Abstract
A C2 cervical spinal cord hemisection (SH) interrupts descending inspiratory-related drive to phrenic motoneurons located between C3 and C5 in rats, paralyzing the ipsilateral hemidiaphragm muscle. There is gradual recovery of rhythmic diaphragm muscle activity ipsilateral to cervical spinal cord injury over time, consistent with neuroplasticity and strengthening of spared, contralateral descending premotor input to phrenic motoneurons. Brainderived neurotrophic factor (BDNF) signaling through the tropomyosin related kinase receptor subtype B (TrkB) plays an important role in neuroplasticity following spinal cord injury. We hypothesized that 1) increasing BDNF/TrkB signaling at the level of the phrenic motoneuron pool by intrathecal BDNF delivery enhances functional recovery of rhythmic diaphragm activity after SH, and 2) inhibiting BDNF/TrkB signaling by quenching endogenous neurotrophins with the soluble fusion protein TrkB-Fc or by knocking down TrkB receptor expression in phrenic motoneurons using intrapleurally-delivered siRNA impair functional recovery after SH. Diaphragm EMG electrodes were implanted bilaterally to verify complete hemisection at the time of SH and 3 days post-SH. After SH surgery in adult rats, an intrathecal catheter was placed at C4 to chronically infuse BDNF or TrkB-Fc using an implanted mini-osmotic pump. At 14 days post-SH, all intrathecal BDNF treated rats (n=9) displayed recovery of ipsilateral hemidiaphragm EMG activity, compared to 3 out of 8 untreated SH rats (p < 0.01). During eupnea, BDNF treated rats exhibited 76 ± 17% of pre-SH root mean squared EMG vs. only 5 ± 3% in untreated SH rats (p < 0.01). In contrast, quenching endogenous BDNF with intrathecal TrkB-Fc treatment completely prevented functional recovery up to 14 days post-SH (n=7). Immunoreactivity of the transcription factor cAMP response element-binding protein (CREB), a downstream effector of TrkB signaling, increased in phrenic motoneurons following BDNF treatment (n=6) compared to artificial cerebrospinal fluid treatment (n=6; p < 0.001). Intrapleural injections of non-sense or TrkB siRNA were administered after SH to specifically target phrenic motoneurons. At 14 days post-SH, none out of 9 TrkB siRNA treated rats displayed functional recovery compared to 5 out of 9 non-sense siRNA treated rats. These results indicate that BDNF/TrkB signaling in phrenic motoneuron pool plays a critical role in functional recovery after cervical spinal cord injury.
Keywords: neurotrophin, neuroplasticity, spinal hemisection, diaphragm muscle, phrenic motoneuron, respiratory, respiration, CREB, siRNA, TrkB-Fc
Introduction
Brain-derived neurotrophic factor (BDNF) signaling through the tropomyosin related kinase receptor subtype B (TrkB) plays an important role in neuroplasticity following spinal cord injury (SCI). At the site of spinal cord injury, BDNF may promote neuroprotection, regeneration of axotomized neurons, and plasticity (Weishaupt, et al., 2012). Indeed, several studies show that BDNF treatment following SCI increases survival of neurons and axonal sprouting when delivered by intrathecal infusion (Bregman, et al., 1997, Novikova, et al., 2000, Novikova, et al., 2002, Ye and Houle, 1997), intraspinal viral transduction (Boyce, et al., 2012, Koda, et al., 2004), or stem cell transplantation (Lu, et al., 2005, Lynskey, et al., 2006). However, the role of BDNF/TrkB signaling on motor pools below the level of injury is not well understood.
A well-established model of incomplete upper cervical SCI involves unilateral C2 spinal cord hemisection (SH). Following SH, the primary ipsilateral descending excitatory input to phrenic motoneurons (located between C3 and C6 in most mammals) is removed and the ipsilateral hemidiaphragm muscle is paralyzed (Goshgarian, et al., 1991, Mantilla, et al., 2012, Mantilla, et al., 2013, Mantilla, et al., 2007, Miyata, et al., 1995, Prakash, et al., 1999, Vinit, et al., 2006, Zhan, et al., 1997). Over time there is spontaneous neuroplasticity and gradual recovery of rhythmic diaphragm activity after SH (Boulenguez, et al., 2007, Golder, et al., 2003, Golder and Mitchell, 2005, Goshgarian, 2003, Mantilla, et al., 2013, Nantwi, et al., 1999, Sieck and Mantilla, 2009), consistent with strengthening of the spared contralateral synaptic input to phrenic motoneurons. We hypothesized that increasing BDNF/TrkB signaling at the level of the phrenic motoneuron pool (i.e., below the level of SCI) enhances recovery of rhythmic phrenic activity after SH. Conversely, we hypothesized that inhibiting TrkB/BDNF signaling by quenching endogenous BDNF with the soluble fusion protein TrkB-Fc or by targeting TrkB receptor knockdown to phrenic motoneurons using intrapleurally-delivered siRNA impair functional recovery after SH. Our results demonstrate that BDNF/TrkB signaling at phrenic motoneurons plays a critical role in functional recovery of rhythmic diaphragm activity after SH.
Materials and Methods
Experimental animals
Adult male Sprague-Dawley rats (colony 236, Harlan, Indianapolis, IN; initial body weight ~300 g) underwent unilateral SH and were randomly assigned to the following groups: untreated (n=9), artificial cerebrospinal fluid treated (n=8), intrathecal BDNF treated (n=18), intrathecal TrkB-Fc treated (n=14), intrapleural TrkB siRNA treated (n=12), and intrapleural non-sense TrkB siRNA treated (n=10). The Institutional Animal Care and Use Committee at Mayo Clinic approved all experimental procedures. All surgical procedures and experimental measurements were conducted under appropriate anesthesia induced intramuscular ketamine (90 mg/kg) and xylazine (10 mg/kg) and maintained by intermittent repeat dosing as needed.
Spinal cord hemisection (SH)
The surgical procedure for SH was previously published (Mantilla, et al., 2012, Mantilla, et al., 2013, Mantilla, et al., 2007, Miyata, et al., 1995, Prakash, et al., 1999). Briefly, a bilateral dorsal laminectomy at C2 exposed the cervical spinal cord and the right half of the spinal cord was transected at C2 anteriorly to the dorsal fissure (i.e., involving the lateral and ventral funiculi, but preserving the dorsal funiculus). All animals were observed daily after surgery and administered intramuscular buprenorphine and oral acetaminophen for the first 3 days.
Chronic diaphragm EMG recordings
Three days prior to the SH surgery, a pair of electrode wires (0.28 mm diameter - model AS631, Cooner Wire Inc., Chatsworth, CA) were stripped about 3 mm at the tip and implanted into both the right and left diaphragm midcostal regions for chronic EMG recordings, as previously described (Dow, et al., 2006, Dow, et al., 2009, Mantilla, et al., 2013, Mantilla, et al., 2011, Trelease, et al., 1982). The uninsulated portion was embedded within the diaphragm. The wires were subcutaneously tunneled to the dorsum of the animal and externalized. Diaphragm EMG activity was recorded in anesthetized rats during SH surgery and at 3 days post-SH (SH 3D) to verify completeness of SH by absence of ipsilateral hemidiaphragm activity. Diaphragm EMG activity was also recorded in anesthetized rats at 7 days post-SH (SH 7D) and 14 days post-SH (SH 14D).
In all cases, the EMG signal was differentially amplified (2000x), band-pass filtered (20–1000 Hz), and analog-to-digital converted at 2 kHz using a LabView data acquisition board and software (National Instruments, Austin, TX). Diaphragm root mean squared (RMS) EMG amplitude was calculated using a moving window of 50 ms (Mantilla, et al., 2013, Mantilla, et al., 2011, Mantilla, et al., 2010). The criteria for classification of functional recovery included evidence of diaphragm EMG signal that: 1) was both rhythmic (i.e., in phase with contralateral side) and periodic (i.e., occurring across multiple eupneic bursts); 2) comprised of more than one motor unit; and 3) exhibited RMS EMG amplitude greater than 10% of the pre-SH amplitude. BDNF ELISA: BDNF expression was measured in a subset of untreated SH and control animals (n=6/group) using the BDNF Emax ImmunoAssay System (Promega, Madison, WI). At SH 14D, the grey matter in the cervical ventral horn region of the spinal cord between C3 and C5 was isolated and frozen in liquid nitrogen. In accordance with the manufacturer’s protocol, tissue extracts were prepared using lysis buffer and added to the wells of a previously coated ELISA plate. BDNF expression was normalized to the total protein concentration determined using the Bio-Rad DC protein assay (Bio-Rad Laboratories, Hercules, CA).
Intrathecal catheter implantation
At the time of SH surgery, an implanted, tunneled polyethylene intrathecal catheter was placed for chronic infusion of BDNF, TrkB-Fc fusion protein, or artificial cerebrospinal fluid (aCSF). The procedure was a modification of techniques previously used to chronically administer intrathecal drugs in rats (Malkmus and Yaksh, 2004, Sakura, et al., 1996, Yaksh and Rudy, 1976). Briefly, an intrathecal catheter (PE-10, I.D. 0.14 mm; O.D. 0.4 mm; Becton Dickinson, Franklin Lakes, NJ) was inserted into the cisternal membrane at the occipital crest and its tip was placed at the C4 level of the spinal cord (by advancing it ~10 mm). After securing the catheter to the skull, a connecting segment of PE-50 tubing was used to provide tension relief by leaving a loose loop of catheter in the neck. A miniosmotic pump (Alzet 2002), Cupertino, CA) was securely implanted subcutaneously in the mid back in order to deliver intrathecal agents for up to 14 days (12 µl/day). BDNF was delivered at 180 ng/day (R&D Systems, Inc., Minneapolis, MN), in agreement with previous studies (Groth and Aanonsen, 2002, Haninec, et al., 2004, Kishino, et al., 1997, Kishino, et al., 2001, Novikova, et al., 2000, Novikova, et al., 2002, Sayer, et al., 2002). The fusion protein TrkB-Fc was delivered at 1.5 µg/day (R&D Systems, Inc.) in order to quench available BDNF (Binder, et al., 1999, Yajima, et al., 2005). The timing of BDNF or TrkB-Fc infusion was controlled to begin at SH 3D by filling the distal end of the catheter with aCSF (10 cm length given the infusion flow rate of the mini-osmotic pump).Thus, absence of diaphragm EMG activity was verified at SH 3D prior to initiating intrathecal BDNF or TrkB-Fc treatment.
TrkB siRNA in vitro optimization
The efficacy of TrkB knockdown with small interfering RNA (siRNA) was determined using C6 glioma cells. Cells were treated with one of four different siRNA constructs (n=4 each): untreated control, si1035, si1348, si2829 and si3051 (dicer-substrate siRNA; Integrated DNA Technologies, Coralville, IA). TrkB mRNA expression was measured following 3 days of siRNA treatment using a modification of a previously-reported mimic internal control, quantitative real-time RT-PCR technique (Geiger, et al., 2006). Internal control RNA was designed for full-length TrkB (TrkB.FL) by flanking a fragment of the specific target gene (~300 bases) with the primer sequences used for real-time PCR. Total RNA was extracted using the RNeasy Mini kit (Qiagen Inc., Valencia, CA) according to the manufacturer’s protocol, followed by an on-column DNase digestion. SUPERSCRIPT III First-Strand Synthesis Supermix for qRT-PCR (Invitrogen, Life Technologies, Grand Island, NY) was used for reverse transcription of a 1 µg aliquot of total RNA from C6 cells and a 1 µl aliquot of ten-fold serial dilutions of internal control cRNA (1 pg – 1 ng). Quantitative analysis of mRNA expression were performed on a LightCycler 2.0 platform (Roche Molecular, Pleasanton, CA) in a reaction mix containing 1X SYBR Green I Master (Roche Molecular,), 2.0 mM MgCl2, 0.5 µM primer and 2 µl cDNA from the reverse transcription reaction. The second derivative of the amplification-fluorescence plot was used to calculate the threshold cycle value for each reaction. Gene product concentration of TrkB-FL was determined from a log-linear plot of the TrkB specific internal control concentrations. All PCR reactions were performed in duplicate for each reverse transcription reaction.
Intrapleural treatment with TrkB siRNA
The optimal construct for TrkB knockdown in vitro, si1035, or a non-sense TrkB siRNA were targeted to phrenic motoneurons by delivery via intrapleural injection, as previously described (Mantilla, et al., 2009). Briefly, TrkB siRNA or non-sense TrkB siRNA (33 pmol/d in 20 µl phosphate-buffered saline - PBS; pH 7.4; Integrated DNA Technologies) were injected using a Hamilton syringe into the pleural space of the right side of the chest (the side of injury) between the 7th and 8th ribs, starting at the time of SH surgery.
Immunohistochemistry
In a subset of SH animals treated with intrathecal BDNF or aCSF (n=6 each), an intrapleural injection of cholera toxin subunit B (CTB; List Biological Laboratories, Campbell, CA) was used to retrogradely label phrenic motoneurons at 3 days before the terminal experiment, as previously reported (Issa, et al., 2010, Mantilla, et al., 2012, Mantilla, et al., 2009). At SH 7D, rats were anesthetized, euthanized by exsanguination after administration of heparin, and transcardially perfused with 4% paraformaldehyde in PBS. The cervical spinal cord was excised and postfixed in 4% paraformaldehyde overnight, followed by 24–72 hours in 24% sucrose in PBS. Longitudinal 50 µm thick sections were cut with a Reichert–Jung Frigocut cryostat (Reichert Microscope Services, Depew, NY). Spinal cord sections were blocked with 10% Donkey Serum in 0.3% Triton tris-buffered saline (TBS), followed by overnight incubation with CTB antibody (goat polyclonal, #703; List Biological Laboratories) and CREB antibody (rabbit polyclonal, sc-186; Santa Cruz Biotechnology, Dallas, TX). Secondary antibodies Cy3-conjugated anti-goat (Jackson Immunoresearch, West Grove, PA) and Alexa 488-conjugated anti-rabbit (Invitrogen) were used. Tissue sections were mounted on slides, dehydrated in graded alcohol concentrations, and coverslipped with DPX mountant (Fluka, Sigma–Aldrich, St. Louis, MO).
Spinal cord sections were imaged using an Olympus FluoView 300 laser scanning confocal microscope (Olympus America Inc., Melville, NY) mounted on an upright Olympus BX50WI microscope with Argon (488 nm) and green HeNe (543 nm) lasers. Acquisition of confocal image stacks has been previously reported (Issa, et al., 2010, Prakash, et al., 2000, Prakash, et al., 1993, Prakash, et al., 1994, Sieck, et al., 1999, Zhan, et al., 2000). Phrenic motoneurons were imaged with a 60× oil immersion lens (NA 1.4), and three-dimensional image stacks were collected in an 800×600 array with a step size of 1 µm. Simultaneous two-color imaging was performed using a 540 nm dichroic and appropriate bandpass filters. Laser intensity, confocal aperture, and photomultiplier gain were kept fixed across all samples. For each primary/secondary antibody pair, additional studies not including the primary antibody (blank) were conducted. Sections from both experimental groups and blank controls were processed in parallel for all immunohistochemical reactions and animals.
Metamorph Imaging Software version 7.6 (Universal Imaging Corporation, Downingtown, PA) was used to quantify the average fluorescence intensity (12-bit) in regions of interest in the cytoplasm and nucleus of CTB-identified phrenic motoneurons (n=421 and n=282 in the SH + aCSF and SH + BDNF groups, respectively), as well as in the surrounding background. For each phrenic motoneuron, a mid-nuclear plane was selected for intensity measurements. The integrated intensity of each region of interest was divided by its area, and the average background intensity in the vicinity of each phrenic motoneuron was subtracted.
Statistical analyses
All statistical evaluations were performed using JMP statistical software (version 9.0.1, SAS Institute Inc., Cary, NC). The proportion of animals displaying functional recovery was compared across groups using Pearson’s chi-square test. Diaphragm RMS EMG amplitude was normalized to the eupneic value before SH for the same animal and differences between treatment groups were examined using one-way analysis of variance (ANOVA). Protein and mRNA measurements and immunoreactivity were compared across groups using t-test or ANOVA as appropriate. The Tukey-Kramer honestly significant difference test was used for post hoc analyses when indicated. Statistical significance was established at the 0.05 level. All experimental data are presented as mean ± SE, unless otherwise specified.
Results
Surgical outcomes after SH
Diaphragm EMG electrodes were successfully implanted in all SH animals at 3 days before SH surgery (n=71) and were used to verify the completeness of SH at the time of surgery. Following SH, there were apparent differences in survival rates across groups, but these differences did not reach statistical significance (Table 1). Differences in survival rates across groups were likely unrelated to the possible additional morbidity associated with intrathecal catheter and mini-osmotic pump implantation, as the survival rate was 70% (28 out of 40) compared to 84% (26 out of 31) in rats not implanted with an intrathecal pump (p = 0.17). The survival associated with SH surgery and repeated intrapleural injections was also not statistically different (82%; 18 out of 22) compared to untreated SH animals (p = 0.63).
Table 1.
Survival after C2 hemisection (SH) across experimental groups
| Experimental Group | n | Survival n (%) |
|---|---|---|
| SH (+ aCSF) | 17 | 14 (82) |
| SH + BDNF | 18 | 15 (83) |
| SH + TrkB-Fc | 14 | 7 (50) |
| SH + non-sense siRNA | 10 | 9 (90) |
| SH + TrkB siRNA | 12 | 9 (75) |
Data presented are the initial number of animals (n) and the number (and percent) that survived in each group at the terminal experiment. The SH 14D group was not different than aCSF treated SH at SH 7D, so these were grouped. There were no statistically significant differences between the treatment groups in pair-wise analyses. Note that six animals in the BDNF treated SH group and eight animals in the aCSF treated SH group had terminal experiments at SH 7D, and the rest of the animals were terminal at SH 14D.
BDNF expression after SH
BDNF protein expression in the cervical ventral horn grey matter in the region of the phrenic motor pool was measured in untreated SH animals (n=6) and control, uninjured animals (n=6) and was normalized to the total protein concentration of the lysate. At SH 14D, BDNF expression was 19.3 ± 1.0 (×10−5 % total protein), compared to 8.7 ± 0.4 (×10−5 % total protein) in uninjured controls (p < 0.05).
Role of BDNF in proportion of animals displaying functional recovery after SH
Diaphragm EMG was recorded at SH 3D to verify the completeness of SH surgery. Mini-osmotic pumps were successfully implanted for intrathecal delivery at the C4 level and functioned properly throughout the 14 day duration of the experiment. All of the animals displayed lack of rhythmic ipsilateral EMG activity during eupnea at SH 3D, indicating complete interruption of ipsilateral descending drive to phrenic motoneurons. Diaphragm EMG recordings were repeated under anesthesia during eupnea at SH 7D and SH 14D. Ipsilateral and contralateral hemidiaphragm EMG activity was rhythmic and occurred in bursts. Representative diaphragm EMG recordings and RMS EMG tracings are shown in Figure 1.
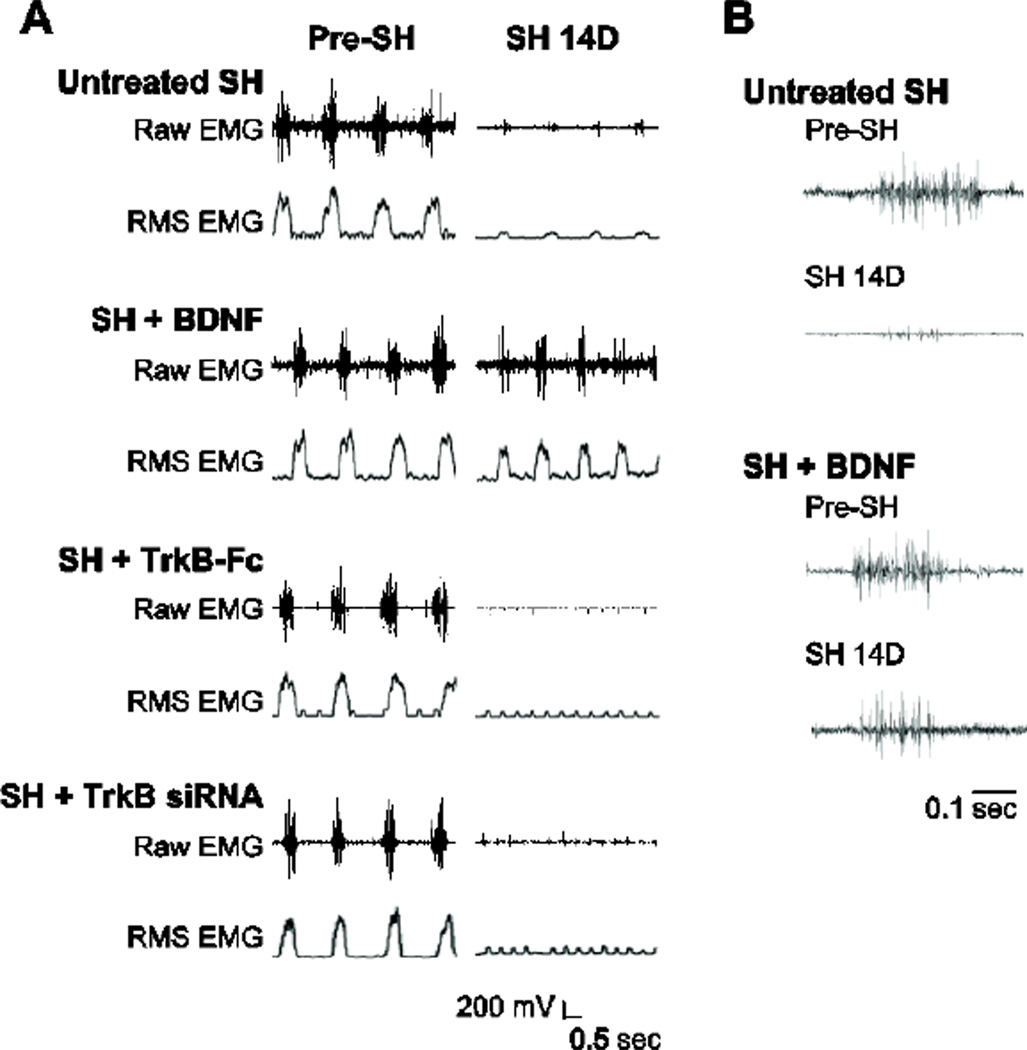
Figure 1.
Representative raw diaphragm EMG recordings and root mean squared (RMS) EMG tracings after C2 hemisection (SH). Untreated SH (SH; n=8), intrathecal BDNF treated SH (SH + BDNF; n=9), intrathecal TrkB-Fc treated SH (SH + TrkB-Fc; n=7), and intrapleural TrkB siRNA treated SH (SH + TrkB siRNA; n=9) animals were monitored using EMG recordings obtained during eupnea via chronically implanted diaphragm electrodes. A) EMG recordings obtained prior to (pre-SH) and 14 days post-SH (SH 14D) for an animal from each group are shown. Diaphragm EMG activity occurred in bursts, reflecting rhythmic inspiration in the anesthetized animals. Notice lack of diaphragm EMG activity in the SH + TrkB-Fc and SH + TrkB siRNA groups. In all groups, there was a lack of non-rhythmic EMG activity suggestive of spasms or tonic activity in either side of the diaphragm muscle at any time point post-SH. Electrocardiographic activity is visible in some recordings. B) Expanded EMG recordings for untreated SH and SH + BDNF groups, prior to SH and at SH 14D. Each tracing is the first burst of the corresponding EMG recording in A. Notice multiple active motor units in the diaphragm EMG signal at SH 14D with intrathecal BDNF treatment.
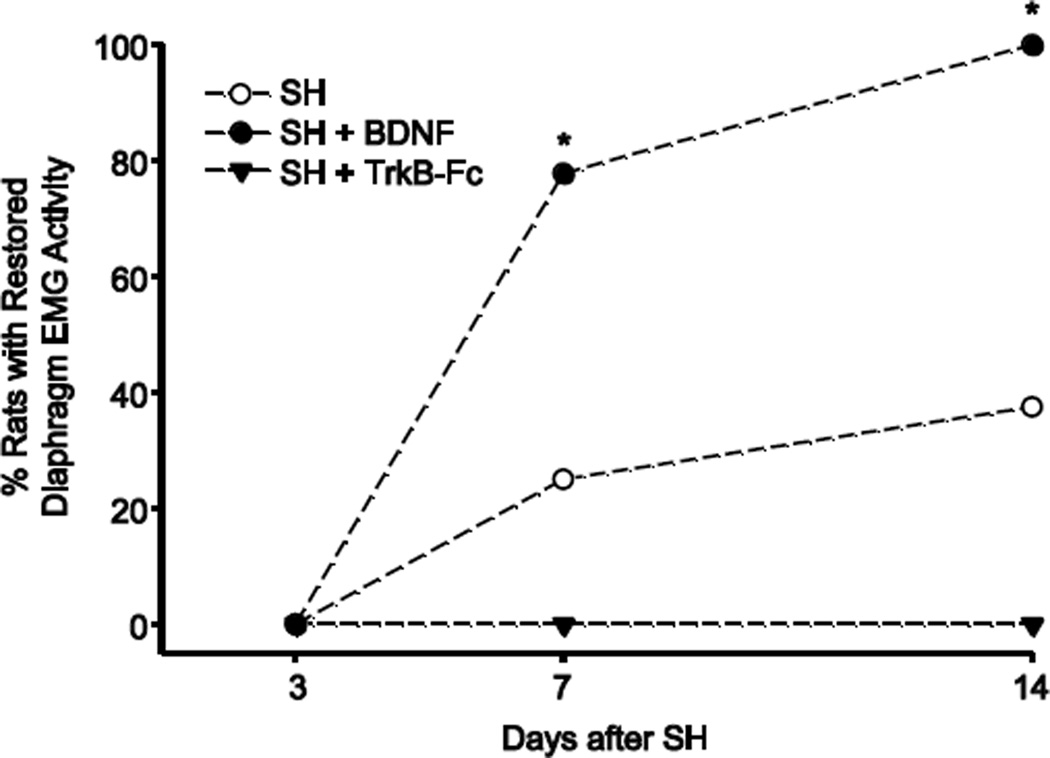
The proportion of animals displaying restoration of ipsilateral hemidiaphragm EMG activity (i.e., functional recovery) after SH was assessed in untreated, BDNF- and TrkB-Fc treated SH groups. Over time after SH, a portion of the untreated SH animals displayed spontaneous recovery of ipsilateral hemidiaphragm EMG activity during eupnea. This activity was in phase with the contralateral (uninjured) side and occurred in over 90% of eupneic bursts. The diaphragm EMG signal consistently comprised more than one motor unit and was at least 10% of the pre-SH RMS EMG amplitude. At SH 7D, two out of 8 untreated SH animals (25%) displayed functional recovery of ipsilateral hemidiaphragm EMG activity. By SH 14D, three untreated SH animals (38%) displayed functional recovery (Figure 2).
Figure 2.
Proportion of animals displaying functional recovery of ipsilateral hemidiaphragm EMG activity after SH. Chronic diaphragm EMG recordings during eupnea were used to determine the proportion of animals displaying functional recovery after SH. The criteria for classification of functional recovery is detailed in the Methods section. At SH 7D, seven out of 9 animals with intrathecal BDNF treatment (SH + BDNF) displayed functional recovery, compared to two out of 8 untreated SH animals (*, p < 0.05). By SH 14D, all of the BDNF treated SH animals displayed functional recovery compared to 3 out of 8 untreated SH animals (p < 0.01). Quenching endogenous BDNF with intrathecal TrkB-Fc (SH + TrkB-Fc; n=7) completely prevented functional recovery in all animals at SH 7D or SH 14D.
Intrathecal BDNF treatment (n=9) increased the proportion of animals displaying ipsilateral hemidiaphragm EMG activity during eupnea after SH. At SH 7D, seven animals (78%) displayed functional recovery (p < 0.05 compared to untreated SH; Figure 2). By SH 14D, all of the BDNF treated SH animals displayed functional recovery (p < 0.01 compared to untreated SH). In contrast, TrkB-Fc treatment completely prevented functional recovery after SH, such that none of the animals treated with TrkB-Fc displayed functional recovery at SH 7D or SH 14D (n=7).
Role of BDNF in extent of eupneic diaphragm EMG activity after SH
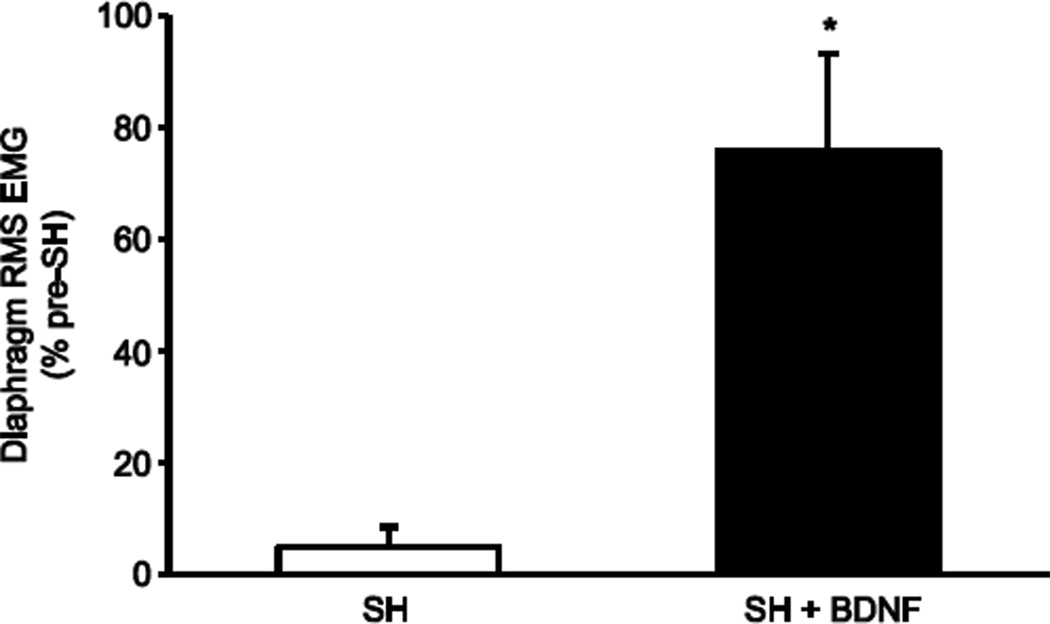
Diaphragm RMS EMG amplitude during eupnea was calculated in order to determine the extent of recovery of ipsilateral rhythmic hemidiaphragm EMG activity. Diaphragm RMS EMG amplitude at SH 14D was normalized to the eupneic value pre-SH for the same animal. Each EMG recording had minimal variability in diaphragm RMS EMG amplitude. Intrathecal treatment with BDNF significantly increased the extent of recovery after SH. At SH 14D, RMS EMG amplitude was 5 ± 3% of the pre-SH value in the untreated SH animals (including both those displaying recovery and those that did not; n=8). In untreated SH animals that displayed recovery, the RMS EMG amplitude was 17 ± 6% of the pre-SH value. In contrast, in BDNF- treated SH rats RMS EMG amplitude was 61% of the pre-SH value at SH 7D (n=2) and 76 ± 17% of the pre-SH value at SH 14D (n=9; p < 0.01 compared to untreated SH rats; Figure 3).
Figure 3.
Extent of functional recovery of ipsilateral rhythmic phrenic activity at SH 14D after BDNF treatment. Diaphragm RMS EMG amplitude was measured during eupnea at various time points after SH and compared to the pre-SH RMS EMG amplitude. In the untreated SH animals, RMS EMG amplitude (mean ± SE) was reduced at SH 14D compared to pre-injury (SH; n=8). Treatment with intrathecal BDNF increased the extent of diaphragm activity after SH (SH + BDNF; n=9) compared to untreated SH (*, p < 0.01).
CREB Translocation after SH
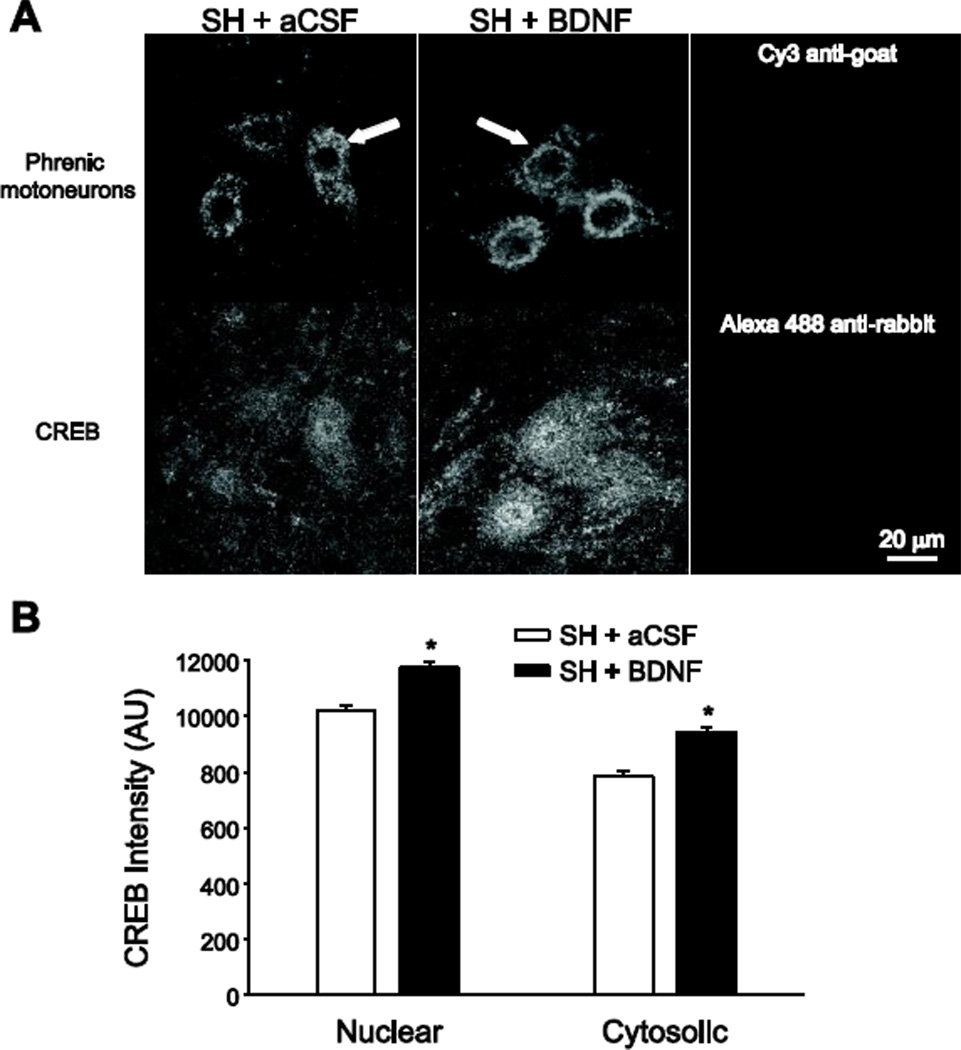
In SH animals treated with intrathecal aCSF or BDNF, the intensity of CREB immunoreactivity in the nucleus and cytosol of phrenic motoneurons was measured at SH 7D. All immunohistochemical processing and image acquisition conditions were carefully controlled to permit comparisons across experimental groups. CREB immunofluorescence was visible in the nucleus and cytosol of all phrenic motoneurons. On average, the amount of CREB immunoreactivity in the nucleus at SH 7D was ~15% greater following BDNF treatment compared to aCSF treatment (n=6; p < 0.001; Figure 4). In addition, the amount of CREB in the cytosol at SH 7D was ~20% greater with BDNF treatment than with aCSF treatment (n=6; p < 0.001).
Figure 4.
Phrenic motoneuron immunoreactivity of the transcription factor cAMP response element-binding protein (CREB) following BDNF treatment. Simultaneous two-color imaging of phrenic motoneurons labeled retrogradely by intrapleural cholera toxin subunit B (CTB) injection (top panels in A) and CREB immunoreactivity (bottom panels in A) was used to quantify CREB immunofluorescence in the nucleus and cytosol of phrenic motoneurons (n=6/group). A) Representative images of CREB immunoreactivity in phrenic motoneurons at SH 7D in animals treated with intrathecal artificial cerebrospinal fluid (SH + aCSF; left panels) or intrathecal BDNF (SH + BDNF; middle panels). Images represent single confocal slices that were midnuclear for the phrenic motoneuron identified with an arrow. Right panels, representative images showing lack of immunofluorescence in tissues treated with secondary antibodies (but no primary antibody) and imaged at the selected acquisition parameters, which were constant throughout all samples. Bar, 20 µm for all images. B) The average fluorescence intensity (12-bit) was calculated in regions of interest in the cytoplasm and nucleus of CTBidentified phrenic motoneurons (n=421 and n=282 in the SH + aCSF and SH + BDNF groups, respectively), as well as in the surrounding background. Average values of CREB immunofluorescence for both nuclear and cytosolic regions (following background subtraction) are plotted. Both nuclear and cytosolic CREB immunoreactivity at SH 7D was greater following BDNF treatment compared to aCSF treatment (*, p < 0.001).
Role of TrkB in proportion of animals displaying functional recovery after SH
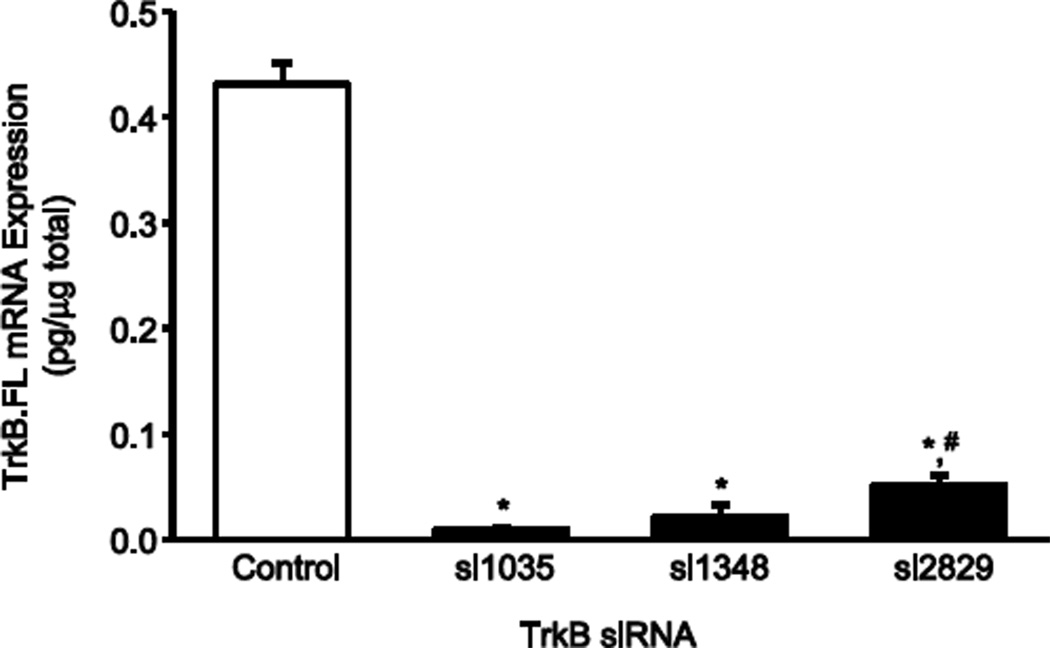
Optimization of TrkB knockdown was performed in vitro with four different TrkB siRNA constructs. Knockdown of TrkB.FL compared to control C6 cells was 98% with construct si1035, 95% with construct si1348, and 88% with construct si2829 (n=4 each construct; p < 0.05 compared to control; Figure 5). Knockdown of TrkB.FL was 26% with construct si3051 (p > 0.05 compared to control). With the goal of targeting TrkB knockdown only in phrenic motoneurons, siRNA constructs were delivered using daily intrapleural injections. Consistent with previous results (Mantilla, et al., 2012, Mantilla, et al., 2009), retrograde transport to phrenic motoneurons was evidenced by appropriate immunofluorescence following intrapleural Cy3-siRNA treatment (results not shown). In pilot studies performed in vivo with daily intrapleural injections of each TrkB siRNA construct starting at the time of SH surgery, animals were treated with each of the four different siRNA constructs tested in vitro in C6 glioma cells. In general agreement with the in vitro studies, animals treated with si1035, si1348, or si2829 did not display rhythmic diaphragm EMG activity at SH 14D whereas the animal treated with construct si3051 displayed rhythmic diaphragm EMG activity at SH 14D.
Figure 5.
TrkB.FL mRNA expression in C6 glioma cells after siRNA treatment determined by quantitative real-time RT-PCR. Optimization of TrkB.FL knockdown was performed in vitro with four different TrkB siRNA constructs si1035, si1348, and si2829 (si3051 not shown), compared to control C6 cells. TrkB.FL knockdown was greatest with construct si1035 and si1348 (n=4 each construct; *, p < 0.05 compared to control). Construct si2829 reduced TrkB.FL mRNA expression but to a lesser extent than construct si1035 (n=4; #, p < 0.05 compared to si1035). In vivo knockdown experiments were conducted using the TrkB construct siRNA si1035 delivered using intrapleural injection.
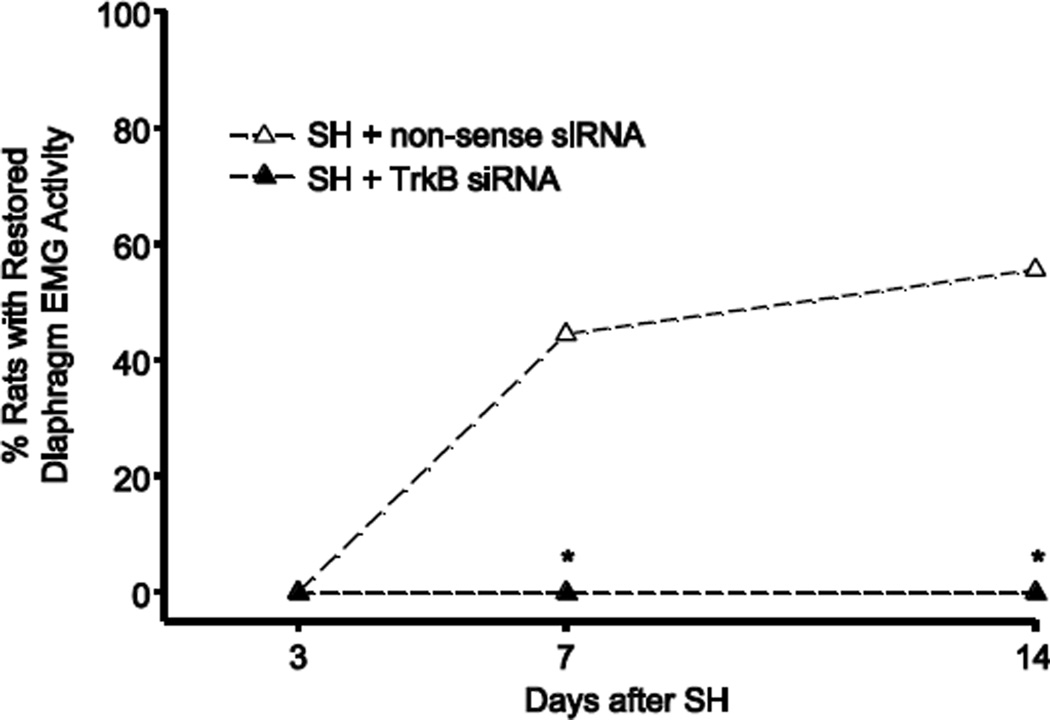
Based on these in vitro and preliminary in vivo results, TrkB siRNA construct si1035 was used for all subsequent studies. Starting at the time of SH surgery, TrkB siRNA or non-sense siRNA was administered intrapleurally daily, and the proportion of animals displaying functional recovery of ipsilateral hemidiaphragm EMG activity after SH was assessed. At SH 7D, four out of nine non-sense siRNA treated SH animals (44%) displayed ipsilateral hemidiaphragm EMG activity. By SH 14D, five non-sense siRNA treated SH animals (56%) displayed functional recovery (Figure 6). The proportion of recovery with non-sense siRNA at SH 14D was not different from that in untreated SH rats, as described above (p > 0.05). In contrast, TrkB siRNA treatment completely prevented functional recovery after SH, such that none of the nine animals treated with TrkB siRNA displayed ipsilateral hemidiaphragm EMG activity at SH 7D (p < 0.05) or SH 14D (p < 0.01), compared to non-sense siRNA treated animals. After non-sense siRNA treatment, diaphragm RMS EMG amplitude was 35 ± 12% of pre-SH values (including both those displaying recovery and those that did not), and was thus not different from untreated SH animals (p > 0.05).
Figure 6.
Proportion of animals displaying functional recovery after intrapleural TrkB siRNA treatment. At SH 7D, none of the 9 animals treated intrapleurally with TrkB siRNA (SH + TrkB siRNA) displayed functional recovery, compared to four out of 9 animals with treated with intrapleural non-sense siRNA (SH + non-sense siRNA; *, p < 0.05). At SH 14D, TrkB siRNA completely prevented functional recovery, compared to 5 out of 9 non-sense siRNA treated animals (p < 0.001).
Respiratory pattern after SH
Respiratory rate was measured from contralateral hemidiaphragm EMG recordings during eupnea. Respiratory rate was unaffected by SH and/or respective treatment. The average respiratory rate across groups was 81 ± 4 min−1 before SH and 85 ± 4 min−1 at SH 14D. Consistent with a small effect of SH on eupneic ventilation, respiratory rate increased slightly in animals that did not display functional recovery at SH 14D (89.5 ± 4.8 min−1; n=19: 4 untreated SH, 8 TrkB siRNA, 5 TrkB-Fc, 2 non-sense siRNA) compared to animals displaying functional recovery (80.1 ± 5.6 min−1; n=14: 3 untreated SH, 8 BDNF, 3 non-sense siRNA), but this difference did not reach statistical significance (p = 0.21). Similarly, there were no differences in contralateral hemidiaphragm RMS EMG amplitude across groups at SH 14D. Contralateral RMS EMG amplitude was 130 ± 25% of the pre-SH value in animals that did not display functional recovery at SH 14D and 161 ± 29% of the pre-SH value in animals that displayed functional recovery (p = 0.43).
Discussion
This study demonstrates that neurotrophins, specifically BDNF, at motor neuron pools below the level of SCI, are important for neuroplasticity and functional recovery. Indeed, recovery of ipsilateral hemidiaphragm EMG activity after SH is enhanced by intrathecally delivering BDNF to the area of the spinal cord containing phrenic motoneurons, below the level of injury. Furthermore, quenching endogenous BDNF with TrkB-Fc in this same region completely prevented functional recovery. BDNF-mediated activation of TrkB receptors likely involves signaling through CREB/ATF-1, since BDNF increased CREB immunoreactivity in phrenic motoneurons following SH, compared to aCSF. Inhibiting TrkB signaling specifically in phrenic motoneurons using targeted intrapleural delivery of TrkB siRNA blocked functional recovery after SH. Taken together, the results of this study demonstrate that BDNF/TrkB signaling at phrenic motoneurons plays a critical role in functional recovery of rhythmic diaphragm activity after SH.
Role of neurotrophin signaling on functional recovery after spinal cord injury
Numerous studies have shown that SCI causes an increase in expression of neurotrophins (Dougherty, et al., 2000, Frisen, et al., 1992, King, et al., 2000, Widenfalk, et al., 2001). At SH 14D, BDNF expression was elevated in the local environment surrounding phrenic motoneurons, compared to uninjured controls, and may contribute to the spontaneous recovery of ipsilateral hemidiaphragm EMG activity that occurs over time after SH.
Increasing BDNF availability within the region of the phrenic motoneuron pool, and subsequent BDNF/TrkB signaling, enhanced recovery of ipsilateral phrenic activity after SH. The importance of BDNF/TrkB signaling to neuroplasticity in motor pools including phrenic motoneurons is highlighted by several observations in other models. For example, facilitation of phrenic motor output induced by episodic hypoxia (an example of neuroplasticity) is associated with increased BDNF expression in the cervical ventral horn (Baker-Herman, et al., 2004). In addition, disruption of BDNF expression (using siRNA) blocks long-term facilitation of phrenic activity, whereas intrathecal administration of BDNF enhances long-term facilitation. In a recent study, partial recovery of respiratory activity after SH induced by orally administered theophylline is accompanied by increased expression of BDNF and increased CREB expression and phosphorylation in ipsilateral C2 to C6 spinal cord segments (Singh, et al., 2012). In the present study, increasing BDNF availability through intrathecal delivery of BDNF at the level of the phrenic motoneuron pool (C4) and thus below the level of injury (C2) resulted in functional recovery of all animals by SH 14D. In addition, ipsilateral hemidiaphragm EMG RMS amplitude was greater in BDNF-treated than untreated SH animals. Reductions in endogenous BDNF availability by intrathecal TrkB-Fc infusion at C4 completely prevented functional recovery at SH 14D. These results indicate that BDNF within the phrenic motor nucleus plays a critical role in functional recovery of ipsilateral hemidiaphragm EMG activity after SH.
Mechanism for effects of TrkB signaling through CREB
In the central nervous system, BDNF, acting through its high-affinity receptor TrkB, plays an important role in mediating synaptic plasticity (Cohen-Cory, 2002, Kafitz, et al., 1999, Kang and Schuman, 1995, Poo, 2001, Schinder and Poo, 2000, Thoenen, 1995). Full-length TrkB (TrkB.FL) signals via phosphorylation (Huang and Reichardt, 2003, Reichardt, 2006). As an index of TrkB activation in phrenic motoneurons, in this study we used the transcription factor cAMP response element-binding proteins CREB and ATF-1, which are downstream effectors of TrkB (Pizzorusso, et al., 2000, Sheng, et al., 1991, Watson, et al., 2001, Watson, et al., 1999) and translocate to the nucleus upon activation (Pizzorusso, et al., 2000, Sheng, et al., 1991, Watson, et al., 2001, Watson, et al., 1999). These transcription factors are implicated in TrkB signaling via phosphorylation and are important regulators of a number of genes including TrkB (Watson, et al., 2001, Watson, et al., 1999). Of note, CREB expression and phosphorylation is increased in the ipsilateral spinal cord at 3 and 5 days post-SH, compared to the contralateral spinal cord (Singh, et al., 2012). The present study examined CREB specifically in phrenic motoneurons and found that CREB immunoreactivity increased following intrathecal BDNF treatment compared to aCSF treatment of SH animals, suggesting that BDNF signaling via TrkB may exert downstream effects through CREB in phrenic motoneurons. This change in CREB expression persists through SH 7D. Although these results are provocative, a detailed time course evaluating CREB changes after SH was not included here. Regardless, both the spontaneous and BDNF-induced recovery of ipsilateral hemidiaphragm EMG activity post-SH involve CREB activation below the level of SCI.
Neurotrophins are important around phrenic motoneurons
To determine if TrkB signaling in phrenic motoneurons plays a role in functional recovery after SH, intrapleural injections of nonsense or TrkB siRNA were administered after SH to specifically target phrenic motoneurons in the cervical spinal cord (Mantilla, et al., 2009). TrkB knockdown in phrenic motoneurons completely blocked functional recovery after SH. In contrast, recovery with non-sense siRNA treatment was not different from that in untreated SH animals. Altering BDNF availability within the region of the phrenic motoneuron pool with intrathecal TrkB-Fc also eliminated functional recovery after SH. Unlike the targeted delivery of TrkB siRNA to phrenic motoneurons via intrapleural injection (Mantilla, et al., 2009), the intrathecal technique does not restrict delivery to phrenic motoneurons but includes other cells in the spinal cord segments containing the phrenic motor pool. Although intrathecal delivery may result in more distant effects, multiple studies document limited visible staining by bromophenol blue dye when injected as repeated bolus injections up to 10 µl (Yaksh and Rudy, 1976, Yaksh and Rudy, 1977). In the present study, intrathecal infusions were limited to 0.5 µl/h (12 µl/d) suggesting that BDNF and TrkB-Fc delivery were very localized to the segments surrounding C4 (catheter tip) and corresponding to the phrenic motor pool in rats (Mantilla, et al., 2009). Regardless, the results of the present study provide novel and convincing evidence that BDNF/TrkB signaling in phrenic motoneurons is important for functional recovery of rhythmic diaphragm activity after SH.
Genetic knockdown models could be used in the future to further evaluate the role of TrkB/BDNF signaling on functional recovery after SH. Genetic homozygous knockdown models that lack TrkB or BDNF die during embryonic or early postnatal development (Snider, 1994), but studying functional recovery after SH in heterozygous models may be useful as they may illuminate issues related to threshold levels of BDNF and TrkB necessary for recovery. In addition, the role of TrkB kinase activity can be evaluated using a chemical-genetic approach. In this regard, TrkBF616A mice expressing knock-in alleles that permit selective, rapid, and reversible inhibition of TrkB kinase activity (Chen, et al., 2005, Mantilla and Ermilov, 2012) may be particularly useful. Based on the results of the present study, we expect that functional recovery after SH will be impaired when TrkB kinase activity is inhibited, in a way similar to the effect of TrkB siRNA or TrkB-Fc treated SH rats. Additional studies will be necessary, however, to determine the time course of any spontaneous recovery of ipsilateral hemidiaphragm activity in mouse models of SH. Consistent with the presence of spontaneous recovery in mice, a previous report documented recovery of ipsilateral hemidiaphragm EMG activity following transection of the contralateral phrenic nerve that became more robust over time (Minor, et al., 2006).
Taken together, this study demonstrates the importance of BDNF/TrkB signaling for local plasticity at the level of the phrenic motor nucleus in a well-established model of incomplete SCI. Furthermore, the results indicate that BDNF/TrkB signaling at phrenic motoneurons plays a critical role in functional recovery of rhythmic diaphragm activity after SH. Selectively targeting delivery of BDNF or TrkB below the level of incomplete SCI may provide an exciting therapeutic option for promoting functional recovery.
Highlights.
Functional recovery post-C2 hemisection (SH) requires motoneuron BDNF/TrkB signaling
Intrathecal BDNF enhances recovery of ipsilateral diaphragm EMG activity after SH
Quenching endogenous BDNF with TrkB-Fc prevents functional recovery after SH
Phrenic motoneurons display increased CREB immunoreactivity after intrathecal BDNF
Delivery of TrkB siRNA to phrenic motoneurons prevents functional recovery after SH
Acknowledgments
The authors wish to thank Jeffrey P. Bailey and Colleen M. Buenz for their technical support. This work was supported by NIH grants R01-HL096750, T32-HL105355 (HMG), and Mayo Clinic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- 2.Binder DK, Routbort MJ, Ryan TE, Yancopoulos GD, McNamara JO. Selective inhibition of kindling development by intraventricular administration of TrkB receptor body. J Neurosci. 1999;19:1424–1436. doi: 10.1523/JNEUROSCI.19-04-01424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boulenguez P, Gauthier P, Kastner A. Respiratory neuron subpopulations and pathways potentially involved in the reactivation of phrenic motoneurons after C2 hemisection. Brain Res. 2007;1148:96–104. doi: 10.1016/j.brainres.2007.02.060. [DOI] [PubMed] [Google Scholar]
- 4.Boyce VS, Park J, Gage FH, Mendell LM. Differential effects of brain-derived neurotrophic factor and neurotrophin-3 on hindlimb function in paraplegic rats. Eur J Neurosci. 2012;35:221–232. doi: 10.1111/j.1460-9568.2011.07950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bregman BS, McAtee M, Dai HN, Kuhn PL. Neurotrophic factors increase axonal growth after spinal cord injury and transplantation in the adult rat. Exp Neurol. 1997;148:475–494. doi: 10.1006/exnr.1997.6705. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Ye H, Kuruvilla R, Ramanan N, Scangos KW, Zhang C, Johnson NM, England PM, Shokat KM, Ginty DD. A chemical-genetic approach to studying neurotrophin signaling. Neuron. 2005;46:13–21. doi: 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Cohen-Cory S. The developing synapse: Construction and modulation of synaptic structures and circuits. Science. 2002;298:770–776. doi: 10.1126/science.1075510. [DOI] [PubMed] [Google Scholar]
- 8.Dougherty KD, Dreyfus CF, Black IB. Brain-derived neurotrophic factor in astrocytes, oligodendrocytes, and microglia/macrophages after spinal cord injury. Neurobiol Dis. 2000;7:574–585. doi: 10.1006/nbdi.2000.0318. [DOI] [PubMed] [Google Scholar]
- 9.Dow DE, Mantilla CB, Zhan WZ, Sieck GC. EMG-based detection of inspiration in the rat diaphragm muscle. Conf Proc IEEE Eng Med Biol Soc. 2006;1:1204–1207. doi: 10.1109/IEMBS.2006.260688. [DOI] [PubMed] [Google Scholar]
- 10.Dow DE, Zhan WZ, Sieck GC, Mantilla CB. Correlation of respiratory activity of contralateral diaphragm muscles for evaluation of recovery following hemiparesis. Conf Proc IEEE Eng Med Biol Soc. 2009;1:404–407. doi: 10.1109/IEMBS.2009.5334892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frisen J, Verge VM, Cullheim S, Persson H, Fried K, Middlemas DS, Hunter T, Hokfelt T, Risling Mg. Increased levels of trkB mRNA and trkB protein-like immunoreactivity in the injured rat and cat spinal cord. Proc Natl Acad Sci USA. 1992;89:11282–11286. doi: 10.1073/pnas.89.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geiger PC, Bailey JP, Mantilla CB, Zhan WZ, Sieck GC. Mechanisms underlying myosin heavy chain expression during development of the rat diaphragm muscle. J Appl Physiol. 2006;101:1546–1555. doi: 10.1152/japplphysiol.00221.2006. [DOI] [PubMed] [Google Scholar]
- 13.Golder FJ, Fuller DD, Davenport PW, Johnson RD, Reier PJ, Bolser DC. Respiratory motor recovery after unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory motor output. J Neurosci. 2003;23:2494–2501. doi: 10.1523/JNEUROSCI.23-06-02494.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci. 2005;25:2925–2932. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goshgarian HG. Plasticity in Respiratory Motor Control: Invited Review: The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol. 2003;94:795–810. doi: 10.1152/japplphysiol.00847.2002. [DOI] [PubMed] [Google Scholar]
- 16.Goshgarian HG, Ellenberger HH, Feldman JL. Decussation of bulbospinal respiratory axons at the level of the phrenic nuclei: a possible substrate for the crossed-phrenic phenomenon. Exp Neurol. 1991;111:135–139. doi: 10.1016/0014-4886(91)90061-g. [DOI] [PubMed] [Google Scholar]
- 17.Groth R, Aanonsen L. Spinal brain-derived neurotrophic factor (BDNF) produces hyperalgesia in normal mice while antisense directed against either BDNF or trkB, prevent inflammation-induced hyperalgesia. Pain. 2002;100:171–181. doi: 10.1016/s0304-3959(02)00264-6. [DOI] [PubMed] [Google Scholar]
- 18.Haninec P, Dubovy P, Samal F, Houstava L, Stejskal L. Reinnervation of the rat musculocutaneous nerve stump after its direct reconnection with the C5 spinal cord segment by the nerve graft following avulsion of the ventral spinal roots: a comparison of intrathecal administration of brain-derived neurotrophic factor and Cerebrolysin. Exp Brain Res. 2004;159:425–432. doi: 10.1007/s00221-004-1969-z. [DOI] [PubMed] [Google Scholar]
- 19.Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 20.Issa AN, Zhan WZ, Sieck G, Mantilla CB. Neuregulin-1 at synapses on phrenic motoneurons. J Comp Neurol. 2010;518:4213–4225. doi: 10.1002/cne.22449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kafitz KW, Rose CR, Thoenen H, Konnerth A. Neurotrophin-evoked rapid excitation through TrkB receptors. Nature. 1999;401:918–921. doi: 10.1038/44847. [DOI] [PubMed] [Google Scholar]
- 22.Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 23.King VR, Bradbury EJ, McMahon SB, Priestley JV. Changes in truncated trkB and p75 receptor expression in the rat spinal cord following spinal cord hemisection and spinal cord hemisection plus neurotrophin treatment. Exp Neurol. 2000;165:327–341. doi: 10.1006/exnr.2000.7480. [DOI] [PubMed] [Google Scholar]
- 24.Kishino A, Ishige Y, Tatsuno T, Nakayama C, Noguchi H. BDNF prevents and reverses adult rat motor neuron degeneration and induces axonal outgrowth. Exp Neurol. 1997;144:273–286. doi: 10.1006/exnr.1996.6367. [DOI] [PubMed] [Google Scholar]
- 25.Kishino A, Katayama N, Ishige Y, Yamamoto Y, Ogo H, Tatsuno T, Mine T, Noguchi H, Nakayama C. Analysis of effects and pharmacokinetics of subcutaneously administered BDNF. Neuroreport. 2001;12:1067–1072. doi: 10.1097/00001756-200104170-00040. [DOI] [PubMed] [Google Scholar]
- 26.Koda M, Hashimoto M, Murakami M, Yoshinaga K, Ikeda O, Yamazaki M, Koshizuka S, Kamada T, Moriya H, Shirasawa H, Sakao S, Ino H. Adenovirus vector-mediated in vivo gene transfer of brain-derived neurotrophic factor (BDNF) promotes rubrospinal axonal regeneration and functional recovery after complete transection of the adult rat spinal cord. J Neurotrauma. 2004;21:329–337. doi: 10.1089/089771504322972112. [DOI] [PubMed] [Google Scholar]
- 27.Lu P, Jones LL, Tuszynski MH. BDNF-expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Exp Neurol. 2005;191:344–360. doi: 10.1016/j.expneurol.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Lynskey JV, Sandhu FA, Dai HN, McAtee M, Slotkin JR, MacArthur L, Bregman BS. Delayed intervention with transplants and neurotrophic factors supports recovery of forelimb function after cervical spinal cord injury in adult rats. J Neurotrauma. 2006;23:617–634. doi: 10.1089/neu.2006.23.617. [DOI] [PubMed] [Google Scholar]
- 29.Malkmus SA, Yaksh TL. Intrathecal catheterization and drug delivery in the rat. Methods Mol Med. 2004;99:109–121. doi: 10.1385/1-59259-770-X:011. [DOI] [PubMed] [Google Scholar]
- 30.Mantilla CB, Bailey JP, Zhan WZ, Sieck GC. Phrenic motoneuron expression of serotonergic and glutamatergic receptors following upper cervical spinal cord injury. Exp Neurol. 2012;234:191–199. doi: 10.1016/j.expneurol.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mantilla CB, Ermilov LG. The novel TrkB receptor agonist 7,8-dihydroxyflavone enhances neuromuscular transmission. Muscle Nerve. 2012;45:274–276. doi: 10.1002/mus.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mantilla CB, Greising SM, Zhan WZ, Seven YB, Sieck GC. Prolonged C2 spinal hemisection-induced inactivity reduces diaphragm muscle specific force with modest, selective atrophy of type IIx and/or IIb fibers. J Appl Physiol. 2013;114:380–386. doi: 10.1152/japplphysiol.01122.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mantilla CB, Rowley KL, Zhan WZ, Fahim MA, Sieck GC. Synaptic vesicle pools at diaphragm neuromuscular junctions vary with motoneuron soma, not axon terminal, inactivity. Neuroscience. 2007;146:178–189. doi: 10.1016/j.neuroscience.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 34.Mantilla CB, Seven YB, Hurtado-Palomino JN, Zhan WZ, Sieck GC. Chronic assessment of diaphragm muscle EMG activity across motor behaviors. Respir Physiol Neurobiol. 2011;177:176–182. doi: 10.1016/j.resp.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantilla CB, Seven YB, Zhan WZ, Sieck GC. Diaphragm motor unit recruitment in rats. Respir Physiol Neurobiol. 2010;173:101–106. doi: 10.1016/j.resp.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mantilla CB, Zhan WZ, Sieck GC. Retrograde labeling of phrenic motoneurons by intrapleural injection. J Neurosci Methods. 2009;182:244–249. doi: 10.1016/j.jneumeth.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minor KH, Akison LK, Goshgarian HG, Seeds NW. Spinal cord injury-induced plasticity in the mouse--the crossed phrenic phenomenon. Exp Neurol. 2006;200:486–495. doi: 10.1016/j.expneurol.2006.02.125. [DOI] [PubMed] [Google Scholar]
- 38.Miyata H, Zhan WZ, Prakash YS, Sieck GC. Myoneural interactions affect diaphragm muscle adaptations to inactivity. J Appl Physiol. 1995;79:1640–1649. doi: 10.1152/jappl.1995.79.5.1640. [DOI] [PubMed] [Google Scholar]
- 39.Nantwi KD, El-Bohy A, Schrimsher GW, Reier PJ, Goshgarian HG. Spontaneous functional recovery in a paralyzed hemidiaphragm following upper cervical spinal cord injury in adult rats. Neurorehab Neural Repair. 1999;13:225–234. [Google Scholar]
- 40.Novikova LN, Novikov LN, Kellerth JO. BDNF abolishes the survival effect of NT-3 in axotomized Clarke neurons of adult rats. J Comp Neurol. 2000;428:671–680. doi: 10.1002/1096-9861(20001225)428:4<671::aid-cne7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 41.Novikova LN, Novikov LN, Kellerth JO. Differential effects of neurotrophins on neuronal survival and axonal regeneration after spinal cord injury in adult rats. J Comp Neurol. 2002;452:255–263. doi: 10.1002/cne.10381. [DOI] [PubMed] [Google Scholar]
- 42.Pizzorusso T, Ratto GM, Putignano E, Maffei L. Brain-derived neurotrophic factor causes cAMP response element-binding protein phosphorylation in absence of calcium increases in slices and cultured neurons from rat visual cortex. J Neurosci. 2000;20:2809–2816. doi: 10.1523/JNEUROSCI.20-08-02809.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 44.Prakash YS, Mantilla CB, Zhan WZ, Smithson KG, Sieck GC. Phrenic motoneuron morphology during rapid diaphragm muscle growth. J Appl Physiol. 2000;89:563–572. doi: 10.1152/jappl.2000.89.2.563. [DOI] [PubMed] [Google Scholar]
- 45.Prakash YS, Miyata H, Zhan WZ, Sieck GC. Inactivity-induced remodeling of neuromuscular junctions in rat diaphragmatic muscle. Muscle Nerve. 1999;22:307–319. doi: 10.1002/(sici)1097-4598(199903)22:3<307::aid-mus3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 46.Prakash YS, Smithson KG, Sieck GC. Measurements of motoneuron somal volumes using laser confocal microscopy: comparisons with shape-based stereological estimations. Neuroimage. 1993;1:95–107. doi: 10.1006/nimg.1993.1003. [DOI] [PubMed] [Google Scholar]
- 47.Prakash YS, Smithson KG, Sieck GC. Application of the Cavalieri principle in volume estimation using laser confocal microscopy. Neuroimage. 1994;1:325–333. doi: 10.1006/nimg.1994.1017. [DOI] [PubMed] [Google Scholar]
- 48.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakura S, Hashimoto K, Bollen AW, Ciriales R, Drasner K. Intrathecal catheterization in the rat. Improved technique for morphologic analysis of drug-induced injury. Anesthesiology. 1996;85:1184–1189. doi: 10.1097/00000542-199611000-00028. [DOI] [PubMed] [Google Scholar]
- 50.Sayer FT, Oudega M, Hagg T. Neurotrophins reduce degeneration of injured ascending sensory and corticospinal motor axons in adult rat spinal cord. Exp Neurol. 2002;175:282–296. doi: 10.1006/exnr.2002.7901. [DOI] [PubMed] [Google Scholar]
- 51.Schinder AF, Poo M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000;23:639–645. doi: 10.1016/s0166-2236(00)01672-6. [DOI] [PubMed] [Google Scholar]
- 52.Sheng M, Thompson MA, Greenberg ME. CREB: a Ca(2+)-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science. 1991;252:1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- 53.Sieck GC, Mantilla CB. Role of neurotrophins in recovery of phrenic motor function following spinal cord injury. Respir Physiol Neurobiol. 2009;169:218–225. doi: 10.1016/j.resp.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sieck GC, Mantilla CB, Prakash YS. Volume measurements in confocal microscopy. Methods Enzymol. 1999;307:296–315. doi: 10.1016/s0076-6879(99)07019-6. [DOI] [PubMed] [Google Scholar]
- 55.Singh LP, Devi TS, Nantwi KD. Theophylline regulates inflammatory and neurotrophic factor signals in functional recovery after C2-hemisection in adult rats. Exp Neurol. 2012;238:79–88. doi: 10.1016/j.expneurol.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 57.Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 58.Trelease RB, Sieck GC, Harper RM. A new technique for acute and chronic recording of crural diaphragm EMG in cats. Electroencephalogr Clin Neurophysiol. 1982;53:459–462. doi: 10.1016/0013-4694(82)90011-6. [DOI] [PubMed] [Google Scholar]
- 59.Vinit S, Gauthier P, Stamegna JC, Kastner A. High cervical lateral spinal cord injury results in long-term ipsilateral hemidiaphragm paralysis. J Neurotrauma. 2006;23:1137–1146. doi: 10.1089/neu.2006.23.1137. [DOI] [PubMed] [Google Scholar]
- 60.Watson FL, Heerssen HM, Bhattacharyya A, Klesse L, Lin MZ, Segal RA. Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nat Neurosci. 2001;4:981–988. doi: 10.1038/nn720. [DOI] [PubMed] [Google Scholar]
- 61.Watson FL, Heerssen HM, Moheban DB, Lin MZ, Sauvageot CM, Bhattacharyya A, Pomeroy SL, Segal RA. Rapid nuclear responses to target-derived neurotrophins require retrograde transport of ligand-receptor complex. J Neurosci. 1999;19:7889–7900. doi: 10.1523/JNEUROSCI.19-18-07889.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weishaupt N, Blesch A, Fouad K. BDNF: the career of a multifaceted neurotrophin in spinal cord injury. Exp Neurol. 2012;238:254–264. doi: 10.1016/j.expneurol.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 63.Widenfalk J, Lundstromer K, Jubran M, Brene S, Olson L. Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid. J Neurosci. 2001;21:3457–3475. doi: 10.1523/JNEUROSCI.21-10-03457.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yajima Y, Narita M, Usui A, Kaneko C, Miyatake M, Yamaguchi T, Tamaki H, Wachi H, Seyama Y, Suzuki T. Direct evidence for the involvement of brain-derived neurotrophic factor in the development of a neuropathic pain-like state in mice. J Neurochem. 2005;93:584–594. doi: 10.1111/j.1471-4159.2005.03045.x. [DOI] [PubMed] [Google Scholar]
- 65.Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- 66.Yaksh TL, Rudy TA. Studies on the direct spinal action of narcotics in the production of analgesia in the rat. J Pharmacol Exp Ther. 1977;202:411–428. [PubMed] [Google Scholar]
- 67.Ye JH, Houle JD. Treatment of the chronically injured spinal cord with neurotrophic factors can promote axonal regeneration from supraspinal neurons. Exp Neurol. 1997;143:70–81. doi: 10.1006/exnr.1996.6353. [DOI] [PubMed] [Google Scholar]
- 68.Zhan WZ, Mantilla CB, Zhan P, Bitton A, Prakash YS, de Troyer A, Sieck GC. Regional differences in serotonergic input to canine parasternal intercostal motoneurons. J Appl Physiol. 2000;88:1581–1589. doi: 10.1152/jappl.2000.88.5.1581. [DOI] [PubMed] [Google Scholar]
- 69.Zhan WZ, Miyata H, Prakash YS, Sieck GC. Metabolic and phenotypic adaptations of diaphragm muscle fibers with inactivation. J Appl Physiol. 1997;82:1145–1153. doi: 10.1152/jappl.1997.82.4.1145. [DOI] [PubMed] [Google Scholar]