Abstract
The role of signaling pathways including the mitogen-activated protein kinases (MAPKs) and phosphatidylinositol 3-kinase (PI3K) during viral infection has gained much recent attention. Our laboratory reported on an important regulatory role for extracellular signal-regulated kinases (ERK1/2), subfamily members of the MAPKs, during coxsackievirus B3 (CVB3) infection. However, the role of the PI3K pathway in CVB3 infection has not been well characterized. CVB3 is the most common known viral infectant of heart muscle that directly injures and kills infected cardiac myocytes during the myocarditic process. In the present study, we investigated the role of protein kinase B (PKB) (also known as Akt), a general downstream mediator of survival signals through the PI3K cascade, in regulating CVB3 replication and virus-induced apoptosis in a well-established HeLa cell model. We have demonstrated that CVB3 infection leads to phosphorylation of PKB/Akt on both Ser-473 and Thr-308 residues through a PI3K-dependent mechanism. Transfection of HeLa cells with a dominant negative mutant of Akt1 or pretreatment of wild-type HeLa cells with the specific PI3K inhibitor LY294002 significantly suppresses viral RNA expression, as reflected in diminished viral capsid protein expression and viral release. Dominant negative Akt1 and LY294002 also increase apoptosis in infected cells, which can be reversed by addition of the general caspase inhibitor benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (zVAD.fmk). Interestingly, blocking of apoptosis by zVAD.fmk does not reverse the viral RNA translation blockade, indicating that the inhibitory effect of dominant negative Akt1 on viral protein expression is not caspase dependent. In addition, we showed that the attachment of virus to its receptor-coreceptor complex is not sufficient for PKB/Akt activation and that postentry viral replication is required for Akt phosphorylation. Taken together, these data illustrate a new and imperative role for Akt in CVB3 infection in HeLa cells and show that the PI3K/Akt signaling is beneficial to CVB3 replication.
Coxsackievirus B3 (CVB3) is a small, nonenveloped, positive-strand RNA Enterovirus in the Picornaviridae family. CVB3 has been known as the most common infectant of the heart that directly injures and kills infected cardiac myocytes (13, 35). CVB3-induced myocarditis was known historically as an immune-mediated disease (25, 33, 41). However, the most recently supported hypothesis is that direct CVB3-induced injury prior to immune cell infiltration is a very important determinant of disease progression (13, 35). In regard to virus-infected cells, the fate of infected cell and the severity of disease are greatly influenced by the balance between antiapoptotic and proapoptotic pathways. Our laboratory has carried out extensive work on the CVB3-triggered death signaling pathway in the context of interaction between this pathway and the virus life cycle. We have characterized apoptotic signaling, and in particular the caspases, following CVB3 infection in HeLa cells (11). Although the interaction between CVB3 and survival cascades has not been investigated in detail, there is mounting evidence to support the idea that a few other viral proteins can modulate such pathways. The X protein of hepatitis B virus activates survival signaling cascades including phosphatidylinositol 3-kinase (PI3K)/Akt and stress-activated protein kinase, resulting in progression of hepatocellular carcinoma (16, 29). It has also been demonstrated that the BHRF1 protein of Epstein-Barr virus and immediate-early proteins IE1 and IE2 of human cytomegalovirus markedly inhibit apoptosis in infected cells (26, 52). However, viral products may have a dual action in infected host cells. A well-characterized example is simian virus 40 large tumor (T) antigen, which may either block or induce apoptosis, depending upon the cell environment (17, 34).
The role of signaling proteins during CVB3 infection and how these proteins can be modulated by the virus are not very well understood. Recently we reported that a biphasic phosphorylation and activation of the extracellular signal-regulated kinase (ERK1/2) participate in the regulation of viral replication and virus-mediated cytopathic effects in infected HeLa cells (31). However, little is known about the role of the other protein kinases during CVB3 infection.
Protein kinases play a critical regulatory role as the second messengers in various intracellular signaling pathways by phosphorylation of proteins that control nearly all features of cell life. One such protein kinase, protein kinase B (PKB), has been intensively studied during the last decade (5, 14, 36, 53). PKB, also known as Akt, is a cytoplasmic serine/threonine kinase containing a pleckstrin homology domain at its amino terminus and acts as a key protein mediator for a wide range of cellular processes (21, 44). Three different isoforms of Akt have been so far characterized: Akt1, Akt2, and Akt3. Each isoform is encoded by a separate gene, but they all carry a pleckstrin homology domain, a catalytic domain, and a putative regulatory domain. Several studies have shown that activation of Akt by various stimuli, including growth factors, insulin, and hormones, is mediated by phosphorylation of both serine 473 and threonine 308 residues through a PI3K-dependent mechanism (3, 36, 47). Upon phosphorylation, Akt modulates diverse downstream signaling pathways associated with cell survival, proliferation, differentiation, migration, and apoptosis. The observation that cell stimulation with growth factors may trigger Akt translocation to the nucleus, alongside findings that Akt activates transcription factors such as Forkhead transcription factors and nuclear factor κB, suggests that Akt may up- or down-regulate the expression of several genes involved in homeostasis (8, 9, 36, 40). The role of PI3K and its downstream effector Akt in regulating cell survival and apoptosis in a number of viral infection models has been a major recent interest (15, 23, 30).
The possibility that the PI3K/Akt pathway participates in the preservation of host cell survival during viral infection and may play a role in providing a supportive milieu for virus replication compelled us to investigate the interaction between CVB3 and this pathway. In the present study, we have shown that Akt can be phosphorylated and activated following CVB3 infection through a PI3K-dependent mechanism. We have also shown, by either using a specific inhibitor of the PI3K/Akt pathway or overexpressing a dominant negative mutant of Akt1, that inhibition of the PI3K/Akt pathway suppresses viral RNA synthesis, viral protein expression, and viral release in CVB3-infected cells.
MATERIALS AND METHODS
Akt1 constructs.
Transfection-grade eukaryotic expression vectors (pUSEamp) encoding a dominant negative mutant Akt1 (expressing the neomycin resistance gene for selection of transfected cells) were purchased from Upstate Biotechnology, Inc., and confirmed by restriction analysis. The dominant negative mutant Akt1 construct (encoding an alanine instead of a serine and a threonine) and the empty vector control were used in stable transfection of HeLa cells.
Cell culture and transfection.
HeLa cells (HeLa S3) were obtained from the American Type Culture Collection. Subconfluent cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated newborn calf serum (Life Technologies, Inc.) at 37°C in a humidified incubator with 5% CO2. Penicillin G (100 μg/ml) and streptomycin (100 μg/ml) (Life Technologies, Inc.) were added to all culture media. For stable transfection of HeLa cells, 2 μg of cDNA was introduced by using Lipofectamine 2000 reagent (Invitrogen Life Technologies) according to the manufacturer's instructions. At 36 h posttransfection, Geneticin (G418) (Sigma Aldrich) was added as a selective marker at the final concentration of 400 μg/ml for selecting the transfected clones and at the final concentration of 200 μg/ml for maintenance of transfection during the course of experiments.
Virus infection.
CVB3 (Nancy strain) was a gift from Reinhard Kandolf and was propagated in HeLa cells and stored at −80°C. The titer of virus was determined routinely prior to each experiment. Subconfluent wild-type or transfected HeLa cells were serum starved for 24 h before the introduction of virus to eliminate the effect of serum growth factors. Cells were infected either with CVB3 at a multiplicity of infection (MOI) of 10 or with DMEM (extracted from HeLa cell culture) for the control group. Following 1 h of incubation at 37°C, cells were washed with serum-free DMEM and replenished with fresh DMEM. For inhibitor experiments, pretreatment with inhibitor was performed 1 h prior to infection and fresh inhibitors at the specified concentrations were added following medium changes.
UV irradiation and inactivation of CVB3.
UV-irradiated virus was prepared as described previously (4, 31). Briefly, 1 ml of diluted virus was transferred to a 1.5-ml tube and then irradiated in a UV Stratalinker 1800 (Stratagene) for a total dose of 15 J/cm2 for 10 min.
Antibodies and inhibitors.
Rabbit polyclonal phospho-Akt antibodies (Ser-473 and Thr-308), rabbit polyclonal Akt antibody, and rabbit polyclonal phosphoglycogen synthase kinase-3 α/β (Ser-21/Ser-9) antibody were purchased from New England BioLabs. Rabbit polyclonal antibody against viral protein VP1 was obtained from Denka Seiken Co., Ltd.; mouse monoclonal caspase-3 antibody, mouse monoclonal β-actin antibody, anti-rabbit immunoglobulin G, and anti-mouse immunoglobulin G conjugated with horseradish peroxidase were purchased from Santa Cruz Biotechnology; and mouse monoclonal antibody against poly(ADP-ribose) polymerase (PARP) was obtained from BioMol Co. The specific PI3K inhibitor 2-morpholino-8-phenyl-4H-1-benzopyran-4-one (LY294002) was obtained from New England BioLabs, and the general caspase inhibitor benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (zVAD.fmk) was obtained from Clontech.
Western blot analysis.
Cells either untreated or treated with different experimental reagents were washed twice with ice-cold phosphate-buffered saline (PBS) containing 5% phosphatase inhibitor (Active Motiff Co.) and kept on ice for 10 min in lysis buffer containing 50 mM pyrophosphate, 50 mM NaF, 50 mM NaCl, 5 mM EDTA, 5 mM EGTA, 100 μM Na3VO4, 10 mM HEPES (pH 7.4), 0.1% Triton X-100, 10 μg of leupeptin/ml, and 1 mM phenylmethylsulfonyl fluoride. Cell lysates were then collected by scraping and centrifuged at 12,000 × g for 10 min at 4°C. The protein concentration was determined by the Bradford assay (Bio-Rad). Twenty to 40 micrograms of extracted protein was fractionated on sodium dodecyl sulfate-10% polyacrylamide gels, electrophoretically transferred to nitrocellulose membranes (Hybond ECL; Amersham Pharmacia Biotech), and blocked with PBS containing 0.1% Tween 20 and 5% nonfat dry milk for 1 h. Afterward, the membrane was incubated with specific primary antibody overnight at 4°C, followed by secondary antibody for 45 min at room temperature. The immunoblots were visualized with an enhanced chemiluminescence detection system according to the protocol of the manufacturer (Amersham Pharmacia Biotech). Densitometry analysis was performed by using the National Institutes of Health ImageJ software, version 1.27z. Density values for proteins were normalized to the level for control groups (arbitrarily set to 1.0-fold).
Viral RNA in situ hybridization.
Subconfluent HeLa cells were grown and maintained on two-chamber culture slides (Becton Dickinson Labware) under serum-free conditions for 24 h prior to CVB3 infection. Cells were infected with either DMEM or CVB3 (MOI of 10). Following 1 h of incubation at 37°C, cells were washed with serum-free DMEM and replenished with fresh DMEM. The culture slides were then washed gently with PBS, fixed with 10% normal formalin buffer for 15 min, washed with PBS, incubated with 70% ethanol for 2 min, and then air dried at room temperature. Culture slides were then subjected to in situ hybridization assays to detect sense and antisense RNAs of CVB3 as previously described (2).
Virus release plaque assay.
Wild-type or transfected HeLa cells either untreated or treated with specific inhibitors were infected with CVB3 at an MOI of 10 for 1 h. Cells were washed and replenished with serum-free DMEM for 9 h. The supernatants were collected and kept at −80°C for the virus release assay. Plaque assays were carried out with HeLa cells in duplicate by standard procedures as described previously (2). Agar overlays were fixed with Carnoy's fixative (25% acetic acid, 75% ethanol) at 3 days after infection. Cells were stained with 1% crystal violet dye to visualize plaques. Images of plates were obtained, and the virus titer was calculated as PFU per milliliter.
Statistical analysis.
Two-way analysis of variance with multiple comparisons and paired Student's t tests were performed. Values shown are the mean ± standard deviation. A P value of <0.05 was considered significant.
RESULTS
CVB3 induces Akt phosphorylation at both serine 473 and threonine 308 residues.
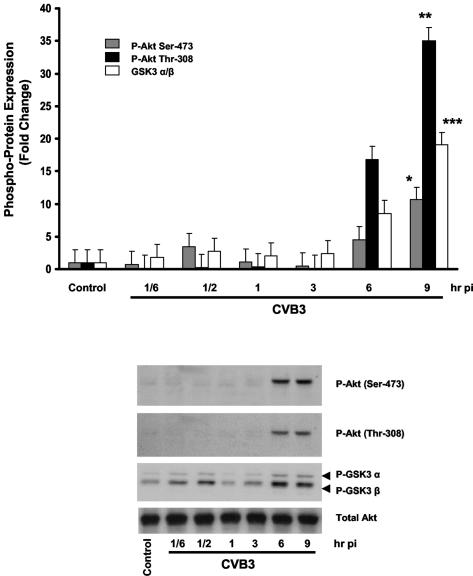
In the present study, we initially determined whether coxsackievirus B3 infection of HeLa cells resulted in phosphorylation of Akt. Serum-starved HeLa cells were infected with CVB3 (MOI of 10) or DMEM. The DMEM used as a control was extracted from HeLa cells in culture under conditions identical to those used for virus propagation to account for the potential effect of proteins within the medium. Cell lysates were then collected at 10 and 30 min and at 1, 3, 6, and 9 h postinfection. As shown in Fig. 1, CVB3 induces Akt phosphorylation at both Ser-473 and Thr-308 residues at 6 to 9 h following infection. The phosphorylation of an Akt-regulated downstream protein, glycogen synthase kinase-3 α/β, was determined as a measure of Akt kinase activity. Total Akt was measured to ensure equal protein loading.
FIG. 1.
CVB3 infection leads to phosphorylation and activation of Akt on both serine 473 and threonine 308 residues. Following serum starvation for 24 h, HeLa cells were either sham or CVB3 infected (MOI of 10) for 1 h. Cell lysates were collected at the specified times postinfection (pi) and subjected to Western blotting to detect phosphorylation of Akt on both Ser-473 and Thr-308 sites. The activity of phospho-Akt was determined based on the phosphorylation of glycogen synthase kinase-3 α/β (GSK3 α/β), a downstream substrate of PKB. Total Akt protein was measured to ensure equal protein loading. Protein density is expressed as the increase in the level of phosphorylated protein with respect to the noninfected control (*, **, and ***, P < 0.01). The data shown are representative of those from quadruplicate experiments. Error bars indicate standard deviations.
CVB3 stimulates Akt phosphorylation via a PI3K-dependent mechanism.
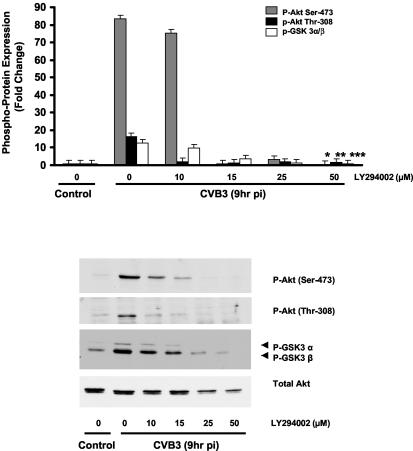
To investigate whether the phosphorylation and activation of Akt upon CVB3 infection occur through the PI3K pathway, HeLa cells were pretreated with increasing doses (5, 10, 25, and 50 μM) of the specific PI3K inhibitor LY294002 1 h prior to virus infection. LY294002 inhibits PI3K activity through a competitive inhibition of the ATP binding site located on the p85α subunit of PI3K (18, 46). Cell lysates were collected at 9 h postinfection and subjected to Western blot analysis to detect the phosphorylation of Akt on Ser-473 and Thr-308. Figure 2 shows LY294002 inhibition of CVB3-induced Akt phosphorylation and activation in a dose-dependent manner. The observation that the phosphorylation of Akt following CVB3 infection was markedly decreased with a higher dose of LY294002 (50 μM) suggests that virus-induced Akt phosphorylation occurs through a PI3K-dependent mechanism.
FIG. 2.
CVB3 induces Akt phosphorylation via a PI3K-dependent mechanism. HeLa cells were serum starved for 24 h. After being treated with LY294002 (50 μM) or vehicle (DMSO), cells were infected with either CVB3 (MOI of 10) or DMEM for 1 h. Total protein was extracted and analyzed by immunoblotting. Akt phosphorylation on both Ser-473 and Thr-308 residues was prevented by the PI3K inhibitor, indicating that Akt activation by CVB3 was mediated via a PI3K-dependent pathway. Total Akt protein was detected to ensure equal loading protein. Density values for phosphoproteins are expressed as the fold change compared to nontreated CVB3-infected cells (*, **, and ***, P < 0.01). GSK 3α/β, glycogen synthase kinase-3 α/β; pi, postinfection. Data are representative of those from two independent experiments. Error bars indicate standard deviations.
UV-irradiated CVB3 is unable to induce Akt phosphorylation and activation.
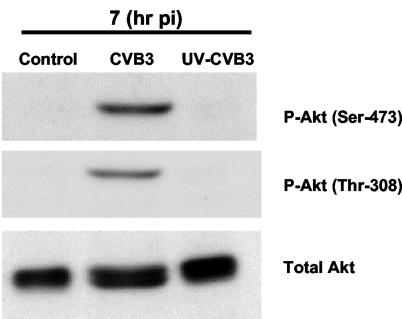
To investigate the mechanism behind the activation of Akt, CVB3 was exposed to UV radiation for 10 min for a total dose of 15 J/cm2. UV irradiation blocks the virus's capability for RNA transcription and protein synthesis but has no effect on virus receptor attachment and subsequent entry into the host cell (4, 19, 28). HeLa cells were infected with either wild-type CVB3 or UV-inactivated virus (MOI of 10) for 1 h. Cell lysates were collected at 7 h postinfection and then subjected to Western blot analysis to detect the phosphorylation of Akt on Ser-473 and Thr-308 sites. As shown in Fig. 3, UV-inactivated CVB3 fails to induce Akt phosphorylation at 7 h postinfection, which implies that Akt phosphorylation is due to postentry viral replication in infected cells.
FIG. 3.
UV-irradiated CVB3, which is capable of receptor binding and endocytosis but not replication, fails to induce Akt phosphorylation. HeLa cells were infected with wild-type or UV-irradiated CVB3, and cell lysates were collected at 7 h postinfection (pi) and subjected to Western blotting to detect the phosphorylation of Akt on Ser-473 and Thr-308 residues. Virus attachment to specific receptor-coreceptor complex is not sufficient for Akt phosphorylation, suggesting that viral postentry replication is required for Akt activation. The results shown are representative of those from triplicate experiments.
LY294002 blocks viral RNA synthesis, viral protein VP1 expression, and virus release in CVB3-infected HeLa cells.
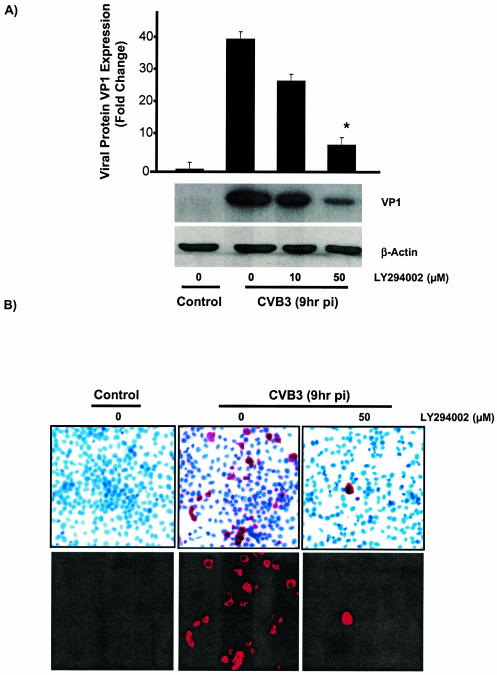
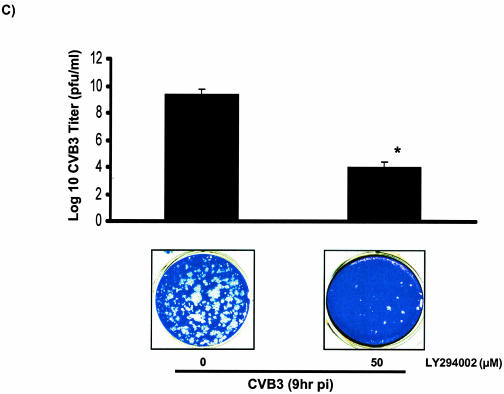
In order to investigate the interaction between the PI3K cascade and CVB3 replication and to understand whether the activation of PI3K plays any role in CVB3 replication, we examined the effect of the specific PI3K inhibitor LY294002 on different stages of the virus life cycle in infected cells. Subconfluent HeLa cells were grown on culture slides and pretreated with either inhibitor or dimethyl sulfoxide (DMSO) prior to infection. The supernatants were collected at 9 h postinfection for the plaque assay experiment to calculate the number of progeny virions released from infected cells. Concurrently, HeLa cells on glass slides were fixed and subjected to in situ hybridization to assess viral RNA expression. The expression of VP1 in the presence or absence of LY294002 was determined by Western blotting. As shown in Fig. 4A, LY294002 blocked viral protein expression in a dose-dependent manner. LY294002 also significantly decreased viral RNA synthesis (Fig. 4B) and viral progeny release (Fig. 4C) compared to control groups. These results suggest that PI3K contributes to the virus life cycle and is beneficial for virus replication.
FIG. 4.
LY294002 blocks CVB3 structural protein (VP1) synthesis, viral RNA expression, and viral release in infected HeLa cells. (A) Viral protein expression in LY294002-treated HeLa cells. CVB3-infected HeLa cells were treated with either DMSO or different doses of LY294002 (10 and 50 μM) for 9 h. Cell lysates were collected for Western blotting to detect viral capsid protein VP1 expression. β-Actin expression was measured to ensure equal protein loading. As shown, LY294002 blocks VP1 synthesis in a dose-dependent manner. (B) In situ hybridization of CVB3-infected HeLa cells at 9 h postinfection (pi), using digoxigenin-labeled viral strand-specific riboprobe (bright-field images in the upper row and fluorescent images in the lower row). Positive signals indicate sense (plus)-strand RNA of CVB3. LY294002 (50 μg/ml) blocks viral RNA transcription and replication in infected cells. (C) Viral release in LY294002-treated HeLa cells. Supernatants from culture slides (from panel B) were collected and assayed for infectious virus by the agar overlay plaque assay method. Comparison of infectious virus particles under the indicated conditions shows that the PI3K inhibitor decreases viral progeny release from infected host cells. Data are averages from three experiments *, P < 0.005 compared to nontreated infected cells. Error bars indicate standard deviations.
A dominant negative mutant of Akt1 also decreases viral RNA synthesis, viral protein VP1 expression, and virus release in infected cells.
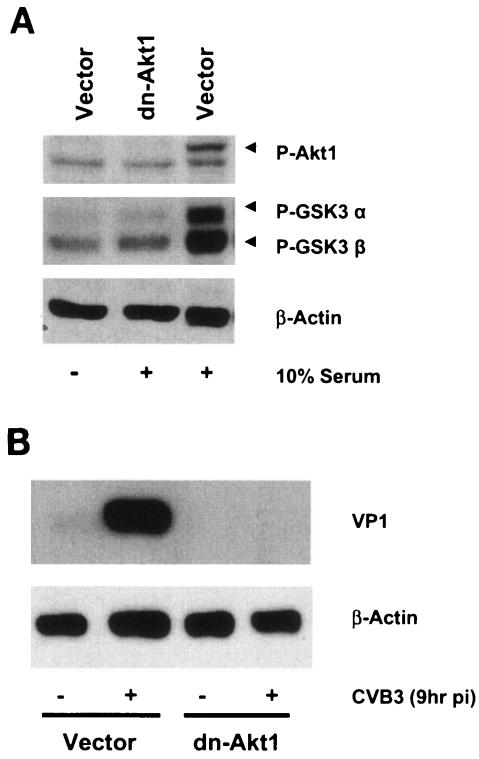
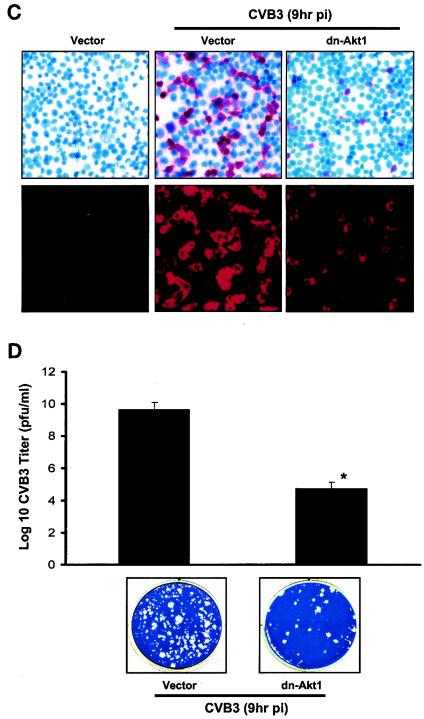
To further study the contribution of Akt as a key downstream effector of PI3K to CVB3 replication, we utilized a different approach by using a dominant negative mutant of Akt1 that is incapable of being phosphorylated in response to different stimuli. The dominant negative Akt1 cDNA contains Myc-His tagged Akt1 (in pUSEamp) under the control of the cytomegalovirus promoter and has substitutions of methionine for lysine and serine and threonine for alanine. In this experiment, HeLa cells were stably transfected with either a dominant negative mutant of Akt1 or a vector control (pUSEamp) plasmid. Akt phosphorylation in the transfected cell line was evaluated prior to the experiment. As expected, in the presence of serum, the dominant negative Akt1 mutant significantly blocked Akt1 phosphorylation and activation in transfected HeLa cells compared to control cells (Fig. 5A). Subsequently, transfected HeLa cells were infected with CVB3 for 1 h and viral protein expression, viral RNA synthesis, and viral progeny release were determined at 9 h postinfection. Our observations showed that the dominant negative mutant of Akt1 blocked CVB3 replication, including viral RNA synthesis (Fig. 5C), viral protein expression (Fig. 5B), and progeny virion release from infected cells (Fig. 5D). These findings, together with our results from inhibitor-based experiments, show that virus-induced PI3K/Akt activation augments CVB3 replication in HeLa cells.
FIG. 5.
Dominant negative mutant of Akt1 blocks CVB3 structural protein (VP1) synthesis, viral RNA expression, and viral release in infected HeLa cells. (A) Phospho-Akt1 expression in stably transfected HeLa cells. Transfected HeLa cells were treated with either serum or PBS for 30 min, and 40 μg of cell lysate was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and probed with anti-phospho-Akt and anti-phospho-glycogen synthase kinase-3 α/β (GSK3 α/β) antibodies. Akt1 phosphorylation and activity are markedly decreased in dn-Akt1 HeLa cells compared to the vector group treated with serum. (B) Viral protein synthesis in HeLa cells overexpressing a dominant negative mutant of Akt1. The dominant negative mutant of Akt1 (dn-Akt1) decreases viral protein synthesis compared to that of the CVB3-infected vector group. (C) In situ hybridization of stably transfected HeLa cells at 9 h postinfection (pi) (bright-field images in the upper row and fluorescent images in the lower row). dn-Akt1 significantly suppresses viral RNA replication. (D) Virus release in dominant negative-transfected HeLa cells. Comparison of CVB3 infectious particles released in the culture medium of the slides indicated in panel C shows that the dominant negative mutant of Akt1 also diminishes virus release from infected HeLa cells. Data are averages from four experiments. *, P < 0.005 compared to CVB3-infected vector. Error bars indicate standard deviations.
A PI3K inhibitor and the dominant negative mutant of Akt1 enhance apoptosis in CVB3-infected HeLa cells through a caspase-dependent mechanism.
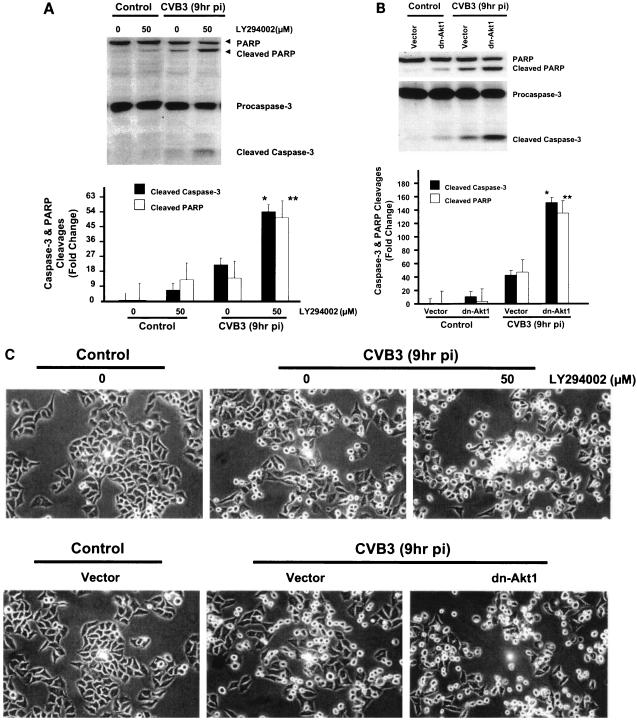
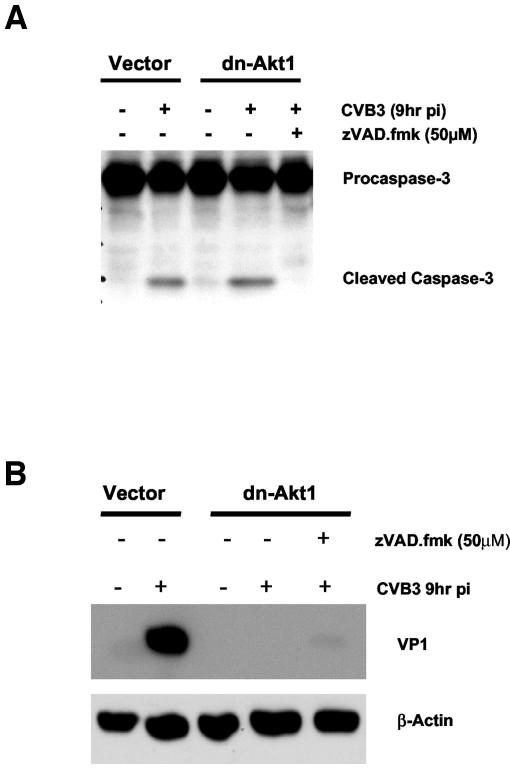
To elucidate the role of Akt in regulating apoptosis in CVB3-infected cells, Akt activation was blocked either by using a specific PI3K inhibitor or by transfecting HeLa cells with a dominant negative mutant Akt1 construct. Apoptosis was assessed by measuring caspase-3 cleavage and PARP cleavage at 9 h postinfection in CVB3-infected cells. The dominant negative mutant of Akt1 (dn-Akt1) and LY294002 both increased caspase-3 and PARP cleavages (Fig. 6A and B) and enhanced cytopathic effects (Fig. 6C) in CVB3-infected cells. Since we have already shown that inhibition of Akt results in a significant decline in CVB3 replication, these findings suggest that increased cytopathic effects in infected cells are due to the inhibition of the antiapoptotic activity of Akt either by LY294002 or by expression of a dominant negative form of Akt1. It is noteworthy that the proapoptotic effect of the PI3K inhibitor or the dominant negative Akt1 construct in CVB3-infected cells is less than that observed in noninfected cells, supporting a role for virus-induced survival pathways in contravening apoptosis in an attempt to initially foster host cell survival and viral replication. The proapoptotic effect of dominant negative Akt1 in infected HeLa cells can be reversed by adding the general caspase inhibitor zVAD.fmk. HeLa cells transfected with either dn-Akt1 or vector control plasmids were pretreated with zVAD.fmk (50 μM) 1 h prior to infection. As shown in Fig. 7A, the caspase inhibitor zVAD.fmk reversed the apoptotic effect of dominant negative Akt1 in CVB3-infected cells by blocking caspase-3 cleavage.
FIG. 6.
LY294002 and the dominant negative mutant of Akt1 induce apoptosis in CVB3-infected cells. (A) Cleavage of caspase-3 and PARP in LY294002-treated HeLa cells. Cells treated with LY294002 or DMSO were either sham infected (DMEM) or infected with virus, and cell lysates were subjected to Western blot analysis for caspase-3 and PARP cleavages. LY294002 increases caspase-3 and PARP cleavages in CVB3-infected cells. The data represent two different experiments. * and **, P < 0.01 compared to nontreated infected cells. Error bars indicate standard deviations. pi, postinfection. (B) Cleavage of casapse-3 and PARP in HeLa cells transfected with the dominant negative Akt1 construct. The dominant negative mutant of Akt1 enhances caspase-3 and PARP cleavages in infected HeLa cells. Density values for caspase-3 and PARP cleavages are expressed as the fold change compared to CVB3-infected cells transfected with vector control (* and **, P < 0.01). The results shown are representative of those from two independent experiments. (C) Phase-contrast microscopic images of wild-type HeLa cells treated with LY294002 and transfected HeLa cells following CVB3 infection. As shown, both LY294002 and the dn-Akt1 construct enhance cytopathic effects and decrease host cell viability in infected HeLa cells as assessed by morphological changes.
FIG. 7.
Akt regulation of CVB3 replication is not caspase dependent. (A) Cleavage of caspase-3 in HeLa cells transfected with the dominant negative Akt1 constructs is reversed by the multicaspase inhibitor zVAD.fmk. Transfected HeLa cells were treated with zVAD.fmk (50 μM) starting 1 h prior to infection. β-Actin expression was measured for equal protein loading. The data represent two independent experiments. pi, postinfection. (B) Inhibition of caspase-3 cleavage by zVAD.fmk does not preserve viral protein expression in infected HeLa cells, indicating that the regulatory effect of Akt on virus replication is not a caspase-dependent process. The data represent two independent experiments. Density values for protein expression are expressed as the fold change compared to nontreated dn-Akt1 cells infected with CVB3.
Inhibition of viral protein expression by dominant negative Akt1 is not caspase dependent.
To clarify whether inhibition of viral protein expression by dominant negative Akt1 is due to acceleration of apoptosis in infected cells, we compared the expression of viral protein VP1 in dn-Akt1-transfected HeLa cells in the presence or absence of the general caspase inhibitor zVAD.fmk. As shown in Fig. 7B, inhibition of caspase-3 cleavage and apoptosis does not reverse the inhibitory effect of dn-Akt1 on viral protein expression. The observation that zVAD.fmk is capable of blocking apoptosis in infected cells (Fig. 7A) without having any effect on viral RNA translation and VP1 expression (Fig. 7B) indicates that the regulatory effect of Akt on virus replication is not a caspase-dependent process.
DISCUSSION
During viral infection, viruses encode highly competent products in order to optimize viral replication, produce huge numbers of progeny virions, and release infectious particles to launch a new round of infection. However, host cells activate prosurvival defenses aimed to preserve host cell viability in response to viral infection. Viruses may evolve strategies to block or delay such host defense mechanisms. How viruses interact with and manipulate host cell signaling pathways to augment their replication is currently the subject of intense study. Our laboratory has previously reported that the phosphorylation and activation of ERK1/2 during CVB3 infection are required for viral replication. We have also shown that CVB3 activates ERK1/2 in a biphasic manner, in early transient and late sustained phosphorylation events. We have recently reported that gamma interferon-inducible GTPase overexpression enhances host cell survival in CVB3-infected cells, likely by inducing the activation of Akt (51). We also revealed that gamma interferon-inducible GTPase overexpression inhibits viral replication, probably by its overwhelming negative effect on viral RNA translation. These finding led our group to further investigate the role of Akt activation in the course of CVB3 infection.
In the present study, we report that CVB3 enhances the phosphorylation of Akt on both serine 473 and threonine 308 residues, resulting in the activation of this protein kinase. The activity of phosphorylated Akt was assessed by phosphorylation of a downstream target, glycogen synthase kinase-3 α/β. Replication-deficient CVB3 (UV irradiated), which is capable of receptor attachment and cell entry but not replication, fails to induce Akt phosphorylation, indicating that virus attachment and entry are not sufficient for Akt activation. This also supports the view that postentry viral RNA synthesis and subsequent steps in viral replication and progeny production are required for Akt phosphorylation and activation in infected cells.
In the present study, we also investigated the regulatory role of PI3K in CVB3-induced Akt phosphorylation by using different dosages of the specific PI3K inhibitor LY294002. The results suggest that CVB3 can trigger Akt activation via a PI3K-dependent mechanism. The observation that LY294002 is capable of blocking virus-induced Akt phosphorylation in a dose-dependent manner strongly supports the importance of PI3K as an intermediate effector in the process of Akt activation by CVB3.
It has previously been shown that PI3K is a major upstream activator of Akt through the production of phosphatidylinositol-3,4,5-triphosphates in response to growth factors and hormones (1, 36). Recent studies revealed that PI3K is commonly stimulated upon activation of membrane receptors that either couple to heterotrimeric GTP proteins or have tyrosine kinase activity (12, 22, 32). Our observation that replication-deficient CVB3 cannot activate the PI3K/Akt pathway supports the idea that activation of PI3K/Akt during CVB3 infection is not a receptor-dependent event, and it is more likely this activation is induced directly or indirectly by viral products. It has also been reported that PI3K can be activated indirectly by the small GTPase Ras, which can bind and activate the p110 subunit of PI3K (27, 39). Our previous findings also revealed that the p21ras GTPase-activating protein RasGAP, a protein which negatively regulates the activation of Ras, is cleaved at 5 h following CVB3 infection (24). Considering these findings, it is suggested that RasGAP cleavage and constitutive activation of Ras following CVB3 infection may participate in the regulation of PI3K/Akt activation in infected cells. However, the actual mechanism behind PI3K/Akt activation in the CVB3 infection model has yet to be fully characterized.
To increase our understanding of the interaction between Akt and virus replication, we also examined the effects of Akt activation at different checkpoints of the virus life cycle, including virus RNA transcription, viral RNA translation and protein expression, and viral release from infected host cells. We observed that inhibition of Akt phosphorylation by either LY294002 or transfection of cells with a dominant negative mutant of Akt1 resulted in a significant decrease in virus RNA transcription and translation as well as virus assembly and release. Although the regulatory mechanism by which Akt controls CVB3 replication is not well understood, there is mounting evidence indicating that viral proteins can be phosphorylated by various protein kinases. Several protein kinases, including ERK1/2 mitogen-activated protein kinase and protein kinase C, have been shown to enhance human immunodeficiency virus type 1 infectivity by direct phosphorylation of viral proteins Vif, Tat, Gag, and Nef (6, 10, 20, 48-50). Hepatitis B virus large envelope protein can be phosphorylated by ERK-type mitogen-activated protein kinases (42). There is also an indication that poliovirus-specific RNA-dependent RNA polymerase can be phosphorylated at serine residues (38). These findings support the idea that Akt may regulate CVB3 replication by direct phosphorylation of viral products, including viral polymerases.
The PI3K pathway including the downstream effector Akt also plays a pivotal role in cell survival and proliferation. It has been reported that the inhibition of the PI3K/Akt pathway results in a significant decrease in host cell viability in different cell environments (7, 37, 43, 45). It is presumable that CVB3 enhances Akt phosphorylation to increase the viability of infected host cells in order for viral replication to take place. Although the downstream effectors of Akt that are involved in cell survival and proliferation have been extensively studied in tumor models, the mechanism and significance of antiapoptotic activity of Akt during CVB3 infection remain obscure.
Our findings show that the inhibition of Akt phosphorylation during CVB3 infection significantly enhances caspase-3 and PARP cleavage, indicating an antiapoptotic role for Akt during CVB3 infection. Since we have already shown that inhibition of Akt results in a substantial decrease in virus replication, it is likely that increased apoptosis in infected cells is a direct consequence of the inhibition of antiapoptotic activity of Akt but not a result of virus replication. We also showed that treating dn-Akt1 cells with the general caspase inhibitor zVAD.fmk blocked caspase-3 cleavage. Although zVAD.fmk blocked caspase-3 cleavage in infected cells, it did not show any effect on viral protein expression. In another words, even by blocking apoptosis, we were unable to reverse the inhibitory effect of dn-Akt1 on viral protein expression and preserve VP1 production. This evidence strongly supports the view that Akt regulates viral replication through a mechanism which is not caspase-dependent.
In summary, our study illustrates an important role for the PI3K/Akt pathway during CVB3 infection in HeLa cells. The present data together with our previous reports suggest that signaling pathways such as ERK1/2 mitogen-activated protein kinase and PI3K/Akt facilitate a productive viral infection. Further studies are required to fully understand the mechanisms by which these protein kinases interact with specific viral components during infection. Our findings provide valuable insights into the mechanism of viral pathogenesis and potential therapeutic targets for enteroviral infections.
Acknowledgments
This work was supported by grants from the Heart and Stroke Foundation of British Columbia and Yukon (to B.M.M.) and the Canadian Institutes of Health Research (to B.M.M.) and by studentships from the Heart and Stroke Foundation of British Columbia and Yukon (to M.E.) and the Michael Smith Foundation for Health Research (to M.E. and B.Y.).
We thank E. Walker for careful review of the manuscript and S. Greene and D. English for graphical support.
REFERENCES
- 1.Alessi, D. R., S. R. James, C. P. Downes, A. B. Holmes, P. R. Gaffney, C. B. Reese, and P. Cohen. 1997. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr. Biol. 7:261-269. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, D. R., J. E. Wilson, C. M. Carthy, D. Yang, R. Kandolf, and B. M. McManus. 1996. Direct interactions of coxsackievirus B3 with immune cells in the splenic compartment of mice susceptible or resistant to myocarditis. J. Virol. 70:4632-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andjelkovic, M., D. R. Alessi, R. Meier, A. Fernandez, N. J. Lamb, M. Frech, P. Cron, P. Cohen, J. M. Lucocq, and B. A. Hemmings. 1997. Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 272:31515-31524. [DOI] [PubMed] [Google Scholar]
- 4.Beck, M. A., N. M. Chapman, B. M. McManus, J. C. Mullican, and S. Tracy. 1990. Secondary enterovirus infection in the murine model of myocarditis. Pathologic and immunologic aspects. Am. J. Pathol. 136:669-681. [PMC free article] [PubMed] [Google Scholar]
- 5.Bellacosa, A., J. R. Testa, S. P. Staal, and P. N. Tsichlis. 1991. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science 254:274-277. [DOI] [PubMed] [Google Scholar]
- 6.Bodeus, M., A. Marie-Cardine, C. Bougeret, F. Ramos-Morales, and R. Benarous. 1995. In vitro binding and phosphorylation of human immunodeficiency virus type 1 Nef protein by serine/threonine protein kinase. J. Gen. Virol. 76:1337-1344. [DOI] [PubMed] [Google Scholar]
- 7.Bondar, V. M., B. Sweeney-Gotsch, M. Andreeff, G. B. Mills, and D. J. McConkey. 2002. Inhibition of the phosphatidylinositol 3′-kinase-AKT pathway induces apoptosis in pancreatic carcinoma cells in vitro and in vivo. Mol. Cancer Ther. 1:989-997. [PubMed] [Google Scholar]
- 8.Borgatti, P., A. M. Martelli, A. Bellacosa, R. Casto, L. Massari, S. Capitani, and L. M. Neri. 2000. Translocation of Akt/PKB to the nucleus of osteoblast-like MC3T3-E1 cells exposed to proliferative growth factors. FEBS Lett. 477:27-32. [DOI] [PubMed] [Google Scholar]
- 9.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857-868. [DOI] [PubMed] [Google Scholar]
- 10.Burnette, B., G. Yu, and R. L. Felsted. 1993. Phosphorylation of HIV-1 gag proteins by protein kinase C. J. Biol. Chem. 268:8698-8703. [PubMed] [Google Scholar]
- 11.Carthy, C. M., D. J. Granville, K. A. Watson, D. R. Anderson, J. E. Wilson, D. Yang, D. W. Hunt, and B. M. McManus. 1998. Caspase activation and specific cleavage of substrates after coxsackievirus B3-induced cytopathic effect in HeLa cells. J. Virol. 72:7669-7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chambliss, K. L., I. S. Yuhanna, C. Mineo, P. Liu, Z. German, T. S. Sherman, M. E. Mendelsohn, R. G. Anderson, and P. W. Shaul. 2000. Estrogen receptor alpha and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ. Res. 87:E44-E52. [DOI] [PubMed] [Google Scholar]
- 13.Chow, L. H., K. W. Beisel, and B. M. McManus. 1992. Enteroviral infection of mice with severe combined immunodeficiency. Evidence for direct viral pathogenesis of myocardial injury. Lab. Investig. 66:24-31. [PubMed] [Google Scholar]
- 14.Cook, S. A., T. Matsui, L. Li, and A. Rosenzweig. 2002. Transcriptional effects of chronic Akt activation in the heart. J. Biol. Chem. 277:22528-22533. [DOI] [PubMed] [Google Scholar]
- 15.Dawson, C. W., G. Tramountanis, A. G. Eliopoulos, and L. S. Young. 2003. Epstein-Barr virus latent membrane protein 1 (LMP1) activates the phosphatidylinositol 3-kinase/Akt pathway to promote cell survival and induce actin filament remodeling. J. Biol. Chem. 278:3694-3704. [DOI] [PubMed] [Google Scholar]
- 16.Diao, J., A. A. Khine, F. Sarangi, E. Hsu, C. Iorio, L. A. Tibbles, J. R. Woodgett, J. Penninger, and C. D. Richardson. 2001. X protein of hepatitis B virus inhibits Fas-mediated apoptosis and is associated with up-regulation of the SAPK/JNK pathway. J. Biol. Chem. 276:8328-8340. [DOI] [PubMed] [Google Scholar]
- 17.Fromm, L., W. Shawlot, K. Gunning, J. S. Butel, and P. A. Overbeek. 1994. The retinoblastoma protein-binding region of simian virus 40 large T antigen alters cell cycle regulation in lenses of transgenic mice. Mol. Cell. Biol. 14:6743-6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galetic, I., M. Andjelkovic, R. Meier, D. Brodbeck, J. Park, and B. A. Hemmings. 1999. Mechanism of protein kinase B activation by insulin/insulin-like growth factor-1 revealed by specific inhibitors of phosphoinositide 3-kinase—significance for diabetes and cancer. Pharmacol. Ther. 82:409-425. [DOI] [PubMed] [Google Scholar]
- 19.Gerba, C. P., D. M. Gramos, and N. Nwachuku. 2002. Comparative inactivation of enteroviruses and adenovirus 2 by UV light. Appl. Environ. Microbiol. 68:5167-5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guy, B., M. P. Kieny, Y. Riviere, C. Le Peuch, K. Dott, M. Girard, L. Montagnier, and J. P. Lecocq. 1987. HIV F/3′ orf encodes a phosphorylated GTP-binding protein resembling an oncogene product. Nature 330:266-269. [DOI] [PubMed] [Google Scholar]
- 21.Haslam, R. J., H. B. Koide, and B. A. Hemmings. 1993. Pleckstrin domain homology. Nature 363:309-310. [DOI] [PubMed] [Google Scholar]
- 22.Haynes, M. P., D. Sinha, K. S. Russell, M. Collinge, D. Fulton, M. Morales-Ruiz, W. C. Sessa, and J. R. Bender. 2000. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ. Res. 87:677-682. [DOI] [PubMed] [Google Scholar]
- 23.He, Y., H. Nakao, S. L. Tan, S. J. Polyak, P. Neddermann, S. Vijaysri, B. L. Jacobs, and M. G. Katze. 2002. Subversion of cell signaling pathways by hepatitis C virus nonstructural 5A protein via interaction with Grb2 and P85 phosphatidylinositol 3-kinase. J. Virol. 76:9207-9217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber, M., K. A. Watson, H. C. Selinka, C. M. Carthy, K. Klingel, B. M. McManus, and R. Kandolf. 1999. Cleavage of RasGAP and phosphorylation of mitogen-activated protein kinase in the course of coxsackievirus B3 replication. J. Virol. 73:3587-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huber, S. A., and P. A. Lodge. 1984. Coxsackievirus B-3 myocarditis in Balb/c mice. Evidence for autoimmunity to myocyte antigens. Am. J. Pathol. 116:21-29. [PMC free article] [PubMed] [Google Scholar]
- 26.Kawanishi, M., S. Tada-Oikawa, and S. Kawanishi. 2002. Epstein-Barr virus BHRF1 functions downstream of Bid cleavage and upstream of mitochondrial dysfunction to inhibit TRAIL-induced apoptosis in BJAB cells. Biochem. Biophys. Res. Commun. 297:682-687. [DOI] [PubMed] [Google Scholar]
- 27.Kodaki, T., R. Woscholski, B. Hallberg, P. Rodriguez-Viciana, J. Downward, and P. J. Parker. 1994. The activation of phosphatidylinositol 3-kinase by Ras. Curr. Biol. 4:798-806. [DOI] [PubMed] [Google Scholar]
- 28.Labrada, L., G. Bodelon, J. Vinuela, and J. Benavente. 2002. Avian reoviruses cause apoptosis in cultured cells: viral uncoating, but not viral gene expression, is required for apoptosis induction. J. Virol. 76:7932-7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee, Y. I., S. Kang-Park, and S. I. Do. 2001. The hepatitis B virus-X protein activates a phosphatidylinositol 3-kinase-dependent survival signaling cascade. J. Biol. Chem. 276:16969-16977. [DOI] [PubMed] [Google Scholar]
- 30.Liu, Y., Y. Wang, M. Yamakuchi, S. Masuda, T. Tokioka, S. Yamaoka, I. Maruyama, and I. Kitajima. 2001. Phosphoinositide-3 kinase-PKB/Akt pathway activation is involved in fibroblast Rat-1 transformation by human T-cell leukemia virus type I tax. Oncogene 20:2514-2526. [DOI] [PubMed] [Google Scholar]
- 31.Luo, H., B. Yanagawa, J. Zhang, Z. Luo, M. Zhang, M. Esfandiarei, C. Carthy, J. E. Wilson, D. Yang, and B. M. McManus. 2002. Coxsackievirus B3 replication is reduced by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway. J. Virol. 76:3365-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin, M. B., T. F. Franke, G. E. Stoica, P. Chambon, B. S. Katzenellenbogen, B. A. Stoica, M. S. McLemore, S. E. Olivo, and A. Stoica. 2000. A role for Akt in mediating the estrogenic functions of epidermal growth factor and insulin-like growth factor I. Endocrinology 141:4503-4511. [DOI] [PubMed] [Google Scholar]
- 33.Mason, J. W., J. B. O'Connell, A. Herskowitz, N. R. Rose, B. M. McManus, M. E. Billingham, T. E. Moon, et al. 1995. A clinical trial of immunosuppressive therapy for myocarditis. N. Engl. J. Med. 333:269-275. [DOI] [PubMed] [Google Scholar]
- 34.McCarthy, S. A., H. S. Symonds, and T. Van Dyke. 1994. Regulation of apoptosis in transgenic mice by simian virus 40 T antigen-mediated inactivation of p53. Proc. Natl. Acad. Sci. USA 91:3979-3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McManus, B. M., L. H. Chow, J. E. Wilson, D. R. Anderson, J. M. Gulizia, C. J. Gauntt, K. E. Klingel, K. W. Beisel, and R. Kandolf. 1993. Direct myocardial injury by enterovirus: a central role in the evolution of murine myocarditis. Clin. Immunol. Immunopathol. 68:159-169. [DOI] [PubMed] [Google Scholar]
- 36.Meier, R., D. R. Alessi, P. Cron, M. Andjelkovic, and B. A. Hemmings. 1997. Mitogenic activation, phosphorylation, and nuclear translocation of protein kinase Bbeta. J. Biol. Chem. 272:30491-30497. [DOI] [PubMed] [Google Scholar]
- 37.Peruzzi, F., M. Prisco, M. Dews, P. Salomoni, E. Grassilli, G. Romano, B. Calabretta, and R. Baserga. 1999. Multiple signaling pathways of the insulin-like growth factor 1 receptor in protection from apoptosis. Mol. Cell. Biol. 19:7203-7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ransone, L. J., and A. Dasgupta. 1989. Multiple isoelectric forms of poliovirus RNA-dependent RNA polymerase: evidence for phosphorylation. J. Virol. 63:4563-4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez-Viciana, P., P. H. Warne, R. Dhand, B. Vanhaesebroeck, I. Gout, M. J. Fry, M. D. Waterfield, and J. Downward. 1994. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature 370:527-532. [DOI] [PubMed] [Google Scholar]
- 40.Rong, R., Q. He, Y. Liu, M. S. Sheikh, and Y. Huang. 2002. TC21 mediates transformation and cell survival via activation of phosphatidylinositol 3-kinase/Akt and NF-kappaB signaling pathway. Oncogene 21:1062-1070. [DOI] [PubMed] [Google Scholar]
- 41.Rose, N. R., L. J. Wolfgram, A. Herskowitz, and K. W. Beisel. 1986. Postinfectious autoimmunity: two distinct phases of coxsackievirus B3-induced myocarditis. Ann. N. Y. Acad. Sci. 475:146-156. [DOI] [PubMed] [Google Scholar]
- 42.Rothmann, K., M. Schnolzer, G. Radziwill, E. Hildt, K. Moelling, and H. Schaller. 1998. Host cell-virus cross talk: phosphorylation of a hepatitis B virus envelope protein mediates intracellular signaling. J. Virol. 72:10138-10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandra, F., N. A. Matsuki, H. Takeuchi, T. Ikebe, T. Kanematsu, M. Ohishi, and M. Hirata. 2002. TNF inhibited the apoptosis by activation of Akt serine/threonine kinase in the human head and neck squamous cell carcinoma. Cell Signal. 14:771-778. [DOI] [PubMed] [Google Scholar]
- 44.Shaw, G. 1996. The pleckstrin homology domain: an intriguing multifunctional protein module. Bioessays 18:35-46. [DOI] [PubMed] [Google Scholar]
- 45.Tsuruta, F., N. Masuyama, and Y. Gotoh. 2002. The phosphatidylinositol 3-kinase (PI3K)-Akt pathway suppresses Bax translocation to mitochondria. J. Biol. Chem. 277:14040-14047. [DOI] [PubMed] [Google Scholar]
- 46.Vlahos, C. J., W. F. Matter, K. Y. Hui, and R. F. Brown. 1994. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J. Biol. Chem. 269:5241-5248. [PubMed] [Google Scholar]
- 47.Welch, H., A. Eguinoa, L. R. Stephens, and P. T. Hawkins. 1998. Protein kinase B and rac are activated in parallel within a phosphatidylinositide 3OH-kinase-controlled signaling pathway. J. Biol. Chem. 273:11248-11256. [DOI] [PubMed] [Google Scholar]
- 48.Yang, X., and D. Gabuzda. 1998. Mitogen-activated protein kinase phosphorylates and regulates the HIV-1 Vif protein. J. Biol. Chem. 273:29879-29887. [DOI] [PubMed] [Google Scholar]
- 49.Yang, X., and D. Gabuzda. 1999. Regulation of human immunodeficiency virus type 1 infectivity by the ERK mitogen-activated protein kinase signaling pathway. J. Virol. 73:3460-3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang, X., J. Goncalves, and D. Gabuzda. 1996. Phosphorylation of Vif and its role in HIV-1 replication. J. Biol. Chem. 271:10121-10129. [DOI] [PubMed] [Google Scholar]
- 51.Zhang, H. M., J. Yuan, P. Cheung, H. Luo, B. Yanagawa, D. Chau, N. Stephan-Tozy, B. W. Wong, J. Zhang, J. E. Wilson, B. M. McManus, and D. Yang. 2003. Overexpression of interferon-gamma-inducible GTPase inhibits coxsackievirus B3-induced apoptosis through the activation of the phosphatidylinositol 3-kinase/Akt pathway and inhibition of viral replication. J. Biol. Chem. 278:33011-33019. [DOI] [PubMed] [Google Scholar]
- 52.Zhu, H., Y. Shen, and T. Shenk. 1995. Human cytomegalovirus IE1 and IE2 proteins block apoptosis. J. Virol. 69:7960-7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zimmermann, S., and K. Moelling. 1999. Phosphorylation and regulation of Raf by Akt (protein kinase B). Science 286:1741-1744. [DOI] [PubMed] [Google Scholar]