Abstract
Addiction to cocaine produces long-lasting, stable changes in brain synaptic physiology that might contribute to the vulnerability to relapse. In humans, exposure to environmental contexts previously paired with drug use precipitates relapse, but the neurobiological mechanisms mediating this relapse are unknown. Initiation of cocaine relapse via re-exposure to a drug-associated context elicited reinstatement of cocaine seeking as well as rapid, transient synaptic plasticity in the nucleus accumbens core (NAcore), measured as an increase in dendritic spine diameter. These results show that rapid context-evoked synaptic potentiation in the NAcore may underpin relapse to cocaine use.
Keywords: Context-induced relapse, cocaine, synaptic potentiation, dendritic spines, nucleus accumbens core
Introduction
Chronic use of cocaine produces enduring neuroadaptations in the corticostriatal brain circuitry involved in the synaptic plasticity contributing to learning behavioral tasks, including morphological changes in dendritic spines of medium spiny neurons (MSNs) of the nucleus accumbens (NA) (Dumitriu et al., 2012; Gipson et al., 2013; Shen et al., 2009). These adaptations are thought to impair the ability of the NA to process information, adaptively regulate reward-seeking behaviors, and thereby contribute to relapse to drug use in substance use disorders. Contextual stimuli associated with drug exposure can serve as occasion setters and initiate craving (Childress et al., 1993) and relapse (Crombag et al., 2008) to drug seeking that is associated with activating the glutamatergic projection from the prefrontal cortex to the nucleus accumbens (Koob and Volkow, 2010).
Contingent cue-induced reinstatement of cocaine seeking produces rapid, transient changes in spine head diameter (dh) of MSNs in the nucleus accumbens core (NAcore) (Gipson et al., 2013). Although discrete, contingent drug-paired conditioned stimuli provoke relapse of drug seeking and elicit synaptic potentiation in NAcore, it is unknown if non-contingent environmental contextual cues associated with the rewarding effects of cocaine also potentiate NAcore MSNs. Thus, the primary objective of the present study was to test the hypothesis that re-exposure to non-contingent drug-paired contextual cues via a contextual renewal paradigm elicits simultaneous cocaine seeking behavior and rapid, transient potentiation of NAcore dendritic spines. To this end, an adapted A-B-A renewal procedure (Bouton and Bolles, 1979; Crombag and Shaham, 2002) of contextual cocaine seeking was employed in which cocaine self-administration occurred in one environment (containing distinct visual, auditory, olfactory, and tactile cues). The animals then underwent extinction training in a saliently distinct environment. Contextual renewal of cocaine seeking was elicited by returning the animal to the original cocaine-paired context, and NAcore synaptic strength was estimated by measuring dendritic spine diameter and density.
Materials and Methods
(See Appendix S1 for Details)
Male Sprague Dawley rats (250 g; Charles River Laboratories) were individually housed with a 12:12 hr dark/light cycle. All experimentation occurred in the dark cycle. Rats received food ad libitum until the day prior to behavioral training, after which food restriction (20 g of rat chow per day) was implemented and maintained throughout the experiment. Rats were allowed 1 week to acclimate to the vivarium before inducing anesthesia and implanting indwelling jugular catheters. All procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Assessment and Accreditation of Laboratory Animal Care.
Cocaine self-administration and extinction training was conducted in one of two environmental contexts (Context A and Context B) which differed in visual, auditory, olfactory and tactile stimuli (see Table S1). Animals were randomly assigned to receive either A-B-A or B-A-B training conditions. Daily 2-hr cocaine self-administration or yoked saline sessions began seven days after surgery, using a Fixed Ratio 1 schedule with a 20-second time out. Active lever presses yielded a 0.2 mg infusion of cocaine as well as a light and tone stimulus. Following 10 consecutive self-administration sessions (≥10 infusions/day), rats began daily extinction training in the alternate context for 14 sessions where an active lever press yielded no programmed consequence. Some rats were immediately taken for spine analysis just before a renewal session (T=0). Renewal was induced by placing the animal back into its original cocaine-paired context for either 15 or 45 minutes, after which rats were anesthetized and transcardially perfused for spine analysis. Yoked saline animals were either placed back in the saline-paired context for 45 min or immediately taken for spine analysis (T=0).
Detailed procedures for dendritic spine procedures are published (Shen et al., 2009). Briefly, a confocal microscope was used to image DiI-labeled sections. Images of DiI-labeled dendrites (Fig. 1b) were acquired via optical sectioning using a 63× oil immersion objective (Plan-Apochromat, Zeiss; NA = 1.4, WD = 90 μm). Images were deconvoluted prior to analysis, and a 3-D perspective was rendered by Imaris software package (Bitplane; Saint Paul, MN). Only spines on dendrites beginning at >75 μm and ending at ≤ 200 μm distal to the soma and after the first branch point were quantified from cells localized to the NAcore. The length of quantified dendrites was 45–55 μm. Five-12 neurons were analyzed from each animal, and the minimum end segment diameter (spine head) was set at ≥0.143 μm. All spine density and dh data were statistically analyzed after averaging the values for all the neurons in each animal. Behavioral data were analyzed using repeated-measures ANOVA, and a Bonferroni-corrected post hoc t test.
Figure 1. Contextual reinstatement of cocaine-seeking rapidly enlarges spine head diameter in NAcore MSNs.
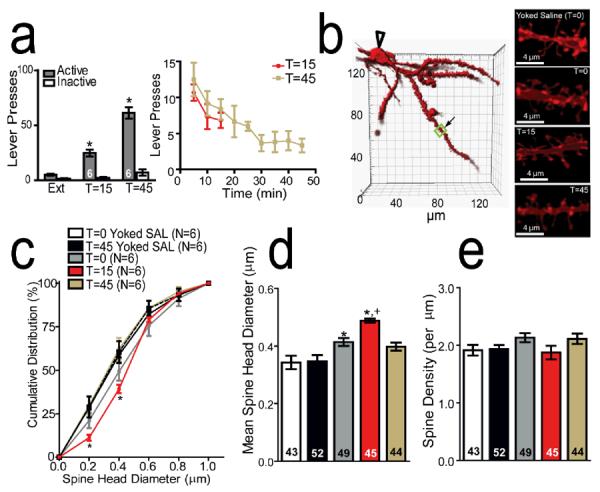
(a) Contextual reinstatement of cocaine-seeking increased active lever pressing at 15 or 45 min prior to euthanizing rats for spine measurements. Time course of active lever pressing during the cue reinstatement session. (b) Example of NAcore medium spiny neuron. Clear arrow - location of soma; boxed region - segment analyzed from a T=0 animal. Sample dendrites from NAcore spiny cells in yoked saline (dh= 0.342 μm) or cocaine-trained rats at T=0 (0.411 μm), T=15 (0.498 μm), or T=45 (0.399 μm) min after contextual reinstatement. (c) Cumulative dh frequency plot. (d) Cocaine self-administration increased dh at T=0 compared to yoked saline. Spine dh was further elevated at T=15, and returned to pre-reinstatement levels at T=45. (e) No change in spine density was found between groups.
*p< 0.05, compared to yoked saline or extinction lever presses, +p< 0.05, compared to T=0 cocaine.
Results
Drug-paired contextual stimuli elicited robust active lever pressing compared to inactive lever pressing during extinction at T=15 and T=45 (F(5,41)= 47.27, P< 0.001; Fig 1a). Akin to the time course of reinstatement to discrete conditioned cues (Gipson et al., 2013), the increase in lever pressing was maximal during the first 10 min of the session, and progressively decreased for the remainder of the session. Cumulative distribution of dh revealed a rightward shift at T=0, and a further shift at T=15 compared to yoked saline (two-way ANOVA, group: F(5,1721)= 7046, P< 0.0001; dh F(4,1721)= 70.57, P< 0.0001; interaction: F(20,1721)= 18.63, P< 0.0001; Fig 1c). At T=0, dh was increased in rats extinguished from cocaine self-administration (0.414 ± 0.014 μm) compared to yoked saline animals at both T=0 (0.343 ± 0.023 μm) and T=45 (0.347 ± 0.022 μm), supporting long-lasting synaptic potentiation after cocaine withdrawal (Figs 1c/d) (Wolf, 2010). Mean dh was further increased at T=15 (0.488 ± 0.007 μm), and returned to pre-reinstatement levels by T=45 (0.398 ± 0.014 μm; one-way ANOVA; F(4,233) = 10.20, p < 0.0001). No change in spine density was found between groups (Fig 1e).
Discussion
The present study shows that akin to discrete cue-induced reinstatement of cocaine seeking (Gipson et al., 2013), initiation of context-induced cocaine relapse elicits rapid, transient increases in NAcore dh. In addition to the NAcore, the nucleus accumbens shell (NAshell) also plays an important role in context-induced cocaine relapse, as both accumbens subregions have been found to facilitate context-induced motivation to seek cocaine (Fuchs et al., 2008). Thus, in the future it will be of interest to examine if rapid plasticity also occurs in the NAshell in context-induced cocaine relapse. Given the importance of environmental contexts previously associated with drug use in precipitating relapse, the current findings extend the possibility that targeting the rapid LTP-like plasticity underlying initiation of relapse to drug seeking may have potential relevance to relapse prevention and pharmacotherapy development.
Supplementary Material
Acknowledgements
We thank Brenton Mahaffey, Charles Thomas, and Megan Hensley-Simon for technical assistance. This work was supported by DA033690 (CDG), and DA03906, DA012513 and DA015369 (PWK) grants from the National Institutes of Health.
References
- Bouton ME, Bolles RC. Role of conditioned contextual stimuli in reinstatement of extinguished fear. Journal of experimental psychology Animal behavior processes. 1979;5:368–378. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O'Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116:169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Laplant Q, Grossman YS, Dias C, Janssen WG, Russo SJ, Morrison JH, Nestler EJ. Subregional, dendritic compartment, and spine subtype specificity in cocaine regulation of dendritic spines in the nucleus accumbens. J Neurosci. 2012;32:6957–6966. doi: 10.1523/JNEUROSCI.5718-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Ramirez DR, Bell GH. Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2008;200:545–556. doi: 10.1007/s00213-008-1234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013;77:867–872. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci. 2009;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci. 2010;33:391–398. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


