, when combined with optogenetic actuators that can be activated at different excitation wavelengths [13], pHTomato facilitates an all-optical strategy for simultaneous controlling and monitoring of neural activity [12•].
While pH sensitive fluorescent proteins remain to be a versatile tool for imaging excytosis, it should be cautioned that expressing fluorescent proteins in granules nevertheless can potentially perturb the biogenesis, trafficking, and/or localization of native secretory granules in cells [14•]. These observations highlight the fact that, at least in certain cells, the behavior of granule-targeted fluorescent cargos may be different from that of native cargos, and hence point out the need of comparing exocytotic behaviors by using different labels and approaches when studying mechanisms of granule fusion.
Fluorescent false neurotransmitters (FFNs) represent a new class of cell specific imaging labels for visualizing neurotransmitter release [15,16••]. FFN102 (Figure 1), for instance, is a water soluble coumarin dye whose cellular uptake relies on the plasma membrane dopamine transporter. Once inside cells, it is enriched in the secretory granule by the vesicular monoamine transporter 2 (VMAT2). In acute brain slices, FFN102 selectively labeled dopaminergic neurons and allowed optical measurements of dopamine release following cell depolarization [16••].
The above fluorescent granular labels for far field measurements of exocytosis can also be used with total internal reflection fluorescence (TIRF) microscopy (or evanescent wave microscopy). TIRF microscopy provides enhanced imaging resolution of the plasma membrane immediately adjacent to the glass cover slip. This attributes to the limited penetration depth (~250 nm) of the excitation light and hence the reduced background fluorescence from the bulk cytoplasm [17]. TIRF microscopy allows monitoring single vesicle movement in cells before exocytosis and granule fusion with very high spatiotemporal resolution but is limited to plasmalemmal events in contact with the glass cover slip — a location that is likely quite different from the physiological release sites normally seen in the interstitial space of tissues.
Besides these three classes of fluorescent granular labels, fluorescent quantum dots [18] and extracellular polar-tracer (e.g. sulforhodamine B) [19] have also been applied to image exocytosis. In the former case, single quantum dots were loaded into individual synaptic vesicles and were used to distinguish kiss-and-run from full fusion events.
Monitoring exocytosis using fluorescent sensors of the released products
The second strategy for imaging exocytosis employs fluorescent indicators responsive to the secreted products. Since this approach does not require filling the secretory granules with exogenous labels, it avoids modifying endogenous granules or altering their cellular behavior and hence should reveal the native characteristics of their exocytotic activity. To achieve higher sensitivity of detection, these sensors are generally targeted to the outer leaflet of the plasma membrane where the local concentration of the released products is highest. In this section we will summarize fluorescent sensors for detecting the release of small molecules including Zn2+, certain amino acids and nucleotides.
Zn2+
A variety of mammalian cells, including pancreatic islet beta cells, prostate epithelial cells, certain glutamatergic neurons in the central nervous system, Paneth cells, pituitary cells, and mast cells, contain a high level of Zn2+ in their secretory granules [20]. During secretion, Zn2+ is co-released with other contents of the secretory granule. As such, the released Zn2+ has been exploited as a surrogate for monitoring the secretion of other biomolecules including, for instance, insulin of islet beta cells. Zn2+ itself may also play important roles in cell signaling and there have been strong interests in understanding how Zn2+ functions as an intercellular messenger in two biological systems, pancreatic islets and central nervous systems [21,22]. Impairments in Zn2+ secretion from the pancreas, prostate, and mammary gland are associated with disorders such as diabetes, infertility, and cancer, respectively [23].
Several fluorescent Zn2+ sensors including FluoZin-3 [24,25], RhodZin-3 and Newport Green DCF [26] have been applied to monitor Zn2+ release. However, since these Zn2+ sensors are applied to the extracellular bath, the sensitivity of detecting local Zn2+ release near the plasma membrane is compromised by the background fluorescence from the bulk solution. To reject bulk fluorescence signal and to improve the sensitivity of detection, TIRF microscopy has been applied [26], but the assay is limited to cultured cells and cannot be extended to tissues. Attempts of imaging Zn2+ release at the synapses using ZnAF-2 or ZP4 were confounded by the report that these probes, despite containing negative charges and a hydrophilic dipicolylamine, were nevertheless cell membrane permeant when added to the brain tissue [27]. This made it difficult to interpret the source of Zn2+ activity when changes in fluorescence intensity were spotted.
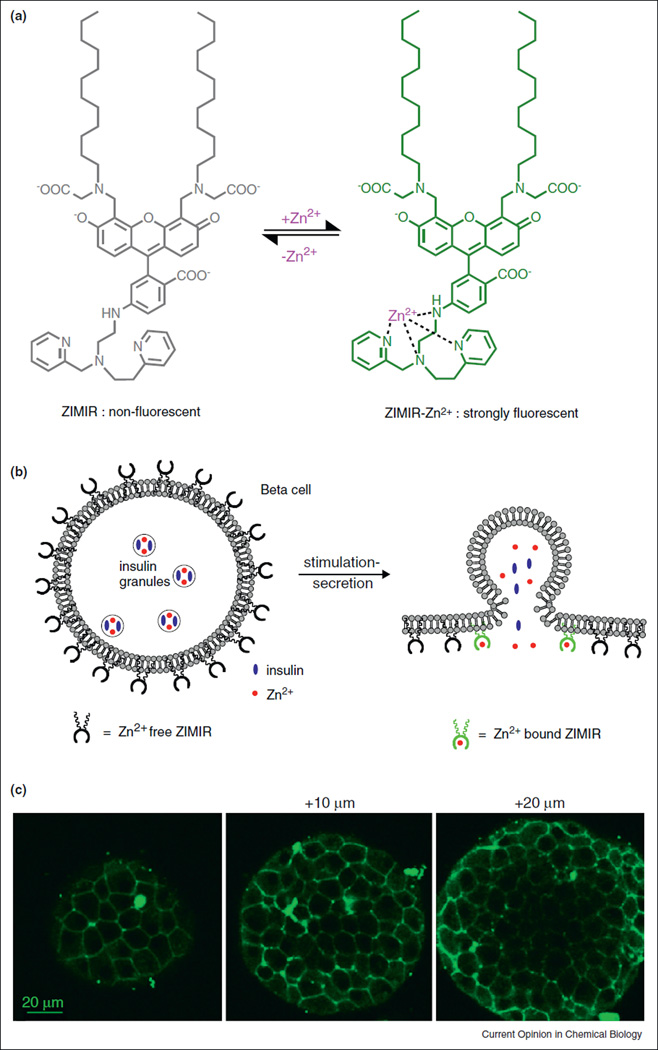
To enhance the sensitivity of detecting Zn2+ secretion and to develop a robust imaging assay compatible with wide-field epifluorescence detection, we recently developed an amphipathic fluorescent zinc indicator for monitoring induced exocytotic Zn + release (ZIMIR, Figure 2) [28••]. ZIMIR contains a pair of dodecyl alkyl chains for membrane tethering, yet it is also highly charged near neutral pH so it cannot diffuse across cell membrane by itself. When ZIMIR is added to cells at low micromolar concentration, the dodecyl chains quickly anchored the molecule to the outer leaflet of the plasma membrane through hydrophobic interactions. The labeling is non-invasive and does not appear to have any observable side effects on cell growth or function. ZIMIR binds Zn 2+ with a Kd of 0.45 µM and displayed a 70-fold enhancement in fluorescence intensity upon Zn2+ binding. A very useful feature of ZIMIR is that the sensor is able to diffuse through interstitial spaces to label both superficial and interior cells in tissues (Figure 2c). This makes it possible to apply confocal or two photon laser scanning microscopy to image insulin/Zn2+ release dynamics in three dimensions with high spatial and temporal resolution. For instance, in isolated rat islets, confocal ZIMIR imaging revealed that islet beta cells were fairly heterogenous in their secretory responses to glucose stimulation, and that small clusters of adjacent islet beta cells act as ‘secretory units to release insulin in synchrony [28••].
Figure 2.
A zinc indicator for monitoring induced exocytotic release (ZIMIR). (a) Chemical structure of ZIMIR in the Zn2+-free (nonfluorescent) and Zn2+-bound (strongly fluorescent) states. (b) Mode of action of ZIMIR for reporting local Zn2+ elevation at the membrane surface during exocytotic insulin granule fusion. The two lipophilic alkyl chains (wavy lines) anchor ZIMIR to the outer leaflet of the membrane lipid bilayer. (c) Confocal images of a ZIMIR-stained mouse pancreatic islet at three imaging depths. Adapted from [28].
Since ZIMIR imaging can be easily applied to cell populations, it also offers an efficient and convenient assay to screen for individual cell clones manifesting robust secretory response or to identify compounds or genes of therapeutic potential for treating diabetes using high-throughput platforms. The former application is of interest to stem cell researchers who are engineering insulin-releasing beta cells from human stem cells for the cell replacement therapy to treat diabetes.
Amino acids
Glutamate is an essential excitatory neurotransmitter in the nervous systems and is used by many neurons to control diverse neuronal signaling and animal behaviors. In glutamatergic neurons, glutamate is stored in synaptic vesicles. Action potential triggers release of glutamate which then acts on glutamate receptors on postsynaptic neurons. Because of its involvement in the formation of synaptic plasticity, glutamate is known to play a role in learning and memory. In addition to neurons, glutamate is also used as an intercellular signaling molecule by other cells including astrocytes and endocrine cells of the islet.
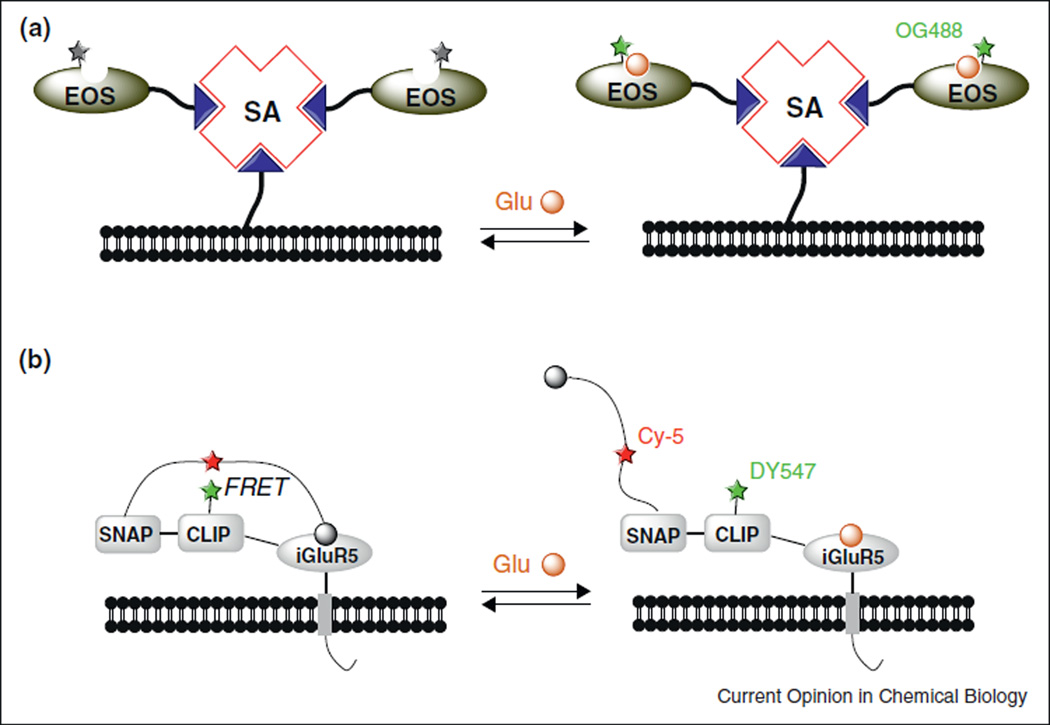
A number of naturally occurring glutamate-binding proteins have been employed as templates for designing fluorescent glutamate sensors. In one approach of combining protein expression with chemical bioconjugation, Hirose, Iino and coworkers developed glutamate (E) optical sensors (collectively termed EOS) that consisted of a mutated AMPA receptor glutamate-binding subunit (GluR2) and a small fluorophore, Oregon Green 488 [29–31]. Oregon Green 488 was conjugated to an amino acid near the glutamate binding pocket to render its signal intensity sensitive to the binding of glutamate. In vitro, EOS bound to glutamate with sub-micromolar affinity [31]. To label cells with EOS, they exploited the multiple binding sites of streptavidin to connect the biotinylated plasma membrane with biotinylated EOS (Figure 3a). Once attached to the cell surface, EOS displayed maximal fluorescence enhancements (ΔF/F0) up to 48% and was able to detect physiologically evoked glutamate dynamics in the extrasynaptic space in both brain slices and in the somatosensory cortex of living rats.
Figure 3.
Semisynthetic fluorescent glutamate sensors consisting of small synthetic fluorophores and a glutamate-binding protein. (a) Biotinylated EOS prepared in vitro was adhered to the cell membrane through streptavidin (SA). Binding of glutamate enhances the signal of the attached fluorophore (green star). Biotin is represented by the blue triangle. (b) A FRET based glutamate sensor, Snifit-iGluR5.
Johnson and coworkers recently reported a semisynthetic glutamate sensor, Snifit-iGluR5 [32•], based on the SNAP-tag labeling technology [33]. Snifit-iGluR5 consisted of four polypeptide chains fused in tandem: a transmembrane domain for membrane anchoring, a glutamate-binding motif from ionotropic glutamate receptor 5 (iGluR5), a SNAP-tag and a CLIP-tag [34]. The CLIP-tag was labeled with a fluorescent dye DY-547, and the SNAP tag was labeled with a Cy-5 derivative containing a glutamate agonist. Since free glutamate in the solution and the covalently linked glutamate agonist compete-etively bound to iGluR5, the fluorescence resonance energy transfer (FRET) efficiency between DY-547 and Cy-5 reflected the level of free glutamate (Figure 3b). When expressed in HEK 293T cells, Sni-fit-iGluR5 displayed a maximal emission ratio (DY-547/Cy-5) change of 56% and a Kd (glutamate) of 15 µM. While the performance of Snifit-iGluR5 remains to be demonstrated in the nervous systems, the concept of glutamate sensing and the modular design of Snifit sensors can in principle be extended to design other FRET indicators of different analytes, for instance, the inhibitory neurotransmitter GABA [35].
Compared to the above semisynthetic glutamate sensors, genetically encoded glutamate sensors employing fluorescent proteins circumvent the requirement of in situ dye labeling and can be more advantageous in biological preparations where the cellular access to the dye is limiting. FLIPE [36] and SuperGluSnFR [37] are genetically encoded FRET indicators with a cyan fluorescent protein (CFP) and a yellow fluorescent protein (YFP) attached to the N-terminus and C-terminus of a bacterial glutamate binding protein ybeJ (GltI). These two indicators have a maximum FRET ratio change of ~45% which limited their ability of detecting glutamate fluctuations in tissues. Recently an intensity-based glutamate sensor, iGluSnFR, has been developed [38•]. iGluSnF R was constructed by inserting GltI into circularly permutated (cp) GFP with optimized linkers. In vitro, purified iGluSnFR displayed a remarkable intensity change (ΔF/F) of 4.5 with a Kd (glutamate) of 107 µM. In cultured neurons, the expression of iGluSnFR on the cell surface altered its Kd(glutamate) to 4.9 µM and its maximum ΔF/F to 1.03. The improved dynamic range and sensitivity of iGluSnFR broadened its application to a variety of biological preparations for detecting glutamate release evoked by physiological means [38•].
ATP
A large variety of cells release ATP through different pathways in response to mechanical or biochemical stimulations. Once secreted, ATP binds to P2 receptors on the cell surface and acts as a paracrine or autocrine signal to modulate cell functions. The most commonly used method for assaying cellular ATP release is based on luciferase-catalyzed, ATP-dependent generation of bio-luminescence from luciferin. Since this reaction only produces weak luminescence, it limits the signal to noise ratio and hence its application in optical imaging. To enhance the sensitivity of detecting local ATP release immediately adjacent to the plasma membrane, luciferase was displayed on the outer cell surface using a variety of methods including heterologous expression with a GPI anchor [39], or affinity labeling using antibody [40]. By imaging bioluminescence of a biotinylated luciferase tethered to the cell surface through streptavidin, Ando and coworkers showed that sheer stress stimulation of vascular endothelial cells provoked local ATP release at caveolin rich regions of the cell membrane [41].
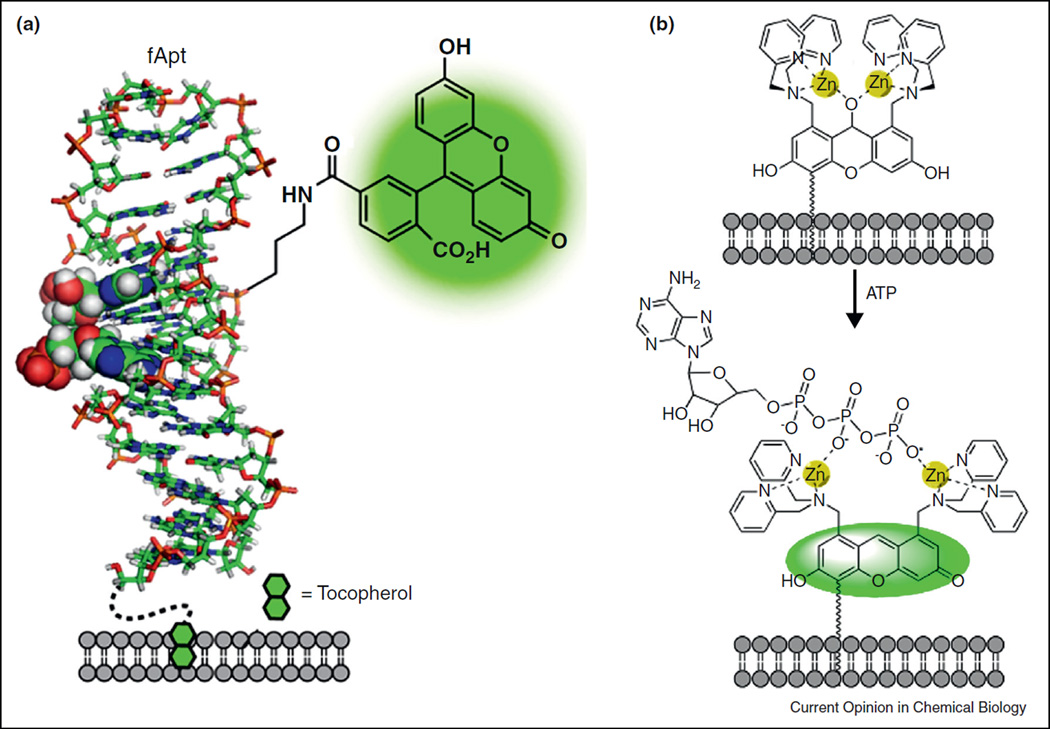
Sando and coworkers applied an adenine-binding DNA aptamer, Apt [42], to develop a fluorescent adenine sensor, fApt [43•]. Labeling Apt with fluorescein at a site close to the adenine binding pocket generated fApt whose fluorescent signal became sensitive to the binding to adenine containing molecules (including ATP). To apply fApt to image cellular ATP release, they labeled the 5’-terminus of fApt with tocopherol (vitamin E), a nontoxic lipophilic molecule that adhered directly to cellular membrane lipids (Figure 4a). In cultured astrocytes, tocopherol-fApt (toc-fApt) was able to detect ATP release evoked by mechanical stimulations [43•].
Figure 4.
Fluorescent sensors for adenine-containing or polyphosphate-containing nucleotides. (a) Toc-fApt contains a fluorescein labeled adenine-binding aptamer (f-Apt) anchored to the cell surface by tocopherol. (b) A Zn2+-binding xanthene derivative adheres to the cell membrane through its hydrophobic tail. Binding to polyphosphate restores its fluorescence.
In a different approach, Hamachi and coworkers demonstrated the feasibility of engineering small synthetic fluorophores for sensing ATP [44]. They prepared a xanthene derivative containing a pair of Zn2+ binding moiety made of dipicolylamine. The chelated zinc ions coordinated with a water molecule and consequently promoted the water attacking the xanthene fluorophore to disrupt its conjugation (Figure 4b). Binding of ATP or other polyphosphates with zinc ions eliminated the water adduct and recovered xanthene conjugation and hence its fluorescence. Using a membrane anchored form of this sensor, the authors reported the detection of cellular ATP leakage after permeabilizing cell membranes with detergents [44].
Finally, genetically encoded fluorescent ATP sensors also represent a promising approach for imaging ATP release. Noji and coworkers created a series of FRET-based biosensors, termed ATeams, that were composed of the ε-subunit of the bacterial FoF1-ATP synthase sandwiched between a pair of fluorescent proteins [45]. ATeams bound to ATP with affinities ranging from 7.4 µM to 3.3 mM and showed high selectivity to ATP over other nucleotides. These sensors have been successfully applied to measure intracellular ATP levels, so in principle ATeams ought to be adapted for imaging ATP secretion when targeted to outer cell surface.
Cell based sensors
Cell based sensors, also termed sniffer cells or biosensors, have been developed for monitoring exocytosis. The assay involves expressing a specific cell surface receptor recognizing an analyte of interest and placing the cell (‘sniffer cell’ or ‘biosensor’) in close juxtaposition to the secretory cells releasing the analyte. For instance, to monitor ATP release, Okada and coworkers expressed a purinergic receptor (P2×2 receptor, a ligand gated cation channel) in PC12 cells [46]. Electrophysiological recording or Ca2+ imaging of PC12 cells was then performed to monitor the activation of P2×2 receptor, which reflected the secretory activity of juxtaposed cells that release ATP [47,48].
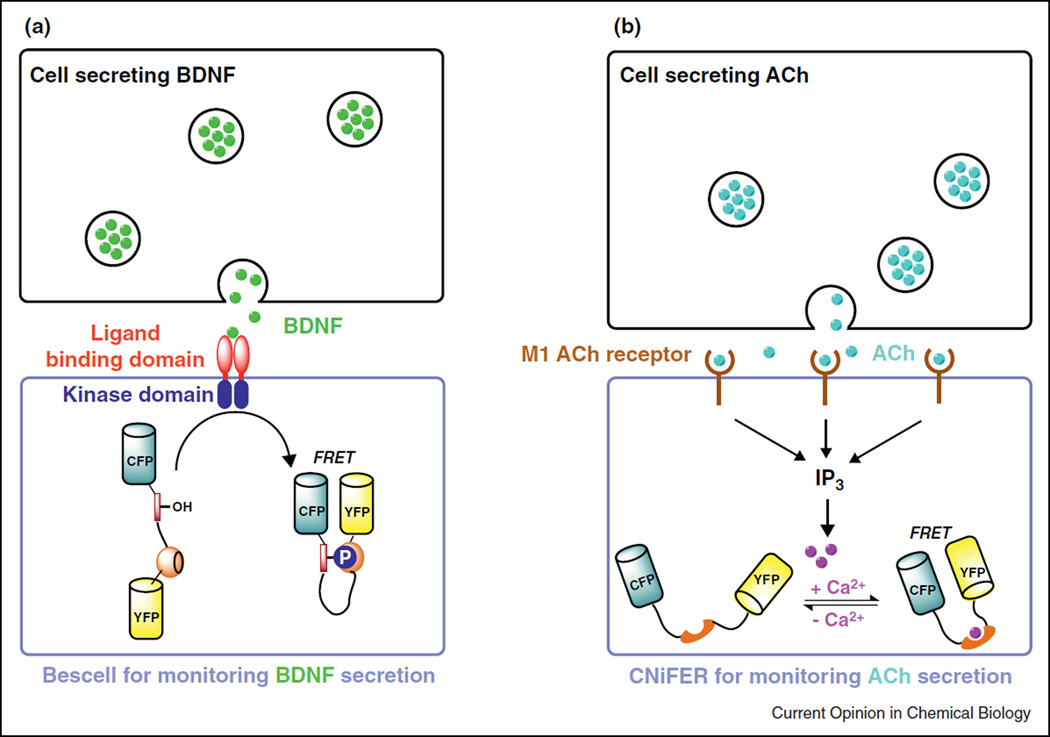
Other recent examples of cell based sensors for imaging secretion include Bescell [49] and CNiFER (Figure 5) [50]. Bescell was developed to follow the release of brain-derived neurotrophic factor (BDNF), a secreted protein that plays a key role in the regulation of growth and survival of neurons. By co-expressing a chimeric receptor tyrosine kinase that bound with BDNF and a genetically encoded fluorescent indicator for visualizing BDNF induced protein phosphorylation (Figure 5a), the authors were able to detect BDNF down to 60 pM and to monitor BDNF secretion from hippocampal neurons [49]. To detect the secretion of a neurotransmitter, acetyl choline (ACh), Kleinfeld and coworkers devised cell-based neurotransmitter fluorescent engineered reporters (CNiFERs) by expressing a metabotropic ACh receptor in HEK293 cells [50]. Activation of muscarinic ACh receptors activated a cascade of cell signaling to raise cytosolic Ca2+ which was detected with a co-expressed Ca2+ sensor.
Figure 5.
Cell based sensors for monitoring BDNF or ACh secretion. (a) Bescell detected BDNF release using a genetically encoded FRET sensor for protein phosphorylation. (b) In CNiFERs, activation of the M1 ACh receptor increased cytosolic Ca2+ via the Gq/inositol triphosphate (IP3) second messenger pathway. Cytosolic Ca2+ was monitored with a genetically encoded FRET Ca2+ sensor, TN-XXL.
These cell based biosensors exploit the exquisite sensitivity and selectivity of a native receptor to its cognate ligand. In principle, the approach can be generalized to design other biosensors by expressing appropriate receptors for virtually every known signaling molecules or secreted products. It is also expected to provide high sensitivity of detection by exploiting the biochemical amplification downstream of the receptor activation in a cellular context. However, the application of these biosensors requires placing a living cell in close proximity to other secretory cells, so the manipulation can be cumbersome especially when assaying secretion in cell populations including tissues.
Conclusions and outlook
Diverse strategies have been developed over the past years for imaging cell secretion. Understanding the physiological regulation of stimulus-secretion coupling would ideally require studying secretion in dissected tissues or living animals where cells reside in their native environments and maintain normal cell-cell interactions. Because tissues typically contain different types of cells, imaging probes that report the exocytotic activity of a given cell type containing their own granules and unique cargos are desirable. While recent progress in engineering fluorescent probes for imaging defined analytes has enabled us to track the exocytosis of several specific granules in physiological preparations [16••,28••,30,38••], there are still enormous needs for devising new sensors to follow the release of many other hormones, cytokines or signaling molecules from a large variety of secretory cells. Fluorescent indicators that bind to a secreted molecule with high specificity, appropriate affinity, fast association and dissociation rates, and display robust signal changes upon binding would be ideal for monitoring the dynamics of regulated secretion in real time.
There is great potential of engineering genetically encoded fluorescent sensors to meet the demands. These protein-based sensors can be designed systematically by fusing different protein modules. Diversified libraries containing either random or biased mutations can be conveniently constructed with the technique of molecular biology, expressed in a suitable system, and efficiently screened by the sensitive detection of fluorescence signal. Genetically encoded sensors, however, do not necessarily replace small indicators prepared from synthetic chemistry. These small chemical sensors can be applied to label cells in situ without genetic expression so they can be particularly useful when studying freshly isolated tissues from higher mammals including primates where the technique of transgenic is not yet practical or cell transfection is not desirable. Moreover, combining small synthetic dyes with various protein tagging strategies further enhance the versatility of their applications [32•]. Finally, DNA aptamers also holds great promise as a general template for designing sensors. Aptamers are specific, versatile, and in principle can be generated for any signaling molecule by an in vitro selection process termed systematic evolution of ligands by exponential enrichment (SELEX) [51].
There has been enormous progress in developing fluorescent sensors for imaging cellular biochemistry [52,53]. The combinatory uses of some of those sensors with the ones reviewed here for imaging regulated secretion would allow a multicolor imaging approach to study mechanisms governing stimulus-secretion coupling. In addition, there have been continuing efforts and success in crafting optogenetic and thermogenetic actuators for manipulating protein activity and controlling cell signaling [13,54]. Future integrations of these new tools will undoubtedly bring forth exciting opportunities to advance our under-standing on how cells control the timing, pattern and sites of vesicular release in complex biological systems to achieve desired physiological output. To follow cell secretion in living animals at the depth where light penetration or scattering becomes limiting, developing probes for whole body imaging techniques such as position emission tomography and magnetic resonance imaging [55•] also presents exciting opportunities and challenges.
Acknowledgements
We thank the financial supports from NIH (grant R01-GM077593) and Juvenile Diabetes Research Foundation (grant 37-2011-21). This work was performed in laboratories constructed with support from NIH grant C06 RR30414.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Simpson JC, Joggerst B, Laketa V, Verissimo F, Cetin C, Erfle H, Bexiga MG, Singan VR, Heriche JK, Neumann B, Mateos A, et al. Genome-wide RNAi screening identifies human proteins with a regulatory function in the early secretory pathway. Nat Cell Biol. 2012;14:764–774. doi: 10.1038/ncb2510. [DOI] [PubMed] [Google Scholar]
- 2.Ponnambalam S, Baldwin SA. Constitutive protein secretion from the trans-golgi network to the plasma membrane. Mol Membr Biol. 2003;20:129–139. doi: 10.1080/0968768031000084172. [DOI] [PubMed] [Google Scholar]
- 3.Burgess TL, Kelly RB. Constitutive and regulated secretion of proteins. Annu Rev Cell Biol. 1987;3:243–293. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez JM, Neher E, Gomperts BD. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. Nature. 1984;312:453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- 5.Kita JM, Wightman RM. Microelectrodes for studying neurobiology. Curr Opin Chem Biol. 2008;12:491–496. doi: 10.1016/j.cbpa.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gaffield MA, Betz WJ. Imaging synaptic vesicle exocytosis and endocytosis with FM dyes. Nat Protoc. 2006;1:2916–2921. doi: 10.1038/nprot.2006.476. A comprehensive and detailed account on the application of FM dyes for imaging vesicle cycling.
- 7.Li D, Herault K, Oheim M, Ropert N. FM dyes enter via a store-operated calcium channel and modify calcium signaling of cultured astrocytes. Proc Natl Acad Sci USA. 2009;106:21960–21965. doi: 10.1073/pnas.0909109106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. The first demonstration of imaging synaptic vesicles release with a genetically encoded and pH-sensitive label.
- 9.Fernandez-Alfonso T, Kwan R, Ryan TA. Synaptic vesicles interchange their membrane proteins with a large surface reservoir during recycling. Neuron. 2006;51:179–186. doi: 10.1016/j.neuron.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez NA, Liang T, Gaisano HY. Live pancreatic acinar imaging of exocytosis using syncollin-phluorin. Am J Physiol Cell Physiol. 2011;300:C1513–C1523. doi: 10.1152/ajpcell.00433.2010. [DOI] [PubMed] [Google Scholar]
- 11.Rutter GA, Loder MK, Ravier MA. Rapid three-dimensional imaging of individual insulin release events by nipkow disc confocal microscopy. Biochem Soc Trans. 2006;34(Pt 5):675–678. doi: 10.1042/BST0340675. [DOI] [PubMed] [Google Scholar]
- 12. Li Y, Tsien RW. Phtomato, a red, genetically encoded indicator that enables multiplex interrogation of synaptic activity. Nat Neurosci. 2012;15:1047–1053. doi: 10.1038/nn.3126. A new color variant of the synaptic vesicle label was developed to facilitate multi-color imaging and integration with optogenetic actuators to study regulated secretion.
- 13.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Michael DJ, Tapechum S, Rohan JG, Johnson JM, Chow RH. Fluorescent cargo proteins in peptidergic endocrine cells cell type determines secretion kinetics at exocytosis. Ann N YAcad Sci. 2009;1152:7–17. doi: 10.1111/j.1749-6632.2008.04006.x. This study and the cited references cautioned the potential pitfall of altering the behavior of secretory granules when they were labeled with foreign protein cargos.
- 15.Gubernator NG, Zhang H, Staal RG, Mosharov EV, Pereira DB, Yue M, Balsanek V, Vadola PA, Mukherjee B, Edwards RH, Sulzer D, et al. Fluorescent false neurotransmitters visualize dopamine release from individual presynaptic terminals. Science. 2009;324:1441–1444. doi: 10.1126/science.1172278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rodriguez PC, Pereira DB, Borgkvist A, Wong MY, Barnard C, Sonders MS, Zhang H, Sames D, Sulzer D. Fluorescent dopamine tracer resolves individual dopaminergic synapses and their activity in the brain. Proc Natl Acad Sci USA. 2013;110:870–875. doi: 10.1073/pnas.1213569110. The authors applied an innovative approach of labeling dopaminergic neurons with fluorescent false neurotransmitter that allowed optical measurements of dopamine release.
- 17.Axelrod D. Total internal reflection fluorescence microscopy in cell biology. Traffic. 2001;2:764–774. doi: 10.1034/j.1600-0854.2001.21104.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q, Li Y, Tsien RW. The dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles. Science. 2009;323:1448–1453. doi: 10.1126/science.1167373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasai H, Kishimoto T, Nemoto T, Hatakeyama H, Liu TT, Takahashi N. Two-photon excitation imaging of exocytosis and endocytosis and determination of their spatial organization. Adv Drug Deliv Rev. 2006;58:850–877. doi: 10.1016/j.addr.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nat Rev Neurosci. 2005;6:449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 21.Sindreu C, Storm DR. Modulation of neuronal signal transduction and memory formation by synaptic zinc. Front Behav Neurosci. 2011;5:68. doi: 10.3389/fnbeh.2011.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardy AB, Serino AS, Wijesekara N, Chimienti F, Wheeler MB. Regulation of glucagon secretion by zinc: lessons from the beta cell-specific znt8 knockout mouse model. Diab Obes Metab. 2011;13(Suppl 1):112–117. doi: 10.1111/j.1463-1326.2011.01451.x. [DOI] [PubMed] [Google Scholar]
- 23.Kelleher SL, McCormick NH, Velasquez V, Lopez V. Zinc in specialized secretory tissues roles in the pancreas, prostate, and mammary gland. Adv Nutr. 2011;2:101–111. doi: 10.3945/an.110.000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gee KR, Zhou ZL, Qian WJ, Kennedy R. Detection and imaging of zinc secretion from pancreatic beta-cells using a new fluorescent zinc indicator. J Am Chem Soc. 2002;124:776–778. doi: 10.1021/ja011774y. [DOI] [PubMed] [Google Scholar]
- 25.Qian J, Noebels JL. Visualization of transmitter release with zinc fluorescence detection at the mouse hippocampal mossy fibre synapse. J Physiol. 2005;566(Pt 3):747–758. doi: 10.1113/jphysiol.2005.089276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michael DJ, Ritzel RA, Haataja L, Chow RH. Pancreatic beta-cells secrete insulin in fast- and slow-release forms. Diabetes. 2006;55:600–607. doi: 10.2337/diabetes.55.03.06.db05-1054. [DOI] [PubMed] [Google Scholar]
- 27.Kay AR, Toth K. Influence of location of a fluorescent zinc probe in brain slices on its response to synaptic activation. J Neurophysiol. 2006;95:1949–1956. doi: 10.1152/jn.00959.2005. [DOI] [PubMed] [Google Scholar]
- 28. Li D, Chen S, Bellomo EA, Tarasov AI, Kaut C, Rutter GA, Li WH. Imaging dynamic insulin release using a fluorescent zinc indicator for monitoring induced exocytotic release (ZIMIR) Proc Natl Acad Sci USA. 2011;108:21063–21068. doi: 10.1073/pnas.1109773109. A small synthetic zinc indicator was shown to label cells rapidly and noninvasively and to provide robust and sensitive readout of hormone secretion in three dimensions in intact pancreatic islets. Potentially useful for high throughput screening in cell populations.
- 29.Namiki S, Sakamoto H, Iinuma S, Iino M, Hirose K. Optical glutamate sensor for spatiotemporal analysis of synaptic transmission. Eur J Neurosci. 2007;25:2249–2259. doi: 10.1111/j.1460-9568.2007.05511.x. [DOI] [PubMed] [Google Scholar]
- 30.Okubo Y, Iino M. Visualization of glutamate as a volume transmitter. J Physiol. 2011;2011;589(Pt 3):481–488. doi: 10.1113/jphysiol.2010.199539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okubo Y, Sekiya H, Namiki S, Sakamoto H, Iinuma S, Yamasaki M, Watanabe M, Hirose K, Iino M. Imaging extrasynaptic glutamate dynamics in the brain. Proc Natl Acad Sci USA. 2010;107:6526–6531. doi: 10.1073/pnas.0913154107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brun MA, Tan KT, Griss R, Kielkowska A, Reymond L, Johnsson K. A semisynthetic fluorescent sensor protein for glutamate. J Am Chem Soc. 2012;134:7676–7678. doi: 10.1021/ja3002277. The authors presented a new approach of designing cell surface FRET sensors for imaging secretion exploiting the principle of competitive binding and techniques of tagging cellular proteins.
- 33.Brun MA, Griss R, Reymond L, Tan KT, Piguet J, Peters RJ, Vogel H, Johnsson K. Semisynthesis of fluorescent metabolite sensors on cell surfaces. J Am Chem Soc. 2011;133:16235–16242. doi: 10.1021/ja206915m. [DOI] [PubMed] [Google Scholar]
- 34.Gautier A, Juillerat A, Heinis C, Correa IR, Jr, Kindermann M, Beaufils F, Johnsson K. An engineered protein tag for multiprotein labeling in living cells. Chem Biol. 2008;15:128–136. doi: 10.1016/j.chembiol.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Masharina A, Reymond L, Maurel D, Umezawa K, Johnsson K. A fluorescent sensor for GABA and synthetic GABA(b) receptor ligands. J Am Chem Soc. 2012;134:19026–19034. doi: 10.1021/ja306320s. [DOI] [PubMed] [Google Scholar]
- 36.Okumoto S, Looger LL, Micheva KD, Reimer RJ, Smith SJ, Frommer WB. Detection of glutamate release from neurons by genetically encoded surface-displayed fret nanosensors. Proc Natl Acad Sci USA. 2005;102:8740–8745. doi: 10.1073/pnas.0503274102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hires SA, Zhu Y, Tsien RY. Optical measurement of synaptic glutamate spillover and reuptake by linker optimized glutamate-sensitive fluorescent reporters. Proc Natl Acad Sci USA. 2008;105:4411–4416. doi: 10.1073/pnas.0712008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marvin JS, Borghuis BG, Tian L, Cichon J, Harnett MT, Akerboom J, Gordus A, Renninger SL, Chen TW, Bargmann CI, Orger MB, et al. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat Methods. 2013;10:162–170. doi: 10.1038/nmeth.2333. Using the techniques of structure guided protein engineering and fluorescence screening of mutations, the authors developed an optimized fluorescent sensor showing robust response to glutamate increase in a variety of biological preparations.
- 39.Pellegatti P, Falzoni S, Pinton P, Rizzuto R, Di Virgilio F. A novel recombinant plasma membrane-targeted luciferase reveals a new pathway for ATP secretion. Mol Biol Cell. 2005;16:3659–3665. doi: 10.1091/mbc.E05-03-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joseph SM, Buchakjian MR, Dubyak GR. Colocalization of ATP release sites and ecto-atpase activity at the extracellular surface of human astrocytes. J Biol Chem. 2003;278:23331–23342. doi: 10.1074/jbc.M302680200. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto K, Furuya K, Nakamura M, Kobatake E, Sokabe M, Ando J. Visualization of flow-induced ATP release and triggering of Ca2+ waves at caveolae in vascular endothelial cells. J Cell Sci. 2011;124(Pt 20):3477–3483. doi: 10.1242/jcs.087221. [DOI] [PubMed] [Google Scholar]
- 42.Jhaveri SD, Kirby R, Conrad R, Maglott EJ, Bowser M, Kennedy RT, Glick G, Ellington AD. Designed signaling aptamers that transduce molecular recognition to changes in fluorescence intensity. J Am Chem Soc. 2000;122:2469–2473. [Google Scholar]
- 43. Tokunaga T, Namiki S, Yamada K, Imaishi T, Nonaka H, Hirose K, Sando S. Cell surface-anchored fluorescent aptamer sensor enables imaging of chemical transmitter dynamics. J Am Chem Soc. 2012;134:9561–9564. doi: 10.1021/ja302551p. The work illustrated the feasibility of using aptamers as a scaffold to design sensors for imaging cell secretion.
- 44.Kurishita Y, Kohira T, Ojida A, Hamachi I. Organelle-localizable fluorescent chemosensors for site-specific multicolor imaging of nucleoside polyphosphate dynamics in living cells. J Am Chem Soc. 2012;134:18779–18789. doi: 10.1021/ja308754g. [DOI] [PubMed] [Google Scholar]
- 45.Imamura H, Huynh Nhat KP, Togawa H, Saito K, Iino R, Kato-Yamada Y, Nagai T, Noji H. Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proc Natl Acad Sci USA. 2009;106:15651–15656. doi: 10.1073/pnas.0904764106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hazama A, Hayashi S, Okada Y. Cell surface measurements of ATP release from single pancreatic beta cells using a novel biosensor technique. Pflugers Arch. 1998;437:31–35. doi: 10.1007/s004240050742. [DOI] [PubMed] [Google Scholar]
- 47.Hayashi S, Hazama A, Dutta AK, Sabirov RZ, Okada Y. Detecting ATP release by a biosensor method. SciSTKE. 2004;2004:114. doi: 10.1126/stke.2582004pl14. [DOI] [PubMed] [Google Scholar]
- 48.Bell PD, Lapointe JY, Sabirov R, Hayashi S, Peti-Peterdi J, Manabe K, Kovacs G, Okada Y. Macula densa cell signaling involves ATP release through a maxi anion channel. Proc Natl Acad Sci USA. 2003;100:4322–4327. doi: 10.1073/pnas.0736323100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakajima T, Sato M, Akaza N, Umezawa Y. Cell-based fluorescent indicator to visualize brain-derived neurotrophic factor secreted from living neurons. ACS Chem Biol. 2008;3:352–358. doi: 10.1021/cb800052v. [DOI] [PubMed] [Google Scholar]
- 50.Nguyen QT, Schroeder LF, Mank M, Muller A, Taylor P, Griesbeck O, Kleinfeld D. An in vivo biosensor for neurotransmitter release and in situ receptor activity. Nat Neurosci. 2010;13:127–132. doi: 10.1038/nn.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang RE, Zhang Y, Cai J, Cai W, Gao T. Aptamer-based fluorescent biosensors. Curr Med Chem. 2011;18:4175–4184. doi: 10.2174/092986711797189637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Newman RH, Fosbrink MD, Zhang J. Genetically encodable fluorescent biosensors for tracking signaling dynamics in living cells. Chem Rev. 2011;111:3614–3666. doi: 10.1021/cr100002u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alford SC, Wu J, Zhao Y, Campbell RE, Knopfel T. Optogenetic reporters. Biol Cell. 2013;105:14–29. doi: 10.1111/boc.201200054. [DOI] [PubMed] [Google Scholar]
- 54.Bernstein JG, Garrity PA, Boyden ES. Optogenetics thermogenetics technologies for controlling the activity of targeted cells within intact neural circuits. Curr Opin Neurobiol. 2012;22:61–71. doi: 10.1016/j.conb.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lubag AJ, De Leon-Rodriguez LM, Burgess SC, Sherry AD. Noninvasive MRI of beta-cell function using a Zn2+-responsive contrast agent. Proc Natl Acad Sci USA. 2011;108:18400–18405. doi: 10.1073/pnas.1109649108. Applying a zinc responsive MRI contrast agent, authors were able to image glucose stimulated insulin secretion in pancreata of live mice.